Abstract
Chronic pain is highly prevalent worldwide and severely affects daily lives of patients and family members. Praeruptorin C (Pra-C) is a main active ingredient derived from Peucedanum praeruptorum Dunn, traditionally used as antibechic, anti-bronchitis and anti-hypertension drug. Here, we evaluated the effects of Pra-C in a chronic inflammatory pain mouse model induced by complete Freund’s adjuvant (CFA) injection. Pra-C (3 mg/kg) treatment for just 3 days after CFA challenge relieved CFA-induced mechanical allodynia and hindpaw edema in mice. In the anterior cingulate cortex (ACC), Pra-C treatment inhibited microglia activation and reduced levels of proinflammatory cytokines, TNF-α and IL-1β, and suppressed upregulation of glutamate receptors caused by CFA injection. In addition, Pra-C attenuated neuronal hyperexcitability in ACC of CFA-injected mice. In vitro studies confirmed the analgesic effect of Pra-C was due to its inhibitory ability on microglial activation. In conclusion, Pra-C administration had a certain effect on relieving chronic pain by inhibiting microglial activation, attenuating proinflammatory cytokine releasing and regulating excitatory synaptic proteins in the ACC of the CFA-injected mice.
Keywords: Praeruptorin C, inflammatory pain, microglia, proinflammatory cytokine
Introduction
Pain has been classified into two major forms, one is acute pain, which lasts a short moment and often serves as protective signals, the other is chronic pain, which lasts for a long time induced by tissue or nerve injury and causes mood disorders, sleep loss and cognitive impairment. Chronic pain severely affects working efficiency and living quality.1 Up to 19% of adults are suffering from chronic pain, which has become an enormous burden to patients, families and society.2 The studies of mechanism and new drugs on chronic pain or pain-related diseases remain as hot topics in current and future medical researches.
According to definition of pain by International Association for the Study of Pain (IASP), one can not experience pain without the central nervous system (CNS).3 Anterior cortical cortex (ACC), along with the somatosensory cortex, prefrontal cortex, insular cortex and amygdala, is a core brain region related to sensory and negative feelings of chronic pain shown by human brain imaging studies.4 ACC stimulation could facilitate spinal sensory excitatory transmission and cause behavioral pain sensitization, suggesting this top-down facilitation may be very important in the process of chronic pain.5 Inhibition of excitatory transmission in ACC could reverse chronic pain, which could also be reduced by blocking the descending facilitation of corticospinal projections.6
Inflammation is one of the main causes of chronic pain, systemic inflammation could lead to hyperalgesia and allodynia, both of which are clinical characterizations of chronic pain.7 Microglia, the resident macrophages of the CNS, has been reported taking part in various brain pathologies.8 Under stimuli like inflammation, microglia would secrete more proinflammatory cytokines, including TNF-α and IL-1β, which mediates synaptic plasticity,9 and plays a critical role in chronic pain.10,11 Drugs inhibiting microglial activation have shown analgesic effect in different animal models,12,13 suggesting a potential target for treating chronic pain.
Praeruptorin C (Pra-C) is a component derived from the root of Peucedanum praeruptorum Dunn, used as an expectorant, antibechic, anti-bronchitis and anti-hypertension drug in traditional Chinese medicine.14 Previous studies have suggested that Pra-C could protect neurons from excitatory toxicity through down-regulating glutamate N-methyl-D-aspartate (NMDA) receptor subtype,15 and inhibit inflammatory responses in lipopolysaccharide (LPS)-stimulated RAW264.7 cells through blocking NF-κB and STAT3 signal pathway.16 Herein, we hypothesized that Pra-C may provide anti-inflammation effect in the CNS especially acting on microglia for treating chronic pain caused by systemic inflammation.
In the present study, we treated the mice with Pra-C (i.p. injection) after complete Freund’s adjuvant (CFA) injection into the hind paws of mice, a classic way to induce inflammatory pain, to investigate the possible role of Pra-C in chronic pain and the involved mechanisms.
Materials and methods
Materials
Complete Freunds adjuvant (CFA), Lipopolysaccharides (LPS), anti-β-actin antibody and Hoechst 33258 were obtained from Sigma (St. Louis, MO, USA). Primary antibody, including, GluN2A, GluN2B, GluA1, GluA2, Iba-1 were purchased from Abcam (Cambridge, UK). All secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Hyclone (Logan, UT, USA), Neurobasal medium, B27, and glutamine were provided by Invitrogen (Carlsbad, CA, USA). In addition, Pra-C was purchased from Shanghai PureOne Biotechnology (Shanghai, China), and was of 98% pure to meet the standard. All of the chemicals and reagents used were of standard biochemical quality.
Animals
C57BL/6 adult male mice (5 weeks) were got from the Experimental Animal Center of the Air Force Military Medical University (Xi’an, China). All animal experiments were carried out under protocols approved by the Animal Care and Use Committee of the Fourth Military Medical University and performed following the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996). They were placed in an environment of alternating light of 12 hours and darkness of 12 hours with a temperature of 24±2°C and a relative humidity of 50-60%. Sufficient food and water were given without regular addition. Mice were allowed to acclimate for 2 weeks of laboratory environment before any experiments.
