Abstract
Background:
Beta blockers act on the beta-adrenergic receptors ADRB1 and ADRB2 to reduce heart rate and blood pressure. Observational studies have revealed strong risk reductions in metastasis and cancer-specific mortality with the use of beta-blockers in patients with some cancers. But observational studies of prostate cancer have reported conflicting results.
Objectives:
We examined the relationship of ADRB1 (Adrenoceptor beta 1) gene expression and ADRB2 (Adrenoceptor beta 2) gene expression with Forkhead box protein A1 (FOXA1) gene expression in prostate cancer. We also analyzed survival data of solid tumor patients with respect to beta 1 (ADRB1) and beta 2 (ADRB2) adrenergic receptor gene expression.
Methods:
We examined the genomics of prostate cancer and other solid primary tumors in the GDC TCGA Prostate Cancer (PRAD) data set. The Cancer Genome Atlas (TCGA) contains the analysis of over 11,000 tumors from 33 of the most prevalent forms of cancer.
Results:
The presence of somatic mutations [Single nucleotide polymorphisms (SNPs) and small insertion/deletion polymorphism (INDELS)] in FOXA1 alters ADRB1 and ADRB2 gene expression. The correlation of FOXA1 gene expression with ADRB1 and ADRB2 gene expression is highly significant. Alterations in FOXA1 genes, ADRB1 genes, and ADRB2 genes are significantly co-occurrent, indicating that they may work in tandem to drive tumor formation and development. Increased ADRB1 and ADRB2 expressions reduce the overall survival of solid tumor patients in the GDC Pan Cancer set.
Conclusions:
FOXA1 signaling may regulate ADRB1 and ADRB2 expression, as well as androgen receptor expression. Analysis of these tumor mutations might indicate whether an individual prostate cancer patient will respond to beta blockers.
Beta blockers have anticancer effects. In vitro and animal studies have shown that activation of beta-adrenergic receptors occurs during tumor cell proliferation. Epinephrine and norepinephrine, which stimulate adrenergic receptors, can induce angiogenesis as well as tumor cell invasion and migration (Zhao and Li, 2019).
Observational studies have revealed strong risk reductions in metastasis and cancer-specific mortality with the use of beta-blockers in patients with some cancers. But observational studies of prostate cancer have reported conflicting results (Assayag et al., 2014).
In the current study, we used the Cancer Genome Atlas (TCGA) to examine the relationship of ADRB1 (Adrenoceptor beta 1) gene expression and ADRB2 (Adrenoceptor beta 2) gene expression with Forkhead box protein A1 (FOXA1) gene expression in prostate cancer. Atenolol, which is beta 1 selective, is associated with a reduction in the risk of prostate cancer (Assayag et al., 2014). FOXA1 regulates androgen receptor (AR) signaling and plays an important role in prostate cancer development (Shah and Brown, 2019). We also looked at the effects of ERG-TMPRSS2 fusion by chr21 deletion, PTEN loss, and SPOP mutations.
In addition, we analyzed survival data of all solid tumor patients with respect to beta 1 (ADRB1) and beta 2 (ADRB2) adrenergic receptor gene expression.
Methods
We examined the genomics of prostate cancer in the GDC TCGA Prostate Cancer (PRAD) data set and the MSKCC/DFCI data set (Wedge et al., 2018). The Cancer Genome Atlas (TCGA) contains the analysis of over 11,000 tumors from 33 of the most prevalent forms of cancer (Hutter and Zenklusen, 2018). To access and analyze the data we used:
- UCSC Xena browser, a web-based visual integration and exploration tool for TCGA data, including clinical and phenotypic annotations (Goldman et al., 2015). Gene expression is quantitated as Fragments Per Kilobase of transcript per Million mapped reads upper quartile (fpkm-uq), which is an RNA-Seq-based expression normalization method (Shahriyari, 2017).
- cBioportal, a web-based interface that enables integrative analysis of complex cancer genomics and clinical profiles (Gao et al., 2013).
Simple statistics were calculated to identify patterns of mutual exclusivity or co-occurrence. For a pair of query genes, an odds ratio (OR) is calculated (Equation 1) that indicates the likelihood that the events in the two genes are mutually exclusive or co-occurrent across the selected cases:
| (1) |
where A = number of cases altered in both genes; B = number of cases altered in gene A but not gene B; C = number of cases altered in gene B but not gene A; and D = number of cases altered in neither gene. Each pair was then assigned to one of three categories indicative of a tendency toward mutual exclusivity, of a tendency toward co-occurrence, or of no association. To determine whether the identified relationship is significant for a gene pair, Fisher’s exact test was performed (Gao et al., 2013).
Survival data of all cancer patients with primary solid tumors related to beta 1 and beta 2 adrenergic receptor gene expression were extracted from the TCGA GDC PanCancer (PANCAN) data set for analysis and generation of Kaplan-Meier curves of overall survival (Nawy, 2018). Survival time was defined as the period from the date of diagnosis to the date of death. If unavailable, the date of the last follow-up was used for KM right censoring. Differences between Kaplan-Meier survival curves were calculated by the log-rank (Mantel-Cox) test.
Results
Prostate cancer patients with primary tumors were age 61 ± 6.8 (mean ± SD). 30% were white, 1.4% African American, 0.4% Asian; and of the remainder, race was not recorded. 97% were of the prostate adenocarcinoma acinar type.
An Oncoprint diagram of ADRB1, ADRB2, FOXA1, and AR in 1,013 primary prostate cancer samples indicates that alterations are present in 2.1% of ADRB1 genes, 1.9% of ADRB2 genes, 9% of FOXA1 genes, and 19% of AR genes (Figure 1). The alterations are significantly co-occurrent (Table 1).
