Abstract
Green nanotechnology has drawn major attention because of its ecofriendly and economical biosynthetic protocols. Synthesis of gold nanoparticles (AuNPs) using plant secondary metabolites is considered as a safer and cheaper option. Plants contain phytochemicals that has been used traditionally for treatment of various diseases, and proved to be non-toxic to healthy tissues. These phytochemicals play an important role in bio-reduction processes as reducing and stabilizing agents, and renders NPs selective toxicity towards diseased tissues. The study reports on the synthesis of AuNPs using Acai berry (AB) and Elderberry (EB) extracts and their anti-cancer properties. Formation of berry-AuNPs was confirmed through measurement of physico-chemical properties. The stability of the AuNPs was tested in biocompatible solutions. Anti-cancer activity of berry extracts and AuNPs was evaluated on the prostate (PC-3) and pancreatic (Panc-1) cancer cells. The berry extracts did not show toxicity to the cells, except for AB extracts on PC-3 cells at higher concentrations. The berry-AuNPs showed potential anti-cancer activities, and these effects could be further exploited for treatment of both the prostate and pancreatic cancers. Further studies are required to study the NP mechanism of action and specificity, as well as identify the phytochemicals involved in the synthesis of AuNPs.
Keywords: Acai berry, anti-cancer properties, elderberry, gold nanoparticles, green nanotechnology
Introduction
Nanotechnology has revolutionized the future of disease diagnostics and treatment using NPs with a diameter size range between 1 nm and 100 nm. These nanomaterials have unique physico-chemical properties (such as tunable size, increased surface area) that make them useful for biomedical applications.1,2 There are a number of NP-based formulations that are approved by Food and Drug Administration (FDA) for clinical treatment of chronic diseases, and several nano-based theranostics that are reported to be undergoing clinical trials.1 However, the methodologies used to synthesize the NPs are limited by the use of toxic chemicals for synthesis of NPs as reducing (organic solvents) and stabilizing (surfactants) agents. These chemicals as well as the byproducts involved in the reaction, are considered as non-friendly and hazardous to the environment. To overcome this challenge, nanotechnology has since advanced to the use of biomaterials such as plants and microorganisms for the biosynthetic production of nanomaterials. Synthesis of metal NPs using green nanotechnology principles has drawn major attention because of its rapid, ecofriendly, non-pathogenic and economical protocol for biosynthetic processes at an industrial scale.2,3
Plant-based synthesis of NPs emerges as a safer option since phytomedicine have been used for decades in traditional medicine and proven to be non-toxic to healthy human tissues. Plants are known to contain phytochemicals that have been used for treating various diseases such as cancer.2,3 These phytochemicals therein play an important role in the bio-reduction of metal salts to produce their respective stable and biocompatible NPs. The most predominant type of nanomaterials that are produced through the green chemistry route is the metallic NPs. Phytochemicals are rich in electrons; therefore have the ability to reduce to Au3+ to AuNPs and subsequently provide a surface coating on the AuNPs. The phytochemicals on the surface of the NPs render them as non-toxic to healthy cells while behaving as treatment of diseased cells.3
The present study reports on the synthesis of AuNPs using AB (Euterpe oleraceae) and EB (Sambucus nigra) extracts. Both AB4,5 and EB6,7 extracts have been reported to have anti-viral, anti-inflammatory and immune modulatory properties that are attributed to their flavonoids and anthocyanins contents.8 In some South American countries, the Acai berries are part of the daily diet, they are incorporated either in beverages or in food for consumption. The acai fruit, pulp and oil contains phytochemicals with various health benefits.8 They are used in traditional medicine as antidiarrheal agents.5 The objective of this study was to synthesize AuNPs using AB and EB extracts, and investigate their anti-cancer properties against prostate and pancreatic cancer cell lines.
Materials and methods
Extraction of bioactive compounds from berry extracts
The AB and EB fruit powders were purchased from Amazon (USA). 100 mg/mL of the berry powders were dissolved in four solvents: distilled water (dH2O), 50% Ethanol (EtOH), 50% Chloroform (Chlorof) and 10% Cyclohexane (Hex). The samples were stirred at room temperature overnight, then centrifuged at 15 000 rpm for 20 min. The supernatants were concentrated to dryness in a rotary evaporator (Rotavapour R-210, BUCHI Corporation, New Castle, USA). Stock solutions of the dried berry extracts were prepared in dH2O.
