Abstract
Purpose of Review
Endothelial cells are of great importance in many types of diseases including the coronary artery diseases in heart and stroke in brain. In this review, we explore the heterogeneity among endothelial cells from an organism-wide, organ-specific, and healthy versus disease perspective.
Recent Findings
Recent studies addressing the cellular heterogeneity between arterial versus venous endothelial cells (ECs) have revealed that arterial ECs have tighter junctions, a decreased immune response, anticoagulant properties while veins have both anticoagulant and procoagulant properties. Blood and lymphatic ECs are quite distinct from each other as well, with the lymphatic ECs being more involved in the immune response and lymphangiogenesis while blood vessel ECs being involved in angiogenesis and maintenance of perfusion throughout the body. ECs from various organs such as the heart, the lung, and especially the brain are quite heterogeneous and provide barriers that prevent small particles to pass through the endothelium when compared with the endothelium of the liver and the kidney that are quite porous. The heart ECs have higher angiogenesis and metabolic rates (oxidation and glycolysis) than lung, liver, and kidney ECs. Ex vivo liver and kidney ECs grow at a moderate pace, while the lung and brain ECs grow very slowly. ECs from within a tumor have fenestrae and large intracellular gaps and junctions leading to increased permeability and tumor cell overgrowth.
Summary
There is a large degree of heterogeneity among organism-wide and organ-specific ECs as well as between healthy and disease-specific ECs. We believe this review will help highlight the EC heterogeneity and further advance our ability to treat cardiovascular disease and other conditions.
Keywords: Endothelial cells, Organism-wide, Organ-specific, Heterogeneity, Blood vessel, Lymphatic
Introduction
Cardiovascular diseases remain the leading causes of death worldwide. Endothelial cells (ECs) play an important role in these diseases, especially coronary artery disease and stroke given that endothelial cells line the coronary arteries and the blood vessels in the brain. In addition, ECs are critical to cancer growth since they are involved in blood vessel formation which the cancer tissue depends on for survival [1]. This EC lining is important to the vessels as it controls the passage of materials such as immune cells and other molecules into and out of the blood vessels. Also, ECs allow the blood vessels to be highly adaptable. For example, ECs can detect shear stress and signal to other molecules to change the diameter and thickness of the blood vessel wall [2].
Another unique aspect of ECs is their heterogeneity among organism-wide ECs, organ-specific ECs, and tumor ECs. Organism-wide EC heterogeneity is exemplified by how arteries express many anticoagulant factors, while veins express a large amount of both anticoagulant and procoagulant factors [3]. Heart-specific ECs have significantly more angiogenic potential and higher metabolic rates than the lung, kidney, and liver ECs [4••]. Also, ECs in cancers are more fenestrated and have enlarged intracellular gaps or junctions over healthy ECs.
In this study, we attempt to discuss this heterogeneity among organism-wide ECs including arterial, venous, capillary and lymphatic ECs; organ-specific ECs including blood brain barrier, liver, heart, kidney, and lung ECs; and healthy versus disease ECs such as those found in tumors. Together, this review offers broad insight into endothelial heterogeneity. Understanding the differences between various EC populations in the body can ultimately help with medical interventions targeting specific EC subtypes.
