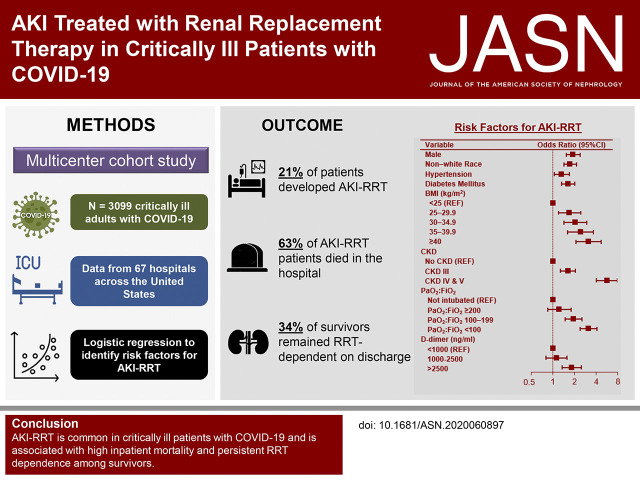
Significance Statement
Although AKI is an important sequela of coronavirus disease 2019 (COVID-19), data on AKI treated with RRT (AKI-RRT) in patients with COVID-19 are limited. In a multicenter cohort study of 3099 critically ill adults with COVID-19 admitted to intensive care units (ICUs) at 67 hospitals across the United States, one in five patients developed AKI-RRT, 63% of whom died during hospitalization. Among patients who survived to hospital discharge, one in three remained RRT dependent at discharge, and one in six remained RRT dependent 60 days after ICU admission. The study identified several patient-and hospital-level risk factors for AKI-RRT and death. AKI-RRT is common among critically ill patients with COVID-19 and is associated with high mortality and persistent RRT dependence.
Keywords: acute renal failure, clinical epidemiology, dialysis, risk factors, COVID-19, renal replacement therapy, acute kidney injury
Visual Abstract
Abstract
Background
AKI is a common sequela of coronavirus disease 2019 (COVID-19). However, few studies have focused on AKI treated with RRT (AKI-RRT).
Methods
We conducted a multicenter cohort study of 3099 critically ill adults with COVID-19 admitted to intensive care units (ICUs) at 67 hospitals across the United States. We used multivariable logistic regression to identify patient-and hospital-level risk factors for AKI-RRT and to examine risk factors for 28-day mortality among such patients.
Results
A total of 637 of 3099 patients (20.6%) developed AKI-RRT within 14 days of ICU admission, 350 of whom (54.9%) died within 28 days of ICU admission. Patient-level risk factors for AKI-RRT included CKD, men, non-White race, hypertension, diabetes mellitus, higher body mass index, higher d-dimer, and greater severity of hypoxemia on ICU admission. Predictors of 28-day mortality in patients with AKI-RRT were older age, severe oliguria, and admission to a hospital with fewer ICU beds or one with greater regional density of COVID-19. At the end of a median follow-up of 17 days (range, 1–123 days), 403 of the 637 patients (63.3%) with AKI-RRT had died, 216 (33.9%) were discharged, and 18 (2.8%) remained hospitalized. Of the 216 patients discharged, 73 (33.8%) remained RRT dependent at discharge, and 39 (18.1%) remained RRT dependent 60 days after ICU admission.
Conclusions
AKI-RRT is common among critically ill patients with COVID-19 and is associated with a hospital mortality rate of >60%. Among those who survive to discharge, one in three still depends on RRT at discharge, and one in six remains RRT dependent 60 days after ICU admission.
The coronavirus disease 2019 (COVID-19) is predominantly a respiratory disorder, but much of the morbidity and mortality associated with severe illness from COVID-19 has been attributed to injury to other organs, including the heart1–3 and kidneys.4,5 AKI is emerging as a common and important sequela of COVID-19, with rates as high as 33%–43% among hospitalized patients.5,6 Mechanisms that have been proposed to account for the high rates of AKI observed in patients with COVID-19 include ischemic acute tubular necrosis,5,7 cytokine storm,8 and direct viral invasion of the renal proximal tubular cells and podocytes.7,9 Additional potential mechanisms include hemodynamic changes associated with invasive mechanical ventilation5 and concomitant use of antibiotics and antiviral medications with nephrotoxic potential.
Existing data on COVID-19–associated AKI are incomplete. Most studies have been single center or have focused on single geographic regions, and therefore, they have limited generalizability.5,10,11 Moreover, few studies have focused on AKI treated with RRT (AKI-RRT),10 the most severe and clinically relevant form of AKI. AKI-RRT is both resource intensive12 and associated with acute mortality rates as high as 63% in patients without COVID-19.13,14 Granular, nationally representative data on AKI-RRT in patients with COVID-19 are urgently needed to inform clinical decision making and resource allocation.
To address these knowledge gaps, we conducted the Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID). STOP-COVID is a multicenter cohort study examining the demographics, comorbidities, organ dysfunction, treatments, and outcomes of critically ill patients with COVID-19 admitted to intensive care units (ICUs) at 67 geographically diverse hospitals across the United States.15 Here, we report the incidence, clinical features, patient- and hospital-level risk factors, and outcomes associated with AKI-RRT among critically ill patients with COVID-19.
Methods
Study Design, Oversight, and Patient Population
We conducted STOP-COVID, a multicenter cohort study that enrolled consecutive adults (≥18 years old) with laboratory-confirmed COVID-19 admitted to ICUs at 67 hospitals across the United States (Supplemental Table 1). STOP-COVID only included patients in the ICU in an effort to focus on the patients at highest risk of acute organ injury and death. The study was approved with a waiver of informed consent by the institutional review board at each participating site and registered on ClinicalTrials.gov (NCT04343898). The primary findings from STOP-COVID are reported elsewhere.15 For this study, we included patients admitted to ICUs between March 4 and April 11, 2020, and we followed patients until the first of hospital discharge, death, or August 1, 2020. We excluded patients with a history of ESKD.
Data Collection
Study personnel at each site collected data by detailed chart review and used a standardized patient report form to enter data into a secure online database (REDCap). Patient-level data included demographics and comorbidities; longitudinal laboratory values, physiologic parameters, medications, treatments, and organ support (including RRT) for the first 14 days following ICU admission; and clinical outcomes, including acute organ injury and death. Baseline serum creatinine (SCr) was defined as the lowest value 365–7 days prior to hospitalization. If a prehospital baseline SCr was not available, we used the SCr value on hospital admission as the baseline. We calculated baseline eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.16
For patients with AKI-RRT, we recorded the initial RRT modality (continuous, intermittent, or other) as well as data on RRT dependence and kidney function at hospital discharge among survivors. We also collected hospital-level data, including the city and state of each hospital, hospital size (assessed by the number of pre–COVID-19 ICU beds, not including surge capacity beds), and the regional density of COVID-19. We assessed regional density of COVID-19 by categorizing hospitals into quartiles according to the regional (county) density of COVID-19 cases present on the median date of ICU admission for the patients enrolled at that hospital. A higher quartile signified a greater regional density of COVID-19. All data were validated through a series of automated and manual verifications (Supplemental Material). A complete list of variables is provided in the patient report form, which is available elsewhere.15
Primary Outcome
The primary outcome was AKI-RRT occurring within 14 days following ICU admission.
Secondary Outcomes
Secondary outcomes included 28-day mortality and kidney function at hospital discharge among patients with AKI-RRT who survived to discharge. Patients who were independent of RRT at hospital discharge were classified as having complete or partial renal recovery if their SCr was ≤0.35 or >0.35 mg/dl above their baseline value, respectively.17,18 Among patients who remained RRT dependent on discharge, we also obtained RRT dependence data 60 days after ICU admission. Other outcomes included AKI and its severity occurring on hospital admission and within 14 days following ICU admission (additional details are provided in Supplemental Material).
Statistical Analyses
Continuous variables are expressed as median and interquartile range, and categorical variables are expressed as count and percentage. We used multivariable logistic regression to identify patient- and hospital-level risk factors for AKI-RRT. We prespecified the following covariates on the basis of clinical knowledge and prior studies4,5,10,11,19: demographics (age, sex, race, body mass index, hypertension, diabetes mellitus, active malignancy, coronary artery disease, congestive heart failure, and CKD; the latter is classified as no CKD [eGFR≥60 ml/min per 1.73 m2], CKD stage 3 [eGFR=30–59 ml/min per 1.73 m2], and CKD stages 4 and 5 [eGFR<30 ml/min per 1.73 m2]); the number of days from hospital to ICU admission (≤3 versus >3 days); acute severity of illness covariates assessed on ICU admission (lymphocyte count, d-dimer, shock, the ratio of the partial pressure of arterial oxygen over the fraction of inspired oxygen [PaO2:FiO2], the coagulation and liver components of the Sequential Organ Failure Assessment score, altered mental status, and secondary infection); and hospital characteristics (the number of pre–COVID-19 ICU beds and the regional density of COVID-19). Covariates are described in further detail in Supplemental Material and Supplemental Table 2.
