Significance Statement
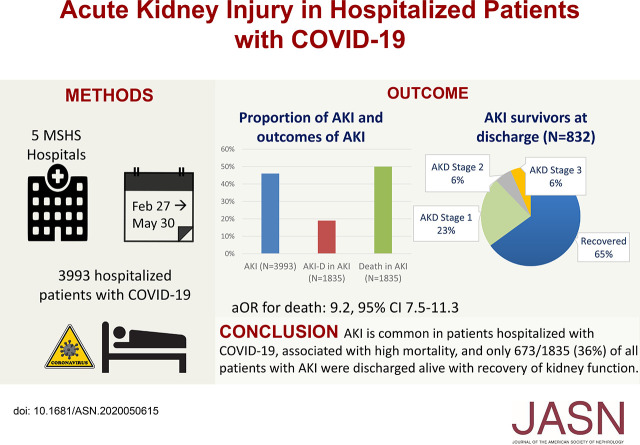
Early reports have indicated that AKI and other kidney abnormalities are associated with coronavirus disease 2019 (COVID-19). Of 3993 hospitalized patients with COVID-19 in a New York City health system, AKI occurred in 1835 (46%) patients; among patients with AKI, 19% required dialysis, and half of them died in the hospital. Among patients who were discharged, 35% had not recovered to baseline kidney function at the time of discharge. AKI is common among patients with COVID-19 and is associated with higher mortality than in patients without AKI; among those who survive, only about a third are discharged with renal recovery. These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function.
Keywords: acute renal failure, clinical nephrology, dialysis, COVID-19
Visual Abstract
Abstract
Background
Early reports indicate that AKI is common among patients with coronavirus disease 2019 (COVID-19) and associated with worse outcomes. However, AKI among hospitalized patients with COVID-19 in the United States is not well described.
Methods
This retrospective, observational study involved a review of data from electronic health records of patients aged ≥18 years with laboratory-confirmed COVID-19 admitted to the Mount Sinai Health System from February 27 to May 30, 2020. We describe the frequency of AKI and dialysis requirement, AKI recovery, and adjusted odds ratios (aORs) with mortality.
Results
Of 3993 hospitalized patients with COVID-19, AKI occurred in 1835 (46%) patients; 347 (19%) of the patients with AKI required dialysis. The proportions with stages 1, 2, or 3 AKI were 39%, 19%, and 42%, respectively. A total of 976 (24%) patients were admitted to intensive care, and 745 (76%) experienced AKI. Of the 435 patients with AKI and urine studies, 84% had proteinuria, 81% had hematuria, and 60% had leukocyturia. Independent predictors of severe AKI were CKD, men, and higher serum potassium at admission. In-hospital mortality was 50% among patients with AKI versus 8% among those without AKI (aOR, 9.2; 95% confidence interval, 7.5 to 11.3). Of survivors with AKI who were discharged, 35% had not recovered to baseline kidney function by the time of discharge. An additional 28 of 77 (36%) patients who had not recovered kidney function at discharge did so on posthospital follow-up.
Conclusions
AKI is common among patients hospitalized with COVID-19 and is associated with high mortality. Of all patients with AKI, only 30% survived with recovery of kidney function by the time of discharge.
Preliminary reports indicate that AKI and kidney abnormalities seem to be associated with coronavirus disease 2019 (COVID-19) severity and outcomes. A recently published study that utilized autopsy specimens from 26 patients who died of COVID-19 in China1 demonstrated that there is evidence of the invasion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into kidney tissue, along with significant acute tubular injury and endothelial damage, as well as glomerular and vascular changes indicative of underlying diabetic or hypertensive disease.
Small studies from China, Europe, and the United States thus far have reported a wide range of the incidence of AKI, ranging between 1% and 42%. The two largest studies published to date show widely disparate rates of AKI. Guan et al.2 reported an incidence rate of AKI of only 0.5% in 1099 patients from 552 hospitals in China. In a study out of Ireland in patients admitted to the intensive care unit (ICU), reported incidence of AKI requiring dialysis was 22.2%, with mortality exceeding 75%.3 In New York, 37% of patients developed AKI, and 14% of patients with AKI required dialysis.4
New York City (NYC) has been at the epicenter of the COVID-19 pandemic in the United States and worldwide and as such, has had the highest number of hospitalizations. The burden of severe AKI in the setting of COVID-19 has led to widespread shortages in NYC of dialysis nurses, machines, replacement fluids and cartridges for continuous RRT, and dialysis.4,5 The number of patients with AKI requiring acute RRT is unprecedented in modern history.
Despite the anecdotal stories regarding the incidence and severity of AKI, the epidemiology of AKI, especially recovery from AKI in hospitalized patients in the United States and particularly NYC, is not well described. Thus, the objective of this report was to describe the incidence, severity, risk factors, and outcomes associated with AKI in the setting of hospitalization of COVID-19 in a major NYC health care system.
Methods
Study Population
The Mount Sinai Health System (MSHS) serves a large racially and ethnically diverse patient population. In this study, patient data came from the electronic health records (EHRs) from five major hospitals that are part of MSHS: Mount Sinai Hospital located in East Harlem, Manhattan; Mount Sinai Morningside located in Morningside Heights, Manhattan; Mount Sinai West located in Midtown and the West Side, Manhattan; Mount Sinai Brooklyn located in Midwood, Brooklyn; and Mount Sinai Queens located in Astoria, Queens. Mount Sinai Beth Israel was not included in this cohort as they used a different EHR.
Inclusion Criteria
We included patients who were at least 18 years old, had a laboratory-confirmed SARS-CoV-2 infection, and were admitted to any of the aforementioned five MSHS hospitals between February 27 and 12 am on May 30, 2020, with follow-up data until June 5, 2020 (time of data freeze). A confirmed case of COVID-19 was defined by a positive RT-PCR assay of a specimen collected via nasopharyngeal swab. The Mount Sinai Institutional Review Board approved this research under a broad regulatory protocol allowing for analysis of patient-level data.
