Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune disease primarily affecting joints but often also associated with lung involvement such as bronchiectasis (BE). The aim of the present systematic review and meta-analysis is to provide an update on the current evidence regarding the prevalence and association between RA and BE. This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines with literature search using the terms ‘Bronchiectasis AND Rheumatoid Arthritis’ without a date limitation on PubMed during May 2020. A total of 28 studies fulfilled the predefined criteria and were included in the present review, with 19 being cross-sectional studies. Twenty-three studies were included in the meta-analysis. The pooled prevalence estimate was 2.69% (95% CI 1.63–4.42) in clinically defined BE, and 24.9% (95% CI 19.21–31.67) in radiologic disease. Many inconsistencies exist regarding potential risk factors for BE in RA patients such as gender, RA duration and severity, as both negative and positive associations have been reported. Although very little is known about possible causative mechanisms between RA and BE, potential pathways might be antigenic stimulation from pulmonary mucus and/or systemic inflammation from joint disease affecting the lungs. At present, the available evidence of bronchiectasis in patients with RA is insufficient to identify RA-associated risk factors for the development of BE, possibly apart from duration of RA, and, consequently, also to fully explore a possible causal relationship between the two disease. However, the increased prevalence of BE in RA patients warrants further studies to explore the association between RA and BE.
Keywords: Bronchiectasis, rheumatoid arthritis, review, underlying mechanisms, prevalence
Introduction
The diagnosis of rheumatoid arthritis (RA) is based on the presence of inflammation of joints, serological tests and duration of symptoms, and the disease is typically diagnosed using the American College of Rheumatology (ACR) criteria.1 However, although RA is a chronic autoimmune disease of the joints, RA is also associated with many well-recognized extra-articular manifestations, such as involvement of the eyes, skin, gastrointestinal tract, heart and the lungs.2 The most common involvement of the lungs in RA is interstitial lung disease, but bronchiectasis (BE) has also been reported in patients with RA as early as half a century ago.3
BE is an irreversible and abnormal dilatation of the airways.4 The diagnosis of BE is based on radiologic findings together with the presence of clinical symptoms such as cough, sputum production, dyspnoea and, and in a limited number of patients, haemoptysis.4 Indeed, both RA and BE significantly increases morbidity and mortality in comparison to the background population5 and older studies even suggest a synergistic effect between the two diseases.6,7
BE is often found as a comorbid condition to other pulmonary diseases such as chronic obstructive pulmonary disease (COPD), severe asthma and interstitial lung disease. Previous studies have found associations between RA and both COPD and asthma, with RA diagnoses both pre- and superseding the pulmonary diseases in a series of large follow-up studies.8,9 Despite the complex interplay suggested between pulmonary disease and RA, the mechanisms at play remain unknown.
Inflammation is a cornerstone of RA10 pathogenesis and a viscous vortex of inflammation has been suggested in BE.11 As such, the underlying chronic inflammatory changes seen in both RA and BE may suggest an association, or perhaps even a causal relationship, between the two diseases. A more detailed understanding of the interaction between the two diseases may pave the way for more targeted therapy for the individual patient. The aim of this systematic review and meta-analysis is to provide an estimate of the prevalence of BE in RA, as well as an update on the current knowledge of a possible association between RA and BE.
Methods
The present review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses.12 The searches were conducted using the terms ‘Bronchiectasis AND Rheumatoid Arthritis’ on the PubMed during May 2020, without a limitation on study publication date. Studies were eligible for inclusion in the present review provided they fulfilled the following criteria: (I) Investigating the prevalence of and/or association with bronchiectasis in patients with RA, and (II) reporting observations from original studies, and none of the following exclusion criteria: (I) non-original research paper i.e. review (II) non-English paper. Studies without prevalence measurements were included to provide contextual evidence towards an association between BE and RA but were not included in crude prevalence estimations. The identified studies were screened for eligibility for this review based on title and abstract, and the full papers possibly fulfilling the criteria were then reviewed for inclusion.
Data was extracted using manual review by RW, and studies included, as well as data extracted, were independently verified by KEJH and CSU.
Variables sought in data extraction were: (I) Study population size and absolute and/or relative prevalence of BE, (II) Definition of BE (including diagnostic modality and/or criteria: Radiological disease or Clinical disease, (III) Definition of RA: diagnostic criteria used (including any serological tests), (IV) Reporting of important confounders: age, gender, smoking status, (V) Timing of disease: Reporting of BE-before-RA or RA-before-BE.
All included studies were individually assessed for risk of bias and quality using the risk of bias assessment tool for prevalence studies by Hoy et al.13 Draft and summary scores are reported in Table 1. Furthermore, an overall assessment of quality of evidence was performed as per GRADE recommendations.52
Table 1.
