Abstract
Background:
Endovascular thrombectomy (EVT) is highly effective but may also lead to hemorrhagic transformation (HT) and edema, which may be more pronounced in severe ischemia. We sought to determine whether glibenclamide can attenuate HT and edema in a severe ischemia-reperfusion model that reflects EVT.
Methods:
Using a transient middle cerebral artery occlusion (tMCAo) rodent model of stroke, we studied two rat cohorts, one without rt-PA and a second cohort treated with rt-PA. Glibenclamide or vehicle control was administered as an intravenous bolus at reperfusion, followed by continuous subcutaneous administration with an osmotic pump.
Results:
Compared to vehicle control, glibenclamide improved neurological outcome (median 7, interquartile range [IQR 6–8] vs. control median 6 [IQR 0–6], p=0.025), reduced stroke volume (323±42 vs. 484±60mm3, p<0.01), swelling volume (10±4 vs. 28±7%, p<0.01) and water content (84±1 vs. 85±1%, p<0.05). Glibenclamide administration also reduced HT based on ECASS criteria, densitometry (0.94±0.1 vs. 1.15±0.2, p<0.01), and quantitative hemoglobin concentration (2.7±1.5 vs. 6.2±4.6uL, p=0.011). In the second cohort with rt-PA coadministration, concordant effects on HT were observed with glibenclamide.
Conclusions:
Taken together, these studies demonstrated that glibenclamide reduced the amount of edema and HT after severe ischemia. This study suggests that co-administration of glibenclamide may be worth further study in severe stroke patients treated with EVT with or without IV rt-PA.
Keywords: Brain edema, hemorrhagic transformation, glibenclamide, ischemic stroke
Introduction
Recanalization after ischemic stroke is associated with reduced brain edema [1–4]. However in some cases, following intravenous (IV) thrombolysis or endovascular thrombectomy (EVT), reperfusion injury from edema and hemorrhagic transformation (HT) can exacerbate the initial ischemic damage [5–8]. Current evidence demonstrates the benefit of endovascular reperfusion in patients with large vessel occlusion within 6 hours of stroke onset [9–12]. Imaging-based selection methods have also identified a subset of patients in later timeframes who also benefit from reperfusion [13–16]. However, reperfusion of severe ischemia may increase the risk of reperfusion injury and limit the clinical benefit of thrombolysis and EVT [8,17,18]. For example, a recent meta-analysis of thrombectomy trials in the HERMES consortium has suggested there may be a benefit in patients with low ASPECT scores, but also demonstrated a higher risk of symptomatic intracerebral hemorrhage relative to EVT patients with a higher ASPECTS score (14% versus 4%) [19]. Co-treatment with IV rt-PA can increase the rate of HT to approximately 20% [20], and the development of HT post-procedure is associated worse outcomes and a higher rate of death [21].
Inhibition of the sulfonylurea receptor 1 (SUR1) is a potential therapeutic target following stroke [22–24]. The channel is upregulated in all cell types of the neurovascular unit after ischemia [22]. Excessive channel opening leads to the influx of sodium and water, cellular swelling, and necrotic cell death [25]. In prior studies, glibenclamide has reduced stroke volume, brain edema and mortality in several rodent model systems [22,26,27].
It is less certain whether glibenclamide can abrogate HT following severe cerebral ischemia. Glibenclamide can reduce brain MMP-9 activity in brain endothelial cells [28,29], but in a rodent middle cerebral artery occlusion (MCAo) model with low dose rt-PA co-administration [26], HT rates were low which limited interpretation. Retrospective human studies suggest an association between sulfonylurea usage and reduced incidence of HT in some [30], but not in all cases [31]. In the current study, we sought to evaluate whether glibenclamide can improve the safety profile of reperfusion in a severe ischemia model using rat transient filament occlusion. We hypothesized that glibenclamide would reduce HT after reperfusion with and without “high dose” IV rt-PA [32,33]. The goal was to inform the design of future clinical studies testing glibenclamide in patients with severe ischemia treated with EVT, with or without rt-PA thrombolysis.
Methods
Animals and experimental groups
All experiments were approved by the Institutional Animal Care and Use Committee at MGH, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 290–330g were used. Briefly, rats were initially anesthetized with 5% isoflurane in 70% nitrous oxide and 30% O2 and then maintained with 1–2% isoflurane in 70% nitrous oxide and 30% O2 by a face mask. Body temperature was maintained at 37°C throughout the surgical procedure by a water heating pad. The right femoral artery was cannulated, and physiologic parameters including rectal temperature, mean arterial blood pressure, and blood gases were monitored.