Exclusion Criteria
We excluded patients with known ESKD prior to admission and patients who were hospitalized for <48 hours (Supplemental Figure 1, Supplemental Table 1).
Data Collection
The dataset was obtained from different sources and aggregated by the Mount Sinai COVID Informatics Center (MSCIC; further description of MSCIC is provided in Supplemental Material). We obtained demographics, diagnosis codes (International Classification of Diseases 9/10 Clinical Modification [ICD-9/10-CM] codes), and procedures as well as vital signs and laboratory measurements during hospitalization. Demographics included age, sex, and language as well as race and ethnicity in the EHR. Racial groups included White, Black, Asian or Pacific Islander, and other. Ethnic groups included non-Hispanic/Latino, Hispanic/Latino, or unknown. Vital signs and laboratory values during the first 48 hours of admissions that were obtained as part of clinical care were included.
Definitions of Preexisting Conditions
We defined a preexisting condition as the presence of ICD-9/10-CM codes associated with specific diseases as per the Elixhauser Comorbidity Software.6,7
Definitions of Outcomes
AKI, the primary end point, was defined as per Kidney Disease Improving Global Outcomes (KDIGO) criteria: a change in the serum creatinine of 0.3 mg/dl over a 48-hour period or 50% increase in baseline creatinine.8 For patients with a previous serum creatinine in the 7–365 days prior to admission, the most recent serum creatinine value was considered the baseline creatinine. For patients without a baseline creatinine in the 7–365 days prior to admission, the admission creatinine was imputed on the basis of a Modification of Diet in Renal Disease (MDRD) eGFR of 75 ml/min per 1.73 m as per the KDIGO AKI guidelines.8 AKI stages were defined using the KDIGO AKI stage creatinine definitions: stage 1 as an increase in serum creatinine of ≥0.3 mg/dl or increase to ≥1.5–1.9 times baseline serum creatinine, stage 2 as an increase to >2–2.9 times from baseline serum creatinine, and stage 3 as an increase to more than three times baseline serum creatinine or a peak serum creatinine ≥4.0 mg/dl or if the patient received RRT during admission. Proteinuria was defined as a protein-creatinine ratio of ≥0.5, 1+ proteinuria or higher on dipstick (trace counted as no proteinuria), or ≥30 mg/dl on urinalysis. Leukocyturia was defined as more than five white blood cells per high-power field. Hematuria was defined as 1+ or higher on dipstick or urinalysis.
We assessed in-hospital mortality defined by survival status at discharge. A small proportion of patients (3%) was still admitted at the time of this manuscript preparation. These patients were not included in mortality analyses. The need for acute dialysis was ascertained by procedure codes and was crossreferenced with nursing flow sheets. For AKI recovery, we compared the last hospital creatinine and postdischarge creatinine with the baseline creatinine and grouped them as recovered or with acute kidney disease (AKD) stage 1, 2, or 3 (Supplemental Table 2).9
Statistical Analyses
Baseline characteristics were reported as medians and interquartile ranges (IQRs) for continuous variables. Categorical variables were summarized as counts and percentages. We used Kruskal–Wallis for continuous variables and chi-squared tests for comparison across groups. For survival analyses, we generated Kaplan–Meier survival curves, and comparison was done using the log-rank test. Patients who were discharged from the hospital were not censored and were still considered at risk for death. Patients who were still hospitalized at the time of data freeze were regarded as having a censored length of stay. Logistic regression models adjusted for covariates (adjusted for age; sex; race; comorbidities including CKD, hypertension, congestive heart failure, diabetes mellitus, liver disease, and peripheral vascular disease; laboratory values including white blood cell count, lymphocyte percentage, hemoglobin, platelets, sodium, potassium, aspartate aminotransferase, alkaline phosphatase, and albumin; and vitals including temperature, systolic BP, heart rate, respiratory rate, and oxygen saturation) were used to estimate the adjusted odds ratio (aOR) for death in patients with AKI versus without AKI. Covariates were chosen on the basis of univariate testing and physician input. We used local regression (loess) for smoothening creatinine values across the interval of 2 weeks posthospital admission to visualize trajectories by AKD category. All analyses were performed with R version 3.4.3 and SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
A consort diagram of included patients and outcomes is depicted in Supplemental Figure 1.
Demographic and Clinical Characteristics
From February 27 to May 30, 2020, 3993 COVID-19–positive patients who fulfilled our inclusion and exclusion criteria were hospitalized at one of five MSHS NYC hospitals. One hundred twenty-four (3%) of patients were still admitted at the time of manuscript drafting. Ninety-two percent of patients had a positive SARS-CoV-2 PCR test on the day of admission. Baseline creatinine was available prior to admission in 1455 (36%); for the patients without baseline creatinine, this was imputed using the MDRD equation as described in Methods. Patient demographics and preexisting conditions as well as vital signs and laboratory values stratified by AKI are displayed in Table 1. Patients who developed incident AKI were older and were more likely to have hypertension, congestive heart failure, diabetes mellitus, and CKD. Patients who developed AKI also had higher white blood cell count, lower lymphocyte percentages, and higher creatinine values. Other variables were statistically significant but likely not clinically significant given small absolute differences.
Table 1.