Overview of studies investigating the association between RA and BE.a
| Authors | Design | Subjects | Methods | Results | Conclusion | BE definition | Study Quality & Risk of Bias |
|---|---|---|---|---|---|---|---|
| Akira et al.14
▴ |
Retrospective cohort | 29 Japanese patients suspected of having RA-associated lung disease (55% women, mean age 59 years, 10 smokers). | HRCT. Follow-up 3–108 months, average of 28 months. | 15/29 (51.7%) had radiologic BE, 1 had progression, 1 new case during follow-up | No clear association between RA and BE could be demonstrated as the study lacked statistical power. | HRCT findings. Fujii et al.15 criteria. | Low quality Moderate risk of bias |
| Allain et al.16
▴ |
Retrospective cross-sectional | 453 patients (77% women, mean age 57.6 years) with a mean RA duration of 8.1 years. | Questionnaire, CXR, review of laboratory tests, CT scan. | 13/453 (2.87%) had BE. No laboratory findings were associated with BE. In 12 of the 13 cases of BE, the respiratory symptoms antedated joint symptoms by a minimum of 16 years. | The results suggest that BE might have an aetiological role in RA disease. | Based on Walker’s criteria 17 clinical findings and/or bronchography. | Low quality Moderate risk of bias |
| Attar et al.18
▴ |
Cross-sectional | 100 patients (85% women, mean age 51 years) with a disease duration of 6.2 years. 12% active smokers. | Clinical assessment, laboratory testing, PFT and HRCT. | 35/100 (35%) had radiologic BE. Anti-CCP positivity was associated with higher risk of BE (OR 3.55 p = 0.052). Age, disease duration and male gender all significantly associated with the development of BE. | The trend for anti-CCP positivity could potentially be a causative link between RA and BE. | HRCT findings. | Low quality Moderate risk of bias |
| Bilgici et al.19
▴ |
Cross-sectional | 54 patients (85% women, mean age 53.6 years) with a mean RA duration of 8.4 years (24% active smokers). | Clinical assessment, PFT and HRCT, laboratory tests and CXR. | 6/54 (11.1%) had radiologic BE. There was a significant correlation in RF values and reduced FEV1 and PEF (p < 0.05 and p < 0.01 respectively). | Because of the positive association between Larsen’s score and abnormal HRCT findings, there is a significant association between disease activity and lung involvement in RA, however not specific to BE. | HRCT based on Muller et al.20 criteria. | Low quality Moderate risk of bias |
| Cortet et al.21
▴ |
Cross-sectional study with a control group | 77 patients (66% women, mean age 57 years) with mean RA duration of 12 years (6 current smokers) | HRCT of the 77 patients that fit the inclusion criteria, no PFT. | 23/77 (29.9%) had radiologic BE, corresponding to 7.9% in asymptomatic (of respiratory disease) patients and 51.2% in symptomatic patients (p = 0.012). | BE in RA patients is most likely a consequence of frequent lung infection (perhaps due to RA treatment), supported by the lower zones of the lungs being affected mostly. | BE defined as abnormal visualization of proximal airways. | Low quality Moderate risk of bias |
| Cortet et al.22
▴ |
Cross-sectional | 68 patients (79% women, mean age 58.8 years) with a mean duration of RA of 12 years, 23.5% current or ex-smokers. | Clinical assessment, HRCT, PFT, laboratory testing. | 18/59 (30.5%) had radiologic BE. No RA activity or disability parameter correlated significantly in the BE patients. | BE is not associated with poor RA disease parameters. | HRCT based on Naidich criteria.23 | Low quality Moderate risk of bias |
| Demir et al.24
▴ |
Cross-sectional | 34 patients (76% women, mean age 45 years) with a mean RA disease duration of 5.4 years | Clinical assessment, CXR, PFT and HRCT, laboratory tests | 9/34 (26.5%) had radiologic BE. No pulmonary abnormalities on HRCT correlated significantly with sex or disease activity, as well as no HRCT finding correlated with RF seropositivity. | No associations nor causative factors could be established between RA disease activity and BE. | HRCT based on Naidich criteria.23 | Low quality Moderate risk of bias |
| Despaux et al.25 | Case study | 14 patients (71% women) were included with a mean disease duration of 11.1 years (2 smokers, both men). | Bronchography, CXR, CT scan, laboratory tests and PFT | Concurrent exacerbations of bronchial and joint symptoms in 6/14 (42.8%) of patients and for almost half of the patients the onset of symptoms of joint disease worsened respiratory symptoms. In 12 out of 14 cases, the BE antedated the RA. No correlation with duration of RA was found. | The results suggest that BE might have an aetiological role in RA disease. | Bronchographic or CT finding. | Low quality High risk of bias |
| Despaux et al.26
▴ |
Prospective cohort | 46 patients (74% women, with a mean age of 60.1 years) | Clinical assessment, laboratory tests, PFT and HRCT | 19/46 (41.3%) had radiologic BE. No statistically significant difference in gender, age of RA onset, RA disease duration or disease severity when compared to RA patients without BE. | Presence of symptomatic or asymptomatic BE did not correlate with either RA disease severity or duration, revealing no specific link between RA and BE. | HRCT findings based on Naidich23 criteria. | Low quality Moderate risk of bias |
| Duarte et al.27
▴ |
Retrospective cohort | 87 patients (75% women, mean age 63 years, mean follow-up time 6.7 years) with a mean RA duration of 14 years, with 14 smokers. | HRCT, PFT, and laboratory tests. | 31/87 (35.6%) had radiologic BE. 27/31 BE patients were female (p = 0.043). Patients with isolated BE had longer RA disease duration (OR = 1.05 95% CI: 1.01–1.09 (p = 0.023)). | Patients with BE tended to have lower anti-CCP positivity, meaning it likely plays a smaller role in BE development. | HRCT findings. | Low quality Moderate risk of bias |
| Hassan et al.28
▴ |
