Abstract
Cryptoglandular perianal fistula is a common benign anorectal disorder that is managed mainly with surgery. A fistula is typically defined as a pathological communication between two epithelialized surfaces. More specifically, perianal fistula manifests as an abnormal tract between the anorectal canal and the perianal skin. Perianal fistulas are often characterized by significantly decreased patient quality of life. The cryptoglandular theory of perianal fistulas suggests their development from the proctodeal glands, which originate from the intersphincteric plane and perforate the internal sphincter with their ducts. Involvement of proctodeal glands in the inflammatory process could play a primary role in the formation of cryptoglandular perianal fistula. The objective of this narrative review was to investigate the current knowledge of the pathogenesis of cryptoglandular perianal fistula with the specific aims of characterizing the potential role of proinflammatory factors responsible for the development of chronic inflammation. Further studies are crucial to improve the therapeutic management of cryptoglandular perianal fistulas.
Keywords: Cryptoglandular perianal fistula, pathogenesis, cytokines, adipokines, proctodeal glands, proinflammatory factors
Introduction
Perianal fistula, or fistula-in-ano, is a common benign anorectal disorder that is treated mainly with surgery. Perianal fistulas are often characterized with significant clinical manifestations, such as local pain, inflammation, purulent drainage, incontinence, and decreased patient quality of life. A fistula is typically defined as a pathological communication between two epithelialized surfaces. More specifically, perianal fistula is a chronic abnormal communication between the epithelialized surface of the anorectal canal and usually, the perianal skin. Perianal fistula resembles a narrow tunnel connecting its internal opening in the anal canal with the external opening in the skin near the anus. The fistula is lined with granulation tissue and typically results from the healing of perianal sepsis. The glandular crypts at the level of the dentate line are the typical origin of fistulas, which is induced by purulent and inflammatory processes.1
Perianal fistula is considered a later, chronic, stage of perianal abscess. Tracing the fistula canal during abscess drainage is possible in approximately 50% of cases. Perianal fistula is quite common and brings patients to a surgeon. Unfortunately, despite proper therapy and management, this disease is characterized by a high recurrence rate.
Significant variation in the epidemiology of cryptoglandular perianal fistula has been observed worldwide, with a wide range of incidence estimates both within and between geographic regions.2 Studies have shown that the incidence of developing a new cryptoglandular perianal fistula is approximately 2 per 10,000 people per year.3 Although perianal cryptoglandular fistula may affect patients at any age, the highest incidence rate is observed between 30 and 50 years of age, and men are affected more often than women.4 The current global estimated incidence rate of perianal fistula is unconfirmed. The estimated incidence in the United States was approximately 20,000 to 25,000 cases per year, and the estimated incidence rate in the Finnish population was 5.5 per 100,000 women and 12.1 per 100,000 men. However, estimated incidence rates are likely significantly lower than the actual numbers owing to patient aversion to seeking medical information or treatment as a result of social embarrassment caused by the symptoms.2,5
Over 90% of perianal fistulas are cryptoglandular in origin and arise from perianal abscesses.6,7 A proportion of patients with anorectal abscess subsequently develop a fistula; however estimating the incidence rate of perianal fistula is difficult, and current data are inconsistent. Sainio et al.2 determined that the incidence of perianal fistula in a single institution between 1969 and 1978 was 8.6 per 100,000 people/year (12.6 for men and 5.6 for women). Zanotti et al.4 reported a higher incidence of perianal fistula, but this was estimated from data from a single year. Subsequently, the authors reviewed European databases and described higher incidences of 1.84/10,000 in the United Kingdom (UK) (2003–2004) and of 2.32/10,000 in Italy (2002). In a study by Sahnan et al.,8 approximately one fifth of all patients with anorectal abscess developed a fistula. Surprisingly, women had the greatest risk, even though both abscess and perianal fistula are more common in men. Also in Sahnan et al.’s study, the independent predictors of fistulation after perianal abscess were female gender, age 41 to 60 years, and ischiorectal or intersphincteric location of the initial abscess.
