Summary
Burn animal models provide substantial insights into burn pathophysiology. Choice of the apt model is important for determining the clinical efficacy of new medicines. Therefore, standardization of burn models is crucial for scientific research. Use of common techniques like hot water, electricity and incandescent instruments to generate animal burn models is widely reported. However, great discrepancy in employed temperature and exposure times demands user-dependent standardization of the animal model prior to research. Establishment of custom generated in vivo burn models giving consideration to reduced use, suffering and risk of the experimental animal is equally crucial. Accordingly, this pilot study demonstrates a novel approach using rabbit and porcine cadaver skin for standardization of burn parameters prior to use in live animal models. Using a custom-made soldering iron coupled to a 16cm2 surface area copper plate, burns at randomly chosen temperatures of 80˚C and 120˚C, with exposure times ranging from 60s to 180s, were produced on rabbit and porcine cadaver skins. On gross and histopathological analysis, parameters required to generate characteristic changes for deep partial and full thickness burn involvement were established. The identified temperature and exposure time parameters were further validated in live animal models. In vivo validation established the success of this approach, highlighting reduced animal use, ease, reproducibility and efficacy in burn model standardization. The findings of this study will hopefully encourage researchers to opt for cadaver skin to determine parameters required to generate a specific degree of burn prior to its use in live animals for burn research.
Keywords: burn model, cadaver skin, rabbit model, porcine model, skin, histology
Abstract
Les modèles animaux permettent des avancées substantielles dans la compréhension de la physiopathologie des brûlures. Le choix du modèle est important pour évaluer l’efficacité de nouvelles thérapeutiques, donc la standardisation de ces modèles est cruciale. Les utilisations de l’eau chaude, de l’électricité et de solides chauds est couramment décrite. Cependant, la grande variabilité des températures et des temps de contact nécessite une calibration avant chaque étude expérimentale. Il est en outre nécessaire de développer des modèles in vivo à façon tout en réduisant le risque d’erreur et la souffrance animale. Cette étude décrit l’utilisation de peaux de lapins et de porcs morts pour la standardisation des modèles de brûlure les utilisant. Nous utilisons un fer à souder couplé à une plaque de cuivre de 16 cm², pouvant délivrer une température réglable entre 80 et 120°C pendant 60 à 180 s sur de la peau de lapin ou de porc morts. Nous avons défini les paramètres permettant de réaliser des brûlures intermédiaires ou profondes, objectivées par études macro- et microscopiques, validés ensuite chez l’animal vivant. Cette étape a permis de confirmer l’efficacité de cette approche qui réduit le nombre d’animaux d’expérience, est aisée et reproductible et bien corrélée à l’approche in vivo. Cette étude devrait conduire les équipes a réaliser une calibration ex vivo avant de réaliser une étude in vivo de brûlure expérimentale.
Introduction
Globally, burn remains a common form of injury and emergency trauma.1 The impact of burns is devastating in nature, affecting both the structure and functionality of multiple organs.1 Current in vitro experiments fail to confine or tackle this complex pathophysiology.2 Based on its close anatomical and physiological resemblance to human skin, the pig model has been widely authenticated for use in burn research.3 Unfortunately, the high cost pertaining to the use of pigs, their handling difficulty in long term studies,4 and the observed variations in wound contraction with respect to the anatomical location of wounds require careful standardization with minimal use of experimental animals. Researchers have also led the way to using rabbit burn models, considering the metabolic similarity to the human skin and affordable size for the generation of large-area burns.4 Nevertheless, in both pig and rabbit models, despite several methods being developed, their standardization is still difficult owing to reported discrepancies in the parameters employed for the generation of the burns. This observed user-dependent variability for the development of burn models is a major concern.2 Hence, the generation of burns with dependable characteristics remains challenging.5 Direct contact with heated metal,5,6 electricity7 and hot water are often described in the creation of experimental animal burn surfaces. This vast inconsistency in burn parameters and the lack of homogenous burns calls for a user-dependent standardization of the animal models prior to use. To specifically address the reduced use of experimental animal numbers, this study proposes a novel approach to using pig and rabbit cadaver skin to determine burn conditions required to create a specific degree of burn in live animals for research.
