Significance Statement
Steroid resistance, relapse, and side effects are common issues in use of high-dose steroids as first-line treatment for adult minimal change nephrotic syndrome. Tacrolimus is used as a steroid-sparing immunosuppressant to reduce adverse effects of long-term or repeated steroid treatment, but no large-scale randomized study has compared combined tacrolimus and low-dose steroid with high-dose steroid in treating minimal change nephrotic syndrome in adults. In this open-label randomized trial, the authors found that treatment with tacrolimus plus low-dose steroid was noninferior to high-dose steroid for complete remission at 8 weeks, and that treatment with a maintenance dose of tacrolimus during steroid tapering reduced the relapse rate, with no clinically-relevant safety differences. This indicates that tacrolimus is an effective alternative to high-dose steroids in this disease, although investigation into long-term safety is warranted.
Keywords: corticosteroid, glomerulonephritis, nephrotic syndrome, immunosuppression, tacrolimus, remission
Visual Abstract
Abstract
Background
Tacrolimus is used as a steroid-sparing immunosuppressant in adults with minimal change nephrotic syndrome. However, combined treatment with tacrolimus and low-dose steroid has not been compared with high-dose steroid for induction of clinical remission in a large-scale randomized study.
Methods
In this 24-week open-label noninferiority study, we randomized 144 adults with minimal change nephrotic syndrome to receive 0.05 mg/kg twice-daily tacrolimus plus once-daily 0.5 mg/kg prednisolone, or once-daily 1 mg/kg prednisolone alone, for up to 8 weeks or until achieving complete remission. Two weeks after complete remission, we tapered the steroid to a maintenance dose of 5–7.5 mg/d in both groups until 24 weeks after study drug initiation. The primary end point was complete remission within 8 weeks (urine protein: creatinine ratio <0.2 g/g). Secondary end points included time until remission and relapse rates (proteinuria and urine protein: creatinine ratio >3.0 g/g) after complete remission to within 24 weeks of study drug initiation.
Results
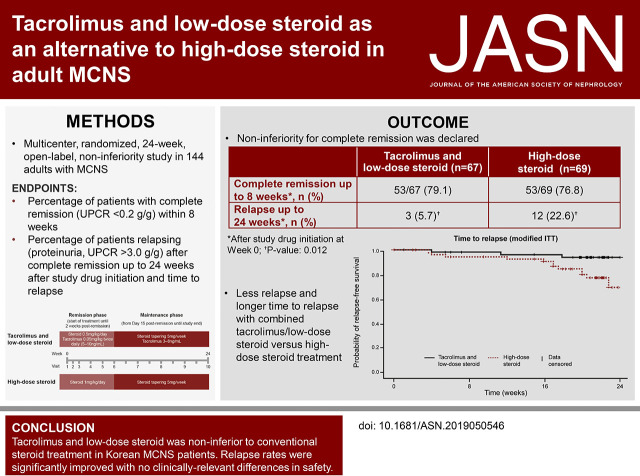
Complete remission within 8 weeks occurred in 53 of 67 patients (79.1%) receiving tacrolimus and low-dose steroid and 53 of 69 patients (76.8%) receiving high-dose steroid; this difference demonstrated noninferiority, with an upper confidence limit below the predefined threshold (20%) in both intent-to-treat (11.6%) and per-protocol (17.0%) analyses. Groups did not significantly differ in time until remission. Significantly fewer patients relapsed on maintenance tacrolimus (3–8 ng/ml) plus tapered steroid versus tapered steroid alone (5.7% versus 22.6%, respectively; P=0.01). There were no clinically relevant safety differences.
Conclusions
Combined tacrolimus and low-dose steroid was noninferior to high-dose steroid for complete remission induction in adults with minimal change nephrotic syndrome. Relapse rates were significantly lower with maintenance tacrolimus and steroid compared with steroid alone. No clinically-relevant differences in safety findings were observed.
Minimal change nephrotic syndrome (MCNS) is a major cause of primary GN.1,2 It is characterized by nephrotic-range proteinuria with minimal abnormality on light microscopy, or deposits on immunofluorescence microscopy and diffuse podocyte foot process loss on electron microscopy.3,4 Whereas the etiology of GN varies by race,5–9 in Korea, adult MCNS accounts for 15.5% of all primary GN and remains the leading cause of nephrotic syndrome.6,10
MCNS is the most common form of pediatric nephrotic syndrome, but in adulthood, other nephropathies predominate and MCNS frequency reduces dramatically.7,11,12 Whereas proteinuria is a key feature of MCNS in both adults and children, hematuria, hypertension, and AKI are features that are more likely to be observed in adults.1,2
Owing to the high incidence of pediatric disease, most clinical data for MCNS originate from studies in children, who are highly responsive to corticosteroids.3,13 The paucity of randomized controlled trials in adults, and lack of high-quality clinical evidence to guide management, results in a continued prominence of “tried and tested” high-dose corticosteroid as first-line therapy.3,14 However, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines acknowledge that therapeutic response is less predictable in adults than in children, with only 75% of adults with MCNS responding to steroid treatment.3 Adults tend to respond more slowly than children, with more than one half experiencing relapse, and up to one third becoming either frequent relapsers or steroid-dependent.1,2 For these patients requiring chronic or repeated courses of steroid treatment, drug-related adverse events (AEs) are common3 and steroid-sparing immunosuppressants, such as cyclophosphamide or calcineurin inhibitors (cyclosporin and tacrolimus), are used to reduce the negative effects of long-term steroids.1,3
Cyclophosphamide and cyclosporin are the most widely used second-line agents.1 However, tacrolimus is a more potent immunosuppressant than cyclosporin15 with a more favorable renal safety profile.16–18 It also compares favorably with cyclophosphamide for inducing remission in steroid-dependent/steroid-resistant adults with MCNS,19–21 and is effective as rescue therapy for patients who have failed treatment with other immunosuppressants.21–23 In addition to its immunosuppressive action, tacrolimus acts directly on podocytes24,25 to stabilize the actin cytoskeleton and reduce levels of angiopoietin-like protein 4, a protein associated with podocyte injury and the pathogenesis of proteinuria in MCNS.24–27
Early cotherapy with combined tacrolimus and low-dose steroid may be beneficial for patients with MCNS with respect to steroid-sparing and podocyte protection.19,25 Tacrolimus monotherapy after short-term intravenous methylprednisolone has been shown to be noninferior to conventional steroid treatment for adult-onset MCNS,28 and a small, noncomparative pilot study (n=14) has shown that combined tacrolimus and low-dose steroid therapy can induce clinical remission rapidly and effectively with a low rate of AEs in adults with MCNS.29 The study presented here aims to build on these preliminary data by comparing, for the first time, the efficacy and safety of combined tacrolimus and low-dose steroid with high-dose steroid alone in a large-scale randomized controlled trial.