The surgical management of cryptoglandular perianal fistulas may be challenging, and the ideal approach remains questionable even among experienced colorectal surgeons. Fistulotomy is the gold standard treatment of perianal fistulas, with a healing rate of > 90%.9 However, patients treated with fistulotomy have a risk of developing postoperative perianal sphincter dysfunction, especially women or patients with complex fistulas, preoperative incontinence, recurrent disease, or previous anorectal surgeries.10 A better understanding of the processes underlying the development of cryptoglandular perianal fistulas may improve treatment; however, currently, the etiology of the disease is unknown. An inflammatory process appears to play a crucial role.11 The aim of this narrative review was to investigate the current knowledge of the etiopathogenesis of cryptoglandular perianal fistula to characterize the role of pathological and inflammatory factors in the development of chronic inflammation. The results and limitations of the studies on this topic were also described, and a discussion of the need for objective parameters for future studies was highlighted.
Methods
Ethics committee approval was not required for this study because it was a review. Patient consent was not required because no patients or patient-identifiable data were involved in the study.
We searched the following databases: PubMed, Embase (OVID version) and Google Scholar. The search query consisted of the combination of the following keywords: “perianal fistula”, “fistula-in-ano”, “pathogenesis of fistula”, “cryptoglandular theory”, “cryptoglandular fistula”, and “perianal abscess”. Results were limited to relevant papers published in English. There were no restrictions on the publication date for the articles cited in all subsections of the manuscript. The first search was performed on 10 January 2020, and the search was updated on 11 August 2020, with a final revision on 9 November 2020. The references in all of the included studies were reviewed for more eligible articles. Each article was reviewed independently by four researchers (MW, JW, ASW, RT) for inclusion according to the inclusion and exclusion criteria, which follow. Disagreements regarding article selection were resolved through discussion until consensus was reached or resolved by discussion between authors LD and JF. Conference abstracts were excluded. Articles were also excluded if they were not in English, the full text was inaccessible, or the studies were preclinical research or commentaries. A standardized form was used to extract data from the included studies. Extracted details were study population and demographics, details of interventions and controls, study methodology, and information to assess bias. Data extraction was performed independently by four authors, and discrepancies were resolved through discussion with the other co-authors.
Pathogenesis of cryptoglandular perianal fistula
The most common theory of the formation of anorectal abscess and subsequent perianal fistula, stated by Parks in 1961, is the pathology of obstructed proctodeal glands and ducts.6,12,13 This statement was established using previous theories describing a glandular mechanism of perianal fistulas reported by the anatomists Desfosses and Herrmann and the researchers Kratzer and Dockerty.6 Parks noted that the perianal glands discharge into the area of transition of the squamous mucosa to the columnar, mucus-secreting, epithelium opening into the base of the perianal crypts. Parks posited the concept that enteric microorganisms begin the process of acute inflammation by infiltrating perianal gland channels and spreading in an inner longitudinal path, causing a perianal abscess or more exceptionally, through the longitudinal muscle and resulting in an ischiorectal abscess. Parks further stated that once a chronic anorectal abscess has developed, infected material will continue to seep through any channel to the skin surface. Summarizing, a perianal fistula is a pathological canal secondary to an inflamed perianal gland; however, the duct opening in the perianal skin makes it a fistula. Therefore, anorectal abscesses and perianal fistulas are continuous phases of a common pathogenic spectrum.
The cryptoglandular theory of perianal fistulas suggests their development from the proctodeal glands, which originate from the intersphincteric plane and perforate the internal sphincter with their ducts. A connection forms between an opening at the level of the dentate line and another in the perianal area. Albeit widely accepted in clinical practice, the cryptoglandular theory popularized by Parks remains controversial.14 However, clinically, Parks’ classification according to the relationship to the sphincter has proved useful. Intersphincteric and distal transsphincteric fistulas are called low fistulas, and proximal transsphincteric and suprasphincteric fistulas are called high fistulas. The most frequently encountered fistulas are uncomplicated distal fistulous tracts.15 Recently, Garg16 proposed an improved classification of perianal fistulas and validated the classification in a cohort of 440 patients (Table 1). Modifications to the previous classification were suggested because earlier classifications, such as those of Parks and the St. James University Hospital17 did not categorize fistulas into simple and complex categories. The new classification proposed by Garg16 divides fistulas into five grades in the order of increasing complexity. Grades I and II are simple fistulas, and fistulotomy can be performed conveniently in these fistulas. Grades III, IV, and V are highly complex fistulas, and fistulotomy should not be attempted in these cases.
