Abstract
Copy number variants at the chromosomal locus 16p11.2 contribute to neurodevelopmental disorders such as autism spectrum disorders, epilepsy, schizophrenia, and language and articulation disorders. Here, we provide detailed findings on the disrupted structural brain connectivity in 16p11.2 deletion syndrome (patients: N = 21, age range: 8–16 years; typically developing (TD) controls: 18, 9–16 years) using structural and diffusion MRI. We performed global short-, middle-, long-range, and interhemispheric connectivity analysis in the whole brain using gyral topology-based cortical parcellation. Using region of interest analysis, we studied bilateral dorsal (3 segments of arcuate fasciculus (AF)) and ventral (inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UF)) language pathways. Our results showed significantly increased axial (AD) and radial (RD) diffusivities in bilateral anterior AF, decreased volume for left long AF, increased mean diffusivity (MD) and RD for right long AF, and increased AD for bilateral UF in the 16p11.2 deletion group in the absence of significant abnormalities in the whole-brain gyral and interhemispheric connectivity. The selective involvement of the language networks may aid in understanding effects of altered white matter connectivity on neurodevelopmental outcomes in 16p11.2 deletion.
Keywords: 16p11.2 deletion, Brain networks, Diffusion tensor imaging, Language pathways, Autism
Introduction
Deletions of the recurrent ~600 kb chromosomal BP4-BP5 region of 16p11.2 are associated with a variety of neurodevelopmental outcomes such as epilepsy, schizophrenia, autism spectrum disorders (ASD), and speech and language disorders (Hanson et al. 2010; Owen et al. 2014; Berman et al. 2015; Hanson et al. 2015; Jenkins et al. 2016; Steinman et al. 2016). About 71% of 16p11.2 deletion patients older than 3 years old are diagnosed with a speech and language related disorder, including phonological processing disorder, as well as expressive and receptive language disorders (Hanson et al. 2015). Moreover, patients with 16p11.2 deletion are reported to have a high incidence of delayed language acquisition and atypical language, regardless of ASD diagnosis (Hanson et al. 2010).
Studies using structural magnetic resonance imaging (MRI) have shown abnormal cortical volume and thickness in extensive brain areas related to language function in patients with 16p11.2 deletion (Maillard et al. 2015; Blackmon et al. 2017). Furthermore, deletion carriers with structural brain abnormalities were found to be associated with significantly worse communication and social skills compared to deletion carriers without any radiologic abnormalities (Owen et al. 2017). Recently, diffusion tensor imaging (DTI) studies have reported atypical structural brain connectivity associated with 16p11.2 deletion (Owen et al. 2014; Berman et al. 2015; Chang et al. 2016). Significantly increased mean diffusivity (MD) of the language related tracts, namely auditory radiations and the arcuate fasciculus (AF), in 16p11.2 deletion patients was shown, where left hemispheric MD and radial diffusivity (RD) values of the AF negatively correlated with clinical language ability in 16p11.2 deletion patients (Berman et al. 2015). However, analysis in that study was confined only to the long segment of the AF, and the auditory radiations, excluding other dorsal or ventral language pathways; further-more, this study did not examine the global effect of 16p11.2 deletion on white matter connectivity throughout the whole brain. Two previous DTI track-based spatial statistics (TBSS) whole brain studies found increased fractional anisotropy (FA), MD, and axial diffusivity (AD) throughout the supratentorial white matter (Owen et al. 2014; Chang et al. 2016) and in several white matter regions such as the bilateral internal and external capsule, and the anterior portion of the corpus callosum in 16p11.2 deletion patients (Owen et al. 2014). In addition, increased AD has been reported in the association, limbic, and projection tracts (Chang et al. 2016). However, these two TBSS studies did not identify and examine specific language related tracts and connections. In addition to the structural and diffusion MRI studies, abnormal auditory processing was revealed by a magnetoencephalography (MEG) study in 16p11.2 deletion patients compared to typically developing (TD) controls (Jenkins et al. 2016), but could not be fully explained by the alterations to white matter structure of the auditory system (Berman et al. 2016), indicating the need of full language pathway analysis.
This study is one of the components of the Simons Variation in Individuals Project (Simons VIP), which is a multi-site project that uses a “genetics-first” approach to the study of ASD and related neurodevelopmental disorders (Simons VIP 2012). Simons VIP project aims to study individuals with specific genetic variations, such as with 16p11.2 deletion, who are at increased risk of developing ASD. ASD is a heterogeneous disorder, which complicates the studies and can lead to contradictory results (Amaral et al. 2008). Similarly, studying a group of participants with language impairments who have a defined genetic etiology is likely to further our understanding of the link between 16p11.2 deletion, whole brain as well as dorsal and ventral language white matter networks, and language ability.
The aim of this study was to determine if involvement of the language network in individuals with 16p11.2 deletion extends beyond the long segment of the AF and to determine if there is a global bias to involve long range connections such as the AF more than short range connections when compared to TD controls. In this project, we studied white matter pathways for the dual stream language model: the dorsal language stream, which is involved in the phonological processing of speech (Saur et al. 2008), and the ventral language stream, which is involved in semantic processing of speech (Saur et al. 2008). It has been shown that in addition to the classical arcuate pathway that connects Broca’s and Wernicke’s areas directly, there is an indirect pathway that is composed of an anterior and posterior segment and runs parallel and lateral to the direct pathway in the dorsal language network (Catani et al. 2005). Previous work on schizophrenia (Catani et al. 2011) and tuberous sclerosis complex (TSC) (Lewis et al. 2013) showed that different portions of the AF could be associated with different clinical outcomes such as the long segment of AF being more selective for language differences in TSC patients (Lewis et al. 2013). We investigated whether changes in the long segment of the AF as shown by Berman and colleagues (Berman et al. 2015) extended to the other segments of the AF as well as the ventral language pathways consisting of the inferior fronto-occipital fasciculus (IFOF), the inferior longitudinal fasciculus (ILF), and the uncinate fasciculus (UF). We also performed a non-biased whole brain analysis of global white matter connectivity (short-, middle- and long-range and interhemispheric connections) using gyral topology-based cortical node parcellations and path length. This method has an ability to capture short association U-fibers between gyri in superficial white matter and long-range connections in deep white matter regions throughout the brain. These short- and long-range connections are subdivided and separately investigated, because disorders may selectively involve short- and long-range connections (Shukla et al. 2011a; Im et al. 2014, 2016).
Methods
Participants
Twenty-one 16p11.2 deletion (mean age 10.91 ± 2.09 years, 11 males, 11 right-handed) and 18 sex- and handedness-matched TD (mean age 12.58 ± 1.99 years, 10 males, 11 right-handed) individuals (Table 1) were included in this study who were enrolled as part of a multicenter study, Simons Foundation Variation in Individuals Project (Simons VIP 2012) (Qureshi et al. 2014). Cognitive testing was performed at Children’s Hospital of Philadelphia and at the University of California San Francisco. All children were administered a battery of neurodevelopmental tests including the Differential Ability Scales-Early Years and School Age, Second Edition (DAS-II) (Elliott 2007), the Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al. 1999), and the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4) (Semel et al. 2003). TD individuals were non-familial control participants who were enrolled in the Simons VIP. 16p11.2 deletion patients had diagnoses of other disorders as well, which can be seen in Table 2. All study procedures were approved by the institutional review boards at the medical centers where the data acquisition and analysis was conducted and are in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008. Imaging and phonotypic data of all participants were extracted from the Simons Foundation Autism Research Initiative Base site (https://sfari.org/resources/sfari-base).
Table 1.
Subject characteristics for the TD and 16p11.2 deletion groups
| TD (n = 18) | 16p (n = 21) | p value | |
|---|---|---|---|
| Age | 12.58 ± 1.99 years | 10.91 ±2.09 years | 0.015* |
| Sex | 10 male; 8 female | 11 male; 10 female | 0.848 |
| Handedness | 11 right, 2 left, 3 ambidextrous, 2 n/a | 11 right, 5 left, 1 ambidextrous, 4 n/a | 0.728 |
| GCA (DAS-II) | 109.28 ±12.88 | 88.00 ±13.66 | <0.0001** |
| SNC (DAS-II) | 107.78 ±11.42 | 92.86 ±12.65 | <0.0001** |
| Verbal (DAS-II) | 110.22 ± 13.46 | 82.95 ±17.13 | <0.0001** |
| Non-word Repetition (CTOPP) | 9.41 ±1.91 | 5.86 ±2.39 | <0.0001** |
| Core Language (CELF-4) | 108.71 ±9.29 | 75.89 ±18.26 | <0.0001** |
| Expressive Language (CELF-4) | 108.35 ±10.31 | 73.72 ±17.06 | <0.0001** |
| Receptive Language (CELF-4) | 101.56 ±8.68 | 78.36 ± 15.48 | 0.001** |
| Language Memory (CELF-4) | 107.11 ±7.91 | 73.71 ±20.78 | <0.0001** |
| ICV (ml) | 1228.37 ±98.14 | 1341.13 ± 135.93 | 0.006** |
significant at p < 0.05
significant at p < 0.01
Table 2.