Endothelial Cell Types
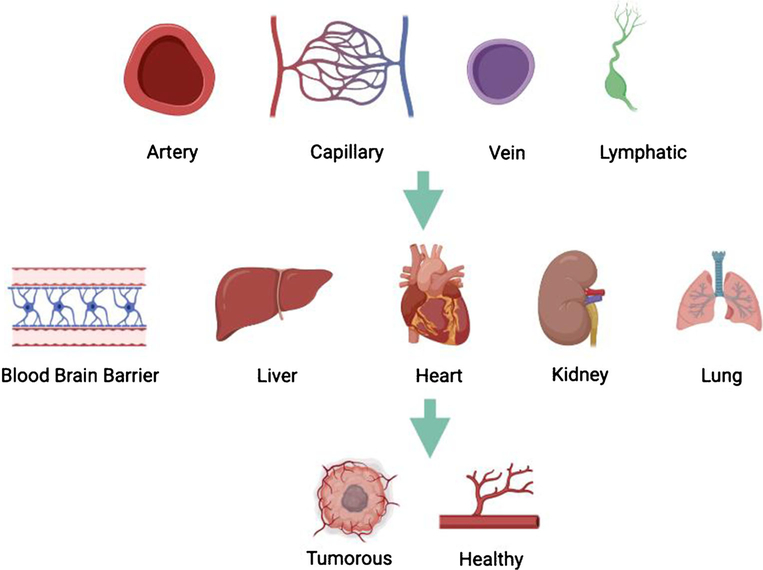
There are four major organism-wide ECs which are arterial, venous, capillary, and lymphatic ECs (Fig. 1) [5].
Fig. 1.
Overview of the various endothelial cells examined for heterogeneity including a comparison of four organism-wide ECs which are arterial, venous, capillary, and lymphatic ECs. Organ-specific ECs including blood brain barrier, liver, heart, kidney, and lung ECs, along with tumor versus healthy ECs. The figure was made in Biorender.
Arterial ECs
Arteries lined with ECs typically carry oxygenated blood away from the heart to deliver oxygen and other nutrients to the tissues [5]. Also, unlike venous ECs, arterial ECs do not develop valves and are invested with smooth muscle cells to generate thick vessel walls which allows them to act as “pressure reserves” that propel the blood rapidly with each heart beat [6]. Arterial ECs are usually flat, from 0.2 μm at the edges to 3 μm in height at the nucleus [7]. They are ellipsoidal or appear elongated and narrow which matches up their high flow rate [5].
Venous ECs
Venous ECs support the transfer of deoxygenated blood back to the heart. ECs in the veins form valves and do not have smooth muscle cell investment. Their thin wall allows them to act as volume reserves [6]. As the blood flow rate in veins is slow in comparison with arteries [5], the venous ECs generally appear short and wide.
Capillary ECs
The capillary ECs are primarily involved in oxygen and nutrient exchange in the circulatory system [6]. The EC lining is very thin to allow for efficient exchange of these substances, while the capillaries are small with a diameter less than 10 μm [6]. The blood flow through the capillaries is slow which facilitates diffusion [6].
Lymphatic ECs
The lymphatic system which these ECs make up is largely responsible for mediating an immune response [8–11]. Lymphatic ECs are encircled by a thin, discontinuous basement membrane; do not have perivascular cells; and entail discontinuous “button-like” cell junctions [12]. They have anchoring filaments to detect changes in interstitial pressure which can modulate the opening and closing of “flap valves” between button junctions to permit fluid and immune cell entry [11].
Endothelial Cells Found Throughout the Organism
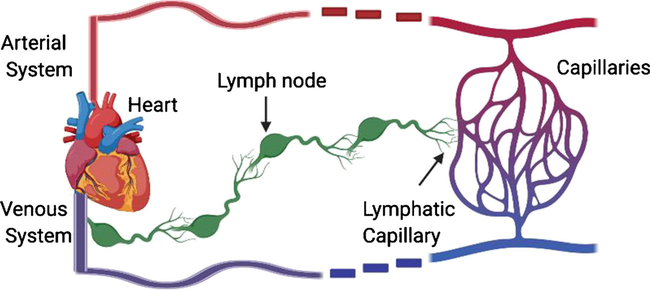
Organism-wide ECs exhibit significant molecular and functional heterogeneity including arterial versus venous and blood versus lymphatic vessels (Fig. 2).
Fig. 2.
Overview of how the circulatory system and lymphatic system work in tandem. The lymphatic capillaries pick up macromolecules, cells, and tissue fluid from capillaries, goes through the lymphatic system through a series of lymph nodes, then enters circulation through the thoracic duct to the vena cava. The vessels in red and in blue represent arteries and veins, respectively. The figure was made in Biorender.
