Significance Statement
In diabetic kidney disease, ascertaining which patients will progress to ESKD is difficult. Efforts are under way to determine whether plasma biomarkers can identify these high-risk individuals; such biomarkers may inform development of therapies and selection of individuals for clinical trials. In this case-cohort study of well-phenotyped individuals with diabetic kidney disease, increased concentrations of plasma biomarkers related to tubular injury, inflammation, and fibrosis (kidney injury molecule 1 [KIM-1], TNF receptor 1 [TNFR-1], TNFR-2, monocyte chemotactic protein-1, soluble urokinase-type plasminogen activator receptor [suPAR], and YKL-40) were associated with increased risk of progression of diabetic kidney disease. After accounting for the other biomarkers, higher TNFR-2 levels were most strongly associated with disease progression. These findings validate the previous literature on TNFR-1, TNFR-2, and KIM-1, and provide new insights on suPAR and YKL-40 as plasma markers of diabetic kidney disease progression that require validation.
Keywords: diabetes, diabetic nephropathy, end stage kidney disease, epidemiology and outcomes, chronic diabetic complications, chronic kidney disease, diabetic kidney disease, biomarker
Visual Abstract
Abstract
Background
Although diabetic kidney disease is the leading cause of ESKD in the United States, identifying those patients who progress to ESKD is difficult. Efforts are under way to determine if plasma biomarkers can help identify these high-risk individuals.
Methods
In our case-cohort study of 894 Chronic Renal Insufficiency Cohort Study participants with diabetes and an eGFR of <60 ml/min per 1.73 m2 at baseline, participants were randomly selected for the subcohort; cases were those patients who developed progressive diabetic kidney disease (ESKD or 40% eGFR decline). Using a multiplex system, we assayed plasma biomarkers related to tubular injury, inflammation, and fibrosis (KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, and YKL-40). Weighted Cox regression models related biomarkers to progression of diabetic kidney disease, and mixed-effects models estimated biomarker relationships with rate of eGFR change.
Results
Median follow-up was 8.7 years. Higher concentrations of KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, and YKL-40 were each associated with a greater risk of progression of diabetic kidney disease, even after adjustment for established clinical risk factors. After accounting for competing biomarkers, KIM-1, TNFR-2, and YKL-40 remained associated with progression of diabetic kidney disease; TNFR-2 had the highest risk (adjusted hazard ratio, 1.61; 95% CI, 1.15 to 2.26). KIM-1, TNFR-1, TNFR-2, and YKL-40 were associated with rate of eGFR decline.
Conclusions
Higher plasma levels of KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, and YKL-40 were associated with increased risk of progression of diabetic kidney disease; TNFR-2 had the highest risk after accounting for the other biomarkers. These findings validate previous literature on TNFR-1, TNFR-2, and KIM-1 in patients with prevalent CKD and provide new insights into the influence of suPAR and YKL-40 as plasma biomarkers that require validation.
Diabetic kidney disease (DKD) develops in approximately 30% of individuals with type 1 diabetes mellitus and 40% of individuals with type 2 diabetes mellitus.1 DKD is the leading cause of ESKD in the United States.2 Pathways and processes leading to progression of DKD are heterogeneous,3 including inflammation, fibrosis, and tubular injury, which makes it difficult to identify patients who will develop DKD progression, and ultimately ESKD. These diverse mechanisms may also contribute to the variability in response to interventions aimed at slowing disease progression.
To date, the established diagnostic and prognostic tools for DKD have limitations. A kidney biopsy is the reference standard for diagnosis, but it is invasive and associated with risk, and provides glomerular and tubulointerstitial histology of modest prognostic value. The best measure of kidney function is eGFR, which carries prognostic information, but is limited due to unreliability.4,5 Albuminuria has also been shown to strongly predict DKD progression, but many individuals with diabetes develop regression of albuminuria.6–8 Furthermore, pathologic lesions3,9,10 and DKD progression occur in the absence or presence of low levels of albuminuria.11–14 Thus, there is an unmet need for discovering better biomarkers that can identify individuals at high risk of progression of DKD who may benefit from more intensified or targeted treatments.
In the past decade, investigations have elucidated specific DKD progression pathways, along with identification of associated biomarkers.15,16 Recent studies have reported associations of both the initiation and the progression of DKD with several proinflammatory and profibrotic plasma markers, such as soluble TNF receptors 1 and 2 (TNFR-1 and TNFR-2),17–21 YKL-40 (a heparin- and chitin-binding glycoprotein),22 monocyte chemotactic protein-1 (MCP-1, also known as the C-C chemokine ligand 2),23 and soluble urokinase-type plasminogen activator receptor (suPAR),24 and biomarkers of tubular injury, including kidney injury molecule 1 (KIM-1).25,26 Prior studies of DKD progression have been limited by relatively small samples, especially of individuals with already established type 2 diabetes, and including those with both normal and impaired kidney function, reporting only one or two biomarker associations, and without determining inter-relationships between biomarkers. Accordingly, we assessed if plasma biomarkers related to inflammation, fibrosis, and tubular injury were associated with the progression of DKD in the well-characterized subpopulation of individuals with mild to moderate kidney disease and diabetes in the Chronic Renal Insufficiency Cohort (CRIC) Study. We hypothesized that plasma concentrations of KIM-1, TNFR-1, TNFR-2, YKL-40, suPAR, and MCP-1 were associated with DKD progression and eGFR decline over time.
Methods
Study Design and Study Population
This study uses a case-cohort design of participants with diabetes within the CRIC Study, a multicenter, prospective, observational cohort study of 3939 men and women with mild to moderate CKD in the United States.27,28 The case-cohort design was chosen to reduce the need to assay biomarkers in the full cohort, while still retaining the validity to conduct prospective analyses. Briefly, CRIC Study participants were recruited from seven clinical centers from 2003 to 2008. The inclusion and exclusion criteria have been detailed previously.27,28 The major eligibility criteria included adults 21–74 years of age with eGFR from 20 to 70 ml/min per 1.73 m2. The CRIC Study protocol was approved by the institutional review boards of all participating centers, and is in accordance with the Declaration of Helsinki. All participants provided written informed consent.
CRIC participants were eligible for this study if they had a diagnosis of diabetes (made on the basis of a fasting glucose of ≥126 mg/dl, a nonfasting glucose of ≥200 mg/dl, or self-reported use of insulin or other medications for glycemic control), an eGFR of <60 ml/min per 1.73 m2, and if their plasma biospecimens had been collected (n=1315). The baseline for each participant was defined as the annual study visit at which the plasma samples were collected. The time between the CRIC baseline visit, at which covariate data were collected, and the visit at which plasma samples were collected was approximately 1 year (median, 370 days; interquartile range [IQR], 0–713 days). A subcohort was constructed as a randomly selected subset of all eligible participants, independent of case status. In addition, all of the individuals who developed the primary outcome of interest, defined as ESKD or 40% decline in baseline eGFR, were included in the study population as cases (see Supplemental Figure 1 for study flow diagram).