Experimental groups
Two cohorts were studied. In the first cohort, we induced 3 hours of transient MCAo (tMCAo) by using an intraluminal vascular occlusion with a silicon-coated filament. Filament occlusion was performed using a 4–0 siliconized suture (Doccol Corp, Sharon, MA) according to standard methods [34,35]. Briefly, the right carotid bifurcation was exposed and the common carotid and distal external carotid arteries were sutured. An arteriotomy was made in the proximal ECA and a siliconized 4–0 suture was then advanced to the level of the middle cerebral artery. Placement was confirmed by the slight resistance that was encountered at approximately 19 mm from the arteriotomy incision. In cohort 1, twenty-eight animals were randomly divided into 2 treatment groups: a control group (n=15) and a glibenclamide group (n=13). This sample size was chosen to achieve 90% power to detect a difference in hemorrhage volume of at least 2.5 μL between placebo and glibenclamide, assuming a standard deviation of 2 μL at a significance level of α=0.05. This was approximately similar in magnitude compared to prior studies of pharmacologic interventions to prevent HT [36]. Five animals died in the placebo group and two animals died in the glibenclamide group, for final group sizes of n=10 and n=11, respectively.
For the second cohort with rt-PA co-administration (n=12 placebo and n=12 glibenclamide), a 90 min ischemia time was used. In a pilot experiment with 3 hours of ischemia time and rt-PA, we observed a 75% mortality, therefore a 90 min ischemia time was chosen. There were 3 deaths in the placebo group and 2 deaths in the glibenclamide group, for final group sizes of n=9 and n=10, respectively. Recombinant rt-PA (Genentech) was administered intravenously at a dose of 10 mg/kg at the end of the ischemia time, coinciding with the time of reperfusion.
Drug administration
The administration of glibenclamide was based on Simard et al. [26], with the following modifications. The glibenclamide group was administered an IV bolus dose (50 μg/kg in 0.9% saline) 5 minutes after MCAo onset, and then continuous glibenclamide was administered via mini-osmotic pump (Alzet 2001, 1.0 μL/hr; DURECT Corporation) primed with 3.3 mM glibenclamide in dimethylsulfoxide (DMSO) that was inserted into the subcutaneous space. In pilot experiments, preparation of glibenclamide with DMSO compared to saline led to a more consistent plasma glibenclamide concentration when delivered from the osmotic pump. This concentration of glibenclamide was chosen based on achieving a target plasma glibenclamide level of ~10 ng/mL, which matched the average level achieved in human studies [24,37]. Control rats were administered an IV bolus of 0.9% saline, followed by subcutaneous insertion of a mini-osmotic pump primed with DMSO.
Neurological deficit evaluation
Neurological function was assessed using the modified Garcia score [38]. Scores were determined shortly after reperfusion and at 48 hours after onset of ischemia. Animals that died were assigned a score of 0. All other animals were sacrificed at 48hr for the measurement of stroke volume, brain edema, and quantification of HT. Approximately 0.5 mL of blood was withdrawn at 48 hours after ischemia onset to measure the blood glucose and glibenclamide plasma level. Animals were euthanized with 5% isoflurane, then transcardially perfused with cold physiological saline until the right atrium outflow was clear. The brain was carefully removed and cut into six 2 mm coronal sections starting from the olfactory bulb-frontal lobe junction.
Measurement of plasma glibenclamide
The plasma glibenclamide concentration was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Briefly, 30 μL of plasma was deproteinized with 70 μL of 75:25 acetonitrile:methanol (v:v). Following centrifugation, 5 μL of supernatant was separated with an XBridge Amide column (2.1mm x 100mm, Waters) using a 1290 Infinity HPLC system (Agilent). The aqueous mobile phase A was 95% water, 5% acetonitrile with 20mM ammonium acetate and 20mM ammonium hydroxide. The organic mobile phase B was 100% acetonitrile. Gradient conditions started at 90% mobile phase B then transitioned to 95% mobile phase A over 6 minutes, followed by 6 minutes of column re-equilibration.
The HPLC system was connected to a 6495 QQQ triple quadrupole mass spectrometer (Agilent), and glibenclamide was monitored in the positive mode with a precursor ion of 495 and a product ion of 169. Serial dilutions of glibenclamide were spiked into a pool of baseline (untreated) rat plasma to generate a standard curve and the plasma level of glibenclamide in the unknown samples was calculated by linear regression.