Figure 1.
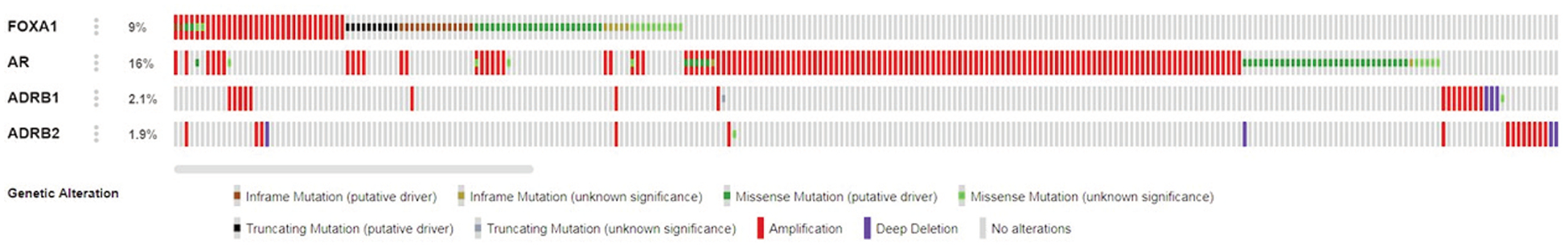
Oncoprint diagram of FOXA1, AR, ADRB1, and ADRB2 in 1,013 primary prostate cancer samples. Alterations are present in 9% of FOXA1 genes, 16% of AR genes, 2.1% of ADRB1 genes, and 1.9% of ADRB2 genes. (via cBioportal)
Table 1.
Significantly co-occurring alterations in prostate cancer patients of ADRB1, ADRB2, AR, and FOXA1. q-value is derived from the Benjamini-Hochberg false discovery rate (FDR) correction procedure for multiple comparisons.
| A | B | Neither | A Not B | B Not A | Both | Log2 Odds Ratio | p-Value | q-Value | Tendency |
|---|---|---|---|---|---|---|---|---|---|
| FOXA1 | ADRB1 | 904 | 88 | 14 | 7 | 2.361 | 0.002 | 0.01 | Co-occurrence |
| FOXA1 | AR | 777 | 69 | 141 | 26 | 1.054 | 0.003 | 0.01 | Co-occurrence |
| FOXA1 | ADRB2 | 904 | 90 | 14 | 5 | 1.843 | 0.026 | 0.052 | Co-occurrence |
| ADRB1 | ADRB2 | 975 | 19 | 17 | 2 | 2.594 | 0.057 | 0.085 | Co-occurrence |
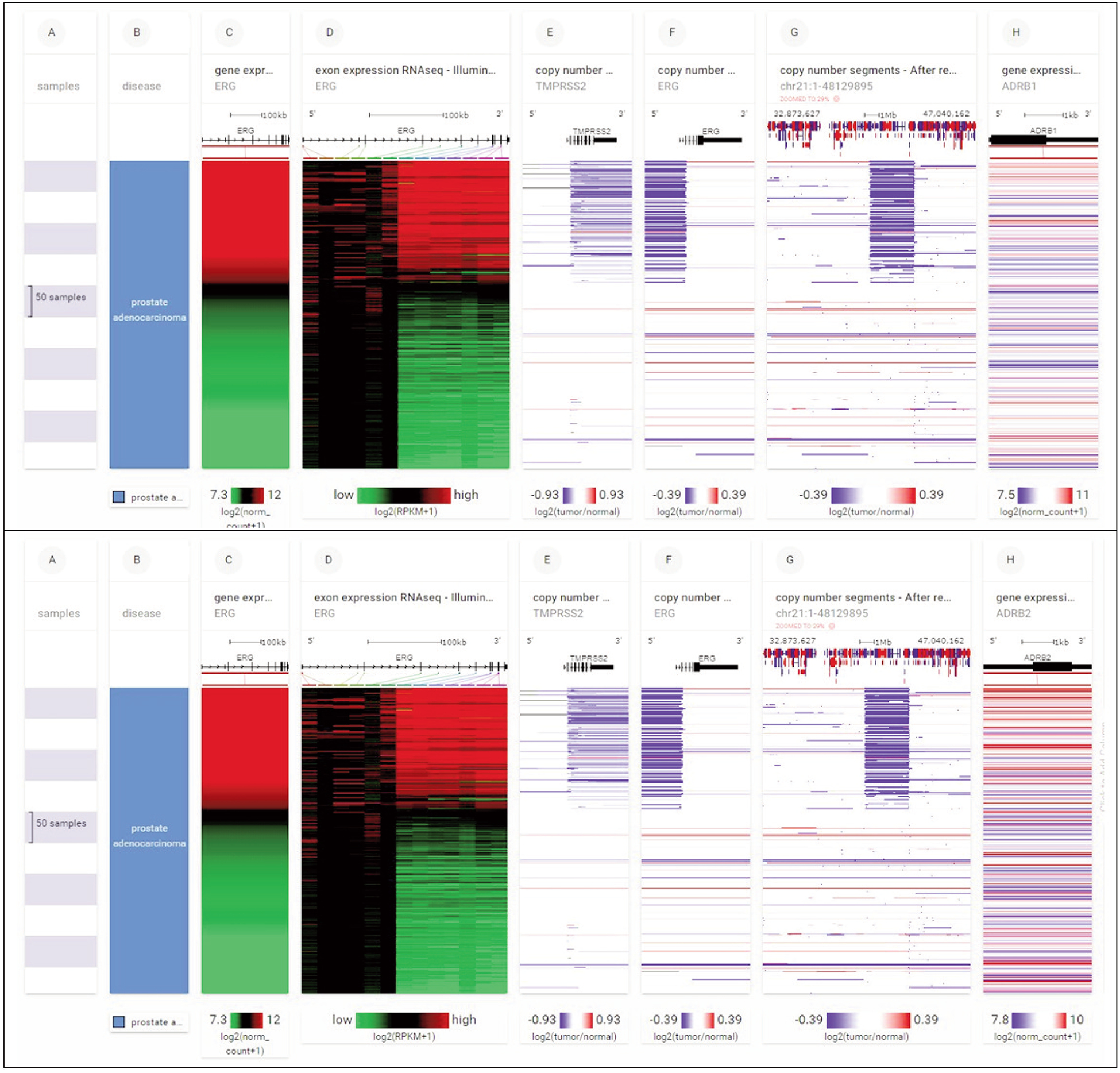
FOXA1 somatic mutations, as well as ADRB1 and ADRB2 gene expression in 492 primary prostate cancer samples, are presented in Figure 2. The presence of somatic mutations — single nucleotide polymorphisms (SNPs) and small insertion/deletion polymorphisms (INDELS) — in FOXA1 alters ADRB1 and ADRB2 gene expression.