For the primary analysis examining risk factors for AKI-RRT, we conducted a series of sensitivity analyses. First, we used generalized estimating equation methods to account for clustering of patients within hospitals.20 Second, we limited the analysis to patients with a prehospital baseline SCr value available. Third, we excluded patients who were transferred from an outside hospital prior to ICU admission. Fourth, because death is a competing risk for AKI-RRT, we limited the analysis to patients who remained alive for the first 14 days following ICU admission. Fifth, as an alternative method to account for death as a competing risk, we examined the composite outcome of AKI-RRT or death (RRT/death) within 14 days following ICU admission.21–24
Among patients with AKI-RRT, we used multivariable logistic regression to examine risk factors for 28-day in-hospital mortality, with the day of ICU admission serving as time 0 (in a sensitivity analysis, we used the day of RRT initiation as time 0). Patients who were discharged alive from the hospital prior to 28 days were considered to be alive at 28 days (we tested the validity of this assumption in a subset of patients described further in Supplemental Material). We selected similar covariates as those described above in addition to oliguria, number of vasopressors, and modality of RRT, each of which has been included in prior risk prediction models for death in critically patients with AKI-RRT.25 These acute severity of illness covariates were assessed on the day of RRT initiation or if unavailable, on the day prior.
To assess interhospital variation in treatments and outcomes, we used multilevel conditional logistic regression modeling with patients nested in hospitals to characterize hospital-level variation and to estimate hospital-specific rates of AKI-RRT. Additional details are provided in Supplemental Material.
Analyses were performed using SAS software version 9.4 (SAS Institute) and Stata 16.1 (StataCorp LLC, College Station, TX).
Results
Patient Characteristics at Baseline
The initial study population included 3193 patients from 67 centers. We excluded 94 patients (2.9%) with ESKD, resulting in 3099 patients who met eligibility criteria (Figure 1). A total of 637 of the 3099 patients (20.6%) developed AKI-RRT within 14 days following ICU admission. Supplemental Figure 1 shows the geographic distribution and density of patients with AKI-RRT among each of the 67 centers.
Figure 1.
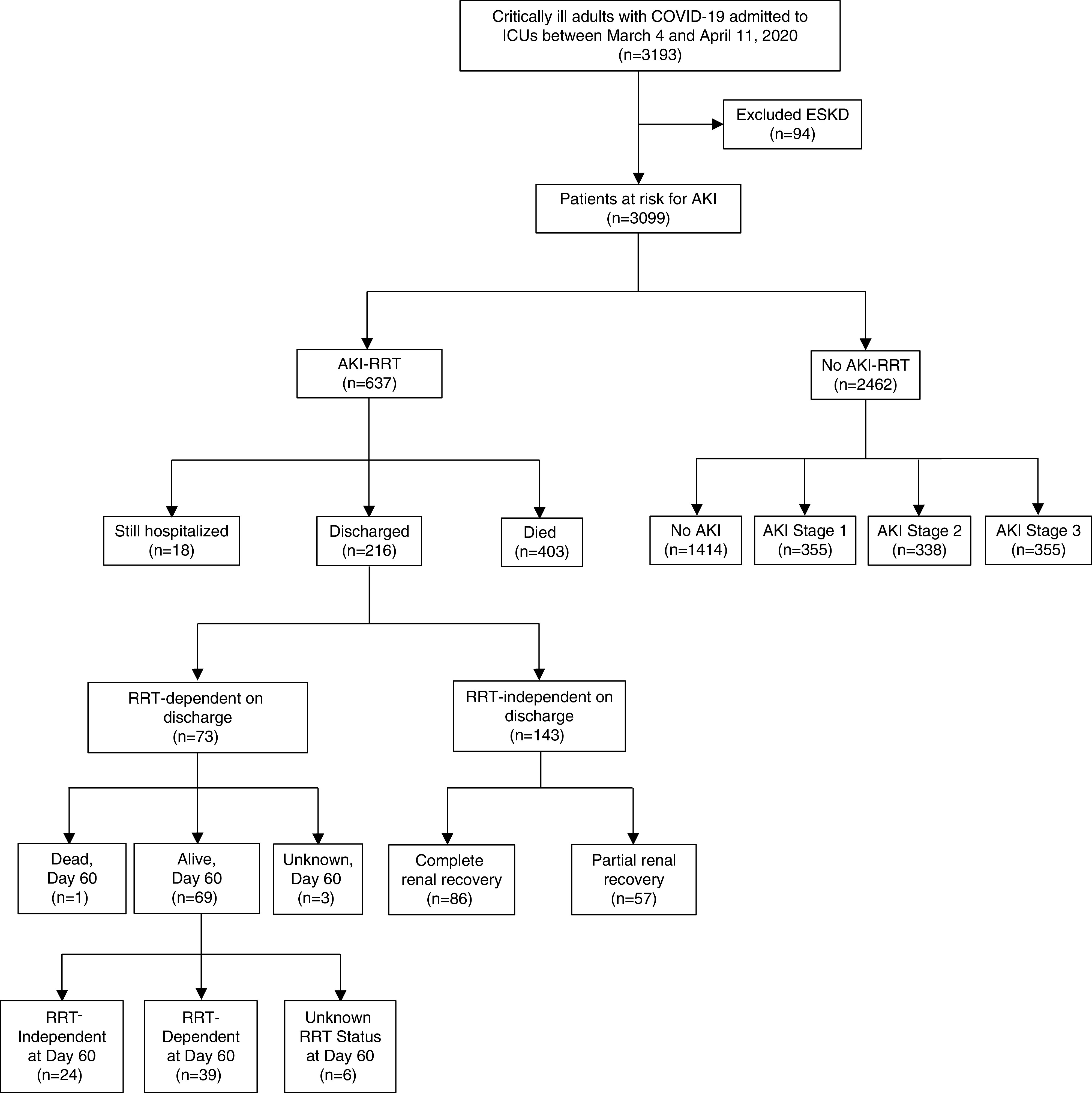
Flowchart of study population. This figure shows the number of patients with and without AKI-RRT and, among those with AKI-RRT, the number of patients who died, were discharged, and were still hospitalized at last follow up.
Baseline characteristics in patients with and without AKI-RRT are shown in Table 1, and a complete list of baseline characteristics across all AKI stages is shown in Supplemental Table 3. Patients with AKI-RRT were similar in age to patients without AKI-RRT, and they were more likely to be men, to be Black, and to have a higher body mass index (Table 1). Patients with AKI-RRT were also more likely to have coexisting conditions, such as diabetes mellitus, hypertension, and CKD, and to have greater severity of illness on arrival to the ICU, including higher rates of invasive mechanical ventilation and treatment with vasopressors (Table 1).
Table 1.