Patient characteristics of all patients and those with and without AKI
| Patient Characteristics | All, n=3993 | AKI, n=1835 | No AKI, n=2158 | P Value |
|---|---|---|---|---|
| Age, yr, median (IQR) | 64 (56–78) | 71 (61–81) | 63 (51–75) | <0.001 |
| Sex, n (%) | ||||
| Women | 1704 (43) | 734 (40) | 970 (45) | 0.002 |
| Race/ethnicity, n (%) | 0.002 | |||
| White | 954 (24) | 439 (24) | 515 (24) | |
| Black | 1054 (36) | 536 (29) | 518 (24) | |
| Hispanic | 1038 (26) | 439 (24) | 599 (28) | |
| Asian | 173 (4) | 74 (4) | 99 (5) | |
| Other or unknown | 774 (19) | 347 (19) | 427 (20) | |
| Comorbidities, n (%) | ||||
| Hypertension | 1527 (38) | 820 (45) | 707 (33) | <0.001 |
| Congestive heart failure | 396 (10) | 244 (13) | 152 (7) | <0.001 |
| Diabetes mellitus | 1019 (26) | 568 (31) | 451 (21) | <0.001 |
| Liver disease | 191 (5) | 99 (5) | 92 (4) | 0.09 |
| Peripheral vascular disease | 362 (9) | 194 (11) | 168 (8) | 0.002 |
| CKD | 420 (11) | 339 (18) | 81 (4) | <0.001 |
| Laboratory values, median (IQR) | ||||
| White blood cell count, 103/μl | 7.55 (5.4–10.9) | 8.68 (6–12.3) | 6.9 (5.1–9.55) | <0.001 |
| Lymphocyte percentage, % | 13.1 (8.1–20.6) | 10.6 (6–12.3) | 15.5 (9.8–23.1) | <0.001 |
| Hemoglobin, g/dl | 12.6 (11.1–13.8) | 12.3 (10.7–13.8) | 12.8 (11.5–13.9) | <0.001 |
| Platelets, 103/L | 214 (160–282) | 207 (151–272) | 221 (165–291) | <0.001 |
| Sodium, mEq/L | 139 (136–142) | 139 (136–143) | 138 (136–141) | <0.001 |
| Potassium, mEq/L | 4.2 (3.9–4.6) | 4.3 (3.9–4.8) | 4.1 (3.8–4.5) | <0.001 |
| Chloride, mEq/L | 104 (101–107) | 104 (100–109) | 103 (101–106) | <0.001 |
| Bicarbonate, mEq/L | 23 (20.5–26) | 22 (19–25) | 24 (21.8–26.1) | <0.001 |
| BUN, mg/dl | 18 (12–32) | 31 (18–51) | 13 (10–19) | <0.001 |
| Serum creatinine, mg/dl | 0.94 (0.72–1.41) | 1.42 (0.95–2.25) | 0.8 (0.67–0.97) | <0.001 |
| AST, U/L | 42 (28–68) | 48 (30–79) | 39 (27–60) | <0.001 |
| ALT, U/L | 30 (18–52) | 29 (18–53) | 30 (19–52) | <0.001 |
| Alkaline phosphatase, U/L | 75 (59–100) | 76 (60–102) | 73 (58–98) | 0.005 |
| Albumin, g/dl | 2.9 (2.6–3.3) | 2.8 (2.5–3.2) | 3 (2.7–3.4) | <0.001 |
| Vital signs, median (IQR) | ||||
| Temperature, °C | 36.9 (36.6–37.5) | 36.9 (36.5–37.4) | 37(36.6–37.5) | <0.001 |
| Systolic BP, mm Hg | 124 (111–140) | 125 (111–141.5) | 124 (112–138) | 0.40 |
| Diastolic BP, mm Hg | 71 (63–79) | 70 (61–80) | 72 (64–79) | <0.001 |
| Heart rate, BPM | 87 (76–98) | 87 (76–99) | 87 (76–97) | 0.08 |
| Respiratory rate, per min | 19 (10–20) | 20 (18–21) | 18 (18–20) | <0.001 |
| Oxygen saturation, % | 96 (94–98) | 96 (94–98) | 96 (95–98) | <0.001 |
| Discharged, n (%) | 3869 (97) | 1750 (95) | 2119 (98) | <0.001 |
| Hospital facility | <0.001 | |||
| Mount Sinai Hospital located in East Harlem, Manhattan | 1507 (38) | 662 (36) | 845 (39) | |
| Mount Sinai Morningside located in Morningside Heights, Manhattan | 699 (18) | 340 (19) | 359 (17) | |
| Mount Sinai West located in Midtown and the West Side, Manhattan | 480 (12) | 167 (9) | 313 (15) | |
| Mount Sinai Brooklyn located in Midwood, Brooklyn | 641 (16) | 345 (19) | 296 (14) | |
| Mount Sinai Queens located in Astoria, Queens | 666 (17) | 321 (18) | 345 (16) |
AST, aspartate transaminase; ALT, alanine transaminase; BPM, beats per minute.
Incidence and Severity of AKI
AKI occurred in 1835 patients (46%), and 347 (19%) of those with AKI required dialysis. In the 976 (24%) patients admitted to intensive care, 745 (76%) experienced AKI. The median time from hospital admission until AKI diagnoses was 1 (IQR, 1–4) day, and the median time from AKI diagnosis to dialysis need was 3 (IQR, 1–6) days. The proportions of patients with AKI in all patients and in the ICU varied by the five MSHS locations (Table 1, Supplemental Table 3). In all admissions, the proportions with stages 1, 2, and 3 AKI were 39%, 19%, and 42%, respectively. In those admitted to the ICU, the proportions were 28%, 17%, and 56%, respectively, and 32% required acute RRT (Figure 1). The median peak serum creatinine was 2.2 (IQR, 1.5–3.6) mg/dl in those who did not receive dialysis and was 8.2 (IQR, 6.1–11) mg/dl in those who did receive dialysis. The proportions of AKI and of AKI stages were not different when limited to only those with a prehospital baseline creatinine or when the nadir hospital creatinine was used as the baseline (data not shown).
Figure 1.
