Abstract
Mesenchymal stem cells derived from bone marrows (BMSCs) and curcumin derived from turmeric were used for osteoarthritis (OA) treatment, respectively. We invested the effects of curcumin supplementation for BMSC therapeutic effects. In vitro, rat BMSCs were identified by dual-immunofluorescent staining of CD44 and CD90, and flow cytometry. Primary articular chondrocytes were identified by toluidine blue staining and immunofluorescent staining of Col2a1. EdU incorporation, migration assay, real-time quantitative polymerase chain reaction, and Western blot analyses were performed to evaluate the alterations of chondrocytes cocultured with BMSCs. In vivo, the rat model of OA was established by monoiodoacetic acid. After intra-articular injection of allogeneic BMSCs, articular cartilage damage and OA progression were evaluated by histological staining, and Osteoarthritis Research Society International and Mankin score evaluation. Although curcumin alone did not improve cell viability of primary articular chondrocytes, it promoted proliferation and migration of chondrocytes when cocultured with BMSCs. Meanwhile, the expression of anabolic genes in chondrocytes was remarkably increased both at mRNA and protein levels. In OA rats, curcumin and BMSCs cooperated to greatly promote articular cartilage repair and retard OA progression. Therefore, curcumin supplementation enhanced the BMSC function for the proliferation and migration of articular chondrocytes, and anabolic gene expression of extracellular matrix in articular chondrocytes in vitro, and the generation of articular cartilage in vivo. Our study shed light on the potential clinical application of curcumin cooperated with BMSCs in cartilage repair for OA treatment.
Keywords: curcumin, BMSCs, OA, chondrocytes, cartilage
Introduction
Osteoarthritis (OA) is a common and unhealthy condition in older adults affecting populations of 250 million worldwide, causing serious economic burden on society and families1–3. This syndrome is becoming more prevalent as the obese population and number of joint injuries increase in the elderly.
The complicated pathogenesis of OA is mainly characterized by articular cartilage degeneration, synovial inflammation, subchondral bone deterioration, osteophyte formation, ligaments degeneration, and hypertrophy of the joint capsule4. Articular cartilage degeneration and the synovitis are the main symptoms at early stages of OA5. Chondrocytes are the unique cell type in articular cartilage tissue implicated in the synthesis and maintenance of the extracellular matrix (ECM), which is responsible for the supply of chondrocyte nutrients and the disposal of metabolic waste, mainly contained type II collagen (Col2) and Aggrecan6–8. The decrease of chondrocyte in elderly people causes the alteration in ECM composition and is contributing to the impaired capability in cartilage repair, therefore resulting in the degeneration of articular cartilage9. In clinic, conventional treatment for early-stage OA mainly included nonsteroidal anti-inflammatory drugs (NSAIDs), hyaluronic acid, and corticosteroid injections. Nevertheless, these therapies did not reverse the OA process, but just alleviated OA symptoms temporarily. The alternative joint replacement surgery was effective for severe OA, however, limited to its complex revision requirement and life span. In addition, the surgery therapy is not suitable for young patients10.
Mesenchymal stem cells (MSCs) are a type of precursor cells with a wide range of sources and can be isolated from bone marrow tissue, adipose tissue, synovium, skeletal muscle, cord blood, and other tissues11. Compared with primary cultured chondrocytes, MSC is easier to culture in vitro, and sufficient cells can be obtained within shorter time. In recent years, MSCs derived from bone marrows (BMSCs) were applied to OA treatment both in animal models and clinical practice by promoting cartilage repair and retarding OA progression10. MSCs in combination with some materials or factors have also been shown to facilitate the articular cartilage repair for OA treatment12,13.
Turmeric derived from Curcuma longa is commonly used in Indian and some Asian cooking as a spice for curry power14. The curcuminoids in turmeric are curcumin, desmethoxycurcumin, and bisdemethoxycurcumin, with curcumin being the main active component15. Curcumin displayed multiple pharmacological activities including anti-inflammatory, antioxidant, and anticancer, and has been employed in different studies involving several pathologies such as cardiovascular disease, depression, Alzheimer’s disease, epilepsy, Parkinson’s disease, cancer, osteoporosis, rheumatic arthritis, and OA15–21. It could inhibit the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ) and synthesis of reactive oxygen species by reducing the production of IL-1, IL-6, IL-8, and tumor necrosis factor α via various pathways, and is emerged as an effective therapeutic agent22,23. For OA treatment, curcumin supplementation exhibited effects mainly based on its anti-inflammation, antioxidant, and antiapoptosis potentials15. In clinical trials, curcumin served as a natural product medicine displayed nearly no side effects, thus making it a potential alternative to NSAIDs and some other medications with known severe adverse effects24,25. The current study aims to explore the pharmacological action of BMSCs supplemented with curcumin on articular chondrocytes in vitro and the therapeutic potential in vivo.
Materials and Methods
Isolation and Culture of BMSCs
Animal experiments in the current study were approved by the Institutional Ethics Committee of Honghui Hospital, Xi’an Jiaotong University. Male Sprague-Dawley (SD) rats, weighing about 200 g, were purchased from the experimental animal center of Xi’an Jiaotong University. Under sterile conditions, the femurs and tibias of SD rats were carefully separated and washed with phosphate-buffered saline (PBS). Then, bone marrow tissues were collected from the cavities of the femurs and tibias with a 10 ml syringe injected into 6 ml culture medium containing Dulbecco’s modified Eagle’s medium/F12 medium (HyClone, Logan, UT, USA), 1.5 mM l-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 10% fetal bovine serum (Gibco, Melbourne, Australia), 100 U/ml penicillin, and 100 μg/ml streptomycin (HyClone). Cells were maintained in an incubator with 5% CO2, 95% humidity, and 37°C environment. The adherent cells were cultured along with medium exchange every 3 days until the confluence reached 80%. Thereafter, cells were passaged at 1:3 and the passage 3 cells were used for subsequent experiments.
