Abstract
In the past few decades, sphingolipids and sphingolipid metabolites have gained attention because of their essential role in the pathogenesis and progression of kidney diseases. Studies in models of experimental and clinical nephropathies have described accumulation of sphingolipids and sphingolipid metabolites, and it has become clear that the intracellular sphingolipid composition of renal cells is an important determinant of renal function. Proper function of the glomerular filtration barrier depends heavily on the integrity of lipid rafts, which include sphingolipids as key components. In addition to contributing to the structural integrity of membranes, sphingolipid metabolites, such as sphingosine-1-phosphate (S1P), play important roles as second messengers regulating biologic processes, such as cell growth, differentiation, migration, and apoptosis. This review will focus on the role of S1P in renal cells and how aberrant extracellular and intracellular S1P signaling contributes to the pathogenesis and progression of kidney diseases.
Keywords: kidney disease, sphingosine-1-phosphate, ceramide, sphingolipid metabolism, sphingolipids
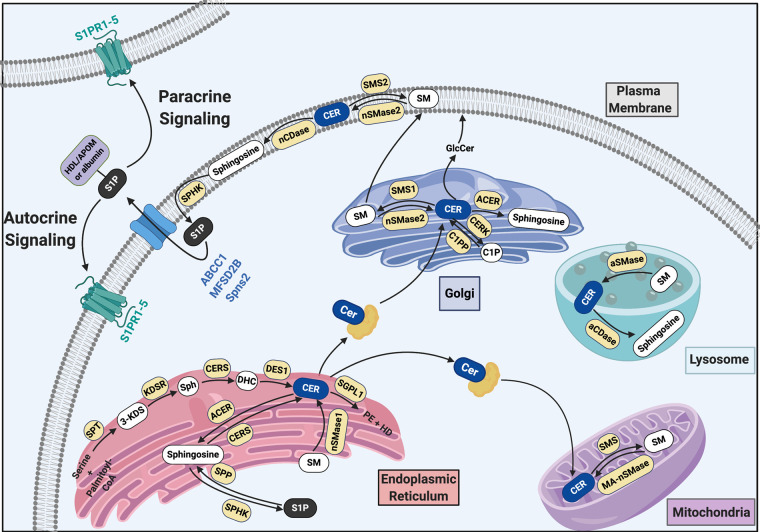
Sphingolipid metabolism is complex and involves a large number of metabolites localized in a variety of cellular compartments and exercising different intracellular functions. Complex sphingolipids are mainly located in the plasma membrane of mammalian cells and exercise structural functions or serve as adhesion sites for proteins. Sphingolipid metabolites, such as ceramide and sphingosine-1-phospate (S1P), are localized in the nucleus, the endoplasmic reticulum (ER), the Golgi apparatus, and lysosomes (Figure 1). It has become clear that a well-balanced sphingolipid metabolism is crucial to the health of a cell.
Figure 1.
Sphingolipid metabolites and enzymes are compartementalized within the cell. ABCC1, ATP-binding cassette transporter C1; aCDase, alkaline ceramidase; ACER, alkaline ceramidase; aSMase, acid sphingomyelinase; CERK, ceramide kinase; CERS, ceramide synthase; C1P, ceramide-1-phosphate; C1PP, ceramide-1-phosphate phosphatase; DES1, dihydroceramide desaturase 1; DHC, dihydroceramide; GlcCer, glucosylceramide; HD, hexadecenal; 3-KDS, 3-ketodihydrosphingosine; KDSR, 3-ketosphingosine; MA-nSMase, mitochondria-associated neutral sphingomyelinase; MFSD2B, Major Facilitator Superfamily Domain Containing 2B; nCDase, neutral ceramidase; nSMase2, neutral sphingomyelinase 2; PE, phosphoethanolamine; SGPL1, sphingosine-1-phosphate lyase 1; SM, sphingomyelin; SMS, sphingomyelin synthase; Sph, sphinganine; SPHK, sphingosine kinase; Spns2, Shingolipid Transporter 2; SPP, sphingosine-1-phosphate phosphatase; SPT, serine-palmitoyl-transferase; S1PR, sphingosine-1-phosphate receptor.
S1P is a bioactive sphingolipid metabolite that regulates many physiologic processes and contributes to the pathogenesis of many diseases, including autoimmune diseases, cancer, atherosclerosis, diabetes, and osteoporosis (reviewed by Maceyka et al.1). The association of S1P with a multitude of cellular processes and diseases results, in part, from its action as both an intracellular and an extracellular signaling molecule. Extracellular sphingosine-1-phospate (eS1P) is a high-affinity ligand for five G protein–coupled receptors, S1PR1 to S1PR5, that mediate a downstream signaling cascade leading to—among other effects—cellular migration and survival. S1P also has functions inside the cell that are independent of S1P receptors and can have similar or opposite downstream effects to eS1P signaling.
This review will focus on the role of S1P and S1P signaling, with a special emphasis on S1P’s role in the pathogenesis of kidney disease.
Sphingolipids and Sphingolipid Metabolism
Sphingolipids are a diverse class of lipids composed of a hydrophobic region (consisting of a long-chain sphingoid base, such as sphingosine, that is linked to a fatty acid by an amide bond) and a hydrophilic region (consisting, in the simplest case, of a hydroxyl group, as observed in ceramide). The fatty acid composition of sphingolipids is variable, but palmitic acid (C16:0) and stearic acid (C18:0) are often present. Complex sphingolipids, such as glycosphingolipids and phosphosphingolipids, are characterized by the presence of sugars and phosphates, respectively, as side chains.
S1P and ceramide are the most studied sphingolipid metabolites, and their biologic functions in cells have been extensively investigated. Their seemingly opposite functions within cells led to the concept that they serve as a “rheostat” of cell fate, with S1P-promoting mechanisms leading to cell survival, inflammation, angiogenesis, and cell invasion and ceramide-promoting mechanisms leading to apoptosis, cell cycle arrest, and senescence. However, it has become clear that sphingolipid metabolism is tightly regulated and highly compartmentalized within cells (Figure 1) and that the roles of ceramide and S1P in regulating cell health and death are more complex. In fact, cell fate is ultimately determined not only by the intracellular levels of S1P, ceramide and other sphingolipid metabolites, but also by their compartmental localization. Thus, the “rheostat” concept solely on the basis of the balance of cellular S1P and ceramide levels requires reconsideration.
Ceramide is the centerpiece of sphingolipid metabolism and is produced either by de novo synthesis or by turnover of other sphingolipids (Figure 1). De novo ceramide synthesis starts at the ER,2 with the synthesis of 3-ketodihydrosphingosine from serine and palmitoyl-CoA catalyzed by serine palmitoyltransferase. Next, 3-ketodihydrosphingosine is reduced to sphinganine (dihydrosphingosine) by 3-ketodihydrosphingosine reductase. Serine palmitoyltransferase3 and 3-ketodihydrosphingosine reductase4 are both localized at the ER, with their catalytic site facing the cytosol. In the next step, sphinganine is N-acylated by one of six ceramide synthases (CerS1 to CerS6) to form dihydroceramide, which is then converted to ceramide. CerS is localized at the ER, with its catalytic activity occurring at the cytosolic surface of the ER.5 Ceramide is then transported to the Golgi apparatus6 or to mitochondria by the soluble ceramide transfer protein.7
In addition to this de novo synthesis pathway of ceramide at the ER, ceramide can be generated directly at the plasma membrane; at the Golgi apparatus; in lysosomes; or in mitochondria, mitochondria-associated membranes,8–10 or both through the hydrolysis of sphingomyelin (SM) to ceramide and phosphocholine through the action of sphingomyelinases (SMases). The latter comprise three subgroups: alkaline SMase, acid SMase, and neutral sphingomyelinase (nSMase), according to their optimal pH activity. To date, three different nSMases (nSMase 1 to nSMase 3) have been identified. nSMase 1 is localized at the ER, whereas nSMase 2 localizes to the plasma membrane and the Golgi apparatus,11 and mitochondria-associated nSMase localizes to mitochondria.12 The generation of ceramide in mitochondria, mitochondria-associated membranes, or both8–10 suggests that it could contribute to mitochondria-mediated apoptosis. Lysosomal acid SMase localizes in the endolysosomal compartment. Finally, ceramide can be converted to sphingosine by ceramidase (CDase), to ceramide-1-phosphate by ceramide kinase, or back to sphingomyelin by sphingomyelin synthase (SMS).
Five different CDases have been identified in different cell compartments and are classified on the basis of their optimal pH activity. Neutral CDase is localized at the plasma membrane,13 whereas acid CDase is found in lysosomes.14 The three different alkaline CDases—ACER1,15 ACER2,16 and ACER317—are localized at the ER, the Golgi apparatus, or both. Ceramide kinase, the enzyme that catalyzes the conversion of ceramide to ceramide-1-phosphate, is localized to the trans-Golgi network.18 The two mammalian SMSs, SMS1 at the Golgi apparatus and SMS2 at the plasma membrane, transfer the phosphorylcholine from phosphatidylcholine onto ceramide to produce sphingomyelin and diacylglycerol.19 Finally, sphingosine can be converted to S1P by sphingosine kinases, SPHK120 and SPHK2.21 S1P can be converted back to sphingosine by phosphatases (SPP122 and SPP223) at the ER, or it can be irreversibly degraded to phosphoethanolamine (PE) and hexadecenal by S1P lyase (SGPL1),24 which is also localized to the ER25 (Figure 1).
Intracellular S1P Signaling
Intracellular sphingosine-1-phosphate (iS1P) levels are tightly regulated by the actions of SPHKs, the release of iS1P via S1P transporters, and the irreversible degradation by SGPL1. SPHK activation increases iS1P levels, which in turn, can inhibit ceramide-induced apoptosis or induce cell migration, proliferation, survival, or differentiation, depending on which SPHK is activated (Figure 2). However, iS1P conversion to sphingosine can also induce apoptosis directly or through increased ceramide synthesis,26,27 underlining the importance for a tight regulation of iS1P levels. Of particular importance here is that the mitogenic and antiapoptotic effects of iS1P do not seem mediated via S1P receptors.28
Figure 2.
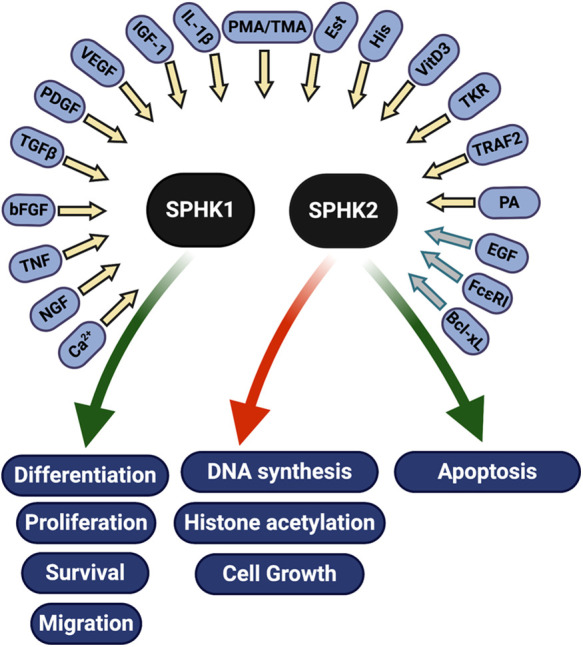
SPHKs are activated by a variety of factors, including cytokines, hormones, growth factors, small GTPases, tyrosine kinase receptors, FcεRI, and others. Following their activation, SPHKs promote (green arrows) or inhibit (red arrow) pathways that regulate cell health and death. Most factors activate SPHK1 (yellow arrows), whereas only a few are known to activate SPHK2 (gray arrows) (reviewed in 247–249). Bcl-xL,40 B cell lymphoma extra large; bFGF,236 fibroblast growth factor; Ca2+, calcium; EGF56,237; Est, estrogen237; FcεRI41, high-affinity IgE receptor; His, histamine238; IGF-1, insulin-like growth factor; IL-1β239, interleukin 1 beta; NGF,240 nerve growth factor; PA, phosphatidic acid; PDGF241, platelet-derived growth factor; phorbol esters TMA238 and PMA242; TGF-β243, transforming growth factor beta; TKR,237 tyrosine kinase receptor; TNF244, tumor necrosis factor alpha; TRAF2,42 TNF-α receptor–associated factor 2; VEGF245, vascular endothelial growth factor; VitD3246, vitamin D3.
Sphingosine Kinases
The phosphorylation of sphingosine to form S1P is catalyzed by SPHKs. The identification of two SPHKs (SPHK1 and SPHK2) with different tissue distribution and expression patterns during development suggests that they have different cellular functions. SPHK1 is expressed in adult lung, spleen, liver, and kidney,20,21 whereas SPHK2 expression is highest in heart and liver; less pronounced in brain, kidney, and testis; and lowest in skeletal muscle and spleen.21 SPHK1 is localized in the cytosol, but when activated, it can translocate to the plasma membrane or nucleus,29–31 where it promotes cell proliferation, migration, differentiation, and survival.32
SPHK1 and SPHK2 have different substrate specificities. Plasma membrane–localized SPHK1 promotes the release of iS1P from the cell, which in turn, can lead to the activation of S1PRs and eS1P signaling. The subcellular localization of SPHK2 is more complex and a determinant of its biologic effect. Nuclear SPHK2 has been associated with the inhibition of DNA synthesis33 and the regulation of histone acetylation,34 whereas SPHK2 localized to mitochondria35 and ER36 promotes apoptosis (Figure 2). In vivo studies suggest that SPHK1 and SPHK2 may have some redundant functions. Sphk1-deficient mice lack a severe phenotype and have dramatically decreased S1P serum levels but normal S1P levels in most tissues.37 Similarly, Sphk2-deficient mice lack a phenotype, whereas mice double deficient in both Sphk1 and Sphk2 are not viable and have no detectable S1P.38 Taken together, these studies suggest that SPHK1 and SPHK2 can, at least partially, compensate for each other and that no other sphingosine kinases exist in mammalian cells.