Methods
Study Design and Objective
This was an open-label, parallel group, multicenter, 24-week, randomized controlled trial conducted at 15 tertiary care teaching institutions located in urban areas in Korea between July 2012 and August 2017 (Clinical Trials.gov: NCT01763580). The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, International Conference on Harmonisation Guideline for Good Clinical Practice and applicable laws and regulations. Institutional review board-approved, written informed consent was obtained from each patient before the study. Parents of study participants aged 16–18 years were required to sign the consent form in addition to the patient.
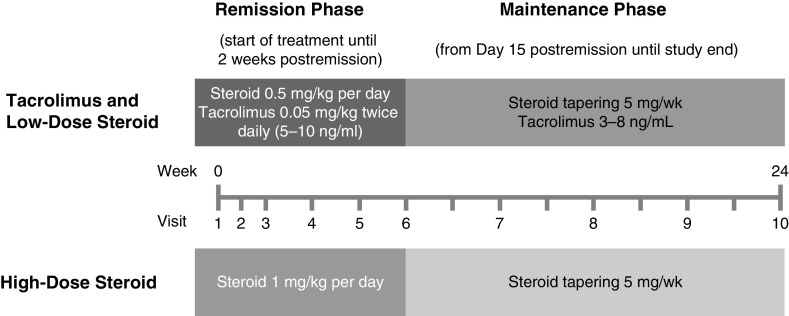
The objective of this study was to investigate if treatment with tacrolimus has any benefits compared with standard therapy for (1) early response and (2) relapse in the longer term. The study included two separate phases. The remission phase comprised Week 0 to Day 14 postremission in patients who achieved remission within 8 weeks and Weeks 0−8 in patients who did not achieve remission. Patients who did not achieve remission within 8 weeks were discontinued from the study. The remission phase compared a combined tacrolimus and low-dose steroid regimen with standard high-dose steroid monotherapy to determine noninferiority for induction of remission. The maintenance phase in patients achieving remission comprised Day 15 after complete remission to within Week 24 after initiating study medication. The steroid dose was tapered during this phase in both treatment arms because of the potential for long-term side effects. The objective of the maintenance phase was to investigate the effect of continued treatment with a maintenance dose of tacrolimus on the rate of relapse during steroid tapering (Figure 1).
Figure 1.
Schematic representation of the study design. This was a 24-week study comprising a remission phase and a maintenance phase. The remission phase comprised Week 0 to Day 14 postremission in patients who achieved remission within 8 weeks, and Weeks 0−8 in patients who did not achieve remission. Remission was defined as urine protein: creatinine ratio <0.2 g/g. Patients who did not achieve remission within 8 weeks were discontinued from the study. The remission phase continued for 2 weeks after remission was achieved, with the steroid dose maintained. Therefore, the end of the remission phase and the start of the maintenance phase varied from patient to patient. For example, a patient achieving remission at Week 2 would start the maintenance phase 2 weeks later at Week 4. By contrast, patients achieving remission at Week 8 would have their dose maintained for an additional 2 weeks before commencing the maintenance phase at Week 10 (these patients would still end the study at Week 24 after initiation of study medication). Blood tacrolimus trough concentration was maintained within the range of 5–10 ng/ml. The maintenance phase was the period from Day 15 after complete remission to within 24 weeks after initiation of study medication; the steroid dose was decreased by 5 mg/wk in both groups to a maintenance dose of 7.5 mg/d for patients weighing ≥80 kg, and 5 mg/d for patients weighing <80 kg. Blood tacrolimus trough concentration was maintained within the range of 3–8 ng/ml throughout the maintenance phase.
Study Patients
Eligible patients were aged 16–79 years with renal biopsy-confirmed primary MCNS (initial or relapsed) and urine protein: creatinine ratio (UPCR) ≥3.0 g/g at screening (spot urine). Patients who fell under at least one of the following criteria were excluded: Modification of Diet in Renal Disease eGFR <30 ml/min per 1.73 m2, treatment with immunosuppressants within 2 weeks of the study, daily administration of >10 mg prednisolone (or equivalent dose of steroid) within 2 weeks of study, current bosentan or potassium-sparing diuretic treatment, live vaccine within the previous 4 weeks, serum bilirubin of >3.6 mg/dl for >1 month or liver test results ≥3× upper limit of normal, hypersensitivity to tacrolimus, prednisolone or macrolide antibiotics, genetic problems (galactose intolerance, Lapp lactose deficiency, or glucose-galactose malabsorption) or significant general disease that made participation inappropriate, pregnant/breastfeeding or planned pregnancy within 6 months of study completion, or failure to consent to the use of contraception.
Randomization and Treatment
Remission phase: at Visit 1 (screening, Week 0), eligible patients were randomized (1:1) to receive either combined treatment with twice-daily 0.05 mg/kg tacrolimus (Prograf; Astellas Pharma Europe Ltd., Addlestone, UK) and once-daily 0.5 mg/kg prednisolone, or once-daily 1 mg/kg prednisolone only (not to exceed 80 mg/d). Blood tacrolimus trough concentration was maintained at 5–10 ng/ml (Figure 1). A randomization schedule for each study center was prepared using SAS 9.1, (SAS Institute Inc., Cary, NC) on the basis of block randomization involving mixing blocks 4 and 6 in each stratum, with study center as the stratification factor. The randomization schedule was provided by the Medical Research Collaborating Center of Seoul National University Medical School/Hospital. Because this was an open-label study, there was no allocation concealment or blinding. The study site pharmacy and the sponsor retained the randomization information for back-up purposes.
Maintenance phase: from Day 15 after complete remission, the steroid dose was decreased by 5 mg/wk in both groups to a maintenance dose of 7.5 mg/d for patients weighing ≥80 kg, and to 5 mg/d for patients who weighed <80 kg. Patients were not required to achieve the target maintenance dose of steroid at the same time in the high- and low-dose steroid groups. Patients received instructions for steroid dose reduction at outpatient visits and through weekly phone calls. The blood tacrolimus level was maintained within the range of 3–8 ng/ml throughout the maintenance phase. The last visit was at Week 24 after initiation of study medication.
End Points
The primary study end point was complete remission (defined as UPCR <0.2 g/g) within 8 weeks. Protocol-designated secondary efficacy end points included the time taken to achieve complete remission, relapse rates (observed proteinuria; UPCR >3.0 g/g) from complete remission to within 24 weeks after initiating study medication, and the time from complete remission to relapse and from first dose of study medication to relapse (after remission). AEs, drug-related AEs, and abnormal clinical findings were assessed over 24 weeks, and classified according to Spiker’s three-level severity scheme. Vital signs (weight, BP, body temperature, and pulse) and laboratory parameters were assessed at each study visit, and chest x-rays were taken to assess cardiothoracic ratio at Visits 3 and 5 (Weeks 2 and 6). Adherence to study medication was assessed by returned capsules.