Isolation and Culture of Articular Chondrocytes
Under sterile conditions, the surface layer of the knee articular cartilage was carefully removed, cut into pieces, digested with 0.25% trypsin (HyClone) for 20 min, and 0.2% type II collagenase (Sigma) at 37°C with shaking for 3 h. The 100-µm filter was then used to remove the debris. After centrifugation, the cells were maintained in the same medium as that used in BMSCs culture.
Immunofluorescent and Toluidine Blue Staining
Passage 3 BMSCs or chondrocytes seeded in coverslips were fixed with paraformaldehyde for 20 min. The cells were incubated with primary detection antibodies against primary antibodies (CD44 and CD90, Abcam, Cambridge, UK, 1:1,000; Col2a1, ZSGB-BIO, Beijing, China, 1:400) at 4°C overnight. Subsequently, cells were incubated with anti-mouse fluorescein isothiocyanate (FITC)-conjugated and/or anti-rabbit CY3-conjugated secondary antibodies (Boster, Beijing, China, 1:200) for 2 h. The cells were then labeled with 4’,6-diamidino-2-phenylindole for 3 min and images were acquired under a fluorescence microscope. For toluidine blue staining, chondrocytes were stained with 1% toluidine blue for 15 min and photographed under an inverted microscope.
Flow Cytometry
Passage 3 BMSCs were digested with 0.25% trypsin, collected, and centrifuged at 1,000 g for 5 min, and the supernatant was discarded. Then the cells were resuspended with 1 × 106 cells in 100 µl PBS and incubated with 10 µl CD90 (eBioscience, San Diego, CA, USA) antibody directly conjugated with FITC and 10 µl CD44 (eBioscience) with PE simultaneously for 30 min at room temperature and in dark. Subsequently, the cells were washed two times with PBS and resuspended in 100 µl PBS to perform flow cytometry analysis (BD Biosciences, San Jose, CA, USA).
Cell Counting Kit-8 Assay
Cell viability was evaluated by Cell Counting Kit-8 (CCK-8) assay following the instructions of manufacturer. Briefly, cells were seeded in a 96-well plate at 3 × 103 cells/well overnight and then treated with curcumin at different concentrations. After 24 h, 10 µl of CCK-8 solution (Boster, Wuhan, China) was added into each well and the mixture was incubated for 2 h at 37°C with 5% CO2. Finally, the absorbance was recorded using a Microplate Reader (Thermo Fisher Scientific) at 450 nm.
EdU Assay
Articular chondrocytes were seeded in the lower chamber of 24-well transwell plates (5 × 104 cells/well) and cocultured with BMSCs (2 × 105 cells/well) seeded in the upper chamber (0.4 µm pore size). After incubation for 24 h at 37°C, chondrocyte proliferation was determined using an EdU assay kit (Solarbio, Beijing, China) according to the manufacturer’s instructions.
Migration Assay
Articular chondrocytes (3 × 104 cells/well) were seeded in the upper chamber (8 µm pore size) of 24-well transwell plates and cocultured with BMSCs (2 × 105 cells/well) seeded in the lower chamber. After incubation for 24 h at 37°C, the inserts were fixed with 4% paraformaldehyde for 20 min and stained with 0.5% crystal violet solution (Solarbio, Beijing, China) for 15 min. Unmigrated cells on the upper surface of the insert membrane were carefully wiped with a cotton swab.
RNA Extraction and Real-time Quantitative Polymerase Chain Reaction
Articular chondrocytes were seeded in the lower chamber of six-well transwell plates (1 × 105 cells/well) and cocultured with BMSCs (2 × 105 cells/well) seeded in the upper chamber (0.4 µm pore size). Total RNA was extracted after 24 h and reverse transcribed to first-strand cDNA according to the manufacturer’s instructions (Thermo Fisher Scientific). Expression of Aggrecan, Col2a1, Sox9, and the internal control gene Gapdh was quantified with quantitative polymerase chain reaction using FastStart Universal SYBR Green Master (Rox, CO, USA). The primers used in the study are listed in Table 1.
Table 1.
The Primer Sequence Information for Quantitative PCR.
| Gene name | Sequence (5′–3′) |
|---|---|
| Aggrecan | For: TCCACATCAGAAGAGCCATAC; Rev: AGTCAAGGTCGCCAGAGG |
| Col2a1 | For: GCTGTGGAAGTGGATGAAGA; Rev: TGAGGAACTGTGGAGAGACG |
| Sox9 | For: CAAGAACAAGCCACACGTCA; Rev: TCGTTCAGCAGTCTCCAGAG |
| Gapdh | For: TGAGGACCAGGTTGTCTCCT; Rev: ATGTAGGCCATGAGGTCCAC |
PCR: polymerase chain reaction.
Protein Extraction and Western Blotting
Articular chondrocytes were cocultured with allogeneic BMSCs in six-well transwell plates. Total proteins were extracted, quantified, separated, and transferred to polyvinylidene difluoride membranes as described previously26. The membranes were incubated with primary detection antibodies against Sox9 (CST, Boston, MA, USA, 1:1,000), Col2a1(Boster, Wuhan, China, 1:200), Aggrecan (Abways, Shanghai, China, 1:400), and Actin (Abcam, 1:1,000); Actin was used as the internal control. Membranes were then incubated with anti-rabbit immunoglobulin G secondary detection antibody (Thermo Fisher Scientific, 1:5000).