Diagnoses in the 16p11.2 deletion carriers
| Disorder | Number of deletion carriers* |
|---|---|
| Attention deficit hyperactivity disorder | 4 |
| Anxiety/obsessive-compulsive disorder/phobia | 2 |
| Articulation disorder | 14 |
| Behavioural disorder | 2 |
| Autism spectrum disorder | 2 |
| Coordination disorder | 8 |
| Enuresis | 5 |
| Language disorder | 5 |
| Learning disorder | 2 |
| Mood disorder | 1 |
| Intellectual disability | 1 |
| Stereotyped motor disorder | 1 |
| Tic/Tourette’s | 2 |
Each carrier could have more than one diagnosis
MRI acquisition
Imaging was performed on two sites, University of California Berkeley and Children’s Hospital of Philadelphia (Qureshi et al. 2014). All MR imaging was performed on 3 T (Tim Trio, Siemens) MRI scanners, one at each site, with identical software, pulse sequences, and 32-channel head coils. The imaging protocol included an axial 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) T1-weighted sequence (TE = 1.64 ms, TR = 2530 ms, TI = 1200 ms, flip angle = 7°, voxel size = 1 × 1 × 1 mm, FOV = 256 mm, and 256 × 256 matrix) and a diffusion-weighted sequence with 30 gradient directions at b = 1000 s/mm2, one b = 0 s/mm2 volume, TR/TE = 10 s/80 ms, voxel size = 2 × 2 × 2 mm, and 128 × 128 matrix (Simons VIP 2012; Owen et al. 2014; Qureshi et al. 2014). To ensure reliability across different imaging sites, five individuals who were not part of the study were scanned at both sites and their data was analyzed separately to show non-significant differences between the two datasets acquired with the two scanners in a previous study that used the same datasets (Qureshi et al. 2014).
Structural and diffusion MRI processing
Quality control of the T1 imaging data was performed previously for other studies that used the same datasets and the data with wrapping, poor head coverage, ringing/striping/blurring, ghosting, inhomogeneities, or susceptibility artifacts were excluded (Owen et al. 2014; Qureshi et al. 2014; Berman et al. 2015). T1-weighted images were processed with FreeSurfer 5.3.0 to extract cortical surfaces (Dale et al. 1999; Fischl et al. 1999). Reconstruction of the cortical models was followed by automatic parcellation into anatomical regions based on gyral/sulcal structure (Fischl et al. 2004a; Desikan et al. 2006). Intracranial volume (ICV) values were extracted from FreeSurfer.
Quality of the diffusion data was checked in previous studies (Owen et al. 2014; Berman et al. 2015). In addition, to assess the degree of head motion in each participant and to test if the 16p11.2 deletion group showed a larger head motion, we conducted a quantitative motion estimation analysis. Diffusion images were processed to correct for minor eddy currents and motion using FSL tools. All diffusion images in the series were aligned to the first b0 image using affine registration (Jenkinson et al. 2002). After extracting the average volume-by-volume translation and rotation from affine transformation of the diffusion volumes, a single value “total motion index” (TMI) was calculated (Yendiki et al. 2014). An independent sample t-test was conducted to compare the TMI values between the TD and 16p11.2 deletion groups.
Fiber tractography
Diffusion data were processed using the DTI FACT (Mori et al. 1999) algorithm with an angle threshold of 45° using Diffusion Toolkit which is a set of tools that reconstruct diffusion-imaging data and generate fiber track data (http://www.trackvis.org/dtk) (Wang et al. 2007). No FA threshold was applied. Instead, a fiber cutoff filter was applied so that fibers shorter than 20 mm and longer than 200 mm were filtered. Fiber tracking was constrained inside the white matter region with the use of a binary mask for the white matter, which was generated by FreeSurfer (http://surfer.nmr.mgh.harvard.edu) (Fischl et al. 2002, 2004b).
Gyral topology-based connectivity analysis
Structural T1-weighted image was registered onto the b0 image using FSL’s intensity-based affine registration tool, FLIRT (http://www.fmrib.ox.ac.uk/fsl), to construct gyral topology-based network (Jenkinson and Smith 2001; Jenkinson et al. 2002). To study global connectivity based on length of connections we used a gyral based parcellation method (Im et al. 2014, 2016), which has the advantage of isolating U-fibers between gyri from middle- and long-range connections based on an individual’s gyral topology. Watershed processing was applied on a sulcal depth map measured on the white matter surface to define gyral node regions. Two gyral node regions were considered to have a structural connection, when at least the end points of two fiber tracts were located <3 mm from each of the two regions. We constructed different fiber groups according to individual gyral topology and defined corticocortical white matter connections between the first, second and third, fourth and more neighboring gyri, and between the hemispheres (corpus callosum) (Fig. 1) (Im et al. 2014, 2016). In the remainder of this paper, the connections between the first neighboring gyri will be referred to as short-range connections; the connections between the second and third neighboring gyri will be referred to as the middle-range connections; and the connections between the fourth and farther gyri will be referred to as the long-range connections. For each of these connections, we measured FA, MD, AD, and RD for the whole brain, and separately for the two hemispheres. There is mostly no overlap among the short-, middle-, and long-range connections, except for the end parts of the fibers at the voxel level in gyral white matter regions where the fiber tracts converge to link to cortical layers, and connectivity measure of one type of connection does not reflect a mixture of all connections.
Fig. 1.
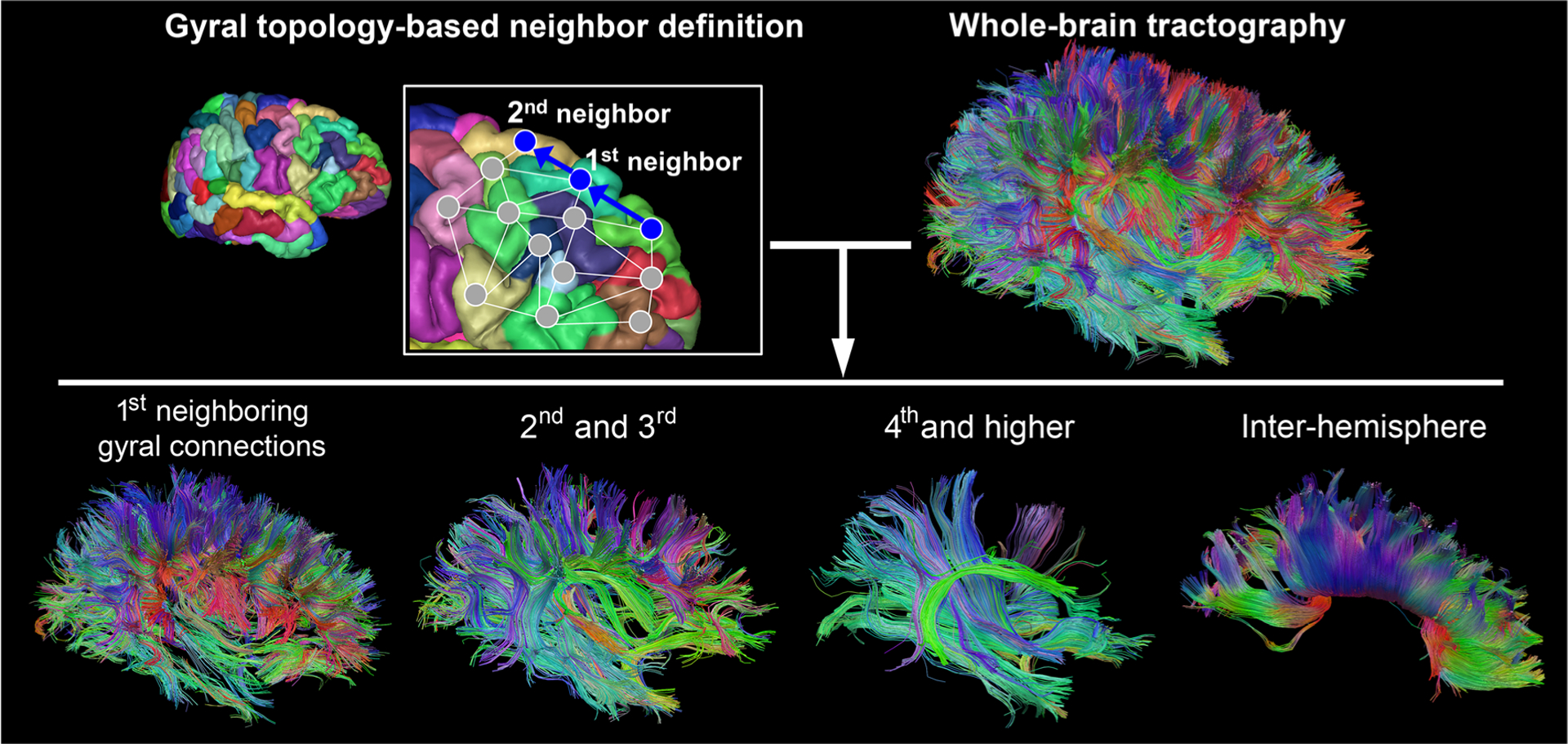
Connectivity analysis based on gyral topology. This figure is partially reproduced from Im et al. (2014)
Region of interest (ROI) analysis
Manual ROI creation and track segmentation was performed using TrackVis, a software tool that can visualize and analyze fiber track data created from MR images by diffusion MR imaging (http://www.trackvis.org). In this project, we manually segmented the white matter pathways for the dual stream language model: the ventral language stream and the dorsal language stream (Fig. 2). Tractography of the ventral and dorsal language network was performed by manually positioning ROIs on each FA color map by three trained individuals who were blinded to subject age, sex, and diagnosis. The few spurious fibers that did not belong to the anatomical pathways being segmented were manually removed using TrackVis. We measured the volume, FA, MD, AD, and RD for each of the fiber tracts that were created in each hemisphere.