Patient characteristics at baseline
| Characteristic | All Patients, n=3099 | No AKI-RRT, n=2462 | AKI-RRT, n=637 | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr, median (IQR) | 62 (51–71) | 62 (51–72) | 62 (52–69) | 0.15 |
| Men, no. (%) | 2003 (64.6) | 1546 (63.8) | 457 (71.7) | <0.001 |
| Race, no. (%) | <0.001 | |||
| White | 1154 (37.2) | 981 (39.8) | 173 (27.2) | |
| Black | 952 (30.7) | 672 (27.3) | 280 (44.0) | |
| Asian | 190 (6.1) | 163 (6.6) | 27 (4.2) | |
| Other/unknown | 803 (25.9) | 646 (26.2) | 157 (24.7) | |
| Hispanic, no. (%) | 1045 (33.7) | 853 (34.6) | 192 (30.1) | |
| Body mass index, median (IQR) | 30.4 (26.6–36.1) | 29.8 (26.1–35.2) | 32.6 (28.2–39.3) | <0.001 |
| Coexisting conditions, no. (%)a | ||||
| Diabetes mellitus, insulin dependent | 419 (13.5) | 304 (12.3) | 115 (18.1) | <0.001 |
| Diabetes mellitus, noninsulin dependent | 811 (26.2) | 588 (23.9) | 223 (35.0) | <0.001 |
| Hypertension | 1869 (60.3) | 1412 (57.4) | 457 (71.7) | <0.001 |
| Chronic obstructive pulmonary disease | 258 (8.3) | 203 (8.2) | 55 (8.6) | 0.75 |
| Current or former smoker | 917 (29.6) | 736 (29.9) | 181 (28.4) | 0.53 |
| Coronary artery disease | 390 (12.6) | 305 (12.4) | 85 (13.3) | 0.50 |
| Congestive heart failure | 270 (8.7) | 200 (8.1) | 70 (11.0) | 0.02 |
| Chronic liver disease | 104 (3.4) | 77 (3.1) | 27 (4.2) | 0.17 |
| Active malignancy | 158 (5.1) | 126 (5.1) | 32 (5.0) | 0.92 |
| Kidney transplant | 58 (1.9) | 37 (1.5) | 21 (3.3) | 0.005 |
| CKD, ml/min per 1.73 m2 | <0.001 | |||
| eGFR≥90 | 1033 (33.3) | 887 (36.0) | 146 (22.9) | |
| eGFR=60–89 | 1164 (37.6) | 948 (38.5) | 219 (34.4) | |
| eGFR=45–59 | 419 (13.5) | 323 (13.1) | 96 (15.1) | |
| eGFR=30–44 | 255 (8.2) | 189 (7.7) | 66 (10.4) | |
| eGFR=15–29 | 159 (5.2) | 96 (3.9) | 64 (10.1) | |
| eGFR<15 | 64 (2.1) | 19 (0.8) | 46 (7.2) | |
| Home medications, no. (%) | ||||
| Immunosuppressive medication | 312 (10.7) | 246 (10.0) | 66 (10.4) | 0.77 |
| ACE-I | 566 (18.3) | 431 (17.5) | 135 (21.2) | 0.03 |
| ARB | 513 (16.6) | 359 (14.6) | 154 (24.2) | <0.001 |
| Mineralocorticoid receptor antagonist | 81 (2.6) | 62 (2.5) | 19 (3.0) | 0.49 |
| Statin | 1156 (37.3) | 885 (35.9) | 271 (42.5) | 0.002 |
| NSAID | 260 (8.4) | 199 (8.1) | 61 (9.6) | 0.23 |
| Aspirin | 664 (21.4) | 510 (20.7) | 154 (24.2) | 0.07 |
| Anticoagulant | 272 (8.8) | 230 (9.3) | 42 (6.6) | 0.03 |
| Vital signs on ICU admission, median (IQR) | ||||
| Temperature, °C | 38.1 (37.3–38.9) | 38.0 (37.3–38.9) | 38.2 (37.4–39.0) | 0.002 |
| Systolic BP, mm Hg | 97 (86–111) | 97 (86–111) | 95 (84–111) | 0.21 |
| Heart rate, beats per min | 104 (90–120) | 104 (90–119) | 108 (93–122) | <0.001 |
| Urine output, ml/d | 725 (350–1250) | 800 (430–1325) | 500 (200–930) | <0.001 |
| Laboratory findings on ICU admission, median (IQR) | ||||
| White cell count, per mm3 | 8.2 (5.9–11.5) | 8.0 (5.8–11.3) | 8.9 (6.5–12.2) | <0.001 |
| Lymphocyte count, per mm3 | 824 (561–1152) | 826 (565–1153) | 796 (541–1148) | 0.26 |
| Hemoglobin, g/dl | 12.7 (11.2–14.1) | 12.7 (11.3–14.1) | 12.6 (10.9–14.0) | 0.02 |
| Platelet count, K/mm3 | 214 (164–272) | 215 (164–274) | 207 (164–260) | 0.08 |
| Creatinine, mg/dl | 1.04 (0.80–1.55) | 1.00 (0.77–1.38) | 1.47 (1.0–2.85) | <0.001 |
| Total bilirubin, mg/dl | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.26 |
| d-dimer, ng/ml | 1310 (700–3263) | 1180 (670–2620) | 2000 (869–6998) | <0.001 |
| C-reactive protein, mg/L | 157 (90–237) | 154 (87–231) | 175 (104–269) | <0.001 |
| IL-6, pg/ml | 56 (19–154) | 50 (18–50) | 100 (29–251) | <0.001 |
| Severity of illness on ICU admission | ||||
| Invasive mechanical ventilation, no. (%) | 2044 (66.0) | 1540 (62.6) | 504 (79.1) | <0.001 |
| FiO2, median (IQR) | 0.8 (0.6–1.0) | 0.8 (0.5–1.0) | 0.9 (0.6–1.0) | <0.001 |
| PEEP, cm of water, median (IQR) | 12 (10–15) | 12 (10–15) | 14 (10–16) | <0.001 |
| PaO2:FiO2, mm Hg, median (IQR)b | 126 (85–194) | 133 (91–204) | 104 (74–104) | <0.001 |
| Noninvasive mechanical ventilation, no. (%) | 54 (1.7) | 46 (1.9) | 8 (1.3) | 0.39 |
| High-flow nasal cannula or NRB, no. (%) | 619 (20.0) | 540 (21.9) | 79 (12.4) | <0.001 |
| Altered mental status on ICU day 1, no. (%) | 681 (22.0) | 552 (22.4) | 129 (20.3) | 0.24 |
| Vasopressors, no. (%) | 1305 (42.1) | 981 (39.8) | 324 (50.9) | <0.001 |
IQR, interquartile range; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; FiO2, fraction of inspired oxygen; PEEP, positive end expiratory pressure; NRB, non-rebreather.
The definitions of the coexisting disorders are provided in Supplemental Material.
PaO2:FiO2 was only assessed in patients receiving invasive mechanical ventilation.
Characteristics of AKI-RRT
The median time from ICU admission to RRT initiation was 4 days (interquartile range, 2–7 days) (Supplemental Figure 2). The most common initial modality was continuous RRT for 24 h/d (52.4%). Other modalities included intermittent hemodialysis (30.0%), continuous RRT for ≤12 h/d (14.9%), and peritoneal dialysis (1.3%). A total of 625 of the 637 patients (98.1%) with AKI-RRT required invasive mechanical ventilation, and AKI-RRT developed at a median of 3 days following intubation (interquartile range, 2–6 days) (Supplemental Figure 3). Patients who initiated RRT on days 1 and 2 following ICU admission were younger, more likely to have CKD stages 4 and 5, and more likely to receive invasive mechanical ventilation on ICU admission compared with patients who initiated RRT on days 3–14 (Supplemental Table 4). Across multiple categories of age, sex, race, comorbidities, and hospital characteristics, patients with AKI-RRT had a higher 28-day mortality than patients without AKI, and patients with AKI that was not treated with RRT had mortality rates that were intermediate between AKI-RRT and no AKI (Figure 2).
Figure 2.
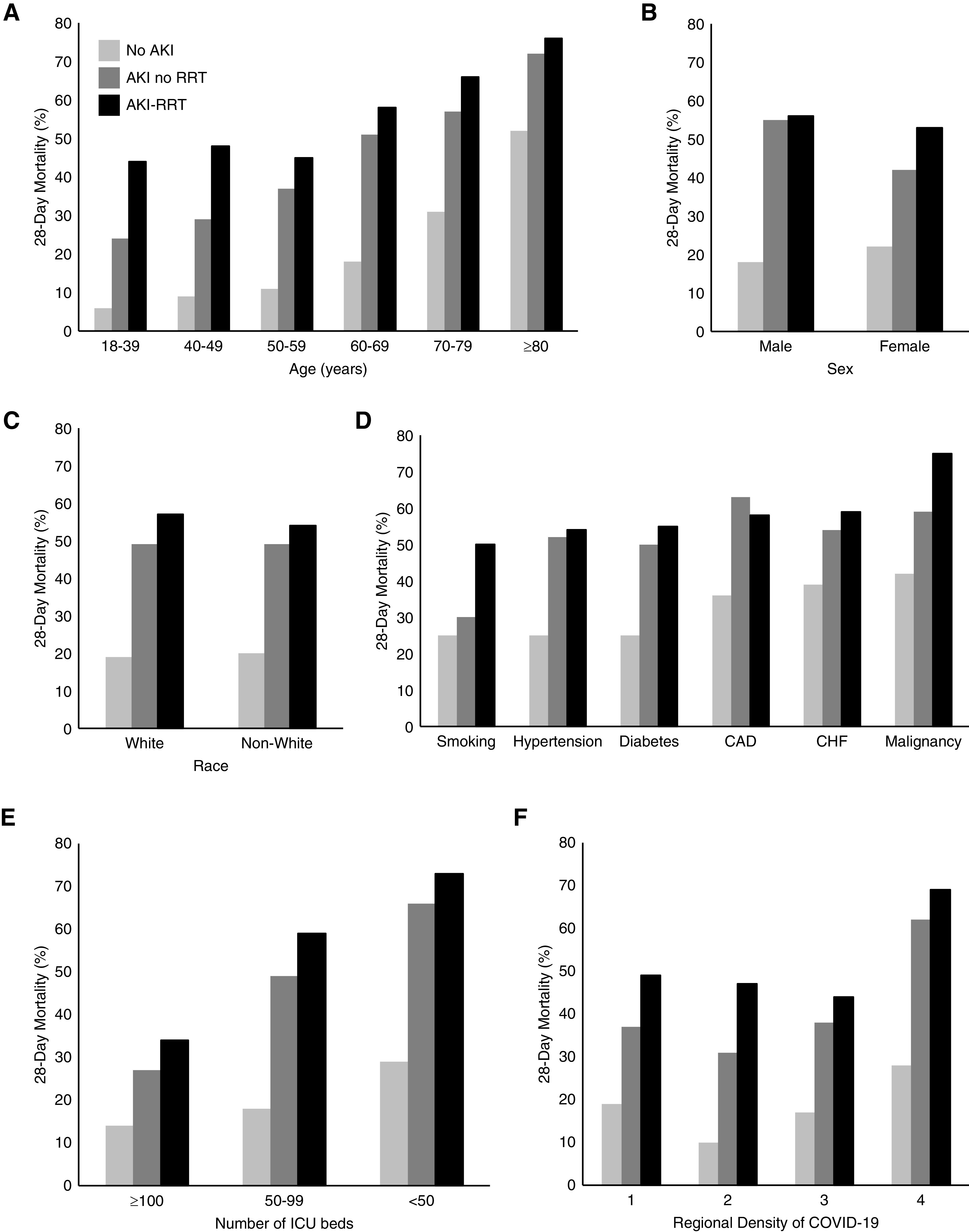
Patients with AKI-RRT have higher 28-day mortality than patients without AKI-RRT. This figure shows the 28-day mortality of patients with AKI-RRT, those with AKI who were not treated with RRT, and those without AKI across categories of age (A), sex (B), race (C), comorbidities (D), and hospital characteristics; number of ICU beds (E) and regional density of COVID-19 (F). CAD, coronary artery disease; CHF, congestive heart failure.