Statistical Analyses
The purpose of this study was to compare complete remission rates with low-dose steroid and tacrolimus versus standard, high-dose steroid therapy, after 8 weeks of treatment. Nakayama et al.30 studied the remission rates in subjects with adult-onset MCNS treated with high-dose steroids and compared them with rates from five other similar studies and found the 8-week complete response rates ranged anywhere from approximately 50% to 95%. Similarly, a study by Park et al.31 resulted in a complete remission rate of 78% after 8 weeks of treatment with high-dose steroids. Since the time of these studies, treatment for MCNS patients has not appreciably changed. Therefore, the true rate of complete remission after 8 weeks of treatment with high-dose steroids is assumed to be 80%. From these estimates, a 20% noninferiority margin was deemed clinically meaningful and the corresponding required sample size was computed to be 64 patients per group, with a target number of 76 per group after adjusting for an assumed 15% drop-out rate. For these calculations, the Power Analysis and Sample Size software was used with a 2.5% significance level (one-sided) and 80% power.
The main efficacy analysis was performed in the modified intent-to-treat (ITT) population, comprising all patients who were randomized and treated with at least one dose of study medication. Supportive analyses were carried out for the per-protocol set (PPS), which included patients who had adhered to treatment (received ≥80% of the anticipated doses and ≤10% more than the anticipated doses; assessed by comparing prescribed and returned study drug products) at every visit and had followed the study protocol. Safety analyses were carried out on the safety set and all patients who received at least one dose of study medication, and was on the basis of the actual treatment administered, irrespective of the randomized treatment. Missing data for the analyses of complete remission and relapse after complete remission to within 24 weeks after initiating study medication were imputed using the last observation carried forward (LOCF) method. Missing values for other efficacy and safety variables were not imputed. Hence, patients with missing values at baseline were excluded from analyses of change from baseline efficacy and safety outcomes.
To demonstrate noninferiority of combined tacrolimus and low-dose steroid to high-dose steroid alone, a one-sided 95% confidence interval (95% CI) was calculated for the difference in remission rates and noninferiority was assessed using the prespecified margin of 20%. If the upper limit of the 95% CI for the difference (high-dose steroid – tacrolimus and low-dose steroid) between treatment groups was <20%, then noninferiority was declared.
Treatment groups were compared using the log-rank test for both the time taken to achieve remission and the time from remission to relapse. Pearson’s chi-square test was used to compare between the treatment groups the percentage of patients who relapsed within 24 weeks after initiating study medication. The time to relapse from complete remission to within 24 weeks after initiating study medication was defined as the period from the complete remission day to the time when relapse was confirmed. Data for patients without relapse up to the last visit day, and those lost to follow-up or withdrawn from the study, were censored in the survival rate analysis. The numbers and percentages of patients with adherence of ≥80% were reported for the modified ITT population and compared between treatment groups using Pearson’s chi-squared test. The percentages of AEs occurring within 24 weeks after initiating study medication were evaluated after the administration of the investigational drug and compared by treatment group using Pearson’s chi-squared test or Fisher’s exact test. For vital signs and laboratory test results, continuous variables were compared with those of Visit 1 (screening visit) in both groups. The changes in value from Visit 1 were compared using a t test or Wilcoxon rank sum test.
Results
Study Population
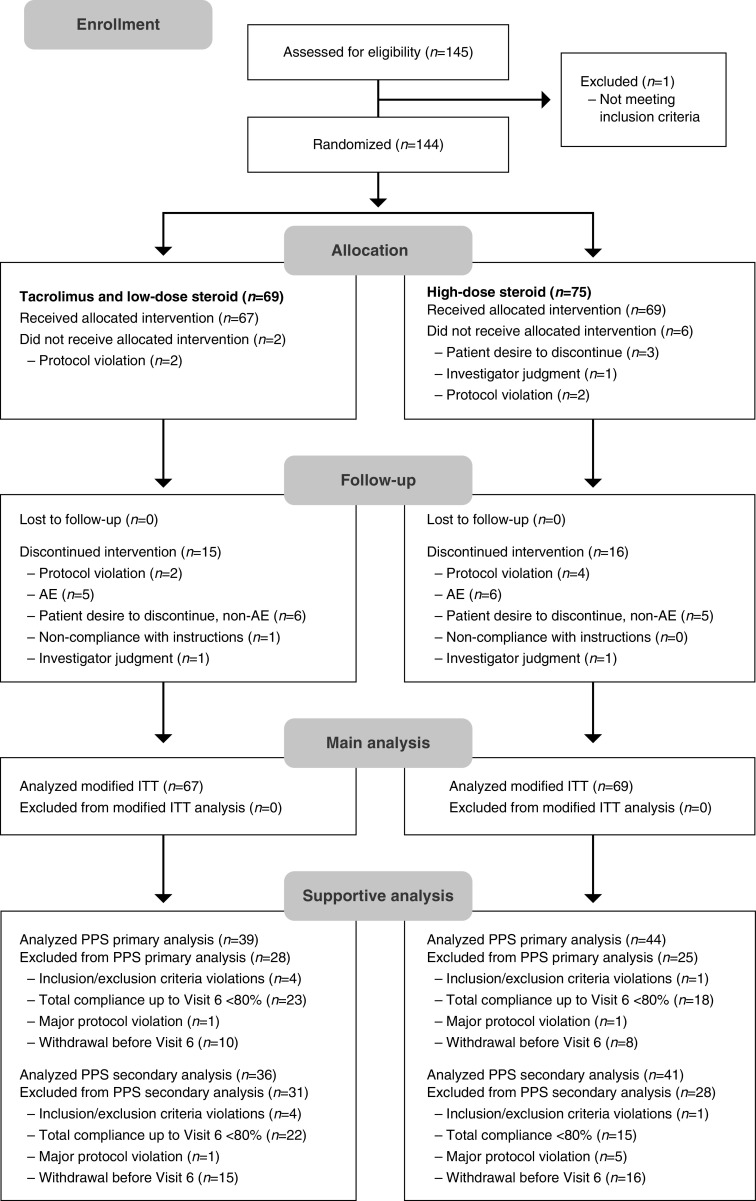
Overall, 144 patients were randomized (tacrolimus and low-dose steroid, 69; high-dose steroid, 75), of whom five were aged <19 years, and 113 patients completed the study (Figure 2). At least one dose of study medication was received by 136 patients (modified ITT; see Supplemental Table 1 for mean dosages). During the study, 31 patients were withdrawn from the modified ITT set (tacrolimus and low-dose steroid, 15; high-dose steroid, 16; Figure 2), and were included in the main analysis using LOCF. Reasons for discontinued intervention during follow-up are shown in Figure 2.
Figure 2.
Flow chart of study populations, including the number of patients who were assessed for eligibility, randomized, received at least one dose of treatment (modified ITT), and followed the study protocol (PPS). Tacrolimus and low-dose steroid: tacrolimus 0.05 mg/kg twice daily combined with prednisolone 0.5 mg/kg per day. High-dose steroid: 1 mg/kg per day prednisolone.