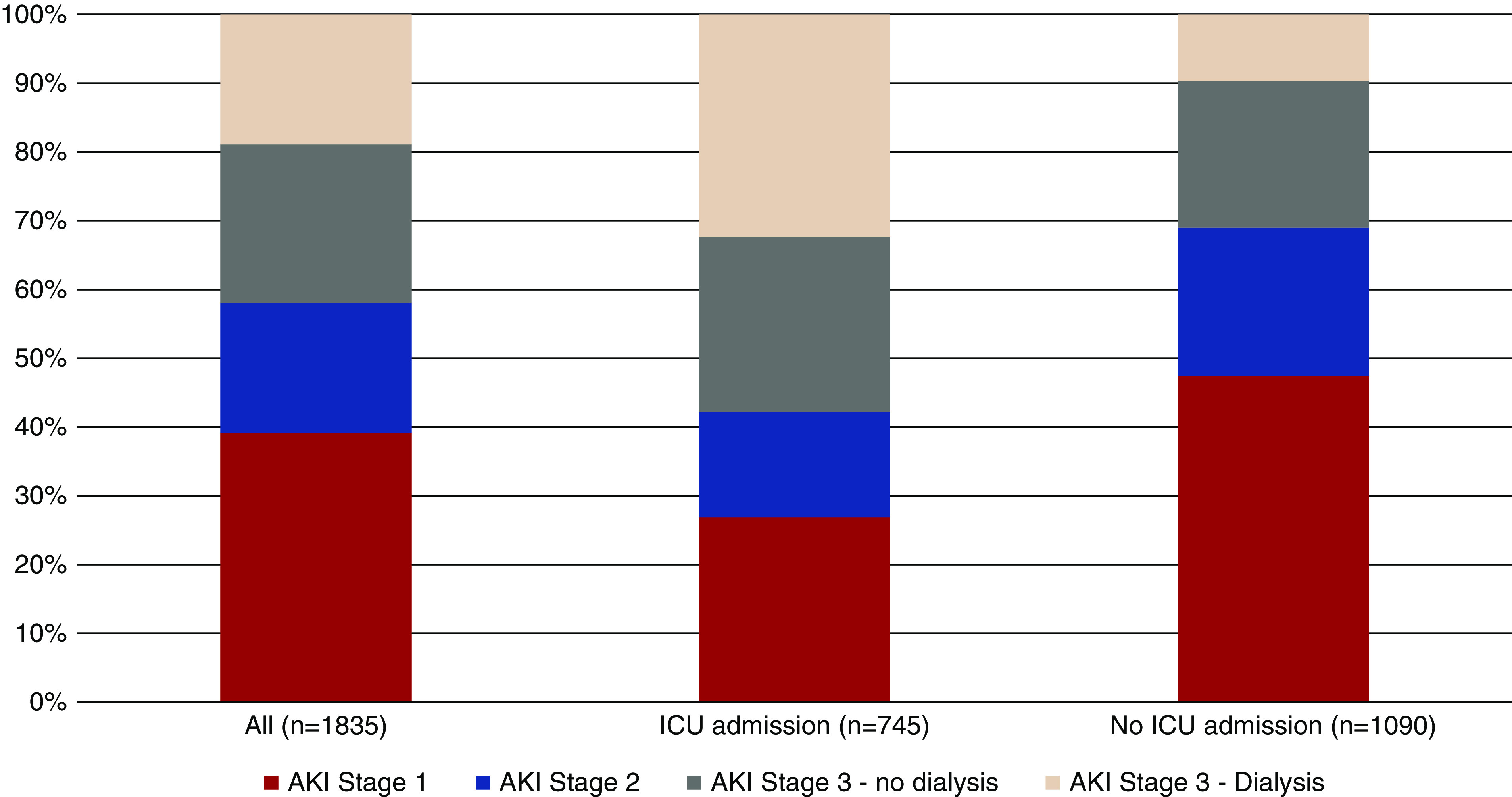
AKI requiring dialysis was common and varied by need for ICU admimssion. While 19% of patients overall required dialysis, 32% of patients who were admitted to the ICU required dialysis.
AKI incidence in patients who had a baseline creatinine was 49%, while in patients who had imputed baseline creatinine it was 45%, P=0.02. Urine studies were available for 656 (16%) patients, of whom 435 (66%) patients had AKI; 639 (97%) of all patients had any urinary abnormalities. Of the 435 patients with AKI and urine studies, 84% had proteinuria, 81% had hematuria, and 60% had leukocyturia. Proteinuria, hematuria, and leukocyturia were significantly more common in patients with AKI than patients without AKI (Supplemental Figure 2).