Plasma Biomarker Measurements
Plasma biomarker concentrations were the primary exposure in this study. Plasma samples were collected at baseline (2003–2010) and stored at −80°C until they were thawed for measurement of the biomarkers KIM-1, MCP-1, TNFR-1, TNFR-2, suPAR, and YKL-40, using a multiplex U-PLEX assay on the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD). All of the following antibodies (capture and detection antibodies) were obtained from R&D Systems (Bio-Techne): KIM-1 (catalog numbers AF1750 and AF1750); YKL-40 (catalog numbers MAB25991 and AF2577); TNFR-1 (catalog numbers MAB625 and MAB225); TNFR-2 (catalog numbers MAB726 and AF726); suPAR (catalog numbers MAB807 and AF807); MCP-1 (catalog numbers MAB679 and AF279). The biomarkers were selected on the basis of prior published reports and preliminary data generated by the CKD Biomarker Consortium investigators.18,24,26,29–31 Biomarker concentrations were expressed as the mean of duplicate measurements. Overall, intra-assay coefficients of variation were all ≤7%, and interassay coefficients of variation were <15.3%. Please see Supplemental Table 1 for quality-control parameters for each biomarker assay. All assays were performed blinded to clinical outcomes.
Other Study Variables
Information on age, sex, race/ethnicity, education, and medical history (including hypertension, prevalent cardiovascular disease, smoking status, and medication use) were collected through self-report. Using a standardized technique, height, weight, and BP were measured. BP was measured in triplicate, and the mean of the latter two measurements was recorded. Hypertension was defined by a systolic BP >140 mm Hg, a diastolic BP >90 mm Hg, or self-reported use of antihypertensive medications.32 Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. Blood samples were used to measure hemoglobin A1c, serum high-sensitivity C-reactive protein (hsCRP), and serum creatinine and cystatin C concentrations for the estimation of GFR with a CRIC-specific equation.33 Proteinuria was assessed using assays of urine collections of creatinine and protein (urine protein-creatinine ratio [UPCR] in milligrams per gram).
Study Outcomes
The primary outcome of interest, DKD progression, was defined as time to incident ESKD (determined through self-report, review of medical records, and linkage to the US Renal Data System) or a ≥40% decline (relative to baseline) during the follow-up period. A secondary outcome of interest included the yearly change in eGFR over time in milliliters per minute per 1.73 m2. Study participants were followed until the occurrence of the primary end point, death, withdrawal from the study, or the end of the follow-up period (mid-2017).
Statistical Analyses
We described the study population using mean (SD) or median (IQR) for continuous variables, and frequency and proportion for categoric variables. The Pearson chi-squared or Fisher exact test and ANOVA or Kruskal–Wallis test were used to compare categoric and continuous variables, respectively. Partial Pearson pairwise correlations, adjusting for age and sex, were assessed to evaluate the relationship between biomarkers, eGFR, and UPCR.
Multivariable, weighted, Cox proportional hazards models that accounted for the case-cohort design were used to assess the association between each plasma biomarker concentration with DKD progression. A weighted regression was used to account for differential sampling probabilities between the subcohort and additional cases to permit estimation of relative risk and rates of the exposure-outcome relationship.34 We performed hierarchic modeling with sequential adjustment for sociodemographic covariates, including sex, race/ethnicity, education, study site; and clinical characteristics, including systolic BP, diastolic BP, BP medications, smoking status, BMI, hsCRP, and hemoglobin A1c; and then by adding eGFR alone, UPCR alone, and finally adjusting for both eGFR and UPCR. We also assessed the interaction between baseline eGFR (<45 versus ≥45 ml/min per 1.73 m2) and DKD progression, expecting that those with a lower baseline eGFR to have enhanced biomarker associations with DKD progression.35
As a sensitivity analysis, to address potential batch effects of the biospecimen assays, we used the ComBat approach, which corrects batch differences in mean and variance of the assay values via empirical Bayes estimation.36 Further, to assess the potential influence of imbalance between the subcohort and additional cases, we limited the Cox proportional hazards models to the subcohort only to assess the consistency of results from the primary analysis using weighted Cox proportional hazards models. As an exploratory analysis, we also fit a multivariable model to evaluate the inter-relationships of the six biomarkers in their association with DKD progression. We used a backward selection algorithm to create a parsimonious model, retaining markers with a significance level of P<0.05.
Mixed-effects models were used to assess the relations of biomarker concentrations with yearly change in eGFR within the subcohort only. All biomarkers were logarithmically transformed to normalize their skewed distributions, and log2 transformed variables were used in all regression analyses. Adjusted hazard ratios (aHRs) with 95% confidence intervals were reported. Analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC). All P values were two-tailed, with an α level of <0.05.
Results
From the 1315 eligible participants in the full CRIC cohort, we studied 894 individuals. Of these 894 individuals, 596 were randomly selected as a subcohort of all eligible CRIC participants (240 of whom subsequently developed DKD progression and thus became cases), and 298 were CRIC participants outside of the subcohort who developed DKD progression and thus became cases during follow-up. Baseline characteristics of the study participants are described in Table 1. The mean age was 60.8 years in the subcohort, and 58.6 years among all cases. A similar frequency of hypertension and current smoking was observed in the subcohort and cases. The mean baseline eGFR was 36.5 ml/min per 1.73 m2 in the subcohort and 32.7 ml/min per 1.73 m2 among cases, with a median baseline UPCR of 0.3 and 1.2 mg/g among the subcohort and all cases, respectively.
Table 1.