Around one third of discontinuations were due to non-AE-related patient desires to discontinue (tacrolimus and low-dose steroid, 6/15; high-dose steroid, 5/16) and another one third (tacrolimus and low-dose steroid, 5/15; high-dose steroid, 6/16) were due to AEs (tuberculosis, alopecia, low abdominal pain, and leg weakness in the combined group; pneumonia, bronchitis, facial edema, worsening of facial edema, palpitations, and herpes zoster in the high-dose steroid group).
The PPS included 83 patients (tacrolimus and low-dose steroid, 39; high-dose steroid, 44) for the analysis of complete remission rate and 77 patients (tacrolimus and low-dose steroid, 36; high-dose steroid, 41) for the analysis of the other efficacy end points. Any patient who discontinued intervention was excluded from the PPS (supportive) analyses. Reasons for exclusion from the PPS are summarized in Figure 2; most exclusions were due to compliance <80%. There were no clinically significant differences in baseline characteristics in the two treatment groups (Table 1). There was no marked difference in the allocation of subjects presenting with MCNS for the first time and with relapsing MCNS across the two treatment groups (Table 1).
Table 1.
Baseline characteristics of adult patients with MCNS in each treatment group (modified ITT)
| Patient Characteristics | Tacrolimus and Low-Dose Steroid (n=67) | High-Dose Steroid (n=69) |
|---|---|---|
| Sex, men, n (%) | 45 (67.2) | 40 (58.0) |
| Age, yr | 41.8±16.7 | 42.2±17.8 |
| Median (min, max) | 40 (18, 78) | 39 (16, 79) |
| (95% CI) | (37.7 to 45.9) | (37.9 to 46.5) |
| Weight, kg | 68.9±13.9 | 64.4±13.0 |
| Median (min, max) | 67 (47, 129.8) | 63.2 (42, 115) |
| (95% CI) | (65.5 to 72.2) | (61.3 to 67.5) |
| BP, mm Hg | ||
| Systolic | 122.2±14.4 | 121.5±13.2 |
| Median (min, max) | 122 (96, 168) | 120.5 (99, 183) |
| (95% CI) | (118.7 to 125.7) | (118.3 to 124.7) |
| Diastolic | 76.1±9.0 | 75.8±9.9 |
| Median (min, max) | 75 (58, 97) | 75 (58, 122) |
| (95% CI) | (73.9 to 78.3) | (73.4 to 78.2) |
| Serum albumin, g/dl | 2.3±0.7 | 2.4±0.8 |
| Median (min, max) | 2.2 (1.1, 4.0) | 2.3 (0.9, 4.1) |
| (95% CI) | (2.2 to 2.5) | (2.3 to 2.6) |
| Creatinine, mg/dl | 0.9±0.3 | 0.9±0.4 |
| Median (min, max) | 0.84 (0.45, 1.8) | 0.82 (0.34, 2.4) |
| (95% CI) | (0.8 to 1.0) | (0.8 to 1.0) |
| MDRD eGFR, ml/min per 1.73 m2a | 95.9±33.7 | 97.6±39.6 |
| Median (min, max) | 90.2 (34.6, 216.6) | 93.3 (34.0, 240.8) |
| (95% CI) | (87.7 to 104.1) | (88.1 to 107.2) |
| UPCR, g protein/g creatinine | 8.4±3.9 | 9.2±6.5 |
| Median (min, max) | 7.58 (3.3, 20.0) | 7.8 (1.1, 34.8) |
| (95% CI) | (7.4 to 9.3) | (7.6 to 10.7) |
| Blood glucose, mg/dl | 100.9±22.0 | 100.8±16.2 |
| Median (min, max) | 96 (77, 203) | 99 (65, 152) |
| (95% CI) | (95.6 to 106.3) | (96.9 to 104.7) |
| LDL cholesterol, mg/dl | 243.9±117.8 | 217.7±102.7 |
| Median (min, max) | 235.5 (27.0, 524.0) | 195 (72.2, 531.0) |
| (95% CI) | (214.5 to 273.4) | (192.3 to 243.1) |
| MCNS first presentation/relapse, n | 32/35 | 36/33 |
Values expressed as mean ± SD, unless stated otherwise. MDRD eGFR, Modification of Diet in Renal Disease estimated glomerular filtration rate.
Four patients had an eGFR <45 ml/min per 1.73 m2 at baseline, and eGFR improved by Week 8 in three of these patients (follow-up data missing for the fourth patient).
Primary Efficacy
For the modified ITT analysis, the numbers of patients achieving complete remission (UPCR <0.2 g/g) within 8 weeks were 53/67 (79.1%) in patients receiving combined tacrolimus and low-dose steroid, and 53/69 (76.8%) in patients receiving high-dose steroid (Table 2). The difference in the 8-week complete remission rates between groups was −2.3%. Because the upper confidence limit (11.6%) was below the predefined threshold of 20%, noninferiority was declared. Similar results were obtained from the PPS analysis, with 33/39 (84.6%) patients and 38/44 (86.4%) patients receiving combined tacrolimus and low-dose steroid, and high-dose steroid, respectively, achieving complete remission. The difference in the 8-week complete remission rates between groups was +1.8%. The upper confidence level limit for the difference between groups in the PPS was 17.0%, below the predefined threshold for noninferiority.
Table 2.
Complete remission rates and relapse after complete remission to within 24 weeks after study drug initiation, by study group (modified ITT and PPS)
| Parameter | Tacrolimus and Low-Dose Steroid | High-Dose Steroid | P Value |
|---|---|---|---|
| Modified ITT set | |||
| Patients who showed complete remission within 8 wk after study drug initiation, n (%) | 53/67 (79.1) | 53/69 (76.8) | |
| Patients who showed relapse after complete remission to within 24 wk after study drug initiation, n (%) | 3/53 (5.7) | 12/53 (22.6) | 0.01a |
| Median time from complete remission to relapse within 24 wk after study drug initiation (d) | –b | –b | 0.02c |
| Time from complete remission to 25% relapse within 24 wk after study drug initiation (d) | –d | 161 | – |
| PPS | |||
| Patients who showed complete remission within 8 wk after study drug initiation, n (%) | 33/39 (84.6) | 38/44 (86.4) | |
| Patients who showed relapse after complete remission to within 24 wk after study drug initiation, n (%) | 1/31 (3.2) | 8/36 (22.2) | 0.02a |
| Median time from complete remission to relapse within 24 wk after study drug initiation (d) | –b | –b | 0.03c |
| Time from complete remission to 25% relapse within 24 wk after study drug initiation (d) | –d | 161 | – |
P value by Pearson’s chi-squared test.
As relapse-free survival rate was not decreased to <50% in both groups, median time from complete remission to relapse could not be estimated.
P value by log-rank test comparing the full relapse time distributions from complete remission to within 24 weeks.
As 25% relapse was not reached in the tacrolimus and low-dose steroid group, time from complete remission to 25% relapse could not be estimated.
