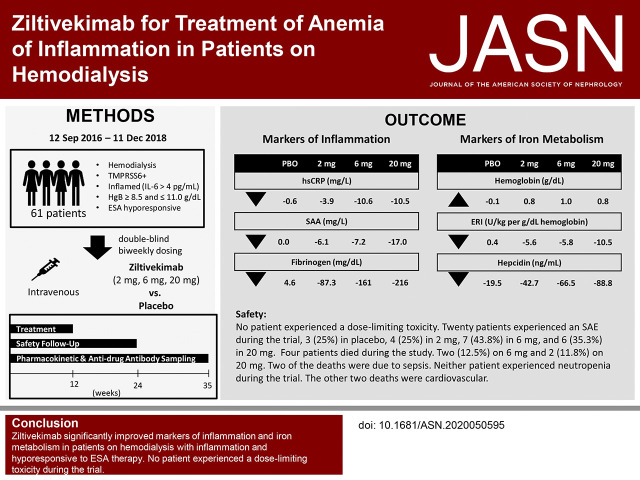
Significance Statement
Patients with CKD who are on hemodialysis are hyporesponsive to erythropoiesis-stimulating agents (ESAs) because of anemia of inflammation mediated by IL-6–induced expression of hepcidin, an iron regulatory hormone. Reducing ESA usage to decrease ESA-related cardiovascular risk, especially with high ESA doses, is a clinical goal of nephrologists. In this randomized, phase 1/2 trial in patients with inflammation on hemodialysis, the authors show that ziltivekimab, a novel anti–IL-6 ligand antibody, reduced markers of inflammation, decreased ESA usage, and increased serum albumin, which might lead to a reduction in overall cardiovascular risk. Because current anemia treatments do not reduce inflammation, the availability of an anti-inflammatory therapy that also improves iron utilization and reduces the need for escalating doses of ESAs could represent an important advancement in the care of patients on hemodialysis.
Keywords: hemodialysis, anemia, chronic kidney disease, cardiovascular disease, erythropoietin stimulating agents, inflammation
Visual Abstract
Abstract
Background
Patients with CKD who are on hemodialysis are hyporesponsive to erythropoiesis-stimulating agents (ESAs) because of anemia of inflammation. Interleukin-6 (IL-6) induced hepcidin expression is a key mediator of such inflammation.
Methods
This phase 1/2, placebo-controlled trial assessed effects of ziltivekimab, a novel anti–IL-6 ligand antibody, in patients on hemodialysis with rs855791, a single nucleotide polymorphism of the TMPRSS6 gene that is hypothesized to heighten susceptibility to IL-6–mediated inflammatory effects. After a screening period documenting stable ESA and iron dosing, we randomized 61 patients with elevated IL-6 (≥4 pg/ml) to receive placebo or ziltivekimab (doses of 2, 6, or 20 mg), administered intravenously every 2 weeks for 12 weeks during hemodialysis. ESA dose adjustments were allowed after 4 weeks. We analyzed safety and effects on inflammation, iron metabolism, serum albumin, and anti-drug antibodies.
Results
No patient experienced dose-limiting toxicity. Four patients (two each in the 6- and 20-mg cohorts) died of a treatment-emergent adverse event. Compared with patients receiving placebo, those receiving ziltivekimab experienced significantly greater reductions of high-sensitivity C-reactive protein, serum amyloid A, and fibrinogen from baseline to end of treatment. Median ESA usage decreased by 15,000, 15,000, or 33,000 IU/wk per patient in the 2-, 6-, and 20-mg ziltivekimab cohorts, respectively, compared with no change in the placebo group. We also noted significant dose responses for decreased ESA resistance index and increased serum iron, total iron binding capacity, transferrin saturation, and serum albumin.
Conclusions
Ziltivekimab significantly improved markers of inflammation, reduced ESA requirements, and increased serum albumin in patients on hemodialysis with inflammation and hyporesponsiveness to ESA therapy.
Clinical Trial registry name and registration number
Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of Multiple Doses of COR-001, NCT02868229
Anemia is common in patients with CKD on hemodialysis and is associated with increased morbidity and mortality. Anemia in patients with CKD is caused by a variety of factors including erythropoietin (EPO) deficiency, due to decreased production in the kidneys, and increased systemic inflammation, known as anemia of inflammation.1 Patients on hemodialysis with inflammation are given erythropoiesis-stimulating agents (ESAs) and supplemental iron to correct iron deficiency and reduce EPO production. However, proinflammatory cytokines cause ESA hyporesponsiveness, which necessitates using higher ESA dosages to maintain hemoglobin levels on target.2–6 Using high doses of ESA is associated with increased cardiovascular risk.7,8
A key mediator of systemic inflammation is IL-6, a pleotropic cytokine with the ability to induce both immune inflammatory–mediated and iron-mediated anemia pathologies, thus allowing a unique interplay between these two conditions. Iron homeostasis is maintained on the basis of the interaction between ferroportin, the cellular iron exporter that transfers iron to blood plasma, and hepatocyte-produced hepcidin, an iron regulatory hormone that inhibits the iron-exporting activity of ferroportin.1 Emergent literature suggests IL-6 has the ability to induce hepcidin transcription alone, or in combination with bone morphogenetic protein receptor type 2 (BMPR2) agonists such as activin and BMP2.1,9 IL-6 induces hepcidin transcription through the signal transducer and activator of transcription 3 binding domain and is one of the predominant proinflammatory cytokines known to induce hepcidin. In vitro, IL-6 also works in concert with endogenous agonists of the BMPR complex in hepcidin induction.9,10 The BMPR complex is a heterotetramer of BMPR1 and BMPR2, which then forms a complex with hemojuvelin. The rs855791 functional single nucleotide polymorphism in TMPRSS6 (prevalence of approximately 80% in the general population)—the gene that encodes a membrane serine protease, Matriptase-2—is responsible for normal variations of serum iron and hemoglobin levels in healthy populations through its modulation of hemojuvelin.11,12 Data suggest rs855791 uniquely sensitizes cellular responsiveness to IL-6 (Corvidia Therapeutics, Inc. internal unpublished data).13 Results from preclinical studies and retrospective analyses of data from multiple populations suggest rs855791 can modulate the degree of IL-6–driven hepcidin expression with subsequent effects on the cellular susceptibility to ischemia reperfusion, and may thereby dictate the effect of serum IL-6 on clinical outcomes. IL-6 is closely correlated with suppression of EPO production and ESA hyporesponsiveness, inversely correlated with serum albumin, and is predictive of cardiovascular and overall mortality in the hemodialysis population.2,6,14–16
Although IL-6–receptor antibody therapies are approved for treatment of rheumatoid arthritis and giant cell arteritis, no anti-inflammatory therapies have been systematically evaluated in patients on hemodialysis to understand their effects on inflammatory anemia and the resulting ESA hyporesponsiveness. Ziltivekimab is a novel human IgG1, κ antibody directed against IL-6 ligand that has been previously investigated in phase 1 trials of patients with rheumatoid arthritis (ClinicalTrials.gov, NCT01559103) and CKD (ClinicalTrials.gov, NCT03126318). This phase 1/2 trial was conducted to examine the safety and pharmacodynamic effects of ziltivekimab in patients on hemodialysis with chronic inflammation. We acknowledge that, simultaneous to their anti-inflammatory potential, anti–IL-6 therapies also run the risk of having immunosuppressive effects. However, it was our hypothesis that inhibiting IL-6 with this neutralizing mAb would be safe and would result in increased hemoglobin and reduced ESA hyporesponsiveness.
Methods
Trial Oversight
The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice, and the trial protocol, which was approved by an institutional review board for each clinical site. A list of the 13 investigators and their sites (all in the United States) is provided in the Supplemental Appendix. All patients provided informed consent before trial entry.