Predictors of AKI-RRT and RRT/Death
In univariate analyses, we found monotonic relationships between each of the following characteristics and a higher risk of AKI-RRT: higher body mass index, higher stages of CKD, lower PaO2:FiO2 ratio on ICU admission, and greater number of vasopressors received on ICU admission (Figure 3).
Figure 3.
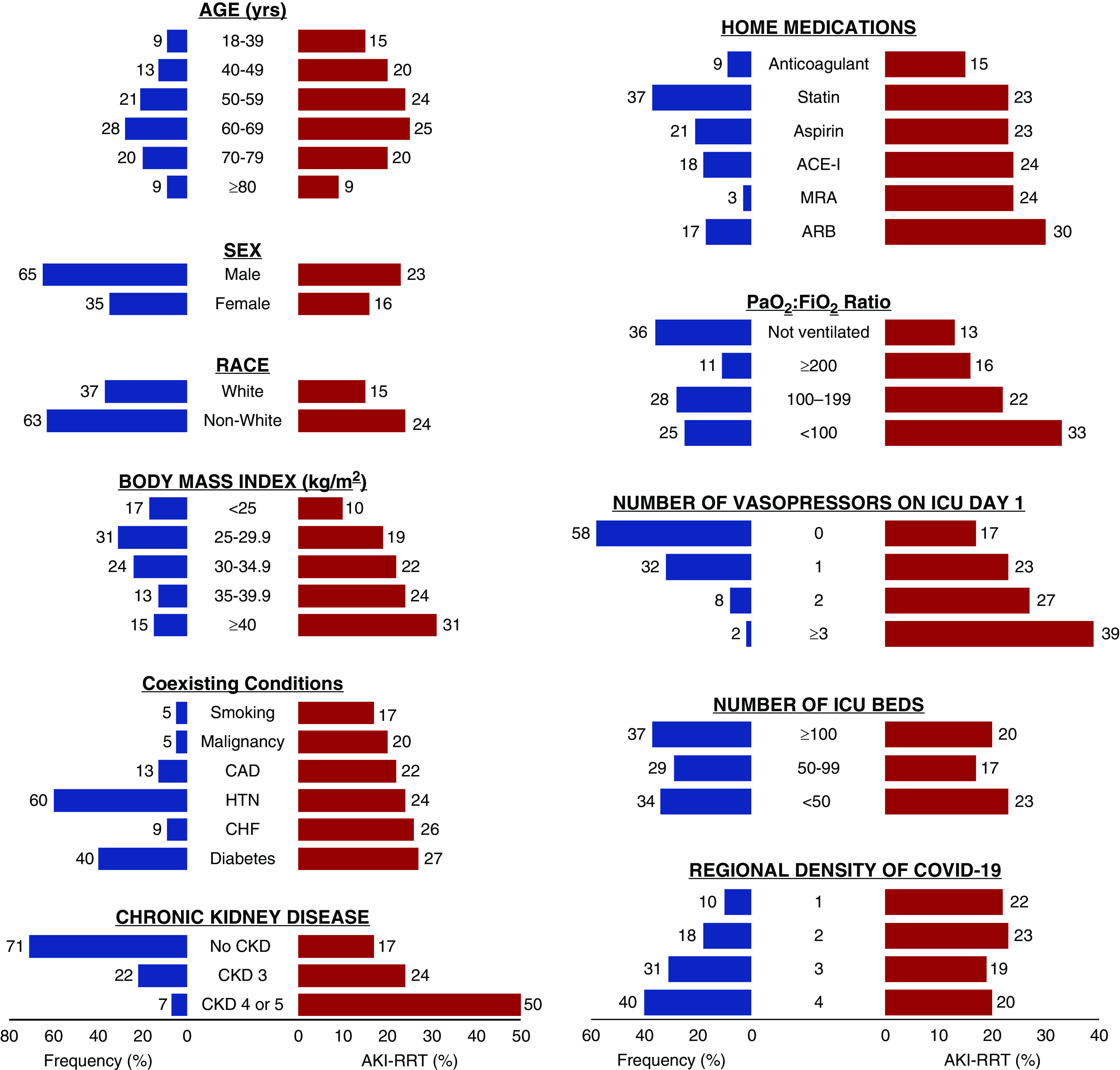
Patient- and hospital-level characteristics and AKI-RRT. The frequency of patient- and hospital-level characteristics in the overall cohort (n=3099) is displayed in blue, and the proportion of patients who developed AKI-RRT within 14 days following ICU admission is displayed in red. Smoking includes both current and former smoking. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CHF, congestive heart failure; HTN, hypertension; MRA, mineralocorticoid receptor antagonist.
In multivariable analyses, CKD was associated with a higher risk of AKI-RRT (odds ratio, 5.63; 95% confidence interval [95% CI], 4.03 to 7.86 for CKD stages 4 and 5 compared with no CKD; odds ratio, 1.62; 95% CI, 1.27 to 2.05 for CKD stage 3 compared with no CKD). Additional patient-level risk factors for AKI-RRT were men, non-White race, hypertension, diabetes mellitus, higher body mass index, lower PaO2:FiO2 ratio on ICU admission, and d-dimer >2500 ng/ml on ICU admission (Figure 4). A shorter interval from hospital to ICU admission (≤3 versus >3 days) was associated with a lower risk of AKI-RRT. Patients admitted to hospitals with greater regional density of COVID-19 had a lower odds of AKI-RRT (odds ratio, 0.66; 95% CI, 0.47 to 0.93 for highest versus lowest quartile) (Figure 4). The finding of lower rates of AKI-RRT in patients admitted to hospitals with greater regional density of COVID-19 persisted in analyses restricted to patients with AKI stage 3 (Supplemental Figure 4). Interpretations were similar in multiple sensitivity analyses (Supplemental Tables 5–8).
Figure 4.
