Abstract
Metabolic syndrome is characterized by insulin resistance/hyperinsulinemia, atherogenic dyslipidemia (elevated triglycerides, low HDL), and hyperglycemia. The high prevalence of metabolic syndrome in pulmonary hypertension leads to the hypothesis that metabolic syndrome may play a contributing role in pulmonary hypertension and heart failure with preserved ejection fraction pathogenesis. We present a 62-year-old woman with morbid obesity, mild pre-capillary pulmonary hypertension, and metabolic syndrome. Her metabolic syndrome was treated with a medically-supervised ketogenic diet delivered by a telehealth healthcare team via a continuous remote care platform. Following one year of treatment, metabolic syndrome was reversed, leading to successful weight loss concurrent with hemodynamic improvement. This case highlights the feasibility of using a nutritional strategy to treat pulmonary hypertension associated with obesity and metabolic syndrome, common contributors to group 2 and 3 pulmonary hypertension. We bring this case and technique to the pulmonary hypertension community to share a tool in our therapeutic toolkit and highlight the importance of nutritional advice extending beyond telling a patient they should lose weight to invoking a rational strategy. We argue that strategic nutritional intervention through reversal of her metabolic syndrome using a medically-supervised ketogenic diet is a safe and effective treatment strategy in metabolic syndrome-associated pulmonary hypertension.
Keywords: pulmonary hypertension, diabetes, obesity and metabolic syndrome, insulin resistance
Background
Metabolic syndrome (MetS) is characterized by insulin resistance/hyperinsulinemia, atherogenic dyslipidemia (elevated triglycerides, low HDL), and hyperglycemia. MetS is a highly morbid disease state associated with obesity and end-organ manifestations including cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).
MetS in pulmonary arterial hypertension (PAH) is quite common and associated with worse outcomes.1–3 MetS is also highly prevalent in pulmonary hypertension (PH) due to left-sided heart disease including heart failure with preserved ejection fraction (HFpEF).4 The high prevalence of MetS in these conditions leads to the hypothesis that MetS may play a contributing role in PH and HFpEF pathogenesis.5 Reversal of MetS through gastric bypass surgery and postoperative weight loss has been associated with an improvement in cardiac function and symptoms in obese patients with HFpEF, and hemodynamics in an obese PAH patient.6,7
For decades, the treatment of type 2 diabetes mellitus (T2DM) and MetS has focused on treating elevated blood glucose levels and dyslipidemia, without addressing the root cause of MetS derangements. In patients with T2DM, the use of a medically-supervised continuous care dietary intervention to sustain nutritional ketosis with remote care monitoring has been shown to be safe, sustainable, and effective.8 After one year on this intervention, 94% of patients on insulin were able to reduce or eliminate insulin, 60% of patients were able to sustain a hemoglobin A1c < 6.5% with no diabetes medication or with only metformin, and average weight loss was 12%. Inflammatory markers, liver enzymes, and triglycerides also improved.8,9
We describe a case of reversal of metabolic syndrome through nutritional ketosis and its association with clinical and hemodynamic improvements.
Case
Our patient is a 62-year-old morbidly obese, former smoker residing at ∼7100 feet elevation who presented with a one-year history of shortness of breath and unexplained hypoxemia, referred for a second opinion. She was initiated on CPAP with oxygen for obstructive sleep apnea with nocturnal hypoxemia. Her baseline oxygen requirements were 3 L/min asleep, 2 L/min at rest, and 5–8 L/min with ambulation at her home elevation. Despite consistent usage of CPAP and supplemental oxygen, she continued to have dyspnea with minimal walking, hypoxemia, and fatigue.
On presentation, body mass index (BMI) was 45.5 kg/m2 and oxygen saturation 93% on 3 L (Table 1). Despite a 90 pack-year-smoking history (quit one year prior to presentation), pulmonary physiology and imaging did not show significant underlying lung disease. Her ventilation/perfusion scan did not reveal thromboembolic disease. Echocardiogram showed an increased estimated right ventricular systolic pressure at 54 mmHg (previously normal) with mild right atrial enlargement and elevated mean left atrial pressure. Despite eight months of CPAP therapy, hemodynamics measured at unforced end-expiration in a resting supine position revealed mild pre-capillary pulmonary hypertension: mean right atrial pressure 12 mmHg, pulmonary artery pressure 46/22/33 mmHg, mean pulmonary artery occlusion pressure (PAOP) 14 mmHg, left ventricular end-diastolic pressure (LVEDP) 15 mmHg, transpulmonary gradient 19 mmHg, diastolic pressure gradient 8 mmHg, thermodilution cardiac output (CO) 5.67 L/min, cardiac index 2.38 L/min/m2, and pulmonary vascular resistance (PVR) 3.35 Wood units.
Table 1.