Study Population
Subjects were recruited into the trial between August 8, 2016 and April 23, 2018; the database was locked and the study was unblinded on August 16, 2018. The main entry criteria are described below; a full listing is available in the Supplemental Appendix. Enrolled patients were ≥18 years of age, were receiving chronic hemodialysis and stable doses of ESA drugs (described below), had IL-6 ≥4 pg/ml, and harbored alanine at position 736 of the Matriptase-2 protein (i.e., homozygous or heterozygous for guanine at rs855791 within the TMPRSS6 gene). Patients also had ferritin >300 ng/ml, transferrin serum iron saturation (TSAT) between 15% and 50%, and hemoglobin between 8.5 and 11.0 g/dl, despite being on higher doses of ESA, as reflected by an ESA resistance index (ERI; calculated as U/kg per g/dl hemoglobin) of more than eight.17 An ERI of more than eight is representative of 58% of the US dialysis population (unpublished observation, Frenova Renal Research, Waltham, MA). Patients were required to have had a continuous ESA prescription at a stable dosage for at least 8 weeks before screening for one of the following ESA drugs: epoetin alfa, darbepoetin alfa, or methoxy propylene glycol-epoetin β. EPO unit equivalent doses for darbopoetin alfa and methoxy polyethylene glycol-epoetin β were calculated by multiplying the dose in micrograms by 300. Subjects had been receiving intravenous (IV) or dialysate iron regularly and continuously (e.g., with each dialysis) during the 3 weeks before randomization, or had received no IV or dialysate iron during that time frame. If the patient had received oral ferric citrate before screening, it had to have been taken for at least 2 weeks prior. Selected key exclusion criteria included the presence of, or anticipated need for, any indwelling vascular catheter (used as a dialysis access or any other indication); clinical evidence or suspicion of an active or smoldering infection, or use of systemic antibiotics, antivirals, or antifungals within 2 weeks before screening; positive blood test for tuberculosis; or serology positive for HIV-1, HIV-2, hepatitis B, or hepatitis C.
Enrollment, Randomization, and Interventions
This was a randomized, double-blind (sponsor/clinical research organization, investigators and patients), placebo-controlled, phase 1/2, multicenter trial of ziltivekimab, administered at 2, 6, or 20 mg IV to sequential cohorts of patients on hemodialysis. Each cohort included eight patients on active treatment and two patients on placebo treatment. Patients were randomized using an Interactive Web Response System (provided by DSG, Inc. Malvern, PA). The randomization list was generated by SAS (Cary, NC), and was concealed by DSG, Inc., and was only available to sites as necessary for emergent unblinding.
The study included a treatment period of 12 weeks (weeks 1–12), a safety follow-up period of 12 weeks (weeks 13–24), and an extended follow-up period of 10 weeks for pharmacokinetic and anti-drug antibody testing (weeks 25–34). Treatment was administered IV during dialysis every 14 days for 12 weeks (6 doses) for each cohort. Each dose of ziltivekimab or placebo was administered via IV infusion with a 0.2-μm, in-line filter over approximately 60 minutes, which started at any time before the last 1 hour of dialysis. Investigators were instructed not to change the patient’s ESA or iron product, or doses or dosing regimens during study weeks 1 through 4. After week 4, investigators were told to follow the provided instructions regarding ESA and parenteral iron dose adjustments as described in the Supplemental Appendix.
End Points
The primary objective of the trial was to evaluate the safety of three ascending dose levels of ziltivekimab through a review of adverse events (AEs) and laboratory results. Before dose escalation, there was a formal review by the safety review committee (including a nephrologist) to determine whether the maximum tolerated dose had been exceeded, on the basis of any dose-limiting toxicities through study day 21. Dose-limiting toxicities included the following: confirmed grade-3 neutropenia and representing a decline of >25% from baseline, serious AEs of infection in the presence of confirmed grade-2 or higher new-onset lymphopenia or new-onset neutropenia, grade-3 or higher alanine aminotransferase or aspartate aminotransferase, grade-4 or higher hematologic toxicity, or grade-3 or higher nonhematologic toxicity. Dose-limiting toxicity was further defined as any toxicity, where a relationship with the investigational agent could not be ruled out, as follows: with the exception of grade-3 nonhematologic events, grade-3 or higher Common Terminology Criteria for Adverse Events toxicity of the above events having an assessed relationship to the study drug of probably, possibly, or unlikely were considered dose-limiting toxicities.
Secondary and exploratory pharmacodynamic end points measured periodically during the study included high-sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA), fibrinogen, lipoprotein lipids, lipoprotein(a), hemoglobin, reticulocyte hemoglobin, TSAT, ERI (calculated as U/kg per g/dl hemoglobin), ESA dose, ferritin, hepcidin, serum prealbumin, and albumin. hsCRP levels were blinded to the investigator throughout the trial.
Statistical Analyses
The primary objective of this study was to determine the maximum tolerated dose of ziltivekimab. The prespecified criterion for determining a maximal tolerable dose was when more than two of the eight patients treated with ziltivekimab (≥25%) in a cohort experienced a dose-limiting toxicity. On the basis of a binomial distribution, the probability of observing two or more patients with dose-limiting toxicity was >63%, and the probability of observing one or more patients with dose-limiting toxicity was 90%. Because of the secondary interest in evaluating the effects of ziltivekimab on inflammation, we estimated that an evaluable sample of 14 patients per group would be required for 80% power to detect a standardized effect size of 1.27 SD, for the difference between placebo and each active treatment group for hsCRP response (a key pharmacodynamic end point),18,19 using a nominal, two-sided α of 0.017 to account for three comparisons to placebo.
The safety analysis included all patients who were randomized and treated with any amount of study medication. The pharmacodynamic population was defined as all randomized patients who were treated with study drug, had any pharmacodynamic assessments, and had no protocol deviations that might have significantly affected the interpretation of the pharmacodynamic end points (e.g., two or more missed doses or two or more incorrect doses). Data from patients on placebo in all cohorts were pooled for analyses.
Pharmacodynamic analyses were conducted using analysis of covariance models, with dose as a factor and baseline value as a covariate. Normality of model residuals was evaluated with the Shapiro–Wilk test, and, if the P value was <0.01, analysis of covariance was performed after rank transformation. Means and SDs are shown for normally distributed variables, and median and interquartile limits are shown for non-normally distributed variables. P values for ordered-dose response (P-DR) were calculated using the Jonckheere–Terpstra test.20,21 An α level of 0.05, two sided, was used to declare statistical significance. Statistical analyses were conducted in SAS.
Results
Patients
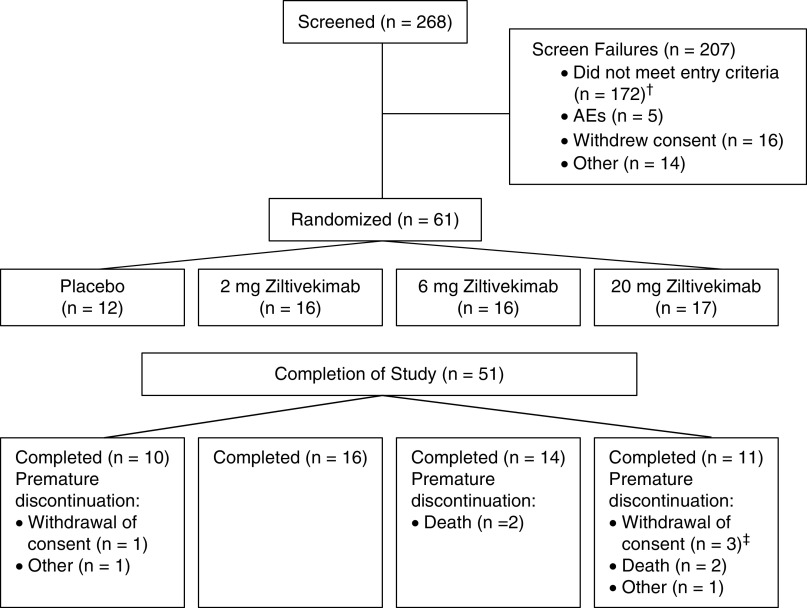
A description of patient flow through the study is presented in Figure 1. Demographic and baseline characteristics of the safety population are summarized in Table 1. There were no marked differences between treatment groups in demographic and baseline characteristics, but subjects in the 20-mg ziltivekimab group tended to be younger and had higher body mass index values. Additionally, more patients in the 6- and 20-mg cohorts were Black. The majority of the patients in the placebo group were male, but there was an even distribution of male and female patients in the ziltivekimab cohorts. Median baseline hsCRP was also higher in the ziltivekimab 2-, 6-, and 20-mg cohorts (6.1, 9.8, and 13.2 mg/L, respectively) than in placebo (4.0 mg/L). Baseline, week 4 (when measured), and end-of-treatment (week 12, or, for parameters with higher variability, the average of weeks 9–12 or weeks 10–12) values for the secondary and exploratory end points are shown in Figures 2, 3, and 4 and Tables 2 and 3.