Fig. 2.
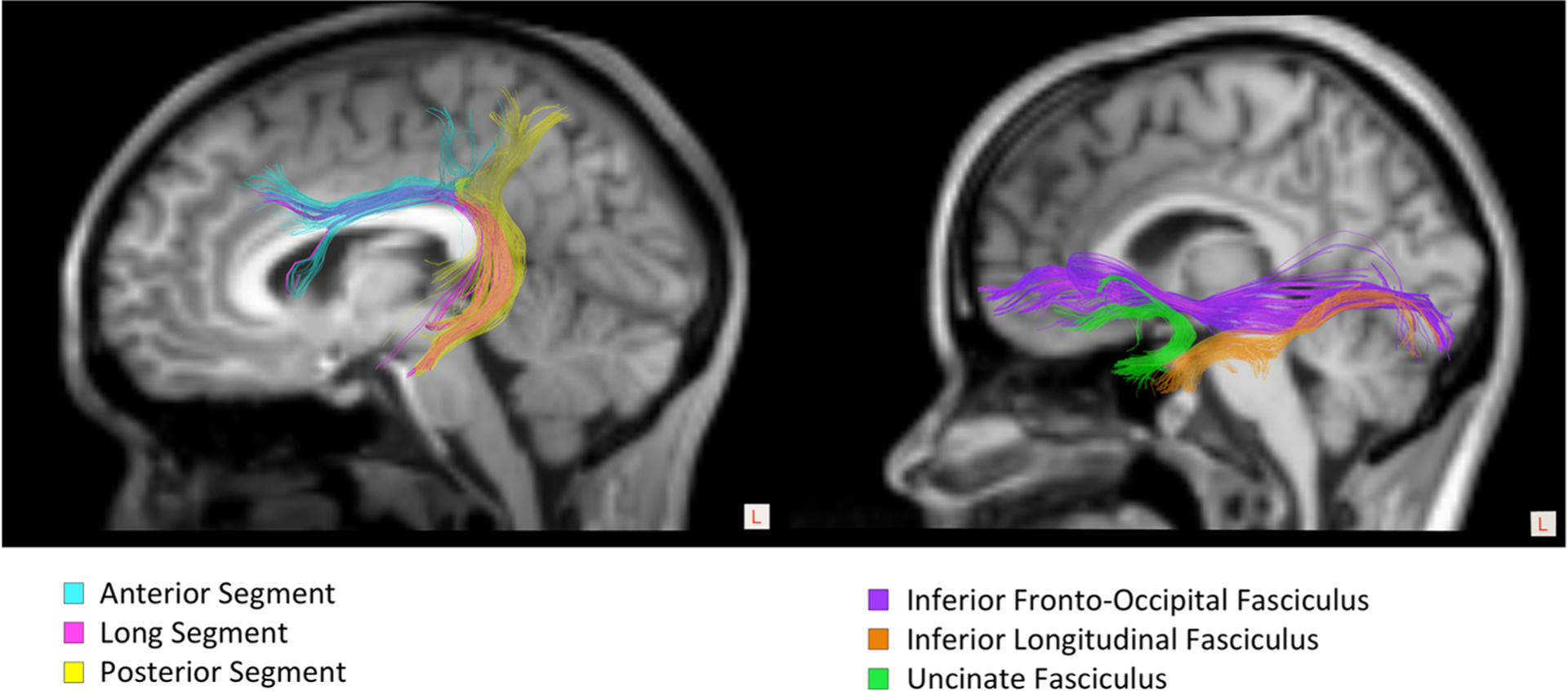
ROI-based segmentation of the dorsal and ventral language pathways. Left: Dorsal language pathways: Anterior segment, long segment, posterior segment of the arcuate fasciculus. Right: Ventral language pathways: Inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, uncinate fasciculus
Dorsal language pathways
The main fiber bundle of the dorsal language pathway is the AF, which is composed by three segments: anterior, long, and posterior (Catani et al. 2005). Manual ROIs were placed to segment the 3 portions of the right and left AF. First, an initial ROI was placed in the most dorsal part of the AF. To create the anterior segment, an additional ROI was placed in the prefrontal/premotor area and an exclusion ROI was placed in the posterior temporal-parietal region to remove fibers from posterior and long AF segments. To create the long segment, two ROIs were added to the initial segmentation, first in the prefrontal/premotor area and second at the junction of the supramarginal and temporal areas. To create the posterior segment, in addition to the initial ROI, another ROI was placed at the junction of the supramarginal and temporal cortices and an exclusion ROI was placed at the prefrontal/premotor area to remove fibers from the anterior and long segments.
Ventral language pathways
The ventral language network is composed of three fiber bundles: the IFOF, ILF, and UF. Manual ROIs were placed to segment these three fiber bundles in the right and left hemisphere. The IFOF was reconstructed using the sagittal section through the lateral putamen to locate the white matter of the external/extreme capsule, where the ROI was placed. The ROI for the ILF was defined by using the sagittal section through claustrum and lateral putamen as means to locate the white matter around the internal capsule and tail of the caudate nucleus. Finally, to reconstruct the UF, the coronal section through the head of the caudate nucleus and putamen was used as reference to place the ROI on the anterior temporal lobe.
Laterality analysis
Laterality index (LI) of all scalar values was calculated for the gyral connections and the ventral and dorsal language pathways. Each lateralization index was calculated as 2 × (xl – xr)/(xl + xr), where xl and xr denote the values for the left and right hemisphere respectively. While a positive value indicates leftward asymmetry, a negative value indicates rightward asymmetry.
Statistical analyses
Preprocessing of the data
The statistical analyses were conducted using the IBM-SPSS software (Version 23, IBM Corp.). In the gyral connectivity analyses, there were no missing values. However, in the ROI analyses, some of the participants were missing certain fiber tracks. Absence of certain fibers has been observed in previous studies (Catani et al. 2007). For example, Verly and colleagues found that the right hemispheric AF was missing in 7/25 TD children and in 10/17 children with ASD who participated in their study (Verly et al. 2014). In our study, out of 18 participants in the TD group, 1 was missing the left hemisphere anterior segment of the AF, 2 were missing the left hemisphere long segment of the AF, and three were missing the right hemisphere long segment of the AF. In the 16p11.2 deletion group, among 21 participants, 1 was missing the right hemisphere anterior segment of the AF, 3 were missing the right hemisphere long segment of the AF.
Group difference analyses using multiple linear regression
Differences in the volume, FA, MD, AD, and RD values between the 16p11.2 deletion and the TD groups for gyral neighbor connectivity and language networks ROI were tested using multiple linear regression analyses with a regression model where the dependent variable (Y) was each measurement (FA, MD, AD, RD), or its LI, the independent variable was the participant group (coded as a dummy variable), and age and ICV were covariates (model fit: Y = b0 + b1group + b2age + b3ICV). Although ICV has not been shown to affect diffusivity values, we used ICV as a covariate in addition to age because the two participant groups significantly differed in ICV values where the 16p11.2 deletion group had larger values and for this reason ICV was used as a covariate in a previous study (Owen et al. 2014). The assumptions of linearity, independence of errors, homoscedasticity, unusual points, and normality of residuals were met for each of the models. The Bonferroni method was used to correct for multiple comparisons. Level of significance (α) was set at 0.0125 for each fiber connection based on gyral topological path length as well as the LI analysis, and at 0.01 for each ROI-based fiber segment due to the multiple tests conducted for each fiber pathway.
Correlational analyses
When significant participant group differences were found in the linear regression analyses, we additionally performed correlation analyses with neurodevelopmental scores. Partial correlational analyses were conducted to examine the correlations of diffusivity values with general conceptual ability (GCA), special nonverbal composite (SNC), and verbal ability (VA) scores as measured with DAS-II; non-word repetition test scores as measured with CTOPP; as well as expressive, receptive, and core language scores as measured with CELF-4 of each participant group. Age and ICV were used as covariates. Additionally, we performed the same correlational analyses for the 16p11.2 deletion patients with and without articulation disorder. The level of significance was set at 0.01 to control for multiple comparisons.
Supplementary analyses
We repeated all of the same regression and correlational analyses by using only age as a covariate.
Results
Participant characteristics
The two participant groups were matched on sex and handedness. The TD group had a mean age of 12.58 ± 1.99 years (age range: 9–16 years) and the 16p11.2 deletion group had a mean age of 10.91 ± 2.09 years (age range: 8–16 years) resulting in the TD being significantly older (p = 0.015). In addition, TD group scored significantly higher than the 16p11.2 deletion group for GCA (p < 0.0001), SNC (p < 0.0001), and VA scores (p < 0.0001), as determined by DAS-II. CTOPP non-word repetition test scores were significantly higher in the TD group than the 16p11.2 deletion group (p < 0.0001). The TD group also had significantly higher scores for the expressive language (p < 0.0001), receptive language (p = 0.001), core language (p < 0.0001), and language memory tests (p < 0.0001), as measured with CELF-4, than the 16p11.2 deletion group. Details on group differences in participant characteristics can be seen in Table 1.
Whole brain volume and motion analysis
Intracranial brain volume values were significantly different between the two participant groups, with the whole brain volume significantly higher for the 16p11.2 deletion group than the TD group (p = 0.006). There was not a significant difference between the two participant groups in their TMI scores (p > 0.05).