Secondary Efficacy
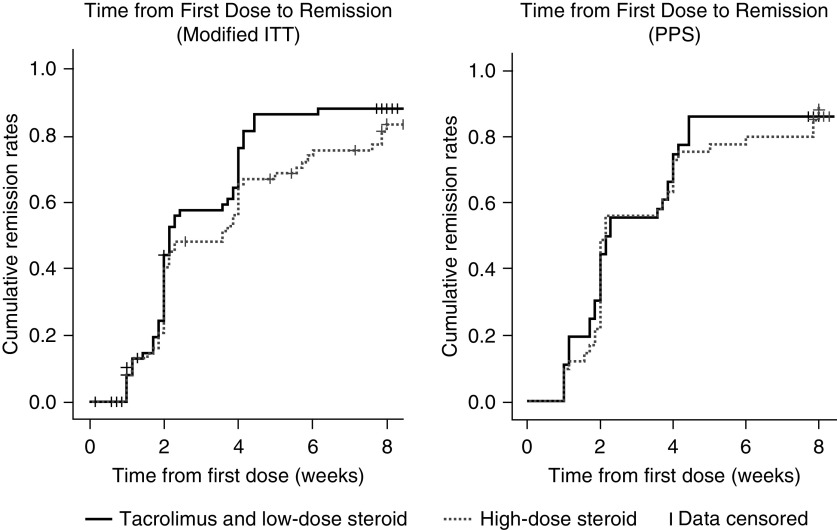
For the modified ITT analysis, the median time to complete remission was 15 days (95% CI, 14 to 27) for patients receiving combined tacrolimus and low-dose steroid, and 25 days (95% CI, 14 to 28) for those receiving high-dose steroid. The log-rank test showed no statistically significant difference between the treatments (P=0.16). For the PPS analysis, the median times to complete remission were 15 days (95% CI, 14 to 27) and 15 days (95% CI, 14 to 28) for patients receiving combined tacrolimus and low-dose steroid, and high-dose steroid, respectively (P=0.79). Kaplan–Meier survival curves of the time to onset of remission for the 24-week study period are shown in Figure 3.
Figure 3.
Kaplan–Meier estimates of time to onset of remission for patients receiving combined tacrolimus and low-dose steroid compared with those receiving high-dose steroid (modified ITT and PPS). Patients were discontinued at Week 8 if they did not achieve remission; therefore, no remission events could occur after Week 8. However, one patient in the tacrolimus group was erroneously considered to have achieved remission by Week 8 and remained in the study. The patient achieved remission at Week 16. This patient was excluded from the PPS analysis. Tacrolimus and low-dose steroid: tacrolimus 0.05 mg/kg twice daily combined with prednisolone 0.5 mg/kg per day. High-dose steroid: 1 mg/kg per day prednisolone.
In the modified ITT population, relapse (UPCR >3.0 g/g among patients with complete remission) occurred in 3/53 (5.7%) patients receiving combined tacrolimus and tapered steroid, and 12/53 (22.6%) of patients receiving tapered steroid alone (Table 2). Pearson’s chi-squared test showed a significant difference between the treatments (P=0.01). Similar results were observed for the PPS; 1/31 (3.2%) versus 8/36 (22.2%) patients receiving tacrolimus and steroid and steroid alone, respectively, experienced relapse (P=0.02).
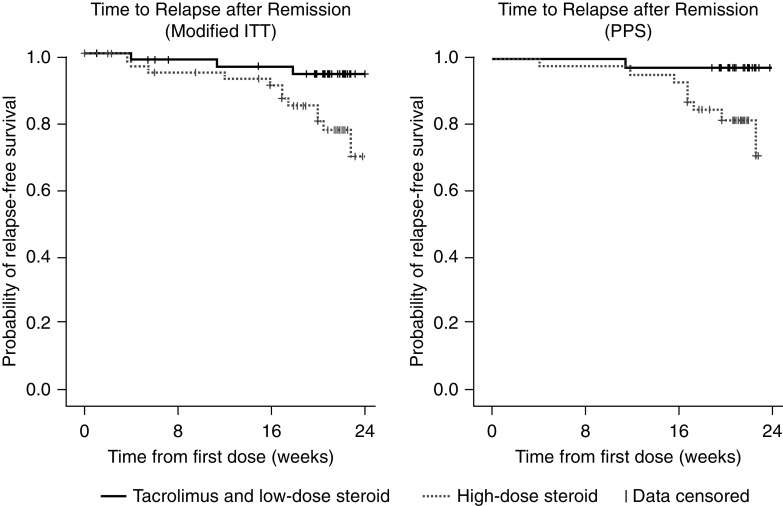
The relapse-free survival rate was >50% in both groups, thereby preventing estimation of the median time to relapse. Kaplan–Meier survival curves of the time from first dose of study medication to relapse are shown in Figure 4. Comparing the full relapse time distributions from complete remission to within 24 weeks, the time to relapse was significantly longer in the low-dose compared with the high-dose steroid group (Table 2; modified ITT, log-rank P=0.02; PPS, P=0.03). For the modified ITT population and PPS, the estimated first quartile of relapse times was 161 days in the high-dose steroid group and was not achieved in the low-dose group. In the low-dose group, only 5.7% of patients relapsed within the 24-week (168-day) follow-up period (modified ITT).
Figure 4.
Kaplan–Meier estimates of the time to relapse from complete remission to within 24 weeks after study drug initiation, by study group (modified ITT and PPS). Curves show the probability of remaining relapse-free at different time points for patients receiving combined tacrolimus and low-dose steroid compared with those receiving high-dose steroid. Data for patients without relapse up to the last visit day, who were lost to follow-up or were withdrawn from the study, were censored. Tacrolimus and low-dose steroid: tacrolimus 0.05 mg/kg twice daily combined with prednisolone 0.5 mg/kg per day. High-dose steroid: 1 mg/kg per day prednisolone.
Medication Adherence
Overall medication adherence (≥80%) in the modified ITT set was not significantly different across the two treatment groups. This was achieved by 45 (67.2%) patients treated with combined tacrolimus and low-dose steroid, and 54 (78.3%) patients treated with high-dose steroid (P=0.15).
Safety
Of the 136 randomized patients in the safety population, 49 patients out of 67 (73.1%) in the combined tacrolimus and low-dose steroid group, and 47 out of 69 (68.1%) in the high-dose steroid group, experienced AEs at least once during the 24-week study period (P=0.52). The total number of AEs was 260 (127, tacrolimus and low-dose steroid; 133, high-dose steroid). Most AEs were classified as mild or moderate, with two cases of severe AEs reported for each treatment group.