Figure 1.
Patient flow through the study. Safety population included all randomized patients. Pharmacodynamic population included 53 patients: placebo (n=12), 2-mg ziltivekimab (n=16), 6-mg ziltivekimab (n=13), and 20-mg ziltivekimab (n=12). †Reasons for screen failures were classified as follows: did not have hemoglobin concentration ≥8.5 and ≤11.0 g/dl before randomization (n=42), did not have serum IL-6 ≥4 pg/ml at screening (n=25), was not genotype positive (GG or AG) (n=24), had a positive tuberculosis test at screening (n=13), hospitalization or outpatient procedures within 2 weeks before screening (n=12), and other (n=91). ‡One of the patients whose discontinuation was formally classified as withdrawal of consent was described by the site as discontinuing the study drug due to low platelets.
Table 1.
Patient demographic and baseline characteristics
| Characteristic | Placebo (n=12) | Ziltivekimab | ||
|---|---|---|---|---|
| 2 mg (n=16) | 6 mg (n=16) | 20 mg (n=17) | ||
| Age (yr), mean (SD) | 62.8 (10.2) | 61.4 (13.0) | 63.8 (11.0) | 57.9 (10.6) |
| Body mass index (kg/m2), mean (SD) | 28.5 (6.6) | 29.5 (8.1) | 30.5 (8.9) | 32.0 (8.4) |
| Prehemodialysis body weight at wk −1 (kg), mean (SD) | 85.9 (20.1) | 84.8 (27.9) | 89.8 (25.6) | 95.0 (28.3) |
| Posthemodialysis body weight at wk −1 (kg), mean (SD) | 83.1 (20.4) | 81.7 (27.1) | 87.2 (25.5) | 92.1 (27.5) |
| Duration of ESKD (d), mean (SD) | 1882 (1204) | 1697 (1302) | 2302 (1182) | 1744 (1402) |
| Single-pool Kt/V, mean (SD) | 1.5 (0.2) | 1.6 (0.3) | 1.7 (0.3) | 1.6 (0.3) |
| Received kidney transplant, mean (SD)a | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (5.9) |
| Sex, n (%) | ||||
| Male | 9 (75.0) | 7 (43.8) | 8 (50.0) | 8 (47.1) |
| Female | 3 (25.0) | 9 (56.3) | 8 (50.0) | 9 (52.9) |
| Race, n (%) | ||||
| White | 7 (58.3) | 10 (62.5) | 4 (25.0) | 5 (29.4) |
| Black | 5 (41.7) | 5 (31.3) | 10 (62.5) | 11 (64.7) |
| Asian | 0 (0.0) | 1 (6.3) | 2 (12.5) | 1 (5.9) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 6 (50.0) | 7 (43.8) | 3 (18.8) | 4 (23.5) |
| Not Hispanic or Latino | 6 (50.0) | 9 (56.3) | 13 (81.3) | 13 (76.5) |
| Diabetes, n (%) | 9 (75.0) | 10 (62.5) | 11 (68.8) | 9 (52.9) |
| Hypertension, n (%) | 12 (100) | 16 (100) | 16 (100) | 16 (94.1) |
| Dyslipidemia/hyperlipidemia, n (%) | 8 (66.7) | 12 (75.0) | 13 (81.3) | 10 (58.8) |
| Congestive heart failure, n (%) | 1 (8.3) | 5 (31.3) | 7 (43.8) | 10 (58.8) |
| Parenteral iron use, n (%) | 4 (33.3) | 9 (56.3) | 8 (50.0) | 8 (47.1) |
| Dialysis access during wk −2, n (%) | ||||
| AV fistula | 11 (91.7) | 10 (62.5) | 10 (62.5) | 10 (58.8) |
| AV fistula, AV graft | 1 (8.3) | 0 (0.0) | 1 (6.3) | 0 (0.0) |
| AV graft | 0 (0.0) | 6 (37.5) | 5 (31.3) | 7 (41.2) |
Kt/v, dialyzer clearance of urea multiplied by time divided by volume of distribution of urea; AV, arteriovenous.
Patient in 20-mg ziltivekimab group had transplanted kidney(s) removed (explanted).
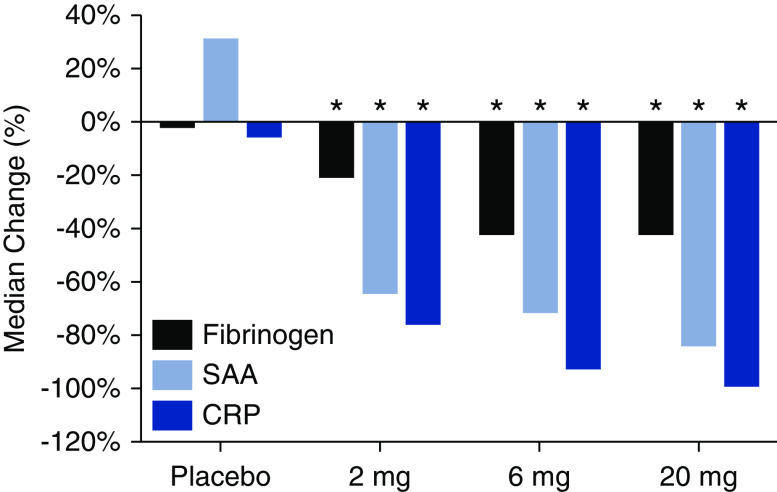
Figure 2.
Median percentage changes from baseline to end of treatment in hsCRP, SAA, and fibrinogen concentrations among patients undergoing hemodialysis receiving placebo or 2-, 6-, and 20-mg ziltivekimab (n=53). *P<0.05 versus placebo.
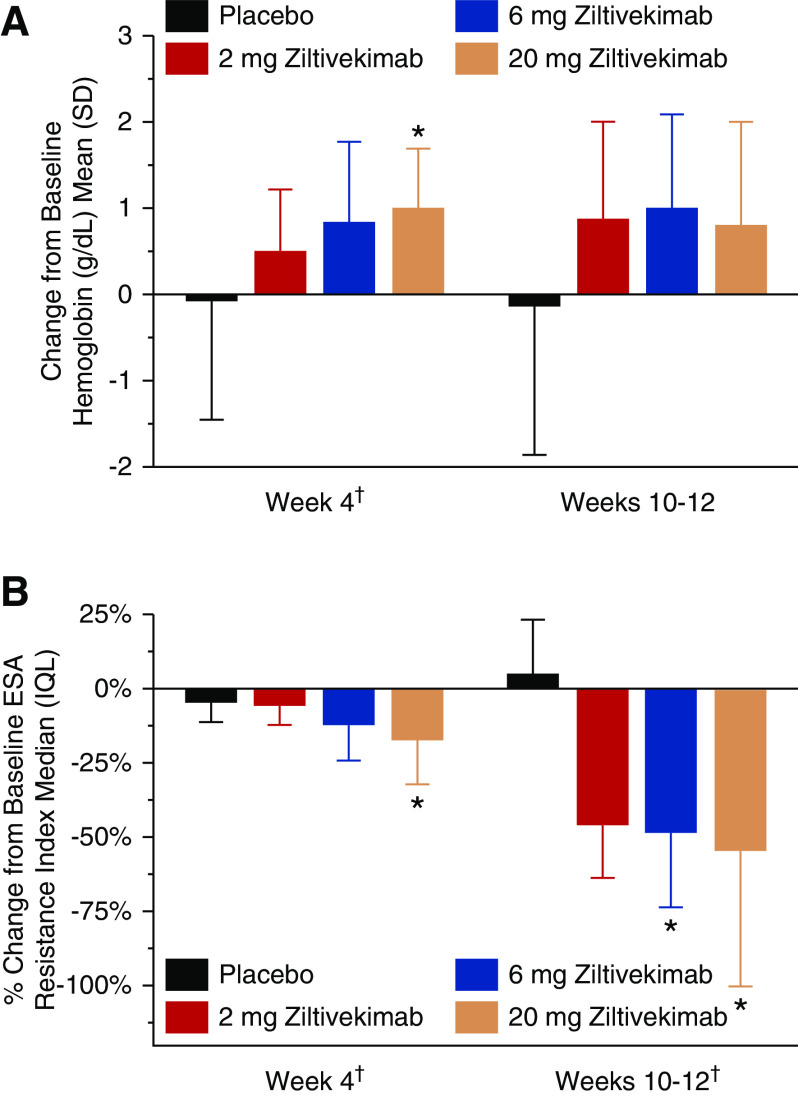
Figure 3.