Green synthesis of berry-AuNPs
The AuNPs were synthesized following an optimized protocol as described by Khoobchandani et al. with slight modifications.2 The berry extracts (four per berry sample) were diluted to 2 mg/mL in dH2O, the solutions were heated to 100°C while stirring. Then, 100 µL 0.1 M NaAuCl4 solution was added in each extract, as soon as the color change, the heat was switched off and allowed the AuNPs to stir for 30 min to 1 h before characterization. The NPs were washed twice with dH2O and centrifuged at 15 000 rpm for 10 min to remove the unreacted extracts. The AuNPs were stored at 4°C until further analysis.
Characterization of berry-AuNPs
The optical properties of the berry-AuNPs were measured from 400 nm to 750 nm using a Varian Cary 50 UV–Vis spectrophotometer (Shimadzu, Columbia, MD, USA). The hydrodynamic size and zeta potential (ζ) of the AuNPs were measured by Zetasizer Nano S90 (Malvern Instruments Ltd., Westborough, MA, USA). The morphology and size distribution of the berry-AuNPs were examined by Transmission Electron Microscope (TEM), JEOL 1400 TEM (JEOL, Tokyo, Japan).
Berry-AuNP stability test
The in vitro stability of the berry-AuNPs was tested on various solutions; distilled water (dH2O), 1× phosphate buffered saline (PBS) (Leinco Technologies, MO, USA), pH7 solution (Thermo Fisher Scientific, MA, USA), 0.5% Bovine Serum Albumin (BSA) (Cell Signaling, MA, USA), 0.2 M Histidine, 0.5% Cysteine (Cys), 0.5% Human Serum Albumin (HSA), and 1% NaCl (Sigma–Aldrich, MO, USA). The NPs were diluted with the selected solutions at 1:10 ratio to a final volume of 2 mL. The solutions were incubated for 24 h at room temperature, thereafter, 100 µL of the AuNP solutions was aliquoted into a 96 well plate and their optical properties (UV–vis) was read at 350–750 nm range.
Evaluation of the effect of berry extracts and AuNPs on cancer cells
Cell culture
Pancreatic (Panc-1) and prostate (PC-3) cancer cell lines were purchased from American Type Culture Collection (VA, USA). The Panc-1 cells were maintained in high glucose (4.5 g/L) Dulbecco’s Modified Eagle Medium (Life Technologies, CA, USA), PC-3 cells were cultured in Roswell Park Memorial Institute medium 1640 (Life Technologies, CA, USA). The media were supplemented with 10% fetal calf serum and 1% Gentamycin (Fresenius Kabi, IL, USA). The cells were grown at 37°C in a humidified incubator with 5% CO2 and observed daily on a Nikon Eclipse TS100 trinocular inverted microscope (Carl Zeiss AG, Oberkochen, Germany).
Cytotoxicity assays
Cells (Panc-1 and PC-3) were seeded in 96-well plates at 1 × 104 cells/mL. The cells were treated with various concentrations of AB and EB extracts (0–200 mg/mL), AB- and EB-AuNPs (0–.4 mg/mL) for 24 h. The cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described by Khoobchandani and colleagues.2 Percentage cell viability was calculated by using the following formula: % cell viability = (T/C) × 100%, where C = absorbance of control (untreated cells), T = absorbance of treated cells.
Statistical analysis
Statistical analysis was carried out using the Student’s t test and Graph Pad Prism software. A p-value less than 0.05 was considered as statistically significant.