Structural connectivity based on gyral topological path length in the whole brain
For the group analysis between 16p11.2 deletion and TD, the statistics for the gyral neighbor connectivity and the corpus callosum (mean, SD, and p-values) are listed in Table 3. There were no significant group differences for any of the values according to the results of the regression analyses.
Table 3.
Statistical results for the group comparisons of gyral connectivity and corpus callosum connectivity between TD and 16p11.2 deletion groups
| Left hemisphere | Right hemisphere | Corpus Callosum | |||||
|---|---|---|---|---|---|---|---|
| First | Second and Third | Fourth and more | First | Second and Third | Fourth and more | ||
| FA | |||||||
| TD | 0.347 ±0.013 | 0.407 ±0.017 | 0.469 ±0.020 | 0.349 ±0.011 | 0.406 ±0.014 | 0.465 ±0.021 | 0.558 ±0.014 |
| 16p | 0.345 ±0.011 | 0.403 ±0.011 | 0.477 ±0.023 | 0.349 ±0.011 | 0.403 ±0.010 | 0.463 ±0.013 | 0.553 ±0.018 |
| p-valuea | 0.624 | 0.690 | 0.523 | 0.453 | 0.525 | 0.555 | 0.410 |
| p-valueb | 0.820 | 0.787 | 0.260 | 0.617 | 0.580 | 0.876 | 0.823 |
| MD (×10−4) | |||||||
| TD | 7.54 ±0.19 | 7.50 ±0.18 | 7.60 ±0.18 | 7.54 ±0.19 | 7.54 ±0.19 | 7.66 ±0.16 | 7.81 ±0.19 |
| 16p | 7.69 ±0.17 | 7.66 ±0.20 | 7.70 ±0.21 | 7.69 ±0.16 | 7.68 ±0.17 | 7.82 ±0.21 | 7.89 ±0.20 |
| p-valuea | 0.209 | 0.115 | 0.283 | 0.233 | 0.385 | 0.140 | 0.786 |
| p-valueb | 0.182 | 0.187 | 0.643 | 0.152 | 0.310 | 0.185 | 0.915 |
| AD (×10−4) | |||||||
| TD | 10.44 ±0.24 | 11.04 ±0.23 | 11.93 ±0.23 | 10.45 ±0.26 | 11.09 ±0.31 | 11.95 ±0.25 | 13.55 ±0.32 |
| 16p | 10.63 ±0.18 | 11.23 ± 0.26 | 12.19 ± 0.38 | 10.67 ±0.20 | 11.24 ±0.22 | 12.18 ± 0.31 | 13.63 ±0.23 |
| p-valuea | 0.087 | 0.145 | 0.122 | 0.105 | 0.716 | 0.350 | 0.631 |
| p-valueb | 0.118 | 0.251 | 0.127 | 0.093 | 0.568 | 0.229 | 0.930 |
| RD (×10−4) | |||||||
| TD | 6.08 ±0.19 | 5.73 ±0.20 | 5.44 ±0.22 | 6.08 ±0.18 | 5.77 ±0.18 | 5.51 ±0.21 | 4.93 ±0.18 |
| 16p | 6.22 ±0.18 | 5.88 ±0.19 | 5.46 ±0.23 | 6.20 ±0.16 | 5.89 ±0.17 | 5.64 ±0.20 | 5.03 ±0.23 |
| p-valuea | 0.406 | 0.181 | 0.793 | 0.482 | 0.303 | 0.208 | 0.482 |
| p-valueb | 0.304 | 0.242 | 0.645 | 0.300 | 0.273 | 0.377 | 0.831 |
Data are denoted as mean ± SD
Age and ICV are used as covariates
Age is used as a covariate
significant at p ≤ 0.0125
Structural connectivity of the dorsal language pathways based on ROI analysis
For the group analysis between 16p11.2 deletion and TD, the statistics (mean, SD, and p-values) for the dorsal language pathways are listed in Table 4.
Table 4.
Statistical results for the group comparisons of bilateral dorsal language networks connectivity between TD and 16p11.2 deletion groups
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| Anterior | Long | Posterior | Anterior | Long | Posterior | |
| Vol (ml) | ||||||
| TD | 6.34 ±3.18 | 7.67 ±3.18 | 5.91 ±2.66 | 6.06 ±2.55 | 5.69 ±2.72 | 4.55 ±2.25 |
| 16p | 5.38 ±2.79 | 5.97 ±2.95 | 6.50 ±2.92 | 5.75 ±3.69 | 5.66 ±3.18 | 5.93 ±2.53 |
| p-valuea | 0.239 | 0.009* | 0.649 | 0.572 | 0.661 | 0.591 |
| p-valueb | 0.515 | 0.064 | 0.290 | 0.756 | 0.440 | 0.106 |
| FA | ||||||
| TD | 0.437 ±0.031 | 0.493 ±0.026 | 0.444 ±0.034 | 0.461 ±0.026 | 0.473 ±0.034 | 0.428 ±0.031 |
| 16p | 0.448 ±0.033 | 0.487 ±0.029 | 0.447 ±0.021 | 0.454 ±0.026 | 0.458 ±0.028 | 0.429 ±0.034 |
| p-valuea | 0.321 | 0.725 | 0.951 | 0.152 | 0.100 | 0.773 |
| p-valueb | 0.209 | 0.882 | 0.457 | 0.998 | 0.176 | 0.854 |
| MD (×10−4) | ||||||
| TD | 7.28 ±0.19 | 7.27 ±0.15 | 7.37 ±0.30 | 7.32 ±0.19 | 7.27 ±0.17 | 7.43 ±0.20 |
| 16p | 7.52 ±0.25 | 7.45 ±0.21 | 7.55 ±0.29 | 7.51 ±0.22 | 7.57 ±0.21 | 7.66 ±0.24 |
| p-valuea | 0.035 | 0.154 | 0.247 | 0.026 | 0.012 | 0.021 |
| p-valueb | 0.021 | 0.083 | 0.496 | 0.083 | 0.001* | 0.042 |
| AD (×10−4) | ||||||
| TD | 10.94 ±0.26 | 11.23 ±1.36 | 11.30 ± 0.47 | 11.32 ±0.33 | 11.37 ±0.52 | 11.21 ±0.42 |
| 16p | 11.38 ± 0.43 | 11.74 ±0.31 | 11.58 ± 0.39 | 11.49 ±0.39 | 11.68 ± 0.32 | 11.55 ±0.49 |
| p-valuea | 0.016 | 0.180 | 0.368 | 0.637 | 0.617 | 0.080 |
| p-valueb | 0.006* | 0.178 | 0.298 | 0.346 | 0.237 | 0.109 |
| RD (×10−4) | ||||||
| TD | 5.45 ±0.28 | 5.06 ±0.37 | 5.43 ±0.31 | 5.30 ±0.22 | 5.23 ±0.19 | 5.54 ±0.23 |
| 16p | 5.59 ± 0.31 | 5.31 ±0.31 | 5.56 ±0.29 | 5.54 ±0.29 | 5.51 ±0.26 | 5.71 ±0.25 |
| p-valuea | 0.530 | 0.133 | 0.372 | 0.010* | 0.019 | 0.193 |
| p-valueb | 0.558 | 0.142 | 0.784 | 0.111 | 0.013 | 0.221 |
Data are denoted as mean ± SD
Age and ICV are used as covariates
Age is used as a covariate
significant at p ≤ 0.01
For the anterior segment of the AF in the left hemisphere, MD (p = 0.035) and AD (p = 0.016) values both approached significance and were higher in the 16p11.2 deletion group. In the right hemisphere, 16p11.2 deletion group had higher RD and MD values; while RD was significant (p = 0.01), the MD values approached significance (p = 0.026).
For the long segment of the AF in the left hemisphere, volume was significantly lower in the 16p11.2 deletion group (p = 0.009), however, there was not a significant group difference for any of the diffusivity values. In the right hemisphere, group differences for the MD (p = 0.012) values and RD values (p = 0.019) approached significance with higher values in the 16p11.2 deletion group.
Posterior segment of the AF in the left hemisphere did not have any significant group differences. In the right hemisphere, 16p11.2 deletion group had higher MD values which approached significance at p = 0.021.
Structural connectivity of the ventral language pathways based on ROI analysis
The statistics for the ventral language pathways are listed in Table 5 for the group analysis between 16p11.2 deletion and TD.
Table 5.