Baseline characteristics of CRIC participants with diabetes overall and by case status
| Characteristics | Eligible Cohort (n=1315) | Random Subcohort (n=596) | All Cases (n=538) |
|---|---|---|---|
| Age, yr (SD) | 61.0 (9.3) | 60.8 (9.3) | 58.6 (9.8) |
| Sex, % | |||
| Male | 57.2 | 58.2 | 60.6 |
| Female | 42.8 | 41.8 | 39.4 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 36.0 | 37.2 | 25.8 |
| Non-Hispanic Black | 41.2 | 40.8 | 45.5 |
| Hispanic | 19.2 | 18.5 | 25.1 |
| Other | 3.7 | 3.5 | 3.5 |
| Education, % | |||
| Less than high school | 27.2 | 28.0 | 31.4 |
| High school and some college | 48.4 | 47.5 | 48.3 |
| College degree and higher | 24.4 | 24.5 | 20.3 |
| Smoking status, % | |||
| Current | 11.2 | 11.4 | 12.3 |
| Former | 45.0 | 43.1 | 45.2 |
| Never | 43.8 | 45.5 | 42.6 |
| Hypertension, % | 95.4 | 96.0 | 96.7 |
| Systolic BP (mm Hg), mean (SD) | 133 (24) | 133 (24) | 140 (24) |
| Diastolic BP (mm Hg), mean (SD) | 68 (13) | 68 (12) | 71 (13) |
| Antihypertensive medication use, % | 98.4 | 98.1 | 98.9 |
| Self-reported cardiovascular disease, % | 45.6 | 43.6 | 45.9 |
| BMI (kg/m2), mean (SD) | 34.1 (7.8) | 33.8 (7.7) | 33.9 (8.0) |
| Hemoglobin A1c, mean (SD) | 7.2 (1.5) | 7.2 (1.5) | 7.4 (1.6) |
| Baseline eGFR (ml/min per 1.73 m2), mean (SD) | 36.6 (12.0) | 36.5 (12.2) | 32.7 (11.9) |
| eGFR (ml/min per 1.73 m2), mean (SD)a | – | −9.0 (12.8) | −19.2 (9.8) |
| UPCR (mg/g), median (IQR) | 0.3 (0.1–1.4) | 0.3 (0.1–1.3) | 1.2 (0.4–3.3) |
| hsCRP (mg/L), median (IQR) | 2.7 (1.1–6.7) | 2.6 (1.1–5.9) | 2.5 (1.0–5.9) |
eGFR: difference between baseline and last eGFR.
Plasma biomarker concentrations were consistently higher among those with lower baseline eGFR values (Figure 1, Supplemental Figure 2, Supplemental Table 2). Partial Pearson pairwise correlations between biomarker concentrations and key predictors of CKD progression (eGFR and UPCR) are shown Table 2. TNFR-1 and TNFR-2 were most correlated (rho=0.82), and the pairwise correlations between MCP-1, suPAR, TNFR-1, TNFR-2 were moderate to high (rho=0.28–0.74). All biomarkers had a consistent inverse correlation with eGFR (rho=−0.22 to −0.73), with the TNFRs and suPAR most strongly correlated. UPCR was positively correlated with all biomarkers (rho=0.07–0.61).
Figure 1.
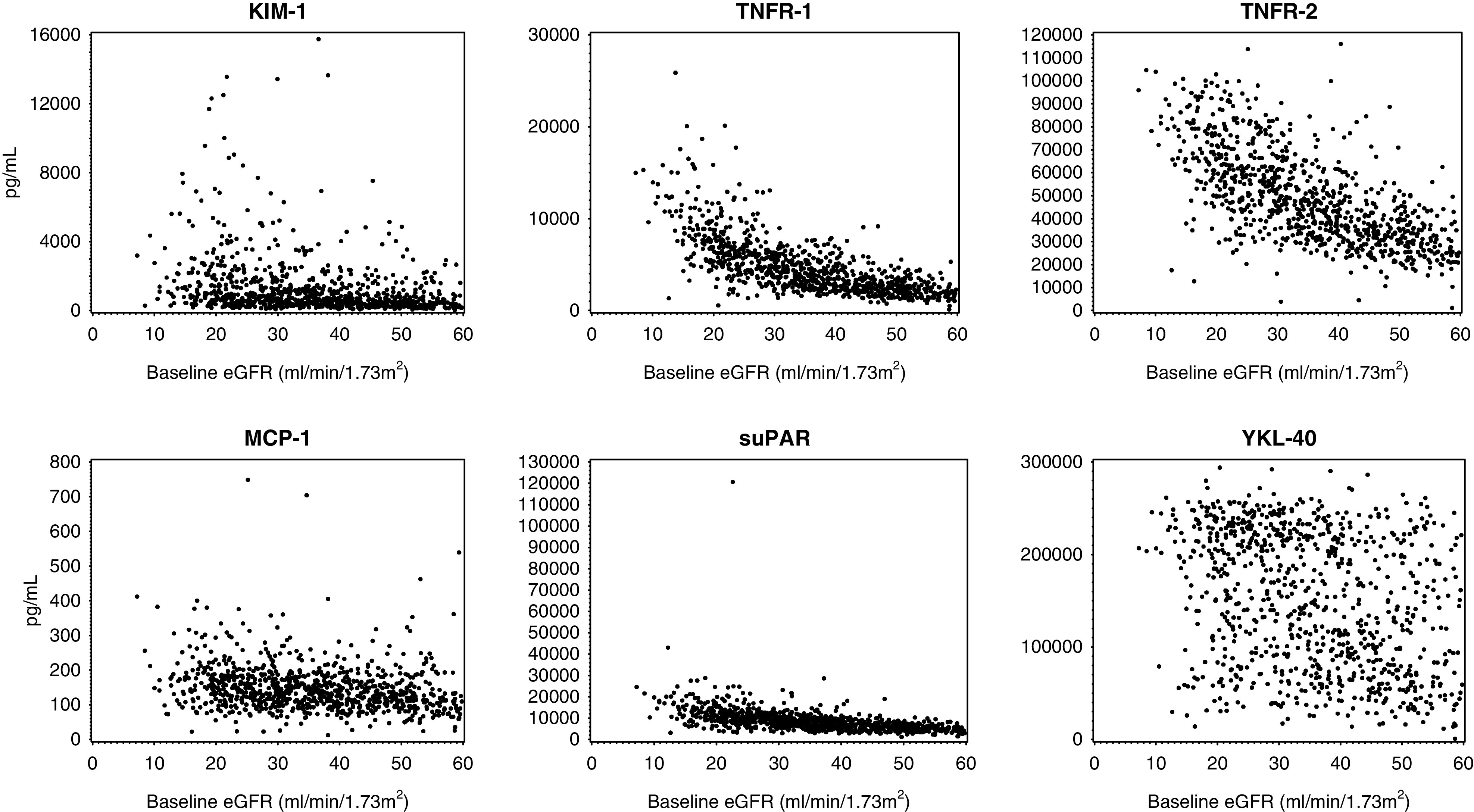
Scatterplot of plasma biomarker concentrations (pg/ml) on y axis were consistenty higher among those with lower baseline eGFR (ml/min per 1.73 m2) values on x axis. Top row (left to right): KIM-1, TNFR-1, and TNFR-2. Bottom row (left to right): MCP-1, suPAR, and YKL-40.