The most frequent treatment-emergent AEs are summarized for each treatment group (Table 3). AEs with the highest incidence in both treatment groups were “gastrointestinal disorders” (Table 3). This was followed by “nervous system disorders” in the combined tacrolimus and low-dose steroid group, and “general disorders and administration-site conditions,” and “infections and infestations” in the high-dose steroid group. Fifteen serious AEs were reported, ten in the combined tacrolimus and low-dose steroid group and five in the high-dose steroid group (Supplemental Table 2). Four of these were considered drug-related; three in the combined tacrolimus and low-dose steroid group (one gastrointestinal disorder; two infections and infestations) and one in the high-dose steroid group (infection and infestations). There was no significant difference in the number of patients who discontinued due to drug-related AEs; five patients (7.5%) discontinued in the combined tacrolimus and low-dose steroid group and six patients (8.7%) in the high-dose steroid group (P=0.79). Six patients (9.0%) in the combined tacrolimus and low-dose steroid group reported treatment-emergent AEs related to renal and urinary disorders: noninfective cystitis, hematuria, pollakiuria and ARF (reported by one patient each), and oliguria (reported by two patients). None of these AEs were considered drug-related and the case of ARF resolved without sequelae. One case of insomnia was reported, and no events related to mood or behavior change occurred.
Table 3.
Treatment-emergent AEs with the preferred term occurring in ≥5% of patients in either treatment group
| System Organ Class | Tacrolimus and Low-Dose Steroid (n=67) | High-Dose Steroid (n=69) |
|---|---|---|
| Gastrointestinal disordersa | 16 (23.9) | 12 (17.4) |
| Abdominal pain | 7 (10.4) | 2 (2.9) |
| Abdominal pain upper | 4 (6.0) | 2 (2.9) |
| Diarrhea | 8 (11.9) | 1 (1.5) |
| Dyspepsia | 2 (3.0) | 8 (11.6) |
| General disorders and administration-site conditions | 9 (13.4) | 11 (15.9) |
| Face edema | 1 (1.5) | 5 (7.2) |
| Generalized edema | 4 (6.0) | 0 |
| Edema | 4 (6.0) | 7 (10.1) |
| Infections and infestationsb | 7 (10.4) | 11 (15.9) |
| Nasopharyngitis | 7 (10.4) | 11 (15.9) |
| Investigations | 1 (1.5) | 4 (5.8) |
| ALT increased | 1 (1.5) | 4 (5.8) |
| Nervous system disorders | 10 (14.9) | 3 (4.3) |
| Headache | 7 (10.4) | 2 (2.9) |
| Paresthesia | 4 (6.0) | 1 (1.5) |
| Respiratory, thoracic, and mediastinal disorders | 4 (6.0) | 4 (5.8) |
| Cough | 4 (6.0) | 4 (5.8) |
Values expressed as number of patients (%). Patients could have more than one AE. ALT, alanine aminotransferase.
Includes one drug-related serious AE in the tacrolimus and low-dose steroid group.
Includes three drug-related serious AEs; two in the tacrolimus and low-dose steroid group, and one in the high-dose steroid group.
There were some statistically significant differences in laboratory test results between the two treatment groups, which were not considered clinically significant (Supplemental Table 3).32 These included changes from baseline to end of study (Week 24) in hemoglobin (P=0.006), red blood cells (P=0.02) and hematocrit (P=0.01), total cholesterol (P=0.004), total protein (P=0.02), albumin (P=0.003), total bilirubin (P<0.001), aspartate aminotransferase (P<0.001), serum creatinine (P=0.04), LDL-cholesterol (P=0.03), and triglyceride (P=0.004). Change in body weight from baseline to Week 24 was also significantly different between the two groups (P=0.002). No other significant differences in laboratory tests, physical examination, chest x-ray/ECG, or vital sign parameters were detected between the two treatment groups at 24 weeks.
Discussion
To our knowledge, this is the first randomized controlled trial to directly compare combined tacrolimus and low-dose steroid treatment with standard high-dose steroid in adult patients with MCNS. Tacrolimus combined with low-dose steroid (0.5 mg/kg per day prednisolone) was noninferior to high-dose steroid (1 mg/kg per day) for complete remission, with a statistically significant reduction in relapse over the 24-week duration of the study. This was observed for both the modified ITT population (n=136) and smaller PPS (n=83).
The 8-week remission rate in the high-dose steroid group (76.8%) was within the expected range for adult MCNS.1,3,30 A similar remission rate of 79.1% was seen in patients who received combined tacrolimus and low-dose steroid. The remission rate in the combined treatment group is also consistent with previous studies in Asian patients where remission rates after combined tacrolimus and steroid (0.5 mg/kg per day) treatment were around 50% at 4 weeks, between 70% and 80% at 12 weeks,19 and 58% at 8 weeks in a study of steroid-resistant patients.20 Median time to remission in our study was 15 days (remission follow-up time 8 weeks). Other trials have reported mean times to remission of 32 and 49 days (remission follow-up time 24 weeks and 24 months, respectively),19,20 and mean time to remission was 2.6 weeks/18 days in a trial of tacrolimus monotherapy after 10 days of intravenous methylprednisolone (remission follow-up 36 weeks).28 However, aside from not reporting median values, the times to remission in these three studies are not directly comparable with our trial because they have very different remission follow-up times. The small-scale pilot study demonstrated a greater cumulative remission rate of 92.9% (13/14) by Week 8.29 However, it should be noted that the pilot study was conducted in 14 patients at a single center.29
The KDIGO guidelines recommend high-dose steroid treatment for up to 16 weeks to achieve complete remission,3 whereas our results suggest that 8 weeks of steroid treatment may be sufficient. However, the effect of longer-term treatment on relapse remains uncertain. Whereas studies in children suggest that extending treatment does not reduce relapse rates,13,33 a recent study in an adult population with MCNS found that shorter treatment duration was an independent predictor of relapse.34 The same study found that combined treatment with calcineurin inhibitors did not show any protective effect on relapse. In contrast, the relapse rate in our study within 24 weeks after initiating study medication was significantly reduced for patients receiving combined tacrolimus and steroid, with a relapse rate just one quarter of that observed for patients receiving steroid alone (5.7% versus 22.6%, respectively, P=0.01). This might be expected because the steroid dose was tapered in both groups, but blood trough concentrations of tacrolimus were maintained at 3–8 ng/ml, which would continue to provide substantial immunosuppression. However, in a previous trial of similar design, comparing combined cyclosporin and prednisolone (0.8 mg/kg per day) with prednisolone alone (1.0 mg/kg per day) in Japanese patients with MCNS,35 no significant difference in remission rate was observed (1, 3, and 6 months) and, in contrast to our study, relapse at 6 months was similar between the two treatment groups (23.1% versus 19.2%, respectively).35 This apparent difference between tacrolimus and cyclosporin in the ability to maintain remission versus standard treatment, and potential differential effects on podocyte protection, warrants further investigation. In a randomized trial of tacrolimus monotherapy (after 10 days of intravenous methylprednisolone) versus standard steroid, high rates of complete remission were observed (92.9% versus 96.2%, respectively), but only around one half of patients in each treatment group maintained remission over the 36-week study (54.5% versus 51.0%).28 Tacrolimus was tapered after 16–20 weeks to maintain blood trough concentrations at 2–5 ng/ml, and the mean time to relapse was around 24 weeks for both treatments. Future comparative studies should further taper or discontinue tacrolimus, and extend the follow-up period beyond 24 weeks, to determine if there is a true reduction in relapse or if the combined tacrolimus and low-dose steroid treatment only delays the time to relapse versus standard treatment.
