Abstract
Background
Craniopharyngiomas are common lesions that occur in the suprasellar region; however, strictly intrinsic third ventricular craniopharyngiomas are rare.
Case Series
We aimed to describe the magnetic resonance imaging features observed in five cases of strictly intrinsic third ventricular papillary craniopharyngiomas, including two cases of mixed cystic and solid tumors and three cases of pure solid masses.
Conclusion
Among the adult population, intrinsic third ventricular papillary craniopharyngiomas should be considered when either solid or mixed cystic and solid masses are observed, in which the solid component shows heterogeneous intensity, heterogeneous and strong enhancement, and is strictly located in the third ventricle.
Keywords: strictly intrinsic third ventricle, craniopharyngioma, magnetic resonance imaging
Introduction
Craniopharyngiomas, which account for 2–4% of all intracranial tumors, originate from the squamous epithelial residual cells in Rathke’s pouch.1,2 Most craniopharyngiomas are located in the suprasellar region, of which 30% extend into the anterior fossa, 23% extending along the midline, and approximately 20% extend into the posterior fossa.2 Strictly intrinsic third ventricular craniopharyngiomas are rare and are more frequently detected in adults than in children. Third ventricular craniopharyngiomas can be divided into two groups: strict intraventricular and non-strict intraventricular craniopharyngiomas, with the former group being less common.3,4 In this paper, we aimed to report the clinical presentation, magnetic resonance imaging (MRI) features, and treatment of five cases of strictly intrinsic third ventricular craniopharyngioma.
Case Series
Cases 1 and 2
Two patients, a 29-year-old female and a 50-year-old male, were admitted to the hospital due to 1-month histories of bifrontal headache and visual disturbance (decreased visual acuity and visual field defects presenting as bitemporal hemianopsia). No focal neurological deficits were revealed for either patient on clinical examination.
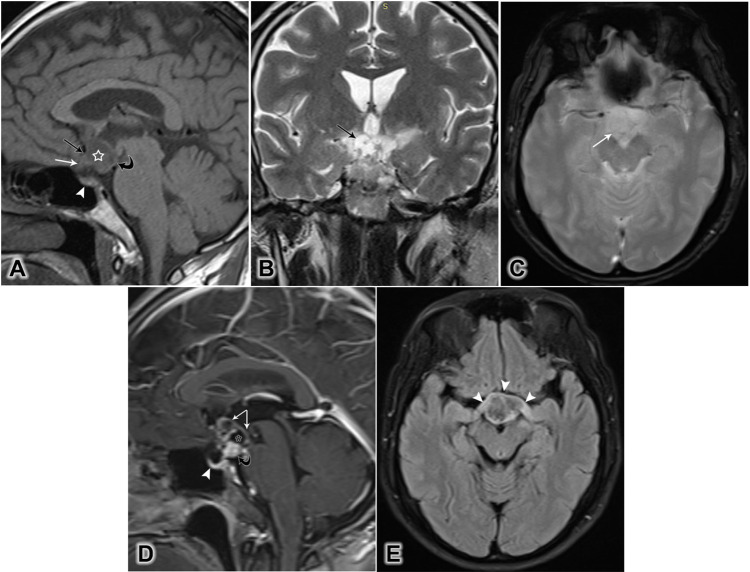
In both cases, a brain MRI with contrast enhancement was performed. MRI revealed strictly intrinsic third ventricular masses that bowed the floor of the third ventricle, expanding into the supraoptic recess, compressing the optic chiasm forward and downward. The pituitary gland, pituitary stalk, and suprasellar cistern remained intact. Both masses had solid components at the central and cystic components in the periphery. In Case 1, the mass was isointense relative to gray matter in T1-weighted images and showed heterogeneous and strong enhancement following the injection of the contrast agent. In Case 2, the mass was hypointense in T1-weighted images and hyperintense on fluid-attenuated inversion recovery (FLAIR) compared to cerebrospinal fluid, revealing rim enhancement following contrast injection. Neither intratumoral calcification nor hydrocephalus was detected in either case (Figure 1).
