Abstract
Multiple sclerosis (MS) is chronic neuroinflammatory condition associated with significant disability. The economic burden of MS is substantial, and high and rising disease-modifying therapy (DMT) prices are the single largest drivers of healthcare expenditures. Over much of the last decade, price increases for most DMTs have surpassed 10% annually. Currently, many MS DMTs exceed US$90,000 a year and their economic value is widely debated. In addition to creating a financial burden for the healthcare system, high DMT costs negatively impact patients through unaffordable out-of-pocket costs and excessive restrictions by insurance companies. The objective of this narrative review is to summarize economic issues related to MS DMTs, including trends in pricing, relative value, and effects on patient care in the United States.
Keywords: pharmacoeconomics, multiple sclerosis, disease-modifying therapies
Introduction
Multiple sclerosis (MS) is a chronic neuroinflammatory disease that leads to central nervous system demyelination, neurologic deficits, and disability. Other clinical symptoms of MS include fatigue, gait difficulties, sensory disturbances, paresthesia, spasticity, pain, and depression.1 There are several different phenotypic subtypes of MS; however, relapsing-remitting MS is the most prevalent. Relapsing forms of MS are characterized by discrete exacerbations that fully or partially resolve. Most individuals with relapsing-remitting MS will convert to a progressive form of MS with continuous disability progression (secondary progressive MS). A smaller proportion (10%) of patients will manifest initially with a primary progressive form that is characterized by progressively worse disability without pronounced remissions. Ultimately, MS is progressive disease with no cure that is a leading cause of non-traumatic disability.
MS is one of the most prevalent progressive neurologic disorders worldwide.2 The incidence of MS is highest for young adults between 20 and 50 years of age.3 Because MS affects individuals early in their life, it has profound consequences on individuals and society. Moreover, MS is a leading cause of non-traumatic disability and results in significant lost productivity, absenteeism, and early retirement.4 About half of patients are unable to perform daily household activities or maintain daily employment within 10 years of onset.5 Over a lifetime, the cumulative costs of MS can exceed $5.6 million [2020 United States (US) dollars] in both direct and indirect costs.6
In addition to costs related loss of productivity, the direct healthcare costs of MS are also substantial. The lifetime direct medical costs of MS are estimated to be $4.8 million (2020 US dollars), making MS the second most expensive chronic condition behind heart failure.7,8 Studies suggest healthcare spending can exceed $68,000 per year (2020 US dollars).7,9
The introduction and proliferation of disease-modifying therapies (DMTs) for MS have revolutionized the prognosis for patients. Despite a growing array of DMT options for patients and neurologists, enthusiasm for these drugs has been diminished because of rising costs. Nationally, prescriptions drugs account for 10–15% of total healthcare spending. However, for individuals with MS, spending on pharmaceuticals accounts for more than two-thirds of total healthcare expenditures.7,9,10 DMTs constitute the single greatest driver of pharmaceutical and total healthcare spending for MS. From 2009 to 2015, total healthcare costs per person nearly doubled from $23,900 to $39,600 annually between 2009 and 2015; MS DMTs spending accounted for 82% of this increase.10 In contrast, spending on inpatient and outpatient professional services increased 11% during this period, and constituted less than 30% of total MS-related healthcare expenditures by 2015.