Cross-sectional | 20 RF-positive patients (90% women, mean age 59 years) with a mean RA duration of 9 years | Clinical assessment, PFT, HRCT, CXR, laboratory testing. | 5/20 (25%) had radiologic BE. No significant difference in the patient group with and without BE based on age, gender, PFT, duration of RA (although a tendency was found towards RA duration). | No definite links could be established between disease parameters of RA and BE. | HRCT based on Naidich criteria.23 | Low quality Moderate risk of bias |
| Izumiyama et al.29
▴ |
Cross-sectional | 186 patients (83% women, mean age 59.8 years) with a mean RA disease duration of 10.2 years (3% smokers) | Clinical assessment, HRCT, CXR, (some underwent PFT). | 21/123 (17.1%) had radiologic BE. Men were at a higher risk of having BE (p < 0.05). |
No features of RA associated with BE. | HRCT findings. | Low quality Moderate risk of bias |
| Koch et al.30
▴ |
Cross-sectional study | 96 patients (89% women, mean age 57.5 years) with a mean RA disease duration of 16.7 years (41.6% smokers) | Clinical assessment, laboratory testing and HRCT. | 25/96 (26.0%) had radiologic BE. Only high CEA and airway changes were significant (p = 0.014) on multivariate analysis | CEA levels can help identify RA patients at risk for development of RA associated lung involvement, however the finding was not specific for BE. | HRCT based on Mori et al.31 | Low quality Moderate risk of bias |
| Matsumoto et al.32
▴ |
Retrospective cohort | 332 patients (85% women, mean age 55 years) with a mean duration of RA of 17 years. 80% never smokers. | HRCT, CXR, clinical assessment and laboratory tests. | 32/332 (9.6%) had radiologic BE. Incidence of lung infection was significantly higher for patients with BE: the HR for infection was 2.14 (95% CI: 1.36–3.65 (p < 0.01.)) | Evidence of airway disease on CT scans increases the risk of lung infection in patients with RA undergoing biological DMARD therapy. | CT finding based on Sokai et al.33 criteria. | Low quality Moderate risk of bias |
| McDonagh et al.34
▴ |
Matched case-control | 40 patients (30% women, median age 66 years) with a median duration of RA 9 years | PFT, clinical assessment, HRCT, laboratory tests, interview. | 6/40 (15%) had radiologic BE. 4/20 (20%) in the control group had radiologic BE. RA patients with BE also had severely reduced PFT. Smoking and antibody titres did not correlate with any HRCT findings. | No features of RA associated with BE. | HRCT finding based on Munro et al. CT criteria 35 | Low quality High risk of bias |
| McMahon et al.6
|
Matched case-control | 32 patients with BE and RA, 32 matched patients with RA but no BE and 31 patients with BE but no RA | Clinical assessment, CXR, PFT, laboratory tests | No differences between RA patients and RA-BE patients in terms of increased extra-articular activity and laboratory findings. No significant differences in circulating antibodies between RA-BE and RA groups. 30 out of 32 patients (93.8%) of the RA-BE group had BE preceding their RA disease. | No features of RA associated with BE. | Based on Walker’s criteria 17 clinical findings and/or bronchography. | Low quality Moderate risk of bias |
| Metafratzi et al.36
▴ |
Cross-sectional | 43 patients (88% women, mean age 56.5 years) with a mean RA disease duration of 10 months | Clinical assessment, laboratory testing, CXR, PFT (of 32/43) and HRCT. | 25/43 (58.1%) had radiologic BE, however the frequency was similar in the control group with 8/18 (44.4%). Severity of BE determined by the HRCT score was higher compared to the control group without RA. | RA is possibly the cause of more severe and extensive BE. | HRCT findings using Fleischner37 and Naidich23 criteria. | Low quality Moderate risk of bias |
| Morrison et al.38
▴ |
Cross-sectional | 104 patients (69% women, mean age 51.1 years) with a mean duration of RA of 12.4 years | Clinical assessment, CXR, laboratory tests and PFT. | 2/104 (1.92%) had BE. | No features of RA associated with BE. | Clinical, CXR and physiological findings. | Low quality Low risk of bias |
| Park et al.39 | Cross-sectional | 83 patients (88% women, mean age 53.3 years, 92.8% non-smokers) | CT scan, PFT and laboratory tests and medical records review. | No reported prevalence of radiologic BE. PFT value of low FEV1/FVC was significantly correlated with bronchiectasis (p = 0.032). No correlations were found between BE and anti-CCP or RF titres. | No association between antibody titres of RA and BE was found, meaning they likely play a smaller role in BE development. | Based on Fleischner Society recommendations40 | Low quality Moderate risk of bias |
| Perez et al.41
▴ |
Cross-sectional | 50 patients (82% women, mean age of 57.8 years) and a mean duration of RA of 14.4 years (22% smokers). | HRCT, clinical assessment, PFT, laboratory tests | 15/50 (30%) had radiologic BE. Of the PFT values for airway disease FEF25–75 correlated with BE but had no correlation with the data from the RA disease. | There was no significant difference in the clinical and laboratory features of RA patients with and without lung involvement – meaning that no RA data (including RA disease severity and duration) and PFT variables correlated. | HRCT criteria by Muller and Miller42 | Low quality Moderate risk of bias |
| Remy-Jardin et al.43
▴ |
Retrospective Cohort | 84 RA patients (66% women, mean age 57 years, 8 smokers) | CT scans. 15 were CT scanned at average 18 months follow-up | 23/84 (29.9%) had radiologic BE. In terms of clinical characteristics (including RA disease severity), comparing those with to those without respiratory symptoms, the only difference was mean duration of articular disease 14 years vs. 11 years (p < 0.05). | RA disease duration is associated with higher risk of having respiratory symptoms, however not specific to BE. | CT findings. | Low quality Moderate risk of bias |
| Robles-Perez et al.44
▴ |
Cross-sectional | 40 patients (75% women, mean age 47 years) with a median disease duration of 12 months (37.5% smokers). | Those with abnormalities in CXR and/or PFT were CT scanned. | 8/40 (20%) had radiologic BE. | No features of RA associated with BE. | HRCT findings. | Low quality Low risk of bias |