Animals, material and methods
Cadaver skin was selected for burn standardization considering its well-preserved skin tissue architecture and suitability for histological studies,88 with the notable advantage of its easy and abundant availability. Additionally, its contribution towards reduced use and suffering of the laboratory animal is to be highlighted. Cadaver skins of multiple in-bred New Zealand white rabbits (from animals aged more than 9 months, weighing between 2500g to 2800g) were harvested after obtaining approval from the Institutional Animal Ethics Committee in line with the CPCSEA guidelines, on the day of euthanization, post their use as sham models in other studies. White Yorkshire pig cadaver skins from multiple animals were collected from the local slaughterhouse immediately after slaughter. Animals subjected to in vivo validation study were housed in individual cages and received food and water (tap water) ad libitum. The care and management of animals followed ISO 10993 – Part II and CPCSEA guidelines.
Standardization of burn injury on cadaver skin
To induce burn, a custom-made burn device was fabricated upon modification of typical soldering equipment. A 16cm2 surface area copper plate was coupled to the soldering iron, with a total weight of 190 grams9 as seen in Fig. 1. On validation of the equipment, temperatures of 80˚C and 120˚C were attained on the copper plate and monitored continuously by an inbuilt temperature detector. Subsequently, rabbit (n=12) and pig (n=24) cadaver skins were harvested in full thickness/depth, collected and transported in phosphate-buffered saline solution. The harvested cadaver skin was immediately placed on a sterile working table top to conduct the experiment. To ensure even burn wounding, the hair on burn sites was shaved off using a sterile blade or a standard electric shaving machine. Commercial de pilatory cream was used to remove any residual hair.10,11,12 Depilatory cream contact time was determined in accordance with the manufacturer’s instructions. On removal of the cream, the skin was rinsed with water, air-dried and disinfected with antiseptic betadine lotion. The experimental design for standardization of partial and full thickness burns on rabbit cadaver skin involved 4 study groups with multiple sites (n=3) of injury: Group 1 (burns at 80˚C for 60s), Group 2 (burns at 120˚C for 60s), Group 3 (burns at 120˚C for 120s), and Group 4 (burns at 120˚C for 180s). Whereas the experimental design for standardization of partial and full thickness burns on pig cadaver skin involved 8 study groups with multiple sites of injury (n=3): Group 1 (burns at 80˚C for 60s), Group 2 (burns at 120˚C for 60s), Group 3 (burns at 120˚C for 120s), Group 4 (burns at 120˚C for 180s), Group 5 (burns at 80˚C for 60s, after removal of epidermis), Group 6 (burns at 120˚C for 60s, after removal of epidermis), Group 7 (burns at 120˚C for 120s, after removal of epidermis), Group 8 (burns at 120˚C for 180s, after removal of epidermis).
Fig. 1. Customized device for infliction of burns: photograph of a custom designed burn device with an electrically controlled internal heater. The device is coupled with the 16cm2 copper plate.

With the aim of attaining better surface contact for injury formation, at the lowest contact temperature and exposure time, we incorporated groups that had burns on sites after removal of the epidermis, as shown in Fig. 2. This was done using a powered dermatome (Zimmer Biomet®) adjusted to a grafting depth of 140μm, by a single plastic surgeon who was involved in this study. All burns were performed by the same evaluator for all the groups as mentioned above; using the heated copper plate placed perpendicularly on the skin, the pressure exerted consisted only of the appliance’s own weight. Multiple replicates were performed on different animals (cadaver skin) to eliminate concerns of possible biological variability within the animals and at different anatomical locations for the same burn condition.