Changes in hemoglobin concentrations and percentage changes in the ERI from baseline to week 4 and weeks 10–12 among patients undergoing hemodialysis receiving placebo or 2-, 6-, and 20-mg ziltivekimab (n=53). Panel A presents results for hemoglobin concentrations and Panel B presents results for the ESA Resistance Index. Data for ERI are shown as median (IQL) because values from one or more of the visits were non-normally distributed. SI conversion for hemoglobin, multiply by ten for grams per liter. IQL, interquartile limits. *P<0.05 versus placebo; †P-DR<0.05 for hemoglobin, week 4; P-DR<0.01 for ERI, for both time points.
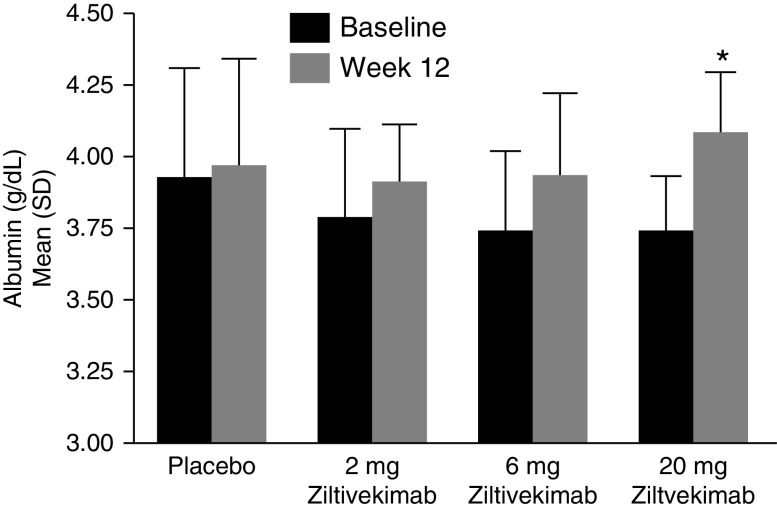
Figure 4.
Baseline and week-12 albumin concentrations among patients undergoing hemodialysis receiving placebo or 2-, 6-, and 20-mg ziltivekimab (n=53). SI conversion, multiply by ten for grams per liter. *P<0.05 for 20-mg ziltivekimab versus placebo in the change from baseline; P-DR<0.001.
Table 3.
Baseline and on-treatment levels and responses of parameters related to iron availability, iron stores, and prealbumin and albumin levels among patients on hemodialysis treated with placebo or 2-, 6-, or 20-mg ziltivekimab
| Parameter | Placebo, Mean (SD) or Median (IQL) (n=12) | Ziltivekimab, Mean (SD) or Median (IQL)a | P-DRb | |||
|---|---|---|---|---|---|---|
| 2 mg (n=16) | 6 mg (n=13) | 20 mg (n=12) | ||||
| Hemoglobin (g/dl) | ||||||
| BL | 10.5 (1.1) | 9.9 (0.6) | 9.8 (0.7) | 10.3 (0.6) | ||
| Wk 4 | 10.5 (1.0) | 10.4 (1.0) | 10.6 (1.1) | 11.3 (0.9) | ||
| ∆ from BL, wk 4 | −0.0 (1.4) | 0.5 (0.8) | 0.8 (1.0) | 1.0 (0.7)c | 0.02 | |
| Wk 10–12 | 10.4 (1.2) | 10.8 (1.1) | 10.8 (1.0) | 11.1 (1.0) | ||
| ∆ from BL, wk 10–12 | −0.1 (1.8) | 0.8 (1.2) | 1.0 (1.1) | 0.8 (1.2) | 0.39 | |
| Reticulocyte hemoglobin (pg) | ||||||
| BL | 32.2 (1.7) | 32.2 (2.0) | 31.9 (2.2) | 31.8 (2.5) | ||
| Wk 4 | 32.4 (1.4) | 33.4 (2.3) | 33.1 (2.1) | 32.3 (2.1) | ||
| ∆ from BL, wk 4 | −0.3 (1.2) | 1.2 (1.2)c | 1.2 (1.1)c | 0.3 (2.3) | 0.48 | |
| Wk 10–12 | 32.2 (1.9) | 33.1 (2.4) | 33.2 (1.5) | 33.0 (1.7) | ||
| ∆ from BL, wk 10–12 | −0.0 (1.3) | 0.9 (1.1) | 1.3 (1.3)c | 1.1 (1.6)c | 0.008 | |
| Serum iron (µg/dl) | ||||||
| BL | 66.9 (30.0) | 60.4 (17.7) | 63.0 (15.6) | 57.1 (12.9) | ||
| Wk 4 | 60.3 (20.1) | 76.1 (32.8) | 76.3 (27.0) | 104 (28.4) | ||
| ∆ from BL, wk 4 | −0.9 (15.0) | 14.8 (26.7) | 11.5 (26.8) | 46.6 (29.8)c | <0.001 | |
| Wk 12 | 64.4 (31.0) | 84.7 (34.1) | 89.9 (37.5) | 89.5 (21.1) | ||
| ∆ from BL, wk 12 | 5.0 (30.3) | 24.3 (26.9) | 26.9 (33.0) | 31.0 (16.8)c | 0.02 | |
| TIBC (µg/dl) | ||||||
| BL | 241 (32.7) | 225 (38.2) | 224 (24.3) | 228 (42.8) | ||
| Wk 4 | 237 (39.1) | 248 (44.5) | 246 (23.4) | 262 (53.2) | ||
| ∆ from BL, wk 4 | −6.4 (11.6) | 21.8 (18.1)c | 21.7 (14.2)c | 33.6 (21.4)c | <0.001 | |
| Wk 12 | 241 (38.0) | 236 (42.3) | 242 (25.2) | 266 (45.2) | ||
| ∆ from BL, wk 12 | −4.8 (15.7) | 10.6 (15.5)c | 17.3 (17.2)c | 33.2 (14.5)c | <0.001 | |
| TSAT (%) | ||||||
| BL | 25.1 (4.5) | 26.1 (5.4) | 28.5 (7.5) | 25.6 (6.2) | ||
| Wk 4 | 25.4 (6.8) | 31.9 (15.8) | 31.2 (10.4) | 41.3 (14.7) | ||
| ∆ from BL, wk 4 | 0.5 (6.7) | 5.6 (12.9) | 1.8 (11.2) | 15.8 (12.4)c | 0.02 | |
| Wk 10–12 | 27.3 (8.5) | 32.5 (10.0) | 37.7 (10.5) | 34.6 (8.6) | ||
| ∆ from BL, wk 10–12 | 2.3 (8.1) | 6.4 (7.2) | 9.2 (8.6)c | 9.0 (6.1)c | 0.005 | |
| ERI (U/kg per g/dl hemoglobin) | ||||||
| BL | 18.2 (10.1–25.3) | 13.4 (11.7–22.0) | 13.5 (8.8–24.7) | 11.9 (9.2–23.5) | ||
| Wk 4 | 17.3 (10.8–21.5) | 12.5 (10.6–19.9) | 12.9 (8.1–18.1) | 8.6 (6.8–11.1) | ||
| ∆ from BL, wk 4 | −0.5 (−2.8 to 0.5) | −0.7 (−1.8 to 0.2) | −1.1 (−5.5 to −0.6)c | −2.4 (−5.5 to −0.6)c | 0.04 | |
| Wk 10–12 | 10.6 (8.2–23.5) | 9.4 (5.6–13.3) | 6.9 (3.8–10.0) | 6.7 (0.0–12.7) | ||
| ∆ from BL, wk 10–12 | 0.4 (−3.5 to 4.9) | −5.6 (−10.6 to −0.3) | −5.8 (−10.0 to −3.5)c | −10.5 (−13.1 to −3.6)c | 0.01 | |
| ESA dose ×103 (U) | ||||||
| BL | 60.0 (39.5–76.0) | 54.0 (33.0–60.0) | 48.0 (36.0–75.0) | 45.0 (33.0–90.0) | ||
| Wk 9–12 | 49.5 (30.0–68.5) | 33.0 (17.3–50.5) | 24.0 (12.0–42.0) | 30.0 (0.0–45.0) | ||
| ∆ from BL, wk 9–12 | 0.0 (−13.5 to 5.5) | −15.0 (−30.0 to 0.0) | −15.0 (−48.0 to −4.0) | −33.0 (−45.0 to −3.8)c | 0.02 | |
| Ferritin (ng/ml) | ||||||
| BL | 830 (510–1323) | 931 (632–1163) | 952 (593–1254) | 903 (801–1193) | ||
| Wk 12 | 896 (471–1205) | 939 (788–1230) | 776 (656–1030) | 838 (689–1095) | ||
| ∆ from BL, wk 12 | −56.7 (−218 to 124) | 96.7 (−108 to 245) | −29.7 (−218 to 86.7) | −7.3 (−167 to 154) | 0.88 | |
| Hepcidin (ng/ml) | ||||||
| BL | 295 (256–333) | 328 (215–489) | 232 (183–352) | 277 (154–353) | ||
| Wk 4 | 239 (195–345) | 200 (161–330) | 149 (120–178) | 155 (87.4–214) | ||
| ∆ from BL, wk 4 | −19.5 (−90.3 to 5.7) | −42.7 (−129 to −17.8) | −66.5 (−119 to −54.2) | −88.8 (−193 to −21.0) | 0.08 | |
| Wk 12 | 298 (161–416) | 340 (244–484) | 219 (111–306) | 181 (160–231) | ||
| ∆ from BL, wk 12 | −18.5 (−132 to 47.7) | −20.8 (−98.8 to 40.6) | −3.0 (−133 to 75.9) | −58.8 (−129 to 82.6) | 0.71 | |
| Prealbumin (mg/dl) | ||||||
| BL | 37.2 (24.5–39.8) | 29.8 (24.9–36.7) | 28.8 (27.1–32.3) | 29.8 (18.8–34.1) | ||
| Wk 10–12 | 39.2 (25.0–40.2) | 35.8 (30.9–44.1) | 39.6 (32.0–45.4) | 39.7 (28.0–48.8) | ||
| ∆ from BL, wk 10–12 | 1.4 (−3.3 to 3.3) | 7.0 (3.7–11.1)c | 7.8 (6.6–11.7)c | 12.1 (8.3–15.2)c | <0.001 | |
| Albumin (g/dl) | ||||||
| BL | 3.9 (0.4) | 3.8 (0.3) | 3.7 (0.3) | 3.7 (0.2) | ||
| Wk 12 | 4.0 (0.4) | 3.9 (0.2) | 3.9 (0.3) | 4.1 (0.2) | ||
| ∆ from BL, wk 12 | 0.0 (0.3) | 0.1 (0.2) | 0.2 (0.2) | 0.3 (0.2)c | <0.001 | |