Experimental designs and Pra-C treatment
Mice were divided into 3 groups, control, CFA and Pra-C groups. The latter two groups of mice were injected subcutaneously with 10 µl CFA (50% in saline) into the left paw of the mice to induce the chronic inflammatory pain, while the control animals were injected with an equal volume of 0.9% saline into the same position. Pra-C was dissolved in olive oil and administrated intraperitoneally (3 mg/kg) to Pra-C group on day 1, 2, 3 after CFA injection. Pra-C dose was selected based on previous study.17 All the behaviors of animals were tested at a fixed time during testing days. Animals were accommodated for 30 min in the testing room before behavioral tests and were sacrificed to collect the ACC after behavior tests on day 21 to examine molecular events during phase of hyperalgesia and effects of Pra-C treatment.
Mechanical allodynia
Mice were placed in individual plastic boxes for 30 min to get acclimatization. The mechanical allodynia was assessed with a set of von Frey filaments (0.008-2g) by using the up-down paradigm before (Day 0) and after the CFA injection (Day 1, 3, 7, 14 and 21). The von Frey filaments were applied to the point of bending on the plantar surface of hindpaws and repeated 6 times at 10-second intervals. The injected hindpaw was tested with intervals longer than 3min. Positive responses included prolonged hindpaw withdrawal followed by licking or scratching. The next force filaments were selected according to the previous results.
Cell culture and treatment
BV2 cell lines were purchased from China Center for Type Culture Collection (CCTCC). BV-2 cells were maintained in DMEM supplemented with 10% Fetal Bovine Serum and 1% penicillin/streptomycin. Cells were cultured in a humidified incubator with 5% CO2 at 37°C and passaged twice every 2–3 days.
Primary cortical neuron cultures were prepared as described previously.18 Briefly, the cortex was isolated from 13– to 15-day-old C57BL/6 mouse embryos and trypsinized for 15 min with 0.25% trypsin. Cells were seeded onto 6-well plates that were precoated with 25 μg/mL poly-d-lysine in water. 24 h after plating with DMEM containing 20% FBS, the medium was completely replaced with neurobasal medium supplemented with 2% B27 (Invitrogen, A3582801). Half of the medium was replaced every 3 days, and neurons were cultured in a humidified incubator with 5% CO2 at 37°C, and used for experiments at 10 days in vitro.
The BV2 cells were seeded in a 6-well plate at a density of 1 × 105cell/well. Then, cells were incubated with Pra-C (10 μM) for 1 h, followed by stimulating with LPS (100 ng/ml) for 24 h. Pra-C dose was selected base on previous study.15 After stimulation, the LPS and Pra-C-containing medium was replaced with fresh medium and the stimulated BV2 cells were cultured for another 24 h. After centrifugation at 150×g for 5 min and removal of cell debris, the supernatants were collected as the conditioned medium (CM). Half of the neuronal culture medium was replaced by the conditioned medium. After 24 h, total proteins were extracted from neurons for the Western-blot analysis.
Western blot
Western blot analysis was conducted as described previously.19 Tissue samples from the ACC were dissected from the brain slices and cell proteins were isolated from cultured cortex neurons. All proteins were quantified by BCA Kits and equal amounts of protein were separated and electrotransferred onto Immun-Blot PVDF membranes. Membranes were blocked with 5% non-fat milk in Tris-phosphate buffer containing 0.05% Tween 20 (TBST) for 1 h and then were incubated with primary antibodies at 4°C overnight. Membranes were washed 3 times with TBST for 5 min of each time and were further incubated with HRP-conjugated secondary antibodies. The following primary antibodies were used: GluN2A (1:500; Abcam, ab133265), GluN2B (1:500; Abcam, ab65783), GluA1 (1:500; Abcam, ab31232), GluA2 (1:1000; Abcam, ab133477), β-actin (1:10000; Sigma, A5441).
Enzyme-linked immunosorbent assay (ELISA)
The ACC tissue samples were isolated from the brain of mice after behavior tests. The supernatants of BV-2 cells were collected as mentioned previously in cell treatment section. The content of TNF-α (R&D Systems, DY410) and IL-1β (R&D Systems, DY401) in both tissue and supernatant samples was detected by double-antibody sandwich method according to the manufacturer’s instructions (R&D Systems).
Immunofluorescence staining
After behavior tests, mice were anesthetized with diethyl ether and perfused with 4% polyformaldehyde. The brain was isolated from the mice and was immersed in 4% paraformaldehyde overnight and then dehydrated through an ascending sucrose series (15% and 30% (w/v) sucrose in 0.1 M PBS) at 4°C overnight respectively. Coronal sections (30 μm) from the ACC were cut by a cryogenic constant sectioning device (Leica, CM1950), immediately mounted on superfrost slides, and stored at 4°C overnight to dry. The next day, all sections were washed with 0.3% Triton X-100 PBS and then were blocked (10% goat serum, 0.1% Triton X-100 in PBS) for 2 hours at 4°C. The brain sections were incubated with goat-anti-Iba-1(1:100; Abcam, ab178847) in blocking solution for 16 h at 4°C and washed with PBS, further incubated with goat anti-rabbit IgG Alexa Fluor 594 (1:200; Abcam, ab150080) in PBS for 1.5 h at room temperature in the dark environment. Nuclei were counter-stained with Hoechst 33258 (Sigma, 94403). The slides were coverslipped with 50% glycerine and were photographed with an Olympus Fluoview FV100 microscope (Olympus, Japan).