Induction of OA Model by Intra-articular Injection of Monoiodoacetic Acid and Treatment
Forty-two male SD rats were used for the in vivo experiment in the current study. Six rats were not subjected to any intervention used as control group. Thirty-six rats were used to induce OA animal model. Briefly, under sterile conditions, the animals were anesthetized with 3% pentobarbital sodium (40 mg/kg) by intraperitoneal injection and intra-articularly injected with 2 mg monoiodoacetic Acid (MIA) dissolved in 50 µl PBS into the patellofemoral joints of right knees using a 26-gauge needle. After 3 days, the rats were randomly divided into three groups, namely, the OA, BMSC, and BMSC + CCM (curcumin) groups. In our previous research, intraperitoneal injection of 50 mg/kg/d curcumin showed some treatment effects on OA rats induced by MIA27. The rats in BMSC + CCM group were intra-articularly injected with 1 × 106 BMSCs suspended in 50 µl PBS into right knee for one time and intraperitoneally injected with 50 mg/kg/d curcumin dissolved in 50 µl dimethyl sulfoxide (DMSO) every day for 2 weeks. Correspondingly, the rats in BMSC group were intra-articularly injected with 1 × 106 BMSCs in 50 µl PBS and intraperitoneally injected with 50 µl DMSO; the rats in OA group were injected 50 µl PBS and 50 µl DMSO. All the rats were sacrificed under anesthesia condition 4 weeks later.
Histological Staining and OA Score Analyses
The femoral condyles were collected from the sacrificed rats, fixed with 4% paraformaldehyde for 48 h, decalcified with 12.5% ethylenediaminetetraacetic acid for 4 weeks, and dehydrated with 30% sucrose solution for 72 h. The articular cartilage was then isolated, frozen, and embedded in OCT compound (Tissue-Tek, Tokyo, Japan). Finally, sections of 7 µm thick were cut in the sagittal plane with a freezing microtome (Leica, Heidelberger, Germany). The frozen sections were fixed again with acetone solution for 10 min, and then stained with hematoxylin and eosin (HE), toluidine blue, and safranin O (Solarbio, Shanghai, China), respectively. The Osteoarthritis Research Society International (OARSI) scoring system was used to determine the extent of cartilage deterioration and the severity of knee OA, with scores ranging from 0 (normal) to 6 (>80% loss of cartilage). The modified Mankin criteria were used to assess cartilage damage, with scores ranging from 0 (normal) to 24 (the most severe OA)28. The evaluations were performed independently by two blinded observers and the scores were evaluated in OA, BMSC, and BMSC + CCM groups.
Statistical Analysis
Data were presented as the mean ± standard error of the mean. All experiments were performed at least three independent occasions. One-way analysis of variance was used to determine statistical differences and post hoc analyses were carried out using the least significance difference tests. SPSS18.0 software was used for statistical analysis. P < 0.05 was considered to indicate statistical significance.
Results
Culture and Identification of BMSCs and Articular Chondrocytes
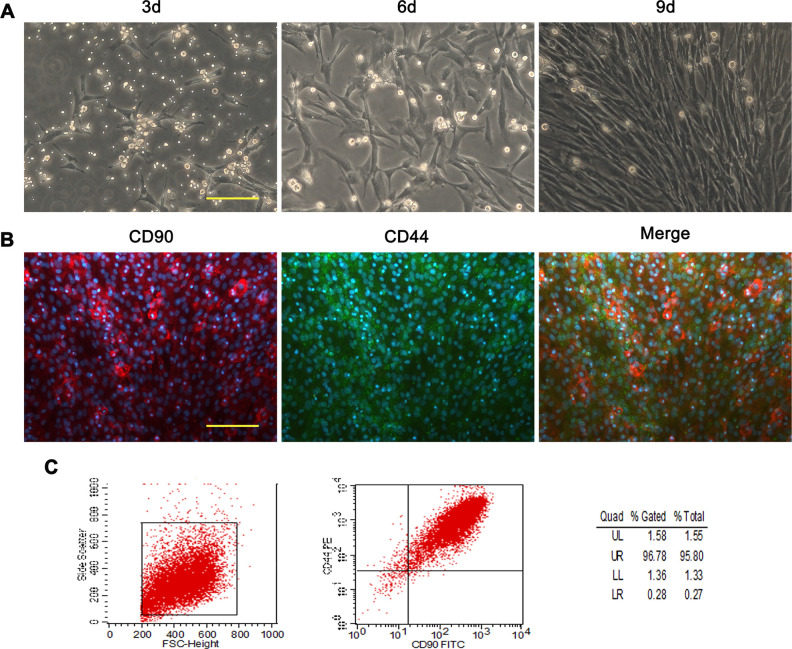
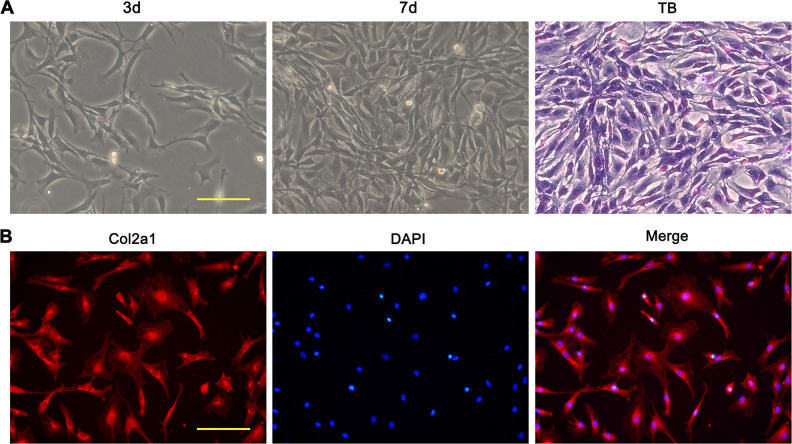
BMSCs and articular chondrocytes of rats were cultured and the media were changed every 2 to 3 days. The cells were imaged under inverted microscope at different time points. At 3 and 5 days, a small number of BMSCs adhered and the cells showed a spindle-shaped appearance with no distinct morphological differences. With the culture period over 9 days, cells multiplied to form a confluent monolayer, exhibiting fibroblast-like appearance and morphologically homogeneous (Fig. 1A). Immunofluorescent staining of CD44 and CD90, which are characteristic markers for BMSCs, demonstrated positive staining for the cells (Fig. 1B). Flow cytometry analysis of BMSC revealed that the percentage of CD44 and CD90 positive cells was over 95% (Fig. 1C). Articular chondrocytes showed long spindle-shaped appearance and the confluence reached about 70% following 7 days of culture (Fig. 2A). The toluidine blue staining and immunofluorescent staining of Col2a1 for the cells were positive (Fig. 2A, B).