In our study, the overall incidence of AEs and the number of patients who discontinued due to AEs were similar across the two treatment groups. Although there were some differences in laboratory test results, these were not deemed to be of any clinical significance and no new safety signals were detected. Drug-induced nephrotoxicity is a major concern for the long-term use of calcineurin inhibitors.18 However, in our study, and a previous study of similar design in patients with nephrotic syndrome and slight mesangial proliferation,36 combined tacrolimus and steroid treatment showed no drug-induced nephrotoxicity, with no increase from baseline in serum creatinine after 24 weeks. Furthermore, a recent trial in patients with MCNS comparing mycophenolate sodium, considered less toxic than tacrolimus, combined with low-dose steroid treatment for 24 weeks showed similar rates of clinical remission and relapse to conventional treatment (high-dose steroid 1 mg/kg per day), with the same rate of serious AEs (15.5% per group).37
A strength of our study was that noninferiority analyses were carried out for both the modified ITT population and PPS. US Food and Drug Administration (FDA) guidance for noninferiority clinical trials emphasizes that ITT analysis may favor the test group, with potential bias toward no treatment difference.38 The fact that both analyses concluded noninferiority adds credibility to our findings, especially because more than one third of patients in the modified ITT analysis were excluded in the PPS analysis, mainly because of low adherence.
Analyses according to MCNS status (first presentation or relapsing) or baseline eGFR were not planned in this randomized study. However, additional post hoc analyses indicated that there was no significant interaction between treatment and either MCNS status or eGFR for remission rates within 8 weeks, in either the modified ITT or PPS (data not shown).
Our study did not demonstrate any clear safety benefits of steroid reduction. This may be because of a relatively low proportion of steroid-resistant/intolerant individuals enrolled, but without better characterization of the patient population it is difficult to speculate. Furthermore, because the timing of initiating dose reduction varied between patients, it was ambiguous to analyze AEs by study phase; this should be addressed in future studies.
This study was associated with the limitations typical of open-label studies, such as reporting bias due to lack of allocation concealment and blinding. The study was also of relatively short duration and did not follow up on patients after tapering and/or discontinuation of tacrolimus. Whereas there was no evidence of any deleterious effect of combined tacrolimus and low-dose steroid (e.g., on renal function), longer-term safety follow-up will be required to check for any emergence of toxic effects and information about remission maintenance in the longer term. Although only patients with MCNS were to be included in this study, four patients had an eGFR <45 ml/min per 1.73 m2 at baseline. Whereas eGFR improved by Week 8 in three of these patients (follow-up data were missing for the fourth patient), no further biopsies were taken, and the pathology of patients who did not respond to treatment was not further investigated. Therefore, FSGS cannot be definitively ruled out in these patients.
The high withdrawal rate (approximately 20% in both study groups) was of some concern, and use of the LOCF method to impute outcomes for >20% of patients in the primary analysis was a limitation. Approximately one third of withdrawals resulted from AEs, one third from withdrawal of patient consent, and the remainder mainly from noncompliance or protocol violation. Unlike most life-threatening diseases, MCNS has no major symptoms except edema, which might lead to lack of motivation among patients to participate in trials or adhere to medication, especially if they have concerns about the safety of steroids. In this study, the upper limit of the one-sided 95% CI was used to determine noninferiority between treatment groups, which is less stringent than using the upper limit of a two-sided 95% CI (equivalent to the upper limit of a one-sided 97.5% CI), as specified in FDA guidance. A further limitation is that the study was conducted in a single country, and thus is largely confined to patients of a single ethnicity (Korean); as such, the findings require confirmation in MCNS patients of other ethnicities. However, the value of this randomized, comparative, controlled trial of >130 patients for adult MCNS should not be underestimated. High-quality evidence on which to base clinical decisions in adult MCNS is sparse and is mainly on the basis of case reports or small-scale observational studies. To put this into context, oral corticosteroids are recommended as the first-line treatment for adult MCNS in the KDIGO guidelines on the basis of two randomized, placebo-controlled trials in around 30 patients.3 In the absence of more large-scale comparative trials to aid evidence-based decision making, dominance of corticosteroids for the initial treatment of MCNS is likely to continue. It is important that strategies are optimized for the induction and maintenance of remission in patients with nephrotic syndrome, particularly as proteinuria is independently associated with low health-related quality of life and high depression scores.39
In summary, our results show that combined tacrolimus and low-dose steroid was noninferior to high-dose steroid treatment for clinical remission in adult patients with MCNS. Relapse rates and relapse-free survival were significantly improved with no clinically relevant differences in safety findings. Thus, combined tacrolimus and low-dose steroid represents an important therapeutic option to limit exposure to steroids, especially for patients with contraindications or intolerance to standard treatment.
Disclosures
H. Jiang and H. Lee are Employees of Astellas Pharma. All remaining authors have nothing to disclose.
Funding
All authors report non-financial support from Astellas Pharma, Inc. and other from Astellas Pharma, Inc., during the conduct of the study.
Data Sharing Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Supplementary Material
Acknowledgments
The authors wish to thank Prof. Suhnggwon Kim and Prof. Kyu Hun Choi for their assistance in designing the study, Ms. Dahae Yim and Ms. Juhee Lee from the Medical Research Collaborating Center (MRCC) of Seoul National University Medical School/Hospital for work on the statistical analysis, Mr. Minsu Kwon (MRCC) for IT management, Ms. Young-shin Kim (MRCC) for designing the database, managing the randomization of the data, and site auditing of the study, Mr. Jin Sang Jung for reviewing the manuscript, and Dr. Lisa O’Rourke and Dr. Anisha Mehra who drafted the initial version of the manuscript for Cello Health MedErgy. Editorial support was provided by Cello Health MedErgy throughout the development of the manuscript, and funded by Astellas Pharma, Inc.
All authors participated in the kick-off meeting in which the content of the manuscript was agreed, critically reviewed the manuscript, and provided direction relating to important intellectual content; all authors approved the final version for submission. Ho Jun Chin, Dong-Wan Chae, Yong Chul Kim, Won Suk An, Chun Gyoo Ihm, Dong-Chan Jin, Sung Gyun Kim, Yong-Lim Kim, Yong-Soo Kim, Yoon-Goo Kim, Ho Seok Koo, Jung Eun Lee, Kang Wook Lee, Jieun Oh, Jung Hwan Park, and Sang Koo Lee undertook the acquisition of data for the work. Hongsi Jiang and Hyuncheol Lee contributed to the conception and design of the study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050546/-/DCSupplemental.
Supplemental Table 1. Daily dosage in the remission and maintenance phases of the study and at each visit.
Supplemental Table 2. Serious treatment-emergent adverse events.