Trends in MS DMT costs
Prices for MS DMTs, which are among the costliest of all medications by disease category, have increased rapidly over the last several decades.11 The US healthcare system spent $18.8 billion on MS DMTs in 2018 – seventh highest by therapeutic class overall.12 Because the incidence of MS is highest among working age individuals, spending on MS DMTs ranks fifth by therapeutic class for employer-sponsored insurance programs.13 However, because of eligibility through disability or old age, the US Medicare program is the single largest purchaser of MS DMTs in the US, covering 25% to 30% of individuals with MS.14 The Medicare Part D program, which pays for outpatient prescription drugs, spent over $5 billion on MS DMTs in 2017. In contrast, the Medicare Part B program, which pays for clinical services from physicians and other providers, paid $1.5 billion to neurologists for clinical services during the same year.15
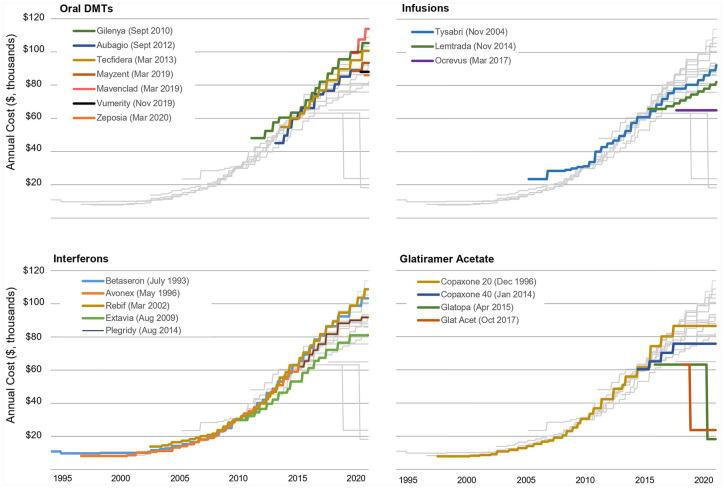
Aggregate spending on DMTs has risen as a consequence of both increasing prices and the introduction of new DMTs.16 The first DMT (interferon beta-1b; Betaseron™) was approved by the US Food and Drug Administration (FDA) in 1993 at an annual cost of $19,509 (2020 US dollars). Since that time, the class has grown substantially in size and diversity. There are currently 20 FDA-approved DMTs that are indicated for several types of MS and differ by route of administration (oral, self-administered injection, infused), efficacy, safety, and tolerability. Despite increases in options and diversity, DMT prices have escalated at rates many times higher than the inflation rate since the early 2000s. In Table 1 and Figure 1, I summarize changes in unadjusted annual price (along with historic inflation rates during the period) for MS DMTs between 1997 and 2020. Between 1993 and 2001, annual prices for available interferon and glatiramer acetate platform therapies were relatively stable. Interferon beta-1a SC (Rebif™) was introduced in 2002 with an acquisition price 30% above the next most expensive DMT – interferon beta-1b. In the following years (2002–2005), the median annual price increased by 11.3% per year. A similar pattern followed the introduction of the first infused DMT natalizumab (Tysabri™) in 2006 and first oral DMT fingolimod (Gilenya™) in 2010. The annual rate of increase has slowed to 5% per year since 2017, perhaps due to increased public and political scrutiny. The introduction of ocrelizumab (Ocrevus™) in 2017 at a relatively lower price, along with a reduction in the price of glatiramer acetate in 2018, may have contributed to this moderation in yearly price increases. Despite this slow down, the median annual cost for available MS DMTs in 2020 is $91,835, and several products exceed $100,000 per year.
Table 1.
Annual price changes for MS DMTs. Annual prices estimated using wholesale acquisition cost (first DataBank). Inflation adjustment and estimates from US bureau of labor statistics CPI.