Fig. 1.
Phase contrast image and identification of rat BMSCs. (A) Light microscopy images showing the morphological characteristic of BMSCs at cultured 3, 6, and 9 days. (B) Photomicrographs of dual-immunofluorescence labeling of passage 3 BMSCs for CD90, CD44, and merge of the two markers. (C) Flow cytometric analysis of passage 3 BMSCs labeled with CD44 and CD90. Scale bars = 100 μm. BMSC: mesenchymal stem cells derived from bone marrow.
Fig. 2.
Phase contrast image and identification of rat primary articular chondrocytes. (A) Light microscopy images of rat primary articular chondrocytes at cultured 3 and 7 days. Light microscopy images of toluidine blue staining for passage 3 chondrocytes. (B) Immunofluorescent images of Col2a1 labeling for passage 3 chondrocytes. Scale bars = 100 μm. TB: toluidine blue.
Curcumin at Low Concentration Showed No Effects on the Proliferation of Articular Chondrocytes
Curcumin is a natural polyphenol product, and the chemical structure of curcumin was shown in Fig. 3A. CCK-8 assay was used to evaluate the effects of curcumin on the proliferation of articular chondrocytes. Curcumin with concentration more than 20 μM displayed cytotoxicity on articular chondrocytes (Fig. 3B). Cell viability was not significantly influenced by curcumin at low concentration (less than 20 μM) and therefore curcumin with 8 μM was used to perform further experiment (Fig. 3B).
Fig. 3.
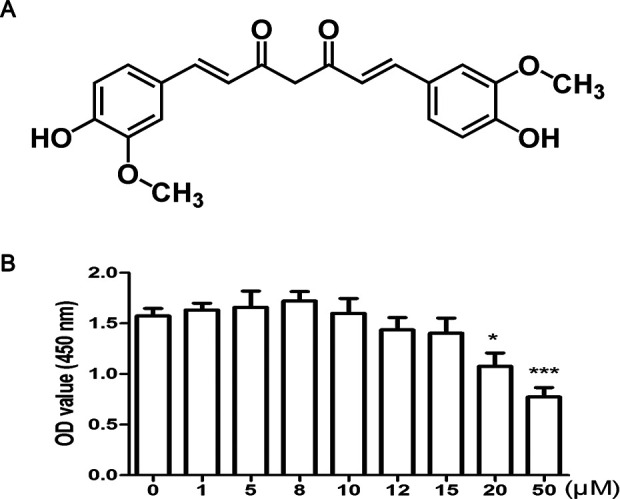
Chemical structure of curcumin and effects of curcumin on the viability of rat primary articular chondrocytes. (A) Chemical structure of curcumin. (B) Cell viability of chondrocytes was detected by CCK-8 assay at indicated concentrations of curcumin. * and *** represent P < 0.05 and P < 0.001, respectively, versus nontreatment group. CCK-8: Cell Counting Kit-8.
Curcumin Supplementation Enhanced Proliferation and Migration of Articular Chondrocytes Cocultured with BMSCs
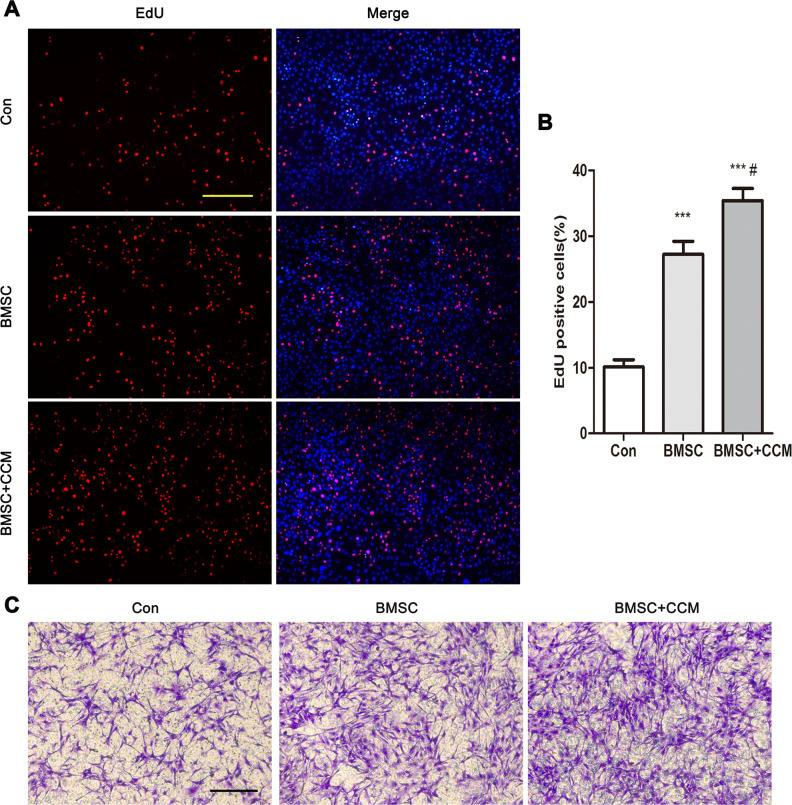
The effect of BMSCs and curcumin supplementation on articular chondrocytes cocultured with BMSCs was assessed by EdU incorporation assays (Fig. 4A). The percentage of EdU-positive cells was significantly increased in BMSC group compared with controls, suggesting that BMSCs promoted the proliferation of articular chondrocyte (Fig. 4B). The EdU assay results in BMSC + CCM compared with control and BMSC group demonstrated that curcumin supplementation enhanced the function of BMSC on chondrocyte proliferation (Fig. 4A, B). In the migration assay, the number of migratory chondrocytes in BMSC group was remarkably increased compared with control group and in BMSC + CCM group was significantly increased compared with control and BMSC group, indicating that BMSCs greatly accelerated chondrocyte migration and curcumin supplementation enhanced this function of BMSC on chondrocytes (Fig. 4C).