Results and discussion
Biosynthesis and characterization of berry-AuNPs
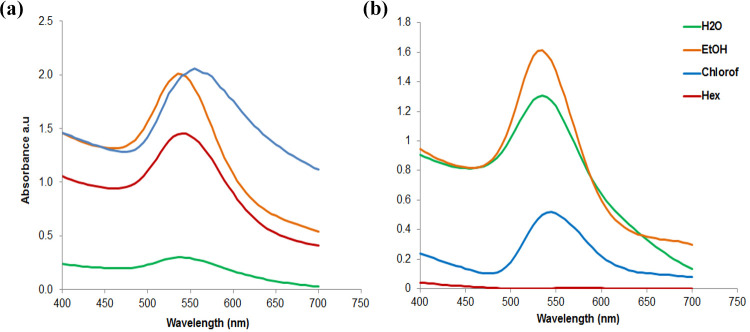
Bioactive compounds were extracted from AB and EB fruit powder with four solvents (dH2O, Hex, Chlorof and EtOH). The solvents were removed and concentrated by evaporation, the dried extracts were resuspended in dH2O and used to synthesize the biogenic AuNPs. Formation of the berry-AuNPs was confirmed by a color change to wine red. The AuNPs were characterized by UV–vis spectroscopy, Zetasizer and TEM. The optical properties of the NPs varied among the different extraction solvents and the extracts from which they were synthesized as shown in Figure 1. All solvents (except Hex extracts from EB) were able to reduce the NaAuCl4 salt for the production of the AuNPs as confirmed by using UV–Visible spectra. Gaussian curves with absorption peaks (Surface Plasmon Resonance, SPR) were obtained between 530 nm and 570 nm for the AB_AuNPs, and between 535 nm and 555 nm for the EB_AuNPs as shown in Table 1. Different berry extracts gave different SPR even when the same solvent was used. For instance, the NPs made from EB dH2O (aqueous) extracts gave a SPR at 555 nm, while the ones from AB aqueous extract was at 535 nm (Table 1). For AB extracts, EtOH and Hex appeared to produce monodispersed NPs while NPs synthesized from aqueous extracts and Chlorof extracts gave a broader peak and are likely to be polydispersed (Figure 1(a)). The EtOH EB extracts produced monodispersed NPs (Figure 1(b)), while the size distribution of NPs from dH2O and Chlorof extracts might not be uniform. Polydispersed NPs always display broader peaks, a characteristic (NP optical properties) that can also be used to estimate the size, concentration and shape of colloidal NPs.2
Figure 1.
Optical properties of the (a) AB and (b) EB AuNPs synthesized from various extracts.
Table 1.
Physico-chemical properties of the berry-AuNPs.
| AB AuNPs | EB AuNPs | |||||
|---|---|---|---|---|---|---|
| Extraction solvent | SPR (nm) | Hydrodynamic size (nm) | ζ-Potential (mV) | SPR (nm) | Hydrodynamic size (nm) | ζ-Potential (mV) |
| dH2O | 535 | 172 ± 15.2 | −36 ± 0.8 | 555 | 195 ± 3.1 | −31 ± 0.4 |
| 50% EtOH | 530 | 168 ± 3.0 | −28 ± 0.6 | 540 | 88 ± 4.1 | −34 ± 0.5 |
| 50% Chlorof | 570 | 197 ± 21.5 | −31 ± 0.8 | 535 | 94 ± 1.4 | −28 ± 0.5 |
| 10% Hex | 535 | 171 ± 2.6 | −26 ± 0.4 | _ | _ | _ |
Note: -, no AuNP synthesis.
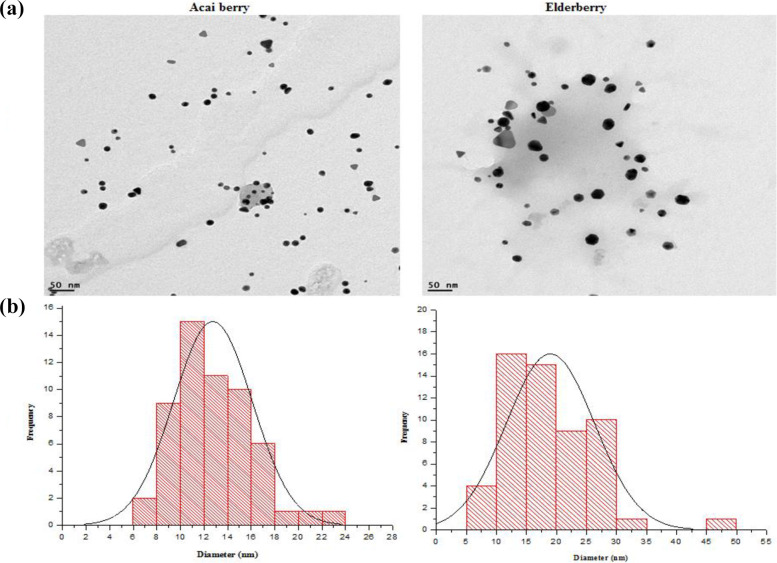
The morphology, size and quality of the biogenic AuNPs were evaluated by TEM. AuNPs of varying shapes and sizes were synthesized using all extraction solvents, i.e. spheres, rod-like and triangular shapes. Figure 2(a) shows the representative micrographs of the berry-AuNPs that were synthesized from the aqueous berry extracts. Similar data was obtained from the other extracts (EtOH and Chlorof), data not shown. Majority of the AuNPs were spheres. The different sizes and shapes of the NPs could be the result of the amount of reducing agents in the plant extracts or various phytochemicals involved in the synthesis. Homogenous NPs can be produced by varying the concentration of the extracts and gold salt, changing temperature or using pure compounds.