Mechanisms of Sphingosine Kinase Activation
SPHKs are activated by growth factors, hormones, cytokines, tyrosine kinase receptors, the high-affinity IgE receptor (FcεRI), and others (Figure 2). Although most of these stimuli activate SPHK1, only a few, including EGF,39 the antiapoptic protein B-cell lymphoma-extra large (Bcl-xL),40 and the high-affinity IgE receptor (FcεRI),41 have been shown to activate SPHK2.39 Increases in intracellular Ca2+ may also contribute to SPHK activation in some cell types, whereas protein-protein interactions (such as SPHK1 with TNF receptor–associated factor 242 or SPHK2 with Bcl-xL40) and phosphatidic acid also regulate SPHK activity (Figure 2). SPHK1 activation seems biphasic, with an initial phase of rapid activation that occurs via post-translational modifications, such as phosphorylation, followed by translocation to the plasma membrane and a second phase that is characterized by increased SPHK1 expression.43,44 SPHK2 activation also involves its phosphorylation,45,46 but the mechanisms leading to its translocation between cell compartments are less known. SPHK2 phosphorylation by protein kinase D leads to nuclear export in response to phorbol 12-myristate 13-acetate (PMA) treatment,47 which may lead to the loss of nuclear SPHK2–associated inhibition of DNA synthesis.33
Pathways Activated by iS1P
Production of iS1P can lead to the activation of a number of downstream pathways, including the extracellular signal–regulated kinase (ERK),48 TNF receptor–associated factor 2,42,49 chromatin-modifying enzyme histone deacetylase-1,34 mitochondrial regulator prohibitin-2,50 atypical protein kinase C,51 and the catalytic subunit of telomere reverse transcription52 and NF-κB pathways. It can also lead to the repression of JNK signaling53 and prevent cAMP accumulation.54 Binding of iS1P to prohibitin-2, a highly conserved protein that regulates mitochondrial assembly and function,50 suggests a role for iS1P in mitochondrial function. iS1P can induce calcium release from the ER.55 In HEK cells, iS1P plays a major role in EGF-mediated calcium signaling.56 On the other hand, iS1P can also be exported from the cell (by cell type–specific S1P transporters) or degraded to phosphoethanolamine (PE) and hexadecenal (by SGPL1), as described in more detail below.
Mechanisms of iS1P Export
Early studies suggested an important role for ATP-binding cassette (ABC) transporters in the export of iS1P from cells. In mast cells, iS1P export is mediated by one member of the ABC transporter superfamily, ABCC1, and is abolished by ABCC1 inhibitors but not inhibitors of ABCB1.57 In rat uterine leiomyoma cells, S1P export is dependent on ABCC1 but not on ABCA1 or ABCB1.58 S1P release was prevented in α-toxin–treated platelets59 by the ABC-protein inhibitor glyburide but not by ABCB1 or ABCC1 inhibitors; it was prevented in rat erythrocytes60 by glyburide and vanadate (a global ATPase inhibitor). However, the ABCC1 inhibitor MK571 did not affect S1P release. Although this study suggests a role for ABC transporters in S1P export, S1P export seemingly does not require ATP hydrolysis.60 Additionally, mice deficient in Abca1, Abca7, and Abcc1 do not have altered serum S1P levels,61 suggesting that ABC transporters might not play a major role in S1P export from cells.
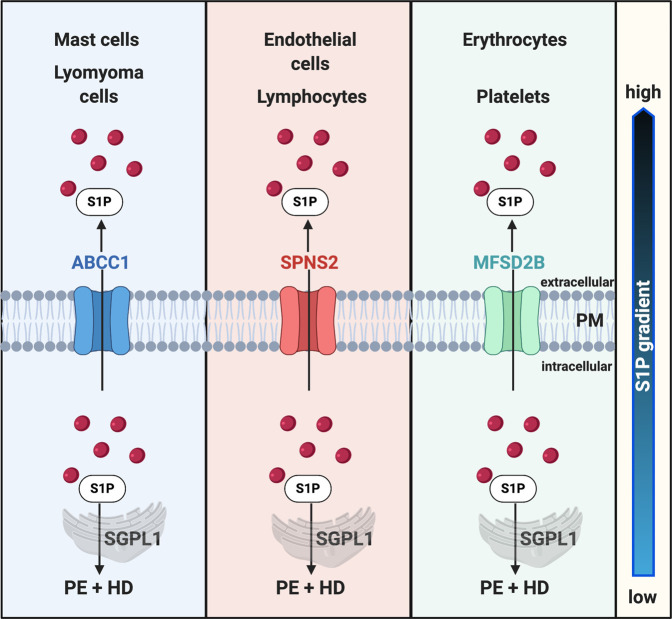
More recently, two other S1P transporters, sphingolipid transporter 2 (SPNS2)62,63 and major facilitator superfamily domain–containing protein 2B (MFSD2B),64 have gained increased attention (Figure 3). Research established a critical role for SPNS2 in S1P transport in Spns2 zebrafish mutants, demonstrating that the cardia bifida phenotype can be rescued by S1P injection and that the export of S1P requires SPNS2.65 In mammalian systems, SPNS2 was also identified as an S1P transporter in human umbilical vein endothelial cells,62 and its overexpression in Chinese ovary hamster cells increases S1P secretion. Spns2-deficient mice are characterized by decreased plasma S1P levels and fewer leukocytes (but not erythrocytes or platelets). Additionally, S1P-dependent lymphocyte trafficking is impaired but they do not have a cardiac phenotype.66 These studies suggest that SPNS2 plays an important role in S1P transport in endothelial cells and in lymphocyte egress but not in S1P transport in erythrocytes and platelets. The S1P transporter in erythrocytes and platelets, Mfsd2b, was recently identified in a study demonstrating reduced S1P plasma levels and red blood cell–specific and platelet-specific S1P accumulation in Mfsd2b-deficient mice.64
Figure 3.
Export of intracellular S1P (iS1P) by cell-specific S1P transporters regulates the S1P gradient. This turnover of intracellularly generated S1P leads to low iS1P and high eS1P concentrations and allows for the formation of a spatial gradient that is crucial for S1P to exercise its biologic activities. ABCC1, ATP-binding cassette transporter C1; MFSD2B, major facilitator superfamily domain containing 2B; PM, plasma membrane; SPNS2, sphingolipid transporter 2.
Thus, S1P export from cells relies on different transporters depending on the cell type. Although S1P export from mast cells and uterine leiomyoma cells might be at least partially mediated by ABCC1, SPNS2 seems to regulate S1P export from endothelial cells and MFSD2B from platelets and erythrocytes (Figure 3). Further studies are needed to identify the transporters that regulate S1P export from other cells, such as renal cells, and to evaluate their potential as new drug targets.
Mechanisms of S1P Degradation
SGPL1 rapidly degrades iS1P to PE and hexadecenal at the ER, leading to low intracellular concentrations of S1P and allowing for the formation of a spatial gradient that is crucial for S1P to exercise its biologic activities (Figure 3). Overexpression of SGPL1 in HEK cells decreases iS1P and sphingosine levels but increases stress-induced ceramide generation and apoptosis24 via the p38 mitogen-activated protein kinase (MAPK), p53, p53-inducible death domain protein, and caspase-2 pathways.67 Consistent with this observation, mice with neural-specific ablation of SGPL1 have increased intracellular iS1P and sphingosine levels and decreased PE levels, leading to blocked autophagy.68 Intestine-specific loss of SGPL1 promotes tumorigenicity and dextran sodium sulfate–associated S1P accumulation.69 On the other hand, inhibition of SGPL1 activity with 4-deoxypyridoxine or with FTY720, a nonselective S1P receptor modulator, reduces the lymphocyte count in mice without affecting iS1P levels.70 Of particular interest is the recent identification of inherited recessive mutations in SGPL1 that have been linked to several human diseases, including congenital Charcot–Marie–Tooth neuropathy71 and steroid-resistant nephrotic syndrome (SRNS).72–74 The role of SGPL1 in SRNS will be discussed in more detail below.
eS1P Signaling
Because of its hydrophobic nature, S1P transport through the circulation requires binding of S1P to carriers, such as HDL–associated apo M (HDL/ApoM) or serum albumin.75 S1P bound to its carriers can then activate S1PRs in an autocrine or paracrine fashion (Figure 1).
eS1P and the S1P Gradient
Approximately 65% of plasma S1P is bound to HDL/ApoM, with the remainder bound to albumin.75 ApoM is a 26-kD protein that belongs to the lipocalin superfamily of apos.76 It is mainly associated with HDL but also associates with triglyceride-rich lipoproteins and LDLs.76 ApoM is exclusively expressed in adult liver and kidney,76–78 suggesting an important physiologic function for ApoM in these two tissues. HDL/ApoM-eS1P is relatively stable, whereas albumin-eS1P is easily degraded. This suggests that HDL/ApoM may play an important role in maintaining the S1P gradient and in keeping plasma concentrations of eS1P high. Levels of iS1P remain in nanomolar concentrations, whereas eS1P levels reach micromolar concentrations in plasma. Serum eS1P levels are even higher (three to four times higher than in plasma)79 because of the release of platelet-associated S1P during coagulation.80 The maintenance of such a gradient is functionally important and regulates essential biologic functions, including the egress of lymphocytes from lymphoid organs, which is blocked when iS1P levels rise.81
The observation that platelets have high SPHK2 activity, produce and store abundant amounts of iS1P,82 and lack activity of SGPL83 led to the belief that eS1P is derived from platelets. However, platelets have little de novo sphingolipid biosynthesis activity84 and rely on the uptake of sphingolipids.85 Research with thrombocytopenic mice demonstrated that platelets do not seem to contribute to the high concentrations of eS1P in the plasma86; instead, red blood cells87,88 and vascular endothelial cells86 provide the main source for plasma eS1P. This observation is important as it highlights the need for standardized, efficient, and highly sensitive methods for the handling of blood samples when determining eS1P levels in biologic materials.80
Although the maintenance of an S1P gradient is functionally important, whether eS1P is bound to HDL/ApoM or albumin is equally important because of their somewhat diverging functions. Albumin-eS1P can partially compensate for HDL/ApoM-eS1P, and both can increase the barrier function of endothelial cells, but the effect of ApoM/HDL-eS1P is more sustained.89,90 Only HDL/ApoM-eS1P can suppress the inflammatory response of endothelial cells91 and regulate lymphopoiesis.92 Albumin-eS1P promotes the egress of lymphocytes, whereas HDL/ApoM-eS1P inhibits it.92 This suggests that eS1P’s protective effects are mostly mediated by HDL/ApoM-eS1P. Interestingly, platelet-released S1P is mainly bound to albumin, which may explain the strong correlation between eS1P and ApoM levels in plasma but not in serum, due to the release of platelet-associated S1P during coagulation.80
S1P Receptor Signaling
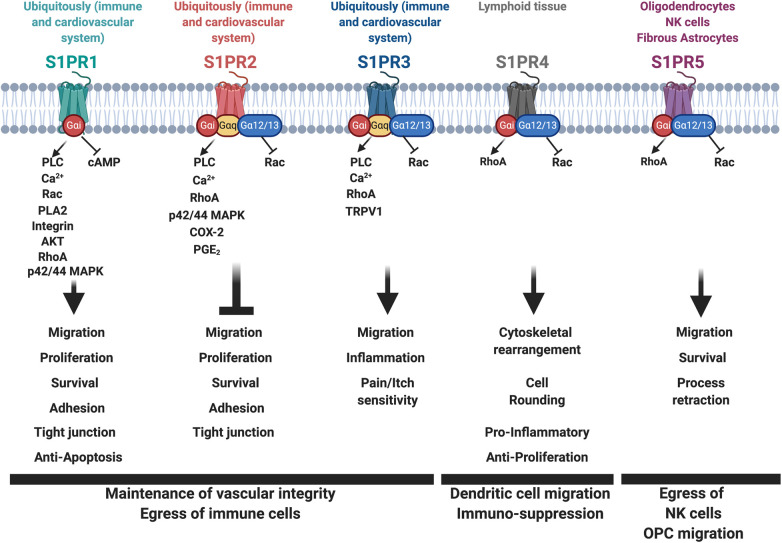
To mediate its cell-specific effects, eS1P binds to one of the five G protein–coupled receptors, named S1PR1 through S1PR5, which bind S1P with high affinity.93 S1PR1 through S1PR3 are mostly expressed ubiquitously but with highest expression in the immune and cardiovascular systems. S1PR4 expression is restricted primarily to lymphoid tissue,94 and S1PR5 expression is restricted to certain cell types, such as oligodendrocytes and/or fibrous astrocytes of the brain95 and natural killer (NK) cells96 (Figure 4). S1PR1 couples exclusively with the inhibitory G-protein α-subunit (Gαi).97,98 S1PR2 and S1PR3 are less selective and can bind to Gαi, Gαq, and Gα12/13,98,99 whereas S1PR4 and S1PR5 couple with Gαi and Gα12/13 subunits.95,100,101 S1P receptor signaling has been extensively studied, particularly due to its important functions in physiologic and pathophysiologic processes (reviewed in Maceyka et al.1 and Pyne et al.102). In this review, we will discuss only some of the key findings with regard to S1PR signaling.
Figure 4.
S1P receptor signaling regulates downstream signaling pathways important for vascular integrity, the egress of immune cells, and cell migration. S1PR1 couples exclusively with the inhibitory Gαi; S1PR2 and S1PR3 bind to Gαi, Gαq, and Gα12/13; whereas S1PR4 and S1PR5 couple with Gαi and Gα12/13 subunits. S1PR coupling to these G proteins is followed by receptor activation and signal transduction leading to the activation of many downstream signaling pathways important in the maintenance of vascular integrity, the egress of immune cells, and cell migration. AKT, AKT/PKB (protein kinase B) kinase; Ca2+, calcium; COX-2, cyclooxygenase-2; OPC, oligodendrocyte progenitor cells; PGE2, prostaglandin E2; PLA2, phospholipase A2; PLC, phospholipase C; TRPV1, transient receptor potential cation channel subfamily V member 1.