Statistical results for the group comparisons of bilateral ventral language networks connectivity between TD and 16p11.2 deletion groups
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| IFOF | ILF | UF | IFOF | ILF | UF | |
| Vol (ml) | ||||||
| TD | 12.99 ±2.58 | 7.08 ±2.09 | 4.67 ±1.78 | 13.32 ±2.26 | 7.08 ±1.97 | 4.83 ±1.11 |
| 16p | 13.70 ±3.24 | 7.21 ±2.26 | 4.91 ±1.56 | 15.22 ±3.44 | 6.24 ±2.01 | 5.43 ±1.63 |
| p-valuea | 0.873 | 0.287 | 0.624 | 0.036 | 0.168 | 0.917 |
| p-valueb | 0.425 | 0.947 | 0.773 | 0.004* | 0.399 | 0.127 |
| FA | ||||||
| TD | 0.499 ±0.041 | 0.476 ±0.027 | 0.419 ±0.026 | 0.502 ±0.029 | 0.475 ±0.028 | 0.421 ±0.024 |
| 16p | 0.508 ±0.034 | 0.483 ±0.023 | 0.425 ±0.021 | 0.493 ±0.032 | 0.457 ±0.043 | 0.435 ±0.019 |
| p-valuea | 0.708 | 0.413 | 0.418 | 0.450 | 0.241 | 0.068 |
| p-valueb | 0.305 | 0.220 | 0.351 | 0.720 | 0.323 | 0.055 |
| MD (×10−4) | ||||||
| TD | 8.01 ±1.44 | 7.88 ±0.24 | 7.78 ±0.22 | 7.74 ±0.23 | 8.08 ±0.27 | 7.78 ±0.26 |
| 16p | 7.74 ±0.21 | 7.93 ±0.27 | 7.98 ±0.22 | 7.85 ±0.17 | 8.07 ±0.24 | 7.89 ±0.23 |
| p-valuea | 0.217 | 0.514 | 0.073 | 0.373 | 0.285 | 0.591 |
| p-valuba | 0.177 | 0.513 | 0.041 | 0.531 | 0.343 | 0.382 |
| AD (×10−4) | ||||||
| TD | 12.42 ±0.72 | 12.37 ±0.36 | 11.59 ±0.33 | 12.56 ±0.47 | 12.65 ±0.48 | 11.60 ±0.33 |
| 16p | 12.61 ±0.31 | 12.55 ±0.50 | 12.01 ±0.30 | 12.61 ±0.35 | 12.41 ±0.62 | 11.96 ±0.31 |
| p-valuea | 0.702 | 0.831 | 0.004* | 0.791 | 0.076 | 0.016 |
| p-valueb | 0.553 | 0.568 | 0.001* | 0.924 | 0.160 | 0.008* |
| RD (×10−4) | ||||||
| TD | 5.35 ±0.28 | 5.62 ±0.32 | 5.86 ±0.27 | 5.34 ±0.26 | 5.78 ±0.33 | 5.85 ±0.30 |
| 16p | 5.29 ±0.31 | 5.61 ±0.27 | 5.98 ±0.24 | 5.47 ±0.28 | 5.90 ±0.37 | 5.84 ±0.23 |
| p-valuea | 0.508 | 0.318 | 0.434 | 0.265 | 0.781 | 0.494 |
| p-valueb | 0.130 | 0.193 | 0.338 | 0.511 | 0.849 | 0.567 |
Data are denoted as mean ± SD
Age and ICV are used as covariates
Age is used as a covariate
significant at p ≤ 0.01
For IFOF and ILF, there were no significant differences between the two participant groups for any of the values.
In the left UF, 16p11.2 deletion group had significantly higher AD values than the TD group (p = 0.004). In the right UF, the group difference in the AD values approached significance (p = 0.016) with higher values in the 16p11.2 deletion group.
Supplementary analysis
When we repeated all the analyses with age as the only covariate, we found that there were still no significant differences between the two groups for any of the values in the gyral connectivity analysis. However, values that showed trends of significance when age and ICV were both used as covariates, now became significant in the ROI analysis. In the dorsal language network, the group differences was significant for the AD value of the left anterior AF (p = 0.006) and the MD value of the right long AF (p = 0.001). For the ventral language network, in the right hemisphere, the volume for IFOF (p = 0.004) and AD values for UF (p = 0.008) were significantly higher for the 16p11.2 deletion group.
Laterality differences
There were no significant group differences in the LI values of the gyral neighbor connections according to the regression analyses. Results of the regression analysis did not show any significant group differences for the LI values of the dorsal or ventral language pathways either.
Supplementary analysis
When we repeated all the regression analyses with age as the only covariate, there were still no significant differences between the two groups for any of the LI values in the gyral connectivity or dorsal language network analysis. However, in the ventral language network, in the IFOF, the LI of RD values were significantly lower in the 16p11.2 deletion group (p = 0.01), with the TD group showing a leftward and the 16p11.2 deletion group showing a rightward asymmetry.
Correlational analyses
We did not perform any correlational analyses for the neurodevelopmental test results and the gyral connectivity diffusivity values due to their non-significant group differences. Among the dorsal and ventral language pathways, which showed significant group differences in the linear regression analyses, there were no significant correlations between the neurodevelopmental test scores and the volume or diffusivity values of these dorsal or ventral language pathways in the TD group at p < 0.01. In the 16p11.2 deletion group, there was a significant positive correlation between the SNC (DAS-II) scores and the volume of the left long AF (p = 0.009). There were no significant correlations between the neurodevelopmental test results and volume or diffusivity values of the language pathways in the 16p11.2 deletion patients with and without articulation disorder at p < 0.01. There were significant correlations at p < 0.05; however, they did not survive multiple comparison correction. These results can be seen in Supplementary Table 1 for comparisons with findings of previous studies where the threshold for significance was p < 0.05 (Berman et al. 2015).
Supplementary analysis
When age was the only covariate used, the results did not change. The only significant result was still the positive correlation between the SNC scores and the volume of the left long AF (p = 0.004) in the 16p11.2 deletion group. There were no significant correlations between the behavioral tests and the diffusivity values in any of the participant groups at p < 0.01. Results where p < 0.05 can be seen in Supplementary Table 1.
Discussion
This is the first study to assess bilateral dorsal (all 3 segments of AF) and ventral (IFOF, ILF, UF) language pathways in the context of global white matter connectivity in 16p11.2 deletion patients. We used a unique gyral pattern-based parcellation scheme that allowed us to examine short-, middle- and long-range white matter connectivity in each hemisphere of the brain. We report new findings of selective white matter abnormalities in the language tracts, specifically the bilateral anterior segment and the right long segment of the AF as well as the bilateral UF, in the absence of significant white matter abnormalities in the whole brain. In addition, we demonstrated significantly different asymmetry between the two participant groups for the RD values in IFOF. Finally, we showed a significant correlation between the volume of the left long segment of the AF and the SNC scores in the 16p11.2 deletion group and showed trends towards significance for correlations between diffusivity scores and language ability in the 16p11.2 deletion group.
1). Structural Connectivity based on Gyral Topological Path Length in Each Hemisphere
Decreased FA and increased MD and RD are very common findings for most of the brain structures in neurodevelopmental disorders such as autism, and disorders with a comorbidity of autism such as TSC, indicating pervasive abnormality in the white matter integrity (for reviews see Travers et al. 2012; Rane et al. 2015). In one of our previous studies, we observed significantly increased MD and RD in patients with TSC compared to TD controls in the intrahemispheric connections (Im et al. 2016). Previous studies also observed significantly increased AD and MD throughout the whole brain, including the corpus callosum, of 16p11.2 deletion patients (Owen et al. 2014; Chang et al. 2016). Although the results of our gyral topology-based global connectivity analysis has the same pattern as most previous studies, our findings did not show any significant differences between the diffusivity values of the two groups for any of these connections, suggesting no global effect of 16p11.2 deletion on the white matter maturation. We did not observe a significant increase in FA in the 16p11.2 deletion group as the previous diffusion studies of the 16p11.2 deletion patients (Owen et al. 2014; Chang et al. 2016). There might be several explanations for this difference between the current and previous studies. The previous studies observed increased FA in deep major white matter tracts (Berman et al. 2015), which could also indicate alterations in most long-range connections, however, the significant increase in FA disappeared once ICV was used as a covariate (Owen et al. 2014), and both of the studies which found increased FA in 16p11.2 deletion patients used the TBSS method. The TBSS method creates a mean FA image across all subjects, performs skeletonization of white matter tracts, uses the nearest maximum FA along a certain tract that is in perpendicular direction from each participant’s FA map, and conducts cross-participant voxelwise stats on skeleton-projected FA (Smith et al. 2006; Ly et al. 2015). The TBSS method does not identify specific tracts/connections in volume image data; therefore, the information of which fiber tracts (in terms of anatomy, function, and length) have altered FA values is not precisely available. On the other hand, we performed deterministic tractography, segmented specific tracts, and then calculated the MD values with all voxels along each tract. Therefore, the discrepancy in the results could also be attributed to the methodology differences between the current and previous studies. Postmortem fetal human brain studies have shown a global temporal pattern of cerebral connectivity development where long-range pathways found in the deep white matter, complete their formation earlier than the short-range pathways located in the superficial white matter (Takahashi et al. 2012, 2014). Since gene expression of the human brain related to connectivity development is temporally dramatically different during fetal life (Dale et al. 1999; Kang et al. 2011), development of long- and short-range pathways might be under different genetic control. Accordingly, we suggest that there is no specific white matter disruption in 16p11.2 deletion compared to TD controls, in terms of temporal or genetic influences for the development of long- and short-range pathways.