Figure 2.
TEM micrographs of (a) AB and EB AuNPs from dH2O extracts and (b) their size distributuion. Scale bar 50 nm.
The hydrodynamic size and the charge of the AuNPs were assessed by dynamic light scattering (Zetasizer). The AuNPs synthesized from the EtOH and Chlorof EB extracts had smaller hydrodynamic size at 88 and 94 nm, respectively, than their counter parts from AB extracts (Table 1). The AuNPs from the EB aqueous extracts had a hydrodynamic size of 194 nm, while the aqueous AB_AuNPs were 172 nm (Table 1). The same AuNPs had a smaller core size, the AB_AuNPs and EB_AuNPs from the aqueous extracts had core with a diameter of 13 and 19 nm, respectively (Figure 2(b)). The difference between the core and the hydrodynamic sizes is due to the fact that the core size accounts for only the metal core while the hydrodynamic size combines both the core and surface composition. The hydrodynamic sizes of 172 nm for AB_AuNPs and 195 nm for EB_AuNPs might then serve an indication that multiple phytochemicals are involved in the synthesis and capping of the berry-AuNPs. Generally, NPs because of their smaller size have a larger surface area which can then allow attachment of multiple molecules on the surface of the AuNP core.2 The NPs had a ζ-potential within a negative range, the AB_AuNPs were more stable compared to EB_AuNPs. The electrokinetic (ζ) potential was used as a sign of stability for the colloidal suspension. NPs with a ζ-potential within −30 mV and +30 mV are considered to be stable while the ones that are outside tis range are unstable.9
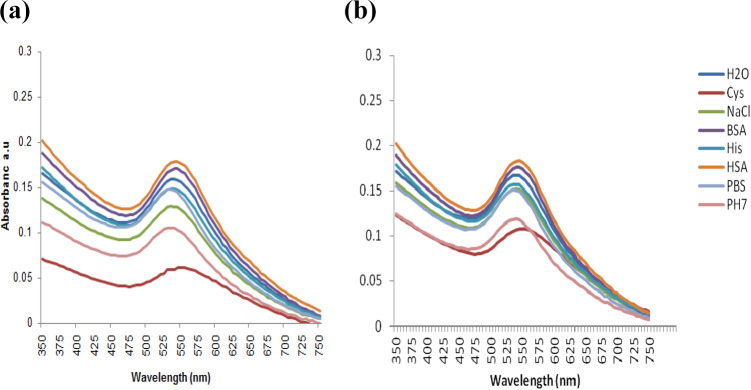
The Stability of the berry-AuNPs was also tested in various buffer systems mostly used in biological tests; the AuNPs were diluted in various buffers at 1:10 ratios and incubated for 24 h. Ideal NPs would retain their optical properties in these buffers, weakly bound capping agents are likely to react with their surrounding environment and this will cause aggregation of the NPs. The NPs with strongly bound capping agents will remain in solution and stable regardless of their environment.3 The AB_NPs in NaCl, cysteine and pH 7 solutions were least stable compared to other buffers as shown in Figure 3(a) by the decrease in the absorbance and a shift in their SPR. The EB_NPs were more stable in the same buffers except in cysteine and pH 7 solutions, as there was no much variation in their SPR (Figure 3(b)).
Figure 3.
The in vitro stability of (a) AB_AuNPs and (b) EB_AuNPs in various buffers. The NPs were diluted 1:10 with the buffers to the final concentration of 1× PBS, 1% NaCl, pH 7 solution, 0.5% HSA, 0.5% BSA, 0.2 M His, and 0.5% Cys.
