Dear Editor,
In response to the COVID-19 pandemic, countries have implemented measures to encourage physical distancing and restricted movement, including shelter-in-place orders, curfews, and travel restrictions. While such directives are expected to slow transmission of COVID-19, they may also result in severe disruption to routine health services.1 In a survey of 108 countries, 78% reported disruption to TB services as a result of COVID-19.2 However, there are limited published data on the actual reduction in TB case notifications as a result of the public health measures related to COVID-19.
Shortly after the first confirmed case of COVID-19 was reported in Uganda on March 21, 2020, the Government of Uganda instituted a ban on public transport (effective March 25, 2020), a full national shelter-in-place order, ban on private transport and an evening curfew order (effective March 30, 2020).3 We estimated the causal effect of these measures on TB case notifications using empirically collected, individual-level data from 58 health facilities across Uganda, a high HIV and TB burden country. We analyzed the number of TB case notifications per week between January 13 and May 2, 2020 using an interrupted time series design. The 58 health facilities were involved in ongoing implementation research studies related to TB case finding or treatment, and included 22 Level III health centers (i.e., the lowest level of the national health system where TB diagnostic services are provided), 21 Level IV health centers, 10 district hospitals and 5 regional referral hospitals across all regions of Uganda.
Ethical approval to extract anonymous TB case notification data from treatment registers had already been obtained for ongoing studies.4-6
We performed linear segmented regression modeling to assess the aggregate change in the number of weekly TB case notifications following the shelter-in-place order. Time (in weeks), an indicator variable representing the periods before (January 13–March 22, 2020; 10 weeks) and after (March 30–May 2, 2020; 5 weeks) the shelter-in-place order, and an indicator variable for the week of March 23 during which the order was issued, were included in the final model. We hypothesized an impact model with a level change and no lag, as the new restrictions were expected to have an immediate effect on patient access to health facilities.7 We tested for autocorrelation using residual plots and the Durbin-Watson test. Seasonality was not accounted for in the model, as the literature suggests there is no significant seasonal variation in TB case notifications in Uganda.8,9 We then modeled the impact of the estimated reduction in TB case notifications on TB-related mortality using a published conversion formula.10 We used national TB incidence and mortality estimates from 2018,11 and assumed a 3-month disruption period, followed by an immediate restoration of TB services to baseline levels.
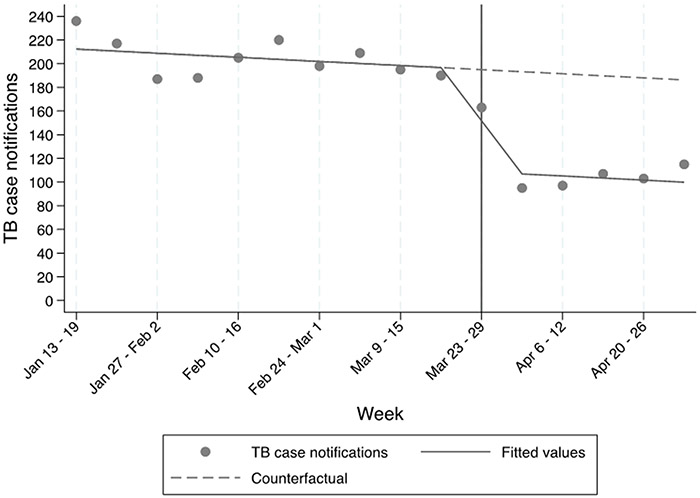
Of 2,725 TB case notifications during the observation period, 2,045 (75%) were before, 163 (6%) during and 517 (19%) after the week in which the shelter-in-place order went into effect. The results of the regression are presented in the Figure and represent the observed weekly TB case notification data and the estimated trend slopes across the 58 facilities before and after the national shelter-in-place order. Weekly TB case notifications were relatively stable before the shelter-in-place order (range 187–236 case notifications/week), declined during the week in which the shelter-in-place order was implemented (163 case notifications), and then remained relatively stable at the lower level for the following 5 weeks (range 95–115 notifications/week), at which time we censored data for this analysis. The immediate effect represented a 43% (95% confidence interval [CI] 34–53; P < 0.001) decline in reported weekly TB case notifications, which was sustained for at least 5 weeks. Visual inspection of residual plots and statistical testing (Durbin-Watson = 1.30) did not show strong evidence of autocorrelation in weekly data.
Figure.