| DMT (approval year) | Approval annual price per patient (2020 US dollars) | 2020 current annual price per patientc | 1997–2001 | 2002–2005 | 2006–2009 | 2010–2016 | 2017–2020 |
|---|---|---|---|---|---|---|---|
| CPI Inflation | 1.7% | 2.8% | 2.4% | 1.6% | 2.1% | ||
| Branded products | |||||||
| Interferon beta-1b (1993) | $19,509 | $103,302 | 0.0% | 11.5% | 17.8% | 15.2% | 6.9% |
| Interferon beta-1a (1996) | $13,608 | $91,835 | 0.0% | 11.3% | 18.2% | 16.1% | 2.0% |
| Glatiramer acetate (1996) | $12,772 | $86,554 | 4.7% | 18.9% | 17.5% | 14.9% | 0.0% |
| Interferon beta-1a SC (2002) | $20,019 | $103,647 | 9.6% | 13.7% | 15.6% | 4.8% | |
| Natalizumab (2006)a | $31,740 | $89,074 | 0.0% | 7.9% | 11.3% | 3.5% | |
| Interferon beta-1b (2009)b | $35,668 | $81,079 | 14.4% | 6.0% | |||
| Fingolimod (2010) | $56,784 | $105,390 | 9.0% | 5.5% | |||
| Teriflunomide (2012) | $50,304 | $93,296 | 16.6% | 5.0% | |||
| Dimethyl fumarate (2013) | $60,677 | $100,690 | 13.4% | 6.0% | |||
| Glatiramer 40 mg (2014) | $66,541 | $75,816 | 7.9% | 0.0% | |||
| Peginterferon beta-1a (2014) | $67,283 | $91,835 | 14.8% | 2.0% | |||
| Alemtuzumab (2014) | $71,916 | $80,320 | 2.5% | 5.1% | |||
| Ocrelizumab (2017) | $68,778 | $65,000 | 0.0% | ||||
| Siponimod (2019) | $89,812 | $93,367 | 5.5% | ||||
| Cladribine (2019) | $100,975 | $107,460 | 8.0% | ||||
| Diroximel fumarate (2019) | $88,000 | $88,000 | NA | ||||
| Median | $58,731 | $91,835 | 0.0% | 11.3% | 17.5% | 14.6% | 5.0% |
| Generic products | |||||||
| Glatopa 20 mg (2015) | $68,901 | $18,250 | −71.1% | ||||
| Glatopa 40 mg (2018) | $67,793 | $19,500 | −70.2% | ||||
| Gen glatiramer 20 (2017) | $66,090 | $23,725 | −62.5% | ||||
| Gen glatiramer 40 (2017) | $68,432 | $25,350 | −61.3% | ||||
Natalizumab was originally approved in 2004, but withdrawn after 2 months to evaluate progressive multifocal leukoencephalopahy risks. It was reintroduced in June 2006.
Novartis’ Extavia™.
January 2020.
CPI, consumer price index; DMT, disease-modifying therapies; MS, multiple sclerosis; NA, not applicable; US, United States.
Figure 1.
Trends in annual price for DMTs for MS by class; 1997–2020. Unadjusted annual price estimated from wholesale acquisition costs (First Databank). The annual price for Lemtrada is based on four 12 mg vials [Package insert dosing: 12 mg/day (5 vials) for five consecutive days in first year; 12 mg/day (3 vials) for 3 days in year 2]. Market introduction date in parenthesis. Updated 12 August 2020 (data through July 2020).
DMT, disease-modifying therapies; MS, multiple sclerosis.
A common critique of reports of drug pricing is that they typically do not reflect net costs to payers because of proprietary discounts and rebates provided by drug manufactures. Analyses that have adjusted for these secret discounts only partially offset the cost of rapidly rising prices for MS DMTs, and net costs are still increasing at rates that exceed 10% per year, substantially more than other therapeutic classes.17,18
Adverse consequences of DMT affordability
The escalating costs of MS DMTs can negatively affect patient care in multiple ways. First, cost-related insurance company exclusions and limitations often create significant access barriers for patients.19 In a recent survey, more than one-third of patients with MS report struggles in getting their DMT covered because of insurance company restrictions.20 Further, nearly half of respondents noted that they have altered how they take their DMT (e.g., skipped doses, delayed treatment) and changed other lifestyle choices (e.g., spend less on entertainment) because of high DMT costs.20 More than half of respondents indicated they were very concerned about being able to afford their DMT in the near future.