Effects of berry extracts on cancer cells
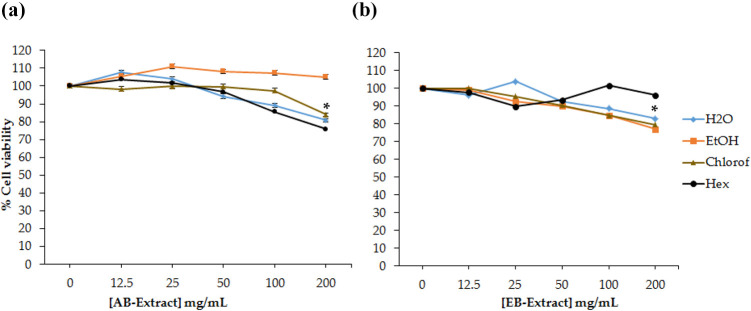
The anti-proliferative activities of AB and EB Extracts were tested against human prostate (PC-3) and pancreatic (Panc-1) cancer cell lines for 24 h. The cells were treated with increasing concentrations (0–200 mg/mL) of the extracts; these concentrations were far higher than the dosage limit (30 µg/mL) allowed by the American National Cancer Institute for clinical treatment of diseases with natural products.10 The extracts demonstrated dose dependent cytotoxicity on the PC-3 cells. Three of AB extracts reduced PC-3 cell viability starting from 50 mg/mL of aqueous (dH2O) and Hex extracts, and only at 200 mg/mL for Chlorof extract (Figure 4(a)). The AB EtOH extract did not have any effect on PC-3 cells even at the highest concentration. The EB extracts showed similar trend on PC-3 cells exposed EtOH, Chlorof and dH2O extracts (Figure 4(b)). Significant effect occurred at 200 mg/mL
Figure 4.
Effects of (a) AB and (b) EB extracts on PC-3 cells. Cell viability was assessed by MTT assay after 24 h exposure to berry extracts. Higher concentrations of AB extracts reduced PC-3 cell viability, while EB did not have effect on the cells. *Indicate statistical significance at p < 0.05.
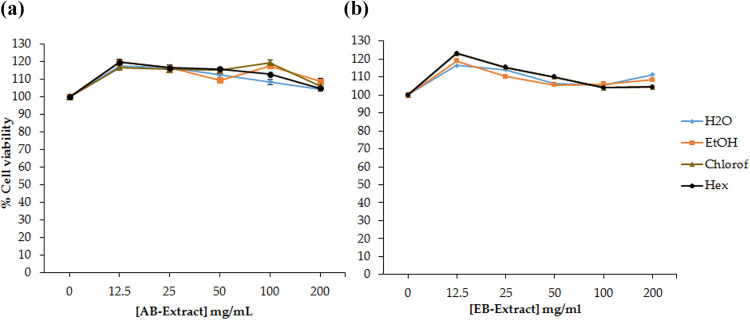
The berry extracts did not show toxicity on Panc-1 cells after 24 h of treatment with both AB and EB (Figure 5), at all the tested concentrations (0−200 mg/mL). Interestingly, the AB extracts that reduced PC-3 cell proliferation, did not show any effect on Panc-1 cells. It is worth investigating which compounds are responsible for this action, and whether this action is disease (PC-3) specific or not. The berries understudy are naturally non-toxic to healthy cells and known to have disease-preventive and curative effects.5 The two berries have a wide history and application in traditional medicine; these medicinal properties are attributed to their phytochemicals such as, flavonoids, polyphenols and anthocyanin. The individual phytochemicals extracted from AB have been reported to have anti-cancer11 and anti-obesity4 properties, and have been proved to reduce the risk of developing cardiovascular diseases and cancer.8 Although some studies have demonstrated that the AB extracts killed the HL-60 leukemia cells through apoptosis11 and that through their anti-inflammatory activity it can inhibit osteoclastogenesis4; they are also capable of using the immune system to fight against diseases.8 Therefore, it is recommended to also study the immunomodulation actions of the individual extracts.
Figure 5.
Effect of (a) AB and (b) EB extracts on Panc-1 cells. Cell viability was assessed by MTT assay after 24 h of treatment. Both the AB and EB extracts had no cytotoxic effects on the Panc-1 cells.