| Shadick et al.45
|
Retrospective cross-sectional | 23 patients (18 women and 5 men), subsequent BE mean age 63.8 and antecedent BE 65.7. | A retrospective analysis of the patients’ records. | 0.6% had BE. The two groups differed in disease duration (the patients with antedated BE had much shorter RA duration (6.4 vs. 28.7 years), but much longer BE duration (22.8 vs. 4 years)). | Bronchiectasis can be a feature of the lung involvement in long-standing nodular RA. | CT scan/bronchogram/chest X-ray, and BE clinical symptoms | Low quality Moderate risk of bias |
| Solanki and Neville46 | Retrospective Cohort | 77 patients with BE and 86 patients with ILD who were under follow-up. | Clinical assessment and yearly control. | The frequency of RA in the BE patients was 4/77 (5.19%). The RA patients all had BE preceding their RA disease (mean 19 years prior) | The results suggest that BE might have an aetiological role in RA disease. | Based on Walker’s criteria.17 | Low quality High risk of bias |
| Treves et al.47
▴ |
Cross-sectional study | 100 RA patients and 88 healthy controls. Mean age of RA patients was 60.1 years and 56.8 in the control group. (19 smokers, 11 were women) | Clinical assessment, bronchoscopy, PFT, CT scan | 6/100 (6%) had radiologic BE. None in the control group had radiologic BE. In 5/6 the BE disease antedated joint disease. | The results suggest that BE might have an aetiological role in RA disease. | Bronchoscopy and/or CT findings. | Low quality Moderate risk of bias |
| Wilsher et al.48
▴ |
Cross-sectional | 60 patients (72% women, median age 54 years) with newly diagnosed RA (disease duration less than 12 months, median 7 months). 28% active smokers. | PFT, HRCT, laboratory tests and clinical assessment | 29/60 (48.3%) had radiologic BE. There was an association between serology (anti-CCP) and reduced levels of DLCO. No significant association between PFT, HRCT findings and RA disease severity. | There is not enough compelling evidence to warrant screening for lung abnormalities in early RA patients, as HRCT, PFT and respiratory symptoms do not correlate with each other. | HRCT findings. Modified Bhalla scoring system 49 | Low quality Moderate risk of bias |
| Zhang et al.50
▴ |
Retrospective cross-sectional | 550 patients (70% women, mean age 61 years) with a mean disease duration of 8 years. | Clinical data and physical examination, medical history, laboratory findings, PFT | 76/550 (13.8%) had radiologic BE. The RA-ILD group showed significantly higher prevalence of radiologic BE 43/237 (18.1%) vs. 33/313 (10.5%) when compared with RA-ILD negative. | The results suggest a correlation between ILD and BE, more-so than RA and BE. No further analysis for associations between BE and RA was conducted. | HRCT findings. | Low quality Moderate risk of bias |
| Zrour et al.51
▴ |
Cross-sectional | 75 patients (84% women, mean age 48 years) with a mean RA duration of 96 months (14.6% smokers, all men) | HRCT, PFT, laboratory tests, clinical assessment, CXR. | 14/75 (18.7%) had radiologic BE. RA duration longer than 2 years associated significantly with BE (p = 0.004). | The results support the idea of systemic chronic inflammation of the joints affecting the lungs, rather than the reverse. | HRCT findings. | Low quality Moderate risk of bias |
a Studies marked ▴ are included in the meta-analysis.
Finally, a meta-analysis of study prevalence measurements was performed. Studies were included if they provided relevant prevalence data, and by that case-control studies and case-series were excluded, unless they reported background prevalence from the case population. For the meta-analysis of prevalence, a generalized linear mixed model with logit transformation was used53 and individual study confidence intervals were estimated using Clopper-Pearson exact binominal intervals. Study estimate heterogeneity was investigated using I2 and corresponding Tau2 scores, and a likelihood-ratio test for heterogeneity. Assessment of publication bias was performed using a funnel plot and Eggers’ test.54 All statistic calculations were performed using the Meta package version 4.9-6 for R 4.0.3 (The R Foundation, AU).
Results
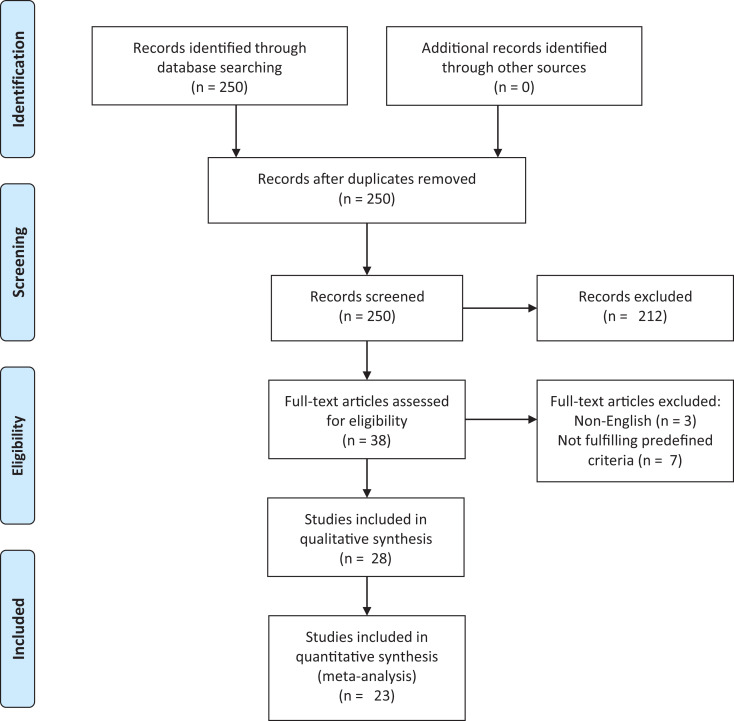
The search algorithm yielded a total of 250 results, 222 of these were excluded based on the predefined criteria (Figure 1). The remaining 28 papers were included and reviewed: 19 cross-sectional studies, 6 prospective or retrospective cohort/follow-up or database studies, 2 case-control studies and 1 case-series study.
Figure 1.
Flow-chart depicting the selection process of the included studies addressing aspects of a possible association between rheumatoid arthritis and bronchiectasis (according to PRISMA).
Individual assessments of studies are available in Supplementary Material 1, and the following results are based on narrative synthesis and meta-analysis of the individual studies.
Included populations and diagnosis of bronchiectasis
The studies included in the present systemic review encompasses 2723 patients with RA, of which 76.3% were reported as female. The crude mean age was 56.1 years (standard deviation (SD) 5.2) and an average of 20% (SD 11.3%) of included patients were reported as current or ex-smokers.
Definitions of BE used varied among the included studies, with four studies.6,16,38,45 using clinical diagnostic criteria. The most commonly used clinical criteria was Walker’s,17 used in three out of four studies. The study performed by Despaux et al.25 did not specify adherence to a specific clinical protocol. In terms of radiologic diagnostic criteria, no less than eight different scoring protocols were used, with the Naidich criteria being the most commonly used.23 Eleven studies did not use a previously published scoring protocol for radiologic diagnosis of BE (Table 1).