Figure 1.
Brain MRI of a 50-year-old male patient, showing a lobulated contour mass with cystic and solid components. (A) Mid-sagittal T1-weighted image showing a hypointense lesion (star), expanding into the supraoptic recess (black arrow), bowing the floor of the third ventricle (curved arrow), and displacing the optic chiasm downward and forward (white arrow). The pituitary gland remains intact (white arrowhead). (B) Coronal T2-weighted image showing the cystic component (arrow). (C) Axial T2-weighted image showing no intratumoral calcification (arrow). (D) Sagittal T1-weighted post-contrast image showing rim enhancement (white arrows) of the cystic component (star), a strongly heterogeneous solid component (black curved arrow), and the normal enhancement of the pituitary gland (arrowhead). (E) Axial fluid-attenuated inversion recovery (FLAIR) showing the anterior deviation and edema of the optic chiasm (arrowheads).
Both patients underwent microsurgery using a frontal transcortical transventricular approach. The histopathological reports revealed both masses to be papillary craniopharyngiomas.
Cases 3 to 5
Three patients, aged 36–68 years, were admitted to our department due to chronic headaches and visual disturbances (decreased visual acuity and field with hemianopsia or quadranopsia). On clinical examinations, the patients presented with no signs of focal neurological deficits.
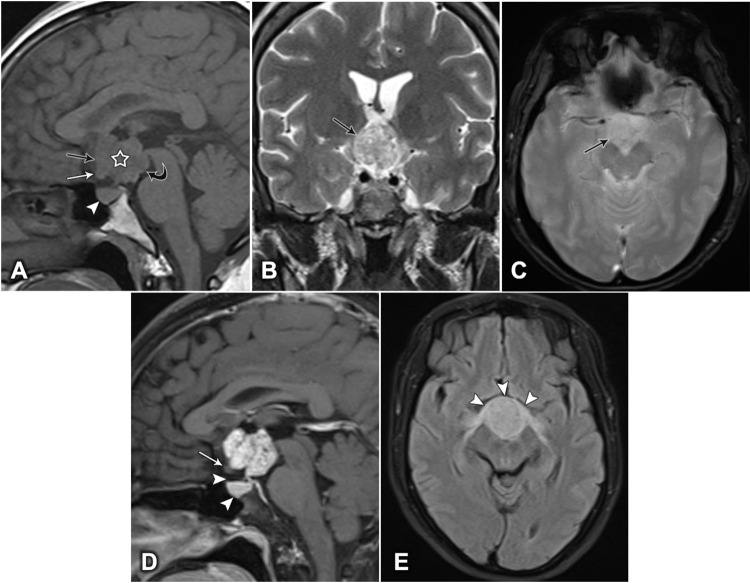
The patients underwent brain MRI with contrast injection. The examinations showed strictly intrinsic third ventricular masses bowing the floor of the third ventricle, expanding into the supraoptic recess. The optic chiasm was deviated forward and downwards. No invasion of the pituitary gland, pituitary stalk, or suprasellar cistern was detected. The masses were lobulated, pure solid, isointense on both T1- and T2-weighted images, and hyperintense on FLAIR compared with the intensity of gray matter. They showed strong and heterogeneous enhancement after contrast injection. No intratumoral calcifications were observed. The lateral ventricles were normal in size (Figure 2).
Figure 2.
Brain MRI of a 46-year-old woman, showing a lobulated, contoured, solid mass. (A) Mid-sagittal T1-weighted image showing a hypointense lesion (white star), expanding into the supraoptic recess (black arrow), bowing the floor of the third ventricle (curved arrow), pushing the optic chiasm downward and forward (white arrow), and the pituitary gland remains intact (white arrowhead). (B) Coronal T2-weighted image showing a heterogeneous, hyperintense lesion (arrow). (C) Axial T2-weighted image showing no intratumoral calcification (arrow). (D) Sagittal T1-weighted image, post-contrast, showing vivid heterogeneous enhancement. The optic chiasm is compressed downward and forward (white arrow), and the suprasellar cistern and the pituitary gland are intact (arrowheads). (E) Axial fluid-attenuated inversion recovery (FLAIR) image showing the anterior deviation and edema of the optic chiasm (arrowheads).