Evaluation of stroke volume and brain edema
Sequential 2 mm thick brain slices were first photographed for imaging evaluation of HT and then immersed in a 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in phosphate buffered saline for 15 minutes at 37°C [39]. After TTC staining, each slice was gently blotted with water absorbing paper to remove small quantities of adsorbent liquid and photographed with a ruler.
Swelling volume was calculated based on previously reported methods [40–42]. Briefly, four regions were individually measured on each brain slice using ImageJ (v1.48, National Institutes of Health). The regions were the contralateral hemisphere volume, the total ipsilateral hemisphere volume, the ipsilateral TTC positive (TTC+) volume, and the TTC negative (TTC-) volume. The contralateral hemisphere volume was defined as the normal hemisphere volume. The “indirect” stroke volume was then calculated according to the following formula:
The swelling volume of the ischemic core was determined as follows:
The swelling volume of the surrounding TTC+ tissue was:
Brain edema was quantified by measuring water content using the wet-dry weight method as described previously, with the following modification [43]. Brain hemispheres were divided and added to 3mL phosphate buffered saline (PBS), followed by homogenization. Homogenization prior to dehydration permitted the simultaneous evaluation of hemoglobin concentration (see below) and brain water content. 0.5 mL of tissue homogenate from each hemisphere was weighed and then dried to constant weight in a vacuum oven at 85°C for 72 hours to obtain the dry weight. Because this method measured the wet weight and dry weight of a portion of the hemisphere, the water content measures represent an estimate of total hemispheric water content. The percent water content was calculated according to the following formula: %H2O = (wet weight – dry weight)/wet weight *100.
Image evaluation of hemorrhagic transformation
Hemorrhagic transformation (HT) was assessed by three methods. Based on the European Cooperative Acute Stroke Studies (ECASS) HT scale [44], brain slices were examined and categorized into H0: no hemorrhage visualized, HI1: hemorrhagic infarct with small petechiae along the periphery of the infarct, HI2: hemorrhagic infarct with confluent petechiae within infarct area, but no mass effect, PH1: parenchymal hemorrhage with a visible blood clot <30% of infarct area and PH2: parenchymal hemorrhage with blood occupying >30% of the infarct area with substantial space-occupying effect.
For color densitometric evaluation of HT, ImageJ software was used to delineate regions of interest (ROI) that circumscribed the hemorrhage. Identically located ROIs were mirrored to the contralateral normal hemisphere. Four separate ROIs within the hemorrhagic lesion were obtained for each animal. The color density of the ROIs was recorded using the measure function in ImageJ software. The mean density ratio was calculated from the four HT ROIs relative to the respective normal ROIs mirrored to the contralateral hemisphere.
To validate the above methods, the mean hemoglobin concentration in the brain of animals from cohort one was quantified using a spectrophotometric assay, as previously described [45,46]. After TTC staining, the brain tissue from each hemisphere was combined, flash frozen, and stored at −80°C until ready for analysis. Hemispheric tissue was added to 3 mL PBS, homogenized for 30 sec with a dounce homogenizer, followed by sonication on ice for 1 min. Following centrifugation at 13,000 rpm for 30 min, 0.2 mL of supernatant was added to 0.8 mL of Drabkin’s reagent (Sigma-Aldrich, St. Louis, MO, USA), and allowed to stand in the dark for 15 minutes at room temperature. Optical density was determined by a spectrophotometer (Spectronix 3000, Milton-Roy, Rochester, NY, USA) at a wave length of 540 nm. A standard curve was created by adding known amounts of blood (incremental volumes of homologous blood: 0, 2, 4, 8, 16 and 32 μL) to naïve hemispheric samples, measuring absorbance following the above protocol, and then performing linear regression analysis of the absorbance versus hemoglobin volume.
Measurement of MMP-9
Whole blood was anticoagulated with potassium ethylenediaminetetraacetic acid (K2EDTA) to achieve a final concentration of 4.5mM K2EDTA. Blood samples were briefly mixed and then centrifuged at 2500xg for 10 min. Plasma supernatant was aliquoted and stored at −80°C until analysis. Rat total MMP-9 was measured using the Quantikine MMP-9 ELISA (R&D Systems) following 1:10 dilution of plasma samples, using the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean ± SD for normal distributions and median and interquartile range for non-normal distributions. The data were analyzed statistically among the groups, employing the unpaired Student’s t-test for normal data and Wilcoxon rank sum for non-normal data. Categorical data were analyzed using Fisher’s exact test or Chi-square test, as appropriate. A p value <0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 8 (San Diego, CA).