Fig. 4.
Effects of curcumin supplementation on the proliferation and migration of articular chondrocytes cocultured with BMSCs. (A) EdU incorporation of chondrocytes cocultured with BMSCs without or with curcumin supplementation. Normal chondrocytes acted as controls. Scale bar = 100 μm. (B) Quantification of the percentage of EdU-positive cells in 3 group of a. (C) Articular chondrocyte migration assays of articular chondrocytes cocultured with BMSCs without or with curcumin supplementation. Scale bar = 100 μm. *** represents P < 0.001 versus Con group, # represents P < 0.05 versus BMSC group. BMSC: mesenchymal stem cells derived from bone marrow.
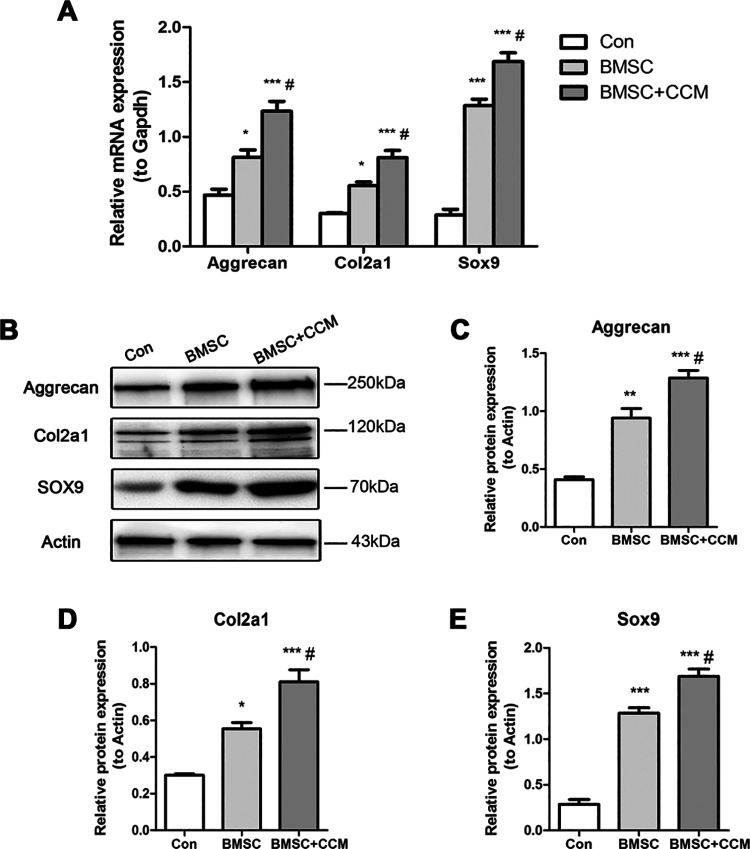
Curcumin Supplementation Increased Anabolic Gene Expression in Articular Chondrocytes Cocultured with BMSCs
Articular chondrocytes synthesize the components of ECM, including collagens and proteoglycans. The balance between anabolic and catabolic mechanisms maintains ECM homeostasis in articular cartilage, and the imbalance is associated with the destruction of OA cartilage29. Col2a1 and Aggrecan are the main anabolic components in the ECM, and Sox9 is a crucial transcription factor for the gene expression30. Cocultured with BMSCs, the expression of Col2a1, Aggrecan, and Sox9 in articular chondrocytes with or without curcumin was significantly increased compared with controls at mRNA level (Fig. 5A). When curcumin was added to the chondrocytes cocultured with BMSCs, the expression of these genes was remarkably increased, suggesting that curcumin supplementation enhances anabolic gene expression in articular chondrocytes cocultured with BMSCs (Fig. 5B). Correspondingly, curcumin supplementation enhanced the expression of these genes in chondrocytes cocultured with BMSCs at protein level (Fig. 5C–E).
Fig. 5.
Effects of curcumin supplementation on the anabolic gene expression of articular chondrocytes cocultured with BMSCs at mRNA and protein levels. (A) Relative mRNA expression of Aggrecan, Col2a1, and Sox9 normalized by Gapdh of articular chondrocytes cocultured with BMSCs without or with curcumin supplementation. Normal chondrocytes acted as controls. (B) Western blot results of Aggrecan, Col2a1, Sox9, and Actin of articular chondrocytes cocultured with BMSCs without or with curcumin supplementation. Normal chondrocytes acted as controls. (C, D, F) Quantification of protein expression of Aggrecan, Col2a1, and Sox9, respectively, normalized to Actin. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively, versus Con group, # represents P < 0.05 versus BMSC group. BMSC: mesenchymal stem cells derived from bone marrow.