Whole-cell patch-clamp recording
The mice were anesthetized by isoflurane in air and then decapitated. Brains were placed for 2 min in an ice-cold artificial cerebrospinal fluid (ACSF, in mM) containing 124 NaCl, 2.5 KCl, 1 MgCl2, 2M CaCl2, 25 NaHCO3, 1 NaH2PO4 and 10 Glucose (310–320 mOsm), continuously bubbling with mixed gas of 95% O2 and 5% CO2. Coronal brain slices containing the ACC were prepared just like previously described.20 Experiment was performed in a recording chamber on the stage of an Olympus microscope with infrared digital interference contrast optics for visualization of whole-cell patch-clamp recordings. Continuous perfusion of ACSF at a rate of 2 ml/min were given during the whole recording time and the room temperature was maintained at 23±1°C. Recording pipettes (3–6 MΩ) were filled with solution containing (mM) 5 NaCl, 145 K-gluconate, 1 MgCl, 10 HEPES, 0.2 EGTA, 2 K-ATP, and 0.1 Na3-GTP, adjusted to pH 7.2 with KOH (280–290 mOsm). Spontaneous excitatory postsynaptic currents (sEPSC) were recorded form layer II-III neurons clamped at –70 mV with an Axon 200B amplifier (Axon Instruments, CA). Access resistance was 15–30 MΩ and monitored throughout the experiment. Data were discarded if access resistance changed more than 20% during an experiment.
Data analyses
All data were presented as mean ± SEM. The statistical significance of differences between groups were analyzed with One-way analysis of variance (ANOVA) followed by LSD and S-N-K(s) t-tests. In all cases, the criterion for statistical significance was p < 0.05.
Results
Effect of Pra-C on CFA-induced hindpaw edema and mechanical allodynia in mice
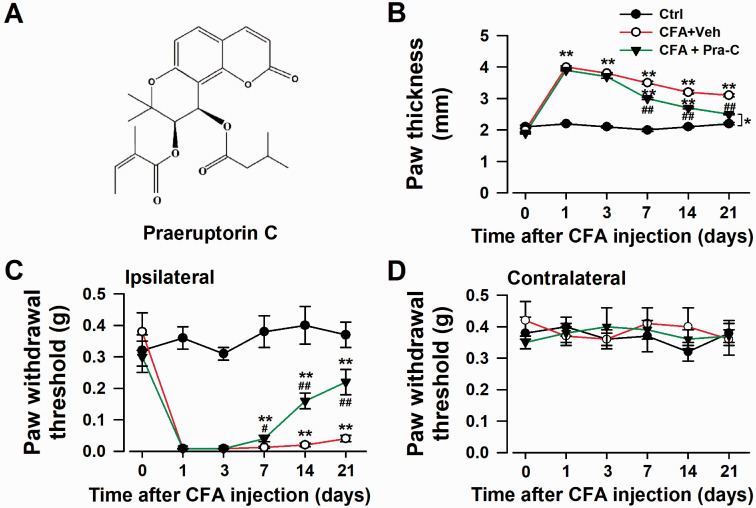
Inflammatory pain is one of the main forms of chronic pain, usually serves as the model for researchers to find the potential treatment targets and new drugs. Intraplantar injection of CFA into the left hind paw produced a significant enhancement of hypersensitivity to mechanical stimuli in the ipsilateral hind paw, and this pain-sensitive feeling could last more than 21 days.21 Pra-C (3 mg/kg) administration from Day 1 to Day 3 could considerably reduce mechanical allodynia (Figure 1(c)). The paw withdrawal threshold didn’t change much in the contralateral hind paw (Figure 1(d)). Moreover, Pra-C treatment significantly decreased the localized swelling caused by CFA injection (Figure 1(b)). These results suggested that Pra-C exerted analgesia and anti-inflammation effects.
Figure 1.
Pra-C relieved the chronic inflammatory pain. (a) Chemical structure of Pra-C. (b) Pra-C reduced the hindpaw edema induced by CFA injection. (c) Pra-C attenuated mechanical allodynia in the ipsilateral hindpaw. Mechanical allodynia was detected on days 0, 1, 3, 7, 14, 21 after CFA injection. (d) No difference in paw withdrawal threshold among groups. Each value represents the mean ± SEM of three independent experiments (n = 6 in each group, *p < 0.05, **p < 0.01 vs. control group, #p < 0.05, ##p < 0.01 vs. CFAvehicle injected group). Ctrl: control; CFA: complete Freund’s adjuvant; Veh: vehicle; Pra-C: Praeruptorin C.