Table 2.
Pearson partial correlations of biomarker levels and eGFR and proteinuria, adjusting for sex and age
| Biomarker | MCP-1 | TNFR-1 | TNFR-2 | suPAR | YKL-40 | eGFR | UPCR |
|---|---|---|---|---|---|---|---|
| KIM-1 | 0.20a | 0.48a | 0.47a | 0.38a | 0.31a | −0.34a | 0.61a |
| MCP-1 | 0.30a | 0.32a | 0.28a | 0.19a | −0.22a | 0.07b | |
| TNFR-1 | 0.82a | 0.74a | 0.49a | −0.73a | 0.44a | ||
| TNFR-2 | 0.71a | 0.53a | −0.64a | 0.39a | |||
| suPAR | 0.47a | −0.64a | 0.29a | ||||
| YKL-40 | −0.30a | 0.27a |
All baseline biomarker values are log2 transformed.
P<0.001.
P<0.05.
Association of Plasma Biomarkers with DKD Progression
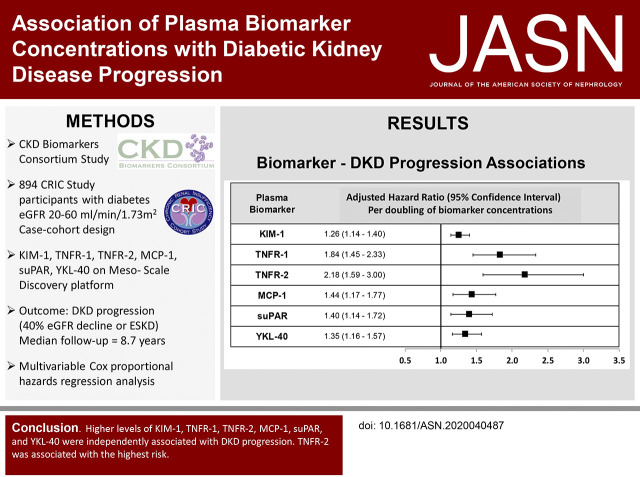
During the median follow-up of 8.67 years (IQR, 6.44–9.31), there were 538 total events of DKD progression, which included 317 ESKD events and 358 instances of a ≥40% decline from the baseline eGFR. When modeling per increment in log2 biomarker values, higher concentrations of each biomarker was associated with a greater risk of DKD progression, even after adjustment for age, sex, race, education, BP, hsCRP, BMI, smoking, eGFR, and UPCR (Figure 2, model 3 in Supplemental Table 3). There was evidence of an interaction between the baseline eGFR (<45 versus ≥45 ml/min per 1.73 m2) and MCP-1 on its association with DKD progression (P interaction=0.01), with higher MCP-1 concentrations associated with increased DKD progression observed for those with eGFR <45 ml/min per 1.73 m2, compared with those with eGFR ≥45 ml/min per 1.73 m2 (HR, 1.35; 95% CI, 1.16 to 1.56, versus HR, 1.03; 95% CI, 0.81 to 1.31).
Figure 2.
Higher plasma biomarker concetrations (per increment in log2 biomarker values) of KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, and YKL-40 were associated with a greater risk of DKD progression, even after adjustment for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), and UPCR. All HRs are adjusted for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), and UPCR.
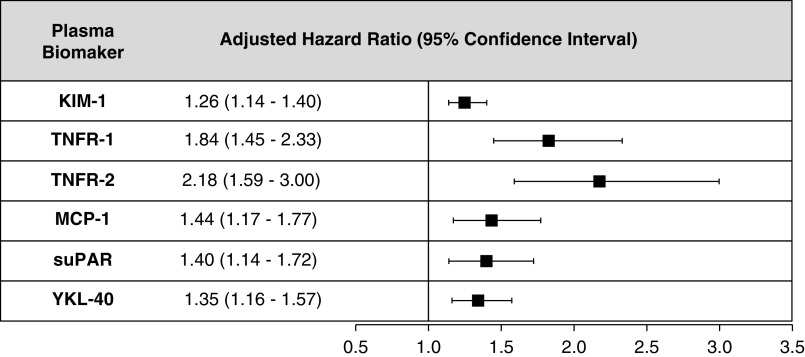
The results of analyses using the ComBat approach to address potential batch effects and limiting the Cox regression models to the subcohort were consistent with the results of the primary analysis using weighted Cox regression (Supplemental Tables 4 and 5). Identification of a panel of markers using Cox regression and backward selection identified three markers, KIM-1, TNFR-2, and YKL-40, that jointly remained associated with an increased risk of DKD progression (KIM-1, aHR, 1.17; 95% CI, 1.05 to 1.30; TNFR-2, aHR, 1.61; 95% CI, 1.15 to 2.26; MCP-1, aHR, 1.20; 95% CI, 0.97 to 1.47; YKL-40, aHR, 1.18; 95% CI, 1.01 to 1.39) (Figure 3, Supplemental Table 6).
Figure 3.
Higher plasma biomarker concetrations (per increment in log2 biomarker values) of KIM-1, TNFR-2, and YKL-40 associated with an increased risk of DKD progression after backward selection of all biomarkers and adjustment for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), and UPCR. All HRs are adjusted for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), baseline UPCR, KIM-1, TNFR-2, MCP-1, and YKL-40. Biomarkers were selected to remain in model via backward selection (P<0.05).
Association of Plasma Biomarkers with Annual Change in eGFR
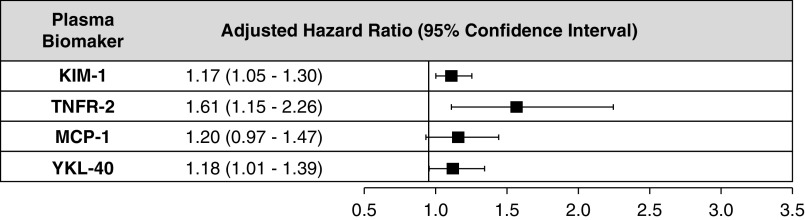
After adjustment for age, sex, race, education, BP, hsCRP, BMI, smoking, eGFR, and UPCR; TNFR-1, TNFR-2, KIM-1, and YKL-40 were significantly associated with the annual rate of decline in eGFR (per change in eGFR slope, in milliliters per minute per 1.73 m2, per log2-biomarker per year) (Figure 4, model 3 in Supplemental Table 7).
Figure 4.