S1PR coupling to G proteins is followed by receptor activation and signal transduction, leading to activation of small GTPases (such as Rho, Rac, and Ras),103–106 to phospholipase C activation followed by mobilization of intracellular calcium signaling,107–109 to AKT and ERK1/2 phosphorylation,106 and to increased intracellular cAMP110 (Figure 4). Evidence suggests that the activation of Gαi proteins and Rac causes S1PR1 phosphorylation and binding to β-arrestin, followed by receptor desensitization and endocytosis.111 In support of this observation, stimulation of HEK293 cells with eS1P or PDGF leads to the formation of a PDGFβ and S1PR1 complex that is constitutively associated with β-arrestin,112 followed by Src recruitment and p42/44 MAPK activation in endosomes.113
Studies of S1PR1 have been driven by its important function in the cardiovascular and immune systems. In the immune system, S1PR1 signaling plays an important role in regulating the egression of immune cells.114,115 S1PR1 coupling to Gi family proteins plays a key role in the development of the cardiovascular system and the maintenance of vascular integrity.116,117 Vascular development is coordinately regulated by S1PR1 through S1PR3 in mice. Although S1PR1 deficiency in mice is embryonic lethal,117 S1PR2 or S1PR3 deficiency is not.118 However, S1PR2 and S1PR3 double deficiency results in partial embryonic lethality and vascular abnormalities, suggesting that S1PR1 to S1PR3 have partially redundant and cooperative functions in vascular development.118
In vitro studies show that activation of S1PR1 results in endothelial cell migration, proliferation, and survival,115,119,120 and S1P treatment of human umbilical vein endothelial cells leads to AKT-mediated migration.103 Overexpression of S1PR1 in HEK293 cells results in its expression primarily at the plasma membrane121; in increased cell-cell aggregation and cadherin expression and RhoA-dependent formation of adherence junctions97; and to S1P-stimulated intracellular Ca2+ accumulation in Chinese hamster ovary cells. In contrast, binding of eS1P to S1PR1 inhibits cAMP accumulation; results in MAPK, ERK2,97 and/or phospholipase A2 activation28,97,122,123; stimulates DNA synthesis; and inhibits apoptosis.28 Finally, eS1P binding to S1PR1 can also activate ανβ3-integrin, leading to enhanced endothelial migration119,120 (Figure 3).
Similarly, S1PR2/Gα13 signaling regulates myocardial migration. Studies show that S1PR2 deficiency in mice and zebrafish leads to cardia bifida resulting from defective S1PR2/Gα13/RhoGEF-mediated migration,124–126 whereas activation of S1PR2 signaling through binding to heterodimeric G12/13 activates Rho, thus inhibiting Rac, cortical actin assembly, and migration.103–105,127 In rat thyroid cells, S1PR2 activation induces phospholipase C–mediated Ca2+ release from intracellular stores and reduces PMA-evoked proliferation.108
S1PR3 activation promotes inflammatory macrophage/monocyte recruitment in atherosclerosis,128 bactericidal effects during sepsis,129 and S1P-evoked acute itch and pain mediated via phospholipase C.130 Interestingly, the inhibitory effect on migration of vascular smooth muscle cells of FTY720, a nonselective S1P receptor modulator, is predominantly mediated through the inhibition of PDGFR-S1PR3 signaling, whereas the effect on the PDGFR-S1PR1 pathway regulates cell growth.131
Much less is known about S1PR4 and S1PR5 signaling. S1PR4 signaling was shown to regulate neutrophil counts in circulation and trafficking to tissues132,133 and dendritic cell function.134 S1PR4 signaling mediates the immunosuppressive effects of S1P by inhibiting proliferation and secretion of cytokines from T cells135 and regulates peritoneal B cell migration.136 Interestingly, S1PR4 deletion in mice profoundly affects dendritic cell function but has only a limited effect on T cell function.134 S1PR4 activation was also shown to induce cytoskeletal rearrangements and cell rounding via RhoA in immune cells.137 S1PR5 expression in NK cells promotes their egress from lymph nodes and bone marrow. S1PR5-deficient mice have defects in the homing of NK cells to blood, spleen, lung, and inflamed liver. However, NK cells are not completely absent from these sites, suggesting that NK cell trafficking also may rely on other pathways.138 Similarly, in Duane mice, which are characterized by a point mutation in the Tbx21 gene encoding the T-bet protein, NK cell numbers are decreased in blood and spleen but are increased in lymph nodes and bone marrow in association with a 30-fold decrease in S1PR5 mRNA expression levels.139 S1P activation of S1PR5 in O4-positive preoligodendrocytes induces process retraction in a Rho kinase/collapsin–mediated pathway.140 Finally, S1PR5 regulates cell migration in oligodendrocyte precursor cells, where engagement of S1PR5 with Gα12/13-coupled Rho/Rho associated protein kinase (ROCK) inhibits migration.141
iS1P and eS1P Signaling in Renal Cells
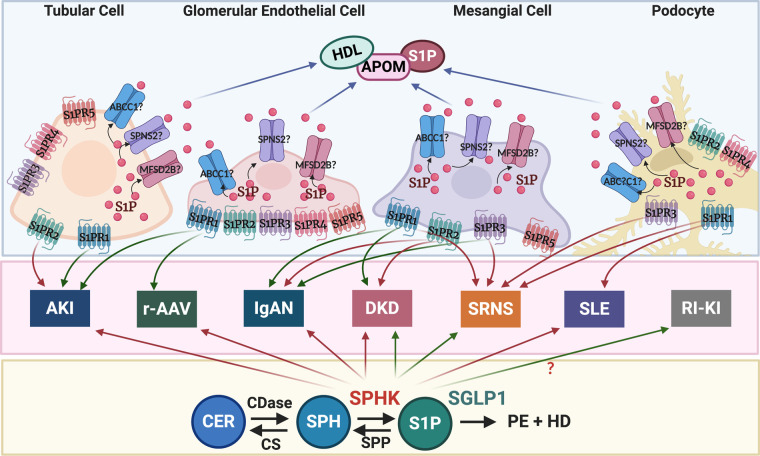
Inflammation and immune system activation contribute to the pathogenesis of AKI and CKD, suggesting an important role for the S1PR pathway in kidney diseases. In the kidney, S1PR1 to S1PR4 are expressed with a rank order of S1PR1 > S1PR3 > S1PR2 > S1PR4, whereas S1PR5 was not found to be expressed.142 However, in vitro studies suggest the expression of S1PR1 to S1PR3 and S1PR5 in glomerular mesangial cells (MCs)143,144; expression of all five S1PRs in glomerular endothelial cells (GEnCs)145 and in imal tubular cell lines, HK-2146,147 and TKPTS147;prox and expression of S1PR1 to S1PR4 in immortalized mouse podocytes148 (Figure 4).
S1P Signaling in MCs
Microarray analysis of MCs isolated from mice with cell-specific GFP expression indicates increased expression of S1PR3 and ACER2 in MCs compared with other renal cells.149 In vitro, S1P treatment of rat MCs induces PDGF-mediated proliferation150,151 and migration,152 phospholipase A2–dependent calcium influx,153 and DNA synthesis. Although MC proliferation is PDGF mediated, SPHK1 inhibition reduces PDGF-induced MC proliferation. Interestingly, PDGF treatment can also induce SPHK expression and activity (Figure 2), suggesting that iS1P, eS1P signaling, or both might contribute to PDGF–induced MC proliferation.151 MC migration is S1PR1 mediated154 or S1PR2 mediated155 and can be inhibited by dexamethasone.154 Extracellular nucleotides that act on purine P2Y receptors promote p42/44 MAPK–mediated proliferation and migration, an effect that can be mimicked by the addition of eS1P.152 Interestingly, MC migration stimulated by ATP and UTP requires SPHK1 activity, whereas MC proliferation is not SPHK1 dependent.152 Depending which SPHK is activated, eS1P and iS1P can also exercise opposite effects. MCs isolated from SPHK2-knockout mice are resistant to apoptosis induced by staurosporin, whereas SPHK1 deficiency sensitizes MCs to staurosporin-induced apoptosis.156 Similarly, eS1P signaling activates the fibrotic response through activation of connective tissue growth factor (CTGF) by TGF-β/Smad, whereas iS1P-mediated CTGF repression attenuates the fibrotic response in MCs.157 Taken together, these studies suggest that in MCs, eS1P signaling exercises a profibrotic effect, whereas iS1P signaling exercises an antifibrotic effect (Figure 5). However, the exact contribution of S1PR1 to S1PR3 in mediating the profibrotic effect requires further investigation.
Figure 5.
Differential iS1P and eS1P signaling in renal cells is associated with different types of kidney disease. Green arrows indicate repression, and red arrows indicate activation of gene expression. ABCC1, ATP-binding cassette transporter C1; CER, ceramide; CS, ceramide synthase; HD, hexadecenal; MFSD2B, major facilitator superfamily domain containing 2B; r-AAV, renal ANCA–associated vasculitis; RI-KI, radiation-induced kidney injury; SPNS2, sphingolipid transporter 2; SGPL1, sphingosine-1-phosphate lyase 1; SPH, sphingosine; SPHK, sphingosine kinase; SPP, sphingosine-1-phosphate phosphatase; S1PR, sphingosine-1-phosphate receptor.
S1P Signaling in GEnCs
Endothelial integrity relies on the balanced expression and activation of different S1PRs. The binding of eS1P to S1PR1 leads to intracellular calcium release and activation of the Rho GTPase Rac1, thus strengthening the assembly of tight junction proteins and F-actin. However, excessive eS1P induces endothelial barrier dysfunction through S1PR2-mediated RhoA/Rho kinase pathway activation, causing disassembly of the tight junctions.158 Single-cell RNA analysis demonstrated that SPHK1/2 and S1PR1 to S1PR4 but not S1PR5 are expressed in GEnCs,159 whereas other studies160,161 did not identify differential expression of genes of the iS1P or eS1P signaling pathways in any renal cell population (Figure 5).
S1P Signaling in Podocytes
Little is known about S1PR signaling in podocytes. Microarray analysis of podocytes isolated from mice with cell-specific GFP expression indicates increased expression of genes of the S1P-S1PR1 and S1P-S1PR3 pathways in podocytes,149 whereas single-cell RNA studies suggest that podocytes express SPHK2 but not SPHK1 or S1PRs.159 In vitro studies demonstrate that TGF-β treatment of podocytes upregulates SPHK1 expression. While SPHK1 overexpression and S1P generation in podocytes is associated with decreased CTGF expression and protects from a fibrotic response, depletion of SPHK1 increases CTGF expression and exacerbates the fibrotic response.157Podocytes cultured in high glucose exhibit increased TNF and vascular endothelial growth factor expression, an effect that can be reduced by the selective S1PR1 agonist, SEW2871, which simultaneously increases nephrin and podocin expression.148 In angiotensin II–infused rats, the nonselective S1P receptor modulator FTY720 attenuates S1P-induced podocyte injury and renal histologic lesions in association with a reduction of inflammatory cytokine production, including TNF and IL-6.162 Taken together, these observations suggest that SPHK1 or S1PR1 agonism may protect from podocyte injury (Figure 5).
S1P Signaling in Tubular Cells
Treatment of TKPTS cells with the selective S1PR1 agonist SEW2871 reduces apoptosis induced by LPS and hypoxia-reoxygenation,147 suggesting that selective S1PR1 agonism may represent a potential treatment for patients with AKI. Similarly, isoflurane anesthesia–induced SPHK1 expression/activity reduces necrosis and inflammation in HK-2 cells, whereas simultaneous treatment with an S1PR1 antagonist (VPC23019) or an SPHK antagonist abrogates isoflurane-mediated renal protection.163 Similarly, knockout or pharmacologic inhibition of SPHK1 increases renal ischemia-reperfusion injury (IRI), whereas mice with SPHK1 overexpression have improved renal function.164 S1PR2 antagonism can also increase SPHK1 activity, thus attenuating hydrogen peroxide–induced necrosis and TNF-α/cycloheximide–induced apoptosis.165 Taken together, studies in tubular cells support the idea that S1PR1 agonism, kidney-specific SPHK1 overexpression, or S1PR2 antagonism may represent a therapeutic option to prevent tubular injury and attenuate the effects of renal IRI (Figure 5). More recently, a role for mir-21 in SPHK1/S1P-mediated TGF-β–stimulated fibrosis in tubular epithelial cells was discovered, suggesting a novel link between iS1P signaling and TGF-β–induced renal fibrosis in tubular epithelial cells.166
S1P Signaling in Intercalated Cells of the Renal Collecting Duct
The renal collecting duct contains intercalated cells and principal cells and plays an important role in the innate immune defense of the kidney. Whole-transcriptome analysis of renal intercalated cells revealed that the S1P signaling pathway is among the top ten activated pathways in intercalated cell reporter mice infected by transurethral inoculation with uropathogenic Escherichia coli,167 supporting a role for S1P signaling in the innate immune response in the kidney.
Considerations when Studying iS1P and eS1P Signaling in Renal Cells
In vitro studies suggest an important role for iS1P and eS1P signaling in MCs, GEnCs, podocytes, and intercalated cells in kidney health and disease. However, many of these studies are hampered by the fact that renal cells in culture do not retain their original characteristics. For example, podocytes in vitro do not form a slit diaphragm or foot processes,168,169 and thus, they are exposed to a different microenvironment. Furthermore, research has clearly indicated the existence of podocyte-endothelial crosstalk in the glomerulus,170,171 and the study of podocytes and endothelial cells in isolation may prove problematic—especially when studying autocrine and paracrine effects of a molecule, as is the case for S1P-mediated signaling. The recent development of kidney organoids,172,173 although constrained by this model’s own limitations, will allow further elucidation of the role of S1P and S1P receptor signaling in kidney health and disease and validation of studies performed in isolated renal cells in culture. Similarly, microarray and single-cell RNA studies confirm an important role for iS1P and eS1P signaling in renal cells in health and disease. However, it is possible that S1P and S1P signaling have not yet been unequivocally implicated in the pathobiology of kidney disease using these methods because many of the enzymes important in S1P metabolism are regulated not by transcriptional levels but rather, by subcellular localization, phosphorylation, or both.
iS1P and eS1P Signaling in Experimental Models of Kidney Disease and in Human Kidney Disease
Although iS1P and eS1P signaling has been extensively studied in experimental models of kidney disease, studies in humans lag behind and mostly focus on determining plasma S1P and ApoM levels, as well as urinary S1P levels.