Arterial Versus Venous Endothelial Cells
Significant heterogeneity exists among ECs from various artery and vein beds in the body. One major component of their heterogeneity is the cellular structure of the blood vessels that is created. Arterial ECs have tighter junctions between them compared with venous ECs. In addition, arterial and venous ECs have different functions and movements; for instance, arteries carry oxygenated blood and extend and contract with each heartbeat, while veins carry deoxygenated blood and do not pulsate [6, 13].
The heterogeneity of the inflammatory response can be explained by analyzing arterial and venous ECs on a microscopic level. As venous ECs mediate a relatively large inflammatory response (e.g., increased permeability to macromolecules and increased expression in genes that accrue leukocytes to the inflammation site), whereas arterial ECs are generally phenotypically unchanged during inflammation [14]. Also, coagulation properties are different between arterial and venous ECs. Two opposing but equally necessary functions of the endothelium are procoagulating and anticoagulating the blood to either prevent blood clots or repair the vascular wall during tissue injury respectively. Procoagulant ECs produce tissue factor, plasminogen activator inhibitor (PAI)-1, von Willebrand factor (vWF), and protease-activated receptors [15, 16], while anticoagulant ECs make tissue factor pathway inhibitor (TFPI), heparan, thrombomodulin, endothelial protein C receptor (EPCR), tissue-type plasminogen activator (t-PA), ecto-ADPase, prostacyclin, and nitric oxide. These different properties are expressed differentially among organism-wide arteries, veins, and capillaries. Arteries and veins appear to be primarily involved in anticoagulation as arteries express thrombomodulin, t-PA, and EPCR; likewise, veins express thrombomodulin and TFPI, while capillaries seem to have more of a mix of the two properties, as they also express procoagulant vWF [16]. However, whether these distinct expression patterns were acquired by “nature (epigenetic modifications) or nurture (environment)” is unknown.
To shed light on the “nature versus nurture” argument, there are various mechanisms that account for endothelial heterogeneity in arteries and veins including epigenetics (nature), microenvironmental (nurture) factors, and biomechanical and biochemical properties [17]. Epigenetics is suggested to be a major mechanism for artery and vein phenotypic heterogeneity, and is potentially involved in distinguishing the different types of arteries and veins. Also, microenvironmental factors as well as biomechanical/biochemical factors are important parts of this heterogeneity. Biomechanical factors play a major role in differentiation particularly when both laminar and turbulent flows occur in arteries while less so for veins. Lastly, biochemical factors have the least effect on artery and vein heterogeneity, which may cause different phenotypes in arteries and veins through differences in O2 levels, CO2 levels, and blood vessel composition. Specifically, for arteries, biochemical factors may play a smaller role in differentiating specific arteries, as blood content across arteries is relatively consistent. In contrast, biochemical factors play a greater role in phenotypic heterogeneity of venous ECs as the content of venous blood is dependent on the exchange of substances from prior capillaries [17]. It is important to note that the “nature vs nurture” debate is still being actively discussed among investigators in the field; thus, these conclusions may be subject to change.
Blood Versus Lymphatic Endothelial Cells
It is important to understand the relationship and similarities between blood and lymphatic ECs before discussing in detail the differences between these vasculatures [11]. The lymphatic system is a linear system that originates from a network of capillaries that collect macromolecules, cells, and tissue fluid from the tissue bed in each organ (Fig. 2)[18]. The lymphatic fluid is then brought through the large collecting lymphatic system that is interspersed with lymph nodes. The lymphatic fluid is then dumped into circulation via the internal thoracic duct into the vena cava [18].