Figure 2.
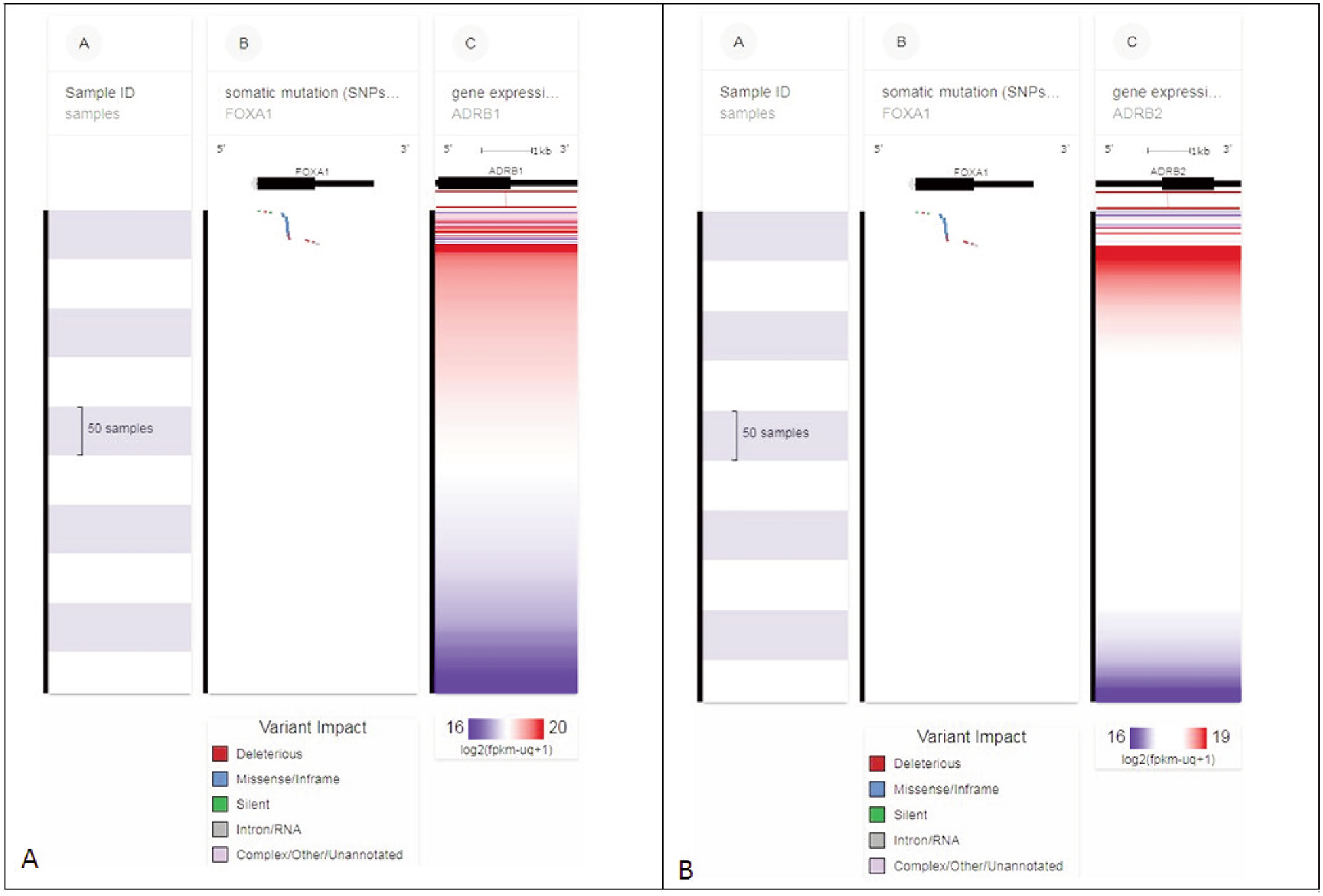
(A) FOXA1 somatic mutations and ADRB1 gene expression in 492 primary prostate cancer samples. Note that the presence of somatic mutations (single nucleotide polymorphisms (SNPs) and small insertion/deletion polymorphisms (INDELS)) in FOXA1 (upper section column B) alters ADRB1 gene expression (multicolored horizontal lines, upper section column C). (B) FOXA1 somatic mutations and ADRB2 gene expression in 492 primary prostate cancer samples. Note that the presence of somatic mutations in FOXA1 (upper section column B) alters ADRB2 gene expression. Each row contains data from a single sample. Row order is determined by sorting the rows by their column values. Column C value is used to sort the rows. (via xenabrowser.net)
FOXA1 gene expression versus ADRB1 gene expression and ADRB2 gene expression is presented in Figure 3. The correlation is highly significant. FOXA1 gene expression also correlates with AR gene expression (Figure 4). ADRB1, ADRB2, FOXA1, and AR are members of a network of neighboring genes shown in Figure 5.