Data availability
All protocols and data will be made available upon request and in accordance with institutional data transfer requirements.
Results
Cohort 1: Effect on blood glucose and neurological outcome
Prior to the onset of ischemia, the baseline blood glucose was similar between the two treatment groups in cohort 1 (178±41 mg/dL in controls vs. 172±52 mg/dL in glibenclamide, p=0.72). At 48 hours, there was a modest but statistically significant reduction in blood glucose in the glibenclamide group (131±24 mg/dL vs. 160±25 mg/dL in controls, p=0.014). No animals demonstrated hypoglycemia, defined as a blood glucose <70mg/dL. The mean plasma glibenclamide level at 48 hours was 13.2±10.4 ng/mL.
Neurological function immediately after reperfusion was similar between the two groups with a median score of 3 (interquartile range [IQR, 3–3]) in controls and a median score of 3 [IQR 3–4] in glibenclamide-treated animals, p=0.46, Figure 1A). At 48 hours, animals treated with glibenclamide had a higher median score (7, IQR [6–8]) relative to control (6, [IQR 0–6]; p=0.025, Figure 1B), consistent with an improvement in neurological function. Mortality by 48 hours was 33% in the control group (Table 1). Although mortality was numerically lower in the glibenclamide group (15%), it was not significant (p=0.40, Fisher’s exact test).
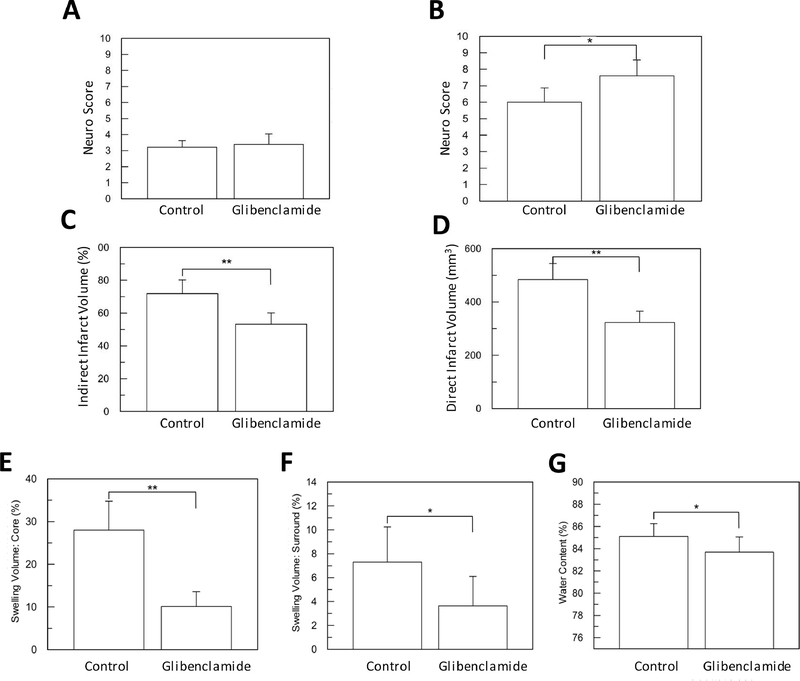
Figure 1. Glibenclamide improves neuro score, stroke volumes and brain edema in cohort 1.
(A) Immediately after reperfusion, there was no difference between the two groups (p=0.46). (B) At 48 hours, glibenclamide improved the neurological score (* p<0.05). Both indirect (C) and direct (D) stroke volume were significantly reduced by glibenclamide (** p<0.01). The swelling volume of ischemic core (E) and the ipsilateral, surrounding TTC positive brain tissue (F) were significantly reduced by glibenclamide (* p<0.05, ** p<0.01, respectively). (G) Concomitantly, estimated total brain water content was reduced by glibenclamide (* p<0.05).
Table 1.