AKI
To date, the role of iS1P and eS1P signaling in kidney disease has been best studied in experimental models of AKI. Both innate and adaptive immunity play an important role in ischemia-reperfusion injury (IRI), a common cause of AKI. Infiltration of the kidney by leukocytes, neutrophils, macrophages, and T cells during IRI occurs as part of the inflammatory response following reperfusion.174 S1PR1 signaling plays an important role in regulating the egression of immune cells.114,115 S1PR4 signaling is important in regulating neutrophil counts in circulation and trafficking/migration to tissues,132,133 as well as in mediating the immunosuppressive effects of S1P by inhibiting proliferation and secretion of cytokines135 (Figure 2). Proximal tubular epithelial cells are prone to necrosis and apoptosis induced by IRI. It has become clear from studies using knockout mice and a variety of agonists and antagonists targeting SPHKs and S1PRs that aberrant SPHK and S1PR1 signaling contributes to AKI (Figure 5, Table 1). Inducible endothelial-specific S1PR1 deletion was shown to cause increased renal tubular necrosis, apoptosis, inflammation, and impaired vascular permeability following IRI in association with reduced expression of heat-shock protein 27.175 Endothelial-specific S1PR1 deletion in iTie2CreERT2S1pr1fl/fl mice exacerbates kidney injury and inflammation induced via IRI, whereas a delayed deletion causes chronic inflammation and progressive fibrosis.176
Table 1.
S1P modulators in experimental models of kidney disease
| Compound Name | Agonist/Antagonist | Target Specificity | Model | Phenotype (A, NE, E) | Reference |
|---|---|---|---|---|---|
| ABC294640 | Antagonist | SPHK2 | LN | Aa | 250 |
| Berberine | Antagonist | SPHK1/S1PR2 | DN | A | 251 |
| CAY10444 | Antagonist | S1PR3 | IRI | NE | 165 |
| CAY10621 | Antagonist | SPHK1 | IRI | E | 164 |
| FTY720 | Agonist | S1PR1/3/4/5 | AngII-induced hypertension in rats | A | 162 |
| FTY720 | Agonist | S1PR1/3/4/5 | STZ-induced DN | A | 148 |
| FTY720 | Agonist | S1PR1/3/4/5 | GN | A | 252, 253 |
| FTY720 | Agonist | S1PR1/3/4/5 | IRI | A | 142 |
| FTY720 | Agonist | S1PR1/3/4/5 | IRI in Sphk2 knockout mice | NE | 254 |
| FTY720 | Agonist | S1PR1/3/4/5 | Cisplatin-induced AKI in S1PR1 knockout mice | NE | 255 |
| FTY720 | Agonist | S1PR1/3/4/5 | PKD | A | 256 |
| FTY720 | Agonist | S1PR1/3/4/5 | AAV | A | 257 |
| FTY720 | Agonist | S1PR1/3/4/5 | IRI | A | 147 |
| FTY720 | Agonist | S1PR1/3/4/5 | Kidney allograft survival (canine) | A | 211 |
| FTY720 | Agonist | S1PR1/3/4/5 | Kidney allograft survival | A | 213 |
| FTY720 | Agonist | S1PR1/3/4/5 | Kidney allograft survival | A | 212 |
| FTY720 | Agonist | S1PR1/3/4/5 | Kidney allograft survival | NE | 214 |
| Isoflurane | Agonist | SPHK1 | IRI | A | 163 |
| JTE-013 | Antagonist | S1PR2 | IgAN | A | 194 |
| JTE-013 | Antagonist | S1PR2 | IRI | A | 165 |
| KRP-203 | Agonist | S1PR1/4 | LN | A | 258 |
| MT-1303 | Antagonist | S1PR1 | LN | A | 259 |
| RP-101075 | Agonist | S1PR1/5 | LN | A | 203 |
| RPC1063 (Ozanimod) | Agonist | S1PR1/5 | LN | A | 203 |
| S1P | Agonist | S1PR1? | Hepatic IRI | A | 260 |
| S1P | Agonist | S1PR1? | IRI in Sphk2 knockout mice | A | 254 |
| S1P | Agonist | S1PR1 | RI nephropathy | A | 182 |
| SEW2871 | Agonist | S1PR1 | STZ-induced DN | A | 148 |
| SEW2871 | Agonist | S1PR1 | IRI | A | 142, 177 |
| SEW2871 | Agonist | S1PR1 | IRI | A | 164 |
| SEW2871 | Agonist | S1PR1 | AAV | NE | 257 |
| SEW2871 | Agonist | S1PR1 | IRI | A | 147 |
| SID46371153 | Agonist | S1PR2 | IRI | E | 165 |
| SK-II | Antagonist | SPHK1/SPHK2 | IRI | E | 164 |
| TY52156 | Antagonist | S1PR3 | AAV | NE | 257 |
| VPC-23019 | Antagonist | S1PR1/3 | IgAN | NE | 194 |
| VPC-23019 | Antagonist | S1PR1/3 | LPS-induced renal injury | A | 261 |
| VPC-23019 | Antagonist | S1PR1/3 | Hepatic IRI | Eb | 260 |
| VPC-44116 | Antagonist | S1PR1 | IRI | Ec | 142 |
| VPC-44116 | Antagonist | S1PR1 | IRI | Ec | 147 |
| W146 | Antagonist | S1PR1 | IRI | NE | 165 |
GN indicates anti–Thy1.1.-induced GN. SPHK, sphingosine kinase; A, attenuation; S1PR, sphingosine-1-phosphate receptor; DN, diabetic nephropathy; NE, no effect; E, exacerbation; PKD, polycystic kidney disease; RI nephropathy, radiation-induced nephropathy.
Improved glomerular pathology but not improved proteinuria.
Reverses S1P-mediated protective effect.
Reverses FTY720-mediated protective effect.
These observations suggest that endothelial S1PR1 is necessary for recovery from IRI and prevention of fibrosis following IRI and that S1PR1 agonism may represent a new therapeutic approach for preventing early injury as well as progressive kidney fibrosis after AKI.176 This is supported by studies showing that the nonselective S1PR modulator FTY720 and S1PR1-specific agonists (such as SEW-2871) have protective effects on renal function in IRI, whereas S1PR1 antagonists, such as VPC-44116, exacerbate the renal phenotype (Table 1).142,177,178 The observation that S1PR1 agonism significantly reduces renal tissue injury, the degree of neutrophil and macrophage infiltration, circulating lymphocytes, and renal T cell infiltration strongly suggests that S1PR1 agonists repress T cell mobilization from renal and extrarenal lymphoid tissue.178 Additionally, studies demonstrated that S1PR2 antagonism protects against renal IRI and that SPHK antagonism or S1PR2 agonism exacerbates it163 (Table 1). These studies clearly demonstrate an opposite role for S1PR1 and S1PR2 signaling in IRI and suggest that therapeutic strategies aimed at activating S1PR1 signaling or inhibiting S1PR2 signaling may be beneficial to prevent IRI (Figure 5). Given that tubular cells express all five S1PRs, the effects of targeting S1PR3 to S1PR5 on IRI warrant further investigation.
Diabetic Kidney Disease
Diabetes mellitus is a major cause of CKD, and approximately 40% of patients with type 2 diabetes will develop diabetic kidney disease (DKD). Single-nucleus RNA sequencing179 of kidney cortex from patients with diabetes and patients without diabetes found differential S1PR3 expression in MCs, whereas SPHK1 expression was found increased in podocytes of patients with DKD compared with normal controls.157 Additionally, as we recently described, sphingomyelinase-like phosphodiesterase 3b (SMPDL3b)–mediated podocyte insulin resistance contributes to the pathogenesis of DKD,180,181 and SMPDL3b overexpression in podocytes leads to iS1P accumulation.182 The finding that SPHK1 expression is increased in podocytes of patients with DKD when compared with patients with renal cancer157 supports our experimental data. Increased glomerular S1P resulting from increased SPHK1 activity is also associated with increased MC proliferation in a model of streptozotocin (STZ)–induced short-term diabetes.183 In MCs exposed to high glucose, SPHK1 activation increases fibronectin expression.184 SPHK1 overexpression can also directly induce fibronectin expression and increase iS1P levels, which can be further exacerbated by high glucose treatment; in contrast, SPHK1 siRNA, as well as SPHK or S1PR2 antagonism, prevents this phenotype.184 These data suggest that iS1P and eS1PR1/S1PR2-mediated signaling may contribute to fibronectin accumulation in MCs exposed to high glucose.
In STZ-injected diabetic rats, S1PR2 antagonism or S1PR1 agonism prevented renal phenotypes (Table 1).148 Mice with alloxan-induced diabetes have increased glomerular SPHK1 expression/activity that leads to S1P accumulation in association with increased TGF-β expression—particularly in MCs—a phenotype that was prevented by berberine (Table 1).185 However, in one study, STZ-injected mice showed increased SPHK1 and CTGF expression in glomeruli, mainly in podocytes and tubules after 30 days, whereas SPHK1 deficiency further aggravated the DKD phenotype and exacerbated CTGF-mediated fibrosis.157
These studies reveal that SPHK1 activity may have opposite effects on different glomerular cells (Figure 5). Although SPHK1 activation in MCs contributes to MC proliferation in STZ-induced short-term diabetes, the activation of SPHK1, particularly in podocytes, might offer protection from STZ-induced renal lesions and from CTGF-mediated fibrosis. Further studies are needed to determine if differential SPHK1 activity in MCs and podocytes differentially contributes to the pathogenesis of DKD, as well as to elucidate the contribution to DKD of eS1P signaling via S1PR1 and/or S1PR2. If proven true that differential SPHK1 activity in glomerular cells contributes differently to the pathogenesis of DKD, targeting iS1P or eS1P signaling as a new therapeutic strategy for patients with DKD may be difficult.
Interestingly, in patients with type 2 diabetes mellitus, plasma S1P levels inversely correlate with the degree of urinary albumin excretion, and reduced plasma S1P levels are observed in patients exhibiting macroalbuminuria compared with those without albuminuria.186 Similarly, compared with healthy controls, plasma ApoM levels are reduced in patients with type 2 diabetes mellitus without DKD and correlate negatively with the degree of insulin resistance in these patients.187,188 However, it remains to be established how much the decrease in plasma eS1P levels or the increase in iS1P—perhaps mediated by the actions of S1PR1 through S1PR3 signaling—may contribute to the development of DKD and diabetes-related complications.
Radiation-Induced Kidney Injury
Radiation-induced kidney injury results from excessive radiation to the kidney during the clinical management of abdominal or paraspinal tumors or total body irradiation.189,190 We recently described an important role for acid SMPDL3b in radiation-induced kidney injury, demonstrating that reduced SMPDL3b mediates radiation-induced damage in human podocytes and is associated with decreased sphingosine and S1P levels and with accumulation of several ceramide species (Figure 5, Table 1).182 Interestingly, overexpression of SMPDL3b or S1P treatment conferred a radioprotective effect in cultured human podocytes (Table 1),182 indicating a potential role for SMPDL3b and S1P signaling in radiation-induced kidney injury. However, which S1PRs specifically contribute to the radioprotective effect in podocytes warrants further investigation.
IgA Nephropathy
About 40% of patients with IgA nephropathy (IgAN), the most common nondiabetic glomerulopathy, progress to ESKD.191 IgAN is characterized by mesangial IgA deposition of immune complexes containing galactose-deficient IgA1, resulting in mesangial proliferation and glomerular inflammation. Microarray analysis of kidneys from the HIGA mouse model for IgAN192 revealed increased expression of S1PRs, particularly of S1PR2 to S1PR4, in 6-week-old HIGA mice when compared with controls, which suggests a role for eS1P signaling in early stages of IgAN. Mechanistically, PDGF treatment of MCs was found to induce S1PR2-mediated MC proliferation.193 These observations were confirmed in another study,194 demonstrating increased S1PR2 expression and increased plasma S1P and ApoM levels in HIGA mice. ApoM overexpression in HIGA mice prevented the development of IgAN and decreased plasma S1P levels and fibrotic gene expression, whereas ApoM knockdown exacerbated the IgAN phenotype. Treatment of HIGA mice with an S1PR2 antagonist (JTE013) ameliorated proteinuria in these mice, whereas S1PR1/S1PR3 (VPC23019) antagonism had no effect194 (Figure 5, Table 1). Similarly, as is observed in HIGA mice, plasma levels of S1P are significantly higher among patients with IgAN compared with healthy controls, and both plasma and urine S1P levels correlate with the degree of proteinuria.162 Interestingly, a low serum ApoM level was an independent predictor for nephritis in patients with IgA vasculitis and correlated with the severity of glomerular lesions.195
SRNS
SRNS is characterized by FSGS and is an important cause of CKD and ESKD. We recently reported an important role for SMPDL3b in the pathogenesis of FSGS, suggesting a role for aberrant sphingolipid metabolism in SRNS.181,196 Whole-exome sequencing has identified recessive mutations in SGPL1, the gene encoding S1P lyase, in several families. These families had a disease phenotype of SRNS, accompanied by such extrarenal manifestations as adrenal insufficiency, ichthyosis-like acanthosis, immunodeficiency, and neurologic abnormalities.72–74 SGPL1 is expressed in podocytes, MCs, and GEnCs. SGPL1 mutations cause decreased SGPL1 protein expression and enzyme activity,73,74 resulting in increased S1P and ceramide levels in one patient.73 Sgpl1 deficiency in mice causes proteinuria and foot process effacement, which may be unrelated to podocyte injury, given that S1P-treated podocytes do not show increased apoptosis or migration. SGPL1 knockdown in MCs leads to reduced CDC42/RAC1-mediated migration; this phenotype is partially reversed by an S1PR1/S1PR3 antagonist,73 suggesting that MC migration is at least partly mediated via S1PR1 and/or S1PR3 signaling and contributes to the pathogenesis of SRNS (Figure 5).