Pra-C attenuated the activation of microglia and reduced inflammatory cytokines in the ACC of mice with inflammatory pain
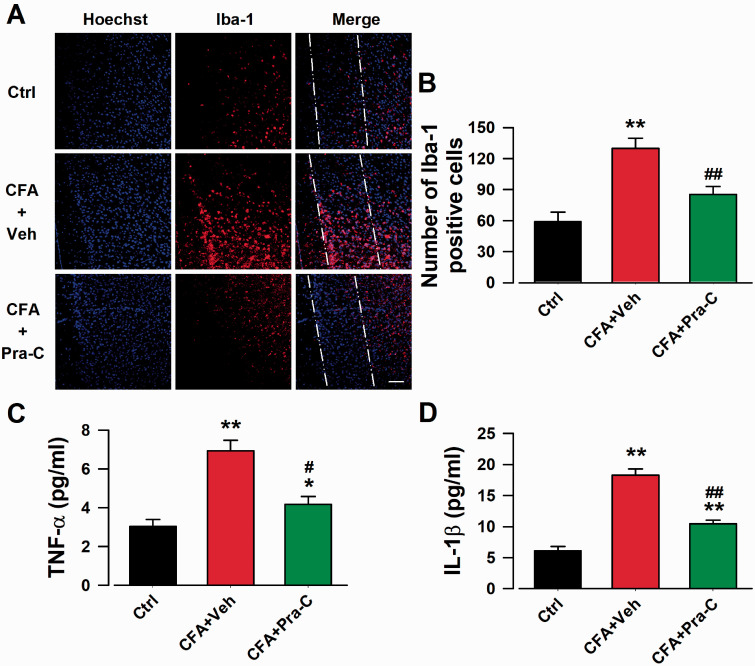
Microglia, the main cells related to inflammatory stimuli, are activated 24 h after tissue damage and could last for 3 months,22 along with increased secretion of inflammatory cytokines, such as TNF-α and IL-1β.23 To further determine the anti-inflammatory effect of Pra-C, we harvested brain slices containing ACC for immunofluorescence staining (Figure 2(a)), and collected ACC tissue for ELISA on day 21 just after behavioral test. After CFA injection, the number of activated microglia was significantly increased (F(2,12) = 19.852, p < 0.01, Figure 2(b)), while Pra-C treatment just for 3 days, which was believed to be the critical window for microglia activation, could dramatically attenuate these increasement (F(2,12) = 19.852, p < 0.01, Figure 2(b)). Systemic inflammation caused by CFA led to a significant upregulation in levels of TNF-α (F(2,12) = 36.386, p < 0.01, Figure 2(c)) and IL-1β (F(2,12) = 33.584, p < 0.01, Figure 2(d)), while the consequence was reversed by Pra-C treatment ((F(2,12) = 36.386, p < 0.05, Figure 2(c); (F(2,12) = 33.584, p < 0.01, Figure 2(d))). These findings suggested that Pra-C attenuated immune cell activation and the release of inflammatory products in the ACC after CFA injection, which may be the mechanism for the analgesic effect of Pra-C.
Figure 2.
Pra-C inhibited microglial activation and inflammatory cytokines release in ACC. (a) ACC slices were immunostained with Iba-1 antibody (red), and nuclei were stained with Hoechst 33258 (Blue). Scale bar = 50 µm. Dash-dot-dot lines were used to indicate layer I (left), II (middle) and III (right) of ACC. (b) Pra-C treatment showed inhibition of microglial activation by reducing the number of Iba-1 positive cells. Pra-C reduced the elevated levels of TNF-α (c) and IL-1β (d) in ACC on day 21 after CFA injection. Each value represents the mean ± SEM of three independent experiments (n = 5 in each group, *p < 0.05, **p < 0.01 vs. control group, #p < 0.05, ##p < 0.01 vs. CFA-vehicle injected group).
Pra-C reversed up-regulation of excitatory synaptic proteins in the ACC
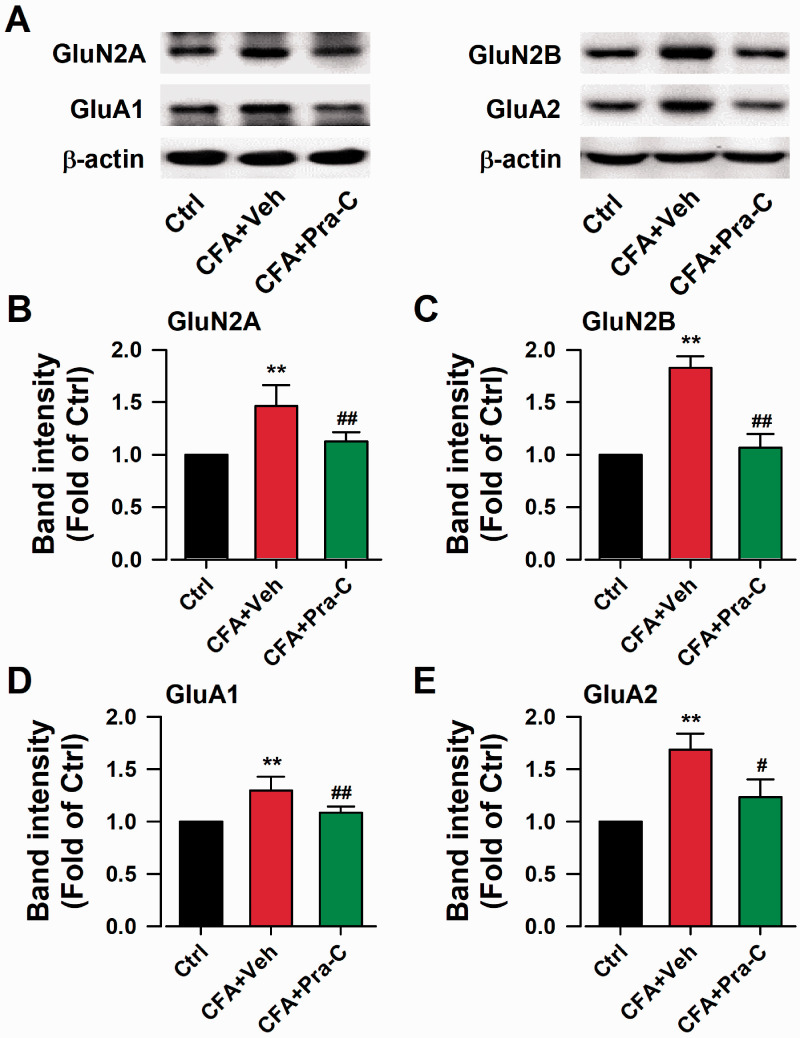
The excitatory protein level upregulation in some brain regions could affect synaptic plasticity and promote nervous disorders.24 Glutamate receptors are most studied excitatory synaptic proteins, such as GluA1 and GluA2-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, GluN2A and GluN2B-containing NMDA receptors.25 To investigate the role of Pra-C in chronic pain mediating, we collected ACC region of mice to perform western blots. The expression of AMPA receptor GluA1 (F(2,6) = 24.611, p < 0.01, Figure 3(d)), GluA2 (F(2,6) = 29.163, p < 0.01, Figure 3(e)) and NMDA receptor GluN2A (F(2,6) = 14.689, p < 0.01, Figure 3(b)), GluN2B (F(2,6) = 31.166, p < 0.01, Figure 3(c)) significantly increased. However, Pra-C treatment for 3 days reversed these upregulated expressions (F(2,6) = 14.689, p < 0.01, Figure 3(b); F(2,6) = 31.166, p < 0.01, Figure 3(c); F(2,6) = 24.611, p < 0.01, Figure 3(d); F(2,6) = 29.163, p < 0.05, Figure 3(e)). These results indicated that Pra-C could reverse the abnormal up-regulated excitatory proteins in CFA-treated mice.