Despite their similarities, blood and lymphatic ECs have many differences as well since they differ in function where blood ECs partake in delivering oxygen and nutrients to tissue along with repairing and regenerating organs, while lymphatic ECs are more involved with the immune response, secreting “lymphangiocrine” molecules such as growth factors and cytokines which have been associated with the immune response [8–11]. Furthermore, studies of ex vivo lymphatic ECs revealed specific expression of a majority of genes involved in lymphangiogenesis, the formation of lymphatic vessels from preexisting lymphatic vessels (ex: PROX-1, podoplanin, FOXC2), and lymphatic EC function (LYVE-1, SLC) [19]. Blood ECs expressed high levels of MEOX1, which has no currently known function; however, the involvement of MEOX2 in endothelial cells suggests it may have a role in angiogenesis [20]. Also, blood vessel ECs but not lymphatic ECs express the full range of MHC class 2 genes for antigen presentation which is involved in immune defense against pathogens [19].
Organ-Specific Endothelial Cells
The vast array of organ functions necessitates the large amount of heterogeneity between different organ-specific ECs (Fig. 2). For example, the liver and kidney ECs have been described to proliferate at a moderate pace while the lung ECs grow slowly. The BBB ECs grow even slower ex vivo which is consistent with the slow turnover rate of these cells [21].
Blood Brain Barrier Endothelial Cells
The blood brain barrier (BBB) is highly selective and maintains the balance of various ions, nutrients, and other macromolecules from entering the CNS [22]. ECs are an integral component of the BBB and exhibit no fenestration or tight junctions between cells. Also, there is low HOX cluster DNA methylation in the brain ECs, which corresponds with the brain being anterior in the human body [23•].
There are also many functions and expression patterns associated with BBB ECs. The BBB ECs express many solute carriers, transporters, and proteins involved in receptor-facilitated processes such as SLC01C1, SLC2A1, and Mfsd2a for the uptake of various molecules and solutes [22, 23•]. BBB ECs also have low pinocytosis activity or expression of multidrug resistance proteins (MRPs) which are involved in immune responses to various drugs [22]. However, brain capillary ECs must also undergo angiogenesis and are known to use angiocrine factors such as Jagged1, EphrinB2, NT-3 PEDF, betacellulin, VEGF-C nitric oxide, and BDNF. These factors aid in the differentiation of repopulating cells, neural stem cells, transit amplifying cells, and neuroblasts [8]. Also, vWF expression in brain ECs is high relative to liver and kidney ECs but similar to that of heart and lung ECs, suggesting that heart and lung ECs may be more readily able to maintain hemostasis [4••].
Liver Endothelial Cells
The liver ECs are an important part of the liver as they help filter blood from the gut and liver and accept 15 to 20% of the cardiac output [6]. The microvessels in the liver exhibit a high degree of fenestration with discontinuous openings of irregular sizes along with convoluted and intercalated junctions between adjacent cells which allows for the passage of large particles [4••]. Furthermore, there is a moderate amount of HOX cluster DNA methylation in liver ECs, associated with the liver’s medial location in the body [23•].
The two main types of ECs in the liver are sinusoid and central vein ECs. Their distinct morphology imparts specific functions and gene expression patterns. Liver sinusoidal ECs expressed scavenger receptors such as Stab2, Clec4g, and Fcgr2b, which help in endocytosis [23•]. The angiogenic potential and metabolic rates (oxygen consumption and glycolysis) of these ECs are moderate as they are similar to other organs such as heart and lung ECs [4••]. Lastly, vWF levels in liver ECs is low relative to heart and lung ECs and is similar to that of kidney ECs [4••].
Heart Endothelial Cells
There are three main types of heart endothelial cells including endocardial, coronary vascular, and aortic endothelial cells [6]. The heart ECs have focal contacts between cells and a continuous layer of ECs in their microvessels with caveolae and stomatal diaphragms [4••]. In addition, heart ECs had higher expression levels of adherens junctions, which further increase its barrier strength [4••, 24]. This structure allows heart ECs to perform barrier functions effectively and avoid many macromolecules or particles going through the endothelium [4••].