AKI Recovery
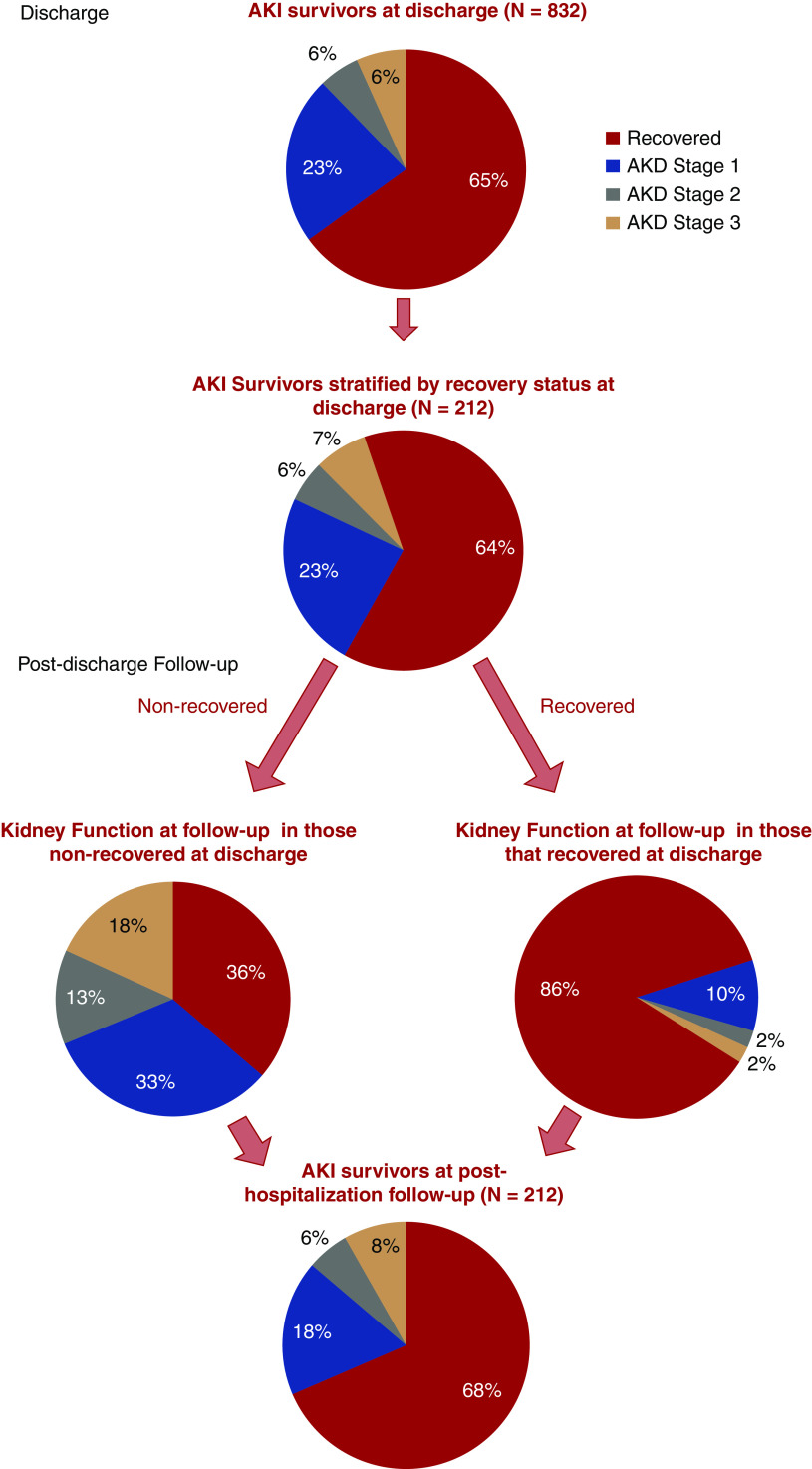
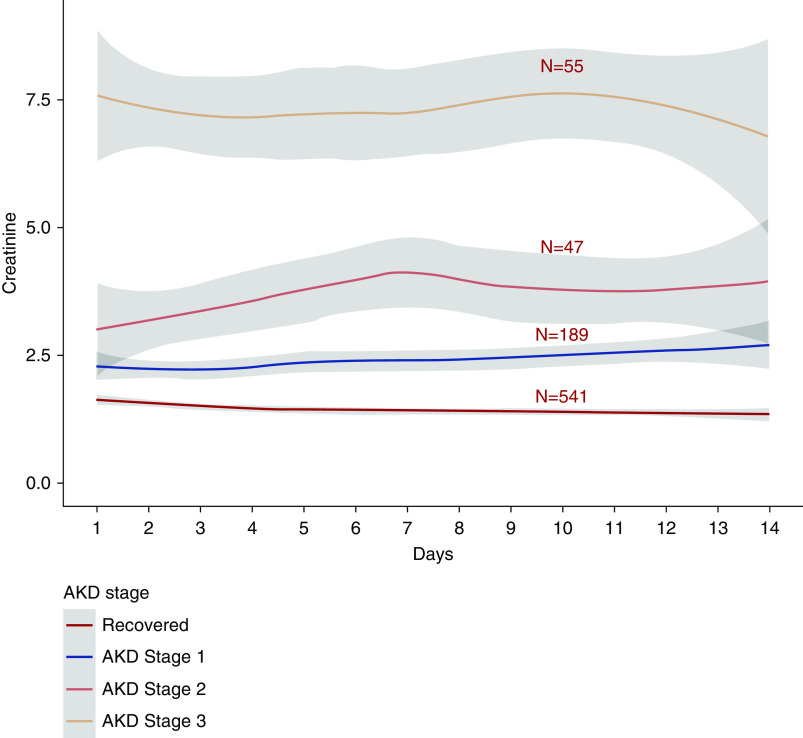
Among 1835 patients with AKI, 832 were discharged from the hospital. At hospital discharge, 541 of 832 (65%) had recovery of AKI, and 291 (35%) had AKD; the median serum creatinine at discharge was 1.1 (IQR, 0.8–1.6 mg/dl) (Figure 2). Only 30% (541 of 1835) of patients with AKI recovered kidney function and were discharged alive; 212 of 1835 (12%) patients who had AKI during their hospitalization had posthospitalization follow-up creatinine data at a median of 21 (IQR, 8–38) days after hospital discharge (Figure 2). On follow-up, of the 77 of 212 (36%) patients with AKD on discharge, 28 of 77 (36%) had recovery of AKD on follow-up. Of the 135 of 212 (64%) patients who had recovery of AKI at discharge, 18 of 135 (14%) had AKD on follow-up (Figure 2). Of the 87 of 832 patients who started on dialysis and were discharged alive, 26 (30%) patients required dialysis within 72 hours of discharge. At 7 days after AKI diagnoses or at discharge or death if it occurred before 7 days, 720 of 1835 (39%) had AKI recovery. The characteristics of patients by recovery and each AKD stage are presented in Supplemental Table 4. Creatinine trends by recovery group are presented in Figure 3 for differing subsets of the population.
Figure 2.