Among individuals with SGPL1 mutations, cell migration in fibroblasts is significantly reduced. Additionally, the plasma and cultured fibroblasts of affected patients exhibited markedly increased levels of S1P, the major endogenous substrate of SGPL1, and its precursors, sphingosine and ceramides.72–74 The extent of organ damage varied between families and may be related to residual functional SGPL1, variants and polymorphisms in other genes involved in S1P metabolism and signaling (including SPHK1, SPHK2, and S1PR1 to S1PR5), or both. Nearly all patients identified with SGPL1 mutations displayed adrenal insufficiency.73,74 This is particularly interesting because the sphingolipid intermediates ceramide and sphingosine are known to reduce steroid hormone synthesis in vitro. The finding of lymphopenia associated with SRNS suggests an alteration in the S1P gradient between tissues and plasma, which is known to affect the egress of T cells from lymphoid organs. Taken together, these findings demonstrate a crucial role of SGPL1 and S1P metabolism in SRNS and suggest that defective S1P metabolism may contribute not only to the renal phenotype observed in SRNS but also, to the phenotypes observed in other tissues and organs. To date, it remains unknown whether the disease manifestations result from an excess of iS1P and imbalance of sphingoid bases, from increased S1P signaling through the S1PRs, or both.
SLE Nephritis
Lupus nephritis (LN) is a form of GN caused by circulating immune complex deposition and complement activation in glomeruli. Podocyte injury contributes to its pathogenesis.197 Experimental studies using the NZB/W F1198 and the MRLlpr/lpr199 mouse models of LN suggest that both FTY720, which can act as a nonselective S1PR agonist200 or functional antagonist,201,202 and ABC294640, which is an SPHK inhibitor, have therapeutic potential in SLE (Table 1). More precisely, S1PR1 signaling, S1PR5 signaling, or both seem to play an important role in LN. Taylor Meadows et al.203 demonstrated that ozanimod (RPC1063), a specific and potent small molecule modulator of S1PR1 and S1PR5, and RP-101075, ozanimod‘s active metabolite, reduced proteinuria and serum BUN, as well as mesangial expansion, glomerular deposits, tubular atrophy, interstitial infiltrates and fibrosis, and endocapillary and exocapillary proliferation in NZB/W F1 mice (Figure 5, Table 1). Further investigations are needed to assess the therapeutic potential of S1PR modulators, particularly of S1PR1 and S1PR5 modulators, for patients with LN.
Increased serum S1P in juvenile SLE204 suggests a role for eS1P signaling in SLE. Compared with healthy controls, patients with LN have higher plasma levels of sphingosine and S1P; they also have elevated plasma levels of specific ceramides compared with patients with SLE without evidence of kidney injury as well as healthy controls. Levels of C24:1 ceramide in particular were higher in patients with LN and were correlated with a reduction in C3 and C4 complement levels, suggesting that ceramide levels may have a role as biomarkers for LN. C16Cer and C20Cer, but not C24:1Cer, positively correlated with albuminuria.205 Another study found the ratio of C16:0 ceramide to S1P to be the best discriminator between patients with SLE and controls and was associated with ongoing disease activity. Levels of C16:0 and C24:1 ceramide correlated with the presence of renal involvement.206 Moreover, dysregulation of sphingolipids appeared to normalize after immunosuppressive treatment. Thus, patients with SLE exhibit an altered sphingolipid profile, and the ratio of circulating ceramides to S1P may serve as a marker of disease activity, including extent of renal injury.
It remains undetermined, however, whether the altered ratio of circulating ceramides to S1P is due to increased biosynthesis of ceramides, enhanced degradation of S1P, or both. Interestingly, CerS5 and S1P lyase were both upregulated in patients with SLE relative to controls. Yet another source of uncertainty is the relative contribution of albumin-bound eS1P and ApoM/HDL-bound eS1P to the presence of S1P dysregulation. In a study by Checa et al.,206 no correlation between plasma S1P and albumin levels was found, indicating that S1P dysregulation was dependent not on albumin-eS1P but rather, the ApoM/HDL-bound S1P fraction.
ANCA-Associated Vasculitis
In vitro, eS1P treatment of GEnCs enhances myeloperoxidase (MPO)-ANCA–positive IgG‐induced disruption of tight junction structure and disorganization of the cytoskeleton and increases the permeability of GEnC monolayers; it also enhances upregulation of MPO‐ANCA–positive IgG‐induced membrane‐bound as well as soluble ICAM‐1/VCAM‐1. Increased ICAM‐1 levels can be reduced by treatment with an S1PR1 agonist or S1PR2 to S1PR4 antagonists, suggesting that eS1P enhances MPO‐ANCA–positive IgG‐mediated GEnC activation through S1PR1 to S1PR4207 (Figure 5).
Interestingly, plasma levels of eS1P are significantly higher in patients with ANCA-associated vasculitis (AAV) compared with healthy controls and in patients with AAV in the active stage compared with those in remission.145,208 Additionally, plasma S1P levels correlated inversely with eGFR. Glomerular expression of S1PR2 to S1PR5, but not S1PR1, was found upregulated in patients with AAV compared with normal controls and was associated with markers of disease activity. A crucial role for S1P in ANCA-mediated recruitment and activation of neutrophils via its interaction with the complement component C5a has been suggested, and blockade of S1P or antagonism of S1PRs was shown to attenuate C5a-induced ANCA-mediated neutrophil migration and activation.208
CKD
Among patients with CKD, those with advanced CKD have lower plasma ApoM levels compared with patients with early CKD and controls without kidney disease. Interestingly, HDL-S1P levels were decreased in patients with more advanced CKD, and HDL-S1P tended to decrease to a greater degree than albumin-bound S1P in all CKD stages except the most advanced stage.209 In a smaller study, a nonsignificant reduction in plasma ApoM levels was accompanied by significantly higher levels of HDL-associated S1P in patients with ESKD compared with controls. These results are unexpected because they contradict other clinical studies suggesting that lower ApoM (or HDL/ApoM-associated S1P) levels are associated with kidney disease.187,188,209 Thus, further studies evaluating plasma S1P and ApoM levels in patients with CKD are needed.
Kidney Allograft Survival
Kidney transplantation is the treatment of choice for patients with ESKD,210 yet mechanisms leading to kidney allograft loss are still elusive. We recently demonstrated decreased podocyte SMPDL3b expression in patients with recurrence of FSGS after kidney transplantation,196 similar to our observations in podocytes with radiation-induced damage.182 Furthermore, we demonstrated in both studies that the mAb rituximab, which recognizes CD20 on B lymphocytes but might also bind to SMPDL3b, preserves podocyte SMPDL3b expression and function. We also showed that patients at high risk for FSGS recurrence who received rituximab during transplantation have a lower incidence of post-transplant proteinuria compared with patients who did not receive the drug,196 suggesting a link between sphingolipids, S1P signaling, and kidney allograft survival. Similarly, research in a canine model of kidney transplantation demonstrated that a combination treatment of FTY720 and cyclosporin significantly prolonged kidney allograft survival,211 and other research in rats showed that FTY720 administration before or directly following transplantation, but not late after transplantation, significantly prolonged kidney allograft survival (Table 1).212–214 Taken together, these studies highlight a potential beneficial effect of S1PR agonism in prolonging kidney allograft survival. However, the exact S1P receptor and the mechanisms by which S1PR activation exercises its protective effects and contributes to allograft survival have not yet been investigated.
Hurdles of Targeting S1P in Human Kidney Disease
A large body of evidence suggests that dysregulation of sphingolipid metabolism contributes to the pathogenesis of many diseases, including kidney disease. However, the development and clinical use of these agonists and antagonists of S1P signaling have proven to be challenging for many reasons, particularly because iS1P and eS1P signaling exercise important physiologic functions and sphingolipid metabolism is tightly regulated and highly compartmentalized within cells. Therefore, targeting iS1P or eS1P signaling though targeting SPHKs or S1PRs proves complex, as it cannot simply rely on modulating the intracellular balance of S1P and ceramide levels.
Drug development is further complicated by the existence of two SPHK isoforms (SPHK1 and SPHK2) that have different, sometimes opposite, functions that are at times compensatory. When activated, these isoforms can have opposite effects depending on the cell type, and their activity and function are determined primarily by post-translational modifications and their compartmental localization. Similarly, S1PRs are widely expressed and have important functions in normal cellular physiology. They also have discrete functions that depend on the cell type, making it necessary to develop drugs targeting a specific receptor depending on the cell type, tissue, or the disease targeted.
With regard to drugs targeting S1PR signaling in disease, the nonselective S1P receptor modulator FTY720 (fingolimod) and more-selective S1PR-targeted agents have been studied in a number of clinical settings (Supplemental Table 1).215 The agent most extensively investigated, FTY720, was approved for the treatment of multiple sclerosis. Intracellularly, FTY720 is phosphorylated to FTY720-P, which can then act as an agonist for S1PR1, S1PR3, S1PR4, and S1PR5 signaling.216,217 Interestingly, SPHK2 phosphorylates FTY720 30-fold more efficiently than SPHK1,218 indicating that efficient treatment with FTY720 relies on the expression of SPHK2 in the targeted cell or tissue. The beneficial effect of FTY720 as an immunomodulator in multiple sclerosis is mostly due to modulation of S1PR1 signaling that results in prevention of lymphocyte egress from secondary lymphoid tissues, in addition to neuroprotective effects mediated by interaction with S1P receptors expressed on neural cells.219 However, when used at higher concentrations, FTY720 also has off-target effects, leading to dysregulation of ceramide, S1P, and sphingosine levels in vitro and in vivo; suppression of protein phosphatase 2A; inhibition of phosphatidylinositol 3-kinase/AKT; modulation of 14-3-3 protein; and augmentation of reactive oxygen species (reviewed in Patmanathan et al.220).
Importantly, FTY720 has been associated with a variety of adverse effects in patients, which vary from mild effects, such as headaches and influenza-like illness, to more severe effects, such as bradycardia, atrioventricular block, hypertension, posterior reversible encephalopathy syndrome, macular edema, skin cancer, and even death.221–223 In an analysis of long-term safety findings in >3500 patients enrolled in phase 2 and 3 studies of fingolimod in multiple sclerosis, the Food and Drug Administration–approved dose of 0.5 mg/d was associated with minor BP increases, transient but rarely symptomatic bradycardia and atrioventricular block in 0.5% of patients, dose-dependent but generally asymptomatic increases in liver transaminases in 9%, and macular edema in 0.4%.224 Fingolimod-associated macular edema seems to be a dose-dependent effect that typically resolves upon therapy discontinuation.221,223,225 Despite the expected reductions in the mean lymphocyte count and an increased incidence of lymphopenia compared with placebo, serious infections have been infrequently observed, and fingolimod has not been associated with an increased incidence of infections at the 0.5-mg dose.224 Progressive multifocal leukoencephalopathy (PML), a rare opportunistic infection caused by reactivation of the JC virus, has been reported in patients with fingolimod-treated multiple sclerosis in the postmarketing setting, including those not previously treated with natalizumab, which is known to increase the risk of PML. However, the risk of PML with fingolimod in the absence of prior natalizumab therapy is low, with an estimated incidence rate of 3.12 per 100,000 patient-years.226 Because of transient dose-dependent effects on heart rate and atrioventricular conduction, initiating fingolimod in patients with cardiac risk factors should be approached with caution; precautions, including close monitoring of vital signs and electrocardiography for 6 hours after the first dose, should be taken.224
Treatment Strategies Targeting iS1P and eS1P Signaling in Human Kidney Disease
As discussed above, FTY720 has demonstrated benefit in several preclinical models of kidney disease (Table 1), but in human kidney disease, it has thus far been studied only for its efficacy in preventing kidney allograft rejection after transplantation. In a phase 1 study in stable kidney transplant recipients maintained on cyclosporin and prednisone, FTY720 produced a significant, dose-dependent, reversible decline in peripheral blood lymphocyte counts,227 consistent with inhibition of lymphocyte egress by FTY720 through modulation of S1PRs. Several promising phase 2 studies demonstrated a dose-dependent reduction in the incidence of biopsy-proven acute rejection with FTY720 plus cyclosporin.228,229 However, phase 3 studies in patients with de novo kidney transplant have shown that although FTY720 2.5 mg plus full-dose cyclosporin is noninferior to mycophenolate mofetil plus cyclosporine for the primary composite outcome of first-treated biopsy-proven acute rejection, graft loss, death, or premature study discontinuation, FTY720 is associated with significantly lower creatinine clearance at 12 months compared with mycophenolate, and FTY720 5 mg plus reduced-dose cyclosporin is associated with an increased incidence of acute rejection.230,231 FTY720 was also associated with a greater incidence of transient bradycardia, lymphopenia, and macular edema. The clinical use of FTY720 has been further hampered by the observations that in rats, bradycardia and hypertension are both mediated by S1PR3, whereas in humans, S1PR1 agonism seems to contribute to bradycardia and S1PR3 activation to the development of hypertension.232–235 The latter finding suggests that S1PR1 selective agonists may deserve further study and may still play a role in kidney transplantation and other treatment of kidney diseases.
Because of these limitations, targeting S1P directly with an anti-S1P mAb, which would result in a lower bioavailability of eS1P, has evolved as an alternative and valid therapeutic strategy. An anti-S1P mAb (sonepcizumab) has been evaluated in a phase 1 trial in patients with advanced solid tumors, where it was well tolerated. However, sonepcizumab did not meet the primary end points when evaluated in phase 2a trials in patients with renal cell carcinoma and in patients with wet age–related macular degeneration (Supplemental Table 1). Although sonepcizumab is not a valid therapeutic strategy for patients with these diseases at this point, it will be important in the future to evaluate sonepcizumab or other anti-S1P mAbs for therapeutic efficacy in other conditions characterized by elevated eS1P. Furthermore, the development and evaluation of alternative strategies to capture and neutralize eS1P in such diseases are warranted.