Supplemental Table 3. Laboratory test changes from baseline showing a significant between-group difference at Week 8 and Week 24 after study drug initiation.
Clinical study protocol.
References
- 1.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al.: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Szeto CC, Lai FM, Chow KM, Kwan BC, Kwong VW, Leung CB, et al.: Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 65: 710–718, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed October 22, 2020 [Google Scholar]
- 4.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, et al.: Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74: 1568–1576, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Chanchlani R, Parekh RS: Ethnic differences in childhood nephrotic syndrome. Front Pediatr 4: 39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al.: Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant 24: 2406–2410, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP; Italian Immunopathology Group, Italian Society of Nephrology: The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S, Leung N, Lager DJ, Melton LJ 3rd, Bergstralh EJ, Rohlinger A, et al.: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Huang JJ, Hsu SC, Chen FF, Sung JM, Tseng CC, Wang MC: Adult-onset minimal change disease among Taiwanese: Clinical features, therapeutic response, and prognosis. Am J Nephrol 21: 28–34, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al.: Mortality and renal outcome of primary glomerulonephritis in Korea: Observation in 1,943 biopsied cases. Am J Nephrol 37: 74–83, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Rivera F, López-Gómez JM, Pérez-García R; Spanish Registry of Glomerulonephritis: Clinicopathologic correlations of renal pathology in Spain. Kidney Int 66: 898–904, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz M, Halon A, Okon K, Wozniak A, et al.; Polish Society of Nephrology: Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 32: ii209–ii218, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Hahn D, Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2015: CD001533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canetta PA, Radhakrishnan J: The evidence-based approach to adult-onset idiopathic nephrotic syndrome. Front Pediatr 3: 78, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi H, Okuyama K, Konno O, Jojima Y, Akashi I, Nakamura Y, et al.: Optimal dose and target trough level in cyclosporine and tacrolimus conversion in renal transplantation as evaluated by lymphocyte drug sensitivity and pharmacokinetic parameters. Transplant Proc 37: 1745–1747, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Prasad N, Manjunath R, Rangaswamy D, Jaiswal A, Agarwal V, Bhadauria D, et al.: Efficacy and safety of cyclosporine versus tacrolimus in steroid and cyclophosphamide resistant nephrotic syndrome: A prospective study. Indian J Nephrol 28: 46–52, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SS, Hafeez F: Comparison of efficacy of tacrolimus versus cyclosporine in childhood steroid-resistant nephrotic syndrome. J Coll Physicians Surg Pak 26: 589–593, 2016 [PubMed] [Google Scholar]
- 18.Nankivell BJ, PʼNg CH, OʼConnell PJ, Chapman JR: Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: Comparison of cyclosporine and tacrolimus eras. Transplantation 100: 1723–1731, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Li X, Li H, Chen J, He Q, Lv R, Lin W, et al.: Tacrolimus as a steroid-sparing agent for adults with steroid-dependent minimal change nephrotic syndrome. Nephrol Dial Transplant 23: 1919–1925, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Li H, Shi X, Shen H, Li X, Wang H, Li H, et al.: Tacrolimus versus intravenous pulse cyclophosphamide therapy in Chinese adults with steroid-resistant idiopathic minimal change nephropathy: A multicenter, open-label, nonrandomized cohort trial. Clin Ther 34: 1112–1120, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Li H, Ye H, Li Q, He X, Zhang X, et al.: Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR. Am J Kidney Dis 54: 51–58, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Li X, Xu N, Li H, Han F, Wang R, He Q, et al.: Tacrolimus as rescue therapy for adult-onset refractory minimal change nephrotic syndrome with reversible acute renal failure. Nephrol Dial Transplant 28: 2306–2312, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Gao X, Bian R, Mei C, Xu C: Tacrolimus improves proteinuria remission in adults with cyclosporine A-resistant or -dependent minimal change disease. Nephrology (Carlton) 22: 251–256, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Liao R, Liu Q, Zheng Z, Fan J, Peng W, Kong Q, et al.: Tacrolimus protects podocytes from injury in Lupus Nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS One 10: e0132724, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JS, Chen X, Peng L, Wei SY, Zhao SL, Diao TT, et al.: Angiopoietin-like-4, a potential target of tacrolimus, predicts earlier podocyte injury in minimal change disease. PLoS One 10: e0137049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, et al.: Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macé C, Chugh SS: Nephrotic syndrome: Components, connections, and angiopoietin-like 4-related therapeutics. J Am Soc Nephrol 25: 2393–2398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Liu Z, Wang L, Wang R, Ding G, Shi W, et al.: Tacrolimus monotherapy after intravenous methylprednisolone in adults with minimal change nephrotic syndrome. J Am Soc Nephrol 28: 1286–1295, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YC, Lee TW, Lee H, Koo HS, Oh KH, Joo KW, et al.: Complete remission induced by tacrolimus and low-dose prednisolone in adult minimal change nephrotic syndrome: A pilot study. Kidney Res Clin Pract 31: 112–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fujimi S: Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis 39: 503–512, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Heo NJ, Lee JP, Park DJ, Seong EY, Ju GU: Adult minimal change nephrotic syndrome: Treatment response and relapse. Kidney Res Clin Pract 23: 419–428, 2004 [Google Scholar]
- 32.Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE: Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat Rev Nephrol 14: 57–70, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teeninga N, Kist-van Holthe JE, van Rijswijk N, de Mos NI, Hop WC, Wetzels JF, et al.: Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 24: 149–159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H, Yoo KD, Oh YK, Kim DK, Oh KH, Joo KW, et al.: Predictors of relapse in adult-onset nephrotic minimal change disease. Medicine (Baltimore) 95: e3179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eguchi A, Takei T, Yoshida T, Tsuchiya K, Nitta K: Combined cyclosporine and prednisolone therapy in adult patients with the first relapse of minimal-change nephrotic syndrome. Nephrol Dial Transplant 25: 124–129, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Miao L, Sun J, Yuan H, Jia Y, Xu Z: Combined therapy of low-dose tacrolimus and prednisone in nephrotic syndrome with slight mesangial proliferation. Nephrology (Carlton) 11: 449–454, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rémy P, Audard V, Natella PA, Pelle G, Dussol B, Leray-Moragues H, et al.; MSN Trial Investigators: An open-label randomized controlled trial of low-dose corticosteroid plus enteric-coated mycophenolate sodium versus standard corticosteroid treatment for minimal change nephrotic syndrome in adults (MSN Study). Kidney Int 94: 1217–1226, 2018 [DOI] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services; Food and Drug Administration; Center for Biologics Evaluation and Research (CBER): Non-Inferiority Clinical Trials to Establish Effectiveness. Guidance for Industry, 2016. Available at: https://www.fda.gov/media/78504/download. Accessed October 22, 2020
- 39.Libório AB, Santos JP, Minete NF, Diógenes CA, Soares AP, Queiroz AL, et al.: Proteinuria is associated with quality of life and depression in adults with primary glomerulopathy and preserved renal function. PLoS One 7: e37763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.