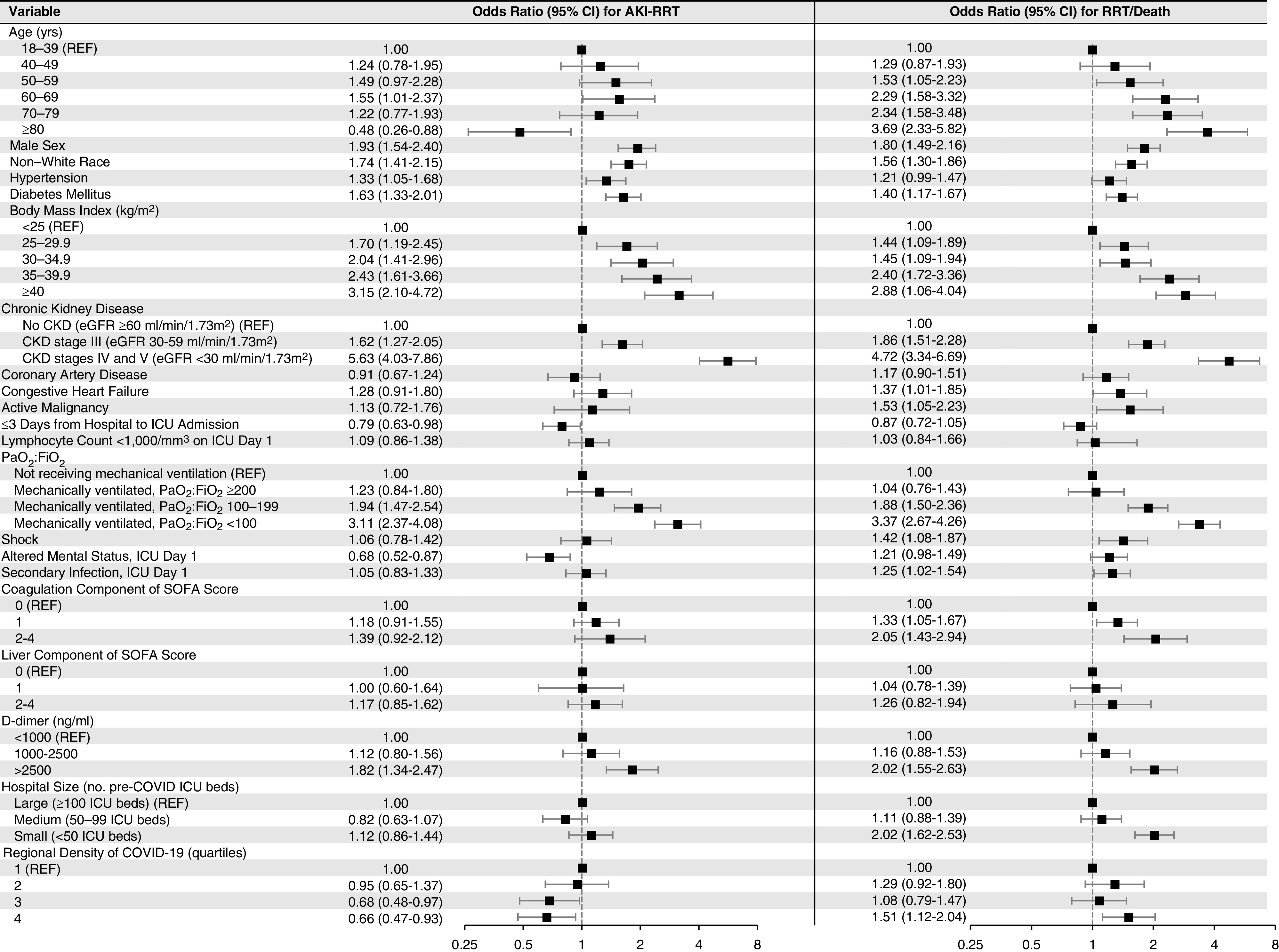
Multivariable model for AKI-RRT and RRT/death. This figure shows (left panel) the odds of AKI-RRT and (right panel) the composite of AKI-RRT or death (RRT/death) within 14 days following ICU admission according to patient- and hospital-level characteristics. A total of 637 of 3099 patients (20.6%) developed AKI-RRT. A total of 1205 of 3099 patients (38.9%) developed RRT/death, of whom 413 (34.3%) developed AKI-RRT and did not die, 568 (47.1%) died without AKI-RRT, and 224 (18.6%) both developed AKI-RRT and died. Unless otherwise indicated, severity of illness covariates were selected as the worst value on ICU day 1 or 2. SOFA, Sequential Organ Failure Assessment.
Factors associated with AKI-RRT were largely similar to those associated with the composite of RRT/death but with several notable exceptions. Whereas age ≥80 years was associated with a lower odds of AKI-RRT, it was associated with a higher odds of RRT/death (Figure 4). Additionally, whereas number of ICU beds was not associated with AKI-RRT, patients admitted to hospitals with fewer ICU beds had a higher odds of RRT/death (Figure 4). Finally, whereas patients admitted to hospitals with greater regional density of COVID-19 had a lower odds of AKI-RRT, they had a higher odds of RRT/death (Figure 4).
Interhospital Variation in AKI-RRT
The incidence of AKI-RRT varied widely between hospitals, ranging from 7.5% at the lowest-risk hospital to 44.6% at the highest (Figure 5A). This variation was attenuated in models adjusted for patient-level characteristics (range, 9.6%–32.7%) (Figure 5B) and was further attenuated in models adjusted for both patient- and hospital-level characteristics (range, 12.4%–29.1%) (Figure 5C).
Figure 5.
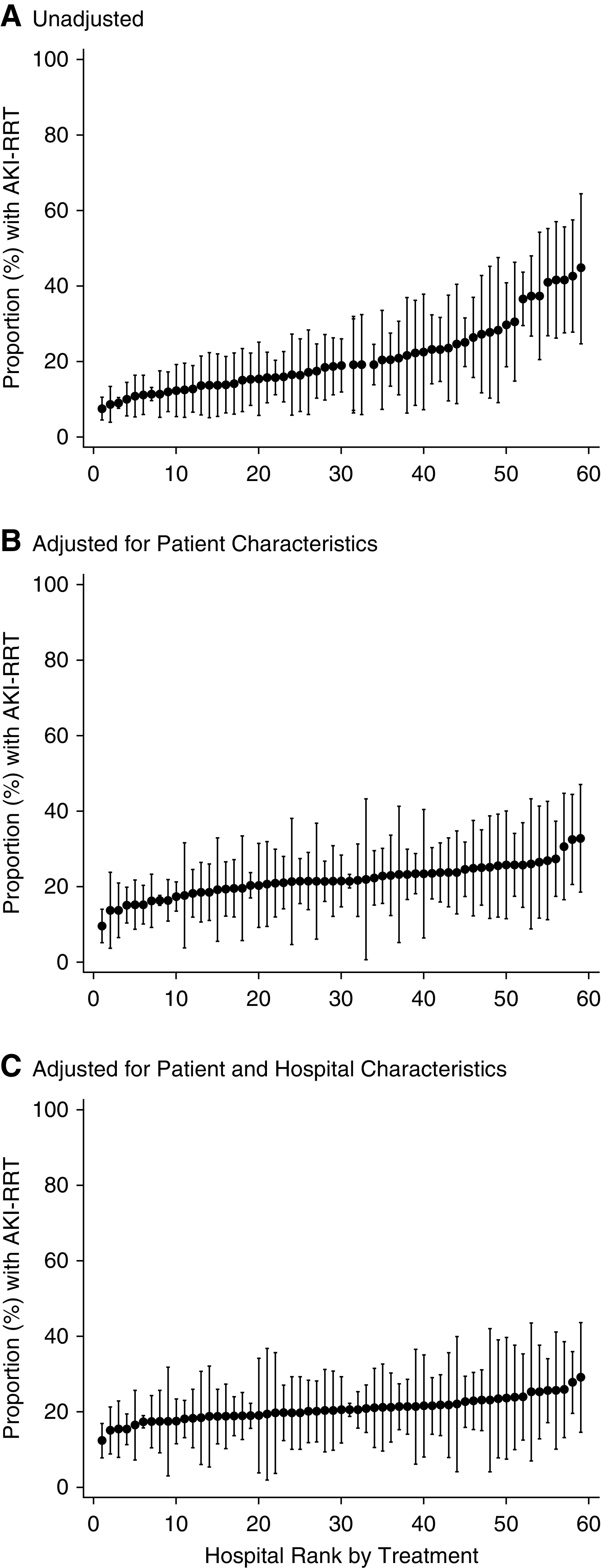
The incidence of AKI-RRT varies by hospital. Interhospital variation in AKI-RRT. Risk- and reliability-adjusted rates of AKI-RRT within 14 days following ICU admission. (A) is unadjusted. (B) is adjusted for patient-level characteristics. (C) is adjusted for both patient- and hospital-level characteristics, including number of ICU beds and regional density of COVID-19.
Predictors of 28-Day Mortality among Patients with AKI-RRT
Among the 637 patients with AKI-RRT, 350 (54.9%) died within 28 days of ICU admission. Older age, receipt of two or more vasopressors at the time of RRT initiation, and severe oliguria (urine output <100 ml/d) at the time of RRT initiation were each associated with a higher risk of 28-day mortality, whereas CKD stage 4 or 5 was associated with a lower risk of 28-day mortality (Figure 6). Patients admitted to hospitals with fewer ICU beds and to those with greater regional density of COVID-19 also had a higher risk of 28-day mortality (Figure 6).
Figure 6.