Table 1.
New classification of perianal fistula with treatment guidelines proposed by Garg.16
| Category | Grade | Description | Treatment guidelines |
|---|---|---|---|
| Simple | I | Low linear intersphincteric and transsphincteric fistulas (less than 1/3 of external sphincter involvement) | Fistulotomy should be possible in almost all of these fistulas (>95%). |
| II | Low intersphincteric and transsphincteric fistulas (less than 1/3 of external sphincter involvement) | Fistulotomy should be possible in the majority of these fistulas (>90%). | |
| Complex | III | High linear transsphincteric fistula (>1/3 of external sphincter involvement) or a fistula associated with Crohn's disease, sphincter injury, post-radiation exposure, or anterior fistula in women | Fistulotomy should not be attempted. FPR or sphincter-preserving procedures (LIFT, VAAFT, AFP, TROPIS, OTSC clip, or FiLaC laser) |
| IV | Complex high (>1/3 external sphincter involvement) transsphincteric fistula with either:IV-A: abscessIV-B: multiple tractsIV-C: horseshoe | Fistulotomy should not be attempted. FPR or sphincter-preserving procedures are recommended (LIFT, VAAFT, AFP, TROPIS, OTSC clip, FiLaC laser). FPR and AFP should be avoided in an abscess. Preferably, refer these fistulas to a fistula expert. | |
| V | Transsphincteric (>1/3 sphincter involvement) with intersphincteric supralevator extension | Fistulotomy should not be attempted. Sphincter-preserving procedures recommended (LIFT, VAAFT, AFP, TROPIS, OTSC clip). Preferably tertiary referral centers. |
FPR, fistulectomy with primary sphincter reconstruction; LIFT, ligation of intersphincteric fistula tract; VAAFT, video-assisted anal fistula treatment; AFP, anal fistula plug; TROPIS, transanal opening of intersphincteric space; OTSC, over-the-scope-clip proctology; FiLaC, fistula-tract laser closure.
Anatomical aspects
Recently, Garg underlined the importance of the intersphincteric space regarding complex anal fistulas stating that almost all fistulas have an element of intersphincteric extension.18,19 Garg stated that the intersphincteric tract in complex fistulas is like an abscess in a closed space. Therefore, managing these fistulas should incorporate the principles of treating abscesses, such as antibiotics, incision, and drainage with deroofing to ensure cavity drainage. Moreover, Garg proposed three cardinal principles when treating complex fistulas: 1) ISTAC – the intersphincteric tract is like an abscess in a closed space; 2) DRAPED – draining all pus and ensuring continuous drainage; and 3) HOPTIC – healing occurs progressively until it is interrupted irreversibly by a collection. Garg’s hypothesis may explain why the ligation of an intersphincteric fistula tract (LIFT) procedure is insufficient in complex fistulas, as this procedure is effective in only 55% of highly complex fistulas.20,21 In complex fistulas, Garg proposed the TROPIS procedure consisting of transanal opening of the intersphincteric space and deroofing the abscess,19 which results in a > 90% healing rate.
Molecular aspects
There have been a small number of studies of the treatment of cryptoglandular fistulas at the molecular and cellular level. A study by Kubota et al.22 confirmed the role of a spray containing basic fibroblast growth factor in the treatment of complex perianal fistulas in infants. Another study revealed that most (75%) cryptoglandular perianal fistulas are lined with granulation tissue, which suggests the role of an inflammatory response.23 However, there are no further reports on whether this type of spray is useful in adults.