Two patients were treated with microsurgery, using a frontal, transcortical, transventricular approach. The third patient was treated with a translaminar terminalis approach. The tumors were completely, and the histological findings revealed papillary craniopharyngiomas.
Discussion
The third ventricle is a rare location for craniopharyngiomas, accounting for only 0.7% to 14% of all cases.3 By 1990, only 22 cases of third ventricular craniopharyngiomas had been reported in the literature.5 By 2004, 105 intraventricular craniopharyngioma cases were included in an overview of the imaging features and surgical approaches used to treat these tumors.1 Craniopharyngiomas originate from the epithelial remnants of Rathke’s pouch. In embryological terms, the pars tuberalis rotates to contact the neuroectodermal layer of the ventral cerebral vesicle, which is the precursor of the third ventricular floor. The pial membrane, which originates from the mesoderm, spreads between the stomodeum and the cerebral vesicle before the rotation of the pars tuberalis. Craniopharyngiomas originate from residual cells of Rathke’s pouch, which spread with the rotation of the pars tuberalis. Behari et al3 supported the hypothesis that the delayed development of the pial membrane resulted in the epithelial crests of Rathke’s pouch forming direct contacts with the neuroectodermal layer of the ventral cerebral vesicle, resulting in the formation of strictly intrinsic third ventricular craniopharyngiomas. However, some authors have suggested that these tumors originate from the existence of a plane between the craniopharyngioma and the third ventricle wall because these tumors attach densely to the infundibulum and the tuber cinereum.6,7
The symptoms of intracranial hypertension, such as headache and nausea, are the most common symptoms associated with craniopharyngioma, accounting for 90.3% of cases. Visual disturbances and endocrinological disorders are typically reported in patients with suprasellar craniopharyngioma that extends into the third ventricle, whereas the patients with strictly intrinsic third ventricular craniopharyngioma generally present with mental, memory, and gait disorders. Sleep and body core temperature disturbances may arise.1,3,4,8 Our five cases presented with headache due to intracranial hypertension and visual disturbance due to optic tract edema.
According to Pascual et al1 using information from necropsies and surgical report, intraventricular craniopharyngiomas are divided into two groups, (1) strictly intrinsic third ventricular craniopharyngiomas and (2) non-strictly intrinsic third ventricular craniopharyngiomas, which included: suprasellar tumors that compress the third ventricle floor; suprasellar tumors that break through the third ventricle floor, developing into the third ventricle cavity; and intraventricular tumors within the third ventricle cavity and floor, which becomes replaced by the tumor, leaving an opening where the tumor replaces the floor.1 Among the 105 cases reported in this retrospective study, 36 were categorized as strictly intrinsic third ventricular craniopharyngiomas, and 69 were categorized as non-strictly intrinsic third ventricular craniopharyngiomas and the available preoperative MRI studies could not discriminate the correct location of all strictly intrinsic third ventricular craniopharyngioma cases, except in the three cases which showed a thin layer of cerebrospinal fluid separating the inferior tumour margin from the third ventricle floor.1 However, Prieto et al9 suggested six characteristics of strictly intrinsic third ventricular tumors based on conventional T1 and T2-weighted images, which included: (i) a typical round shape, (ii) the downward deviation of the optic chiasm, (iii) a well-observed pituitary stalk, (iv) free chiasmic cistern, (v) mammillary body angle (MBA) is 30–60° and (vi) the hypothalamus region being situated below the lower third of the tumor. In our study, all cases had features that were compatible with the categorization of strictly intrinsic third ventricular tumors in Prieto’s study and these were confirmed by surgical description reports.