Conclusions and Future Perspectives
Research efforts over the past two decades have led to a better understanding of the role of iS1P and eS1P signaling in different renal cells and have elucidated their contribution to, or association with, a variety of different kidney diseases. It has become clear that the consequences of iS1P and eS1P signaling may differ depending on the renal cell type. Similarly, iS1P and eS1P signaling may differ in their contribution to, or association with, specific kidney diseases. The identification in several families with SRNS of mutations in SGPL1, the gene encoding S1P lyase, together with experimental data demonstrating that Sgpl1 deficiency in mice causes proteinuria and foot process effacement strongly support a causative role for iS1P signaling in SRNS. However, despite a consensus that differential iS1P and eS1P signaling in various renal cells can be observed in a number of kidney diseases, a causative role in disease pathogenesis, disease progression, or both remains to established.
Although S1PR1, S1PR2, and S1PR3 signaling has important roles in renal cell injury, the parts played by S1PR4 and S1PR5 signaling are less well understood and need further investigation, particularly when focusing on kidney diseases. Targeting iS1P signaling as a new therapeutic strategy for patients with kidney diseases may prove difficult given the observation that increased SPHK1 activity can be observed in some kidney diseases and decreased SPHK1 activity may be important in others and given the fact that SPHK1 activation may have different effects in different renal cells. Similarly, a possible differential contribution of eS1P signaling in early and late stages of disease—such as described in experimental models of DKD—suggests that targeting eS1P as a new therapeutic strategy may prove equally difficult.
Therefore, more studies are needed to investigate both signaling pathways and their potential as therapeutic targets. Recent efforts aimed at lowering the bioavailability of eS1P have evolved as an alternative therapeutic strategy. This kind of strategy might offer a valid approach to treat patients with kidney diseases in which high plasma S1P levels are detected, such as in IgAN and LN. However, this treatment approach would not be applicable to patients with CKD featuring reduced plasma S1P levels. Whether CKD with decreased plasma S1P levels reflects a different mechanism of S1P-mediated renal injury or whether this reduction is attributable to the loss of albumin-bound S1P into the urine remains to be determined.
Interestingly, clinical studies indicate a correlation between reduced serum ApoM levels and markers of disease progression in a variety of kidney diseases. Because ApoM is an important modulator of eS1P metabolism, reconstitution of serum or cellular ApoM levels to regulate eS1P and iS1P signaling may represent another valid new therapeutic strategy for the treatment of patients with kidney disease. Further research is warranted to confirm such therapeutic potential.
Disclosures
A. Fornoni is Vice-President of L & F Health LLC and is a consultant for ZyVersa Therapeutics, Inc.; ZyVersa Therapeutics, Inc. has licensed worldwide rights to develop and commercialize hydroxypropyl-β-cyclodextrin from L & F Research for the treatment of kidney disease. A. Fornoni is also the founder of LipoNexT LLC. A. Fornoni and S. Merscher are supported by F. Hoffman-La Roche and by Boehringer Ingelheim. A. Fornoni and S. Merscher are inventors on pending or issued patents (PCT/US11/56272, PCT/US12/62594, PCT/US2019/041730, PCT/US2019/032215, PCT/US13/36484 and PCT 62/674,897) aimed at diagnosing or treating proteinuric kidney diseases. They stand to gain royalties from their future commercialization of these patents. S. Merscher is a consultant for Kintai Therapeutics, Inc. and holds equity interest in L & F Research. S. Merscher is also a Review Editor for Frontiers in Medicine, section nephrology; a Guest Editor for Frontiers in Medicine, section nephrology; and has licensed a patent to L & F Health LLC. A. Mitrofanova reports scientific advisor or membership with the Multidisciplinary Digital Publishing Institute as an editorial board member and other interests/relationships with the American Society of Nephrology as a member and the American Heart Association as a member. All remaining authors have nothing to disclose.
Funding
Y. Drexler is supported by National Center for Advancing Translational Sciences grant UL1TR002736 (Miami Clinical Translational Science Institute). A. Fornoni and S. Merscher are supported by National Institutes of Health grants R01DK117599, R01DK104753, and R01CA227493, and by Boehringer Ingelheim grant GR014854. A. Fornoni is supported by National Institutes of Health grants U54DK083912, UM1DK100846, U01DK116101 and by National Institute on Minority Health and Health Disparities grant UL1TR000460.
Supplementary Material
Acknowledgments
We give a special thanks to the Katz family for their continuous support.
Figures were created with BioRender.com.
Y. Drexler, A. Fornoni, S. Merscher, and J. Molina contributed to the writing and editing of the manuscript; and S. Merscher and A. Mitrofanova designed the figures.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050697/-/DCSupplemental.
Supplemental Table 1. S1P modulators currently in clinical trials.
References
- 1.Maceyka M, Harikumar KB, Milstien S, Spiegel S: Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter VP, Sweeney K, Morré DJ: Neutral lipid precursors for gangliosides are not formed by rat liver homogenates or by purified cell fractions. Biochim Biophys Acta 750: 346–352, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K: Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem 267: 11144–11148, 1992 [PubMed] [Google Scholar]
- 4.Kihara A, Igarashi Y: FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem 279: 49243–49250, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Hirschberg K, Rodger J, Futerman AH: The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J 290: 751–757, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, et al.: Molecular machinery for non-vesicular trafficking of ceramide. Nature 426: 803–809, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Beutel O, Ebell K, Korneev S, Holthuis JC: Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J Cell Sci 130: 360–371, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T: Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids 33: 601–605, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Shimeno H, Soeda S, Yasukouchi M, Okamura N, Nagamatsu A: Fatty acyl-Co A: Sphingosine acyltransferase in bovine brain mitochondria: Its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull 18: 1335–1339, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Vance JE: Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256, 1990 [PubMed] [Google Scholar]
- 11.Airola MV, Hannun YA: Sphingolipid metabolism and neutral sphingomyelinases. Handb Exp Pharmacol 215: 57–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA: Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem 285: 17993–18002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tani M, Okino N, Mitsutake S, Tanigawa T, Izu H, Ito M: Purification and characterization of a neutral ceramidase from mouse liver. A single protein catalyzes the reversible reaction in which ceramide is both hydrolyzed and synthesized. J Biol Chem 275: 3462–3468, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bernardo K, Hurwitz R, Zenk T, Desnick RJ, Ferlinz K, Schuchman EH, et al.: Purification, characterization, and biosynthesis of human acid ceramidase. J Biol Chem 270: 11098–11102, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Mao C, Xu R, Szulc ZM, Bielawski J, Becker KP, Bielawska A, et al.: Cloning and characterization of a mouse endoplasmic reticulum alkaline ceramidase: An enzyme that preferentially regulates metabolism of very long chain ceramides. J Biol Chem 278: 31184–31191, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, et al.: Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J 20: 1813–1825, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM: Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem 276: 26577–26588, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, et al.: Ceramide kinase uses ceramide provided by ceramide transport protein: Localization to organelles of eicosanoid synthesis. J Lipid Res 48: 1293–1304, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC: Identification of a family of animal sphingomyelin synthases. EMBO J 23: 33–44, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S: Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem 273: 23722–23728, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al.: Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 275: 19513–19520, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Le Stunff H, Peterson C, Thornton R, Milstien S, Mandala SM, Spiegel S: Characterization of murine sphingosine-1-phosphate phosphohydrolase. J Biol Chem 277: 8920–8927, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Mechtcheriakova D, Wlachos A, Sobanov J, Kopp T, Reuschel R, Bornancin F, et al.: Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal 19: 748–760, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Reiss U, Oskouian B, Zhou J, Gupta V, Sooriyakumaran P, Kelly S, et al.: Sphingosine-phosphate lyase enhances stress-induced ceramide generation and apoptosis. J Biol Chem 279: 1281–1290, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Stoffel W, LeKim D, Sticht G: Distribution and properties of dihydrosphingosine-1-phosphate aldolase (sphinganine-1-phosphate alkanal-lyase). Hoppe Seylers Z Physiol Chem 350: 1233–1241, 1969 [DOI] [PubMed] [Google Scholar]
- 26.Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD: Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet beta-cells. Endocrinology 147: 4705–4712, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Mandala SM, Thornton R, Galve-Roperh I, Poulton S, Peterson C, Olivera A, et al.: Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1- phosphate and induces cell death. Proc Natl Acad Sci U S A 97: 7859–7864, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, et al.: Dual actions of sphingosine-1-phosphate: Extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol 142: 229–240, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kihara A, Anada Y, Igarashi Y: Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J Biol Chem 281: 4532–4539, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kleuser B, Maceyka M, Milstien S, Spiegel S: Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett 503: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al.: Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J 22: 5491–5500, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengst JA, Guilford JM, Fox TE, Wang X, Conroy EJ, Yun JK: Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Arch Biochem Biophys 492: 62–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S: Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 278: 46832–46839, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al.: Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325: 1254–1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al.: Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148: 988–1000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al.: SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280: 37118–37129, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al.: Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL: Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, et al.: IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem 281: 2515–2525, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al.: Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278: 40330–40336, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al.: Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199: 959–970, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, et al.: Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem 277: 7996–8003, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, et al.: Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med 201: 49–54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM: The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem 281: 11693–11701, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Dai L, Qi Y, Chen J, Kaczorowski D, Di W, Wang W, et al.: Sphingosine kinase (SphK) 1 and SphK2 play equivalent roles in mediating insulin’s mitogenic action. Mol Endocrinol 28: 197–207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S: Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem 282: 12058–12065, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Jahangeer S, et al.: Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem 282: 27493–27502, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S: Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res 281: 115–127, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al.: Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465: 1084–1088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al.: Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25: 600–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajimoto T, Caliman AD, Tobias IS, Okada T, Pilo CA, Van AN, et al.: Activation of atypical protein kinase C by sphingosine 1-phosphate revealed by an aPKC-specific activity reporter. Sci Signal 12: eaat6662, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panneer Selvam S, De Palma RM, Oaks JJ, Oleinik N, Peterson YK, Stahelin RV, et al.: Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal 8: ra58, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S: Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem 76: 1573–1584, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Goodemote KA, Mattie ME, Berger A, Spiegel S: Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem 270: 10272–10277, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Ghosh TK, Bian J, Gill DL: Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science 248: 1653–1656, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Meyer zu Heringdorf D, Lass H, Kuchar I, Alemany R, Guo Y, Schmidt M, et al.: Role of sphingosine kinase in Ca(2+) signalling by epidermal growth factor receptor. FEBS Lett 461: 217–222, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S: Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A 103: 16394–16399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanfin Z, Serrano-Sanchez M, Leiber D: ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cell Signal 23: 1997–2004, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, et al.: Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res 47: 614–621, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T: Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem 284: 21192–21200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T: A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat 84: 154–162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T: Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7: e38941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC, et al.: Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J 27: 1001–1011, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM, et al.: Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550: 524–528, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N: The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323: 524–527, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L, et al.; Sanger Mouse Genetics Project: The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J Immunol 189: 102–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al.: Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci U S A 103: 17384–17389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitroi DN, Karunakaran I, Gräler M, Saba JD, Ehninger D, Ledesma MD, et al.: SGPL1 (sphingosine phosphate lyase 1) modulates neuronal autophagy via phosphatidylethanolamine production. Autophagy 13: 885–899, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Degagné E, Pandurangan A, Bandhuvula P, Kumar A, Eltanawy A, Zhang M, et al.: Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest 124: 5368–5384, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemdan NY, Weigel C, Reimann CM, Gräler MH: Modulating sphingosine 1-phosphate signaling with DOP or FTY720 alleviates vascular and immune defects in mouse sepsis. Eur J Immunol 46: 2767–2777, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Atkinson D, Nikodinovic Glumac J, Asselbergh B, Ermanoska B, Blocquel D, Steiner R, et al.: Sphingosine 1-phosphate lyase deficiency causes Charcot-Marie-Tooth neuropathy. Neurology 88: 533–542, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janecke AR, Xu R, Steichen-Gersdorf E, Waldegger S, Entenmann A, Giner T, et al.: Deficiency of the sphingosine-1-phosphate lyase SGPL1 is associated with congenital nephrotic syndrome and congenital adrenal calcifications. Hum Mutat 38: 365–372, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, et al.: Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest 127: 912–928, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, et