Approximately a third of patients did not have renal recovery at discharge or post-hospitalization follow up. Proportion of patients with AKI stratified by recovery versus nonrecovery at discharge and discharge follow-up. Recovery of kidney function was stratified into recover and AKD defined as (1) recovery: difference in creatinine is ≤0.3 and creatinine change in percentage is ≤25%; (2) stage 1: difference in creatinine is >0.3 and creatinine change in percentage is ≤25% or creatinine change in percentage is >25% and ≤100%; (3) stage 2: creatinine change in percentage is >100% and ≤200%; and (4) stage 3: creatinine change in percentage is >200% or requiring dialysis 72 hours prior to discharge.
Figure 3.
Trajectory of creatinine during the first 14 days of hospitalizations in patients who were discharged by recovery and nonrecovery which demonstrate the severity and prolonged nature of the AKI. We used local regression (loess) for smoothening creatinine values across the interval of 2 weeks posthospital admission to visualize trajectories by AKD category.
Independent Predictors of Severe AKI
Independent predictors of incident stage 3 AKI in patients (n=731) admitted to the hospital with COVID-19 were men (aOR, 1.46; 95% confidence interval [95% CI], 1.2 to 1.8), admission potassium (aOR, 1.7; 95% CI, 1.6 to 2.0), and CKD (aOR, 2.8; 95% CI, 2.1 to 3.7) (Supplemental Figure 3).
AKI and Outcomes
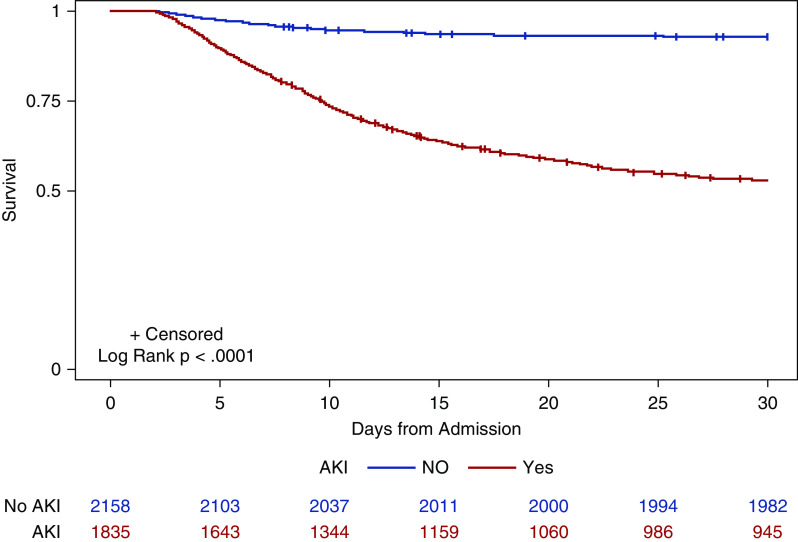
Patients with AKI were more likely to have ICU admissions, have mechanical ventilation, and require vasopressors administration (Supplemental Table 5). In-hospital mortality in patients who experienced AKI was 50%, and it was 8% in patients without AKI (P<0.001). In-hospital mortality percentages of patients with AKI in the ICU (42%), and non-ICU setting (62%) were markedly higher than those without AKI (ICU, 7% and non-ICU, 13%). The results of Kaplan–Meier survival analysis stratified by AKI status are presented in Figure 4. Patients with AKI had significantly lower survival than patients without AKI (log-rank P value <0.001). After adjustment for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 (95% CI, 7.2 to 18) for ICU with AKI versus no AKI, and the aOR for death was 9.2 (95% CI, 7.5 to 11.3) for all patients with AKI versus no AKI (Supplemental Table 6). There was an increasing risk of death with increasing stages of AKI, with the highest risk seen in patients with AKI stage 3 requiring dialysis (Supplemental Table 6).
Figure 4.
Survival probability is lower in patients with AKI. Kaplan–Meier survival curves for patients with and without AKI. Red line indicates patients with AKI, and blue line is for those without AKI. All patients were censored at 30 days. Patients who were discharged alive before 30 days were treated as still at risk and not censored at discharge.
Discussion
In nearly 4000 patients with COVID-19 admitted to MSHS, AKI occurred in nearly half of patients, and nearly a quarter of those patients required acute dialysis. AKI was independently associated with higher mortality. Many survivors (35%) did not recover kidney function by hospital discharge, and of all patients with AKI, only 30% survived and recovered kidney function.
Although the current COVID-19 pandemic is caused by a coronavirus like the severe acute respiratory syndrome coronavirus 1 outbreak in 2005, the reported incidence of AKI associated with COVID-19 disease seems to be substantially higher.10 Additionally, the incidence of AKI associated with COVID-19 is higher than reported in patients hospitalized with community-acquired pneumonia (34%) and severe sepsis (22%) in the United States.11,12 During the last pandemic in recent history, the 2009 pandemic influenza A (H1N1), AKI incidence was found to be high (34%–67%).13–15 Although the incidence of AKI may be similarly high between the two pandemics, the spread of COVID-19 far exceeds than that of the H1N1 pandemic: 2 million people with confirmed COVID-19 in the United States over the first 6 months compared with 1 million with confirmed H1N1 over a 10-year period. Reports suggest that rhabdomyolysis may have been a contributing factor to AKI from H1N1. Although we do not have creatinine kinase values, the presence of proteinuria, leukocyturia, and hematuria in patients with COVID-19–associated AKI suggests a different mechanism of injury.
The incidence of AKI in this study is higher than what has been reported in China and Italy and similar to the incidence in another NYC health care system.16 There are several key differences in the MSHS cohort, including higher proportions of patients with comorbidities of hypertension, diabetes, and CKD, which are risk factors for developing AKI.2 In our analysis, there was no association between hypertension and diabetes with severe AKI, but CKD was independently associated with severe AKI. As others have reported, patients who were admitted to the ICU had a higher incidence of AKI likely reflective of more severe disease.