Prevalence of bronchiectasis in patients with rheumatoid arthritis
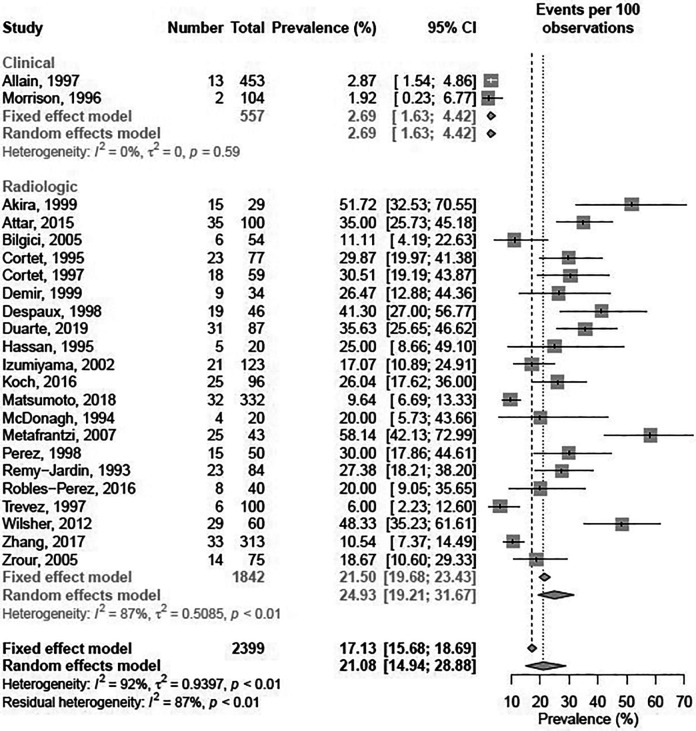
The reported prevalence in the included studies ranged from 1.92%38 to 58.1%.36 In the meta-analysis, the 23 studies marked in Table 1 were included. Five studies were excluded due to missing data on population size or ii) no usable relative measurement reported due to study design i.e. matched case-control or case-series studies. Before meta-analysis, studies were stratified by diagnostic criteria, that is Clinical or Radiologic.
In the pooled prevalence estimates, the prevalence of BE in RA was 21.1% (95% CI: 15.0%–28.9%) using a random effects model (Figure 2). Significant differences in prevalence were seen between Clinical (2.69% (95% CI: 1.63%–4.42%)) and Radiologic (24.9% (95% CI: 19.2%–31.7%)) diagnostic modalities (Q = 63.33, (p < 0.0001)).
Figure 2.
Pooled prevalence estimates of bronchiectasis in patients with rheumatoid arthritis.
Overall, significant heterogeneity was observed in reported prevalence with an I2 of 92% and a Tau2 of 0.94; with a corresponding significant Likelihood-Ratio test for heterogeneity (p < 0.001).
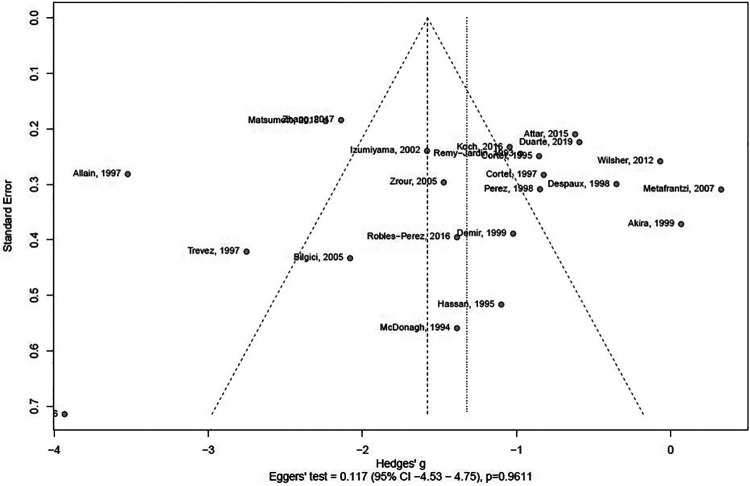
When assessed for publication bias using a funnel plot, a large spread in effect size was seen as a large spread in Hedges’ g. However, Eggers’ test performed without subgrouping was insignificant (Figure 3).
Figure 3.
Assessment of potential publication bias of studies addressing the possible association between bronchiectasis and rheumatoid arthritis.
An overall GRADE quality of evidence-rating of ‘low quality’ was found in the present review and meta-analysis due to the observational nature of included study as well as several methodologic weaknesses including differing diagnostic criteria for BE with large effect on estimated endpoints (clinical versus radiologic disease prevalence).
Temporality and causality between rheumatoid arthritis and bronchiectasis
Five studies reported on temporal association between symptoms/diagnosis of BE and RA.6,16,25,46,47 In all five studies, BE was found to antedate RA in 53 out of 69 cases, corresponding to 91.3%. Additionally, five studies18,27,43,45,51 reported exposure time (to either BE or RA) to be a significant predictor of BE-in-RA, while four found inconclusive results or no association between exposure time and BE-in-RA.25,26,28,41 Additionally, Despaux et al.25 reported simultaneously occurring arthritic flare-ups and exacerbations of BE in 42.8% of included patients.