al.: Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest 127: 942–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, Sevvana M, et al.: Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A 108: 9613–9618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu N, Dahlbäck B: A novel human apolipoprotein (apoM). J Biol Chem 274: 31286–31290, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Faber K, Axler O, Dahlbäck B, Nielsen LB: Characterization of apoM in normal and genetically modified mice. J Lipid Res 45: 1272–1278, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Zhang XY, Dong X, Zheng L, Luo GH, Liu YH, Ekström U, et al.: Specific tissue expression and cellular localization of human apolipoprotein M as determined by in situ hybridization. Acta Histochem 105: 67–72, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Hla T, Venkataraman K, Michaud J: The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta 1781: 477–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frej C, Andersson A, Larsson B, Guo LJ, Norström E, Happonen KE, et al.: Quantification of sphingosine 1-phosphate by validated LC-MS/MS method revealing strong correlation with apolipoprotein M in plasma but not in serum due to platelet activation during blood coagulation. Anal Bioanal Chem 407: 8533–8542, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG: Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309: 1735–1739, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Urtz N, Gaertner F, Legate KR, Petzold T, Lorenz M, et al.: Sphingosine kinase 2 (Sphk2) regulates platelet biogenesis by providing intracellular sphingosine 1-phosphate (S1P). Blood 122: 791–802, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Yatomi Y, Yamamura S, Hisano N, Nakahara K, Igarashi Y, Ozaki Y: Sphingosine 1-phosphate breakdown in platelets. J Biochem 136: 495–502, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Tani M, Sano T, Ito M, Igarashi Y: Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res 46: 2458–2467, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Engelmann B, Kögl C, Kulschar R, Schaipp B: Transfer of phosphatidylcholine, phosphatidylethanolamine and sphingomyelin from low- and high-density lipoprotein to human platelets. Biochem J 315: 781–789, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al.: Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hänel P, Andréani P, Gräler MH: Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J 21: 1202–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al.: Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316: 295–298, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Swendeman SL, Xiong Y, Cantalupo A, Yuan H, Burg N, Hisano Y, et al.: An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal 10: eaal2722, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM: Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: High density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem 287: 44645–44653, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, et al.: HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation [published correction appears in Sci Signal 8: er8, 2015]. Sci Signal 8: ra79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, et al.: HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523: 342–346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spiegel S: Sphingosine 1-phosphate: A ligand for the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci 905: 54–60, 2000 [DOI] [PubMed] [Google Scholar]
- 94.Gräler MH, Bernhardt G, Lipp M: EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics 53: 164–169, 1998 [DOI] [PubMed] [Google Scholar]
- 95.Im DS, Heise CE, Ancellin N, O’Dowd BF, Shei GJ, Heavens RP, et al.: Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem 275: 14281–14286, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Drouillard A, Mathieu AL, Marcais A, Belot A, Viel S, Mingueneau M, et al. : S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J Allergy Clin Immunol 141: 2265–2268.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 97.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al.: Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR: Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem 274: 27351–27358, 1999 [DOI] [PubMed] [Google Scholar]
- 99.Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y: EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun 260: 203–208, 1999 [DOI] [PubMed] [Google Scholar]
- 100.Van Brocklyn JR, Gräler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S: Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood 95: 2624–2629, 2000 [PubMed] [Google Scholar]
- 101.Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, et al.: Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca(2+) signaling pathway. Biochem Biophys Res Commun 268: 583–589, 2000 [DOI] [PubMed] [Google Scholar]
- 102.Pyne S, Adams DR, Pyne NJ: Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog Lipid Res 62: 93–106, 2016 [DOI] [PubMed] [Google Scholar]
- 103.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al.: Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell 8: 693–704, 2001 [DOI] [PubMed] [Google Scholar]
- 104.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al.: Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res 90: 325–332, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y: Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol 23: 1534–1545, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al.: S1P1-selective in vivo-active agonists from high-throughput screening: Off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 12: 703–715, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Im DS, Tomura H, Tobo M, Sato K, Okajima F: Enhancement of sphingosine 1-phosphate-induced phospholipase C activation during G(0)-G(1) transition in rat hepatocytes [published correction appears in J Pharmacol Sci 95: 402, 2004]. J Pharmacol Sci 95: 284–290, 2004 [DOI] [PubMed] [Google Scholar]
- 108.Björklund S, Palmberg S, Rask S, Westerdahl AC, Törnquist K: Effects of sphingosine 1-phosphate on calcium signaling, proliferation and S1P2 receptor expression in PC Cl3 rat thyroid cells. Mol Cell Endocrinol 231: 65–74, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Im DS, Fujioka T, Katada T, Kondo Y, Ui M, Okajima F: Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am J Physiol 272: G1091–G1099, 1997 [DOI] [PubMed] [Google Scholar]
- 110.Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, et al.: Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282: 10576–10584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferguson SS, Downey WE 3rd, Colapietro AM, Barak LS, Ménard L, Caron MG: Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366, 1996 [DOI] [PubMed] [Google Scholar]
- 112.Alderton F, Rakhit S, Kong KC, Palmer T, Sambi B, Pyne S, et al.: Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem 276: 28578–28585, 2001 [DOI] [PubMed] [Google Scholar]
- 113.Waters CM, Connell MC, Pyne S, Pyne NJ: c-Src is involved in regulating signal transmission from PDGFbeta receptor-GPCR(s) complexes in mammalian cells. Cell Signal 17: 263–277, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL: S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med 207: 1113–1124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, et al.: Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 274: 35343–35350, 1999 [DOI] [PubMed] [Google Scholar]
- 116.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, et al.: Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119: 1871–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al.: Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al.: The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279: 29367–29373, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Paik JH, Chae Ss, Lee MJ, Thangada S, Hla T: Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem 276: 11830–11837, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Wang L, Lee JF, Lin CY, Lee MJ: Rho GTPases mediated integrin alpha v beta 3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol 129: 579–588, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T: Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell 10: 1179–1190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee MJ, Evans M, Hla T: The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem 271: 11272–11279, 1996 [DOI] [PubMed] [Google Scholar]
- 123.van Koppen C, Meyer zu Heringdorf M, Laser KT, Zhang C, Jakobs KH, Bünemann M, et al.: Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem 271: 2082–2087, 1996 [DOI] [PubMed] [Google Scholar]
- 124.Kupperman E, An S, Osborne N, Waldron S, Stainier DY: A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406: 192–195, 2000 [DOI] [PubMed] [Google Scholar]
- 125.Osborne N, Brand-Arzamendi K, Ober EA, Jin SW, Verkade H, Holtzman NG, et al.: The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol 18: 1882–1888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye D, Lin F: S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence. Development 140: 789–799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, et al.: Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol 20: 9247–9261, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, Heusch G, et al.: Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res 108: 314–323, 2011 [DOI] [PubMed] [Google Scholar]
- 129.Hou J, Chen Q, Wu X, Zhao D, Reuveni H, Licht T, et al.: S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am J Respir Crit Care Med 196: 1559–1570, 2017 [DOI] [PubMed] [Google Scholar]
- 130.Hill RZ, Morita T, Brem RB, Bautista DM: S1PR3 mediates itch and pain via distinct TRP channel-dependent pathways. J Neurosci 38: 7833–7843, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mousseau Y, Mollard S, Richard L, Nizou A, Faucher-Durand K, Cook-Moreau J, et al.: Fingolimod inhibits PDGF-B-induced migration of vascular smooth muscle cell by down-regulating the S1PR1/S1PR3 pathway. Biochimie 94: 2523–2531, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, et al.: Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem 286: 7348–7358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.CHARGE Consortium Hematology Working Group: Meta-analysis of rare and common exome chip variants identifies S1PR4 and other loci influencing blood cell traits. Nat Genet 48: 867–876, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schulze T, Golfier S, Tabeling C, Räbel K, Gräler MH, Witzenrath M, et al.: Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J 25: 4024–4036, 2011 [DOI] [PubMed] [Google Scholar]
- 135.Wang W, Graeler MH, Goetzl EJ: Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J 19: 1731–1733, 2005 [DOI] [PubMed] [Google Scholar]
- 136.Kleinwort A, Lührs F, Heidecke CD, Lipp M, Schulze T: S1P signalling differentially affects migration of peritoneal B cell populations in vitro and influences the production of intestinal IgA in vivo. Int J Mol Sci 19: 391, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gräler MH, Grosse R, Kusch A, Kremmer E, Gudermann T, Lipp M: The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J Cell Biochem 89: 507–519, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, et al.: Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol 8: 1337–1344, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al.: T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med 206: 2469–2481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, et al.: Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 25: 1459–1469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI: Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J 21: 1503–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 142.Awad AS, Ye H, Huang L, Li L, Foss FW Jr, Macdonald TL, et al.: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 143.Imasawa T, Kitamura H, Ohkawa R, Satoh Y, Miyashita A, Yatomi Y: Unbalanced expression of sphingosine 1-phosphate receptors in diabetic nephropathy. Exp Toxicol Pathol 62: 53–60, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Koch A, Völzke A, Puff B, Blankenbach K, Meyer Zu Heringdorf D, Huwiler A, et al.: PPARγ agonists upregulate sphingosine 1-phosphate (S1P) receptor 1 expression, which in turn reduces S1P-induced [Ca(2+)]i increases in renal mesangial cells. Biochim Biophys Acta 1831: 1634–1643, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Sun XJ, Wang C, Zhang LX, Yu F, Chen M, Zhao MH: Sphingosine-1-phosphate and its receptors in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 32: 1313–1322, 2017 [DOI] [PubMed] [Google Scholar]
- 146.Kim M, Kim M, Park SW, Pitson SM, Lee HT: Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, et al.: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, et al.: Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 79: 1090–1098, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brunskill EW, Potter SS: Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol 13: 70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hanafusa N, Yatomi Y, Yamada K, Hori Y, Nangaku M, Okuda T, et al.: Sphingosine 1-phosphate stimulates rat mesangial cell proliferation from outside the cells. Nephrol Dial Transplant 17: 580–586, 2002 [DOI] [PubMed] [Google Scholar]
- 151.Katsuma S, Hada Y, Ueda T, Shiojima S, Hirasawa A, Tanoue A, et al.: Signalling mechanisms in sphingosine 1-phosphate-promoted mesangial cell proliferation. Genes Cells 7: 1217–1230, 2002 [DOI] [PubMed] [Google Scholar]
- 152.Klawitter S, Hofmann LP, Pfeilschifter J, Huwiler A: Extracellular nucleotides induce migration of renal mesangial cells by upregulating sphingosine kinase-1 expression and activity. Br J Pharmacol 150: 271–280, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen PF, Chin TY, Chueh SH: Ca2+ signaling induced by sphingosylphosphorylcholine and sphingosine 1-phosphate via distinct mechanisms in rat glomerular mesangial cells. Kidney Int 54: 1470–1483, 1998 [DOI] [PubMed] [Google Scholar]
- 154.Koch A, Jäger M, Völzke A, Grammatikos G, Zu Heringdorf DM, Huwiler A, et al.: Downregulation of sphingosine 1-phosphate (S1P) receptor 1 by dexamethasone inhibits S1P-induced mesangial cell migration. Biol Chem 396: 803–812, 2015 [DOI] [PubMed] [Google Scholar]
- 155.Völzke A, Koch A, Meyer Zu Heringdorf D, Huwiler A, Pfeilschifter J: Sphingosine 1-phosphate (S1P) induces COX-2 expression and PGE2 formation via S1P receptor 2 in renal mesangial cells. Biochim Biophys Acta 1841: 11–21, 2014 [DOI] [PubMed] [Google Scholar]
- 156.Hofmann LP, Ren S, Schwalm S, Pfeilschifter J, Huwiler A: Sphingosine kinase 1 and 2 regulate the capacity of mesangial cells to resist apoptotic stimuli in an opposing manner. Biol Chem 389: 1399–1407, 2008 [DOI] [PubMed] [Google Scholar]
- 157.Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, et al.: Transforming growth factor-beta2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-beta2 by impeding CTGF expression. Kidney Int 76: 857–867, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Li Q, Chen B, Zeng C, Fan A, Yuan Y, Guo X, et al.: Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Exp Physiol 100: 95–107, 2015 [DOI] [PubMed] [Google Scholar]
- 159.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al.: Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liao J, Yu Z, Chen Y, Bao M, Zou C, Zhang H, et al.: Single-cell RNA sequencing of human kidney. Sci Data 7: 4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fu J, Akat KM, Sun Z, Zhang W, Schlondorff D, Liu Z, et al.: Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J Am Soc Nephrol 30: 533–545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Su K, Zeng P, Liang W, Luo Z, Wang Y, Lv X, et al.: FTY720 attenuates angiotensin II-induced podocyte damage via inhibiting inflammatory cytokines. Mediators Inflamm 2017: 3701385, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim M, Kim M, Kim N, D’Agati VD, Emala CW Sr, Lee HT: Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Park SW, Kim M, Kim M, D’Agati VD, Lee HT: Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int 80: 1315–1327, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT: Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 266–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu X, Hong Q, Wang Z, Yu Y, Zou X, Xu L: Transforming growth factor-β-sphingosine kinase 1/S1P signaling upregulates microRNA-21 to promote fibrosis in renal tubular epithelial cells. Exp Biol Med (Maywood) 241: 265–272, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saxena V, Fitch J, Ketz J, White P, Wetzel A, Chanley MA, et al.: Whole transcriptome analysis of renal intercalated cells predicts lipopolysaccharide mediated inhibition of retinoid X receptor alpha function. Sci Rep 9: 545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, et al.: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 169.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 170.Daehn IS: Glomerular endothelial cell stress and cross-talk with podocytes in early [corrected] diabetic kidney disease [published correction appears in Front Med (Lausanne) 5: 113, 2018 10.3389/fmed.2018.00113]. Front Med (Lausanne) 5: 76, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Siddiqi FS, Advani A: Endothelial-podocyte crosstalk: The missing link between endothelial dysfunction and albuminuria in diabetes. Diabetes 62: 3647–3655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al.: Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015 [DOI] [PubMed] [Google Scholar]