SI conversions: hemoglobin, multiply by ten for g/L; ferritin, multiply by 2.247 for pmol/L; albumin, multiply by ten for g/L. IQL, interquartile limit; BL, baseline; ∆, change; TIBC, total iron binding capacity.
ERI, ESA dose, ferritin, hepcidin, and prealbumin were non-normally distributed (Shapiro–Wilk P value was <0.01), so analyses were performed on the ranked values and median (IQL) values are shown. Hemoglobin, reticulocyte hemoglobin, TSAT, and albumin were normally distributed and results are reported as mean (SD).
No adjustments were made for multiple comparison groups or time points.
P<0.05 versus placebo.
Maximum Tolerable Dose and Other Safety Parameters
No patient experienced dose-limiting toxicity; therefore, a maximum tolerable dose was not established. Overall, there was a total of 233 treatment-emergent AEs reported by 46 of the 61 patients. A summary of AEs is presented in Table 4. A total of 20 patients experienced one or more serious AEs during the study: three patients (25.0%) in the placebo group, four patients (25.0%) in the 2-mg cohort, seven patients (43.8%) in the 6-mg cohort, and six patients (35.3%) in the 20-mg cohort. None of the AEs were judged by the investigator to be related to the study drug. Four patients died due to a treatment-emergent AE during the study: two patients (12.5%) in the 6-mg cohort, both with sepsis and sudden cardiac death; and two patients (11.8%) in the 20-mg cohort, one due to sepsis and one due to cardiac arrest. Two of the deaths (n=1 cardiac arrest and n=1 sudden cardiac death) occurred during the safety follow-up period after the 12-week treatment period. None of the deaths were considered by the investigator to be related to the study drug.
Table 4.
Summary of safety among patients on hemodialysis taking placebo or 2-, 6-, or 20-mg ziltivekimab
| AE Term | Placebo, n (%) (n=12) | Ziltivekimab, n (%) | ||
|---|---|---|---|---|
| 2 mg (n=16) | 6 mg (n=16) | 20 mg (n=17) | ||
| Serious AEs leading to death | 0 (0.0) | 0 (0.0) | 2 (12.5) | 2 (11.8) |
| Any serious AE | 3 (25.0) | 4 (25.0) | 7 (43.8) | 6 (35.3) |
| AE leading to discontinuation of treatment regimen | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (17.6) |
| Patients with one or more treatment-emergent AEs | 7 (58.3) | 12 (75.0) | 15 (93.8) | 12 (70.6) |
| AEs occurring in 10% of patients in any treatment cohort | ||||
| Cardiac disorders | 0 (0.0) | 1 (6.3) | 6 (37.5) | 4 (23.5) |
| Cardiac failure, congestive | 0 (0.0) | 0 (0.0) | 4 (25.0) | 0 (0.0) |
| Tachycardia | 0 (0.0) | 1 (6.3) | 1 (6.3) | 2 (11.8) |
| Endocrine disorders | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyperparathyroidism | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 3 (25.0) | 5 (31.3) | 3 (18.8) | 3 (17.6) |
| Hematochezia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.8) |
| Vomiting | 1 (8.3) | 3 (18.8) | 1 (6.3) | 0 (0.0) |
| Infections and infestations | 4 (33.3) | 6 (37.5) | 4 (25.0) | 6 (35.3) |
| Osteomyelitis acute | 2 (16.7) | 1 (6.3) | 0 (0.0) | 0 (0.0) |
| Injury/poisoning/procedural complications | 1 (8.3) | 3 (18.8) | 2 (12.5) | 6 (35.3) |
| Fall | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.8) |
| Metabolism and nutrition disorders | 1 (8.3) | 4 (25.0) | 7 (43.8) | 3 (17.6) |
| Diabetes mellitus, inadequate control | 0 (0.0) | 1 (6.3) | 2 (12.5) | 0 (0.0) |
| Fluid overload | 0 (0.0) | 0 (0.0) | 2 (12.5) | 0 (0.0) |
| Hyperkalemia | 0 (0.0) | 2 (12.5) | 2 (12.5) | 0 (0.0) |
| Hypoglycemia | 0 (0.0) | 2 (12.5) | 3 (18.8) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 2 (12.5) | 1 (5.9) |
| Musculoskeletal/connective tissue disorders | 2 (16.7) | 2 (12.5) | 3 (18.8) | 4 (23.5) |
| Arthralgia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.8) |
| Neck pain | 0 (0.0) | 0 (0.0) | 1 (6.3) | 2 (11.8) |
| Pain in extremity | 2 (16.7) | 1 (6.3) | 1 (6.3) | 1 (5.9) |
| Psychiatric disorders | 0 (0.0) | 1 (6.3) | 2 (12.5) | 1 (5.9) |
| Mental status changes | 0 (0.0) | 1 (6.3) | 2 (12.5) | 0 (0.0) |
| Respiratory/thoracic/mediastinal disorders | 2 (16.7) | 4 (25.0) | 6 (37.5) | 4 (23.5) |
| Dyspnea | 1 (8.3) | 0 (0.0) | 4 (25.0) | 1 (5.9) |
| Vascular disorders | 2 (16.7) | 3 (18.8) | 3 (18.8) | 3 (17.6) |
| Hypertension | 1 (8.3) | 0 (0.0) | 2 (12.5) | 1 (5.9) |
| Hypertensive emergency | 2 (16.7) | 0 (0.0) | 1 (6.3) | 0 (0.0) |
| Hypotension | 0 (0.0) | 1 (6.3) | 0 (0.0) | 2 (11.8) |
An analysis of platelet and neutrophil counts showed that grade-2 thrombocytopenia (50,000–75,000/mm3) was experienced by one (8.3%), zero (0.0%), two (12.5%), and one (5.9%) of the patients in the placebo and 2-, 6-, and 20-mg ziltivekimab groups, respectively. Grade-2 neutropenia (1000–1500/mm3) was experienced by two (16.7%), three (18.8%), one (6.3%), and zero (0.0%) of the patients in the placebo and 2-, 6-, and 20-mg ziltivekimab groups, respectively; grade-3 neutropenia (500–1000/mm3) was experienced by zero (0.0%), one (0.0%), one (6.3%), and three (17.6%) of the patients in the placebo and 2-, 6-, and 20-mg ziltivekimab groups, respectively. Reductions in platelets were not associated with AEs of bleeding; instances of grade-2 or -3 neutropenia were not associated with AEs of infection. None of the patients in the study who experienced neutropenia (at least grade 1 or higher) experienced a serious AE of sepsis or death. Of the 20 patients who experienced neutropenia, two patients also had AEs of infections during the trial. In both instances, the neutropenia did not occur at the time of the event. One instance was 3 weeks before the onset of the AE (gastroenteritis), and the other instance occurred 1 month before the onset of the AE (fungal skin infection).