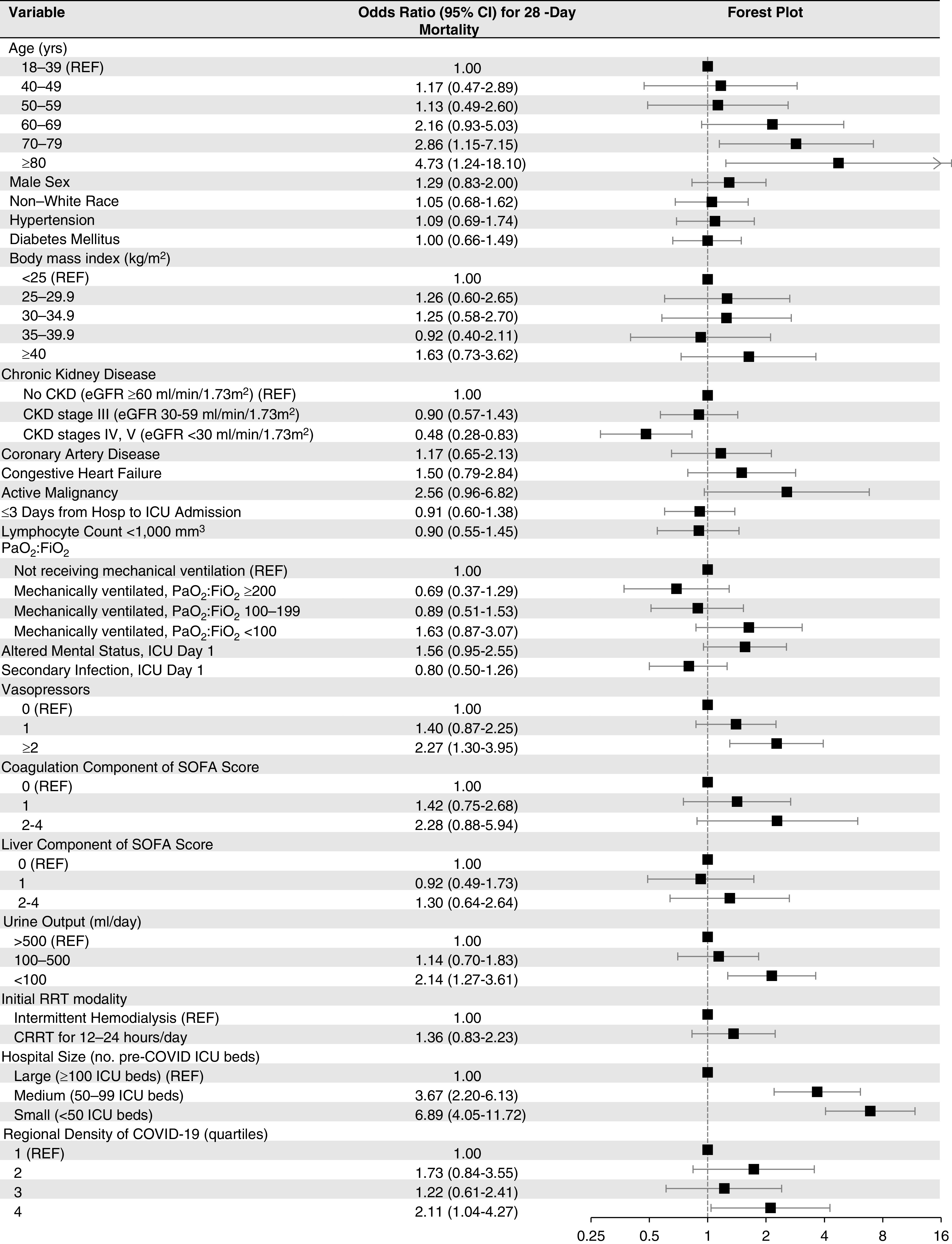
Multivariable model for 28-day mortality in patients with AKI-RRT. Unless otherwise indicated, severity of illness covariates were selected on the day of RRT initiation or if unavailable, the day prior. CRRT, continuous RRT; SOFA, Sequential Organ Failure Assessment.
When 28-day mortality was assessed from the date of RRT initiation (rather than from ICU admission), 365 patients (57.3%) had died, and the results were overall similar (Supplemental Table 9).
We also examined variation in 28-day mortality among patients with AKI-RRT according to geographic region. As shown in Supplemental Figure 5, unadjusted 28-day mortality was highest among patients admitted to hospitals in the northeastern part of the United States. Patients admitted to hospitals in the Northeast had similar median age and burden of comorbidities compared with patients admitted elsewhere but had higher rates of receipt of invasive mechanical ventilation on admission to the ICU. Regional density of COVID-19 was highest in the Northeast.
Renal Recovery
Among the 637 patients with AKI-RRT, during a median follow-up of 17 days (interquartile range, 9–30 days; range, 1–123 days), 403 patients (63.3%) had died, 216 (33.9%) were discharged, and 18 (2.8%) were still hospitalized. Among the 216 patients discharged, 73 (33.8%) remained RRT dependent on discharge. When these 73 patients were followed to 60 days from ICU admission, 69 (94.5%) were still alive, one (1.4%) had died, and three (4.1%) had unknown survival status. Of the 69 patients still alive at day 60, 39 (56.5%) remained RRT dependent at day 60, 24 (34.8%) became independent of RRT by day 60, and six (8.7%) had unknown RRT dependence data on day 60 (Figure 1).
AKI Not Treated with RRT
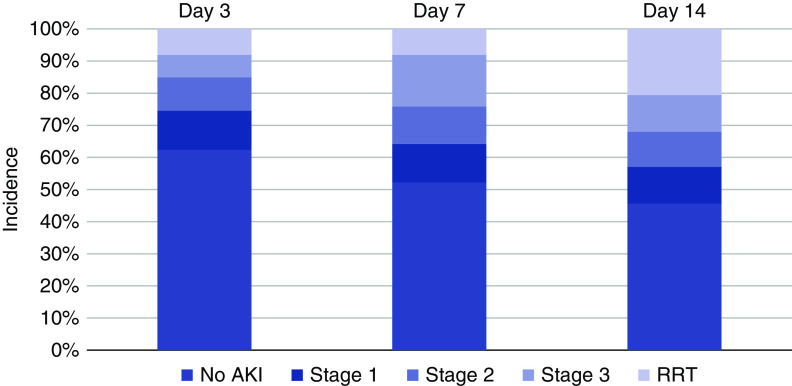
The incidence, severity, and outcomes of AKI not treated with RRT, both on hospital admission and within 14 days following ICU admission, are shown in Supplemental Figures 6 and 7 and Supplemental Table 10. The cumulative incidence of AKI by stage at days 3, 7, and 14 is shown in Figure 7.
Figure 7.
Incidence of AKI at 3, 7, and 14 days from ICU admission. For each time period, patients were categorized according to the highest stage of AKI achieved.
Discussion
In this multicenter cohort study of over 3000 critically ill adults with COVID-19 admitted to ICUs at 67 hospitals across the United States, we found that over one in five patients developed AKI-RRT. CKD, higher body mass index, and greater severity of hypoxemia on ICU admission are each independently associated with a higher risk of AKI-RRT. Among patients with AKI-RRT, 63% died during hospitalization. Predictors of death in patients with AKI-RRT include older age, oliguria, and admission to a hospital with fewer ICU beds or one with greater regional density of COVID-19. Finally, among patients with AKI-RRT who survived to hospital discharge, 34% remained RRT dependent on discharge. Cumulatively, these findings indicate that AKI-RRT is common among critically ill patients with COVID-19 and that it is associated with high acute mortality and high rates of continued RRT dependence among survivors.
Several studies have examined the incidence of AKI in hospitalized patients with COVID-19,3–5 but few have focused on AKI-RRT. The United Kingdom–based Intensive Care National Audit and Research Centre found that among 10,168 critically ill patients with COVID-19, 26.6% developed AKI-RRT,26 similar to the 21% that we report here.
We identified several patient-level risk factors for AKI-RRT, including CKD, higher body mass index, men, non-White race, diabetes mellitus, and hypertension. CKD and obesity have each been associated with a higher risk of AKI in patients with H1N1 influenza27–29 and in critically ill patients in non–COVID-19 settings.30 CKD is a well-known risk factor for AKI in general,31,32 and a recent study in hospitalized patients with COVID-19 in New Orleans found that patients with AKI-RRT had a higher median body mass index compared with patients without AKI-RRT.10 We also found that lower PaO2:FiO2 ratio and shock on ICU admission are each independent risk factors for AKI-RRT. Similar to Hirsch et al.,5 we found that AKI-RRT commonly occurs around the time of intubation, suggesting a role for alterations in hemodynamics as a potentially important mechanism for AKI in COVID-19 because vasopressors are often initiated at the time of intubation.
We found that older age is associated with a lower risk of AKI-RRT but a higher risk of RRT/death. It is possible that informed decision making about poor outcomes among older patients with AKI-RRT in non–COVID-19 settings may have led to the decision to not initiate RRT in patients ≥80 years of age.33,34
We found that admission to a hospital with greater regional density of COVID-19 is associated with a lower risk of AKI-RRT but a higher risk of RRT/death. Multiple potential explanations could account for this finding. Patients admitted to hospitals with greater regional density of COVID-19 may have presented at a more advanced stage of acute illness or may have differed from patients admitted to hospitals with less regional density of COVID-19 in other ways that were not captured by our data. Variation in the risk- and reliability-adjusted use of RRT in critically ill patients has been described in settings outside of COVID-19,35 consistent with heterogeneity in severity of illness and in patient and provider preferences. Because we did not capture data on the reasons for RRT initiation or noninitiation, including data on goals of care as well as the availability of RRT equipment, nurses, and physicians, caution is needed in interpreting our findings.
We also identified several patient- and hospital-level risk factors for death among patients with AKI-RRT. Patient-level risk factors included older age and severe oliguria, whereas hospital-level risk factors included greater regional density of COVID-19 and fewer ICU beds. Both older age and oliguria have been associated with death in patients with AKI-RRT in non–COVID-19 settings.25 Although CKD was a predictor of AKI-RRT, we found that CKD was inversely associated with 28-day mortality among patients with AKI-RRT. One possible explanation for these findings is that patients with relatively intact baseline kidney function would have required a larger insult to develop AKI-RRT, and may therefore have been sicker, than patients with advanced underlying CKD.