Insurance companies often operate through pharmacy benefit managers (PBM), who are middlemen in the drug distribution chain and negotiate contracts between payers (insurers, employers), drug companies and wholesalers, and pharmacies. As part of these negotiations, PBMs employ strategies such as formulary exclusions, prior authorizations (PA), and step therapy to manage utilization and costs for payers. The most prevalent utilization management strategy is PA, which involves requiring patients meet certain clinical criteria as prerequisite for payment. Within the Medicare program, coverage of MS DMTs has declined from near universal coverage of interferon or glatiramer-based DMT in 2007 to rates of coverage between 54% and 89% in 2016.21 Over the same period, Medicare drug plans (Part D) also increased the use of PA or step therapy policies. Although there are no studies among individuals with MS, systematic reviews clearly and consistently show PAs and other coverage restrictions are detrimental to medication adherence and worsen clinical outcomes across a number of clinical conditions.22–24 Delays or treatment interruptions likely contribute to adverse clinical outcomes because evidence from randomized clinical trials show that timely and ongoing treatment with DMT in patients with relapsing forms of MS can reduce relapse rates, decrease inflammatory brain lesions, and slow (but not stop or reverse) disability progression.25–29
In addition to insurance company restrictions, patients are increasingly required to pay significant amounts out-of-pocket (OOP) for their medications. The most common manifestation of prescription drug cost-sharing arrangements include copayments, which are set dollar amounts charged per prescription, and co-insurance, where patients pay a proportion of the cost of the prescription. Co-insurance models are especially problematic because the percentage that patients are required to pay OOP is related directly to the drug’s list price, before any negotiated rebates or discounts. Some data suggest that OOP costs might be one of the most important features that affect DMT preferences. Using conjoint analyses, Hincapie et al. found that OOP costs were the most important factor patients considered when selecting a MS DMT, surpassing other medication attributes such as route, frequency of administration, and efficacy.30
For patients enrolled in Medicare, OOP for high-priced specialty medications such as those used for MS can be particularly high. This is because of Part D’s unique benefit structure where OOP costs are incurred year-round without an OOP maximum.31 Annual OOP costs for Medicare beneficiaries using a DMT can exceed $6000 a year.32 OOP costs for patients with MS are also rising among other coverage groups. In a study of patients insured through United Healthcare from 2004 to 2016, Callaghan et al. estimated changes in OOP costs for patients with MS, and several other neurologic conditions and found the mean OOP cost increased more than 20-fold from $15 to $309 per month; the mean cumulative OOP was $2238 in the 2 years following initial diagnosis.33 The authors also noted that OOP costs exceeded $600 a month in 2016 for patients enrolled in high-deductible plans, which are increasingly common.33,34
A growing body of evidence consistently indicates that cost-sharing can have a negative effect on DMT adherence.20,35–39 Among commercially insured patients with MS, Romley et al. found that those facing high cost-sharing amounts were 12.7% less likely to initiative a DMT in the 2 years following initial diagnosis relative to those without cost-sharing.35 Gleason et al. also show that patients with OOP costs exceeding $200 had a seven-fold increased odds of abandoning their prescription at the pharmacy relative to those who faced OOP costs under $100.36 Other studies in both commercial and Medicare populations with MS have consistently shown higher OOP costs are associated with reduced ongoing DMT adherence.37–39 Although there are no studies that quantify the effects of DMT cost-sharing on MS-related outcomes, several studies have shown that sub-optimal DMT adherence associates with higher rates of MS-relapse, increased hospitalizations and emergency department visits, and higher medical costs.40–45