Colloid cyst, germinoma, lymphoma, choroid plexus papilloma and glioma are the main differential diagnoses of intrinsic third ventricular craniopharyngiomas. Based on anatomical localization, components intensity and enhancement of lesions, MRI is regarded to be the most valuable imaging technique for diagnosis.2
Craniopharyngiomas have two histopathological subtypes, adamantinomatous and papillary. Adamantinomatous craniopharyngiomas are found predominantly in children, typically presenting as a cystic component containing hyperintense fluid on T1-weighted images due to the presence of intratumoral proteins, triglycerides, or methemoglobins. Calcification is frequently detected, and these tumors tend to compress or invade adjacent structures.5,10 Papillary craniopharyngiomas are observed exclusively in adults. The lesions typically contain a solid component that is either hypointense or isointense on T1-weighted images and hyperintense on T2-weighted images. However, some areas within the solid component may appear hypointense on both T1- and T2-weighted images due to the deposition of hemosiderin or keratin nodules. Heterogeneous enhancement after the injection of contrast media is another characteristic finding. These lesions may contain cystic components that are hypointense on T1-weighted images, although intratumoral calcification is uncommon. These tumors occasionally invade the adjacent cerebral arteries and parenchyma.5,10–12 Behari et al3 described six cases of strictly intrinsic third ventricular craniopharyngiomas, including four patients featuring mixed cystic and solid components and two patients with pure solid tumors. Pascual et al1 reported no difference in the distribution rates between adamantinomatous and papillary subtypes. In our report, five adult cases, including two patients with mixed components and three cases with pure solid components, without calcification were all diagnosed as the papillary subtype. On histological staining, the presence of well-differentiated stratified squamous epithelium as the predominant type of epithelium and absence of wet keratin nodules that was compatible with papillary craniopharyngiomas.
Surgical management is the strategic treatment for strictly intrinsic third ventricular craniopharyngiomas. The consideration of a tumorous approach and the potential of tumorous removal remain challenging for neurological surgeons. The most common approaches are the frontal transcortical, transcallosal, or translaminar terminalis approaches. The application of microscope-based neuronavigation can enhance surgical results.6,8,13–16 According to Jung et al’s study,17 improvements in visual and endocrinological conditions were observed after surgical treatment. In our case series, the improvement in both visual acuity and field was seen without aggravation after surgery. Gamma knife radiosurgery can also be considered for recurrent or residual cases. Some short-term reactions, such as nausea, fatigue, or skin reactions, have been reported following radiotherapy.17,18
Conclusion
Strictly intrinsic third ventricular craniopharyngiomas are rare tumors, commonly found in adults. Imaging features showing a mixed cystic and solid or pure solid, heterogeneous mass with strong enhancement that is strictly located in the third ventricle are typical characteristics of these tumors.
Acknowledgment
Two authors contributed equally to this article as co-first authors: Nguyen Duy Hung and Nguyen Minh Duc.
Funding Statement
This study received no funding.