2). Increased AD and RD Values in the Bilateral Anterior Segment of the AF in the 16p11.2 Deletion Group
AF is part of the dorsal language stream, which is responsible for mapping auditory speech sounds to articulatory representations (Dick and Tremblay 2012). The anterior segment of the AF is part of the indirect pathway of the dorsal language network and it is suggested to have a role in vocalizing semantic content (Catani et al. 2005), in speech fluency (Fridriksson et al. 2013; Basilakos et al. 2014), and language learning abilities (Kepinska et al. 2017). In the current study, we showed significantly increased AD values for the left anterior AF and significantly increased RD values for the right anterior AF in the 16p11.2 deletion group. RD values of anterior segment of the right AF negatively correlated, showing a trend towards significance, with verbal and non-verbal ability scores in 16p11.2 deletion. We also showed that the negative correlation between the AD values of the anterior segment of the left AF and non-verbal ability scores approached significance in the 16p11.2 deletion group, particularly in those with an articulation disorder indicating better diffusivity with increased performance (Supplementary Table 1). Previous studies also showed negative correlations between AD values and non-verbal ability scores (Owen et al. 2014) as well as between RD values and non-verbal ability scores in 16p11.2 deletion patients (Chang et al. 2016). Few studies have examined the anterior segment of the AF in neurodevelopmental disorders and showed abnormalities in the white matter microstructure of the anterior segment. Billeci and colleagues found increased MD for the anterior segment of the AF in the right hemisphere in autism when compared to TD controls (Billeci et al. 2012). The expressive language abilities of individuals with ASD were negatively correlated with MD and AD of the left anterior segment of the AF (Billeci et al. 2012). Another study showed that FA of the anterior segment of AF was significantly decreased in the TSC patients with ASD compared to TSC patients without ASD as well as TD controls (Lewis et al. 2013). Moreover, the same study found that the MD values decreased with age more quickly in TD controls than TSC patients with ASD in the left anterior segment of the AF (Lewis et al. 2013). It has also been shown that the severity of auditory hallucinations in schizophrenia negatively correlated with the white matter integrity of the anterior segment of the left AF (Curcic-Blake et al. 2015). Significantly increased diffusivity values in the anterior segment of the AF in the 16p11.2 deletion group could be associated with the speech and articulation impairments that are observed in majority of the 16p11.2 deletion patients (Hanson et al. 2010; Rosenfeld et al. 2010; Hanson et al. 2015).
3). Increased MD and RD Values for the Long Segment of the AF in the Right Hemisphere for the 16p11.2 Deletion Group
The long segment of the AF is the direct pathway of the dorsal language network, and it plays a role in word learning (Lopez-Barroso et al. 2013), phonological processing (Catani et al. 2005), and speech processing (Wan et al. 2012). We found decreased volume of the long segment of the AF in the left hemisphere and increased MD and RD values for the long segment of the AF in the right hemisphere in the 16p11.2 deletion group compared to the TD group. Furthermore, the volume of the long segment of the left AF was significantly positively correlated with non-verbal ability, measured with DAS-II, in the 16p11.2 deletion group, which was a finding that approached significance in patients with 16p11.2 deletion who had a diagnosis of articulation disorder. There was a trend towards significance for the positive correlation between volume of the left long segment of AF and language ability, measured with CELF-4, in 16p11.2 deletion group. Volume of the left long segment of AF also showed a positive correlation, which approached significance, with general cognitive ability scores in the 16p11.2 deletion group, and also the 16p11.2 deletion patients without articulation disorders. Such correlations were not observed for the right long segment of the AF in the 16p11.2 deletion group. On the other hand, in the TD group, MD values of the long segment of the right AF showed a negative correlation, which approached significance, with language memory scores (Supplementary Table 1). Our correlational findings between the left long segment of the AF and language ability are consistent with the previous studies showing a link between abnormal microstructure of language pathways and impaired language ability in 16p11.2 deletion (Berman et al. 2015).
Long segment of the AF has been found to be abnormal in groups of patients who have language impairments. Berman and colleagues found that the 16p11.2 deletion patients had significantly increased MD and RD values for bilateral long segment of the AF and increased AD values for the right long segment of the AF compared to TD controls (Berman et al. 2015). Increased MD in the left long segment of the AF were shown in a group of adolescents with high functioning autism (Fletcher et al. 2010) and also in TSC patients with ASD (Lewis et al. 2013) compared to TD controls. Autism and language impairment both showed increased MD, compared to TD and language impairment, in the left long segment of the AF (Nagae et al. 2012; Roberts et al. 2014), however, while autism was a main affect on the increased AD, language impairment had a main affect on the increased RD values indicating different mechanisms for the white matter abnormalities associated with these two disorders (Roberts et al. 2014). Moreover, the long segment AF volume, especially in the right hemisphere, was reduced in autism than in TD controls (Moseley et al. 2016).
Berman and colleagues showed significant negative correlations between MD and RD values of the left long segment of the AF and language ability in 16p11.2 deletion patients, and a significant positive correlation between AD values and language ability in TD control participants (Berman et al. 2015). A previous study found that TD children who used more complex syntax in their spoken narratives also had lower FA in the right long segment of the AF and higher MD values in the left long segment of the AF (Mills et al. 2013). The absence of the right long AF has been associated with a lower language performance in ASD (Verly et al. 2014). Furthermore, children with autism who are completely non-verbal showed a rightward asymmetry of the long segment of the AF, opposite of what has been found in TD children (Wan et al. 2012). Previous studies that examined the long segment of the AF in 16p11.2 deletion, autism, and language impaired patient groups support our findings regarding the loss of structural integrity of the right AF (Berman et al. 2015; Moseley et al. 2016; Roberts et al. 2014). These results indicate a neurobiological abnormality in the long segment of the AF of the 16p11.2 deletion group and are in parallel with clinical findings of phonological processing disorder commonly observed in this patient group (Hanson et al. 2010, 2015). The abnormal microstructure of the long segment of AF might be implicated in the speech and language deficits in 16p11.2 deletion (Wan et al. 2012).
4). Increased AD Values for the Bilateral UF in the 16p11.2 Deletion Group
Among the ventral language pathways, we found significantly increased AD values in the 16p11.2 deletion group in bilateral UF. We also showed that the negative correlation between the AD values of bilateral UF and language ability scores as measured with CELF-4 approached significance in the 16p11.2 deletion group and especially in those with an articulation disorder. Furthermore, in the TD group AD values of the bilateral UF showed negative correlations that approached significance with verbal and non-verbal ability scores measured with DAS-II (Supplementary Table 1). The most common finding of the previous studies that examined the microstructure of UF is decreased FA or increased MD in bilateral (Bloemen et al. 2010; Jou et al. 2011; Shukla et al. 2011b) or right UF (Pugliese et al. 2009; Sahyoun et al. 2010) in ASD than TD individuals. Cheon and colleagues (Cheon et al. 2011) found decreased FA and increased MD and RD in the left UF in individuals with ASD. Participants with specific language impairment had significantly lower FA and higher RD in bilateral UF and higher AD in left UF than TD participants (Vydrova et al. 2015). A study of TSC children also found significantly higher AD values for the UF in the patient group than the TD group (Zikou et al. 2016), which supports our findings. Decreased FA of bilateral UF has been observed in patients with schizophrenia compared to TD controls (Singh et al. 2016). UF continues developing into the third decade of life; this long-range association pathway is one of the last white matter tracts that complete its maturation (Olson et al. 2015). Even though the functional role of UF is still debated, in addition to its roles for emotional processing, memory, and empathy (Oishi et al. 2015), it has been suggested that it might also be associated with semantic processing, lexical retrieval, and auditory working memory/sound recognition, as well as speech fluency (Lu et al. 2002; Frey et al. 2004; Grossman et al. 2004; Hickok and Poeppel 2004, 2007; Basilakos et al. 2014). Our results suggest that increased AD for UF might be a sensitive biomarker for speech and language problems observed in 16p11.2 deletion and its comorbid disorders such as TSC and ASD (Owen et al. 2014) and are consistent with previous findings of negative correlations between AD values and non-verbal ability scores in 16p11.2 deletion (Owen et al. 2014).
5). Laterality Analyses and Asymmetry of the IFOF in the 16p11.2 Deletion Group
Our results did not show any significant differences between the TD and 16p11.2 deletion groups for the LI of whole-brain gyral and interhemispheric connectivity or dorsal language network diffusivity values. On the other hand, there was a significant group difference for the LI of RD values of the IFOF, where the 16p11.2 deletion group showed a rightward asymmetry and the TD group showed a leftward asymmetry. We also showed that the volume of the right IFOF positively correlated, with a trend towards significance, with language ability scores as measured with CELF-4 as well as verbal and non-verbal ability scores as measured with DAS-II, in the 16p11.2 deletion group (Supplementary Table 1). The role of IFOF is suggested to involve reading and writing (Catani and Mesulam 2008) as well as semantic processing (Almairac et al. 2015; Mandonnet et al. 2007; Duffau et al. 2005) and semantic working memory (Turken and Dronkers 2011). Asymmetry of IFOF findings is variable in existing literature. In TD individuals bilateral (Hickok and Poeppel 2007), stronger leftward asymmetry (Parker et al. 2005), and stronger rightward asymmetry (Thiebaut de Schotten et al. 2011) has been observed. A TBSS study found significantly reduced orientation dispersion index in the left IFOF of patients with 16p11.2 deletion compared to TD controls (Owen et al. 2014). Another study found increased rightward volumetric laterality of the IFOF in children with specific language impairment compared to TD controls (Vydrova et al. 2015). The same study also showed increased MD and RD values in the left IFOF in children with specific language impairment compared to TD children (Vydrova et al. 2015). Other studies showed lower FA values in the left IFOF in individuals with early onset of schizophrenia (Epstein et al. 2014) and individuals with autism (Jou et al. 2011) compared to TD individuals. Language function lateralization is influenced by genetic and prenatal environmental effects on the brain development where possible disruptions may result in atypical lateralization of white matter seen in some disorders such as ASD (Knaus et al. 2010; Lange et al. 2010), which is not necessarily related to the increased left-handedness found in this patient group (Markou et al. 2017). Our findings suggest that there might be developmental alterations of the IFOF in 16p11.2 deletion patients resulting in atypical lateralization and impairments in cognitive and language function, independent of atypical handedness observed in 16p11.2 deletion.