Inflammatory and Cardiovascular Biomarkers
Ziltivekimab administration resulted in significant decreases in hsCRP, SAA, and fibrinogen concentrations (Figure 2, Table 2). Ziltivekimab treatment led to median decreases in hsCRP of 3.5, 12.1, and 13 mg/L (P-DR<0.001) in the 2-, 6-, and 20-mg cohorts, compared with a median decrease of 0.2 mg/L in the placebo group by week 4. Similarly, large decreases were observed in the median percentage change from baseline to the end of treatment for hsCRP (−74.8%, −91.7%, and −98.1% for 2-, 6-, and 20-mg doses, respectively, versus −4.5% for placebo), demonstrating a positive dose response across the dose groups of ziltivekimab (P-DR<0.001) (Figure 2). A median decrease in SAA was observed at week 4 and end of treatment (P-DR<0.005). Ziltivekimab decreased SAA by medians of 6.1, 7.2, and 17.0 mg/L (−63.3%, −70.4%, and −83.1%) at the end of treatment in the 2-, 6-, and 20-mg cohorts, respectively, whereas there was no change in the placebo group (Figure 2, Table 2). Mean decreases of 87.3, 161, and 216 mg/dl (−19.8%, −41.3%, and −41.1%) in fibrinogen were noted at the end of treatment in the three ziltivekimab-treated arms, respectively, whereas there was a mean increase of 4.6 mg/dl in the placebo arm (Figure 2, Table 2). There was a positive dose response (P-DR<0.001) for fibrinogen at the end of treatment across the ziltivekimab-treated groups. A slight elevation of mean LDL cholesterol was noted at the end of treatment in the 20-mg cohort, whereas no elevation was observed in the placebo or the 2-mg or 6-mg cohorts (Table 2). Mean HDL cholesterol increased at the end of treatment with a positive dose response (P-DR<0.05) for ziltivekimab (Table 2). Ziltivekimab treatment resulted in median decreases at the end of treatment in lipoprotein(a) of 3, 16, and 13 nmol/L in the 2-, 6-, and 20-mg cohorts, respectively, exhibiting a positive dose response (P-DR=0.02) (Table 2).
Table 2.
Baseline and on-treatment levels and responses of inflammatory, cardiac, and cardiovascular biomarkers among patients on hemodialysis treated with placebo or 2-, 6-, or 20-mg ziltivekimab
| Parameter | Placebo, Mean (SD) or Median (IQL) (n=12) | Ziltivekimab, Mean (SD) or Median (IQL)a | P-DRb | ||
|---|---|---|---|---|---|
| 2 mg (n=16) | 6 mg (n=13) | 20 mg (n=12) | |||
| hsCRP (mg/L) | |||||
| BL | 4.0 (1.5–8.1) | 6.1 (3.8–25.0) | 9.8 (3.8–19.6) | 13.2 (4.7–21.5) | |
| Wk 4 | 4.5 (1.3–13.5) | 2.7 (0.8–4.5) | 0.5 (0.3–3.3) | 0.6 (0.2–1.0) | |
| ∆ from BL, wk 4 | −0.2 (−0.9 to 1.3) | −3.5 (−18.5 to −2.4)c | −12.1 (−18.8 to 3.5)c | −13.0 (−20.6 to −4.3)c | <0.001 |
| Wk 10–12 | 3.0 (1.8–6.3) | 3.5 (0.8–6.9) | 0.8 (0.5–2.2) | 0.3 (0.2–0.4) | |
| ∆ from BL, wk 10–12 | −0.6 (−2.0 to 1.0) | −3.9 (−16.7 to −1.8)c | −10.6 (−15.8 to −4.7)c | −10.5 (−20.8 to −4.3)c | <0.001 |
| SAA (mg/L) | |||||
| BL | 6.5 (2.8–21.1) | 8.2 (5.0–39.3) | 10.4 (6.9–15.2) | 19.1 (9.3–42.6) | |
| Wk 4 | 6.0 (2.3–13.8) | 3.3 (1.8–9.3) | 2.8 (1.5–5.4) | 2.7 (2.1–6.9) | |
| ∆ from BL, wk 4 | −1.5 (−2.6 to 0.3) | −6.2 (−23.9 to −2.1)c | −9.0 (−13.3 to −4.2)c | −17.0 (−41.6 to −6.1)c | 0.004 |
| Wk 10–12 | 5.1 (3.2–12.4) | 6.5 (1.8–11.9) | 2.5 (1.4–3.7) | 1.9 (1.7–4.2) | |
| ∆ from BL, wk 10–12 | 0.0 (−10.1 to 0.7) | −6.1 (−25.3 to −1.6)c | −7.2 (−11.6 to −4.3)c | −17.0 (−32.8 to −7.4)c | 0.002 |
| Fibrinogen (mg/dl) | |||||
| BL | 447 (101) | 498 (100) | 484 (85.1) | 486 (96.5) | |
| Wk 12 | 453 (133) | 410 (92.4) | 323 (68.8) | 266 (64.4) | |
| ∆ from BL, wk 12 | 4.6 (53.3) | −87.3 (67.1)c | −161 (90.1)c | −216 (83.7)c | <0.001 |
| LDL cholesterol (mg/dl) | |||||
| BL | 72.8 (41.1) | 69.6 (36.4) | 64.4 (34.2) | 67.1 (38.6) | |
| Wk 12 | 67.2 (29.6) | 61.4 (31.4) | 63.8 (32.7) | 68.1 (39.6) | |
| ∆ from BL, wk 12 | −5.6 (22.8) | −6.5 (15.0) | −1.2 (12.5) | 4.7 (14.8) | 0.24 |
| HDL cholesterol (mg/dl) | |||||
| BL | 51.3 (18.1) | 46.9 (17.1) | 46.4 (14.5) | 45.9 (13.5) | |
| Wk 12 | 51.6 (16.5) | 50.1 (18.1) | 54.2 (17.2) | 52.5 (15.1) | |
| ∆ from BL, wk 12 | 0.3 (4.0) | 3.2 (7.2) | 7.8 (6.1)c | 6.1 (6.9)c | 0.002 |
| Lipoprotein(a) (nmol/L) | |||||
| BL | 21.5 (13.5–41.0) | 63.5 (36.5–148) | 60.0 (40.0–118) | 57.0 (27.0–109) | |
| Wk 12 | 22.0 (13.5–46.0) | 52.0 (37.5–138) | 57.0 (30.0–121) | 29.0 (21.0–97.0) | |
| ∆ from BL, wk 12 | −0.5 (−5.5 to 3.5) | −3.0 (−13.0 to 1.0) | −16.0 (−26.0 to −3.0) | −13.0 (−35.0 to 3.0) | 0.02 |
SI conversions: fibrinogen, multiply by 0.0294 for µmol/L; LDL and HDL cholesterol, multiply by 0.0259 for mmol/L; lipoprotein (a), multiply by 0.0357 for µmol/L. IQL, interquartile limit; BL, baseline; ∆, change.
hsCRP, SAA, and lipoprotein(a) were non-normally distributed (Shapiro–Wilk P value was <0.01), so analyses were performed on the ranked values and median (IQL) values are shown. Fibrinogen and LDL and HDL cholesterol were normally distributed and results are reported as mean (SD).