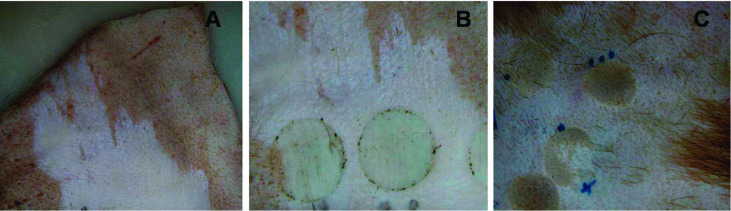
Fig. 2. Burn infliction on pig cadaver skin: (A) Skin showing removed epidermis using a powered dermatome; (B) Burn injuries created on epidermis removed skin; (C) Burn injuries created on hair shaved skin.
In vivo demonstration and assessment of the depth of burn
To validate the in vitro standardized burn injury parameters, in vivo demonstration of second-degree burn wounds was carried out on the rabbit model. As our aim was to use as few animals as possible, for the in vivo demonstration four different lesions were induced on a single animal. The animal was anesthetized using ketamine hydrochloride [50mg/kg] and xylazine [5mg/kg] (I/M). The burns were induced on the dorsal skin of the rabbits, which were positioned in sternal recumbence. The caudal border of the field of operation was 10 cm from the iliac crest, while the cranial border was 14 cm from the seventh cervical vertebra as described. 13 Using a standard electric shaving machine, the dorsal region was clean-shaved. As mentioned earlier, residual hairs were also removed with commercial depilatory cream. Standardized protocol and parameters for second-degree deep rabbit burns, i.e. 120˚C for 120s as described above, were adopted to induce four individual, 16cm2 surface area burns on an animal. After recovery from anesthesia, the animal was kept in a cage and fed normal diet and water ad libitum. Ampicillincloxacillin injection (10mg/kg) was given twice and meloxicam (0.6 mg/Kg) was given once. On the day after burn induction, the animal was euthanized by injecting an overdose of thiopentonesodium 1% w/v solution as intravenous injection after anesthetizing with xylazin (5mg/kg) and ketamine (50 mg/kg) i/m. The burn sites along with normal skin were excised; gross pictures were taken and were fixed in 10% neutral buffered formaldehyde. The samples were paraffin embedded and ~5m thick sections were stained with hematoxylin and eosin stain. Light microscopy (Nikon E 600, Nikon, Japan) aided histopathologic examinations for degree of burn injury and photomicrographs were captured using a camera (Nikon DsR1, Nikon, Japan) attached to the microscope.
Histopathological analysis
Tissue samples were collected upon excision of the burn injury sites along with surrounding normal skin and were subjected to gross and histopathologic examinations. On blinding all information pertaining to the study group, a single pathologist involved in the study performed the gross and histopathologic examination of the tissues. Tissue specimens were fixed in a 10% neutral buffer formalin solution, processed routinely in an automatic tissue processor (ASP300, Leica, Germany) and then embedded in paraffin. Five micron thin sections were cut using a rotary microtome (RM2550, Leica, Germany) and stained with hematoxylin and eosin stain. Histopathologic examinations for depth of burn injury were assessed by light microscope (Nikon E 600, Nikon, Japan) and photomicrographs were captured using a camera (Nikon DsR1, Nikon, Japan) attached to the microscope. The criteria for histological assessment were based on observing the extent of collagen alteration, endothelial injury, and follicular and epithelial injury upon comparison with noninjured control skin.
Data analysis
Only descriptive statistics were used in the observational study.