In terms of causality, no studies claim to present proof of causality between BE and RA. Sixteen of the included studies self-report as negative studies,6,14,22,24,26,28,29,30,34,38,39,41,43,44,48,50 while five studies suggested associations based on biological/biochemical processes,18,21,27,45,51 four based on (temporality of) symptoms16,25,46,47 and three based on prevalence of radiographic findings.19,32,36
Discussion
Prevalence of BE based on clinical findings compared to the radiologic finding of BE
The prevalence of BE in these studies varies greatly based on how BE is defined. Of the 28 studies included, only 5 used clinical symptoms to diagnose BE, the remaining used radiology, such as computed tomography, exclusively. The prevalence in the studies using clinical BE definitions varied from 0.6%45 to 2.87%.16 In the studies where BE was defined by CT scan the prevalence varied from 6%47–58.1%,36 suggesting that the overwhelming majority of BE found radiologically does not present clinically, but longitudinal studies are needed to determine the importance of these findings. In the Dong et al.55 pilot study for patients with early RA (average of 1 year RA disease duration and using CT for BE definition) they found a prevalence of 6%, however patients were excluded if they had existing respiratory diagnoses, and thus likely underestimating the true prevalence. In the cross-sectional study by Metafratzi et al.36 they found a prevalence of 58.1% BE in early RA patients (without respiratory symptoms), however this was not different from their healthy control group of 18 non-smokers, but the extent of BE was greater in the RA patients when using their semiquantitative grading system. The findings from this study suggest that the origin of BE is not RA, but it is somehow exacerbated by the disease. This is further supported by the finding from Despaux et al.25 that exacerbations of bronchial and joint symptoms coincided in 43% of patients, as well as the fact that symptoms of joint disease worsened these respiratory symptoms in over half of the study group.
In our meta-analysis, the pooled prevalence estimate ranged from 2.69% when using clinical parameters for defining BE presence, and an almost 10-fold increase to 24.9% when using purely radiological features. The differing prevalence estimates underlines the need for unified definition of BE in research when studying prevalence going forward and cements the increased prevalence, regardless of radiologic or clinical definitions, when compared to approximately 0.5% clinical BE in the background population.5
Is there a causal relationship between RA and BE?
Early childhood lung infections and exposure to smoke and other pollutants are known risk factors of developing bronchiectasis56 and are not unique to RA patients, however, their RA disease severity and other RA-related parameters seemingly are. Most of the studies included in this review used an RA classification system to determine disease severity of their patients, as well as blood sampling to determine antibody titres and clinical parameters such as RA disease duration. However, not all these studies then analysed their findings regarding the risk of BE. Of the ones that looked at BE specifically, Attar et al.18 found anti-CCP positivity was associated with higher risk of BE with an OR of 3.55, (p = 0.052), and while disease severity was not significantly associated with BE development, another study showed that age, male gender and disease duration was.29 In contrast to the findings of Izumiyama et al.,29 other studies found that female gender was significantly associated with BE in these RA patients (p = 0.043), while also finding a significant correlation with longer disease duration (OR = 1.05 95% CI: 1.007–1.095, p = 0.023),27 but no association with any antibody titres and BE development.39 In the study by McMahon et al.6 in 1993 they found essentially no differences in disease severity and antibody titres between patients with RA and BE compared to patients with RA alone. The lack of differences between the two groups is contradictory to later findings but might be explained by the relatively small group consisting of only 64 patients. Additionally, in the study by Despaux et al.26 regardless of statistical significance, the clinical significance of a decrease in lung functionality should be considered as 80% of those with radiologic BE and respiratory symptoms had abnormal PFT, whereas only 62% of those with no respiratory symptoms (but still radiologic BE) had abnormal PFT. Larsen’s score to determine RA disease severity was used in the study by Bilgici et al.,19 and they found high Larsen’s score statistically increased risk of an abnormal HRCT finding (OR 2.04 95% CI: 1.14–3.66, p < 0.01). This was however not specific to BE, as it included findings such as pulmonary fibrosis. Metafratzi et al.36 found no such correlation between HRCT abnormalities and RA disease severity using the same scoring system. In a similar vein, Despaux et al.26 found no such correlation to BE specifically, although they did use a different method to determine disease severity, in this case Steinbrocker Functional Classification, signalling the importance of standardized classification systems to improve comparability of future studies.
Potential causal pathways linking BE and RA
An emerging theory regarding the possible connection between RA and BE is based on antigenic stimulation from the mucus in patients with BE. This hypothesis reverses the causality, so that BE is ultimately the cause of RA. Some studies support this hypothesis by having most of their RA patients, if not all, with antecedent BE, ranging from 83.3%47 to 100%,46 while others have only 21.7% of their RA patients having preceding BE.45 Allain et al.16 had 92.3% with preceeding BE and Despaux et al.25 and McMahon et al.6 had 85.7% and 93.8% with preceding BE, respectively. The potential causative mechanism that could explain this has yet to be adequately established, however, it fails to explain why some RA-BE patients develop RA before their BE.
Another possible theory is based on the idea that the systemic inflammation of the joints in RA disease somehow also affects the lungs, which would in-part explain antedated RA in RA-BE. Studies have investigated the importance of the autoantibodies associated with RA, specifically RF and anti-CCP. None of the included studies have found a connection between RF and increased prevalence or severity of BE. In fact, Duarte et al.27 found a trend that RA-BE patients had lower anti-CCP positivity when compared to those with no BE (p = 0.074), and Park et al.39 found that there was no such association. Attar et al.18 did, however, find an association between anti-CCP and higher risk of BE (OR 3.55, p = 0.052) as the only positive association, suggesting that these autoantibodies likely play a very small role in BE development in RA patients.
Genetic factors play a role in RA development, but very few studies have investigated this in relation to BE development. Bilgici et al.19 found that HLA-DR1, which is associated with RA, was not significantly increased in RA-BE patients, although this might have been due to the small subject size of the study. Other genetic factors such as the cystic fibrosis transmembrane regulator (CFTR) gene mutations potentially play a role for heterozygous RA-BE patients, as a study has shown allele mutations in 20 out of 55 BE patients,57 potentially predisposing to BE. However, Solanki and Neville46 argued that patients with cystic fibrosis and BE, who later develop RA, are affected by a unique seronegative variant, and thus is a different mechanism of association than the one connecting isolated BE and RA.