Interpretation of results
In the current study, in addition to FA, we also examined MD, AD, and RD indices to acquire more detailed information about the microstructural properties of the dorsal and ventral language pathways as well as the short-, middle-, and long-range pathways as defined by gyral topology in the brain of 16p11.2 deletion subjects. Our findings show a consistent pattern of increased MD, AD, and RD in the 16p11.2 deletion group. MD is directly related to interstitial space between axonal fibers (Billeci et al. 2012). Therefore, an increase in MD might suggest increased water mobility between structures such as myelin, axonal membranes, and glial cells that can be caused by decreased myelin or reduced axonal density (Berman et al. 2015). AD can be affected by changes in axonal integrity and diameter (Budde et al. 2009). Increased AD could result from axonal injury and damage, which leads to reduced axonal density or caliber, or axonal loss, increasing the extra-axonal space by allowing faster water molecule movement parallel to axons (Song et al. 2003; Kumar et al. 2008). An increase in RD might be due to less dense and/or thicker axons (Travers et al. 2012) or may represent disordered myelin sheaths, axonal depletion, or extracellular changes (Peters et al. 2012). Therefore, our results suggest white matter alterations that are more pronounced in specific language pathways in the 16p11.2 deletion group. Abnormal development of language pathways would explain the impaired language functions observed in 16p11.2 deletion patients (Berman et al. 2015). Moreover, our findings are supported by previous studies that reported similar white matter abnormalities (e.g. decreased FA and increased MD) in individuals with ASD, TSC, or language impairment suggesting a common link between brain structure and language function among these comorbid disorders (Berman et al. 2015). Some variability between our findings and those of previous studies might be due to different data processing strategies or differences in the participant groups.
Limitations
There are a number of limitations in the present study. First, the participant groups were not age matched. However, the age difference was very small between the participant groups and we used age as a covariate in our statistical analyses to prevent our results from showing a bias for age difference. Second, in 16p11.2 deletion syndrome the rates of left-handedness and ambidexterity are higher than the rates found in the TD population (Hanson et al. 2015). Although, the approach of matching the two participant groups on handedness was taken in order to test that the neuroanatomical abnormalities were associated with the 16p11.2 deletion and not with left-handedness, it is important to note that selecting a handedness-matched TD control group caused our TD control sample to be not perfectly representative of a TD population in terms of non right-handedness rates, where left-handedness and ambidexterity are found in lower numbers. Furthermore, there were insufficient participants in the 16p11.2 deletion group with a diagnosis of ASD (N = 2), which made it impossible for us to perform statistical analyses to make a comparison of the 16p11.2 deletion patients with and without a diagnosis of ASD. However, the similar findings between studies of ASD and 16p11.2 deletion patients suggest a common pathophysiological link between the brain structure and language function of the two disorders (Berman et al. 2015). Moreover, to understand the genetic etiology of the 16p11.2 deletion disorder and its link to ASD or language disorders, future studies must combine brain imaging data with genetic and behavioural data (Berman et al. 2015). In particular, the addition of behavioral testing measuring language performance related to each language specific fiber pathway would be more informative (Fletcher et al. 2010).
Even though our results suggest that the language tracts are selectively involved, this conclusion is currently based on the comparison of white matter of short-, middle-, and long-range whole brain gyral connections with dorsal and ventral language pathways without the inclusion of other specific tracts or systems. Our method, gyral connectivity analysis only analyzes the corticocortical white matter tracts, and not the long-range projectional tracts such as the corticospinal tracts. In addition, in this study the ROI analyses were performed only for the tracts of interest. In order to confirm our finding of selective involvement of language tracts in 16p11.2 deletion, future studies would benefit from the involvement of other tracts such as corticospinal tracts, somatosensory radiation, or the cingulum to provide a basis of comparison. Finally, as all corticocortical white matter fibers are projected and connected to the boundary between gray and white matter, there would be more crossing fibers in the superficial outer part of the white matter regions. Hence, a part of short-range fiber pathways might not be captured in DTI-based tractography. More advanced acquisition sequences, such as the high angular resolution diffusion imaging methods including diffusion spectrum imaging, where crossing fibers are included in the model (Wedeen et al. 2008), would enhance the reconstruction of the short-range fibers.
Conclusions
This is the first study to examine white matter alterations of the bilateral dorsal (all 3 segments of the AF) and ventral (IFOF, ILF, UF) language pathways in the context of whole brain in a group of 16p11.2 deletion patients as well as TD controls. Our results show that there is no global selective bias for involvement of long, medium or short connections in the whole brain. However, our results suggest a selective involvement of the dorsal and ventral language pathways, specifically the bilateral anterior and right long segments of the AF and bilateral UF, as well as different asymmetry in the IFOF in 16p11.2 deletion patients. The selective involvement of the language networks we report may help better understand the effects of altered white matter connectivity on neurodevelopmental outcomes in 16p11.2 deletion.
Supplementary Material
Acknowledgements
This work is submitted on behalf of the Simons Variation in Individuals Project (Simons VIP) investigators. We are grateful to all off the families at the participating Simons Variation in Individuals Project (Simons VIP) sites, as well as the investigators in the Simons VIP Consortium (Simons VIP Consortium, 2012). We are thankful to be given access to the phenotypic data on SFARI Base. We would also like to acknowledge Marie Drottar and Ashley Curran for their assistance.
Funding This study was funded by R01HD079484, R01EB014947, R01HD076258, R01HD065762, and R21HD083956.
Footnotes
Conflict of interest Authors Banu Ahtam, Naira Link, Erikson Hoff, P. Ellen Grant, and Kiho Im declare that they have no conflict of interest.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11682-018-9859-3) contains supplementary material, which is available to authorized users.
References
- Almairac F, Herbet G, Moritz-Gasser S, de Champfleur NM, & Duffau H (2015). The left inferior fronto-occipital fasciculus sub-serves language semantics: A multilevel lesion study. Brain Structure & Function, 220, 1983–1995. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, & Nordahl CW (2008). Neuroanatomy of autism. Trends in Neurosciences, 31, 137–145. [DOI] [PubMed] [Google Scholar]
- Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, & Fridriksson J (2014). Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Frontiers in Human Neuroscience, 8, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Chudnovskaya D, Blaskey L, Kuschner E, Mukherjee P, Buckner R, Nagarajan S, Chung WK, Spiro JE, Sherr EH, & Roberts TP (2015). Abnormal auditory and language pathways in children with 16p11.2 deletion. Neuroimage Clinics, 9, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Chudnovskaya D, Blaskey L, Kuschner E, Mukherjee P, Buckner R, Nagarajan S, Chung WK, Sherr EH, & Roberts TP (2016). Relationship between M100 auditory evoked response and auditory radiation microstructure in 16p11.2 deletion and duplication carriers. AJNR. American Journal of Neuroradiology, 37, 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci L, Calderoni S, Tosetti M, Catani M, & Muratori F (2012). White matter connectivity in children with autism spectrum disorders: A tract-based spatial statistics study. BMC Neurology, 12, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon K, Thesen T, Green S, Ben-Avi E, Wang X, Fuchs B, Kuzniecky R, & Devinsky O (2017). Focal cortical anomalies and language impairment in 16p11.2 deletion and duplication syndrome. Cerebral Cortex, 1–9. 10.1093/cercor/bhx143 [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK, van Amelsvoort TA, Schmitz N, Robertson D, Murphy KC, & Murphy DG (2010). White matter integrity in Asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Research, 3, 203–213. [DOI] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, & Song SK (2009). Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: A quantitative pixelwise analysis. The Journal of Neuroscience, 29, 2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the dis-connection theme in language and aphasia: History and current state. Cortex, 44, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57, 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, & Jones DK (2007). Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America, 104, 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG, & McGuire P (2011). Altered integrity of perisylvian language pathways in schizophrenia: Relationship to auditory hallucinations. Biological Psychiatry, 70, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Pojman NJ, Thieu T, Bukshpun P, Wakahiro ML, Marco EJ, Berman JI, Spiro JE, Chung WK, Buckner RL, Roberts TP, Nagarajan SS, Sherr EH, & Mukherjee P (2016). Reciprocal white matter alterations due to 16p11.2 chromosomal deletions versus duplications. Human Brain Mapping, 37, 2833–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, Nair A, Koh YJ, Jang DP, Kim YB, Leventhal BL, Cho ZH, Castellanos FX, & Schultz RT (2011). Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A diffusion tensor imaging study. Brain Research, 1417, 77–86. [DOI] [PubMed] [Google Scholar]
- Curcic-Blake B, Nanetti L, van der Meer L, Cerliani L, Renken R, Pijnenborg GH, & Aleman A (2015). Not on speaking terms: Hallucinations and structural network disconnectivity in schizophrenia. Brain Structure & Function, 220, 407–418. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Dick AS, & Tremblay P (2012). Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain, 135, 3529–3550. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, & Capelle L (2005). New insights into the anatomo-functional connectivity of the semantic system: A study using corticosubcortical electrostimulations. Brain, 128, 797–810. [DOI] [PubMed] [Google Scholar]