In addition to issues of affordability, the value of DMTs has also been questioned. Value in healthcare is typically measured using cost-effectiveness analyses. Cost-effectiveness studies use a methodology that seeks to quantify both the magnitude of health benefits and costs for competing healthcare interventions to determine which option is most economically efficient – that is, which intervention results in the largest health gains per dollar spent. Because assessments of health gains differ by clinical condition (e.g., reduced disability, years of life extended, improved quality of life), cost-effectiveness studies often employ a metric termed the quality-adjusted life year (QALY) as a common denominator capturing both quantity and quality of life changes. Cost-effectiveness analyses are often based on mathematical decision models which are used to simulate lifetime costs and benefits (QALYs gained or losses) of two or more interventions to estimate the incremental cost-effectiveness ratio (ICER) of one option relative to another. In the US, ICERs less than $150,000 per QALY are typically economically attractive.46,47
Although the use cost-effectiveness is common in other industrialized countries, its use to support policy or coverage decision has been slow to gain traction in the US. Resistance to the use of cost-effectiveness data in the US has lagged other parts of the globe for several reasons.48,49 Unlike most industrialized countries, the US lacks a centralized single payer system to demand such economic data. In the US, where healthcare industries are less constrained by government regulation, there is a strong political aversion to the adoption of financial parameters for allocating healthcare resources. There is also an ingrained cultural resistance to explicitly acknowledging cost constraints in healthcare among patients, clinicians, and policy makers. Although the US lacks a centralized governmental health technology appraisal program, the non-profit Institute for Clinical and Economic Review was established in 2006 with the aim of conducting objective and transparent evidence and economic appraisals sensitive to the needs of a wide variety of stakeholders across the healthcare system. Its reports influence coverage decisions for many large payers, such as the Department of Veterans Affairs, some state Medicaid programs, and certain insurers.50
In 2017, the Institute published a comprehensive clinical evidence appraisal and economic analysis for DMTs for relapsing (RRMS) and primary progressive (PPMS) MS.51 Their analysis evaluated the lifetime effectiveness and costs of 14 DMTs relative to best supportive care using a validated model of disability progression and relapse (RRMS only) to estimate the value of each DMT.51 Because the acquisition costs for ocrelizumab were not yet disclosed at the time of the report, separate analyses were performed to estimate a cost that would make this DMT good value (<$150,000/QALY) for both RRMS and PPMS. For RRMS, the effect of each DMT on disability progression and relapse rates was based on a network meta-analysis indicating that alemtuzumab and natalizumab were the most effective [relative risk (RR) for disability progression 0.47 to 0.56, relapse rate 0.28–0.65], followed by fingolimod and dimethyl fumarate (RR for disability progression 0.62–0.68, relapse rate 0.46–0.53), and finally teriflunomide, glatiramer, and interferons (RR for disability progression 0.63–0.86, relapse rate 0.63–0.83). Costs included annual acquisition costs of DMTs (including payer discounts and rebates and administrative costs for infusion), costs for laboratory and clinic visits for monitoring, disability-related costs, and second-line treatment assuming discontinuation. A secondary analysis also considered indirect societal costs related to disability and reduced workplace productivity. Alemtuzumab was the only DMT to yield an economically attractive ICER, at $38,277 per QALY ($48,787 per relapse avoided). Incremental costs per QALYs for the other DMTs ranged from $183,240/QALY for interferon beta-1b (Extavia™) to $355,115/QALY for interferon beta 1a IM (Rebif™). Costs per relapse avoided ranged $227,149 for natalizumab to $942,036 for interferon beta-1a (Avonex™). For ocrelizumab, net acquisition costs would need to be $58,608 and $14,367 for RRMS and PPMS, respectively, to achieve a cost per QALY under $150,000; both estimates fall below the current acquisition price for this drug. A follow-up report determined the cost-effectiveness of siponimod for secondary progressive MS to $1.15 million per QALY.52 The Institute’s review largely comports with other older US-based economic analyses, which commonly report ICERs exceeding $150,000 per QALY.53,54 In sum, these data strongly suggest that, with the exception of alemtuzumab and ocrelizumab, most MS DMTs are overpriced relative to the health benefits they deliver to patients.