Currently, the exact mechanisms of AKI due to COVID-19 are unclear. A postmortem patient series reported by Su et al.1 found significant acute tubular injury in all patients. A separate study found a high frequency of urinalysis abnormalities (hematuria, proteinuria, and leukocyturia) despite normal creatine values; which suggest renal pathology is not limited to acute tubular injury (Zhou H, Zhang Z, Fan H, Li J, Li M, Dong Y, et al.: Urinalysis, but not blood biochemistry, detects the early renal-impairment in patients with COVID-19. https://www.medrxiv.org/content/10.1101/2020.04.03.20051722v1.full.pdf, 2020). In this study, of patients who had urine studies sent, nearly all patients had urine abnormalities. Although urine abnormalities were more frequent in patients with AKI diagnosis, a large proportion of patients without AKI by creatinine criteria also had urine abnormalities. These findings are in contrast to studies in critically ill patients and patients undergoing cardiac surgery that demonstrated that only 33%–48% of patients had proteinuria and that 65% had hematuria.17–19 Given the high incidence of AKI and lack of full recovery at and after discharge, identification of potential mechanisms of COVID-19–related AKI would allow for potential interventions to reduce this devastating complication.
This study is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI. A third of patients with AKI in the setting of COVID-19 did not recover kidney function back to baseline. The low recovery rate is expected given the overall severity of AKI (high peak creatinine, need for dialysis), as well as the knowledge that many patients with COVID-19 have extensive acute tubular injury on tissue examination, potential microthrombi, and a high prevalence of proteinuria.1 A study in a Chinese cohort reports similar findings, with less than half of patients fully recovering kidney function.20 This is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days.21 It remains to be seen what the implications for post-AKI CKD, progression of prior CKD, and ESKD are for COVID-19–associated AKI.22–27 This will require long-term follow-up and further investigation.
Our study should be interpreted in light of the following limitations. Over half of patients did not have a baseline creatinine values. However, we used MDRD imputation as suggested by KDIGO guidelines, and the incidence was comparable for those with known baseline serum creatinine versus those who we imputed because of missing serum creatinine prior to admission.8 Although we presented data stratified by those who did and did not have ICU admissions, we did not examine the temporal relationship between the development of AKI and ICU admission. We did not have data regarding acute respiratory distress syndrome or ICU severity scores (e.g., Acute PHysiology and Chronic Health Evaluation or Sequential Organ Failure Assessment), which are often associated with AKI and need for dialysis. However, many features that make up these scores (age, vital signs, and laboratory values) were features that we included in our multivariable model. Recovery was assessed at time of discharge, and we only had postdischarge creatinine values in a subset of patients. As patients could receive dialysis care outside of the MSHS, we are unable to determine dialysis dependence at postdischarge follow-up. As the COVID-19 pandemic is still evolving, few long term follow-up laboratory test have been performed. We did not have sufficient data on inflammatory makers (e.g., IL-6, ferritin, and fibrinogen) in a large proportion of patients because they were not routinely checked early in the pandemic and a majority of patients did not have these values (Paranjpe I, Russak A, Freitas JKD, Lala A, Miotto R, Vaid A, et al.; Mount Sinai Covid Informatics Center [MSCIC]: Clinical characteristics of hospitalized Covid-19 patients in New York City. medRxiv:2020.04.19.20062117, 2020). Additionally, urinalysis was also missing in the majority of patients. Although we provide analysis of the subset of patients who did have urine studies obtained, this is likely a bias subset as the urine samples were obtained for a clinical reason. Lastly, baseline medications of interest, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and other nonsteroidal anti-inflammatory drugs, were not included.
In conclusion, in a diverse cohort of patients hospitalized with COVID-19 in NYC, we described a very high incidence of AKI, severe AKI requiring dialysis, and risk of death associated with AKI. We identified several predictors associated severe AKI and found that a third of patients did not recover kidney function at time of discharge. The results of this study may be useful to other centers for resource planning during the COVID-19 pandemic, for potential additional waves of COVID-19, and for preparing for the increased load of patients with COVID-19 and CKD due to severe AKI and lack of recovery.
Disclosures
S. Coca, J.C. He, B. Murphy, and G. Nadkarni receive financial compensation as consultants and advisory board members for RenalytixAI and own equity in RenalytixAI. Z. Fayad has a patent Trained Therapeutix discovery patents with royalties paid to Trained Therapeutix Discovery. B. Murphy is a nonexecutive director of RenalytixAI. B. Murphy reports personal fees from Renalytix AI, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
To all of the nurses, physicians, and providers who contributed to the care of these patients. To the patients and their family members who were affected by this pandemic.
Dr. Lili Chan, Dr. Steven Coca, and Dr. Girish Nadkarni conceptualized and designed the study; Dr. Lili Chan, Dr. Kumardeep Chaudhary, Dr. Steven Coca, and Dr. Giurish Nadkarni provided acquisition, analysis, or interpretation of data; Dr. Lili Chan, Dr. Kumardeep Chaudhary, Dr. Steven Coca, Dr. Girish Nadkarni, and members of MSCIC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; Dr. Lili Chan, Dr. Steven Coca, and Dr. Girish Nadkarni drafted the manuscript; Dr. Kumardeep Chaudhary, Dr. Kinsuk Chauhan, Dr. Aparna Saha, and Dr. Akhil Vaid provided statistical analysis; members of MSCIC provided administrative, technical, or material support; Dr. Steven Coca and Dr. Girish Nadkarni provided supervision; and all authors critically revised the manuscript for important intellectual content.