Figure 3.
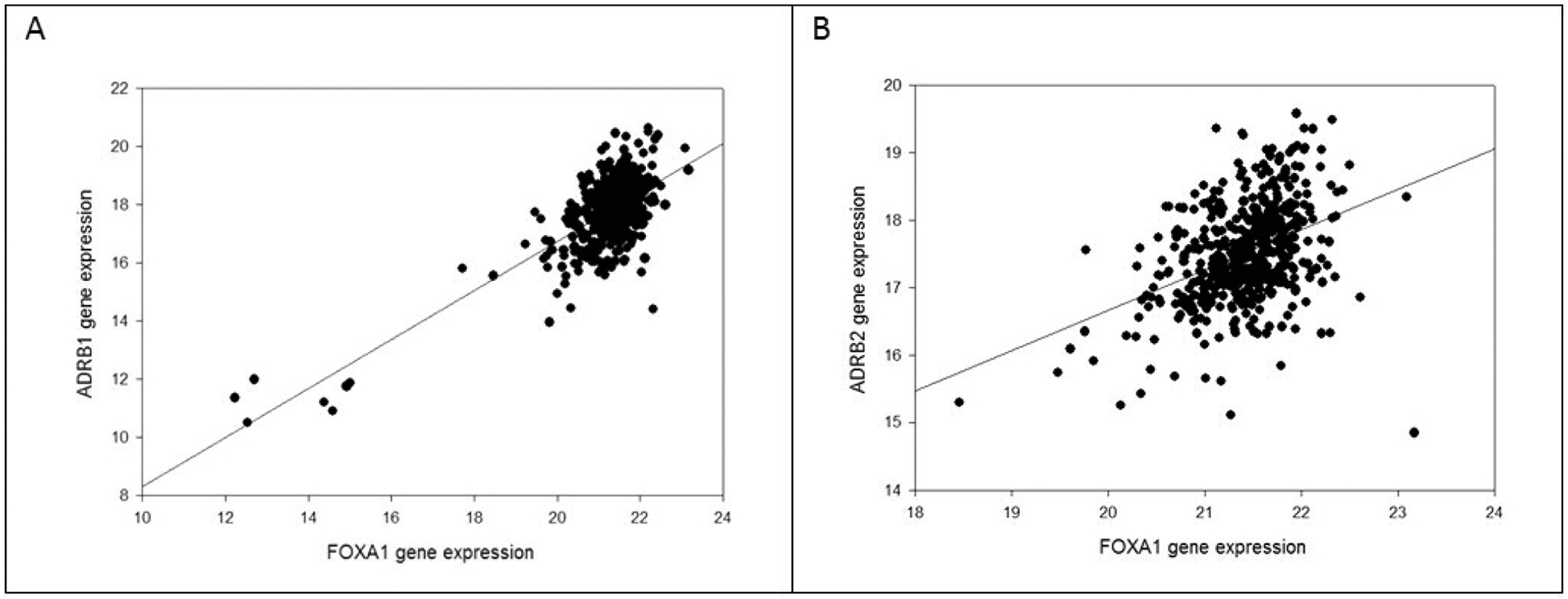
(A) FOXA1 gene RNA expression (log2(fpkm-uq+1)) and ADRB1 gene RNA expression in 504 primary prostate cancer samples. The correlation is highly significant (r = 0.72, p < 0.0001). (B) FOXA1 gene expression and ADRB2 gene expression in 504 primary prostate cancer samples. The correlation is highly significant (r = 0.40, p < 0.0001).
Figure 4.
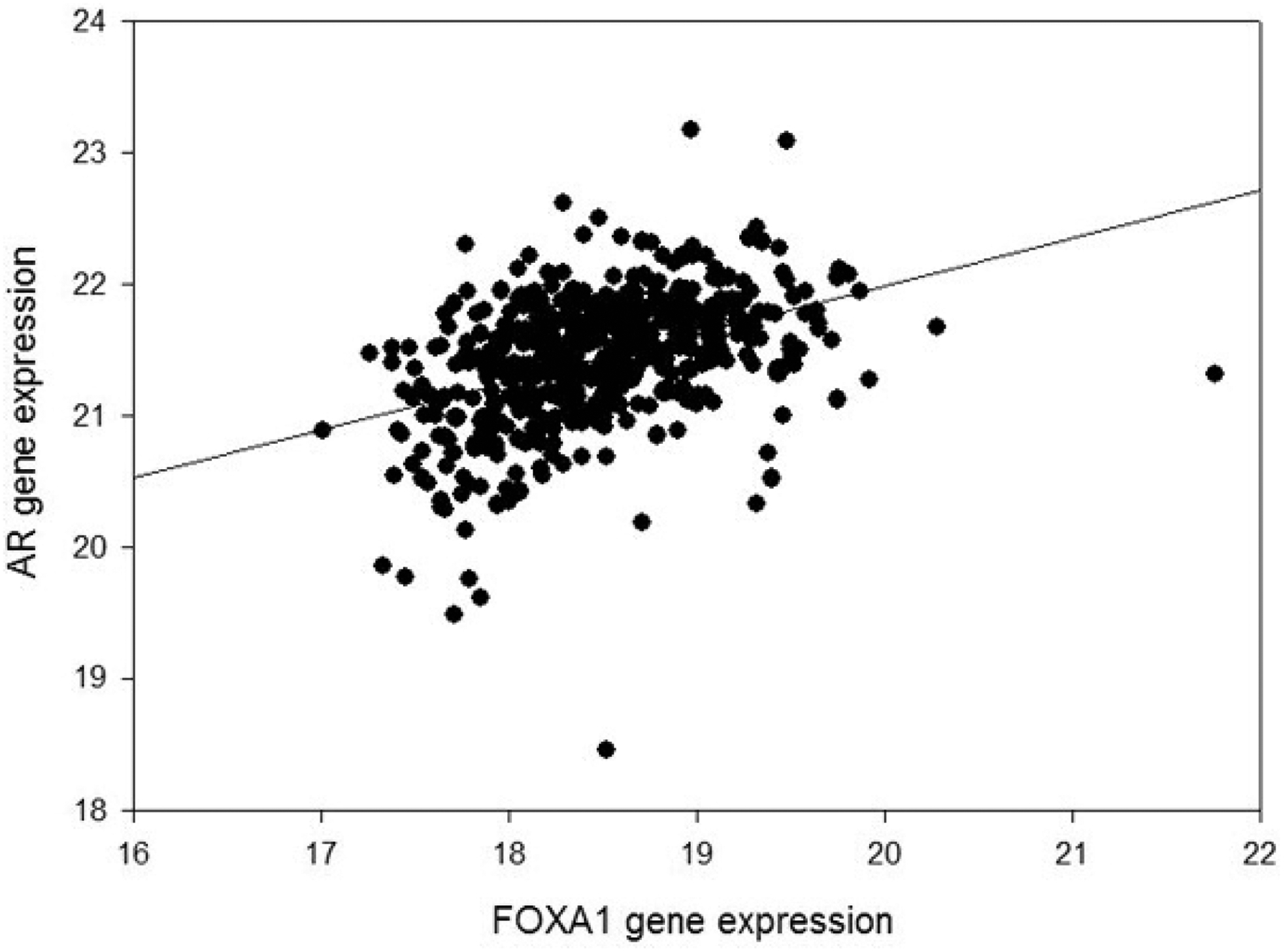
FOXA1 gene RNA expression and AR gene RNA expression in 504 primary prostate cancer samples. The correlation is highly significant (r = 0.42, p < 0.0001).
Figure 5.
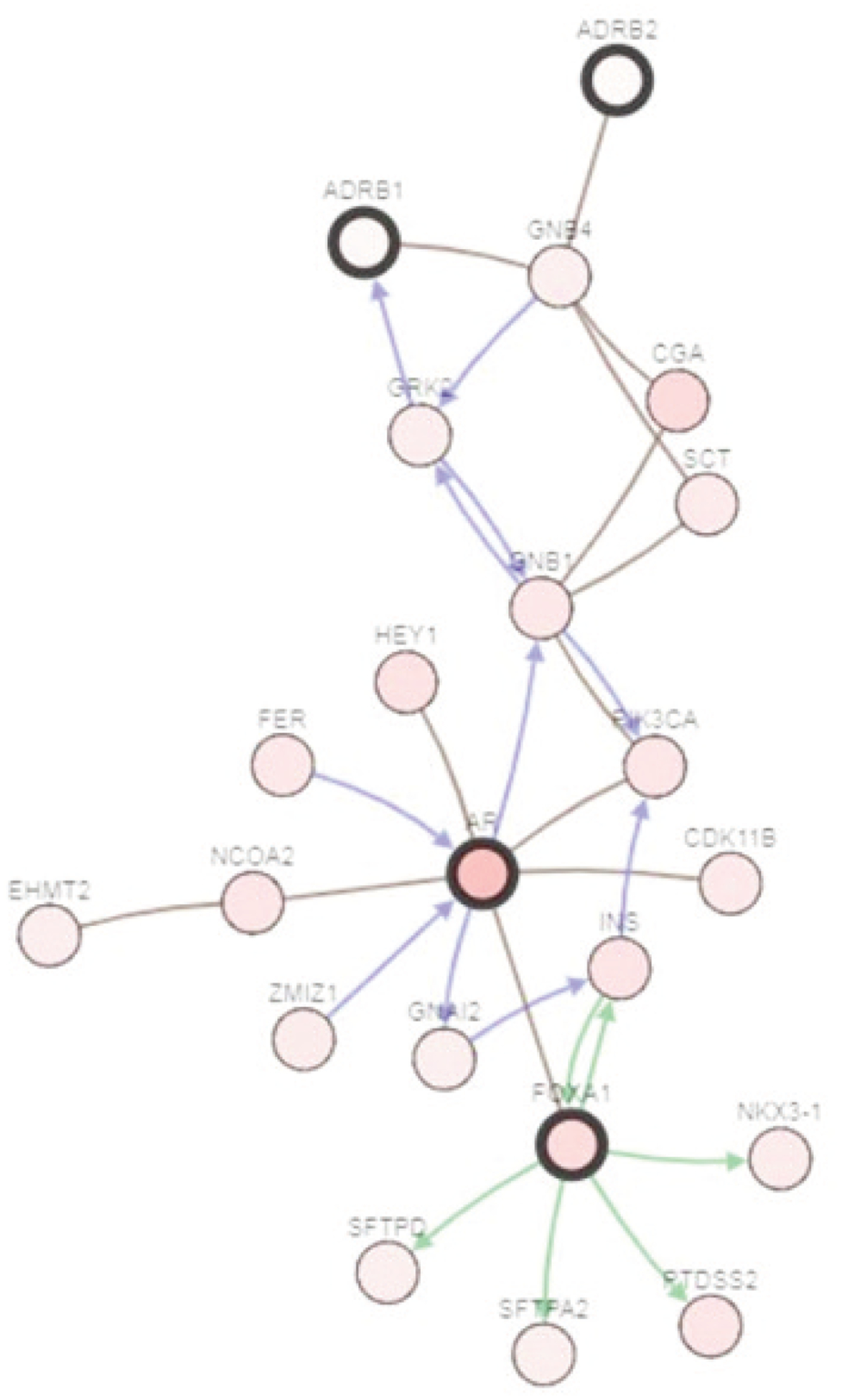
FOXA1 and its network of neighboring genes, including AR, ADRB1, and ADRB2, are implicated in the genesis of prostate cancer. An arrow means a directed interaction and a line means an undirected interaction. The green arrow means control of phosphorylation. A blue arrow means control of the state of change (i.e., genetic alterations). The brown lines mean targeted by drugs. (via cBioportal.org)
FOXA1 mutation is highly prevalent (~11% in primary prostate cancer). We investigated whether ADRB1 or ADRB2 expression is correlated with AR expression and whether AR expression is independently correlated with FOXA1, to rule out whether ADRB1 and ADRB2 are not predicted by AR signaling alone. For this analysis, we used the multivariate general linear model of SPSS, AR as a fixed factor, and ADRB1, ADRB2, and FOXA1 as dependent variables. AR expression was independently correlated with ADRB1 expression (p = 0.007), ADRB2 expression (p = 0.010), and FOXA1 expression (p = 0.002).