Curcumin Supplementation Assisted BMSCs to Promote Articular Cartilage Repair and Alleviate OA Progression
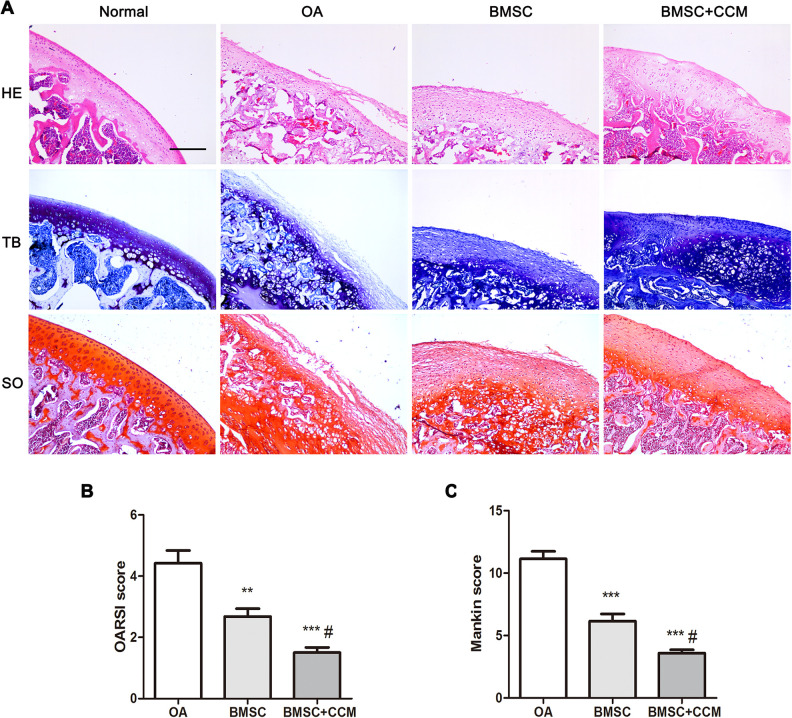
MIA was used to induce OA model in SD rats. Four weeks later, HE staining results demonstrated that the articular cartilage layer was thin and the number of articular chondrocytes was reduced in OA rats (Fig. 6A). Further toluidine and safranin O staining indicated that glycosaminoglycan and Aggrecan content was decreased in articular cartilage of OA rats (Fig. 6A). Treated by BMSCs, the damage of articular cartilage was greatly improved and curcumin supplementation further promoted the repair process (Fig. 6A). Accordingly, the OARSI and Mankin scores for OA and treated rats demonstrated that BMSCs could alleviate OA progression and curcumin supplementation further improved the treatment effects (Fig. 6B, C).
Fig. 6.
Effects of curcumin supplementation on articular cartilage repair in OA rats intra-articularly injected with BMSCs. (A) Histological staining in control, OA, BMSC without or with curcumin group. (B) OARSI score analysis for OA, BMSC without or with curcumin group. (C) Mankin score analysis for OA, BMSC without or with curcumin group. Scale bar = 100 μm. ** and *** represent P < 0.01 and P < 0.001, respectively, versus Con group, # represents P < 0.05 versus BMSC group. HE: hematoxylin and eosin; OA: osteoarthritis; OARSI: Osteoarthritis Research Society International; SO: safranin O; TB: toluidine blue.
Discussion
OA is a chronic musculoskeletal degenerative and ageing related disease. In recent years, MSCs as a regeneration medicine showed promising potential in OA treatment. In the current study, although curcumin alone did not improve cell viability of primary articular chondrocytes, it enhanced proliferation and migration of chondrocytes when cocultured with BMSCs. Meanwhile, the expression of anabolic genes in chondrocytes related to ECM synthesis was remarkably increased both at mRNA and protein levels. In OA rats, curcumin and BMSCs cooperating to greatly promote articular cartilage regeneration and retard OA progression.
OA could be simulated in vitro by stimulating primary articular chondrocytes with IL-1β, which serve important roles in articular cartilage damage31. Curcumin demonstrated anti-inflammation potential by inhibiting the IL-1β-induced activation through regulating NF-κB signal pathway in rat or human articular chondrocytes32–34. In OA rats, TLR4 activation was involved in OA mechanism and intraperitoneal injection of curcumin reduced inflammation through blocking TLR4/MyD88/NF-κB signal pathway35,36. In OA patients, the released proinflammatory cytokine IL-1β could further stimulate the synthesis of more inflammatory cytokines and induce chondrocyte apoptosis37. These cytokines could further induce matrix degradation by producing catabolic enzymes such as matrix metalloproteinases (MMPs)38. IL-1β also inhibited the expression of cartilage-specific ECM components including COL2A and cartilage-specific Aggrecans39. Curcumin could inhibit IL-1β-induced apoptosis and caspase-3 activation and promote autophagy through activation of ERK1/2 signaling pathways in rat articular chondrocytes40. In spontaneous and surgically induced OA mice models, curcumin treatment could enhance autophagy and reduce apoptosis and articular cartilage loss. Meanwhile, the in vitro experiment demonstrated that oral administration of curcumin inhibited apoptosis and promoted autophagy through regulating Akt/mTOR pathway41. The pathophysiology of OA was also related with mitochondrial function in articular chondrocytes of OA patients, and curcumin could suppress endoplasmic reticulum (ER) stress in chondrocytes induced by tert-Butyl hydroperoxide via promoting SIRT1 expression to inhibit PERK-eIF2α-CHOP axis42,43. In OA rats induced by surgical transection of right anterior cruciate ligament, intraperitoneal injection of curcumin inhibited ER stress and ameliorated cell apoptosis in articular cartilage43. Therefore, curcumin exerts anti-inflammatory, antiapoptotic, and antioxidant by regulating specific signal pathways and has chondroprotective effects.
In recent years, increasing evidence showed that most intra-articularly injected MSCs were rarely or not engrafted in the articular cartilage and the therapeutic function of the injected MSCs is mainly attributed to their paracrine effects44,45. MSCs could secrete extracellular vesicles (EV) containing abundant trophic factors to modulate the injured tissue microenvironment for cartilage regeneration via proliferation, differentiation, and ECM synthesis46. Exosomes and microvesicles are the two main categories of EV according to their size and biogenesis47. Intra-articular injection of microvesicles and exosomes of BMSCs exerted similar chondroprotective and anti-inflammatory function in vitro and protected mice from developing OA in vivo, suggesting that either exosomes or microvesicles reproduced the main therapeutic effect of BMSCs48. The study of Wang et al. confirmed that embryonic MSCs alleviate cartilage destruction and ECM degradation, and exosomes derived from embryonic MSCs also successfully impeded cartilage destruction in OA mice49. Li et al. found that exosomes derived from MSCs of various sources regulate specific signaling pathways such as p38/ERK/Akt, TGF-ß1/miR-135b/Sp1, and autophagy-related mTOR, and lead to increased synthesis of Col2 and proteoglycans and decreased production of ADAMTS-5 and MMP-13 in vitro and in vivo. Exosomes could restore the homeostasis of the articular microenvironment and remodel the stability of cartilage for knee OA therapies50. In the current study, coculture chondrocytes with BMSCs in transwell may imply the paracrine effects of BMSCs on chondrocytes.