Recently, there has been enthusiasm for the use of mesenchymal stem cells in the treatment of complex perianal fistulas, primarily in Crohn’s disease (CD). In a randomized controlled trial of 49 patients with complex perianal fistula (35 cryptoglandular, 14 with CD) by Garcia-Olmo et al.,24 a comparison of treatment with autologous adipose-derived stem cells (ASC) plus fibrin glue vs. treatment with fibrin glue alone showed higher healing rates in the ASC group vs the fibrin glue only group (71% vs. 16%, respectively). A subgroup analysis of patients with cryptoglandular fistula also showed significantly greater efficacy with ASC treatment than with fibrin glue alone (71% vs. 17%, respectively). However, a main limitation of the study was that the authors used autologous stem cells, which take approximately 4 months to obtain and process before they are ready for injection during the surgical procedure. Thus, more recent studies of complex perianal fistulas in CD have used allogeneic stem cells, which are ready to use as soon as they are thawed.25 However, the role of stem cell therapy in the treatment of patients with complex cryptoglandular perianal fistulas has yet to be elucidated. Furthermore, the mechanism of action behind stem cell therapy in humans remains incompletely understood, although preclinical studies proved that ASC act as immunomodulators.26 In the presence of inflammatory mediators, stem cells increase the production of interleukin-10 (IL-10) and the number of regulatory T cells, and also act as T-lymphocyte inhibitors.26 Recent findings further support the hypothesis that cytokines and immune cells contribute to the development and persistence of perianal fistulas.11,27
In a recent study, higher secretion of proinflammatory cytokines, such as IL-1beta and IL-8 were observed in perianal fistulous tracts compared with normal perianal tissue.11 Abundant expression of the proinflammatory cytokine IL-1beta was detected in 93% of the perianal fistulas in the study. Additionally, the cytokines IL-12 and tumor necrosis factor (TNF)-alpha were overexpressed in a high number of patients with perianal fistulas.
The role of epithelial-to-mesenchymal transition (EMT) has been proven in normal and neoplastic processes related to wound healing.28 EMT and abnormal integrity of the extracellular matrix (ECM) have been seen in several human physiological and pathological processes, and a possible role has been confirmed in the pathogenesis of cryptoglandular perianal fistula and CD-related perianal fistula.29–31 The gene overexpression of transforming growth factor (TGF)-beta, as a crucial molecule in EMT, and other typical EMT indicators, was seen in tissue from cryptoglandular perianal fistulas. Further support for EMT and abnormal ECM integrity has been confirmed by the lack of expression of E-cadherin and a high overexpression of protein kinase (RNA)-like endoplasmic reticulum kinase (PERK) and nuclear factor B (NFkB), two TGF-beta-inducible intracellular agents associated with EMT and ECM.32
The lipopolysaccharide (LPS) TGF-beta and other bacterial remnants, play a role in the development of chronic inflammatory lesions and cause the release of proinflammatory cytokines and other molecules by triggering EMT and causing abnormal ECM integrity.33 Therefore, EMT may promote an increased migration of epithelial cells, which could contribute to fistula canal development. Abnormal ECM integrity significantly impairs fistula healing. Additionally, in EMT, the secretion of other enzymes, such as matrix metalloproteinases (MMP), by macrophages and fibroblasts increases, which could affect the formation of fistulous tracts. MMPs facilitate fistula progression by degrading the ECM and promoting cell migration. MMP overexpression in perianal fistulas associated with CD has been proven.34 Once developed, a fistula canal could allow entry of other pathogens, which may promote further progression of chronic inflammation. Recent immunological evidence for the pathogenesis of fistulas in CD patients indicates that an epithelial defect caused by inflammation or injury allows pathogen-associated patterns to develop and induce various pathways mediated by TNF-a, TGF-beta, IL-13, MMPs, and integrin alpha v beta (avb).35 These factors drive EMT, which allows cell invasion and migration, resulting in a penetrating fistulous tract lined by transitional cells. However, this theory is mainly based on the study of fistulas in CD patients, and it is difficult to safely transfer this theory to cryptoglandular fistula disease.
According to a study by Haddow et al.,36 the cytokine and phosphoprotein profiles of idiopathic and CD-related perianal fistulas are broadly similar. Detailed profiling of 30 cytokines and chemokines and 39 phosphoproteins at four biopsy sites showed that the idiopathic and CD groups were strongly comparable with only a few discrepancies. Only two measurements showed a significant difference between the groups: IL-12 and the IL-1RA/IL-1β ratio, both at the internal opening. Tozer et al.37 found that cryptoglandular and CD perianal fistulas have similar levels of IL-2, IL-4, IL-6, interferon (IFN)‐γ‐inducible protein 10 (IP-10), TNF-α, and IFN-γ in tissue culture supernatants, but also reported higher numbers of T cells, lower expression of dendritic cell homing markers, and fewer cluster of differentiation 65 (CD65)-positive macrophages in CD-related perianal fistulas. Data provided by Haddow et al. contributes to an emerging theory that cryptoglandular and CD-related perianal fistulas may not be as immunologically distinct as previously supposed, which introduces the possibility that biological agents used in CD-related perianal disease may also have a role in cryptoglandular perianal fistulas.