Effect of ADRB1 and ADRB2 expression on overall survival of patients in the TCGA GDC Pan Cancer set with primary solid tumors is shown in Figure 6. ADRB1 underexpression is the best prognostic indicator.
Figure 6.
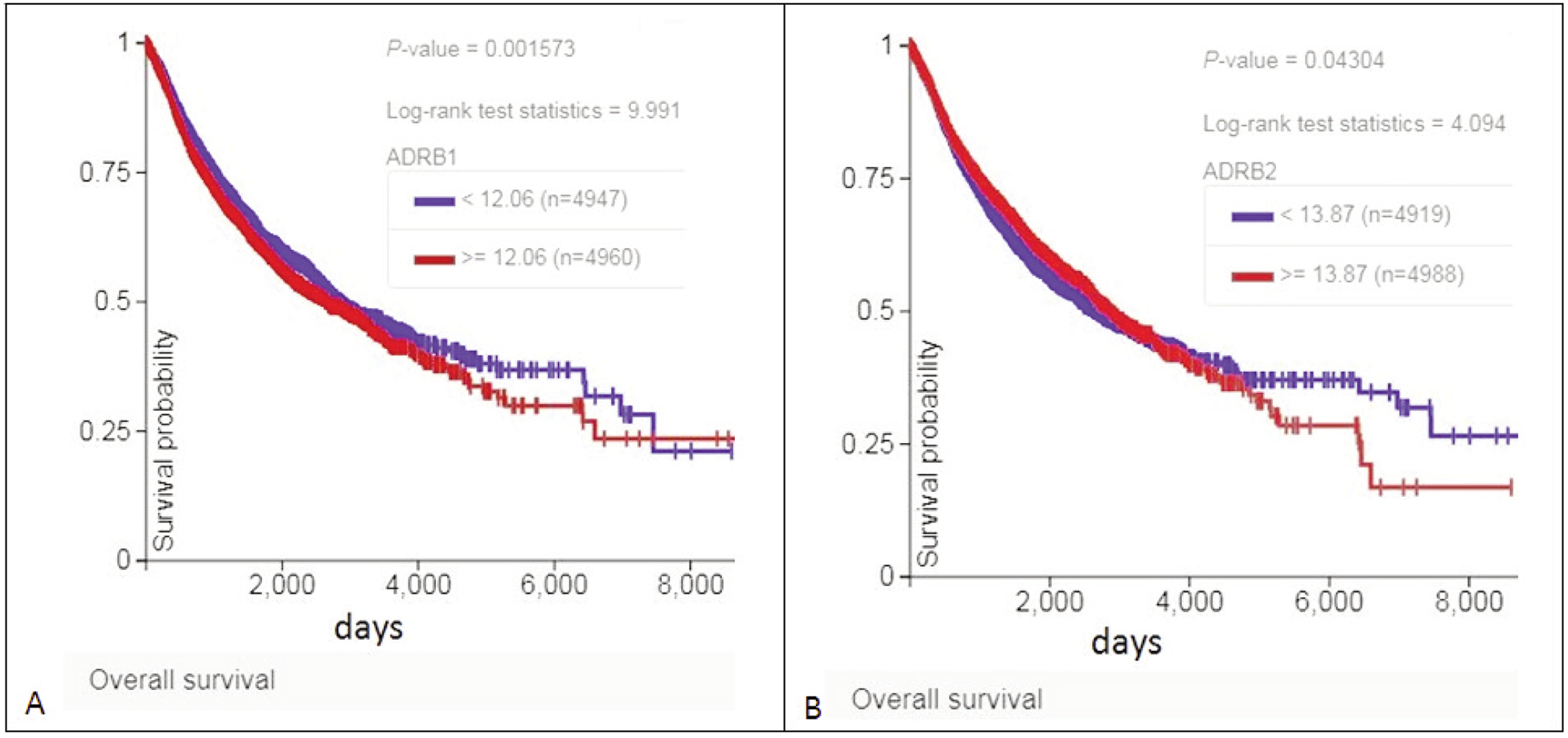
Effect of ADRB1 and ADRB2 expression on overall survival of patients in the TCGA GDC Pan Cancer data set with primary solid tumors. Reduced expression (blue curves) is associated with better survival, especially in the case of ADRB1 (A). In (B), survival associated with increased expression deteriorates with the passage of time.