Higher plasma biomarker concetrations (per increment in log2 biomarker values) of TNFR-1, TNFR-2, KIM-1, and YKL-40 were significantly associated with the annual rate of decline in eGFR (ml/min per 1.73 m2 per year) after adjustment for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), and UPCR. All β-coefficients are adjusted for age, sex, race/ethnicity, education, clinical center, systolic BP, diastolic BP, BMI, hsCRP, hemoglobin A1c, antihypertensive medication use, smoking status, baseline eGFR (ml/min per 1.73 m2), and UPCR.
Discussion
In a cohort of individuals with diabetes mellitus and mild to moderate CKD, we observed that plasma TNFR-1, TNFR-2, KIM-1, MCP-1, suPAR, and YKL-40 concentrations were each associated with DKD progression, even after adjustment for established clinical risk factors; all but MCP-1 and suPAR were also associated with eGFR decline. These findings validate the previous literature on TNFR-1, TNFR-2, and KIM-1, and provide new insights into plasma suPAR and YKL-40 as markers that need validation. Further, higher plasma concentrations of TNFR-2 seem to be most strongly associated with DKD progression after accounting for the other plasma biomarkers.
Worldwide, the number of people with type 2 diabetes mellitus is increasing, and it is estimated that DKD will affect approximately 40% of these individuals,37 and DKD contributes to nearly 50% of new ESKD cases each year.38 The latter observation highlights the importance of identifying and confirming better predictive and prognostic biomarkers for DKD. The pathophysiology of DKD progression is multifactorial and involves inflammation, fibrosis, endothelial dysfunction, tubulointerstitial damage, podocyte injury, extracellular matrix remodeling, and angiogenesis.39 A key pathway noted to be active in DKD progression in humans is inflammation, which is associated with upregulated secretion of several proinflammatory cytokines, including TNFR proteins (e.g., TNF-R1) and ILs (e.g., IL-1, IL-6, IL-18).40,41 At the cellular level, these cytokines have been linked to increasing vascular endothelial cell permeability, glomerular hypercellularity, glomerular basement membrane thickening, inducing apoptosis of endothelial cells, and direct toxicity to kidney cells.42–44 Plasma concentrations of TNFR-1 and TNFR-2 are reflective of activation of the TNF pathway. In this investigation, higher concentrations of TNFR-1 and TNFR-2 were associated with DKD progression. These findings are in agreement with prior studies of populations with type 1 or type 2 diabetes mellitus, with normal and decreased baseline eGFR, that demonstrated elevated circulating concentrations of TNFR-1 and TNFR-2 were associated with decline in kidney function (e.g., development of ESKD, macroalbuminuria, or eGFR decline).17–21,29 The consistent, strong associations of TNFR-1 and TNFR-2 with DKD progression support their important role in the chronic inflammation of DKD. Only TNFR-2 was associated with DKD progression because TNFR-1 was not retained in the exploratory model that considered all markers jointly, likely because the two biomarkers overlap to such an extent that only one could fit in the model. This finding supports the key role of inflammation in DKD progression.
MCP-1 (or C-C chemokine ligand 2), another inflammatory biomarker, is a member of the C-C chemokine family that leads to the recruitment of monocytes and transformation to macrophages. To date, studies investigating MCP-1 and kidney disease in humans have mostly focused on urine, rather than plasma or serum, MCP-1 concentrations. Urinary MCP-1 has been associated with allograft failure, cardiovascular disease, and death in recipients of kidney transplants45,46; it is also associated with kidney function decline, in individuals with type 2 diabetes who have preserved kidney function,47 and macroalbuminuria.23 However, serum MCP-1 concentrations have not been associated with eGFR decline among those with type 2 diabetes and relatively preserved kidney function.22 This study observed an increased risk of DKD progression with higher plasma MCP-1 concentrations, but only among those with eGFR <45 ml/min per 1.73 m2 at baseline, making this the first study to report an association of plasma MCP-1 concentrations and DKD progression among individuals with moderate to severe CKD. Due to the limited investigation of the relationship of blood concentrations of MCP-1 with kidney disease, more research in other study populations with diabetes is warranted to further investigate its role in DKD.
Circulating suPAR, the soluble form of the urokinase-type plasminogen activator receptor which is expressed mainly on immune and endothelial cells, is released into the circulation during inflammation.48,49 High suPAR levels have been suggested to activate podocytes in pathologic conditions.50 Higher suPAR concentrations were significantly associated with a larger annual decline in eGFR and an increased risk of incident CKD, defined by eGFR <60 ml/min per 1.73 m2, in a study population largely without diabetes (68%).24 In a prospective study of 667 individuals with type 1 diabetes and various degrees of albuminuria, a higher level of suPAR was an independent risk factor for cardiovascular events, ≥30% eGFR decline, and mortality.51 More recently, suPAR was found to correlate with incident ESKD in a Chinese CKD cohort, of which 20% were diabetic.52 Consistent with these reports, in this investigation, higher plasma levels of suPAR were associated with an increased risk of DKD progression, even after adjustment for baseline eGFR and albuminuria. In contrast to prior studies, suPAR was not associated with rate of eGFR decline or DKD progression after accounting for competing biomarkers. Importantly, this is the first time that suPAR was measured in the same study cohort as TNFR-1 and TNFR-2. Distinctions between our study and others that examined an association between suPAR and eGFR decline include different assays and mean baseline eGFR (35 ml/min per 1.73 m2 in our study versus 50–82 ml/min per 1.73 m2).24,51,52 The lack of a correlation between suPAR and the rate of eGFR decline was also noted by Hayek et al.24 in the subpopulation with baseline eGFR <60 ml/min per 1.73 m2, suggesting the suPAR association may be less prominent or absent in patients with prevalent CKD.
Recurrent or sustained tubular injury is hypothesized to contribute to DKD progression. KIM-1, a marker of acute and chronic proximal tubular injury in both animal models and humans, is detectable in blood and urine after shedding of the KIM-1 ectodomain, consistent with this hypothesis.26 However, previous data on circulating KIM-1 as a biomarker for kidney disease progression has been inconsistent. Serum KIM-1 has been observed to increase across CKD stages and to be associated with eGFR decline and progression to ESKD in some,26,29,53,54 but not all,55,56 studies. Prior studies have been limited by small sample sizes and by including those with all levels of eGFR, and individuals without diabetes. In this investigation, higher plasma KIM-1 concentrations were associated with DKD progression and yearly decline in eGFR, which further elucidates the relationship of circulating KIM-1 and kidney function over time. Additionally, even when adjusting for other biomarkers, KIM-1 remained associated with DKD progression. Further research should be conducted to validate the association of KIM-1 with DKD progression.