Summary
The high cost of prescription drugs is a uniquely American problem and reflects the confluence of a relatively unregulated, purposely opaque consumer market and an inherently high-risk, and high reward, industry. Addressing the root cause will likely require large structural changes to the way pharmaceuticals in the US are purchased. Policies enacted at both the state and federal level being considered include pricing transparency reforms, bulk purchasing, caps on price increases, limits on patient OOP costs, importation from other countries, and enhanced federal authority to negotiate directly with industry for lower prices.55 There are also actions that individual neurologists can take. First, there may be opportunities to reduce costs for patients by preferentially lower cost DMTs where clinically appropriate. Several generic versions of glatiramer acetate are now priced considerably lower than most other DMTs. The use of the monoclonal anti-CD20 antibody rituximab is an effective, but substantially less expensive, alternative to ocrelizumab.56,57 Neurologists can also work with professional organizations on advocacy efforts to reduce the burden of high prescription drug costs for patients. For instance, the American Academy of Neurology has designated prescription drug pricing as a priority issue in 2020.58
High and rising costs for MS DMTs are a major concern for neurologists, payers, patients, and society. Although DMTs can be life-changing for patients with MS, the costs of these drugs continue to escalate at rates well beyond inflation and affordably is a major challenge. For many with MS, high DMT costs lead to considerable anxiety and emotional distress about their ability to access these medications in a timely manner. Additionally, excessive OOP costs can often deter initiation and continued adherence which can increase the risk for relapse and subsequent disability progression. Issues of affordability are compounded by the fact that cost-effectiveness studies have generally shown that clinical benefits delivered by DMTs do not appear to justify their high costs.
Footnotes
Conflict of interest statement: Research support from AbbVie Pharmaceuticals Research and consulting from NMSS.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Daniel M. Hartung  https://orcid.org/0000-0002-0685-773X
https://orcid.org/0000-0002-0685-773X
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019; 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodin DS. The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol 2014; 122: 231–266. [DOI] [PubMed] [Google Scholar]
- 4. Kobelt G, Berg J, Atherly D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006; 66: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 5. Carroll CA, Fairman KA, Lage MJ. Updated cost-of-care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Serv Res 2014; 14: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whetten-Goldstein K, Sloan FA, Goldstein LB, et al. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler J 1998; 4: 419–425. [DOI] [PubMed] [Google Scholar]
- 7. Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ 2013; 16: 639–647. [DOI] [PubMed] [Google Scholar]
- 8. Owens GM, Olvey EL, Skrepnek GH, et al. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm 2013; 19(1 Suppl. A): S41–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim Y, Krause TM, Blum P, et al. Disease modifying therapies continue to drive up health care cost among individuals with multiple sclerosis. Mult Scler Relat Disord 2019; 30: 69–75. [DOI] [PubMed] [Google Scholar]
- 10. Johnson B. The rising cost of specialty drugs drove spending increases for people with multiple sclerosis. Washington, DC: Health Care Cost Institute, 2018. [Google Scholar]
- 11. Hartung DM, Bourdette DN, Ahmed SM, et al. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology 2015; 84: 2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aitken M. Medicine use and spending in the U.S. a review of 2018 and outlook to 2023, https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. (2019, accessed 19 January 2021).
- 13. Cubanski J, Rae M, Young K, et al. How does prescription drug spending and use compare across large employer plans, Medicare Part D, and Medicaid? San Francisco, CA: Kaiser Family Foundation, 2019. [Google Scholar]
- 14. NMSS. Medicare. National Multiple Sclerosis Society, https://www.nationalmssociety.org/Living-Well-With-MS/Work-and-Home/Insurance-and-Financial-Information/Health-Insurance/Medicare (2020, accessed 29 June 2020). [Google Scholar]
- 15. CMS Part D Public Use File. Baltimore, MD: U.S. Centers for Medicare & Medicaid Services; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data (2017, accessed 19 January 2021). [Google Scholar]
- 16. Petruzzo M, Palladino R, Nardone A, et al. The impact of diagnostic criteria and treatments on the 20-year costs for treating relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2020; 38: 101514. [DOI] [PubMed] [Google Scholar]
- 17. Hernandez I, San-Juan-Rodriguez A, Good CB, et al. Changes in list prices, net prices, and discounts for branded drugs in the US, 2007-2018. JAMA 2020; 323: 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Examination of Health Care Cost Trends and Cost Drivers. Commonwealth of Massachusetts, Office of the Attorney General, 2016. [Google Scholar]
- 19. Bourdette D, Patti F. US health insurance is an obstacle to disease-modifying treatments in MS. Neurology 2016; 87: 346–347. [DOI] [PubMed] [Google Scholar]
- 20. Quantifying the Effect of the High Cost of DMTs. National Multiple Sclerosis Society, https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Advocacy/NMSS-Research-Report-Full-Access-to-MS-Medications.pdf (2019, accessed 17 June 2020).