Results
Standardization of burns on rabbit cadaver skin
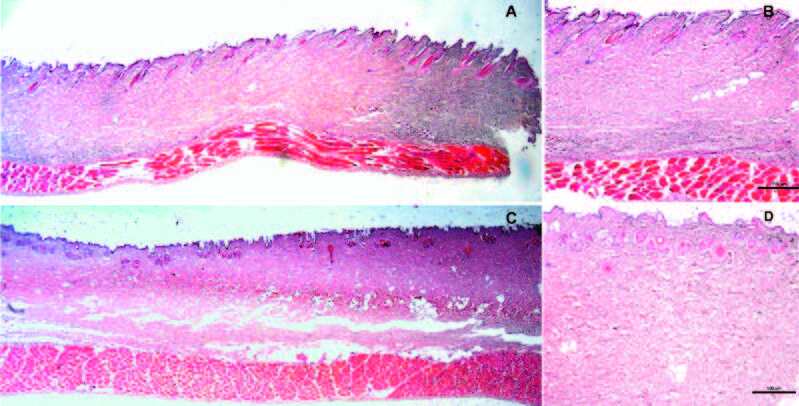
Gross and histopathological observations of rabbit burn cadaver skin determined second-degree superficial burn wounds at 120˚C for 60s. The epidermis was degenerated and appeared coagulated. Superficial collagen up to the end of hair follicle base was also seen coagulated. At 120˚C for 120s, second-degree deep burn wounds were generated as shown in Fig.3. Histological analysis showed the epithelial layer, including keratin, degenerated completely and coagulated. Within the dermis, superficial and deep dermal collagen appeared coagulated. Hair follicles were degenerated and appeared coagulated. Burns at 80˚C for 60s showed no characteristics of a second-degree deep burn wound (data not shown). Burns at 120˚C for 180s showed third-degree full thickness burns with completely damaged epidermis and dermis, full length coagulation of collagen, vacuolization of fatty tissues and degeneration cells were observed in the deep subcutaneous fat layer.
Fig. 3. Histological assessment of inflicted burns on rabbit cadaver skin: (A) Second-degree superficial burn wounds at 120˚C for 60s (1x); (B) Second-degree superficial burn wounds at 120˚C for 60s (4x); (C) Second-degree deep burn wounds at 120˚C for 120s (1x); (D) Seconddegree deep burn wounds at 120˚C for 120s (4x), showed epithelial layer, including keratin, degenerated completely and coagulated. Within the dermis, superficial and deep dermal collagen appeared coagulated. Hair follicles were degenerated and appeared coagulated, (n=3).
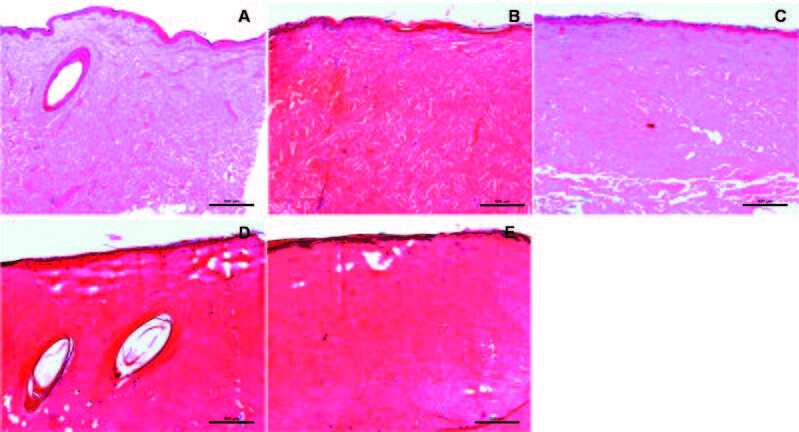
Standardization of burns on porcine cadaver skin Gross and histopathological observations of pig burn cadaver skin determined no significant difference in depth of burn injury upon removal of the epidermis (Fig. 4A). First-degree burn wounds were observed at 80˚C for 60s, showing superficial dermal collagen damaged and coagulated (1/5th depth of the dermis) (Fig. 4B). Second-degree burn wounds were generated at 120˚C for 60s, showing completely damaged epidermis, and half of dermal collagen damaged and coagulated; the remaining half of the dermis was left unaffected (Fig. 4C). Third-degree burn wounds were observed at 120˚C for 120s (Fig. 4D) and at 120˚C for 180s (Fig. 4E), showing complete epidermal and dermal damage, vacuolated areas with full length, with degenerated subcutaneous fat.
Fig. 4. Histological assessment of inflicted burns on pig cadaver skin: (A) Histology of normal porcine skin; (B) First-degree burn wounds observed at 80˚C for 60s, showing superficial dermal collagen damaged and coagulated (1/5th depth of the dermis); (C) Second-degree burn wounds were generated at 120˚C for 60s, showing completely damaged epidermis, half of dermal collagen damaged and coagulated; remaining half of the dermis left unaffected, (D) Third-degree burn wounds were observed at 120˚C for 120s and (E) at 120˚C for 180s, (n=3).