- 173.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD: Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ham A, Kim M, Kim JY, Brown KM, Fruttiger M, D’Agati VD, et al.: Selective deletion of the endothelial sphingosine-1-phosphate 1 receptor exacerbates kidney ischemia-reperfusion injury. Kidney Int 85: 807–823, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perry HM, Huang L, Ye H, Liu C, Sung SJ, Lynch KR, et al.: Endothelial sphingosine 1-phosphate receptor-1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol 27: 3383–3393, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW: S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006 [DOI] [PubMed] [Google Scholar]
- 178.Lai LW, Yong KC, Igarashi S, Lien YH: A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 71: 1223–1231, 2007 [DOI] [PubMed] [Google Scholar]
- 179.Wilson PC, Wu H, Kirita Y, Uchimura K, Ledru N, Rennke HG, et al.: The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A 116: 19619–19625, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, et al.: SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun 10: 2692, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, et al.: Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol 26: 133–147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, et al.: Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J 31: 771–780, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Geoffroy K, Troncy L, Wiernsperger N, Lagarde M, El Bawab S: Glomerular proliferation during early stages of diabetic nephropathy is associated with local increase of sphingosine-1-phosphate levels. FEBS Lett 579: 1249–1254, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Lan T, Liu W, Xie X, Xu S, Huang K, Peng J, et al.: Sphingosine kinase-1 pathway mediates high glucose-induced fibronectin expression in glomerular mesangial cells. Mol Endocrinol 25: 2094–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lan T, Shen X, Liu P, Liu W, Xu S, Xie X, et al.: Berberine ameliorates renal injury in diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P signaling pathway. Arch Biochem Biophys 502: 112–120, 2010 [DOI] [PubMed] [Google Scholar]
- 186.Bekpinar S, Yenidunya G, Gurdol F, Unlucerci Y, Aycan-Ustyol E, Dinccag N: The effect of nephropathy on plasma sphingosine 1-phosphate concentrations in patients with type 2 diabetes. Clin Biochem 48: 1264–1267, 2015 [DOI] [PubMed] [Google Scholar]
- 187.Kurano M, Tsukamoto K, Shimizu T, Kassai H, Nakao K, Aiba A, et al.: Protection against insulin resistance by apolipoprotein M/Sphingosine-1-Phosphate. Diabetes 69: 867–881, 2020 [DOI] [PubMed] [Google Scholar]
- 188.Memon AA, Bennet L, Zöller B, Wang X, Palmér K, Dahlbäck B, et al.: The association between apolipoprotein M and insulin resistance varies with country of birth. Nutr Metab Cardiovasc Dis 24: 1174–1180, 2014 [DOI] [PubMed] [Google Scholar]
- 189.Breitz H: Clinical aspects of radiation nephropathy. Cancer Biother Radiopharm 19: 359–362, 2004 [DOI] [PubMed] [Google Scholar]
- 190.Cassady JR: Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys 31: 1249–1256, 1995 [DOI] [PubMed] [Google Scholar]
- 191.Coppo R: Clinical and histological risk factors for progression of IgA nephropathy: An update in children, young and adult patients. J Nephrol 30: 339–346, 2017 [DOI] [PubMed] [Google Scholar]
- 192.Miyawaki S, Muso E, Takeuchi E, Matsushima H, Shibata Y, Sasayama S, et al.: Selective breeding for high serum IgA levels from noninbred ddY mice: Isolation of a strain with an early onset of glomerular IgA deposition. Nephron 76: 201–207, 1997 [DOI] [PubMed] [Google Scholar]
- 193.Katsuma S, Shiojima S, Hirasawa A, Suzuki Y, Takagaki K, Murai M, et al.: Genomic analysis of a mouse model of immunoglobulin A nephropathy reveals an enhanced PDGF-EDG5 cascade. Pharmacogenomics J 1: 211–217, 2001 [DOI] [PubMed] [Google Scholar]
- 194.Kurano M, Tsuneyama K, Morimoto Y, Nishikawa M, Yatomi Y: Apolipoprotein M suppresses the phenotypes of IgA nephropathy in hyper-IgA mice. FASEB J 33: 5181–5195, 2019 [DOI] [PubMed] [Google Scholar]
- 195.Wu J, He L, Bai L, Tan L, Hu M: Apolipoprotein M serum levels correlate with IgA vasculitis and IgA vasculitis nephritis. Dis Markers 2019: 1825849, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al.: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bomback AS, Markowitz GS: Lupus podocytopathy: A distinct entity. Clin J Am Soc Nephrol 11: 547–548, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Alperovich G, Rama I, Lloberas N, Franquesa M, Poveda R, Gomà M, et al.: New immunosuppresor strategies in the treatment of murine lupus nephritis. Lupus 16: 18–24, 2007 [DOI] [PubMed] [Google Scholar]
- 199.Okazaki H, Hirata D, Kamimura T, Sato H, Iwamoto M, Yoshio T, et al.: Effects of FTY720 in MRL-lpr/lpr mice: Therapeutic potential in systemic lupus erythematosus. J Rheumatol 29: 707–716, 2002 [PubMed] [Google Scholar]
- 200.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, et al.: Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458: 524–528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chiba K, Matsuyuki H, Maeda Y, Sugahara K: Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol 3: 11–19, 2006 [PubMed] [Google Scholar]
- 202.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al.: Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360, 2004 [DOI] [PubMed] [Google Scholar]
- 203.Taylor Meadows KR, Steinberg MW, Clemons B, Stokes ME, Opiteck GJ, Peach R, et al.: Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 13: e0193236, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Watson L, Tullus K, Marks SD, Holt RC, Pilkington C, Beresford MW: Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. J Clin Immunol 32: 1019–1025, 2012 [DOI] [PubMed] [Google Scholar]
- 205.Patyna S, Büttner S, Eckes T, Obermüller N, Bartel C, Braner A, et al.: Blood ceramides as novel markers for renal impairment in systemic lupus erythematosus. Prostaglandins Other Lipid Mediat 144: 106348, 2019 [DOI] [PubMed] [Google Scholar]
- 206.Checa A, Idborg H, Zandian A, Sar DG, Surowiec I, Trygg J, et al.: Dysregulations in circulating sphingolipids associate with disease activity indices in female patients with systemic lupus erythematosus: A cross-sectional study. Lupus 26: 1023–1033, 2017 [DOI] [PubMed] [Google Scholar]
- 207.Sun XJ, Chen M, Zhao MH: Sphingosine-1-phosphate (S1P) enhances glomerular endothelial cells activation mediated by anti-myeloperoxidase antibody-positive IgG. J Cell Mol Med 22: 1769–1777, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hao J, Huang YM, Zhao MH, Chen M: The interaction between C5a and sphingosine-1-phosphate in neutrophils for antineutrophil cytoplasmic antibody mediated activation. Arthritis Res Ther 16: R142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sørensen IM, Bertelsen M, Freese E, Lindhard K, Ullum H, Feldt-Rasmussen B, et al.: Apolipoprotein M in patients with chronic kidney disease. Atherosclerosis 275: 304–311, 2018 [DOI] [PubMed] [Google Scholar]
- 210.Suthanthiran M, Strom TB: Renal transplantation. N Engl J Med 331: 365–376, 1994 [DOI] [PubMed] [Google Scholar]
- 211.Suzuki S, Kakefuda T, Amemiya H, Chiba K, Hoshino Y, Kawaguchi T, et al.: An immunosuppressive regimen using FTY720 combined with cyclosporin in canine kidney transplantation. Transpl Int 11: 95–101, 1998 [DOI] [PubMed] [Google Scholar]
- 212.Ueda H, Takahara S, Azuma H, Kusaka M, Suzuki S, Katsuoka Y: Effect of a novel immunosuppressant, FTY720, on allograft survival after renal transplant in rats. Eur Surg Res 32: 279–283, 2000 [DOI] [PubMed] [Google Scholar]
- 213.Ueda H, Takahara S, Itoh S, Nomi H, Shibahara N, Katsuoka Y: Preoperative administration of FTY720 prolonged renal allograft survival. Transpl Immunol 14: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 214.Koch M, Poehnert D, Nashan B: Effects of FTY720 on peripheral blood lymphocytes and graft infiltrating cells in a rat model of chronic renal allograft rejection. Transpl Immunol 35: 12–17, 2016 [DOI] [PubMed] [Google Scholar]
- 215.Cartier A, Hla T: Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 366: eaar5551, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al.: The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002 [DOI] [PubMed] [Google Scholar]
- 217.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al.: Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349, 2002 [DOI] [PubMed] [Google Scholar]
- 218.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T: Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278: 47408–47415, 2003 [DOI] [PubMed] [Google Scholar]
- 219.Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C: The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323: 626–635, 2007 [DOI] [PubMed] [Google Scholar]
- 220.Patmanathan SN, Yap LF, Murray PG, Paterson IC: The antineoplastic properties of FTY720: Evidence for the repurposing of fingolimod. J Cell Mol Med 19: 2329–2340, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al.; TRANSFORMS Study Group: Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415, 2010 [DOI] [PubMed] [Google Scholar]
- 222.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al.; FTY720 D2201 Study Group: Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355: 1124–1140, 2006 [DOI] [PubMed] [Google Scholar]
- 223.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al.; FREEDOMS Study Group: A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362: 387–401, 2010 [DOI] [PubMed] [Google Scholar]
- 224.Kappos L, Cohen J, Collins W, de Vera A, Zhang-Auberson L, Ritter S, et al.: Fingolimod in relapsing multiple sclerosis: An integrated analysis of safety findings. Mult Scler Relat Disord 3: 494–504, 2014 [DOI] [PubMed] [Google Scholar]
- 225.Jain N, Bhatti MT: Fingolimod-associated macular edema: Incidence, detection, and management. Neurology 78: 672–680, 2012 [DOI] [PubMed] [Google Scholar]
- 226.Berger JR, Cree BA, Greenberg B, Hemmer B, Ward BJ, Dong VM, et al.: Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 90: e1815–e1821, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kahan BD, Karlix JL, Ferguson RM, Leichtman AB, Mulgaonkar S, Gonwa TA, et al.: Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: A multicenter, randomized, placebo-controlled, phase I study. Transplantation 76: 1079–1084, 2003 [DOI] [PubMed] [Google Scholar]
- 228.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, et al.: FTY720, a novel immunomodulator: Efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 77: 1826–1833, 2004 [PubMed] [Google Scholar]
- 229.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, et al.: FTY720, a novel immunomodulator: Efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 79: 1553–1560, 2005 [PubMed] [Google Scholar]
- 230.Salvadori M, Budde K, Charpentier B, Klempnauer J, Nashan B, Pallardo LM, et al.; FTY720 0124 Study Group: FTY720 versus MMF with cyclosporine in de novo renal transplantation: A 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant 6: 2912–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 231.Tedesco-Silva H, Pescovitz MD, Cibrik D, Rees MA, Mulgaonkar S, Kahan BD, et al.; FTY720 Study Group: Randomized controlled trial of FTY720 versus MMF in de novo renal transplantation. Transplantation 82: 1689–1697, 2006 [DOI] [PubMed] [Google Scholar]
- 232.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, et al.: Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 309: 758–768, 2004 [DOI] [PubMed] [Google Scholar]
- 233.Fryer RM, Muthukumarana A, Harrison PC, Nodop Mazurek S, Chen RR, Harrington KE, et al.: The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS One 7: e52985, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. : The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol 167: 1035–1047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al.: Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004 [DOI] [PubMed] [Google Scholar]
- 236.Xu CB, Zhang Y, Stenman E, Edvinsson L: D-erythro-N,N-dimethylsphingosine inhibits bFGF-induced proliferation of cerebral, aortic and coronary smooth muscle cells. Atherosclerosis 164: 237–243, 2002 [DOI] [PubMed] [Google Scholar]
- 237.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, et al.: Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: The role of sphingosine kinase-1. J Cell Biol 173: 301–310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huwiler A, Döll F, Ren S, Klawitter S, Greening A, Römer I, et al.: Histamine increases sphingosine kinase-1 expression and activity in the human arterial endothelial cell line EA.hy 926 by a PKC-alpha-dependent mechanism. Biochim Biophys Acta 1761: 367–376, 2006 [DOI] [PubMed] [Google Scholar]
- 239.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T: Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: Function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 17: 1203–1217, 2005 [DOI] [PubMed] [Google Scholar]
- 240.Edsall LC, Pirianov GG, Spiegel S: Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci 17: 6952–6960, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Olivera A, Spiegel S: Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365: 557–560, 1993 [DOI] [PubMed] [Google Scholar]
- 242.Buehrer BM, Bardes ES, Bell RM: Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim Biophys Acta 1303: 233–242, 1996 [DOI] [PubMed] [Google Scholar]
- 243.Miller AV, Alvarez SE, Spiegel S, Lebman DA: Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol 28: 4142–4151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Osawa Y, Banno Y, Nagaki M, Brenner DA, Naiki T, Nozawa Y, et al.: TNF-alpha-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J Immunol 167: 173–180, 2001 [DOI] [PubMed] [Google Scholar]
- 245.Shu X, Wu W, Mosteller RD, Broek D: Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol 22: 7758–7768, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kleuser B, Cuvillier O, Spiegel S: 1Alpha,25-dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res 58: 1817–1824, 1998 [PubMed] [Google Scholar]
- 247.Maceyka M, Payne SG, Milstien S, Spiegel S: Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 1585: 193–201, 2002 [DOI] [PubMed] [Google Scholar]
- 248.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S: Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 249.Neubauer HA, Pitson SM: Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J 280: 5317–5336, 2013 [DOI] [PubMed] [Google Scholar]
- 250.Snider AJ, Ruiz P, Obeid LM, Oates JC: Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS One 8: e53521, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huang K, Liu W, Lan T, Xie X, Peng J, Huang J, et al.: Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One 7: e43874, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Peters H, Martini S, Wang Y, Shimizu F, Kawachi H, Krämer S, et al.: Selective lymphocyte inhibition by FTY720 slows the progressive course of chronic anti-thy 1 glomerulosclerosis. Kidney Int 66: 1434–1443, 2004 [DOI] [PubMed] [Google Scholar]
- 253.Martini S, Krämer S, Loof T, Wang-Rosenke Y, Daig U, Budde K, et al.: S1P modulator FTY720 limits matrix expansion in acute anti-thy1 mesangioproliferative glomerulonephritis. Am J Physiol Renal Physiol 292: F1761–F1770, 2007 [DOI] [PubMed] [Google Scholar]
- 254.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, et al.: Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, et al.: Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol 26: 908–925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li X, Wu M, Chen L, Lu J, Li G, Fu L, et al.: A sphingosine-1-phosphate modulator ameliorates polycystic kidney disease in han:SPRD rats. Am J Nephrol 51: 1–10, 2020 [DOI] [PubMed] [Google Scholar]
- 257.Wang LY, Sun XJ, Wang C, Chen SF, Li ZY, Chen M, et al.: Sphingosine-1-phosphate receptor modulator FTY720 attenuates experimental myeloperoxidase-ANCA vasculitis in a T cell-dependent manner. Clin Sci (Lond) 134: 1475–1489, 2020 [DOI] [PubMed] [Google Scholar]
- 258.Wenderfer SE, Stepkowski SM, Braun MC: Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int 74: 1319–1326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sugahara K, Maeda Y, Shimano K, Murase M, Mochiduki S, Takemoto K, et al.: Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 modulator, potently inhibits the progression of lupus nephritis in two murine SLE models. J Immunol Res 2019: 5821589, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lee SY, Kim DH, Sung SA, Kim MG, Cho WY, Kim HK, et al.: Sphingosine-1-phosphate reduces hepatic ischaemia/reperfusion-induced acute kidney injury through attenuation of endothelial injury in mice. Nephrology (Carlton) 16: 163–173, 2011 [DOI] [PubMed] [Google Scholar]
- 261.Kurano M, Tsuneyama K, Morimoto Y, Shimizu T, Jona M, Kassai H, et al.: Apolipoprotein M protects lipopolysaccharide-treated mice from death and organ injury. Thromb Haemost 118: 1021–1035, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.