The finding that mortality differed considerably according to number of ICU beds is intriguing. It is well known that ICU organization, experience, and staffing may affect outcomes in critically ill patients in non–COVID-19 settings. For example, studies have demonstrated the beneficial effects of being admitted to an ICU with mandatory staffing (or consultation) by intensivists.36 The importance of experience is highlighted by studies revealing a “volume-outcome relationship” for mechanically ventilated patients37 and patients receiving RRT,38–40 where patients who receive these interventions have better outcomes if admitted to hospitals that care for a larger number of such patients.
When analyzing rates of renal recovery among patients with AKI-RRT who survived to hospital discharge, we found that >30% remained RRT dependent on discharge. Although this number decreased considerably when patients were followed to day 60, studies of AKI-RRT in critically ill patients without COVID-19 have generally found lower rates of RRT dependence at hospital discharge among survivors, ranging from 7% to 18% in most studies.12,41,42 Potential reasons for the high rate of persistent RRT dependence in COVID-19 include severe or ongoing renal tubular injury due to direct viral invasion7,9 and cytokine storm.8 Further studies are needed to determine long-term renal outcomes in these patients.
Our study has several strengths. First, the large number of patients in our cohort allowed us to focus on AKI-RRT, the most severe and clinically relevant form of AKI. Second, we included patients from 67 geographically diverse sites across the United States, thereby increasing the generalizability of our findings and allowing us to examine interhospital variability in outcomes. Third, all data were obtained by detailed chart review rather than reliance on administrative or billing codes, which allowed us to capture granular and reliable data. Fourth, over 97% of the patients with AKI-RRT had a definitive outcome (dead or discharged) by the end of follow-up.
We also acknowledge several limitations. First, data on AKI-RRT were only captured for the first 14 days following ICU admission. Accordingly, we may have underestimated the true incidence of AKI-RRT in critically ill patients with COVID-19, although rates of AKI-RRT declined precipitously over time, with 80% of the known cases occurring in the first 7 days of ICU admission (Supplemental Figure 2). Second, data on SCr prior to hospitalization were missing for a large proportion of the cohort. In these patients, we used SCr on hospital admission as a surrogate for baseline SCr, which may have overestimated the prevalence of CKD in our cohort. Third, we did not collect data on urinalysis/urine sediment or volume status at the time of RRT initiation or during the course of RRT. Fourth, the majority of cases of AKI-RRT were from the Northeast and Midwest regions of the United States, reflecting the regional surge in COVID-19 cases in March and April 2020, and we did not include data from international sites. Thus, our findings may not be generalizable beyond the United States or to regions of the United States that were not well-represented in this study. Fifth, as with all observational studies, there may be residual confounding, which may have particularly affected our findings with respect to hospital-level characteristics and outcomes. For example, our models may not sufficiently account for resource availability across hospitals, as we did not capture data on hospital or ICU patient volume or the availability of RRT machines, physicians, and nurses. Sixth, we did not determine the reasons for RRT initiation and noninitiation.
In this multicenter, nationally representative cohort study of critically ill adults with COVID-19 in the United States, we found that more than one in five patients developed AKI-RRT, over 60% of whom died within 28 days. Among those with AKI-RRT who survived to hospital discharge, nearly one in three remained RRT dependent. We identified several patient- and hospital-level risk factors for AKI-RRT and death. Future studies are needed to investigate the long-term outcomes of patients with AKI-RRT in the setting of COVID-19, as well as to explore the underlying etiologies of AKI in this setting so that targeted therapies can be developed.
Disclosures
The authors of the writing committee are supported by National Institutes of Health (NIH) grants F32HL149337 (to A. Admon), R01AG066471 (to L. Chan), U01DK106962 (to S. Coca), R01DK115562 (to S. Coca), R01HL85757 (to S. Coca), R01DK112258 (to S. Coca), U01OH011326 (to S. Coca), R01DK126477 (to S. Coca), F32DC17342 (to S. Gupta), R01HL144566 (to D. Leaf), R01DK125786 (to D. Leaf), UL1TR001998 (to J. Neyra), R01HL085757 (to C. Parikh), and K23DK120811 (to A. Srivastava). L. Chan reports grants from NIH and Renal Research Institute, outside the submitted work. D. Charytan reports personal fees from AstraZeneca, Douglas and London, Fresenius, GSK, Merck, PLC Medical, and Zoll; grants from BioPorto; grants and personal fees from Amgen, Medtronic, Gilead, and NovoNordisk; personal fees and other from Jannssen; and other from Daichi-Sankyo, outside the submitted work. M. Christov is currently employed by New York Medical College, Regeneron Pharmaceuticals; and reports ownership interest in Regeneron Pharmaceuticals. S. Coca is a cofounder and a member of the advisory board of RenalytixAI, and owns equity in the same. In the past 3 years, he has received consulting fees from Bayer, Boehringer-Ingelheim, CHF Solutions, pulseData, Quark Biopharma, Relypsa, RenalytixAI, and Takeda Pharmaceuticals as well as personal fees from inRegen. J. Flythe reports other from American Renal Associates, the American Society of Nephrology, AstraZeneca, Fresenius Medical Care, North America, the National Kidney Foundation, and NxStage Medical; and grants from Renal Research Institute and Fresenius Medical Care, outside the submitted work. A. Friedman is currently a member of the scientific advisory board for GI Dynamics and has consulted for DSMB Watermark. S. Gupta is a scientific coordinator for the Anaemia Studies in CKD: Erythropoiesis via a Novel PHI Daprodustat (ASCEND) trial (GlaxoSmithKline); and reports personal fees from GlaxoSmithKline and grants from NIH, outside the submitted work. S. Hayek is funded by National Heart, Lung, and Blood Institute (NHLBI) grant 1R01HL153384-01; the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor (U-M G024231) award; and reports personal fees from Trisaq, outside the submitted work. S. Hedayati reports honoraria from the American College of Physicians for participation in Nephrology Medical Knowledge Self-Assessment Program (MKSAP) and the American Society of Nephrology Post-Graduate Education Program; is a scientific advisor for or reports membership in the American Heart Association; and reports study sections in American College of Physicians (ACP), MKSAP Nephrology Committee and the American Society of Nephrology In-Training Examination Committee. D. Leaf received research support from BioPorto. K. Liu reports grants from NIH: NHLBI and NIH: National Institute of Diabetes and Digestive and Kidney Disease (NIDDK); personal fees from the American Thoracic Society, Astra Zeneca, Baxter, Biomerieux, Durect, Potrero Med, Quark, Theravance, and UpToDate; and other from Amgen and the National Policy Forum on Critical Care and ARF, outside the submitted work. M. Melamed reports personal fees from the American Board of Internal Medicine and Icon Medical Consulting, outside the submitted work. M. Molnar reports personal fees from Abbvie, CareDx, and Natera; and grants from CareDx and Viracor, outside the submitted work. J. Neyra consults for Baxter Healthercare, Inc. and Renibus Therapeutics, Inc. C. Parikh serves on the board of Renalytix and is a Data Safety Monitoring Board (DSMB) member for Genfit. C. Parikh reports consultancy agreements with Genfit Biopharmaceutical Company; ownership interest in Renaltix AI; research funding from NHLBI and NIDDK; and scientific advisor for or membership in Genfit Biopharmaceutical Company and Renalytix. C. Schulze is a medical director for Davita-Century City home hemodialysis program. M. Sise receives research funding from Abbvie, Gilead, MEDSerono, and Merck; honoraria from the International Society of Hemodialysis–Hemodialysis University Lecture; consults for BioPorto; and serves on the scientific advisory board for Abbvie and Gilead. A. Srivastava received grants from NIH/NIDDK and personal fees from Horizon Pharma, Public Limited Company, AstraZeneca, and CVS Caremark. J. Velez is currently employed by Ochsner Clinic Foundation; reports consultancy agreements with Mallinckrodt Pharmaceuticals; reports honoraria from Mallinckrodt Pharmaceuticals and Otsuka; is a scientific advisor for or reports membership in Mallinckrodt Advisory Board and Retrophin Advisory Board; and speakers bureau: Otsuka Pharmaceuticals. A. Vijayan reports consultancy agreements with Boehringer Ingelheim, NxStage, and Sanofi; reports research funding from Astellas; reports spectral honoraria from Astute and Sanofi; is a scientific advisor for or reports membership in NxStage; and is a member of the National Kidney Foundation. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