Clinical, metabolic, and hemodynamic markers at baseline and after one year treatment with a medically-supervised ketogenic diet (MSKD) delivered through a continuous remote care platform.
| Baseline | After 1 year MSKD treatment | |
|---|---|---|
| Weight (kg) | 134 | 100 |
| BMI (kg/m2) | 45.5 | 34.1 |
| Blood pressure (mmHg) | 110/62 | 119/73 |
| Heart rate (bpm) | 63 | 64 |
| SpO2 (%) | 95 | 91 |
| Supplemental oxygen | 2 L rest, 3–5 L ambulation | Room air |
| Hemoglobin A1c (%) | 6.2 | 6.1 |
| Triglycerides (mg/dL) | 256 | 194 |
| Cholesterol (mg/dL) | 262 | 141 |
| HDL (mg/dL) | 41 | 44 |
| LDL-C (mg/dL) | 170 | 58 |
| HDL/TG ratio | 6.2 | 4.4 |
| FEV1/FVC (%) | 74.5 | 74.5 |
| FEV1 (L (% pred)) | 1.81 (63) | 2.30 (84) |
| FVC (L (% pred)) | 2.43 (65) | 3.09 (88) |
| DLCO (ml/min/mmHg (% pred)) | 19.22 (71) | 24.59 (108) |
| VA (L) | 3.93 (73) | 5.45 (98) |
| DL/VA (ml/min/mmHg/L)Total lung capacity (L (%pred)) Residual volume (L (%pred)) | 4.89 (97) 4.98 (89) 2.46 (113) | 4.13 (100)–– |
| RVSP (mmHg) | 54 | 44 |
| Right atrial size (cm) | 5.3 | 5.7 |
| Right ventricular diameter (cm) | 3.9 | 4.3 |
| Left atrial volume Index (ml/m2) | 34 | 36 |
| TAPSE (cm) | 2.4 | 2.7 |
| RA pressure (mmHg) | 12 | 11 |
| PAP (s/d/m, mmHg) | 46/22/33 | 40/19/28 |
| Mean PAOP (mmHg) | 14 | 12 |
| LVEDP (mmHg) | 15 | - |
| TPG (mmHg) | 19 | 16 |
| DPG (mmHg) | 8 | 7 |
| Cardiac output (L/min) | 5.67 | 6.0 |
| Cardiac index (L/min/m2) | 2.38 | 2.9 |
| PVR (Wood Units) | 3.35 | 2.67 |
BMI: body mass index; SpO2: oxygen saturation; HDL: high density lipoprotein cholesterol; LDL-C: calculated low density lipoprotein cholesterol; FEV1/FVC: ratio of forced expiratory volume in one second to forced vital capacity; FEV1: forced expiratory volume in one second; pred: predicted; FVC: forced vital capacity; DLCO: diffusing capacity of carbon monoxide; VA: alveolar volume; DL/VA: diffusing capacity adjusted for alveolar volume; R: right; RVSP: right ventricular systolic pressure; TAPSE: tricuspid annular plane systolic excursion; PAP: pulmonary arterial pressure; s/d/m: systolic/diastolic/mean; PAOP: pulmonary artery occlusion pressure; TPG: transpulmonary gradient; DPG: diastolic pressure gradient; PVR: pulmonary vascular resistance.
Given medically complicated obesity with MetS (hemoglobin A1c 6.2%, triglycerides 256 mg/dL, HDL 41 mg/dL), radiographic fatty liver disease, and high-normal PAOP and LVEDP, we classified her as having a Group 3 phenotype (hypoxemia and sleep apnea), with a probable Group 2 (diastolic dysfunction) component and counseled her on aggressive lifestyle changes to reverse her MetS.
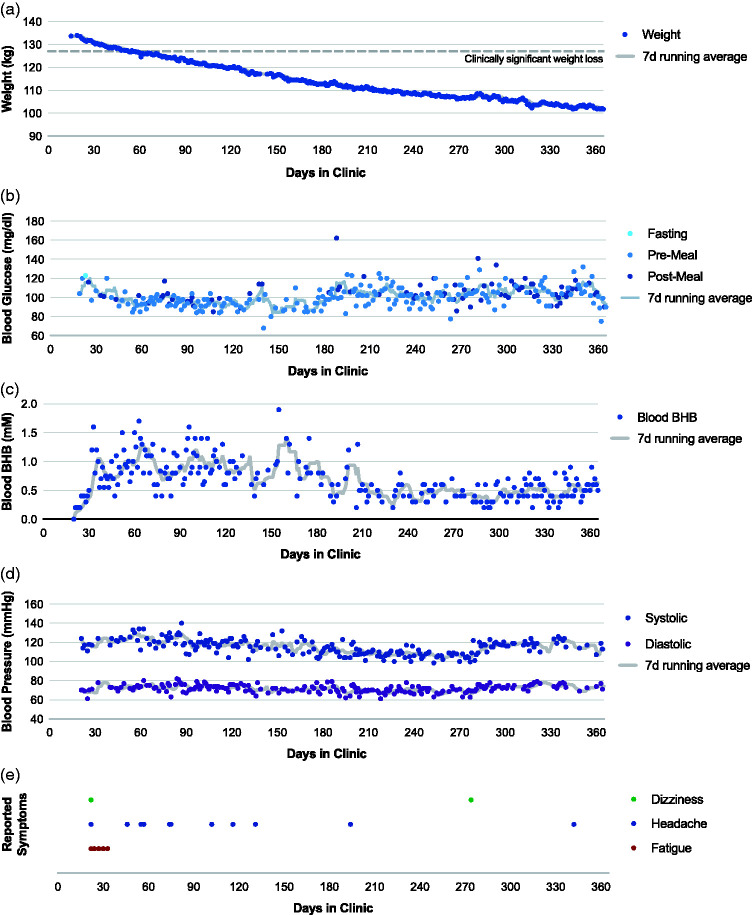
After an initial intake, the patient was prescribed atorvastatin and participated in a medically-supervised ketogenic diet (MSKD) treatment via a continuous care platform delivered by a telehealth team (endocrinologist and health coach).8 This MSKD treatment utilizes carbohydrate-restriction therapy including nutritional ketosis (blood β-hydroxybutyrate ≥0.5 mM) to reduce hyperglycemia and weight in patients with insulin resistance. Through the platform, patients receive education and practical resources alongside frequent communication and medical supervision from the care team to initiate and sustain nutritional and behavioral changes and also can engage with a peer community for social support. Our patient retained her primary care physician and pulmonologist for any issues unrelated to glycemia and weight change. As part of her care plan, she recorded daily glucose, ketone, weight, blood pressure, and symptoms, and the care team adjusted the care plan over time according to health needs and the patient’s goals/values (Fig. 1).