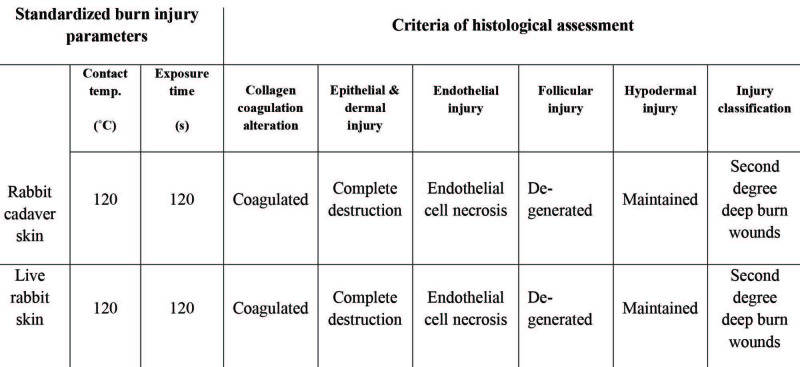
Assessment of in vivo inflicted burns Following in vivo application of the standardized parameters using cadaver skin (Fig. 5A) for generation of second-degree burns in rabbit models (Fig. 5B), the depth of burn injury was observed to be second-degree upon histological analysis (Fig. 5C). The second-degree burn wounds at 120˚C for 120s showed the epithelial layer, including keratin, to be degenerated completely and coagulated. Within the dermis, superficial and deep dermal collagen appeared coagulated. Hair follicles were degenerated and also appeared coagulated. Table I shows the reproducibility of the approach based on descriptive histopathological analysis of the obtained injury, using the same parameters in cadaver and live animal skin.
Fig. 5. Assessment of inflicted burns on live rabbit skin: (A) Creation of burn injury using standardized parameters obtained from rabbit cadaver skin; (B) Burns inflicted on live rabbit skin; (C) Representative H&E stained section of burn generated using the standardized parameters, showing achievement of second degree burns (4x), (number of injured sites=4, performed in one animal).
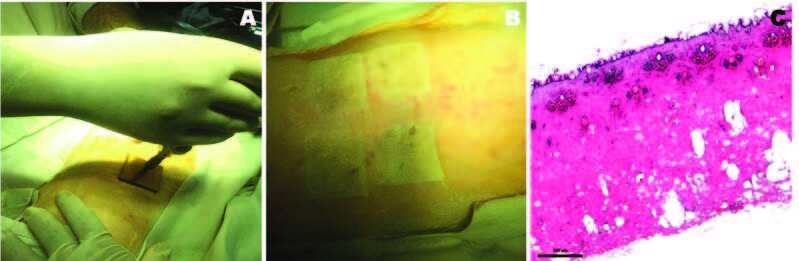
Table I. Comparative histopathological analysis in cadaver and live rabbit skin.