Impact of national shelter-in-place orders on TB case notifications
The drastic reduction in TB case notifications observed in our study has serious implications for projected TB mortality. If applied across Uganda, the observed 43% (95% CI 34–53) reduction in case detection would result in an increase of approximately 14% (95% CI 11–16) in TB-related deaths. This translates to an excess of 2,670 deaths beyond the estimated 19,600, bringing the total to 22,270 deaths, i.e., back to the levels observed in 2015.12 Of note, these numbers represent an overestimate if TB case notifications return to or exceed pre-intervention levels in less than 3 months, or an underestimate if disruption continues beyond 3 months, or should future disruption occur.13
Although Uganda’s response to COVID-19 appears to have been successful (total notified cases remain below 1,400 at the time of reporting), it has taken a significant toll on TB care. On a global level, assuming a reduction in TB case notifications similar to that observed in Uganda, a modeling study estimated an excess of 350,000–1.4 million TB deaths between 2021 and 2025, depending on the duration of shelter-in-place orders (2–3 months) and time to restoration of normal TB services (2–10 months).14 Our findings suggest that the impact of COVID-19 on TB control at the country and global level is a major setback to the progress made toward End TB Strategy targets.
Along with further efforts to slow COVID-19 transmission, delivery of TB services should be prioritized, as mortality caused by the disruption to routine TB services may exceed that of COVID-19 itself.15 To compensate for the high numbers of missed diagnoses during the shelter-in-place period, an urgent restoration of normal services, plus an additional emphasis on enhanced active case finding and scale-up of contact tracing, will be essential.14 This response might even provide unique opportunities for innovative approaches that benefit both TB and COVID-19 control through effective contact tracing and infection control measures.10 In summary, TB case detection and treatment services should be considered among the most critical of essential healthcare services. It is vital that we ensure continued availability of these services—and ideally these should be enhanced—during any potential subsequent waves of COVID-19.
Acknowledgements
The authors thank the administration and staff at the participating 58 health facilities. The work was supported by the National Heart, Lung, and Blood Institute (Bethesda, MD, USA; R01HL130192 and K12HL138046); Stop TB Partnership’s TB REACH initiative (Geneva, Switzerland; W6–37); the Swedish Research Council (Stockholm, Sweden); and Parker B Francis Foundation (Kansas City, MO, USA; P0523744).
Footnotes
Conflicts of interest: none declared.
References
- 1.Parpia AS, et al. Effects of response to 2014–2015 Ebola outbreak on deaths from malaria, HIV/AIDS, and tuberculosis, West Africa. Emerg Infect Dis 2016; 22(3): 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Global Fund. Global Fund survey: majority of HIV, TB and Malaria Programs face disruptions as a result of COVID-19. Geneva, Switzerland: The Global Fund, 2020. https://www.theglobalfund.org/en/covid-19/news/2020-06-17-global-fund-survey-majority-of-hiv-tb-and-malaria-programs-face-disruptions-as-a-result-of-covid-19/. [Google Scholar]
- 3.US Government. Public Health (Control of COVID-19) Rules, 2020. Washington DC, USA: US Government, 2020. [Google Scholar]
- 4.Evaluating the impact of cash transfers on tuberculosis (TB) care: ExaCT TB Study https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=6051. Pan African Clinical Trials Registry. Tygerberg, South Africa: 2019. [Google Scholar]
- 5.Reza TF, et al. Study protocol: a cluster randomized trial to evaluate the effectiveness and implementation of onsite GeneXpert testing at community health centers in Uganda (XPEL-TB). Implement Sci 2020; 15(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.From directly-observed therapy (DOT) to digital adherence technology (DAT) for TB treatment https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=3567. Pan African Clinical Trials Registry. Tygerberg, South Africa: 2018 [Google Scholar]
- 7.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46(1): 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaganath D, et al. Seasonality of childhood tuberculosis cases in Kampala, Uganda, 2010–2015. PLoS One 2019; 14(4): e0214555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabaera B, et al. Seasonal variation among tuberculosis suspects in four countries. Int Health 2009; 1(1): 53–60. [DOI] [PubMed] [Google Scholar]
- 10.Glaziou P Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv 10.1101/2020.04.28.20079582 [DOI] [Google Scholar]
- 11.World Health Organization. Global tuberculosis report, 2019. WHO/CDS/TB/2019.15. Geneva, Switzerland: WHO, 2019. [Google Scholar]
- 12.World Health Organization. WHO’s global tuberculosis database. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 13.Hogan AB, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health 2020; 10.1016/S2214-109X(20)30288-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stop TB Partnership, Imperial College, Avenir Health, Johns Hopkins University. The potential impact of the COVID-19 response on tuberculosis in high-burden countries: a modelling analysis. Geneva, Switzerland: Stop TB Partnership, 2020. [Google Scholar]
- 15.Bell D, et al. Predicting the impact of COVID-19 and the potential impact of the public health response on disease burden in Uganda. Am J Trop Med Hyg 2020; 10.4269/ajtmh.20-0546 [DOI] [PMC free article] [PubMed] [Google Scholar]