Ethical Approval
The need for institutional ethics approval for this case report was waived. All patients provided informed consent for their case details and images to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pascual JM, González-Llanos F, Barrios L, Roda JM. Intraventricular craniopharyngiomas: topographical classification and surgical approach selection based on an extensive overview. Acta Neurochir (Wien). 2004;146(8):785–802. doi: 10.1007/s00701-004-0295-3 [DOI] [PubMed] [Google Scholar]
- 2.Tayari N, Etemadifar M, Hekmatnia A, Mahzouni P, Maghzi AH, Rouzbahani R. Intrinsic third ventricular craniopharyngioma: a case report. Int J Prev Med. 2011;2(3):178–185. [PMC free article] [PubMed] [Google Scholar]
- 3.Behari S, Banerji D, Mishra A, et al. Intrinsic third ventricular craniopharyngiomas: report on six cases and a review of the literature. Surg Neurol. 2003;60(3):245–252. doi: 10.1016/S0090-3019(03)00132-0 [DOI] [PubMed] [Google Scholar]
- 4.Rambarki O, Rajesh A. Third ventricular craniopharyngioma. Neurol India. 2016;64(4):834–835. doi: 10.4103/0028-3886.185370 [DOI] [PubMed] [Google Scholar]
- 5.da Cunha M, Pletz ALB. Strictly intraventricular craniopharyngioma: case report and literature review. Arquivos Brasileiros De Neurocirurgia. 2018;37(2):113–118. doi: 10.1055/s-0035-1564825 [DOI] [Google Scholar]
- 6.Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: trans-lamina terminalis approach. Neurosurgery. 2000;47(4):857–63;discussion 863–5. doi: 10.1097/00006123-200010000-00014 [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki K, Kondo A, Takahashi JB, Yamanobe K. Intraventricular craniopharyngioma: report of two cases and review of the literature. Surg Neurol. 1992;38(4):294–301. doi: 10.1016/0090-3019(92)90045-o [DOI] [PubMed] [Google Scholar]
- 8.Pan J, Qi S, Lu Y, et al. Intraventricular craniopharyngioma: morphological analysis and outcome evaluation of 17 cases. Acta Neurochir (Wien). 2011;153(4):773–784. doi: 10.1007/s00701-010-0938-5 [DOI] [PubMed] [Google Scholar]
- 9.Prieto R, Pascual JM, Barrios L. Topographic diagnosis of craniopharyngiomas: the accuracy of MRI findings observed on conventional T1 and T2 images. AJNR Am J Neuroradiol. 2017;38(11):2073–2080. doi: 10.3174/ajnr.A5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol. 1997;18(1):77–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Ikezaki K, Fujii K, Kishikawa T. Magnetic resonance imaging of an intraventricular craniopharyngioma. Neuroradiology. 1990;32(3):247–249. doi: 10.1007/BF00589123 [DOI] [PubMed] [Google Scholar]
- 12.Urbach H, Behrens E, von Deimling A, Reul J. Solides Kraniopharyngeom im III. Ventrikel–Differentialdiagnostische Aspekte [Solid craniopharyngioma in the 3rd ventricle–differential diagnostic aspects]. Aktuelle Radiol. 1998;8(2):95–97. [PubMed] [Google Scholar]
- 13.Sipos L, Vajda J. Craniopharyngioma of the third ventricle. Acta Neurochir (Wien). 1997;139(1):92–93. doi: 10.1007/BF01850877 [DOI] [PubMed] [Google Scholar]
- 14.Ciurea AV, Saceleanu V, Mohan A, Moreanu MS, Toader C. Craniopharyngiomas in children - experience of consecutive 152 operated cases. Acta Endocrinol (Buchar). 2020;16(1):103–109. doi: 10.4183/aeb.2020.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YQ, Ma ZY, Wu ZB, Luo SQ, Wang ZC. Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg. 2008;44(6):435–443. doi: 10.1159/000172965 [DOI] [PubMed] [Google Scholar]
- 16.Xu JG, You C, Cai BW, et al. Microsurgical resection of craniopharyngioma of the third ventricle via an improved transventricular approach. Chin Med J (Engl). 2005;118(10):806–811. [PubMed] [Google Scholar]
- 17.Jung TY, Jung S, Jang WY, Moon KS, Kim IY, Kang SS. Operative outcomes and adjuvant treatment of purely third ventricle craniopharyngioma after a transcallosal approach. Br J Neurosurg. 2012;26(3):355–360. doi: 10.3109/02688697.2011.631615 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed SI, Javed G, Laghari AA, et al. Third ventricular tumors: a comprehensive literature review. Cureus. 2018;10(10):e3417. doi: 10.7759/cureus.3417 [DOI] [PMC free article] [PubMed] [Google Scholar]