Discussion
Lack of user-independent, reproducible and consistent burn injury animal model parameters has restrained research and development in burn therapy. 14,15 Extensive reports demonstrate rabbit and porcine burn models generated with either scaldinghot water, heated metal or glass, or heat radiation.16 However, in models of partial and full thickness burns, huge inconsistency in the temperatures used, in addition to contact time with regard to the size and region of the injury, is reported upon use of aluminum coupled soldering irons.9,17 Preheated aluminum bars at 80°C for 20 or 30 seconds demonstrated porcine partial thickness burns.16 Whereas a dry-heated brass rod at 90°C for 10 and 20 seconds demonstrated deep-partial thickness burns in rabbits.18 Use of 90˚C for 40 seconds with a constant pressure of 0.064 kg/cm2 also demonstrated mini-pig full-thickness dermal burns.19 Considering the observed discrepancy in the parameters, research groups are mandated to pre-optimize their own customized animal models which again requires the use of large numbers of laboratory animals. This adds to the suffering of the animal as well as the cost involved in burn model standardization. This pilot study was specifically designed to help reduce the use of laboratory animals during burn model standardization by using cadaver skin. A set of temperatures and exposure times were selected that reliably produced a mid-dermal, partial thickness burn and full thickness burns on rabbit and porcine skin using our customized heating mantle. With the help of histological evaluation, our standardization approach demonstrated the use of 120˚C at 120s and 120˚C at 60s to be effective and reproducible in multiple repeated attempts for generating partial thickness burns on rabbit and porcine cadaver skin, respectively. Meanwhile, using 120˚C at 180s and 120˚C at 120s was effective in generating full thickness burns in rabbit and porcine cadaver skin, respectively. Furthermore, to validate the standardized in vitro parameters attained using cadaver skin, we randomly opted to demonstrate infliction of second-degree burns on rabbit models, favored by the low maintenance cost and ease in handling. Histological analysis of the in vivo wounds implicated that in vitro standardization using cadaver skin was an effective approach for standardizing burns, highlighting reduced animal usage. It may be noted that even though cadaver skin is limited by lack of blood supply, the standardized parameters attained using the cadaver skin were effective and reproducible in wounding live animal models with blood flow in the underlying tissue showing no differential effect on heat dissipation and degree of injury. In order to encourage experimental animal research determined by maximum translational relevance to humans, outweighing the economic considerations,20 this study explored in vitro standardization of burn injury conditions on porcine cadaver skin, which may be adapted for in vivo application by other research groups. The described method in this study is simple, cost-effective and reproducible. Multiple replicates were performed on different animals (cadaver skin) to eliminate concerns of possible biological variability within the animals and at different anatomical locations for the same burn condition. We do acknowledge that this study is limited by its sample size. However, the demonstrated findings are intended to serve as a simple feasibility guide for standardizing pre-defined burn injury models for research in relation to in-house customized heat mantle characteristics (i.e. metal used, metal size, metal shape, metal thickness etc.), available heating equipment and preferred animals for research using cadaver skin, highlighting minimal animal usage.
Limitations of the study
This pilot study has several limitations that merit further discussion. Though multiple animal cadaver skins were harvested and used for standardization, the validation of the parameter was performed in vivo on only one animal model using one rabbit. The assessment of the reliability of our scale is further limited by the fact that intra-observer reliability is based on only one dermatopathologist. We recognize that the burn depth analysis performed in this study was limited to standard H & E staining. Future studies should evaluate the use of our method with other histological stains. It is recommended that researchers in other institutions and perhaps using other animal models perform external verification of our approach using correlation coefficients and Bland Altman analysis to compare injury on cadaver and live skin. This demonstrated approach could also be assessed for other types of non-thermal burns.
Conclusion
This study casts light on the use of cadaver skin as an efficient strategy for minimizing the use of animals for burn standardization studies. This strategy could prove useful for determining burn conditions required prior to using live animals. Furthermore, successful establishment of in vivo burn models using cadaver skin standardized parameters seems cost-effective and promising to evaluate several potential therapeutic agents in reducing/treating burn injury progression. We hope this study will contribute to medical and scientific communities’ own investigations of partial and full thickness burn treatments.
Acknowledgments
Acknowledgements.The authors acknowledge The Head, BMT Wing and The Director of SCTIMST for their encouragement and support throughout this study.
Funding.The authors received no specific funding for this work.