Although the effects of BMSCs on chondrocyte has been well documented, few studies have reported the effect of curcumin on BMSCs. Buhrmann et al. reported that although curcumin alone did not have chondrogenic effects on MSCs, it inhibits IL-1β-induced activation of NF-κB, caspase-3, and COX2 in MSCs as well as in chondrocytes. Curcumin further promoted chondrogenic differentiation, which was suppressed by the proinflammatory factor when the cells received a chondrogenic stimulus or mixedly cultured with articular primary chondrocytes51. They speculated that curcumin treatment might help to establish a microenvironment in which the effects of proinflammatory cytokines are antagonized, thus facilitating chondrogenesis of MSC-like progenitor cells in vivo 51. Cao et al. further validated that curcumin had no effects on the expression of chondrogenic markers in the process of chondrogenic differentiation in MSCs. Their further exploration demonstrated that curcumin inhibited chondrocyte hypertrophy through Indian hedgehog homolog and Notch signal pathways in MSCs52. In our study, we found that curcumin enhanced the effects of BMSCs on primary articular chondrocytes, including cell proliferation and migration, and on the expression of anabolic markers at mRNA and protein levels for chondrogenesis, indicating that curcumin might interact with trophic factors released from BMSCs, and improve the cultured condition for articular chondrocytes.
The in vivo experiment in OA rats induced by MIA further proved that curcumin supplementation enhanced the therapeutic effects of BMSCs for OA disease. MIA has an inhibitory effect on the activity of glyceraldehydes-3 phosphate dehydrogenase in chondrocytes resulting in disruption of glycolysis, reduces quantity and synthesis of proteoglycans, and eventually leads to cell death53. Intra-articular injection of MIA caused histological and biochemical changes in articular cartilage of the knee joint resembling human knee OA symptoms54. Combining intra-articular injection of BMSCs and localized application of curcumin, we treated the MIA induced rats equivalent to human OA at early stage55,56. Localized treatment of curcumin in OA was minimally invasive and had effect on pain relief21,57. The results in current study demonstrated that BMSCs promoted cartilage repair and retarded OA progression at early stage, which was greatly enhanced by curcumin supplementation. This enhanced effect may correlate with the interaction between curcumin and trophic factors secreted from BMSCs, which could regulate the microenvironment of the joint cavity for articular cartilage regeneration and thereby promoting the therapeutic effect for OA treatment. Further studies regarding the interaction and molecular mechanisms are warranted. In addition, BMSCs primed with proinflammatory cytokines could improve their homing and immunomodulatory functions58. Whether BMSCs primed with curcumin could improve their function and promote their therapeutic effects for OA treatment is very interesting and deserves further studying.
Conclusion
In conclusion, the balance between anabolic and catabolic of ECM is vital for articular cartilage. BMSCs provide great promising potential for repairing damaged cartilage in OA disease. In the current study, curcumin enhanced the BMSC function for the proliferation and migration of articular chondrocytes, and anabolic gene expression of ECM in articular chondrocytes in vitro, and the regeneration of articular cartilage in vivo. These results indicated potential clinical application of curcumin cooperation with BMSCs in cartilage repair for OA treatment.
Footnotes
Ethical Approval: This study was approved by the Medical Ethics Committee of Honghui Hospital, Xi’an Jiaotong University, China.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Animal Care guidelines of Xi’an Jiaotong University, China, and approved by the Administration Committee of Experimental Animals, Shaanxi Province, China.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current study was supported by the Natural Science Foundation of Shaanxi Province (No. 2018JM7039, No. 2018JM7081) and the National Natural Science Foundation of China (No. 81601913).
ORCID iD: Rui Zhang  https://orcid.org/0000-0002-8664-6915
https://orcid.org/0000-0002-8664-6915
References
- 1. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. [DOI] [PubMed] [Google Scholar]
- 2. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73(9):1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. [DOI] [PubMed] [Google Scholar]
- 4. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35(4):401–404. [DOI] [PubMed] [Google Scholar]
- 7. Fosang AJ, Beier F. Emerging Frontiers in cartilage and chondrocyte biology. Best Pract Res Clin Rheumatol. 2011;25(6):751–766. [DOI] [PubMed] [Google Scholar]
- 8. Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, Im HJ. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, Atzeni F, Canesi B. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(1 suppl 1):1–10. [DOI] [PubMed] [Google Scholar]
- 10. Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 11. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 12. Zhang K, Zhang Y, Yan S, Gong L, Wang J, Chen X, Cui L, Yin J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013;9(7):7276–7288. [DOI] [PubMed] [Google Scholar]
- 13. Xia Q, Zhu S, Wu Y, Wang J, Cai Y, Chen P, Li J, Heng BC, Ouyang HW, Lu P. Intra-articular transplantation of atsttrin-transduced mesenchymal stem cells ameliorate osteoarthritis development. Stem Cells Transl Med. 2015;4(5):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo EJ, Efferth T, Panossian A. Curcumin downregulates expression of opioid-related nociceptin receptor gene (OPRL1) in isolated neuroglia cells. Phytomedicine. 2018;50:285–299. [DOI] [PubMed] [Google Scholar]
- 15. Yang M, Akbar U, Mohan C. Curcumin in autoimmune and rheumatic diseases. Nutrients. 2019;11(5):1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin S, Huang L, Gong J, Shen S, Huang J, Ren H, Hu H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J. 2017;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naqvi F, Saleem S, Naqvi F, Batool Z, Sadir S, Tabassum S, Ahmed S, Liaquat L, Haider S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak J Pharm Sci. 2019;32(Suppl 4):1893–1900. [PubMed] [Google Scholar]