Summary of outcomes evaluated in Cohorts 1 and 2.
| Cohort 1 | Control | Glibenclamide | p value |
| neuro score | 6 [0– 6] | 7 [6–8] | 0.025 |
| mortality | 5 (33%) | 2 (15%) | 0.400 |
| direct stroke volume | 483±60 | 323±42 | <0.001 |
| indirect stroke volume | 72±8% | 53±7% | <0.001 |
| swelling, % | 28±7% | 10±4% | <0.001 |
| MMP-9, 48 hr, (ng/mL) | 11.4±2.5 | 8.5±3.0 | 0.042 |
| glucose, 48 hr, (mg/dL) | 160±25 | 131±24 | 0.014 |
| HT densitometry | 1.15±0.16 | 0.94±0.05 | <0.001 |
| Hemorrhage volume, μL | 6.2±4.6 | 2.7±1.5 | 0.011 |
| HT categories | <0.001 | ||
| None | 1 | 8 | |
| HI1 | 1 | 4 | |
| HI2 | 9 | 1 | |
| PH1 | 4 | 0 | |
| Cohort 2 | Control | Glibenclamide | p value |
| neuro score | 7 [6–8] | 8 [8–10] | 0.021 |
| mortality | 3 (25%) | 2 (17%) | 0.999 |
| direct stroke volume, mm3 | 335±161 | 183±76 | 0.028 |
| indirect stroke volume, % | 45±11% | 36±11% | 0.107 |
| swelling, % | 20±13% | 5±5.5% | 0.003 |
| MMP-9, 48 hr, (ng/mL) | 41.3±19.6 | 31.6±6.7 | 0.209 |
| glucose, 48 hr, (mg/dL) | 191±35 | 155±19 | 0.012 |
| HT categories | <0.01 | ||
| None | 1 | 8 | |
| HI1 | 1 | 2 | |
| HI2 | 5 | 0 | |
| PH1 | 2 | 0 | |
Stroke volume and brain edema
Among all animals in cohort 1, indirect stroke volumes corresponded to 62±12% of the ipsilateral hemisphere. Comparing treatment arms, glibenclamide administration reduced both the indirect stroke volume relative to control (Figure 1C, 53±7% versus 72±8%, respectively, p<0.01) as well as direct stroke volume (Figure 1D and Table 1, p<0.01).
When examining edema, the swelling volume of the ischemic core (corresponding to the unstained TTC lesion) was also reduced by glibenclamide (Figure 1E, 10±4% versus 28±7% in controls, p<0.01). There was a similar reduction in swelling volume in the surrounding ipsilateral brain tissue that was TTC+ (Figure 1F, glibenclamide 4±2% versus control 7±3%, p<0.05). Consistent with the swelling volume measurements, the estimated water content of the ipsilateral hemisphere was reduced by glibenclamide (Figure 1G, 85±1% vs. 84±1%, p=0.04).
Hemorrhagic transformation
Hemorrhagic transformation within the ischemic core was common. Fourteen out of 15 control rats exhibited HT in cohort 1. In contrast, 5 out of 13 rats in the glibenclamide group exhibited HT. Figure 2A shows examples of the type of HT, and the improvement in the distribution of HT categories between control and glibenclamide treatment groups (p<0.001). Administration of glibenclamide also reduced hemorrhage severity based on the mean density ratio measured on the brain slice images (Figure 2B, glibenclamide 0.94±0.05 versus control 1.15±0.16, p<0.001).
Figure 2. Hemorrhagic transformation is reduced by glibenclamide in cohort 1.
(A) Examples of the different HT categories are provided. HI1: small petechiae along the periphery of the infarct; HI2: confluent petechiae within infarct area; PH1: blood visible within <30% of infarct area. There was less severe HT in the animals treated with glibenclamide (white bars) compared to control (gray bars; p<0.001). (B) Quantitative assessment of HT density on brain slice photographs also showed that glibenclamide reduced the mean density ratio (*** p<0.001). (C) The mean density ratio on photographs correlated with brain hemorrhage volume measured using spectrophotometry (r=0.76, p<0.001). (D) Accordingly, glibenclamide reduced brain hemorrhage volume relative to control (**, p=0.01).
To further validate the HT imaging analysis and confirm the effect of glibenclamide on hemorrhagic transformation, we compared the image density ratio with hemoglobin concentration using spectrophotometry. There was a strong correlation between the mean density ratio and hemoglobin concentration (spearman r=0.76; p<0.001, Figure 2C). Accordingly, the amount of hemoglobin extravasated into the brain was reduced by glibenclamide compared to control (Figure 2D, 2.7±1.5 μL versus 6.2±4.6 μL, p=0.011).