A. Bansal, S. Brenner, L. Chan, D. Charytan, S. Coca, R. Dy, J. Flythe, A. Friedman, N. Goyal, S. Hayek, S. Hedayati, M. Joo, E. Judd, O. Kamal, A. Leonberg-Yoo, K. Liu, M. Mallappallil, M. Melamed, J. Neyra, H. Nguyen, S. Puri, A. Rashidi, R. Redfern, E. Schenck, C. Schulze, S. Sharma, A. Shehata, M. Sosa, A. Srivastava, A. Sutherland, J. Velez, A. Vijayan, and J. Zhang collected data; A. Admon, J. Boateng, S. Gupta, D. Leaf, C. Parikh, S. Short, and M. Sise analyzed data; S. Gupta and D. Leaf interpreted the results and wrote the manuscript; all authors contributed to revision of the final version of the manuscript, approved the final version submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; D. Leaf acts as guarantor for the work; and D. Leaf attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Carl P. Walther, Samaya J. Anumudu, Justin Arunthamakun, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen, Shahzad Shaefi, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia, Sushrut S. Waikar, Zoe A. Kibbelaar, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Adam Green, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea, Daniel L. Edmonston, Christopher L. Mosher, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams, Patricia Walters, Ronaldo C. Go, Keith M. Rose, Rebecca Lisk, Amy M. Zhou, Ethan C. Kim, Kusum S. Mathews, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis, Afshin Ahoubim, Leslie F. Thomas, Dheeraj Reddy Sirganagari, Pramod K. Guru, Yan Zhou, Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta, Princy N. Kumar, Deepa G. Lazarous, Seble G. Kassaye, Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar, Ritesh Raichoudhury, Akshay Athreya, Mohamed Farag, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki, Amey Bhivshet, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner M.B. Mohamed, Rupali S. Avasare, David Zonies, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Heather Yang Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller, Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley, Chris Rowan, Farah Madhani-Lovely, Vasil Peev, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta, Jared Radbel, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson, Shuchi Anand, Joseph E. Levitt, Pablo Garcia, Suzanne M. Boyle, Rui Song, Ali Arif, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter, Moh’d A. Sharshir, Vadym V. Rusnak, Muhammad Imran Ali, Terri Peters, Kathy Hughes, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, Rana Hejal, Laura Latta, Ashita Tolwani, Timothy E. Albertson, Jason Y. Adams, Steven Y. Chang, Rebecca M. Beutler, Etienne Macedo, Harin Rhee, Vasantha K. Jotwani, Jay L. Koyner, Chintan V. Shah, Vishal Jaikaransingh, Stephanie M. Toth-Manikowski, James P. Lash, Nourhan Chaaban, Yahya Ahmad, Madona Elias, Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Alessia Fornoni, Hayley B. Gershengorn, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O.’ Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Abbas Bitar, Elizabeth Anderson, John P. Donnelly, Matthew J. Tugman, Emily H. Chang, Brent R. Brown, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen, Csaba P. Kovesdy, Ambreen Azhar, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett, Kyle B. Enfield, Pavan K. Bhatraju, A. Bilal Malik, Matthew W. Semler, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao, Greg L. Schumaker, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, Perry Wilson, Tanima Arora, and Ugochukwu Ugwuowo
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060897/-/DCSupplemental.
Supplemental Material. List of the STOP-COVID Investigators and their affiliations.
Supplemental Figure 1. Number of patients with AKI-RRT by state among contributing sites.
Supplemental Figure 2. Time to AKI-RRT.
Supplemental Figure 3. Timing of AKI-RRT in relation to intubation.
Supplemental Figure 4. Proportion of patients with AKI stage 3 treated with RRT by quartile of regional density of COVID-19.
Supplemental Figure 5. The 28-day mortality of patients with AKI-RRT by region.
Supplemental Figure 6. AKI on hospital admission and within 14 days following ICU admission.
Supplemental Figure 7. Flowchart of outcomes of patients without AKI-RRT.
Supplemental Table 1. List of participating sites.
Supplemental Table 2. Definitions of baseline characteristics, comorbidities, treatments, and outcomes.
Supplemental Table 3. Complete list of patient characteristics according to AKI stage.
Supplemental Table 4. Characteristics of patients with AKI-RRT on days 1 and 2 versus days 3–14.
Supplemental Table 5. Multivariable model for AKI-RRT, accounting for clustering.
Supplemental Table 6. Multivariable model for AKI-RRT among patients with a prehospital baseline serum creatinine available.
Supplemental Table 7. Multivariable model for AKI-RRT excluding patients transferred from an outside hospital.
Supplemental Table 8. Multivariable model for AKI-RRT among patients who survived 14 days.
Supplemental Table 9. Multivariable model for 28-day mortality from the day of RRT initiation.
Supplemental Table 10. AKI by stage and 28-day mortality among patients with a prehospital baseline SCr available.
References
- 1.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al.: COVID-19 and cardiovascular disease. Circulation 141: 1648–1655, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al.: Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 5: 802–810, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-J, Ni Z-Y, Hu Y, Liang WH, Ou CQ, He JX, et al.: China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al.: Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan L, Coca S: Acute kidney injury in the time of COVID-19. Kidney360 1: 588–590, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al.: COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group : Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 31: 1683–1687, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al.: Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360 1: 614–622, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan Lili, Chaudhary Kumardeep, Saha Aparna, Chauhan Kinsuk, Vaid Akhil, Zhao Shan, et al.: AKI in Hospitalized Patients with COVID-19. JASN 31[9]: 2145–2157, 2020. 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manns B, Doig CJ, Lee H, Dean S, Tonelli M, Johnson D, et al.: Cost of acute renal failure requiring dialysis in the intensive care unit: Clinical and resource implications of renal recovery. Crit Care Med 31: 449–455, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Metnitz PGH, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al.: Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30: 2051–2058, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Allegretti AS, Steele DJR, David-Kasdan JA, Bajwa E, Niles JL, Bhan I: Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: A cohort study. Crit Care 17: R109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al.: Factors associated with death among critically ill patients with coronavirus disease 2019 in the US [published online ahead of print July 15, 2020]. JAMA Intern Med 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA: Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewitte A, Joannès-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P, et al.: Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol 10: 1900–1910, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al.: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al.: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22, 1986 [Google Scholar]
- 21.Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al.: Tissue inhibitor metalloproteinase-2 (TIMP-2) • IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int 95: 1486–1493, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, et al.: Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 87: 1046–1054, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIlroy DR, Bellomo R, Billings FT 4th, Karkouti K, Prowle JR, Shaw AD, et al.: Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: Renal endpoints. Br J Anaesth 121: 1013–1024, 2018. [DOI] [PubMed] [Google Scholar]
- 24.Leaf DE, Waikar SS: End points for clinical trials in acute kidney injury. Am J Kidney Dis 69: 108–116, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirjian S, Chertow GM, Zhang JH, O’Connor TZ, Vitale J, Paganini EP, et al.: VA/NIH Acute Renal Failure Trial Network : Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol 6: 2114–2120, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intensive Care National Audit & Research : ICNARC report on COVID-19 in critical care, 2020. Available at: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed August 5, 2020
- 27.Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, et al.: Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): Report from a Canadian province. Am J Kidney Dis 55: 848–855, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung JY, Park BH, Hong SB, Koh Y, Suh GY, Jeon K, et al.: Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: A multicenter study. J Crit Care 26: 577–585, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Demirjian SG, Raina R, Bhimraj A, Navaneethan SD, Gordon SM, Schreiber MJ Jr., et al.: 2009 influenza A infection and acute kidney injury: Incidence, risk factors, and complications. Am J Nephrol 34: 1–8, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Danziger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, et al.: Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med 44: 328–334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoste EAJ, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JMA, Colardyn FA: Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol 14: 1022–1030, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al.: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valley TS, Nallamothu BK, Heung M, Iwashyna TJ, Cooke CR: Hospital variation in renal replacement therapy for sepsis in the United States. Crit Care Med 46: e158–e165, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampieri FG, Salluh JIF, Azevedo LCP, Kahn JM, Damiani LP, Borges LP, et al.: ORCHESTRA Study Investigators : ICU staffing feature phenotypes and their relationship with patients’ outcomes: An unsupervised machine learning analysis. Intensive Care Med 45: 1599–1607, 2019. [DOI] [PubMed] [Google Scholar]
- 37.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD: Hospital volume and the outcomes of mechanical ventilation. N Engl J Med 355: 41–50, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen YL, Wallace DJ, Yordanov Y, Trinquart L, Blomkvist J, Angus DC, et al.: The volume-outcome relationship in critical care: A systematic review and meta-analysis. Chest 148: 79–92, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaara ST, Reinikainen M, Kaukonen K-M, Pettilä V; Finnish Intensive Care Consortium : Association of ICU size and annual case volume of renal replacement therapy patients with mortality. Acta Anaesthesiol Scand 56: 1175–1182, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Chimunda T, Silver SA, Kuwornu JP, Li L, Nash DM, Dixon SN, et al.: Hospital case volume and clinical outcomes in critically ill patients with acute kidney injury treated with dialysis. J Crit Care 48: 276–282, 2018. [DOI] [PubMed] [Google Scholar]
- 41.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al.: Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med 33: 1563–1570, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Cole L, Bellomo R, Silvester W, Reeves JH: A prospective, multicenter study of the epidemiology, management, and outcome of severe acute renal failure in a “closed” ICU system. Am J Respir Crit Care Med 162: 191–196, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.