Figure 3.
Effects of Pra-C on excitatory synaptic proteins in ACC. (a) Representative results of Western blot analysis showed the expression of GluN2A, GluN2B, GluA1, GluA2. Pra-C treatment significantly decreased the upregulated expression of GluN2A (b), GluN2B (c), GluA1 (d) and GluA2 (e) on the day 21 after CFA injection. Each value represents the mean ± SEM of three independent experiments (n = 3 in each group, **p<0.01 vs. control group, #p<0.05, ##p<0.01 vs. CFA-vehicle injected group).
Effects of Pra-C on excitatory synaptic transmission in the ACC
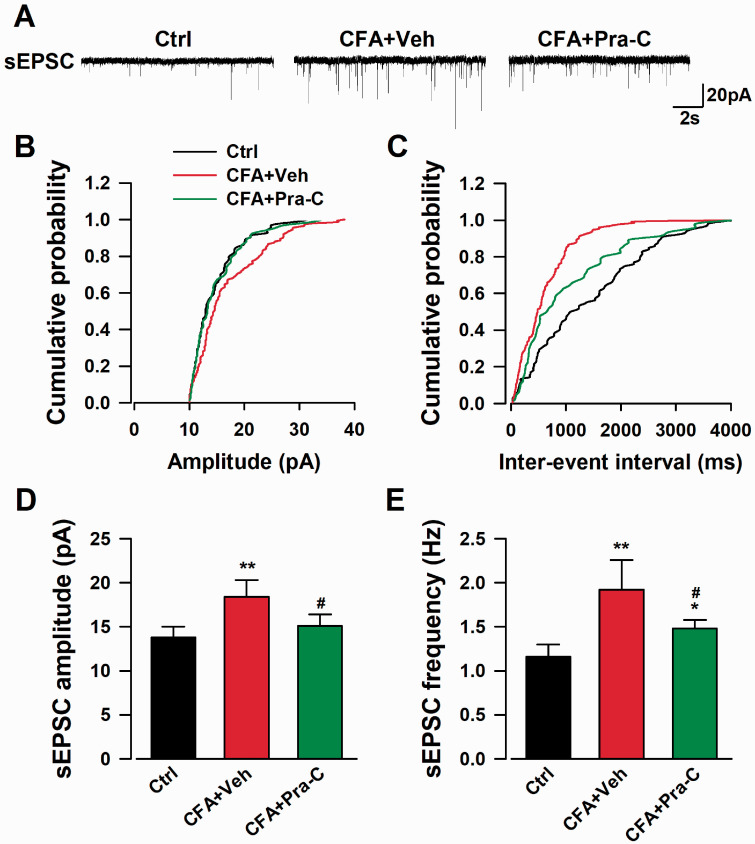
Since the expressions of excitatory synaptic proteins in the ACC were changed after CAF-injection, we wondered whether the function of neurons was disturbed. We performed whole-cell patch-clamp recordings and recorded spontaneous excitatory postsynaptic currents (sEPSC) in the neurons of ACC 21 days later since the CAF injection. Both the amplitude (F(2,21) = 8.274, p < 0.01, Figure 4(d)) and frequency (F(2,21) = 8.324, p < 0.01, Figure 4(e)) of sEPSC were significantly increased in the ACC neurons of CFA-injected mice, whereas Pra-C treatment could dramatically reverse the enhancement of both amplitude (F(2,21) = 8.274, p < 0.05, Figure 4(d)) and frequency (F(2,21) = 8.324, p < 0.05, Figure 4(e)) of sEPSC induced by CFA injection (Figure 4). These results strongly showed the regulatory effect on excitability of ACC neurons by Pra-C.
Figure 4.
Pra-C treatment abolished the enhanced excitatory transmission in neurons of ACC after CFA injection. (a) The sample showed the sEPSCs in pyramidal neurons of the ACC in each group. Cumulative amplitude (b) and frequency (c) histogram of sEPSC from slices in different groups. The amplitude (d) and frequency (e) of sEPSC analysis showed Pra-C treatment remarkably reversed both increased indicates in the ACC neurons after CFA injection. All graph represented mean ± SEM (n=8 neurons/4 mice, *p < 0.05, **p < 0.01 vs. control group, #p < 0.05 vs. CFA-vehicle injected group) .sEPSC: spontaneous excitatory postsynaptic current.