No adjustments were made for multiple comparison groups or time points. For the key pharmacodynamic end point (hsCRP at wk 4), a nominal P value <0.017 should be considered statistically significant.
P<0.05 versus placebo.
Markers of Iron Availability/Iron Stores, and Serum Prealbumin and Albumin Levels
In the first 4 weeks of treatment, during which ESA and iron doses were kept constant, ziltivekimab increased mean hemoglobin concentrations by 0.5, 0.8, and 1.0 g/dl in the 2-, 6-, and 20-mg cohorts, respectively, compared with no change in the placebo group. There was a positive dose response (P-DR<0.05) at week 4 across ziltivekimab doses, and hemoglobin remained elevated through the end of treatment (Figure 3A, Table 3). Similarly, mean reticulocyte hemoglobin increased by 0.9, 1.3, and 1.1 pg in the 2-, 6-, and 20-mg ziltivekimab cohorts at the end of treatment, with a positive dose response (P-DR<0.05) compared with no change in the placebo group. There were also dose-related increases in serum iron and total iron-binding capacity at both week 4 and at the end of treatment (P-DR<0.05) (Table 3). Mean percentage of transferrin saturation also increased at the end of treatment by 2.3%, 6.4%, 9.2%, and 9.0% in the placebo, 2-mg, 6-mg, and 20-mg ziltivekimab cohorts, respectively. At week 4 and the end of treatment, there was a positive dose response (P-DR<0.05) for change from baseline in TSAT (Table 3). There was a dose-related decrease in median hepcidin at week 4; hepcidin decreased by 42.7, 66.5, and 88.8 ng/ml in the 2-, 6-, and 20-mg ziltivekimab-treated groups, compared with a decrease of 19.7 ng/ml in the placebo group. However, there were no significant differences at the end of treatment in median hepcidin. No changes in ferritin were observed with ziltivekimab at any dose versus placebo at the end of treatment (Table 3).
A dose response (P-DR=0.02) toward a decrease from baseline in median ESA equivalent dose with increasing ziltivekimab dose was observed at the end of treatment (Table 3). The median ESA usage decreased by 15,000, 15,000, and 33,000 IU/wk per patient in the patients treated with 2-, 6-, and 20-mg ziltivekimab, respectively, compared with no change in ESA utilization in the placebo group. At least half of the patients in the 2-mg (eight of 16 patients; 50%), 6-mg (seven of 13 patients; 54%), and 20-mg (seven of 12 patients; 58%) ziltivekimab groups had a 50% reduction from baseline to the end of treatment in their ESA dose, compared with two of 12 patients (17%) in the placebo group. Consistent with this, there was a >50% change in median ERI in the 6-mg and 20-mg ziltivekimab-treated arms during this time frame. The median ERI decreased by 5.6, 5.8, and 10.5 U in the 2-, 6-, and 20-mg ziltivekimab-treated cohorts, respectively, at the end of treatment, compared with an increase of 0.4 U in the placebo group. A positive dose response (P-DR<0.05) for a decrease was observed for ERI (Table 3) or percent change in ERI (Figure 3B) at week 4 and the end of treatment across ziltivekimab groups. In the 20-mg ziltivekimab group, 33% of the patients stopped using ESAs and were EPO independent at the end of the treatment period. Ziltivekimab improved both serum prealbumin and albumin levels in a dose-dependent manner by the end of treatment (P-DR<0.001 for each) (Figure 4, Table 3).
Immunogenicity
Anti-drug antibodies were detected for one or more time point in two (16.7%) patients on placebo, two (12.5%) patients on 2-mg ziltivekimab, one (6.3%) patient on 6-mg ziltivekimab, and two (12.5%) patients on 20-mg ziltivekimab. There was no apparent effect of anti-drug antibodies on hsCRP, hemoglobin, platelets, neutrophils, or serum albumin.
Discussion
This study with ziltivekimab, a potent human anti–IL-6 antibody, was the first placebo-controlled trial of an anti-inflammatory agent conducted in patients on hemodialysis with chronic inflammation. No patient experienced dose-limiting toxicity, the primary outcome of this trial, with ziltivekimab at 2-, 6-, or 20-mg doses. In this study of patients on hemodialysis with increased inflammation, and who were genetically predisposed to the negative effects of IL-6 on erythropoiesis and hyporesponsive to ESAs, administration of ziltivekimab for 12 weeks was associated with decreased ESA usage and ERI; improved inflammatory burden, as measured by decreased hsCRP and SAA; and increased serum albumin. In the high-dose group of 20-mg ziltivekimab, ESAs were stopped in 33% of the patients after 12 weeks of therapy, and 58% of patients had at least a 50% reduction. It is well established that patients on hemodialysis with inflammation require higher ESA dosages to maintain hemoglobin levels on target.3–5 The Correction of Hemoglobin and Outcomes in Renal Insufficiency trial7 and the Trial to Reduce Cardiovascular Events with Aranesp Therapy8 highlight the cardiovascular risks associated with using higher doses of ESA to correct the anemia of CKD. Therefore, reducing ESA usage to decrease the potential cardiovascular risk associated with its use, in particular at high doses, is a clinical goal of nephrologists. Ziltivekimab may provide an opportunity to reduce ESA usage, which could lead to a reduction in the overall cardiovascular risk that is associated with the use of high doses of ESA in patients who are hyporesponsive.
Inflammation-driven ESA hyporesponsiveness in patients on hemodialysis is largely mediated by increased hepcidin,22,23 resulting from direct stimulation by the proinflammatory cytokine IL-6.24,25 Previously, it has been shown that patients with chronic inflammation, characterized by elevated IL-6, have elevated hepcidin.9 In this study, ziltivekimab administration resulted in dose-dependent decreases in hepcidin at week 4, during which time ESA and iron usage was kept stable. Consistent with decreased hepcidin, patients treated with ziltivekimab exhibited increased serum iron, total iron binding capacity, and TSAT levels, presumably due to the release of iron from cellular sources and potentially increased intestinal absorption. These data support that ziltivekimab reduced IL-6–induced iron sequestration by decreasing hepcidin levels, which led to increased serum iron, TSAT, and hemoglobin, and, thus, improved ERI. Hepcidin levels are difficult to interpret after week 4, when ESA and iron doses were allowed to be changed, because both ESA and iron can influence hepcidin expression through the BMPR. Because serum ferritin is suggested to be a marker of inflammation, levels would have been expected to decrease with ziltivekimab therapy. The lack of changes in ferritin could be due to continuation of iron therapies, but other mechanisms for the lack of observed effect on serum ferritin will need to be explored because serum ferritin levels may also be related to cellular iron mobilization and turnover of red blood cells.
Four patients died during the study; two of these deaths occurred during the safety follow-up period after treatment had been discontinued. Although it is notable that these deaths occurred at the higher ziltivekimab doses (6 and 20 mg), the study was not powered to detect differences in mortality between active treatment and placebo. A major cause of morbidity and mortality for patients on hemodialysis is infections. As with any anti-inflammatory intervention, ziltivekimab has the potential to be immunosuppressive, even at the low doses administered in our study. Ziltivekimab treatment resulted in dose-dependent, but relatively low rates of, neutropenia and thrombocytopenia, compared with the high doses of anti–IL-6 receptor mAbs used for rheumatoid arthritis and other systemic inflammatory conditions. The ziltivekimab-induced decreases in neutrophil count generally resolved by 14 weeks after drug cessation. The annual rates of mortality, all-cause hospitalizations, hospitalizations for sepsis, infection, and cardiovascular events in this study were all below the annual rates for the general US dialysis population published by the US Renal Data System.26 Much larger trials in patients on hemodialysis will be required to evaluate the potential increased risk of infection compared with the benefits of mitigating the inflammatory sequelae with ziltivekimab therapy.