The latest studies describe a significant role of white adipose tissue (WAT), which apart from its ability to respond to afferent signals from the endocrine system and the central nervous system, is also involved in the expression and secretion of factors with important functions.38 In fact, WAT produces and releases a wide range of pleiotropic proteins, and those released entirely by WAT are collectively named “adipokines”. Among these, leptin, resistin, adiponectin, and secreted frizzled‐related protein 5 (SRFP5) may play promising key roles in the development and suppression of the inflammatory response, and their role has been confirmed in other inflammatory disorders, such as CD.39 Abnormal adipokine secretion appears to be involved in the pathogenesis of different inflammatory diseases. Fat wrapping or “creeping fat”, defined as WAT hypertrophy and extension, is positively correlated with increased inflammatory processes, and macrophage and lymphocyte perivascular infiltration.38 These findings and similar cytokine profiles of cryptoglandular and CD perianal fistulas identified by Haddow et al. suggest that perianal fat tissue may play a crucial role in fistula treatment. In a recent randomized clinical trial by Garcia-Arranz et al.,40 a significant positive role was observed for autologous ASC for treating complex cryptoglandular perianal fistulas.
Microbial aspects
The development of anal fistulas may depend on the presence of specific gut bacteria in anorectal abscesses. Patients in whom Escherichia coli and gut-specific Bacteroides are found in abscess are more likely to present later with anal fistulas.41 Moreover, Toyonaga et al.42 stated that gut-derived microorganisms within anorectal abscesses are more likely to be associated with the development of anal fistulas than skin-derived organisms. One study proposed that local bacterial replication might be an inflammatory factor in cryptoglandular perianal fistula development.11 In a study by van Onkelen et al.,43 a significant level of proinflammatory peptidoglycans was detected in most of the cryptoglandular perianal fistulas. Peptidoglycan, as a main component of the cell wall of both Gram-negative and Gram-positive bacteria, provides structural strength and enables the cell walls to resist osmotic pressure. Peptidoglycan expresses its proinflammatory properties by increasing the secretion of IL-1beta. This indicates that even in the absence of local living bacteria, bacterial remnants promote chronic inflammation. Recent studies of the inflammatory pathogenesis of chronic diseases, such as CD, confirm that peptidoglycans are powerful stimulators of inflammation.44 However, Tozer et al.45 suggested that there is scant bacterial colonization of the luminal fistulous tracts, which was proven by the very marked paucity of mucosa-associated bacteria in the study’s samples. The authors’ theory, in contrast to what was previously thought, implies a less significant role of bacteria in anal fistula pathology. Moreover, the authors proposed that bacteria are unimportant in the persistence of anal fistula. Data confirming this thesis are insufficient; however, this shift in the etiology of anal fistula would have a strong impact on treatment; in particular, regarding the use of antibiotics.
Conclusions and future perspectives
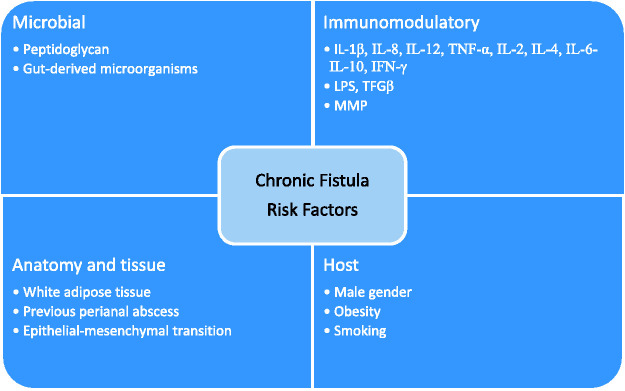
The underlying reasons for the development and persistence of cryptoglandular perianal fistulas remain incompletely understood. Recent studies suggest a crucial role of multiple elements, such as histological, microbiological, and molecular factors (Figure 1). A better understanding of the pathogenesis of cryptoglandular perianal fistulas is fundamental to helping surgeons achieve better treatment outcomes and could also lead to the development of novel therapies. Therefore, further studies in this area are important to improve the therapeutic management of cryptoglandular perianal fistulas. Future research following observations from recent studies might contribute to a comprehensive explanation of the role of adipokines in the pathogenesis of perianal fistula and provide reliable markers for the disease course and treatment efficacy, as well as implicate novel, more rational therapeutic approaches.