- 21. Hartung DM, Johnston KA, Irwin A, et al. Trends in coverage for disease-modifying therapies for multiple sclerosis in medicare Part D. Health Aff (Millwood) 2019; 38: 303–312. [DOI] [PubMed] [Google Scholar]
- 22. Happe LE, Clark D, Holliday E, et al. A systematic literature review assessing the directional impact of managed care formulary restrictions on medication adherence, clinical outcomes, economic outcomes, and health care resource utilization. J Manag Care Spec Pharm 2014; 20: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergeson JG, Worley K, Louder A, et al. Retrospective database analysis of the impact of prior authorization for type 2 diabetes medications on health care costs in a medicare advantage prescription drug plan population. J Manag Care Pharm 2013; 19: 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seabury SA, Goldman DP, Kalsekar I, et al. Formulary restrictions on atypical antipsychotics: impact on costs for patients with schizophrenia and bipolar disorder in Medicaid. Am J Manag Care 2014; 20: e52–e60. [PubMed] [Google Scholar]
- 25. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 26. Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 2018; 90: 789–800. [DOI] [PubMed] [Google Scholar]
- 27. Hartung HP, Graf J, Kremer D. Long-term follow-up of multiple sclerosis studies and outcomes from early treatment of clinically isolated syndrome in the BENEFIT 11 study. J Neurol 2020; 267: 308–316. [DOI] [PubMed] [Google Scholar]
- 28. Lizak N, Lugaresi A, Alroughani R, et al. Highly active immunomodulatory therapy ameliorates accumulation of disability in moderately advanced and advanced multiple sclerosis. J Neurol Neurosurg Psychiatry 2017; 88: 196–203. [DOI] [PubMed] [Google Scholar]
- 29. Chalmer TA, Baggesen LM, Nørgaard M, et al. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol 2018; 25: 1262–e110. [DOI] [PubMed] [Google Scholar]
- 30. Hincapie AL, Penm J, Burns CF. Factors associated with patient preferences for disease-modifying therapies in multiple sclerosis. J Manag Care Spec Pharm 2017; 23: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trish E, Xu J, Joyce G. Medicare beneficiaries face growing out-of-pocket burden for specialty drugs while in catastrophic coverage phase. Health Aff (Millwood) 2016; 35: 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hartung DM, Johnston K, Irwin A, et al. Trends in coverage for disease modifying therapies for multiple sclerosis in medicare Part D. Paper presented at AcademyHealth Annual Research Meeting, 24 June 2018, Seattle, WA. [Google Scholar]
- 33. Callaghan BC, Reynolds E, Banerjee M, et al. Out-of-pocket costs are on the rise for commonly prescribed neurologic medications. Neurology 2019; 92: e2604–e2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esper GJ, Hartung D, Avitzur O. The patient protection and affordable care act and chronic neurological illnesses: benefits and challenges. JAMA Neurol 2015; 72: 739–740. [DOI] [PubMed] [Google Scholar]
- 35. Romley J, Goldman D, Eber M, et al. Cost-sharing and initiation of disease-modifying therapy for multiple sclerosis. Am J Manag Care 2012; 18: 460–464. [PubMed] [Google Scholar]
- 36. Gleason PP, Starner CI, Gunderson BW, et al. Association of prescription abandonment with cost share for high-cost specialty pharmacy medications. J Manag Care Pharm 2009; 15: 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shao H, Stoecker C, Monnette AM, et al. Cost sharing of Disease-Modifying Treatments (DMTs) as policy lever to improve DMTs’ access in multiple sclerosis. Value Health 2018; 21: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 38. Dor A, Lage MJ, Tarrants ML, et al. Cost sharing, benefit design, and adherence: the case of multiple sclerosis. Adv Health Econ Health Serv Res 2010; 22: 175–193. [DOI] [PubMed] [Google Scholar]