YKL-40 is an indicator of tubular injury severity and may play a role in limiting tubular cell apoptosis during the repair phase of AKI.30 To date, YKL-40 has mainly been studied as a kidney disease biomarker in the urine and has not yet been extensively studied in blood samples, or in populations with diabetes. Elevated urine levels of YKL-40 have been associated with need for dialysis in kidney transplantation,30 but urinary YKL-40 levels were not associated with eGFR decline in a population with type 2 diabetes.47 Blood concentrations of YKL-40 have not been shown to be associated with kidney function changes over time. In one study of participants with type 2 diabetes and varying levels of baseline eGFR (mean eGFR 78 ml/min per 1.73 m2) and albuminuria, plasma YKL-40 was not associated with eGFR decline.22 In this investigation, elevated plasma concentrations of YKL-40 were observed to associate with DKD progression and decline in eGFR over time, even after adjustment for potential confounders and other plasma biomarkers. The panel of biomarkers that was significantly associated with DKD progression, which included inflammatory (TNFR-2) and tubular injury (KIM-1 and YKL-40) molecules, reflects the complex pathobiology of DKD. Further, although the biomarkers had mostly moderate to high intercorrelations, each biomarker alone was associated with DKD progression, suggesting different loci of injury or pathways may be involved in determining outcomes. Ultimately, a multibiomarker approach to risk prediction of DKD progression may be necessary.
Rigorous technical and clinical validation studies are necessary before novel biomarkers can enter clinical practice. Several of the biomarkers examined in this study have previously been shown to be associated with kidney disease progression, although the studies were often small and included heterogeneous etiologies of CKD. To date, many phase-3 clinical trials for DKD have failed,57 which may relate to insufficient power, due to low overall rates of progression to end points (e.g., doubling of serum creatinine, onset of ESKD, or death).58 Biomarkers may be used in the design of clinical trials to identify patients who are at high risk for developing DKD progression and targeting them for enrollment. Furthermore, novel biomarkers may provide additional, clinically meaningful outcomes, particularly in phase-2 trials. The use of rigorously validated biomarkers for prognostic enrichment of trial populations would reduce trial costs, size, and duration.59 Such trials may also be more attractive to potential participants who are identified by virtue of their high risk of disease progression. We assessed the potential utility of the plasma biomarkers assessed in this study to enrich clinical trial enrollment using the open-source software, Biomarker Prognostic Enrichment Tool for Survival Outcomes (http://prognosticenrichment.com/surv/), which is similar to the previously published BioPET for binary outcomes60 (Supplemental Material, Supplemental Figures 3 and 4, Supplemental Table 8). As an illustrative example, plasma TNFR-2 could be used to enrich enrollment by excluding individuals at varying concentrations of plasma TNFR-2, such as below the 75th percentile. Assuming no interaction between baseline level of TNFR-2 and treatment effect, it is estimated that the sample size needed to detect a 20% reduction in DKD progression over a 5-year period could be reduced by nearly 50%, with a nearly similar percentage reduction in cost. A similar effect, but to a lesser magnitude, was estimated for KIM-1 (Supplemental Figures 3 and 4, Supplemental Table 8).
The results of this investigation need to be interpreted in the context of some limitations and study strengths. First, due to the observational nature of the study, residual confounding by eGFR is possible because correlations of the biomarkers with eGFR were strong. To address confounding, we leveraged the existence of a well-characterized cohort by adjusting for an extensive set of clinical covariates. Second, participants with elevated biomarker levels may have been recruited into the CRIC Study later in their disease, highlighting the importance of assessment of earlier DKD to avoid index-event bias, which is not fully addressed with adjustment for the baseline eGFR. Third, misclassification is a concern because we did not follow participants after they reached the primary outcome, which would preclude an assessment of an improvement in eGFR. However, 40% eGFR decline represents a large change, which reliably predicts ESKD61 and conveys the low probability that enough subjects would achieve improved eGFR to substantially influence the results. Fourth, comparison of current and prior results for a specific biomarker may be complicated by ELISA assays using antibodies that recognize different peptide epitopes. Previous studies evaluating biomarkers and DKD have been limited by small sample sizes, especially in the case of participants with type 2 diabetes, and have included participants without kidney disease, which limits the interpretation regarding disease-related pathways. Additionally, all plasma biomarkers were measured in the same laboratory with detailed quality control measures. Additional strengths of this study include the assessment of the relationship of key biomarkers that are likely indicative of pathways important for DKD progression. Sensitivity analyses accounting for batch effects were also consistent with the primary analyses. Furthermore, we assessed these associations in a large, carefully phenotyped, prospective cohort with moderate to severe CKD from clinical centers across the United States that included representation of diverse races and both sexes. These features add to the generalizability of our findings to a typical DKD population.
In summary, higher plasma concentrations of KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, and YKL-40 were independently associated with increased risk of DKD progression, even after adjusting for known risk factors. These findings validate the previous literature on TNFR-1, TNFR-2, and KIM-1, and provide new insights on plasma suPAR and YKL-40 as markers that need validation. Further, TNFR-2 seems to be most strongly associated with DKD progression after accounting for the other plasma biomarkers. These biomarkers may help in the development of novel therapies to prevent DKD progression and the selection of individuals for clinical trials that will be needed to test their effectiveness.