Figure 1.
Factors involved in the healing of cryptoglandular perianal fistulas.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by the Polish National Science Center (2016/23/N/NZ5/02564, to MW).
ORCID iD: Marcin Włodarczyk https://orcid.org/0000-0001-9118-4623
References
- 1.Dziki Ł, Mik M, Trzciński R, et al . Treatment of perianal fistulas in Poland. Polish J Surg 2015; 87: 614–619. [DOI] [PubMed] [Google Scholar]
- 2.Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol 1984; 73: 219–224. [PubMed] [Google Scholar]
- 3.Ommer A, Herold A, Berg E, et al . Cryptoglandular anal fistulas. Dtsch Arztebl Int 2011; 108: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanotti C, Martinez-Puente C, Pascual I, et al. An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis 2007; 22: 1459–1462. [DOI] [PubMed] [Google Scholar]
- 5.Nelson R. Anorectal abscess fistula: what do we know? Surg Clin North Am 2002; 82: 1139–1151, v–vi. [DOI] [PubMed] [Google Scholar]
- 6.Parks AG. Pathogenesis and treatment of fistula-in-ano. BMJ 1961; 1: 463–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik AI, Nelson RL, Tou S. Incision and drainage of perianal abscess with or without treatment of anal fistula. Cochrane Database Syst Rev 2010: CD006827. [DOI] [PubMed] [Google Scholar]
- 8.Sahnan K, Askari A, Adegbola SO, et al. Natural history of anorectal sepsis. Br J Surg 2017; 104: 1857–1865. [DOI] [PubMed] [Google Scholar]
- 9.Garg P. Is fistulotomy still the gold standard in present era and is it highly underutilized?: An audit of 675 operated cases. Int J Surg 2018; 56: 26–30. [DOI] [PubMed] [Google Scholar]
- 10.Kunitake H, Poylin V. Complications following anorectal surgery. Clin Colon Rectal Surg 2016; 29: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Onkelen RS, Gosselink MP, Van Meurs M, et al. Pro-inflammatory cytokines in cryptoglandular anal fistulas. Tech Coloproctol 2016; 20: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann G, Desfosses L. [Sur la muqueuse la la region cloacale du retum.] CR Acad Sci. 1880; 90: 1301. [Google Scholar]
- 13.Kratzer GL, Dockerty MB. Histopathology of the anal duct. Surg Gynecol Obstet. 1947; 80: 333–338. [PubMed] [Google Scholar]
- 14.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976; 63: 1–12. [DOI] [PubMed] [Google Scholar]
- 15.Roig JV, García-Armengol J, Jordán JC, et al. Fistulectomy and sphincteric reconstruction for complex cryptoglandular fistulas. Color Dis 2009; 12: e145–e152. [DOI] [PubMed] [Google Scholar]
- 16.Garg P. Comparing existing classifications of fistula-in-ano in 440 operated patients: is it time for a new classification? A retrospective cohort study. Int J Surg 2017; 42: 34–40. [DOI] [PubMed] [Google Scholar]
- 17.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000; 20: 623–635. [DOI] [PubMed] [Google Scholar]
- 18.Garg P. A new understanding of the principles in the management of complex anal fistula. Med Hypotheses; 132. DOI: 10.1016/j.mehy.2019.109329. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Garg P. Transanal opening of intersphincteric space (TROPIS) - a new procedure to treat high complex anal fistula. Int J Surg 2017; 40: 130–134. [DOI] [PubMed] [Google Scholar]
- 20.Garg P. Supralevator extension in fistula-in-ano is almost always in the intersphincteric plane. Dis Colon Rectum 2016; 59: e41–e42. [DOI] [PubMed] [Google Scholar]
- 21.Garg P. Understanding and treating supralevator fistula-in-ano: MRI analysis of 51 cases and a review of literature. Dis Colon Rectum 2018; 61: 612–621. [DOI] [PubMed] [Google Scholar]