Studies that found that longer RA disease duration was associated with the development of BE support the theory of systemic joint inflammation affecting the lungs. For example, Zrour et al.51 found that RA duration over 2 years associated with BE (p = 0.004) and Duarte et al.27 found that patients with isolated BE had longer RA disease duration (OR = 1.05 95% CI: 1.01–1.09 (p = 0.023)) compared to patients with ILD. It is not unlikely, however, as most of these patients were undergoing treatment for RA with immunosuppressants, that this is partly (if not entirely) because of their increased susceptibility to recurrent infection, and thus at an increased risk of developing BE,57 and much in the same way, this would not explain why some develop BE prior to their RA.
The different diagnostic criteria for RA and BE
There are many different scoring systems, classification methods and diagnostic criteria for both RA and BE, and a major limitation during analysis of the present studies is the lack of validation and the low comparability between the different methods used. Many of the included studies did not specify how they defined diagnosis of BE, other than stating that it was identified using HRCT/CT.18,27,29,43,44,50,51 Although explicitly defined, the rest of studies that used exclusively radiological criteria for BE diagnosis also had varying classification systems, further increasing the heterogeneity of the studies. This can also be said for RA disease severity scoring, as DAS-28, Larsen score, Stoke Index and Steinbrocker Functional Classification all measure this parameter, and simultaneously display the need for standardization.
Conclusion
Based on a meta-analysis of the currently available literature, there is an increased prevalence of both radiologic and symptomatic BE in patients with RA compared to the general population, most likely due to an increased risk of recurrent respiratory infections. Despite the many hypothesized causal pathways, such as mucus-antigenic stimulation, there is not enough evidence to suggest a single causal link between BE and RA. Standardization ranging from classification systems to diagnostic criteria of RA and BE, as well as further long-term prospective studies with unified diagnostic criteria are direly needed to uncover the risk of developing BE in
Supplemental material
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121994565 for A causal relationship between rheumatoid arthritis and bronchiectasis? A systematic review and meta-analysis by Rafal Wiater, Kjell Erik Julius Håkansson and Charlotte Suppli Ulrik in Chronic Respiratory Disease
Footnotes
Authors’ note: RW and KEJH share first authorship.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kjell Erik Julius Håkansson  https://orcid.org/0000-0001-5804-0740
https://orcid.org/0000-0001-5804-0740
Charlotte Suppli Ulrik  https://orcid.org/0000-0001-8689-3695
https://orcid.org/0000-0001-8689-3695
Supplemental material: Supplemental material for this article is available online.
References
- 1. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford, England) 2012; 51(6): vi5–vi9. [DOI] [PubMed] [Google Scholar]
- 2. Cojocaru M, Cojocaru IM, Silosi I, et al. Extra-articular manifestations in rheumatoid arthritis. Maedica 2010; 5: 286. [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan JD. Cardiopulmonary manifestations of rheumatoid disease in childhood. South Med J 1964; 57: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 4. King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 5. Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMahon MJ, Swinson DR, Shettar S, et al. Bronchiectasis and rheumatoid arthritis: a clinical study. Ann Rheum Dis 1993; 52: 776–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi IA, Lee JS, Song YW, et al. Mortality, disability, and healthcare expenditure of patients with seropositive rheumatoid arthritis in Korea: a nationwide population-based study. PLoS One 2019; 14: e0210471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford JA, Liu X, Chu SH, et al. Asthma, chronic obstructive pulmonary disease, and subsequent risk for incident rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol (Hoboken, NJ) 2020; 72: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaccardelli A, Liu X, Ford JA, et al. Elevated anti-citrullinated protein antibodies prior to rheumatoid arthritis diagnosis and risks for chronic obstructive pulmonary disease or asthma. Arthritis Care Res (Hoboken) [Internet]. 2020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/31961487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (London, England) 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 11. Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev 2019; 28: 190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62(10): e1–e34. [DOI] [PubMed] [Google Scholar]
- 13. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65: 934–939. [DOI] [PubMed] [Google Scholar]
- 14. Akira M, Sakatani M, Hara H. Thin-section CT findings in rheumatoid arthritis-associated lung disease: CT patterns and their courses. J Comput Assist Tomogr 1999; 23: 941–948. [DOI] [PubMed] [Google Scholar]
- 15. Fujii M, Adachi S, Shimizu T, et al. Interstitial lung disease in rheumatoid arthritis: assessment with high-resolution computed tomography. J Thor Imaging 1993; 8(1): 54–62. [PubMed] [Google Scholar]
- 16. Allain J, Saraux A, Guedes C, et al. Prevalence of symptomatic bronchiectasis in patients with rheumatoid arthritis. Revue du Rhumatisme (English Ed) 1997; 64: 531–537. [PubMed] [Google Scholar]
- 17. Walker WC. The lung in rheumatoid arthritis. MD Thesis, University of Edinburgh, 1966. [Google Scholar]
- 18. Attar SM, Alamoudi OS, Aldabbag AA. Prevalence and risk factors of asymptomatic bronchiectasis in patients with rheumatoid arthritis at a tertiary care center in Saudi Arabia. Ann Thor Med 2015; 10: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bilgici A, Ulusoy H, Kuru O, et al. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 2005; 25: 429–435. [DOI] [PubMed] [Google Scholar]
- 20. Muller NL, Staples CA, Miller RR, et al. Disease activity in idiopathic pulmonary fibrosis: CT and pathologic correlation. Radiology 1987; 165(3): 731–734. [DOI] [PubMed] [Google Scholar]