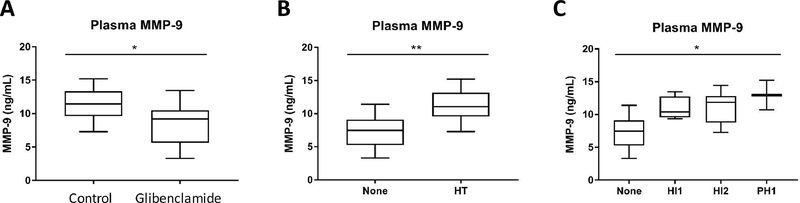
We next measured plasma MMP-9 in the plasma samples collected at 48 hours. The total plasma MMP-9 level was modestly lower in the glibenclamide group compared to vehicle control (Figure 3A, 8.5±3.0 ng/mL versus 11.4±2.5 ng/mL, p=0.042). Animals that did not have any evidence of HT had a lower plasma MMP-9 level compared to those animals who did exhibit HT (Figure 3B, 7.3±2.7 ng/mL versus 11.4±2.3 ng/mL, p=0.004). When examining HT by individual categories, there was also a stepwise increase in plasma MMP-9 (Figure 3C, p=0.021).
Figure 3. Plasma MMP-9 is related to HT and reduced by glibenclamide in cohort 1.
(A) Plasma total MMP-9 was lowered by continuous glibenclamide administration (* p<0.05). (B) Plasma MMP-9 was elevated in tMCAO animals that had HT compared to those that did not (** p<0.01), and (C) there was a stepwise relationship with the severity of HT categories (* p<0.05).
Cohort 2: co-administration of rt-PA
In cohort 2, we examined the effect of continuous glibenclamide compared to vehicle control in the setting of 10 mg/kg IV rt-PA co-administration. Like cohort 1, animals treated with glibenclamide had the same median neurological score as control animals immediately after reperfusion (median 4 [IQR 4–5] versus median 4 [IQR 4–5], p=0.60). At 48 hours after reperfusion, glibenclamide-treated animals had better neurological scores (median 8 [IQR 8–10]) compared to controls (median 7 [IQR 6–8], p=0.021).
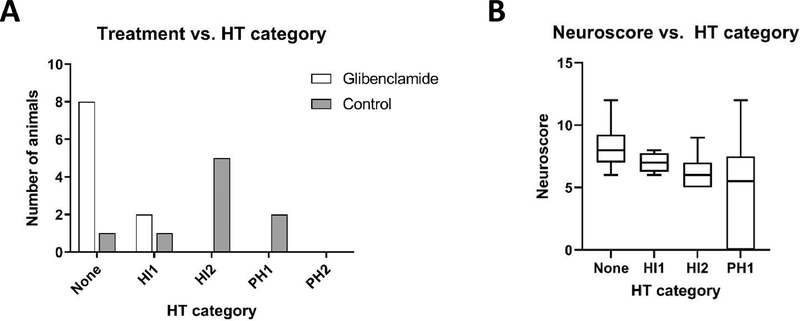
The indirect stroke volume was not different between the two treatment arms (glibenclamide 36±11% versus placebo 45±11%, p=0.11). However, the amount of swelling was reduced to 5±5% with glibenclamide, compared to 20±13% in placebo (p=0.003). Similar to cohort 1, glibenclamide also reduced the severity of HT (Figure 4A, p<0.01). Moreover, the presence and severity of HT was correlated with the neurological score (Figure 4B, p=0.01). Finally, we examined the relationship between treatment and plasma MMP-9, and although there was a numerical reduction in plasma MMP-9 (31.6±6.7 ng/mL in glibenclamide group versus 41.3±19.6 ng/mL in the control group), this was not a significant difference (p=0.21).
Figure 4. Glibenclamide reduces hemorrhagic transformation in cohort 2.
(A) The severity of HT was less in animals treated with glibenclamide (white bars) compared to placebo treated animals (gray bars; p<0.01). (B) The neurological scores are also worse in animals with increasing HT severity (p=0.01)
Discussion
In this study, we examined the effect of glibenclamide in a filament reperfusion model of stroke. We studied an animal model of stroke that mimics severe ischemia, with and without IV rt-PA, to determine whether glibenclamide might be considered as an adjunct to patients treated with thrombectomy. We selected a model that may reflect a patient population where reperfusion injury and HT may limit the benefit from EVT [8]. We found that continuous glibenclamide reduced stroke volume, brain edema and hemorrhagic transformation.