Heart ECs also display various epigenetic patterns, consistently with their heterogeneous functions. These ECs have high angiogenic potential and metabolic rates (oxygen consumption and glycolysis) relative to the lung, liver, and kidneys. These ECs also use distinct angiocrine factors such as neureguilin-1, endothelin-1, and nitric oxide [8]. In addition, vWF expression is high relative to liver and kidney ECs but similar to that of lung ECs [4••].
Kidney Endothelial Cells
Two main types of kidney ECs exist, glomerular ECs and vasa recta ECs [6]. The kidney ECs are vital to maintaining homeostasis as they are involved in filtration for excretion and take around 20% of the cardiac output [6]. Kidney ECs’ microvessels have complicated and intercalated junctions between them and have many fenestrae with diaphragms of uniform anatomy and magnitude [4••]. HOX cluster DNA methylation is moderately high in kidney ECs associated with their low position in the body relative to most organs [23•].
The kidney ECs also have epigenetic factors that correspond with various functions. Their angiocrine and metabolic rates are like other organs such as the lungs and liver [4••]. Likewise, their vWF expression is similar to that of liver ECs, however, is low relative to lung and heart ECs [4••].
Lung Endothelial Cells
The three primary lung ECs are pulmonary microvascular, pulmonary artery, and pulmonary vein ECs [6]. The lungs are not only important in absorbing oxygen into the blood stream and eliminating carbon dioxide, but are also involved in immunity. The ECs in microvessels from the lung contain cell-matrix adhesions between cells, and the endothelium is continuous with caveolae and stomatal diaphragms [4••]. Given their anatomical location, the lung ECs also exhibit a modest amount of HOX cluster DNA methylation [23•].
Beyond regulating the transit of solutes and gases, lung ECs expressed angiotensin-converting enzyme (ACE), which is involved in regulating blood pressure, and phospholipase A2 group XII, a catalysis used in secreted surfactant and development, and two developmental regulators, secreted frizzled-related protein 1 (sFRP1), and osteoglycin [23•, 25]. Pulmonary capillary ECs regenerate various epithelial cells with angiocrine factors MMP14-mediated EGF-receptor ligand release to help differentiate lung alveolar epithelial cells and type 2 alveolar ECs along with BMP4-NFAT/c-Thrombospondin1 to assist in differentiating epithelial progenitor cells.
Healthy Versus Tumorous Endothelial Cells
ECs that line tumor blood vessels are distinctively different from normal ECs [26]. These tumorous ECs in blood vessels are wider, convoluted with many twists and turns, form arteriovenous shunts, and lack the standard continuum of artery to capillary to vein [27]. These blood vessels have increased permeability, varying diameter, greater degree of branching, and disordered flow patterns. These tumor ECs can sometimes be overgrown, project more into the lumen, have increased numbers of fenestrae, and have enlarged intercellular gaps and junctions [26].
The mechanisms that account for the differences in architecture and function between tumor and normal blood vessels and their associated ECs vary greatly [26]. One key mechanism is from the release of various growth factors and cytokines by the tumor and their associated ECs. The tumor ECs can also release factors that affect the growth of tumors [28, 29]. The tumor blood vessels lack an ordered basement membrane and are therefore quite disorganized with abnormal signaling interactions [26]. It is important to note that not all tumor ECs are the same, as tumor blood vessels from different organs in the body have organ-specific epigenetic markers which enable them to maintain diverse EC phenotypes [30, 31].