- 22.Kubota M Hirayama Y, andOkuyama N.. Usefulness of bFGF spray in the treatment of perianal abscess and fistula-in-ano. Pediatr Surg Int 2010; 26: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 23.Mitalas LE, Van Onkelen RS, Monkhorst K, et al. Identification of epithelialization in high transsphincteric fistulas. Tech Coloproctol 2012; 16: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula. Dis Colon Rectum 2009; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- 25.Panés J, García-Olmo D, Van Assche G, et al . Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 2018; 154: 1334–1342.e4. [DOI] [PubMed] [Google Scholar]
- 26.Gao F, Chiu SM, Motan DAL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016; 7: e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugrue J, Nordenstam J, Abcarian H, et al. Pathogenesis and persistence of cryptoglandular anal fistula: a systematic review. Tech Coloproctol 2017; 21: 425–432. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratto C, Litta F, Lucchetti D, et al. Immunopathological characterization of cryptoglandular anal fistula: a pilot study investigating its pathogenesis. Colorectal Dis 2016; 18: O436–O444. [DOI] [PubMed] [Google Scholar]
- 30.Siegmund B, Feakins RM, Barmias G, et al. Results of the fifth scientific workshop of the ECCO (II): pathophysiology of perianal fistulizing disease. J Crohns Colitis 2016; 10: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bataille F, Rohrmeier C, Bates R, et al . Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohnʼs disease. Inflamm Bowel Dis 2008; 14: 1514–1527. [DOI] [PubMed] [Google Scholar]
- 32.Ratto C, Litta F, Lucchetti D, Parello A, et al. Immunopathological characterization of cryptoglandular anal fistula: a pilot study investigating its pathogenesis. Colorectal Dis. 2016; 18: O436–O444. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Li Y, Liu D, et al. LPS promotes epithelial–mesenchymal transition and activation of TLR4/JNK signaling. Tumor Biol 2014; 35: 10429–10435. [DOI] [PubMed] [Google Scholar]
- 34.Scharl M, Rogler G, Biedermann L. Fistulizing Crohn’s disease. Clin Transl Gastroenterol 2017; 8: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddow JB, Musbahi O, MacDonald TT, et al. Comparison of cytokine and phosphoprotein profiles in idiopathic and Crohn’s disease-related perianal fistula. World J Gastrointest Pathophysiol 2019; 10: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tozer PJ. Clinical and experimental studies in idiopathic and Crohn’s-related anal fistula PhD Thesis, Imperial College London, UK, 2012.
- 38.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355. [DOI] [PubMed] [Google Scholar]
- 39.Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin - implications for inflammatory bowel disease. Mol Nutr Food Res 2008; 52: 855–866. [DOI] [PubMed] [Google Scholar]
- 40.Garcia‐Arranz M, Garcia‐Olmo D, Herreros MD, et al. Autologous adipose‐derived stem cells for the treatment of complex cryptoglandular perianal fistula: a randomized clinical trial with long‐term follow‐up. Stem Cells Transl Med 2020; 9: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eykyn SJ, Grace RH. The relevance of microbiology in the management of anorectal sepsis. Ann R Coll Surg Engl 1986; 68: 237–239. [PMC free article] [PubMed] [Google Scholar]
- 42.Toyonaga T, Matsushima M, Tanaka Y, et al. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fistula in acute anorectal sepsis. Int J Colorectal Dis 2007; 22: 209–213. [DOI] [PubMed] [Google Scholar]
- 43.Van Onkelen RS, Mitalas LE, Gosselink MP, et al. Assessment of microbiota and peptidoglycan in perianal fistulas. Diagn Microbiol Infect Dis 2013; 75: 50–54. [DOI] [PubMed] [Google Scholar]
- 44.Reingold L, Rahal K, Schmiedlin-Ren P, et al. Development of a peptidoglycan–polysaccharide murine model of Crohnʼs disease. Inflamm Bowel Dis 2013; 19: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 45.Tozer PJ, Rayment N, Hart AL, et al. What role do bacteria play in persisting fistula formation in idiopathic and Crohn’s anal fistula? Colorectal Dis 2015; 17: 235–241. [DOI] [PubMed] [Google Scholar]