Prior studies have demonstrated that glibenclamide, a sulfonylurea antidiabetic drug and selective SUR1 inhibitor, can prevent brain edema [22,27,47]. The effect on HT has not been as clearly established, with prior studies reporting mixed results. For example, one study examined the effect of a lower dose rt-PA (0.9 mg/kg) with glibenclamide, but the overall rate of HT was ~10% which limited the ability to detect any differences [26]. In retrospective clinical studies, there was an association between baseline sulfonylurea use with less symptomatic hemorrhage [30], however, another study did not confirm this finding [31]. Studies in patients have also suggested that glibenclamide reduces MMP-9 level [28,48], a protease associated with the development of HT [49].
Our study was therefore designed to further evaluate the effect of glibenclamide on HT using several distinct methods that included a categorical HT designation [44] and quantitative assessments of hemorrhage [45]. Although we note concordance between the current study and patients with respect to the effect of glibenclamide, it is important to note that the rate of HT was not reduced in the GAMES-RP trial [24]. Because BBB permeability was not assessed in these patients, it is not known whether the different HT results were due to a differential effect on BBB or whether alternative pathways may contribute to HT in patients relative to rodent models. Moreover, we did not find the same reduction in plasma MMP-9 when rt-PA was co-administered in the rodent model. The augmentation of plasma MMP-9 by rt-PA is a well described phenomenon [50,51], and may have obscured any potential effect of glibenclamide in this setting. Finally, we note that in our study we initiated glibenclamide treatment at the time of occlusion, which may limit the generalizability of our findings. However, the beneficial effect of glibenclamide has been previously shown to extend out to 10 hours after stroke onset [26].
Prior studies have shown expression of SUR1 in the multiple cell types that constitute the neurovascular unit [22,27], and that glibenclamide can reduce MMP-9 secretion specifically in brain endothelial cells [28,29]. Since endothelial cells resides at the interface between the brain and circulation, it is also notable that circulating plasma MMP-9 is related to HT [49]. Based on these data and the observations in this study, we hypothesize that the brain microvascular endothelium is a mediator of HT and the likely target of glibenclamide for this effect.
Taken together our data extend prior findings to show that glibenclamide can have beneficial effects in a stroke model that mimics reperfusion of severe ischemia, with or without IV tPA co-administration. These data raise the possibility that glibenclamide can be studied in combination with EVT in patients with severe ischemia, such as those who present with a low Alberta Stroke Program Early CT (ASPECTS) Score.
Acknowledgments
Grant Support: AHA 14GRNT19060044 and NIH R01 NS099209.
Funding: This study was funded by AHA 14GRNT19060044 (W.T.K.), and NIH R01 NS099209 (W.T.K.).
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Authors TI, CS, and ZW declare they have no conflict of interest. WTK has received research grants from NINDS, AHA, Remedy Pharmaceuticals, and Biogen, and is co-lead for the CHARM trial supported by Biogen.
Statement of the welfare of animals: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. 2019;142(5):1399–407. [DOI] [PubMed] [Google Scholar]
- 2.Irvine HJ, Ostwaldt A-C, Bevers MB, Dixon S, Battey TWK, Campbell BCV, et al. Reperfusion After Ischemic Stroke is Associated with Reduced Brain Edema. J Cereb Blood Flow Metab. 2018. July;38(10):1807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorén M, Dixit A, Escudero-Martínez I, Gdovinová Z, Klecka L, Rand V-M, et al. Effect of Recanalization on Cerebral Edema in Ischemic Stroke Treated With Thrombolysis and/or Endovascular Therapy. Stroke. 2020. January;51(1):216–23. [DOI] [PubMed] [Google Scholar]
- 4.Kimberly WT, Dutra BG, Boers AMM, Alves HCBR, Berkhemer OA, Van Den Berg L, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: A secondary analysis of the MR CLEAN Trial. JAMA Neurol 2018;75(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada Y, Yamaguchi T, Minematsu K, Miyashita T, Sawada T, Sadoshima S, et al. Hemorrhagic transformation in cerebral embolism. Stroke. 1989;20:598–603. [DOI] [PubMed] [Google Scholar]
- 6.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997;28(5):957–60. [DOI] [PubMed] [Google Scholar]
- 7.Larrue V, von Kummer RR, Müller a, Bluhmki E, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438–41. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42(1):93–7. [DOI] [PubMed] [Google Scholar]
- 9.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N Engl J Med 2015;372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Goyal M, Bonafe A, Diener H-CC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372(24):2285–95. [DOI] [PubMed] [Google Scholar]
- 11.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372(11):1009–18. [DOI] [PubMed] [Google Scholar]
- 12.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015. June;372(24):2296–306. [DOI] [PubMed] [Google Scholar]