Summary and Future Perspectives
Understanding EC heterogeneity is important not only for our interest in basic biology but also to facilitate organ-specific treatments of various diseases. However, much of our current knowledge of EC gene expression is complicated by the fact that most of the prior published studies were performed using bulk cell analysis. This approach cannot ascertain the expression of genes specific to each cell type in the tissue sample. However, the advent of single cell expression technologies including single cell mRNA sequencing and single cell epigenetic profiling that looks at molecular signatures of each cell individually can now address this problem, allowing for an improved delineation of the high degree of heterogeneity among organism-wide, organ-specific, and healthy versus disease ECs. As the ability to determine gene expression for each cell individually minimizes confounding issues such as contamination from cells that are not ECs, we hope single-cell RNA sequencing will provide more accurate data with a better understanding of the biology of ECs and their heterogeneity.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 2006;116(5):1284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach R, Plendl J, Kusha B. Endothelial cell heterogeneity and differentiation Angiogenesis in health and disease: Springer; 1992: 55–62. [Google Scholar]
- 4.Marcu R, Choi YJ, Xue J, Fortin CL, Wang Y, Nagao RJ, et al. Human organ-specific endothelial cell heterogeneity. iScience. 2018;4:20–35•• This study compares endothelial cells from 4 out of 5 organs disscussed in this review based not only on morphological differences but also differences in gene expression and cellular functionality.
- 5.dela Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res 2009;335(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100(2):174–90. [DOI] [PubMed] [Google Scholar]
- 7.Florey L The endothelial cell. Br Med J 1966;2(5512):487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafii S, Butler JM, Ding B-S. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357(6353):eaal2379. [DOI] [PubMed] [Google Scholar]
- 10.Potente M, Mäkinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 2017;18(8):477. [DOI] [PubMed] [Google Scholar]
- 11.Petrova TV, Koh GY. Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med. 2018;215(1):35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007;204(10):2349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol 1976;68(3):705–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson EE, Karlof E, Lundmark K, Rotzius P, Hedin U, Xie X. Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler Thromb Vasc Biol 2005;25(4):723–8. [DOI] [PubMed] [Google Scholar]
- 15.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med 2001;29(7):S28–34. [DOI] [PubMed] [Google Scholar]
- 16.Aird WC. Phenotypic heterogeneity of the endothelium: I. structure, function, and mechanisms. Circ Res 2007;100(2):158–73. [DOI] [PubMed] [Google Scholar]
- 17.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Am Heart Assoc. 2006. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Choi I, Hong Y-K, editors. Heterogeneity and plasticity of lymphatic endothelial cells Seminars in thrombosis and hemostasis; 2010: © Thieme Medical Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amatschek S, Kriehuber E, Bauer W, Reininger B, Meraner P, Wolpl A, et al. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 2007;109(11):4777–85. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 2005;11(9):959–65. [DOI] [PubMed] [Google Scholar]
- 21.Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res 1998;55(1):65–76. [DOI] [PubMed] [Google Scholar]
- 22.Gastfriend BD, Palecek SP, Shusta EV. Modeling the blood–brain barrier: beyond the endothelial cells. Curr Opin Biomed Eng 2018;5:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabbagh MF, Heng JS, Luo C, Castanon RG, Nery JR, Rattner A, et al. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. Elife. 2018;7:e36187.• Finds differences between ECs from 4 out of 5 organs analyzed in this study using genome-wide analyses on the transcriptome, chromatin structure, and DNA methylation.
- 24.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004;84(3):869–901. [DOI] [PubMed] [Google Scholar]
- 25.Chi J-T, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci 2003;100(19):10623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aird WC. Endothelial cell heterogeneity. Cold Spring Harbor perspectives in medicine. 2012;2(1):a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy JA, Chang S-H, Shih S-C, Dvorak AM, Dvorak HF, editors. Heterogeneity of the tumor vasculature Seminars in thrombosis and hemostasis; 2010: © Thieme Medical Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burridge KA, Friedman MH. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am J Phys Heart Circ Phys 2010;299(3): H837–H46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med 2011;3(66):66ra5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumura D, Yuan F, Monsky WL, Chen Y, Jain RK. Effect of host microenvironment on the microcirculation of human colon adenocarcinoma. Am J Pathol 1997;151(3):679. [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts WG, Delaat J, Nagane M, Huang S, Cavenee WK, Palade GE. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol 1998;153(4): 1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]