References
- 1.Jeschke MG, van Baar ME, Choudhry MA, Chung K. Burn injury. Nat Rev Dis Primer. Nature Publishing Group. 2020;6(1):11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life SciCMLS. 2014;71:32–41. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S-J, Sartika D, Fan G-Y, Cherng J-H, Wang Y-W. Animal models of burn wound management. Anim Models Med Biol. [cited 2020 Jul 10];Intech Open, 2019. Available from: https://www.intechopen.com/books/animal-models-in-medicineand-biology/animal-models-of-burn-wound-management . [Google Scholar]
- 4.Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018;138:2095. doi: 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Andrade ALM de, Parisi JR, Brassolatti P, Parizotto NA. Alternative animal model for studies of total skin thickness burns. Acta Cir Bras. 2017;32:836. doi: 10.1590/s0102-865020170100000005. [DOI] [PubMed] [Google Scholar]
- 6.Derakhshanfar A, Moayedi J, HashemiS-S X, Vahedi M, Valizadeh A. Comparative study on the effects of heated brass bar and scald methods in experimental skin burn in rat. Comp Clin Pathol. 219;28:1381. [Google Scholar]
- 7.Campelo APBS, Campelo MWS,, Britto GA de C,, Ayala AP. An optimized animal model for partial and total skin thickness burns studies. Acta Cir Bras. 2011;26 Suppl 1:38. doi: 10.1590/s0102-86502011000700008. [DOI] [PubMed] [Google Scholar]
- 8.Gupta T, Gauba K. Cadaveric tissue histology: a viable alternative. J Clin Diagn Res. 2011;5:5. [Google Scholar]
- 9.Venter NG, Monte-Alto-Costa A, Marques RG. A new model for the standardization of experimental burn wounds. Burns. 2015;542:41. doi: 10.1016/j.burns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Abdeldjelil MC, Messai A, Boudebza A, Beghoul S. Practical aspects to generate cutaneous experimental burns in a rat model. Pharm Lett. 2017;9(1):70–84. [Google Scholar]
- 11.Ozcelik U, Ekici Y, Bircan HY, Aydogan C. Effect of topical platelet-rich plasma on burn healing after partial-thickness burn injury. Med Sci Monit Int Med J Exp Clin Res. 2016;22:1903. doi: 10.12659/MSM.895395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Landén NX. Mol Dermatol Methods Protoc [Internet] Springer, New York, NY: 2020. [cited 2020 Jul 11]. Investigation of skin wound 12 healing using a mouse model. Available from: https://doi.org/10.1007/978-1-0716-0648-3_20 . [Google Scholar]
- 13.Knab JS, Bayer GS, Bauer WA, Schwendenwein I. Controlled partial skin thickness burns: an animal model for studies of burn wound progression. Burns J Int Soc Burn Inj. 1999;25:229. doi: 10.1016/s0305-4179(98)00172-7. [DOI] [PubMed] [Google Scholar]
- 14.Andrews CJ, Kempf M, Kimble R, Cuttle L. Development of a consistent and reproducible porcine scald burn model. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0162888. e0162888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla SK, Sharma AK, Shaw P, Kalonia A. Creation of rapid and reproducible burn in animal model with a newly developed burn device. Burns. 2020;46:1142. doi: 10.1016/j.burns.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Singer AJ, Hirth D, McClain SA, Crawford L. Validation of a vertical progression porcine burn model. J Burn Care Res Off Publ Am Burn Assoc. 2011;32:638. doi: 10.1097/BCR.0b013e31822dc439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busuioc CJ, Mogoşanu GD, Popescu FC, Lascăr I. Phases of the cutaneous angiogenesis process in experimental third-degree skin burns: histological and immunohistochemical study. Romanian J Morphol Embryol Rev. 2013;54:163. [PubMed] [Google Scholar]
- 18.Friedrich EE, Niknam-Bienia S, Xie P, Jia S-X. Thermal injury model in the rabbit ear with quantifiable burn progression and hypertrophic scar. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2017;25:327. doi: 10.1111/wrr.12518. [DOI] [PubMed] [Google Scholar]
- 19.Deng X, Chen Q, Qiang L, Chi M. Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of shikonin on hypertrophic scar remediation. [cited 2019 Jan 7];Front Pharmacol [Internet] 2018 9 doi: 10.3389/fphar.2018.00590. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5996232/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2010 PROGRAM: Animal Welfare and Scientific Research: 1985 to 2010 [Internet] [cited 2019 Jan 7]; Available from: https://grants.nih.gov/grants/olaw/seminar/index.html . [Google Scholar]