Disclosures
A. Anderson consulted for Kyowa Hakko Kirin. J. Bonventre declares ownership interests (stock or stock options, excluding diversified mutual funds) with Goldfinch Bio (cofounder and equity holder) and Medibeacon; being a coinventor on KIM-1 patents assigned to Partners Healthcare and thus receives patent rights and royalty payments for intellectual property; and consultancy with Renalytix. J. Bonventre also holds equity in DX Now, Innoviva, Medibeacon, Rubius, Sensor‐Kinesis, Sentien, and Thrasos; has received consulting income from Aldeyra, Biomarin, Cadent, Esperion, Praxis, Sarepta, Seattle Genetics, and Sumitomo Dainippon; and has received grant funding from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). J. Bonventre’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. S. Coca declares providing consultancy for Bayer, CHF Solutions, Relypsa, RenalytixAI, and Takeda; receiving personal fees from Boehringer-Ingelheim, inRegan, and Quark; and being on the advisory committee of and having ownership interests (stock or stock options, excluding diversified mutual funds) in RenalytixAI. S. Coca also has the patent “Derivation and Validation of a Machine Learning Risk Score using Biomarker and Electronic Patient Data To Predict Rapid Progression of Diabetic Kidney Disease” licensed to RenalytixAI. H. Feldman reports being a scientific advisor or having membership of the steering committee of the CRIC Study; consultancy agreements with DLA Piper, LLP; InMed, Inc.; Kyowa Hakko Kirin Co., Ltd. (ongoing); and National Kidney Foundation; being editor-in-chief of the American Journal of Kidney Diseases (member of advisory board); receiving research funding from Regeneron; and receiving honoraria from Rogosin Institute (as invited speaker). O. Gutierrez has received grant funding and consulting fees from Akebia and Amgen, grant funding from GlaxoSmithKline, and personal fees from QED. J. Ix is the principal investigator of an investigator-initiated research grant supported by Baxter International. P. Kimmel reports being an editor of Chronic Renal Disease, and being a scientific advisor to or a member of the board of directors of Washington Academy of Medicine. C. Parikh declares having a consulting arrangement with Genfit and Tricida, and having ownership interests (stock or stock options, excluding diversified mutual funds) in and consultancy with Renalytix. V. Sabbisetti declares KIM-1 patents assigned to Partners Healthcare (patent rights). M. Sarnak declares being on the steering committee for Akebia, being on the advisory board for Bayer, and being a consultant for Cardurian. M. Shlipak has served as a consultant for Cricket Health and Intercept Pharmaceuticals, and is a scientific advisor for TAI Diagnostics. S. Waikar reports receiving grants and personal fees from Allena Pharmaceuticals; and personal fees from Barron and Budd (versus Fresenius), Bunch and James, Cerus, CVS, GE Healthcare, GlaxoSmithKline, Harvard Clinical Research Institute (also known as Baim), Johnson & Johnson, Kantum Pharma, Mallinckrodt, Mass Medical International, Pfizer, Public Health Advocacy Institute, Roth Capital Partners, Strataca, Takeda, Venbio, and Wolters Kluewer; outside the submitted work. All remaining authors have nothing to disclose.
Funding
S. Schrauben is supported by the NIDDK career development grant K23DK118198-01A1. S. Coca and C. Parikh are members and have salary support from the CKD Biomarker Consortium via NIDDK grant U01DK106962. S. Coca is also supported, in part, by National Heart, Lung, and Blood Institute grants R01DK115562, R01HL85757, and R01DK112258; and by National Institute for Occupational Safety and Health grant U01OH011326. J. Bonventre is supported by National Heart, Lung, and Blood Institute grants R37DK039773 and R01DKD072381; and by NIDDK contract U01DK085660. V. Sabbisetti and S. Waikar are supported by the NIDDK contract U01DK085660. J. Ix, M. Sarnak, O. Gutierrez, and M. Shlipak were supported by the NIDDK contract U01DK102730. J. Schelling is supported by NIDDK contract U01DK106965. J. Greenberg is supported by the NIH career development grant K08DK110536, and by the Charles H. Hood Foundation (Boston, MA). A. Anderson has received support from NIDDK grants U01DK103225 and U01DK060990, and R01DK104730 related to this project. C. Rebholz was supported by NIDDK mentored research scientist development award K01 DK107782, and a National Heart, Lung, and Blood Institute grant R21 HL143089. Funding for the CRIC Study was obtained under a cooperative agreement from the NIDDK under grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902, and U24DK060990. In addition, this work was supported, in part, by National Center for Advancing Translational Sciences (NCATS) grants UL1TR000003 (via the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award), UL1 TR-000424 (via Johns Hopkins University), M01 RR-16500 (via University of Maryland General Clinical Research Center), UL1TR000439 (Case Western Reserve University/Cleveland Clinic CTSA), UL1TR000433 (via Michigan Institute for Clinical and Health Research), and UL1RR029879 (via University of Illinois at Chicago Clinical and Translational Science Awards); NIH grant P20 GM109036 (via Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases); National Center for Research Resources grant NCRR UCSF-CTSI UL1 RR-024131 (via Kaiser Permanente); and National Heart, Lung, and Blood Institute grant R01DK119199 (via Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM).
Supplementary Material
Acknowledgments
Dr. Amanda Hyre Anderson, Ms. Xiaoming Zhang, Dr. Haochang Shou, Dr. Joseph V. Bonventre, Dr. Steven Coca, Dr. Susan L. Furth, Dr. Orlando M. Gutierrez, Dr. Joachim H. Ix, Dr. Chirag R. Parikh, Dr. Casey M. Rebholz, Dr. Mark J. Sarnak, Dr. Michael G. Shlipak, Dr. Sushrut S. Waikar, Dr. Paul L. Kimmel, Dr. Ramachandran S. Vasan, Dr. Harold I. Feldman, and Dr. Jeffrey R. Schelling designed the study; Dr. Joseph V. Bonventre and Dr. Venkata Sabbisetti carried out assays; Dr. Sarah J. Schrauben, Dr. Haochang Shou, Dr. Xiaoming Zhang, and Dr. Harold I. Feldman analyzed the data; Dr. Sarah J. Schrauben made the figures; Dr. Sarah J. Schrauben, Dr. Haochang Shou, Dr. Jeffrey R. Schelling, and Dr. Harold I. Feldman drafted the paper; and all authors revised the paper and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Lawrence J. Appel, Alan S. Go, Jiang He, Robert G. Nelson, Panduranga S. Rao, Mahboob Rahman, Vallabh O. Shah, Raymond R. Townsend, Mark L. Unruh, Michelle Denburg, Bradley Warady, Josef Coresh, Morgan Grams, Alison Abraham, Eugene Rhee, Ruth Dubin, Tom Hostetter, Rajat Deo, Dawei Xie, Shawn Ballard, Krista Whitehead, Heather Collins, and Peter Ganz
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040487/-/DCSupplemental.
Supplemental Table 1. Quality control parameters of biospecimen assays.
Supplemental Table 2. Plasma values of biomarkers according to baseline eGFR category (n=894).
Supplemental Table 3. Association of plasma biomarkers with risk of DKD progression in staged weighted Cox proportional hazards regression models.
Supplemental Table 4. Association of plasma biomarkers with risk of DKD progression using ComBat approach.
Supplemental Table 5. Association of plasma biomarkers with risk of DKD progression in proportional hazards regression model in the subcohort only.
Supplemental Table 6. Association of plasma biomarkers with risk of DKD progression in proportional hazards regression model adjusting for biomarkers.
Supplemental Table 7. Association of plasma biomarkers with annual change in eGFR (ml/min per 1.73 m2) in staged linear mixed effects models.
Supplemental Table 8. BioPETsurv analysis of modest to highly prognostic biomarkers for a 5-year clinical trial of DKD progression.
Supplemental Figure 1. Flow diagram of sample selection.
Supplemental Figure 2. Scatterplot of plasma biomarkers (log2-transformed) on y-axis by baseline eGFR (ml/min per 1.73 m2) on x-axis.