Inhibition of cytokines released from microglia by Pra-C attenuated abnormal neuronal excitability
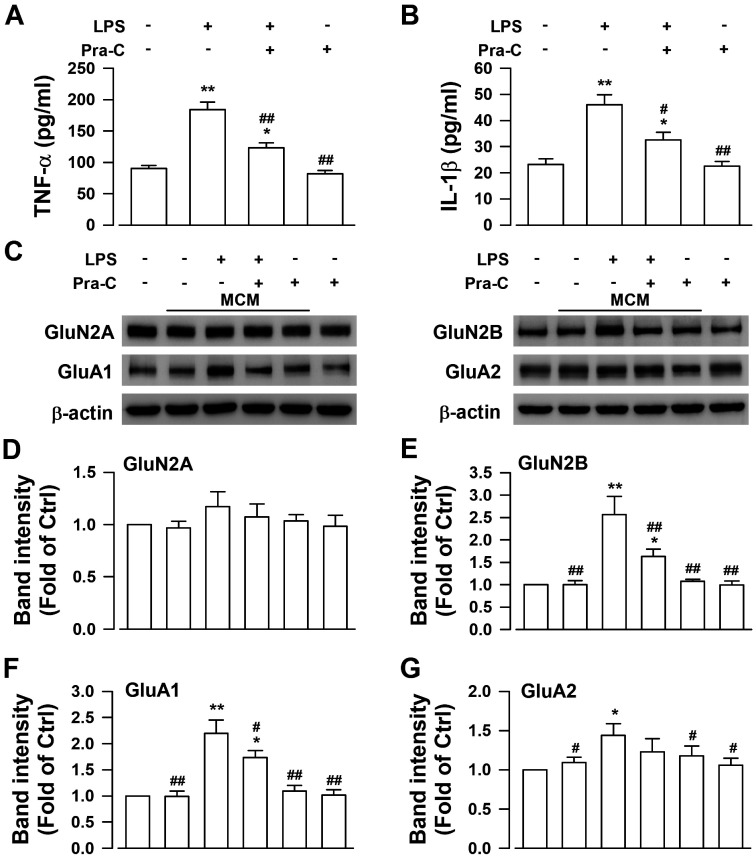
To confirm the target of Pra-C since it exhibited excitability regulatory effect under chronic pain condition, we cultured and treated BV-2 cells and primary cortical neurons respectively. After pre-treatment with the dose of 10 μM Pra-C or vehicle, BV-2 cells were stimulated with 100 ng/ml of LPS for activation. Supernatant medium was collected and the concentration of cytokine was tested by ELISA. As was shown in Figure 5(a) and (b), LPS increased the release of TNF-α (F(3,16) = 29.940, p < 0.01, Figure 5(a)) and IL-1β (F(3,16) = 18.938, p < 0.01, Figure 5(b)). However, pretreatment with Pra-C could inhibit the effects of LPS (F(3,16) = 29.940, p < 0.01, Figure 5(a); F(3,16) = 18.938, p < 0.01, Figure 5(b)). Then we mixed the same volume of BV-2 supernatant (drug-free because the whole medium was changed 24 h before collection) with neurobasal to get microglial conditioned medium (MCM). Incubation with MCM from LPS-stimulated BV-2 increased excitatory synaptic proteins GluA1 (F(5,18) = 41.706, p < 0.01, Figure 5(c) and (f)), GluA2 (F(5,18) = 5.647, p < 0.05, Figure 5(c) and (g)) and GluN2B (F(5,18) = 34.796, p < 0.01, Figure 5(c) and (e)) significantly, but Pra-C pretreatment attenuated the upregulation of GluA1 (F(5,18) = 41.706, p < 0.05, Figure 5(c) and (f) and GluN2B (F(5,18) = 34.796, p < 0.01, Figure 5(c) and (e)) dramatically. Incubation with none-treated MCM or directly treatment with Pra-C showed no significant change in these excitatory synaptic proteins compared with control groups (Figure 5(c) to (g)). These results indicated that of Pra-C regulated neuronal excitability through inhibition of microglial activation.
Figure 5.
Pra-C attenuated increased cortical neuronal excitability by inhibiting cytokines released from microglia. Elevated levels of TNF-α (a) and IL-1β (b) released from BV-2 cells after LPS stimulation were significantly abolished by pretreatment with Pra-C. (c) Representative results of Western blot analysis showed expression levels of GluN2A, GluN2B, GluA1, GluA2 in primary cultured cortical neurons under different treatment. (d) GluN2A levels didn’t show significant change among these groups. (e–g) MCM from BV-2 activated by LPS with pretreatment with Pra-C notably prevented upregulation of GluN2B (e) and GluA1 (f) in cortical neurons, but no significant change in GluA2 levels. None treated or Pra-C treated MCM, and Pra-C directly incubation didn’t change these excitatory synaptic proteins in cortical neurons (d–g). Each value represents the mean ± SEM of three independent experiments (n = 5 in each group of ELISA assay and n = 4 in each group of Western blot, *p < 0.05, **p < 0.01 vs. control group, #p < 0.05, ##p < 0.01 vs. LPS stimulated group). LPS: Lipopolysaccharides; MCM: microglial conditional medium.
Discussion
In this study, we first showed that Pra-C treatment effectively relieved CFA-induced chronic inflammatory pain on mechanical allodynia and paw edema in mice. In addition, we further discovered the inhibition of microglial activation and reduced levels of released inflammatory cytokine from microglia might be responsible for the analgesic effect of Pra-C.