Common mutations in prostate cancer affect ADRB1 and/or ADRB2 expression. ERG-TMPRSS2 fusion by chr21 deletion is correlated with ADRB2 expression (r = 0.18, p = 0.00004485) but not ADRB1 expression (r = 0.007, p = 0.87, Figure 7). PTEN loss is correlated with ADRB2 expression (r = −0.18, p = 0.00006801) but not with ADRB1 expression (r = −0.06, p = 0.17). SPOP mutations alter unpredictably the expression of ADRB1 and ADRB2 (Figure 8).
Figure 7.
A common mutation in prostate cancer, ERG-TMPRSS2 fusion by chr21 deletion, is correlated with ADRB2 expression (r = 0.18, p = 0.00004485, lower panel) but not ADRB1 expression (r = 0.007, p = 0.87, upper panel). The blue blocks (columns E, F, G) represent samples with the ERG-TMPRSS2 fusion by chr21 deletion.
Figure 8.

SPOP mutations (column C, upper section) alter unpredictably the expression of ADRB1 (horizontal lines, column D, upper panel) and ADRB2 (horizontal lines, column D, lower panel).
Discussion
The prostate gland is rich in nerves and neural growth factors. Prostate cancer cells directly interact with nerves, and nerve density in the prostate is highest in areas with cancer (Ayala et al., 2008). Neural progenitor cells, from a region of the brain in which the normal process of nerve-cell formation (neurogenesis) occurs, migrate to the site of prostate cancer. These cells invade the tumor microenvironment and give rise to new nerve cells (Mauffrey et al., 2019). Therefore, drugs that target the nervous system might help treat prostate cancer, as well as other cancers.
One common class of drugs that acts on the nervous system, beta blockers, has been used since the 1960s to reduce blood pressure, treat cardiovascular disease, and sometimes manage short-term anxiety. These drugs block the beta-adrenergic receptors ADRB1 and ADRB2 to reduce heart rate (Servick, 2019).
The incidence of treatment-related neuroendocrine prostate cancer (t-NEPC) is rising as more potent drugs targeting the androgen signaling axis are introduced. Neuroendocrine transdifferentiation (NEtD), an initial step in t-NEPC development, is induced by androgen-deprivation therapy (ADT) or anti-androgens, and by activation of ADRB2 in prostate cancer cell lines (Braadland et al., 2019).
In a recent retrospective review of more than 4,182 men who had undergone a biopsy for prostate cancer, a cohort of 669 men had taken a beta blocker - either atenolol (Tenormin), metoprolol (Lopressor/Toprol XL), or carvedilol (Coreg) - within one year of their biopsy. Atenolol, which is beta 1 selective, was associated with a reduction in intermediate-risk prostate cancer of approximately 50% compared to no beta blocker. Moreover, a significant reduction in low-risk disease on biopsy was noted in men taking atenolol. In contrast, metoprolol and carvedilol had no effect (Zahalka et al., 2019). Metoprolol is beta 1-selective while carvedilol is a non-selective beta-blocker (Stoschitzky et al., 2001).
The anti-cancer effect of atenolol may be due in part to its high water solubility, as opposed to metoprolol and carvedilol, which are poorly water-soluble but highly lipid-soluble (Gengo et al., 1987). The lack of good aqueous solubility has been frequently identified as a key obstacle to the development and clinical use of anti-cancer compounds (Narvekar et al., 2014). Water flow in the prostate is mediated by a channel mechanism, termed the aquaporins, some of which allow a selective permeation of water (Wang et al., 2008).
Forkhead box protein A1 (FOXA1) is commonly mutated in prostate cancer, as was mentioned above (Yang and Yu, 2015). FOXA1 mutations alter lumen structure and increase androgen receptor signaling. FOXA1 mutations in prostate cancer predict a worse clinical outcome, according to two new studies (Shah and Brown, 2019).
One study found an increased prevalence of FOXA1 mutations by using RNA sequencing data from approximately 1,546 prostate cancers (Parolia et al., 2019). The mutations fall into three classes and drive the development of the disease in at least three ways:
- Class 1 FOXA1 mutations cause the FOXA1 transcription factor to travel more quickly through the DNA, allowing the androgen receptor to activate the expression of cancer-promoting genes. These mutations are seen in early-stage prostate cancer and are likely what triggers the disease.
- Class 2 FOXA1 mutations cause a part of the FOXA1 molecule to be cut off. The truncated molecule binds strongly to DNA, preventing normal FOXA1 from binding. Class 2 mutations are often found in lethal hormone-therapy resistant prostate cancer and promote tumor spread to different sites.
- Class 3 FOXA1 mutations result in complex rearrangements of the FOXA1 genomic position, creating duplications and overexpression.
A second study of FOXA1 analyzed sequencing data from 3,086 patients with primary or metastatic prostate cancer. 11% had mutations in FOXA1. Mutated FOXA1 was associated with higher Gleason Scores, reduced time to biochemical recurrence, and more rapid metastasis. The effects of the mutations are dependent on their position. More than 50% of FOXA1 mutations were in the Wing2 region of the forkhead DNA-binding domain. Mutations clustered more infrequently at R219 (Adams et al., 2019).
Biological processes or pathways in cancer are often deregulated through different genes or by multiple different mechanisms. But cancer gene alterations usually do not occur at random. Alterations of certain cancer genes tend to co-occur, indicating that they may work in tandem to drive tumor formation and development (Gao et al., 2013). This may be the case with the co-occurring alterations in Table 1 of FOXA1, AR, ADRB1, and ADRB2.