Effects of berry-AuNPs on cancer cells
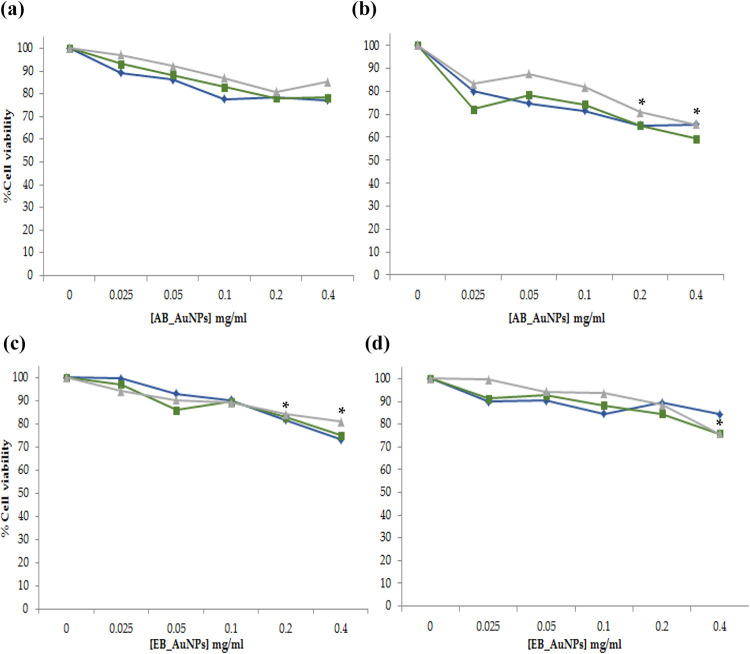
AuNPs synthesized from both AB and EB extracts were used to treat the PC-3 and Panc-1 cells, the cellular response was assessed through MTT assay after 24 h. These NPs were synthesized using 2 mg/mL of the extracts, 100 times less diluted when compared to the highest dose (200 mg/mL) of extracts that was used to treat the cells (Figures 4 and 5). The berry-AuNPs were further diluted (1:5) with the media during treatment of cells, assuming the entire extracts were involved in NP synthesis, the highest concentration of the extracts will now become 0.4 mg/mL. Therefore, the cells would have been treated with NPs containing 0.025–0.4 mg/mL of the berry extracts, of note at this concentration all the extracts were not cytotoxic to the two cell lines tested. As shown in Figure 4(a), the berry-extracts only showed toxicity on PC-3 cells from 50 mg/mL. After 24 h treatment, all the berry-AuNPs reduced PC-3 and Panc-1 cell viability as shown in Figure 6. The anti-proliferative activity of AB-AuNPs was more pronounced on Panc-1 cells, the berry-AuNPs synthesized from the three (dH2O, EtOH, Chlorof) extracts resulted in more than 30% cell death. High cellular toxicity was observed in Panc-1 cells treated with EtOH AB_NPs, causing 41% cell death. The EB_AuNPs reduced proliferation of PC-3 and Panc-1 cells by ∼20% with the highest concentration (0.4 mg/mL). Interestingly, their respective extracts had negligible effect on the same cell lines. This effect might be attributed to the properties of NPs. NPs have a large surface area that in turn allows for a large loading capacity; by attaching more active phytochemicals on the NPs, minute (smaller) dosage can have enhanced and significant effects. Moreover, NPs due to their small size are capable of taking advantage of the enhanced permeability and retention effect on diseased cells and pass through cellular barrier that would be otherwise impossible for the bulk material.12
Figure 6.
Effect of berry-AuNPs on PC-3 and Panc-1 cells after 24 h exposure. (a) and (b) The AB_AuNPs and (c) and (d) the EB_AuNPs, (a) and (c) reduced PC-3, and (b) and (d) Panc-1 cell viability. The concentrations of the NPs are based on the concentration of the extract. *Indicate statistical significance at p < 0.05.