A large number of studies have illuminated the pivotal role of ACC in the process of chronic pain.5,26 ACC acts as nociceptive information collector from amygdala, thalamus and other pain-related regions in mammalian brain.27 Besides, in vivo and in vitro electrophysiological studies have demonstrated ACC neurons respond to noxious stimuli, and the excitability of ACC neurons increases with intensity of pain.28 Inhibition of ACC activity with epigenetic or pharmacologic methods is analgesic in different animal models of chronic pain,29,30 and surgical ablation of ACC can reduce pain sensitation.31 In this case, drugs that could inhibit ACC activity right after injury or other noxious stimuli may have potential analgesic functions. Previous studies have indicated the non-neuronal cells especially microglia were involved in the initiation of pain, not only in spinal cord but also in the ACC.32 We administered Pra-C to mice after CFA injection, and the Pra-C treatment lasted only for the first 3 days, which is just the time window as microglial activation and before astrocytic activation.33 As resident immune cells in CNS, under autoimmune inflammation conditions, activated microglia releases and responses to several cytokines, including TNF-α and IL-1β, which contribute the maintenance of pain. Our result has shown that Pra-C treatment remarkably inhibited activation of microglia in ACC of CFA-injected mice, and significantly reduced inflammatory cytokine release in ACC. This suggested that Pra-C could relieve pain through its microglial inhibitory effect.
Glutamate is the major excitatory transmitter in the CNS, activating glutamate receptors, including AMPARs and NMDARs. These synaptic proteins are key molecules responsible for the development of pain hypersensitivity.34 Upregulation of these excitatory glutamate receptors have been found in many studies within different animal models.35,36 Inhibition of the ACC glutamatergic system, especially reducing AMPAR and NMDAR accumulation, reversed chronic pain.6 We found that the reversed elevation of GluA1, GluA2, GluN2A, GluN2B in ACC of CFA-injected mice caused by Pra-C treatment may correct abnormal excitability changes in ACC, which was responsible for pain relief. And our electrophysiological experiment result confirmed the ACC neuronal excitability had been reversed by Pra-C treatment after CFA-injection, partially demonstrated the function of Pra-C on hyperactivity in ACC. Future studies were needed to reveal the direct effect of Pra-C in synaptic plasticity.
Glial cells support and affect neurons all the time. Neuron-glia crosstalk participate in many CNS disorders like stroke,37 neurodegenerative diseases38 as well as pain state.39 The activation of microglia leads to inflammatory cascade responses, releasing high levels of pro-inflammatory cytokines, acting on neurons around and results in abnormal excitability enhancement. In a previous study, we observed that Pra-C exerts neuroprotection effect against excitatory toxicity through inhibiting GluN2B of cortical neurons.15 Whether the Pra-C reduced neuronal excitability through inhibition of microglial activation or directly targeting neurons needs to be clarified. We performed in vitro tests on BV-2, a widely used mouse microglial cell line. After pretreatment with Pra-C and stimulation with LPS, the medium was changed totally to avoid drug existance in MCM affecting neurons. Our results indicated that Pra-C alone or MCM with Pra-C treatment didn’t affect levels of excitatory synaptic proteins in cortical neurons, while Pra-C pretreatment remarkably reduced these levels in LPS-stimulating MCM group. Additionally, abnormal expression of these excitatory proteins was highly related with inflammatory cytokines like TNF-α and IL-1β in MCM. These findings, in line with previous studies, indicate that the analgesic effect of Pra-C is associated with the inhibition of microglial activation.
To conclude, the present results showed that Pra-C relieves inflammatory pain induced by CFA challenge in mice. It also confirmed that microglial inhibition, proinflammatory cytokine releasing blockage and excitatory synaptic protein regulation attribute to the analgesic effect of Pra-C. Further studies need to assess the effects of Pra-C in other pain models. In short, Pra-C should be considered as a potential agent for further development in chronic pain treatment.
Acknowledgment
Thanks for Dr. Yun-Qing Li providing the lab and equipment in the Fourth Military Medical University for the whole process during immunofluorescence staining.
Footnotes
Author Contributions: LY conceived and designed the study. MG-Z assisted in designing the study. DJ-S, SY-W performed the animal experiments and analyzed the data, LF-L performed the cell experiments and analyzed the data. YJ-W and Q-Y did literature research, data acquisition and analysis. DJ-S and LY wrote the manuscript, and MG-Z proofread it. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China under Grant 31800887; Research Foundation from Social Development Science and Technology Project of Shaanxi Province under Grant 2018SF-305; and Young Talent Project in Tangdu Hospital.