The generalizability of these results is limited by the selection of patients having genotypic variations in TMPRSS6 (prevalence of approximately 80% in the general population) that was hypothesized to heighten susceptibility to IL-6–induced inflammation. This was the first clinical trial conducted in patients with the rs855791 genotype. Additional studies are needed to investigate whether this marker, high levels of IL-6, or high ESA requirements will define patients who respond better to ziltivekimab. Another limitation is that the study did not evaluate other factors associated with ESA hyporesponsiveness, such as hyperparathyroidism or vitamin-D deficiency.27,28
Current anemia treatments do not reduce inflammation; advancement of an anti-inflammatory therapy that improves iron utilization and reduces the need for escalating doses of ESAs would be a paradigm shift in the care of patients on hemodialysis. Several compounds are under development for the treatment of anemia of inflammation; most are in preclinical or phase 1 stages.1,29 Hypoxia-inducible factor prolyl hydroxylase inhibitors, which are being investigated for the treatment of anemia in CKD, have been shown to correct anemia despite levels of inflammatory markers, but, to date, there is no evidence that they decrease inflammation. These results support that ziltivekimab could lead to a reduction in overall cardiovascular risk in patients with increased inflammation on hemodialysis by reducing inflammation; decreasing ESA usage; improving serum albumin; and decreasing SAA, fibrinogen, and hsCRP.
Disclosures
M. Chonchol reports receiving grants and personal fees from Corvidia Therapeutics, Inc., and from Kadmon, Otsuka, Sanofi, and National Institute of Diabetes and Digestive and Kidney Diseases outside the submitted work. M. Davidson, M. Devalaraja, K. Herzog, and L. Lo are employees of Corvidia Therapeutics, Inc. M. Davidson has the patent “Methods for Treating IL-6 Mediated Inflammation without Immunosuppression” pending. M. Devalaraja has the patent “Methods for Treating IL-6 Mediated Inflammation without Immunosuppression” pending. R. Kakkar is currently employed by Pandion Therapeutics and has ownership interest in Corvidia Therapeutics, Inc. and Pandion Therapeutics. L. Lo is an employee of, and has ownership interest in, Corvidia Therapeutics, Inc. V. Mathur was a consultant to Corvidia Therapeutics, Inc.; and reports receiving personal fees from Equillium, Intarcia, Myovant, PTC Bio, Rigel, Sanifit, Trevi Therapeutics, and Tricida, outside the submitted work. P. Pergola received research support from Corvidia Therapeutics, Inc. and was a study investigator. M. Smith has consultancy agreements with Bayer Aktiengesellschaft; has received research funding from Corvidia Therapeutics, Inc.; and is currently employed by Nephrology Associates, P.C. and Southeastern Clinical Research Institute LLC. S. Fishbane reports receiving Cara Therapeutics grants and others during the conduct of the study.
Funding
This study was funded by Corvidia Therapeutics, Inc.
Data Sharing Statement
Data will be shared according to Novo Nordisk’s data sharing policy, as described at https://www.novonordisk-trials.com/ed/sharing-results.
Supplementary Material
Acknowledgments
The authors thank Debbie Dillon, consultant to Corvidia Therapeutics, Inc. for clinical operational assistance, and Douglas Kling of Corvidia Therapeutics, Inc. and Dr. Mary R. Dicklin of Midwest Biomedical Research for manuscript preparation including editorial and clerical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Will Targeting Interleukin-6 in the Anemia of CKD Change Our Treatment Paradigm?” on pages 6–8.
Supplemental Material
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050595/-/DCSupplemental.
References
- 1.Ganz T: Anemia of inflammation. N Engl J Med 381: 1148–1157, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Revised European Best Practices Guidelines for the Management of Anaemia in Patients with Chronic Renal Failure: Section IV. Failure to respond to treatment. Nephrol Dial Transplant 19[Suppl 2]: ii32–ii36, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Smrzova J, Balla J, Barany P: Inflammation and resistance to erythropoiesis-stimulating agents—What do we know and what needs to be clarified? Nephrol Dial Transplant 20[Suppl 8]: viii2–viii7, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Priyadarshi A, Shapiro JI: Erythropoietin resistance in the treatment of the anemia of chronic renal failure. Semin Dial 19: 273–278, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Panichi V, Rosati A, Bigazzi R, Paoletti S, Mantuano E, Beati S, et al.; RISCAVID Study Group: Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: Results from the RISCAVID study. Nephrol Dial Transplant 26: 2641–2648, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Kuragano T, Kitamura K, Matsumura O, Matsuda A, Hara T, Kiyomoto H, et al.: ESA hyporesponsiveness is associated with adverse events in maintenance hemodialysis (MHD) patients, but not with iron storage. PLoS One 11: e0147328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al.; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al.; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ganz T, Nemeth E: Iron sequestration and anemia of inflammation. Semin Hematol 46: 387–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrighting DM, Andrews NC: Interleukin-6 induces hepcidin expression through STAT3. Blood 108: 3204–3209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, et al.: Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet 41: 1170–1172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nai A, Pagani A, Silvestri L, Campostrini N, Corbella M, Girelli D, et al.: TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood 118: 4459–4462, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Kakkar R, Lo L, Kling D, Devalaraja M, Davidson MH: Effects of ziltivekimab (ZILTI), a novel anti-interleukin-6 monoclonal antibody, on markers of inflammation and cardiovascular risk in patients with chronic kidney disease on hemodialysis. Presented at American Heart Association Scientific Sessions, Philadelphia, PA, November 16–18, 2019
- 14.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Rao M, Guo D, Perianayagam MC, Tighiouart H, Jaber BL, Pereira BJ, et al.: Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 45: 324–333, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, et al.; European Uremic Toxin Work Group (EUTox): Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 77: 550–556, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Fishbane S, Kowalski EA, Imbriano LJ, Maesaka JK: The evaluation of iron status in hemodialysis patients. J Am Soc Nephrol 7: 2654–2657, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, et al.; DRIVE Study Group: Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sieper J, Braun J, Kay J, Badalamenti S, Radin AR, Jiao L, et al.: Sarilumab for the treatment of ankylosing spondylitis: Results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann Rheum Dis 74: 1051–1057, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpstra TJ: The asymptotic normality and consistency of Kendall’s test against trend, when ties are present in ranking. Indag Math 14: 327–333, 1952 [Google Scholar]
- 21.Jonckheere AR: A distribution-free k-sample test against ordered alternatives. Biometrika 41: 133–145, 1954 [Google Scholar]
- 22.Eleftheriadis T, Kartsios C, Liakopoulos V, Antoniadi G, Ditsa M, Papadopoulos C, et al.: Does hepcidin affect erythropoiesis in hemodialysis patients? Acta Haematol 116: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Eleftheriadis T, Liakopoulos V, Antoniadi G, Kartsios C, Stefanidis I: The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial 22: 70–77, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD: Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis 42: 761–773, 2003 [DOI] [PubMed] [Google Scholar]
- 25.van der Putten K, Braam B, Jie KE, Gaillard CA: Mechanisms of disease: Erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol 4: 47–57, 2008 [DOI] [PubMed] [Google Scholar]
- 26.United States Renal Data System 2011. Atlas of chronic kidney disease and end stage renal disease. Available at https://www.usrds.org/annual-data-report/previous-adrs/. Accessed October 29, 2019
- 27.Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M: Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: The potential role of inflammation. Nephrol Dial Transplant 28: 1672–1679, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Amnuay K, Srisawat N, Wudhikarn K, Assanasen T, Polprasert C: Factors associated with erythropoiesis-stimulating agent hyporesponsiveness anemia in chronic kidney disease patients. Hematol Rep 11: 8183, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begum S, Latunde-Dada GO: Anemia of inflammation with an emphasis on chronic kidney disease. Nutrients 11: 2424, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.