Green synthesis of metallic NPs using AB and EB extracts and their remarkable bio-activities have been previously reported. EB_AuNPs have shown potential as anti-diabetic therapy by reducing blood glucose levels, oxidative stress and inflammation in rats.13 EB_AgNPs showed enhanced in vitro anti-proliferative activity and exhibited anti-inflammatory effects on HaCaT keratinocytes when compared to EB extracts. Topical application of EB_AgNPs cream on patients (humans) with psoriasis vulgaris skin lesions, presented better anti-inflammatory effect when compared to commercial hydrocortisone.6 Encapsulating lipophilic or poorly water soluble compounds from EB extracts in liposomes,7 and AB extracts in either chitosan14 or oil nanoemulsion15 greatly enhanced their activity. The use of AB oil nanoemulsion in photothermal therapy was also reported to have significantly improved anti-cancer properties in melanoma cells and reduced tumor size in mice models.15
Conclusions
Phytochemicals extracted from AB and EB showed potential to reduce the gold salt and produce AuNPs. The berry extracts did not show anti-proliferative effects on Panc-1 cells after 24 h of treatment, significant cytotoxic effects on PC-3 cells were obtained at very high concentrations of the AB extracts. Interestingly, the AB_AuNPs and EB_AuNPs were effective in killing the prostate and pancreatic cancer cells. Studies to identify the different bioactive compounds in the extracts as well as the phytochemicals responsible for producing the berry-AuNPs are underway. The active compounds will be isolated and used to synthesize the metallic NPs. Certain parameters (e.g. pH, temperature and extract concentration) need to be optimized for a controlled synthesis of homogeneous NPs. Furthermore, the mechanisms involved in berry-AuNPs anti-cancer activities and their specificity also need to be explored.
Acknowledgments
We would like to acknowledge the “University of Missouri South African Education Program (UMSAEP)” for funding the research visit of Dr Nicole Sibuyi to the “Institute of Green Nanotechnology and Cancer Nanotechnology (University of Missouri, Department of Radiology, Columbia, USA) where this research was conducted.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was funded by the UMSAEP) and the DSI/Mintek Nanotechnology Innovation Centre.
ORCID iD: Nicole Remaliah Samantha Sibuyi  https://orcid.org/0000-0001-7175-5388
https://orcid.org/0000-0001-7175-5388
References
- 1. Bobo D, Robinson KJ, Islam J, et al. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 2016; 33: 2373–2387. [DOI] [PubMed] [Google Scholar]
- 2. Khoobchandani M, Katti K, Maxwell A, et al. Laminin receptor-avid nanotherapeutic EGCG-AuNPs as a potential alternative therapeutic approach to prevent restenosis. Int J Mol Sci 2016; 17(3): 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh P, Kim YJ, Zhang D, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 2016; 34(7): 588–599. [DOI] [PubMed] [Google Scholar]
- 4. Brito C, Stavroullakis AT, Ferreira AC, et al. Extract of acai-berry inhibits osteoclast differentiation and activity. Arch Oral Biol 2016; 68: 29–34. [DOI] [PubMed] [Google Scholar]
- 5. Hassimotto NMA, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem 2005; 53: 2928−2935. [DOI] [PubMed] [Google Scholar]
- 6. David L, Moldovan B, Vulcu A, et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf B Biointerfaces 2014; 122: 767–777. [DOI] [PubMed] [Google Scholar]
- 7. Bryła A, Lewandowicz G, Juzwa W. Encapsulation of elderberry extract into phospholipid nanoparticles. J Food Eng 2015; 167: 189–195. [Google Scholar]
- 8. Nile SH, Park SW. Edible berries: bioactive components and their effect on human health. Nutrition 2014; 30: 134–144. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharjee S. DLS and zeta potential – what they are and what they are not? J Control Release 2016; 10(235): 337–351. [DOI] [PubMed] [Google Scholar]
- 10. Wang XY, Liu L, Zhu R, Kang T, et al. Cytotoxic activities of some selected medicinal plants of the genus Euphorbia. J Med Plant Res 2011; 5(31): 6766–6769. [Google Scholar]
- 11. Del Pozo-Insfran D, Percival SS, Talcott ST. Açai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J Agric Food Chem 2006; 54(4):1222–1229. [DOI] [PubMed] [Google Scholar]
- 12. Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol 2014; 1477(3155): 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Opris R, Tatomir C, Olteanu D, et al. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf B Biointerfaces 2017; 150: 192–200. [DOI] [PubMed] [Google Scholar]
- 14. Shim HR, Lee JS, Nam HS, et al. Nanoencapsulation of synergistic combinations of acai berry concentrate to improve antioxidant stability. Food Sci Biotechnol 2016; 25: 1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monge-Fuentes V, Muehlmann LA, Longo JP, et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: a potential treatment for melanoma. J Photochem Photobiol B 2017; 166: 301–310. [DOI] [PubMed] [Google Scholar]