ORCID iDs: Ming-gao Zhao https://orcid.org/0000-0002-7539-3887
References
- 1.Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Progr Neuro-psychopharmacol Biol Psychiatry 2018; 87: 159–167. [DOI] [PubMed] [Google Scholar]
- 2.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, Zhang N, Luo MH, Tao W, Wang H, Li J, Li J, Qiu BS, Zhou JN, Li X, Xu H, Wang K, Zhang X, Liu Y, Richter-Levin G, Xu L, Zhang Z. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 2019; 22: 1649–1658. [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat RevNeurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li ZH, Lu YC, Inoue K, Tsuda M, Li YQ, Nakatsuka T, Zhuo M. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun 2018; 9: 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Wang W, Dong YL, Zhang MM, Wang J, Koga K, Liao YH, Li JL, Budisantoso T, Shigemoto R, Itakura M, Huganir RL, Li YQ, Zhuo M. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol Brain 2014; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci 2018; 19: 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013; 155: 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micaroni M, Stanley AC, Khromykh T, Venturato J, Wong CX, Lim JP, Marsh BJ, Storrie B, Gleeson PA, Stow JL. Rab6a/a' are important Golgi regulators of pro-inflammatory TNF secretion in macrophages. PLoS One 2013; 8: e57034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain J Neurol 2018; 141: 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popiolek-Barczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM, Pilat D, Mika J. Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast 2015; 2015: 676473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang TT, Xue R, Fan SY, Fan QY, An L, Li J, Zhu L, Ran YH, Zhang LM, Zhong BH, Li YF, Ye CY, Zhang YZ. Ammoxetine attenuates diabetic neuropathic pain through inhibiting microglial activation and neuroinflammation in the spinal cord. J Neuroinflamm 2018; 15: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkhail P, Shafiee A, Sarkheil P. Biological activities and pharmacokinetics of praeruptorins from Peucedanum species: a systematic review. BioMed Res Int 2013; 2013: 343808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Li XB, Yang Q, Zhang K, Zhang N, Guo YY, Feng B, Zhao MG, Wu YM. The neuroprotective effect of praeruptorin C against NMDA-induced apoptosis through down-regulating of GluN2B-containing NMDA receptors. Toxicol In Vitro 2013; 27: 908–914. [DOI] [PubMed] [Google Scholar]
- 16.Yu PJ, Jin H, Zhang JY, Wang GF, Li JR, Zhu ZG, Tian YX, Wu SY, Xu W, Zhang JJ, Wu SG. Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-kappaB and STAT3 activation. Inflammation 2012; 35: 967–977. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wang J, Yang L, Zhou SM, Guan SY, Yang LK, Shi QX, Zhao MG, Yang Q. Effect of Praeruptorin C on 3-nitropropionic acid induced Huntington's disease-like symptoms in mice. Biomed Pharmacother 2017; 86: 81–87. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Yang Q, Zhang K, Li YJ, Wu YM, Liu SB, Zheng LH, Zhao MG. Neuroprotective effects of daphnetin against NMDA receptor-mediated excitotoxicity. Molecules 2014; 19: 14542–14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Yang ZM, Zhang N, Tian Z, Liu SB, Zhao MG. Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons. Mol Cell Biochem 2014; 386: 251–258. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q, Zhang K, Liu SB, Zhao MG, Wu YM. Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun 2016; 56: 352–362. [DOI] [PubMed] [Google Scholar]
- 21.Bravo L, Llorca-Torralba M, Suarez-Pereira I, Berrocoso E. Pain in neuropsychiatry: insights from animal models. Neurosci Biobehav Rev 2020; 115: 96–115. [DOI] [PubMed] [Google Scholar]
- 22.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005; 115: 71–83. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Fan G, Guo G, Kang W, Wang D, Xu B, Zhao J. FK506 attenuates the inflammation in rat spinal cord injury by inhibiting the activation of NF-kappaB in microglia cells. Cell Mol Neurobiol 2017; 37: 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo L, Sun T, Yang L, Liu A, Liu QQ, Tian QQ, Wang Y, Zhao MG, Yang Q. Scopoletin ameliorates anxiety-like behaviors in complete Freund's adjuvant-induced mouse model. Mol Brain 2020; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo M. Ionotropic glutamate receptors contribute to pain transmission and chronic pain. Neuropharmacology 2017; 112: 228–234. [DOI] [PubMed] [Google Scholar]
- 26.Meda KS, Patel T, Braz JM, Malik R, Turner ML, Seifikar H, Basbaum AI, Sohal VS. Microcircuit mechanisms through which mediodorsal thalamic input to anterior cingulate cortex exacerbates pain-related aversion. Neuron 2019; 102: 944–959 e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A, Patel D, Li A, Hu L, Zhang Q, Liu Y, Guo X, Robinson E, Martinez E, Doan L, Rudy B, Chen ZS, Wang J. Mapping cortical integration of sensory and affective pain pathways. Curr Biol 2020; 30: 1703 e1705–1715 e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, Navratilova E. Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 2018; 159: 2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Um SW, Kim MJ, Leem JW, Bai SJ, Lee BH. Pain-relieving effects of mTOR inhibitor in the anterior cingulate cortex of neuropathic rats. Mol Neurobiol 2019; 56: 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto K, Kume K, Ohsawa M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J Pharmacol Sci 2017; 134: 158–165. [DOI] [PubMed] [Google Scholar]
- 33.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol 2013; 716: 106–119. [DOI] [PubMed] [Google Scholar]
- 34.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Investig 2011; 121: 1608–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gui Y, Chen L, Duan S, Li G, Tang J, Li A. Methyl cinnamate alleviated CCI-induced upregualtion of spinal AMPA receptors and pain hypersensitivity by targeting AMPK. Eur J Pharmacol 2018; 833: 183–189. [DOI] [PubMed] [Google Scholar]
- 37.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol 2017; 13: 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griciuc A, Patel S, Federico AN, Choi SH, Innes BJ, Oram MK, Cereghetti G, McGinty D, Anselmo A, Sadreyev RI, Hickman SE, El Khoury J, Colonna M, Tanzi RE. TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer’s disease. Neuron 2019; 103: 820 e827–835 e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain MZ, Unno S, Ando H, Masuda Y, Kitagawa J. Neuron-glia crosstalk and neuropathic pain: involvement in the modulation of motor activity in the orofacial region. Int J Mol Sci 2017; 18: 2051. [DOI] [PMC free article] [PubMed] [Google Scholar]