- 39. Li P, Hu T, Yu X, et al. Impact of cost-sharing increases on continuity of specialty drug use: a quasi-experimental study. Health Serv Res. Epub ahead of print 24 July 2017. DOI: 10.1111/1475-6773.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen BA, Coyle PK, Leist T, et al. Therapy optimization in multiple sclerosis: a cohort study of therapy adherence and risk of relapse. Mult Scler Relat Disord 2015; 4: 75–82. [DOI] [PubMed] [Google Scholar]
- 41. Ivanova JI, Bergman RE, Birnbaum HG, et al. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ 2012; 15: 601–609. [DOI] [PubMed] [Google Scholar]
- 42. Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 2010; 30: 89–100. [DOI] [PubMed] [Google Scholar]
- 43. Oleen-Burkey MA, Dor A, Castelli-Haley J, et al. The relationship between alternative medication possession ratio thresholds and outcomes: evidence from the use of glatiramer acetate. J Med Econ 2011; 14: 739–747. [DOI] [PubMed] [Google Scholar]
- 44. Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011; 28: 51–61. [DOI] [PubMed] [Google Scholar]
- 45. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res 2017; 9: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-QALY threshold. New Eng J Med 2014; 371: 796–797. [DOI] [PubMed] [Google Scholar]
- 47. 20-2023 Value Assessment Framework. Institute for Clinical and Economic Review, https://icer.org/our-approach/methods-process/value-assessment-framework/ (2020, accessed 19 January 2021). [Google Scholar]
- 48. Neumann PJ, Greenberg D. Is the United States ready for QALYs? Health Aff (Millwood) 2009; 28: 1366–1371. [DOI] [PubMed] [Google Scholar]
- 49. Neumann PJ, Weinstein MC. Legislating against use of cost-effectiveness information. New Eng J Med 2010; 363: 1495–1497. [DOI] [PubMed] [Google Scholar]
- 50. Roland D. Obscure model puts a price on good health—and drives down drug costs. Wall Street Journal, 4 November 2019. [Google Scholar]
- 51. Institute for Clinical and Economic Review. Disease-modifying therapies for relapsing-remitting and primary-progressive multiple sclerosis: effectiveness and value. Boston, MA: Institute for Clinical and Economic Review, 2017. [Google Scholar]
- 52. Institute for Clinical and Economic Review. Siponimod for the treatment of secondary progressive multiple sclerosis: effectiveness and value. Boston, MA: Institute for Clinical and Economic Review, 2019. [Google Scholar]
- 53. Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune Dis 2012; 2012: 784364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iannazzo S, Iliza A-C, Perrault L. Disease-modifying therapies for multiple sclerosis: a systematic literature review of cost-effectiveness studies. PharmacoEconomics 2018; 36: 189–204. [DOI] [PubMed] [Google Scholar]
- 55. Hartung DM, Bourdette D. Addressing the rising prices of disease-modifying therapies for multiple sclerosis. JAMA Neurol. Epub ahead of print 26 August 2019. DOI: 10.1001/jamaneurol.2019.2445. [DOI] [PubMed] [Google Scholar]
- 56. Kister I, Corboy JR. Reducing costs while enhancing quality of care in MS. Neurology 2016; 87: 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bourdette D. Rituximab for treating multiple sclerosis: off-label but on target. Neurology 2016; 87: 2070–2071. [DOI] [PubMed] [Google Scholar]
- 58. American Academy of Neurology. AAN position statement: prescription drug prices, https://www.aan.com/policy-and-guidelines/policy/priority-issues/ (2020, accessed 19 January 2021).