- 13.Jovin TG, Liebeskind DS, Gupta R, Rymer M, Rai A, Zaidat OO, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: Retrospective multicenter analysis of 237 consecutive patients. Stroke. 2011;42(8):2206–11. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol 2012;11(10):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2017. November;NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 16.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378(8):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomalla G, Sobesky J, Kohrmann M, Fiebach JB, Fiehler J, Zaro Weber O, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38(2):313–8. [DOI] [PubMed] [Google Scholar]
- 18.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 2016;15(9):925–33. [DOI] [PubMed] [Google Scholar]
- 19.Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018;17(10):895–904. [DOI] [PubMed] [Google Scholar]
- 20.Panni P, Gory B, Xie Y, Consoli A, Desilles J-P, Mazighi M, et al. Acute Stroke With Large Ischemic Core Treated by Thrombectomy. Stroke. 2019. May;50(5):1164–71. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira RG, Gupta R, Jovin TG, Levy EI, Liebeskind DS, Zaidat OO, et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: a multicenter retrospective analysis of 1122 patients. J Neurointerv Surg 2015. January;7(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simard JM, Chen M, Tarasov K V., Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 2006;12(4):433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simard JMM, Sheth KNKN, Kimberly WTT, Stern BJBJ, Del Zoppo GJGJ, Jacobson S, et al. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2014;20(2):319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016;15(11):1160–9. [DOI] [PubMed] [Google Scholar]
- 25.Simard JM, Kent TA, Chen M, Tarasov K V, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 2007;6(3):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, Yurovsky V, Ivanova S, et al. Glibenclamide-10-h Treatment Window in a Clinically Relevant Model of Stroke. Transl Stroke Res 2012;3(2):286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide Is Superior to Decompressive Craniectomy in a Rat Model of Malignant Stroke. Stroke. 2010;41(3):531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, et al. Does inhibiting Sur1 complement rt-PA in cerebral ischemia? Ann N Y Acad Sci 2012;1268(1):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerzanich V, Kwon MS, Woo SK, Ivanov A, Marc Simard J. SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One. 2018. DOI: 10.1371/journal.pone.0195526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol 2012;72(5):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favilla CG, Mullen MT, Ali M, Higgins P, Kasner SE. Sulfonylurea use before stroke does not influence outcome. Stroke. 2011;42(3):710–5. [DOI] [PubMed] [Google Scholar]
- 32.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost 1981 [PubMed] [Google Scholar]
- 33.El Amki M, Lerouet D, Coqueran B, Curis E, Orset C, Vivien D, et al. Experimental modeling of recombinant tissue plasminogen activator effects after ischemic stroke. Exp Neurol 2012. DOI: 10.1016/j.expneurol.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 34.Tatlisumak T, Fisher M. Handbook of experimental neurology : methods and techniques in animal research. Cambridge ; New York: Cambridge University Press; 2006. [Google Scholar]
- 35.Sicard KM, Fisher M. Animal models of focal brain ischemia. Exp Transl Stroke Med 2009;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39(12):3372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Mandava P, Yoo AJJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014;45(1):281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods. 2006;156(1–2):161–5. [DOI] [PubMed] [Google Scholar]
- 39.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–8. [DOI] [PubMed] [Google Scholar]
- 40.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24(1):117–21. [DOI] [PubMed] [Google Scholar]
- 41.Overgaard K, Meden P. Influence of different fixation procedures on the quantification of infarction and oedema in a rat model of stroke. Neuropathol Appl Neurobiol 2000;26(3):243–50. [DOI] [PubMed] [Google Scholar]
- 42.Hosomi N, Ban CR, Naya T, Takahashi T, Guo P, Song XR, et al. Tumor necrosis factor-alpha neutralization reduced cerebral edema through inhibition of matrix metalloproteinase production after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2005;25(8):959–67. [DOI] [PubMed] [Google Scholar]
- 43.Keep RF, Hua Y, Xi G. Brain Water Content: A Misunderstood Measurement? Transl Stroke Res 2012;3(2):263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 45.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28(11):2296–302. [DOI] [PubMed] [Google Scholar]
- 46.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab 2000;20(12):1681–9. [DOI] [PubMed] [Google Scholar]
- 47.Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009. February;40(2):604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimberly WT, Battey TWK, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38(2 Suppl):748–52. [DOI] [PubMed] [Google Scholar]
- 50.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–9. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 2003;9(10):1313–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All protocols and data will be made available upon request and in accordance with institutional data transfer requirements.