Supplemental Figure 3. BioPETsurv analysis of KIM-1 as a modest prognostic biomarker for a 5-year clinical trial of DKD progression.
Supplemental Figure 4. BioPETsurv analysis of TNFR-2 as a highly prognostic biomarker for a 5-year clinical trial of DKD progression.
References
- 1.Reutens AT: Epidemiology of diabetic kidney disease. Med Clin North Am 97: 1–18, 2013. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System (USRDS) : Chapter 1: Incidence, prevalence, patient characteristics, and treatment modalities. Available at: https://www.usrds.org/media/1688/v2_c01_incprev_17.pdf. Accessed June 20, 2019
- 3.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes 55: 1832–1839, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Porrini E, Ruggenenti P, Luis-Lima S, Carrara F, Jiménez A, de Vries APJ, et al.: Estimated GFR: Time for a critical appraisal. Nat Rev Nephrol 15: 177–190, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al.: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabaei BP, Al-Kassab AS, Ilag LL, Zawacki CM, Herman WH: Does microalbuminuria predict diabetic nephropathy? Diabetes Care 24: 1560–1566, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, et al.: Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: Inception cohort study. BMJ 328: 1105, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramori ML, Fioretto P, Mauer M: Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 52: 1036–1040, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS: In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 77: 57–64, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CH, Lam KS: Biomarkers of progression in diabetic nephropathy: The past, present and future. J Diabetes Investig 6: 247–249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, et al.: ERA-EDTA diabesity working group : Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 3: 382–391, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes 55: 1832–1839, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Kramer HJ, Nguyen QD, Curhan G, Hsu CY: Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289: 3273–3277, 2003. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy MI: Painting a new picture of personalised medicine for diabetes. Diabetologia 60: 793–799, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder JL, Hodgin JB, Kretzler M: Integrative biology of diabetic kidney disease. Kidney Dis 1: 194–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavkov ME, Weil EJ, Fufaa GD, Nelson RG, Lemley KV, Knowler WC, et al.: Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int 89: 226–234, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al.: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al.: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA: Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 87: 812–819, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G; DCCT/EDIC Research Group : Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 36: 2317–2323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pena MJ, Heinzel A, Heinze G, Alkhalaf A, Bakker SJ, Nguyen TQ, et al.: A panel of novel biomarkers representing different disease pathways improves prediction of renal function decline in type 2 diabetes. PLoS One 10: e0120995, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titan SM, Vieira JM Jr., Dominguez WV, Moreira SR, Pereira AB, Barros RT, et al.: Urinary MCP-1 and RBP: Independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications 26: 546–553, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al.: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waikar SS, Sabbisetti V, Ärnlöv J, Carlsson AC, Coresh J, Feldman HI, et al.: Chronic Kidney Disease Biomarkers Consortium Investigators : Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant 31: 1460–1470, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al.: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al.: Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, et al.: Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol 24: 309–319, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH: Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50: 471–480, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al.: National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee : The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al.: CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schemper M, Wakounig S, Heinze G: The estimation of average hazard ratios by weighted Cox regression. Stat Med 28: 2473–2489, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Panduru NM, Sandholm N, Forsblom C, Saraheimo M, Dahlström EH, Thorn LM, et al.: FinnDiane Study Group : Kidney injury molecule-1 and the loss of kidney function in diabetic nephropathy: A likely causal link in patients with type 1 diabetes. Diabetes Care 38: 1130–1137, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Johnson WE, Li C, Rabinovic A: Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8: 118–127, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MC, Cooper ME, Zimmet P: Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 12: 73–81, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Espinel CH: The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 236: 579–581, 1976. [DOI] [PubMed] [Google Scholar]
- 39.Colhoun HM, Marcovecchio ML: Biomarkers of diabetic kidney disease. Diabetologia 61: 996–1011, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al.: A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25: 805–813, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA: Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am J Physiol 257: L399–L410, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Navarro-González JF, Mora-Fernández C: The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Pichler R, Afkarian M, Dieter BP, Tuttle KR: Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol 312: F716–F731, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M, Katz R, Shlipak MG, Weiner D, Tracy R, Jotwani V, et al.: Urinary markers of fibrosis and risk of cardiovascular events and death in kidney transplant recipients: The FAVORIT Trial. Am J Transplant 17: 2640–2649, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ix JH, Katz R, Bansal N, Foster M, Weiner DE, Tracy R, et al.: Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: A case-cohort ancillary study of the FAVORIT Trial. Am J Kidney Dis 69: 410–419, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, et al.: Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: The ACCORD trial. Clin J Am Soc Nephrol 11: 1343–1352, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahdi F, Shariat-Madar Z, Todd RF 3rd, Figueroa CD, Schmaier AH: Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood 97: 2342–2350, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Blasi F, Carmeliet P: uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol 3: 932–943, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Zeier M, Reiser J: suPAR and chronic kidney disease-a podocyte story. Pflugers Arch 469: 1017–1020, 2017. [DOI] [PubMed] [Google Scholar]
- 51.Rotbain Curovic V, Theilade S, Winther SA, Tofte N, Eugen-Olsen J, Persson F, et al.: Soluble urokinase plasminogen activator receptor predicts cardiovascular events, kidney function decline, and mortality in patients with type 1 diabetes. Diabetes Care 42: 1112–1119, 2019. [DOI] [PubMed] [Google Scholar]
- 52.Lv L, Wang F, Wu L, Wang JW, Cui Z, Hayek SS, et al.: Soluble urokinase-type plasminogen activator receptor and incident end-stage renal disease in Chinese patients with chronic kidney disease. Nephrol Dial Transplant 35: 465–470, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, et al.: Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89: 459–467, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, et al.: SUMMIT Investigators : Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int 88: 888–896, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen SE, Andersen S, Zdunek D, Hess G, Parving H-H, Rossing P: Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 79: 1113–1118, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Conway BR, Manoharan D, Manoharan D, Jenks S, Dear JW, McLachlan S, et al.: Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int 82: 812–818, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Chan GC, Tang SC: Diabetic nephropathy: Landmark clinical trials and tribulations. Nephrol Dial Transplant 31: 359–368, 2016. [DOI] [PubMed] [Google Scholar]
- 58.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al.: VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Heerspink HJL, List J, Perkovic V: New clinical trial designs for establishing drug efficacy and safety in a precision medicine era. Diabetes Obes Metab 20[Suppl 3]: 14–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerr KF, Roth J, Zhu K, Thiessen-Philbrook H, Meisner A, Wilson FP, et al.: Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials 14: 629–638, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al.: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.