The co-occurrence of alterations may not be causative. Additional studies in cell lines would therefore be worthwhile: 1) Measuring ADRB expression in FOXA1 enhanced and FOXA1 knockout or knockdown cell lines; 2) measuring ADRB expression in FOXA1 cell lines with added testosterone versus no testosterone given the association with AR; 3) measuring a surrogate marker in the pathway, e.g., PIK3CA (after AR in the cascade) or INS (insulin gene, before AR in the cascade) as presented in Figure 5; 4) performing a growth assay of FOXA1 in β-blocker solution versus control that shows restricted growth in FOXA1 mutated prostate cancer. These studies could confirm the genetic relationships presented here.
In summary, data presented suggest that FOXA1 signaling, along with other common prostate cancer genetic mutations (ERG-TMPRSS2 fusion, PTEN loss, SPOP mutations), may regulate ADRB1 and ADRB2 expression, as well as androgen receptor expression. Analysis of these tumor mutations might indicate whether an individual prostate cancer patient will respond to beta blockers.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Adams EJ, Karthaus WR, Hoover E, Liu D, Gruet A, Zhang Z, Cho H, Diloreto R, Chhangawala S, Liu Y, Watson PA, Davicioni E, Sboner A, Barbieri CE, Bose R, Leslie CS, Sawyers CL. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571(7765):408–412, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assayag J, Pollak MN, Azoulay L. Post-diagnostic use of beta-blockers and the risk of death in patients with prostate cancer. Eur J Cancer 50(16):2838–2845, 2014. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14(23):7593–7603, 2008. [DOI] [PubMed] [Google Scholar]
- Braadland PR, Ramberg H, Grytli HH, Urbanucci A, Nielsen HK, Guldvik IJ, Engedal A, Ketola K, Wang W, Svindland A, Mills IG, Bjartell A, Tasken KA. The beta2-Adrenergic Receptor Is a Molecular Switch for Neuroendocrine Transdifferentiation of Prostate Cancer Cells. Mol Cancer Res DOI: 10.1158/1541-7786.MCR-1118-0605, 2019. [DOI] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):l1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo FM, Huntoon L, Mchugh WB. Lipid-soluble and water-soluble beta-blockers. Comparison of the central nervous system depressant effect. Arch Intern Med 147(1):39–43, 1987. [DOI] [PubMed] [Google Scholar]
- Goldman M, Craft B, Swatloski T, Cline M, Morozova O, Diekhans M, Haussler D, Zhu J. The UCSC Cancer Genomics Browser: update 2015. Nucleic Acids Res 43(Database issue):D812–D817, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter C, Zenklusen JC. The Cancer Genome Atlas: Creating Lasting Value beyond Its Data. Cell 173(2):283–285, 2018. [DOI] [PubMed] [Google Scholar]
- Mauffrey P, Tchitchek N, Barroca V, Bemelmans A, Firlej V, Allory Y, Romeo PH, Magnon C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569(7758):672–678, 2019. [DOI] [PubMed] [Google Scholar]
- Narvekar M, Xue HY, Eoh JY, Wong HL. Nanocarrier for poorly water-soluble anticancer drugs — barriers of translation and solutions. AAPS PharmSciTech 15(4):822–833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy T. A pan-cancer atlas. Nat Methods 15(6):407, 2018. [DOI] [PubMed] [Google Scholar]
- Parolia A, Cieslik M, Chu SC, Xiao L, Ouchi T, Zhang Y, Wang X, Vats P, Cao X, Pitchiaya S, Su F, Wang R, Feng FY, Wu YM, Lonigro RJ, Robinson DR, Chinnaiyan AM. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 571(7765):413–418, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K. War of nerves. Science 365(6458):1071–1073, 2019. [DOI] [PubMed] [Google Scholar]
- Shah N, Brown M. The Sly Oncogene: FOXA1 Mutations in Prostate Cancer. Cancer Cell 36(2):119–121, 2019. [DOI] [PubMed] [Google Scholar]
- Shahriyari L. Effect of normalization methods on the performance of supervised learning algorithms applied to HTSeq-FPKM-UQ data sets: 7SK RNA expression as a predictor of survival in patients with colon adenocarcinoma. Brief Bioinform, 2017. [DOI] [PubMed] [Google Scholar]
- Stoschitzky K, Koshucharova G, Zweiker R, Maier R, Watzinger N, Fruhwald FM, Klein W. Differing beta-blocking effects of carvedilol and metoprolol. Eur J Heart Fail 3(3):343–349, 2001. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanji N, Sasaki T, Kikugawa T, Song X, Yokoyama M. Androgens upregulate aquaporin 9 expression in the prostate. Int J Urol 15(10):936–941, 2008. [DOI] [PubMed] [Google Scholar]
- Wedge DC, Gundem G, Mitchell T, Woodcock DJ, Martincorena I, Ghori M, Zamora J, Butler A, Whitaker H, Kote-Jarai Z, Alexandrov LB, Van Loo P, Massie CE, Dentro S, Warren AY, Verrill C, Berney DM, Dennis N, Merson S, Hawkins S, et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet 50(5):682–692, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YA, Yu J. Current perspectives on FOXA1 regulation of androgen receptor signaling and prostate cancer. Genes Dis 2(2):144–151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka A, Fram E, Lin W, Mohn L, Frenette P, Agalliu I, Watts K. Atenolol reduces incident low and intermediate risk prostate cancer. Journal of Urology 201(Supplement 4):e703–e703, 2019. [Google Scholar]
- Zhao Y, Li W. Beta-adrenergic signaling on neuroendocrine differentiation, angiogenesis, and metastasis in prostate cancer progression. Asian J Androl 21(3):253–259, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