Fig. 1.
Daily clinical markers during one year treatment with a MSKD. Daily (a) weight, (b) blood glucose, (c) blood ketones (beta-hydroxybutyrate), (d) blood pressure, and (e) symptoms of the patient monitored via a continuous remote care platform.
On this MSKD program, our patient lost 34 kg in one year (–25% initial body weight), decreased her BMI to below 40 kg/m2, normalized her triglycerides, and improved her oxygen requirement and symptoms. Her NYHA functional class improved from III to II, and one year later she is able to walk her dog one mile at her home elevation without oxygen. Her spirometry showed significant improvement, and repeat hemodynamics after one year of MSKD showed improvement with mPAP 28 mmHg, mean PAOP 12 mmHg, CO 6.0 L/min, and PVR 2.67 Wood units.
This case report was limited by lack of saline bolus or exercise challenge in our hemodynamics, which would have helped further characterize whether there was a significant HFpEF phenotype. We speculate that her hemodynamic improvements were a downstream effect of weight loss with resultant improvement in lung volumes, hypoxemia, and therefore improvement in the Group 3 component of her disease. We cannot ascertain from this case whether independent of weight loss, the induction of nutritional ketosis, and the attendant metabolic and metabolomic alterations improved PVR and/or cardiac function.
Conclusion
This case highlights the feasibility of using a nutritional strategy to treat PH associated with MetS and obesity with successful improvement in clinical status and hemodynamics. In patients with Group 2 or 3 PH, it is paramount to treat the underlying condition. We bring this representative case of an obese patient with Group 3 PH and the MSKD technique to the PH community to share a tool in our therapeutic toolkit and highlight the importance of nutritional advice extending beyond the recommendation to lose weight by invoking a rational strategy. We argue that strategic nutritional intervention to address the root cause of this patient’s PH, through reversal of MetS using a MSKD, is a safe and effective treatment.
Footnotes
Authors’ contributions: Kim and George did primary writing and editing.
McKenzie provided data, analysis and editing and patient consent form.
Roberts provided editing.
Conflict of Interest: George works as a non-promotional speaker for Actelion and Bayer Pharmaceuticals, and has served on advisory boards for Actelion, Altavant, Bayer, and United Therapeutics. She has an investigator-initiated grant from Actelion and is the PI on clinical trials with United Therapeutics and Complexa. Dr. McKenzie and Dr. Roberts are employed by Virta Health Corp and have been offered stock options.
Ethical approval: The patient gave written consent for this case study to be published.
Guarantor: MG.
Funding: This publication was supported by the Rose Community Foundation.
References
- 1.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Resp J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pugh ME, Robbins IM, Rice TW, et al . Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ussavarungsi K, Thomas CS, Burger CD. Prevalence of metabolic syndrome in patients with pulmonary hypertension. Clin Resp J 2017; 11: 721–726. [DOI] [PubMed] [Google Scholar]
- 4.Von Bibra H, Ströhle A, St. John Sutton M, et al. Dietary therapy in heart failure with preserved ejection fraction and/or left ventricular diastolic dysfunction in patients with metabolic syndrome. Int J Cardiology 2017; 234: 7–15. [DOI] [PubMed] [Google Scholar]
- 5.Ranchoux B, et al. Metabolic syndrome exacerbates pulmonary hypertension due to left heart disease. Circ Res 2019; 125: 449–466. [DOI] [PubMed] [Google Scholar]
- 6.Mikhalkova D, Holman SR, Jiang H, et al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity 2018; 26: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugh ME, Newman JH, Williams DB, et al. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: potential role for metabolic regulation. Diabetes Care 2013; 36: e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther 2018; 9: 583–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: and open-label, non-randomized controlled study. BMJ Open 2019; 9: e023597. [DOI] [PMC free article] [PubMed] [Google Scholar]