- 18. Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, et al. Protective effects of Indian spice curcumin against amyloid-beta in Alzheimer’s disease. J Alzheimers Dis. 2018;61(3):843–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adami R, Bottai D. Curcumin and neurological diseases. Nutr Neurosci. 2020:72(2):1–21. [DOI] [PubMed] [Google Scholar]
- 20. Chen Z, Xue J, Shen T, Mu S, Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med. 2016;37(2):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M, Kim SJ, Hirsh DM, Hardin JA, Cobelli NJ, Friedman JM, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. [DOI] [PubMed] [Google Scholar]
- 24. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016;24(6):377–388. [DOI] [PubMed] [Google Scholar]
- 25. Sterzi S, Giordani L, Morrone M, Lena E, Magrone G, Scarpini C, Milighetti S, Pellicciari L, Bravi M, Panni I, Ljoka C, et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur J Phys Rehabil Med. 2016;52(3):321–330. [PubMed] [Google Scholar]
- 26. Wu S, Zhang R, Nie F, Wang X, Jiang C, Liu M, Valenzuela RK, Liu W, Shi Y, Ma J. MicroRNA-137 inhibits EFNB2 expression affected by a genetic variant and is expressed aberrantly in peripheral blood of schizophrenia patients. EBioMedicine. 2016;12:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou Z, Zhou J, Xiao K, Meng F, Zhu T, Rui Z. Therapeutic effects of curcumin on rats with knee osteoarthritis in duced by monoiodocetic acid. J XJTU (MS). 2020;41(2):299–302. [Google Scholar]
- 28. Huesa C, Ortiz AC, Dunning L, McGavin L, Bennett L, McIntosh K, Crilly A, Kurowska-Stolarska M, Plevin R, van ‘t Hof RJ, Rowan AD, et al. Proteinase-activated receptor 2 modulates OA-related pain, cartilage and bone pathology. Ann Rheum Dis. 2016;75(11):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alcaraz MJ, Megias J, Garcia-Arnandis I, Clerigues V, Guillen MI. New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol. 2010;80(1):13–21. [DOI] [PubMed] [Google Scholar]
- 30. Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211(2):109–121. [DOI] [PubMed] [Google Scholar]
- 31. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Ma J, Gu JH, Wang FY, Shang XS, Tao HR, Wang X. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol Med Rep. 2017;16(2):1837–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11(6):R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim L, Kim JY. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1beta via an anti-inflammatory mechanism. Food Sci Biotechnol. 2019;28(2):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan D, He B, Guo J, Li S, Wang J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir Bras. 2019;34(6):e201900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-kappaB signal pathway. Drug Dev Res. 2019;80(3):353–359. [DOI] [PubMed] [Google Scholar]
- 37. Blanco FJ, Guitian R, Moreno J, de Toro FJ, Galdo F. Effect of antiinflammatory drugs on COX-1 and COX-2 activity in human articular chondrocytes. J Rheumatol. 1999;26(6):1366–1373. [PubMed] [Google Scholar]
- 38. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275(5):3687–3692. [DOI] [PubMed] [Google Scholar]
- 40. Li X, Feng K, Li J, Yu D, Fan Q, Tang T, Yao X, Wang X. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. 2017;9(4):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang G, Cao J, Yang E, Liang B, Ding J, Liang J, Xu J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci Rep. 2018;38(4):BSR20171691. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Liu H, Li Z, Cao Y, Cui Y, Yang X, Meng Z, Wang R. Effect of chondrocyte mitochondrial dysfunction on cartilage degeneration: a possible pathway for osteoarthritis pathology at the subcellular level. Mol Med Rep. 2019;20(4):3308–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X, Li H, Tang T, Yang F, Wang X. Curcumin inhibits the PERK-eIF2alpha-CHOP Pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid Med Cell Longev. 2019;2019:8574386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One. 2016;11(2):e0149835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuroda K, Kabata T, Hayashi K, Maeda T, Kajino Y, Iwai S, Fujita K, Hasegawa K, Inoue D, Sugimoto N, Tsuchiya H. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet Disord. 2015;16:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toh WS, Foldager CB, Pei M, Hui JH. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev Rep. 2014;10(5):686–696. [DOI] [PubMed] [Google Scholar]
- 47. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. [DOI] [PubMed] [Google Scholar]
- 48. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Z, Li M, Xu P, Ma J, Zhang R. Compositional Variation and functional mechanism of exosomes in the articular microenvironment in knee osteoarthritis. Cell Transplant. 2020;29:963689720968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-kappaB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12(4):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao Z, Dou C, Dong S. Curcumin inhibits chondrocyte hypertrophy of mesenchymal stem cells through ihh and notch signaling pathways. Chem Pharm Bull (Tokyo). 2017;65(8):762–767. [DOI] [PubMed] [Google Scholar]
- 53. Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9(1):R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Naveen SV, Ahmad RE, Hui WJ, Suhaeb AM, Murali MR, Shanmugam R, Kamarul T. Histology, glycosaminoglycan level and cartilage stiffness in monoiodoacetate-induced osteoarthritis: comparative analysis with anterior cruciate ligament transection in rat model and human osteoarthritis. Int J Med Sci. 2014;11(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Udo M, Muneta T, Tsuji K, Ozeki N, Nakagawa Y, Ohara T, Saito R, Yanagisawa K, Koga H, Sekiya I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: proposed model-specific scoring systems. Osteoarthritis Cartilage. 2016;24(7):1284–1291. [DOI] [PubMed] [Google Scholar]
- 56. Dai L, Zhang X, Hu X, Liu Q, Man Z, Huang H, Meng Q, Zhou C, Ao Y. Silencing of miR-101 prevents cartilage degradation by regulating extracellular matrix-related genes in a rat model of osteoarthritis. Mol Ther. 2015;23(8):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ratanavaraporn J, Soontornvipart K, Shuangshoti S, Shuangshoti S, Damrongsakkul S. Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model. Inflammopharmacology. 2017;25(2):211–221. [DOI] [PubMed] [Google Scholar]
- 58. Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]