Dr. Lili Chan reports grants from the National Institutes of Health and Renal Research Institute, outside the submitted work. In the past 3 years, Dr. Steven Coca has received consulting fees from Bayer, Boehringer-Ingelheim, CHF Solutions, Goldfinch Bio, inRegen, pulseData, Quark, Relypsa, and Takeda Pharmaceuticals, and National Institutes of Health grants U01DK106962, R01DK115562, R01HL085757, U01OH011326, R01DK112258, and RRTI UG 2019 outside the submitted work. Dr. Zahi Fayad reports grants from Amgen, Bristol Myers Squibb, Daiichi Sankyo, and Siemens Healthineers and personal fees from Alexion, GlaxoSmithKline, and Trained Therapeutix Discovery, outside the submitted work. Dr. John C. He reports fees from RenalytixAL and grants from Shangpharma, outside the submitted work. In the past 3 years, Dr. Girish Nadkarni has received consulting fees from AstraZeneca, BioVie, GLG Consulting, Pensieve Health, and Reata, grant support from Goldfinch Bio, National Institutes of Diabetes and Digestive and Kidney Diseases Career Development Award K23DK107908, and National Institutes of Health grants R01DK108803, U01HG007278, and U01DK116100, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Lili Chan, Kumardeep Chaudhary, Aparna Saha, Kinsuk Chauhan, Akhil Vaid, Shan Zhao, Ishan Paranjpe, Sulaiman Somani, Felix Richter, Riccardo Miotto, Anuradha Lala, Arash Kia, Prem Timsina, Li Li, Robert Freeman, Rong Chen, Jagat Narula, Allan C. Just, Carol Horowitz, Zahi Fayad, Carlos Cordon-Cardo, Eric Schadt, Matthew A. Levin, David L. Reich, Valentin Fuster, Barbara Murphy, John C. He, Alexander W. Charney, Erwin P. Böttinger, Benjamin S. Glicksberg, Steven G. Coca, and Girish N. Nadkarni
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050615/-/DCSupplemental.
Supplemental Figure 1. Study flow diagram.
Supplemental Figure 2. Proportion of patients with hematuria, leukocyturia, and proteinuria by AKI status in a subset of patients with urine studies (N=656).
Supplemental Figure 3. Patient characteristics that were associated with severe AKI (stage 3).
Supplemental Summary 1. Supplemental methods: Mount Sinai COVID Informatics Center data platform and investigators.
Supplemental Table 1. ICD codes used for identification of ESKD cohort; ESKD was defined as having both an ESKD diagnosis code and an ESKD procedure code.
Supplemental Table 2. Definitions of acute kidney disease.
Supplemental Table 3. Incidence of AKI in patients admitted to the ICU by MSHS hospital (P<0.06).
Supplemental Table 4. Patient characteristics by kidney recovery and acute kidney disease stages excluding patients who were still admitted to the hospital.
Supplemental Table 5. Outcomes in patients with AKI and without AKI and the full cohort (n=3993).
Supplemental Table 6. Adjusted and unadjusted OR of in-hospital mortality in patients with outcomes data available.
References
- 1.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. : Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intensive Care National Audit & Research Centre : ICNARC report on COVID-19 in critical care, 2020. Available at: https://www.icnarc.org/DataServices/Attachments/Download/c31dd38d-d77b-ea11-9124-00505601089b. Accessed April 21, 2020
- 4.Abelson R, Fink S, Kulish N, Thomas K: An overlooked, possibly fatal coronavirus crisis: A dire need for kidney dialysis. New York Times, 2020. Available at: https://www.nytimes.com/2020/04/18/health/kidney-dialysis-coronavirus.html. Accessed April 24, 2020
- 5.Goldfarb DS, Benstein JA, Zhdanova O, Hammer E, Block CA, Caplin NJ, et al. : Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol 15: 880–882, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality : Elixhauser Comorbidity Software, Version 3.7. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 25, 2020
- 7.Package comorbidity, 2020. Available at: https://cran.r-project.org/web/packages/comorbidity/comorbidity.pdf. Accessed April 15, 2020
- 8.Kidney Disease: Improving Global Outcomes (KDIGO): Acute Kidney Injury (AKI). Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed April 10, 2020
- 9.Duff S, Murray PT: Defining early recovery of acute kidney injury [published online ahead of print April 1, 2020]. Clin J Am Soc Nephrol doi:10.2215/CJN.13381019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. : Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67: 698–705, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, et al. ; Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators : Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77: 527–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Pettilä V, Webb SA, Bailey M, Howe B, Seppelt IM, Bellomo R: Acute kidney injury in patients with influenza A (H1N1) 2009. Intensive Care Med 37: 763–767, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NKJ; Canadian Critical Care Trials Group H1N1 Collaborative : Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: Cohort study. BMC Nephrol 14: 123, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirjian SG, Raina R, Bhimraj A, Navaneethan SD, Gordon SM, Schreiber MJ Jr., et al. : 2009 influenza A infection and acute kidney injury: Incidence, risk factors, and complications. Am J Nephrol 34: 1–8, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. ; and the Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar AO, Parikh CR, Sint K, Coca SG, Koyner J, Patel UD, et al. : Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin J Am Soc Nephrol 7: 1749–1760, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SS, Ahn SY, Ryu J, Baek SH, Chin HJ, Na KY, et al. : Proteinuria and hematuria are associated with acute kidney injury and mortality in critically ill patients: A retrospective observational study. BMC Nephrol 15: 93, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeter H, Yıldırım T, Eyüpoğlu D, Paşayev T, Aslan A, Çetik S, et al. : Mild to moderate proteinuria is a heralding sign for acute kidney injury and mortality for intensive care unit patients. Turk J Med Sci 49: 543–550, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. : Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, et al. ; Centers for Disease Control and Prevention CKD Surveillance Team : Acute kidney injury recovery pattern and subsequent risk of CKD: An analysis of veterans health administration data. Am J Kidney Dis 67: 742–752, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basile DP: Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, et al. : Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol 288: F953–F963, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP: Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, et al. ; for the TRIBE-AKI Consortium : Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25: 1063–1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.