- 21. Cortet B, Flipo RM, Remy-Jardin M, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis 1995; 54: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortet B, Perez T, Roux N, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis 1997; 56: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naidich DP, McCauley DI, Khouri NF, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr 1982; 6(3): 437–444. [DOI] [PubMed] [Google Scholar]
- 24. Demir R, Bodur H, Tokoglu F, et al. High resolution computed tomography of the lungs in patients with rheumatoid arthritis. Rheumatol Int 1999; 19: 19–22. [DOI] [PubMed] [Google Scholar]
- 25. Despaux J, Polio JC, Toussirot E, et al. Rheumatoid arthritis and bronchiectasis. A retrospective study of fourteen cases. Revue du Rhumatisme (English Ed) 1996; 63: 801–808. [PubMed] [Google Scholar]
- 26. Despaux J, Manzoni P, Toussirot E, et al. Prospective study of the prevalence of bronchiectasis in rheumatoid arthritis using high-resolution computed tomography. Revue Du Rhumatisme (English Ed) 1998; 65: 453–461. [PubMed] [Google Scholar]
- 27. Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients – an overview of different types of involvement and treatment. Rheumatology (Oxford, England) 2019; 58: 2031–2038. [DOI] [PubMed] [Google Scholar]
- 28. Hassan WU, Keaney NP, Holland CD, et al. High resolution computed tomography of the lung in lifelong non-smoking patients with rheumatoid arthritis. Ann Rheum Dis 1995; 54: 308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Izumiyama T, Hama H, Miura M, et al. Frequency of broncho-bronchiolar disease in rheumatoid arthritis: an examination by high-resolution computed tomography. Modern Rheumatol 2002; 12: 311–317. [DOI] [PubMed] [Google Scholar]
- 30. Koch MC, Pereira IA, Nobre LFS, et al. Computed tomography of pulmonary changes in rheumatoid arthritis: carcinoembryonic antigen (CEA) as a marker of airway disease. Rheumatol Int 2016; 36: 531–539. [DOI] [PubMed] [Google Scholar]
- 31. Mori S, Cho I, Koga Y, et al. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol 2008; 35(8): 1513–1521. [PubMed] [Google Scholar]
- 32. Matsumoto T, Iwano S, Takahashi N, et al. Association between chest computed tomography findings and respiratory adverse events in rheumatoid arthritis patients undergoing long-term biological therapy. Int J Rheum Dis 2019; 22: 626–635. [DOI] [PubMed] [Google Scholar]
- 33. Sokai R, Ito S, Iwano S, et al. Respiratory mechanics measured by forced oscillation technique in rheumatoid arthritis-related pulmonary abnormalities: frequency-dependence, heterogeneity and effects of smoking. Springerplus 2016; 5: 335–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDonagh J, Greaves M, Wright AR, et al. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Brit J Rheumatol 1994; 33: 118–122. [DOI] [PubMed] [Google Scholar]
- 35. Munro NC, Cooke JC, Currie DC, et al. Narrow section computed tomography in the diagnosis of bronchiectasis. Thorax 1988; 43(10): P812(A). [Google Scholar]
- 36. Metafratzi ZM, Georgiadis AN, Ioannidou CV, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scand J Rheumatol 2007; 36: 338–344. [DOI] [PubMed] [Google Scholar]
- 37. Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996; 200(2): 327–331. [DOI] [PubMed] [Google Scholar]
- 38. Morrison SC, Mody GM, Benatar SR, et al. The lungs in rheumatoid arthritis—a clinical, radiographic and pulmonary function study. S Afr Med J 1996; 86: 829–833. [PubMed] [Google Scholar]
- 39. Park WH, Kim SS, Shim SC, et al. Visual assessment of chest computed tomography findings in anti-cyclic citrullinated peptide antibody positive rheumatoid arthritis: is it associated with airway abnormalities? Lung 2016; 194: 97–105. [DOI] [PubMed] [Google Scholar]
- 40. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246(3): 697–722. [DOI] [PubMed] [Google Scholar]
- 41. Perez T, Remy-Jardin M, Cortet B. Airways involvement in rheumatoid arthritis. Am J Respir Crit Care Med 1998; 157: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 42. Muller NL, Miller RR. Diseases of the bronchioles: CT and histopathologic findings. Radiology 1995; 196(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 43. Remy-Jardin M, Remy J, Cortet B, et al. Lung changes in rheumatoid arthritis: CT findings. Radiology 1994; 193: 375–382. [DOI] [PubMed] [Google Scholar]
- 44. Robles-Perez A, Luburich P, Rodriguez-Sanchon B, et al. Preclinical lung disease in early rheumatoid arthritis. Chron Respir Dis 2016; 13: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shadick NA, Fanta CH, Weinblatt ME, et al. Bronchiectasis. A late feature of severe rheumatoid arthritis. Medicine 1994; 73: 161–170. [PubMed] [Google Scholar]
- 46. Solanki T, Neville E. Bronchiectasis and rheumatoid disease: is there an association? Rheumatology 1992; 31: 691–693. [DOI] [PubMed] [Google Scholar]
- 47. Treves R, Pugnere N, Bonnet C, et al. A prospective study of pulmonary symptoms in 188 patients with rheumatoid arthritis. Revue du Rhumatisme (English Ed) 1997; 64: 435. [PubMed] [Google Scholar]
- 48. Wilsher M, Voight L, Milne D, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir Med 2012; 106: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 49. Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000; 55(3): 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Li H, Wu N, et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 2017; 36: 817–823. [DOI] [PubMed] [Google Scholar]
- 51. Zrour SH, Touzi M, Bejia I, et al. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Prospective study in 75 patients. Joint Bone Spine 2005; 72: 41–47. [DOI] [PubMed] [Google Scholar]
- 52. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 53. Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 54. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dong H, Julien PJ, Demoruelle MK, et al. Interstitial lung abnormalities in patients with early rheumatoid arthritis: a pilot study evaluating prevalence and progression. Eur J Rheumatol 2018; 6: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morrissey BM. Pathogenesis of bronchiectasis. Clin Chest Med [Internet] 2007; 28(2): 289–296. [DOI] [PubMed] [Google Scholar]
- 57. Wilczynska MM, Condliffe AM, McKeon DJ. Coexistence of bronchiectasis and rheumatoid arthritis: revisited. Respir Care 2013; 58: 694–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121994565 for A causal relationship between rheumatoid arthritis and bronchiectasis? A systematic review and meta-analysis by Rafal Wiater, Kjell Erik Julius Håkansson and Charlotte Suppli Ulrik in Chronic Respiratory Disease