- Elliott CD (2007). Differential ability scales (2nd Ed.) Canadian Journal of School Psychology, 22, 128–132. [Google Scholar]
- Epstein KA, Cullen KR, Mueller BA, Robinson P, Lee S, & Kumra S (2014). White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 53(362–372), e361–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage, 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004a). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, & Dale AM (2004b). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl 1), S69–S84. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, & Lainhart JE (2010). Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage, 51, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Kostopoulos P, & Petrides M (2004). Orbitofrontal contribution to auditory encoding. NeuroImage, 22, 1384–1389. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Guo D, Fillmore P, Holland A, & Rorden C (2013). Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain, 136, 3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, & Gee J (2004). What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain, 127, 628–649. [DOI] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, Lian A, Hundley R, Shen Y, Wu BL, Holm IA, Miller DT, & p11.2 Study Group C. (2010). Cognitive and behavioral characterization of 16p11.2 deletion syndrome. Journal of Developmental and Behavioral Pediatrics, 31, 649–657. [DOI] [PubMed] [Google Scholar]
- Hanson E, Bernier R, Porche K, Jackson FI, Goin-Kochel RP, Snyder LG, Snow AV, Wallace AS, Campe KL, Zhang Y, Chen Q, D’Angelo D, Moreno-De-Luca A, Orr PT, Boomer KB, Evans DW, Kanne S, Berry L, Miller FK, Olson J, Sherr E, Martin CL, Ledbetter DH, Spiro JE, Chung WK, & Simons Variation in Individuals Project C. (2015). The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biological Psychiatry, 77, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2004). Dorsal and ventral streams: A frame-work for understanding aspects of the functional anatomy of language. Cognition, 92, 67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Im K, Paldino MJ, Poduri A, Sporns O, & Grant PE (2014). Altered white matter connectivity and network organization in polymicrogyria revealed by individual gyral topology-based analysis. NeuroImage, 86, 182–193. [DOI] [PubMed] [Google Scholar]
- Im K, Ahtam B, Haehn D, Peters JM, Warfield SK, Sahin M, & Ellen Grant P (2016). Altered structural brain networks in tuberous sclerosis complex. Cerebral Cortex, 26, 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J 3rd, Chow V, Blaskey L, Kuschner E, Qasmieh S, Gaetz L, Edgar JC, Mukherjee P, Buckner R, Nagarajan SS, Chung WK, Spiro JE, Sherr EH, Berman JI, & Roberts TP (2016). Auditory evoked M100 response latency is delayed in children with 16p11.2 deletion but not 16p11.2 duplication. Cerebral Cortex, 26, 1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Jackowski AP, Papademetris X, Rajeevan N, Staib LH, & Volkmar FR (2011). Diffusion tensor imaging in autism spectrum disorders: Preliminary evidence of abnormal neural connectivity. The Australian and New Zealand Journal of Psychiatry, 45, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, & Sestan N (2011). Spatio-temporal transcriptome of the human brain. Nature, 478, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinska O, Lakke E, Dutton EM, Caspers J, & Schiller NO (2017). The perisylvian language network and language analytical abilities. Neurobiology of Learning and Memory, 144, 96–101. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, & Tager-Flusberg H (2010). Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain and Language, 112, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, & Harper RM (2008). Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatric Research, 64, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, & Lainhart JE (2010). Atypical diffusion tensor hemispheric asymmetry in autism. Autism Research, 3, 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WW, Sahin M, Scherrer B, Peters JM, Suarez RO, Vogel-Farley VK, Jeste SS, Gregas MC, Prabhu SP, Nelson CA 3rd, & Warfield SK (2013). Impaired language pathways in tuberous sclerosis complex patients with autism spectrum disorders. Cerebral Cortex, 23, 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barroso D, Catani M, Ripolles P, Dell’Acqua F, Rodriguez-Fornells A, & de Diego-Balaguer R (2013). Word learning is mediated by the left arcuate fasciculus. Proceedings of the National Academy of Sciences of the United States of America, 110, 13168–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A, Gilmore RL, Bauer RM, & Roper SN (2002). Category-specific naming deficits for objects and actions: Semantic attribute and grammatical role hypotheses. Neuropsychologia, 40, 1608–1621. [DOI] [PubMed] [Google Scholar]
- Ly MT, Nanavati TU, Frum CA, & Pergami P (2015). Comparing tract-based spatial statistics and manual region-of-interest labeling as diffusion analysis methods to detect white matter abnormalities in infants with hypoxic-ischemic encephalopathy. Journal of Magnetic Resonance Imaging, 42, 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard AM, Ruef A, Pizzagalli F, Migliavacca E, Hippolyte L, Adaszewski S, Dukart J, Ferrari C, Conus P, Mannik K, Zazhytska M, Siffredi V, Maeder P, Kutalik Z, Kherif F, Hadjikhani N, Beckmann JS, Reymond A, Draganski B, Jacquemont S, & p11.2 European C. (2015). The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Molecular Psychiatry, 20, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, & Duffau H (2007). Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain, 130, 623–629. [DOI] [PubMed] [Google Scholar]
- Markou P, Ahtam B, & Papadatou-Pastou M (2017). Elevated levels of atypical handedness in autism: Meta-analyses. Neuropsychology Review, 27, 258–283. [DOI] [PubMed] [Google Scholar]
- Mills BD, Lai J, Brown TT, Erhart M, Halgren E, Reilly J, Dale A, Appelbaum M, & Moses P (2013). White matter microstructure correlates of narrative production in typically developing children and children with high functioning autism. Neuropsychologia, 51, 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, & van Zijl PC (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45, 265–269. [DOI] [PubMed] [Google Scholar]
- Moseley RL, Correia MM, Baron-Cohen S, Shtyrov Y, Pulvermuller F, & Mohr B (2016). Reduced volume of the arcuate fasciculus in adults with high-functioning autism Spectrum conditions. Frontiers in Human Neuroscience, 10, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae LM, Zarnow DM, Blaskey L, Dell J, Khan SY, Qasmieh S, Levy SE, & Roberts TP (2012). Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. AJNR. American Journal of Neuroradiology, 33, 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Hsu J, Tippett D, Mori S, & Hillis AE (2015). Critical role of the right uncinate fasciculus in emotional empathy. Annals of Neurology, 77, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, & Vyas G (2015). Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental Cognitive Neuroscience, 14, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Chang YS, Pojman NJ, Bukshpun P, Wakahiro ML, Marco EJ, Berman JI, Spiro JE, Chung WK, Buckner RL, Roberts TP, Nagarajan SS, Sherr EH, Mukherjee P, & Simons VIPC (2014). Aberrant white matter microstructure in children with 16p11.2 deletions. The Journal of Neuroscience, 34, 6214–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Bukshpun P, Pojman N, Thieu T, Chen Q, Lee J, D’Angelo D, Glenn OA, Hunter JV, Berman JI, Roberts TP, Buckner R, Nagarajan SS, Mukherjee P, & Sherr EH (2018). Brain MR imaging findings and associated outcomes in carriers of the reciprocal copy number variation at 16p11.2. Radiology, 286, 217–226. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, & Lambon Ralph MA (2005). Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage, 24, 656–666. [DOI] [PubMed] [Google Scholar]
- Peters JM, Sahin M, Vogel-Farley VK, Jeste SS, Nelson CA 3rd, Gregas MC, Prabhu SP, Scherrer B, & Warfield SK (2012). Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Academic Radiology, 19, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell’Acqua F, Thiebaut de Schotten M, Murphy C, Robertson D, Deeley Q, Daly E, & Murphy DG (2009). The anatomy of extended limbic pathways in Asperger syndrome: A preliminary diffusion tensor imaging tractography study. NeuroImage, 47, 427–434. [DOI] [PubMed] [Google Scholar]
- Qureshi AY, Mueller S, Snyder AZ, Mukherjee P, Berman JI, Roberts TP, Nagarajan SS, Spiro JE, Chung WK, Sherr EH, Buckner RL, & Simons VIPC (2014). Opposing brain differences in 16p11.2 deletion and duplication carriers. The Journal of Neuroscience, 34, 11199–11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy DN, & Frazier JA (2015). Connectivity in autism: A review of MRI connectivity studies. Harvard Review of Psychiatry, 23, 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TP, Heiken K, Zarnow D, Dell J, Nagae L, Blaskey L, Solot C, Levy SE, Berman JI, & Edgar JC (2014). Left hemisphere diffusivity of the arcuate fasciculus: Influences of autism spectrum disorder and language impairment. AJNR. American Journal of Neuroradiology, 35, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JA, Coppinger J, Bejjani BA, Girirajan S, Eichler EE, Shaffer LG, & Ballif BC (2010). Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. Journal of Neurodevelopmental Disorders, 2, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, & Mody M (2010). White matter integrity and pictorial reasoning in high-functioning children with autism. Brain and Cognition, 73, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, & Weiller C (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105, 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. 2003. Clinical evaluation of language fundamentals, fourth edition (CELF-4). Toronto: Canada: The Psychological Corporation/A Harcourt Assessment Company. [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, & Muller RA (2011a). Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia, 49, 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, & Muller RA (2011b). Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 52, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons Vip C (2012). Simons variation in individuals project (Simons VIP): A genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron, 73, 1063–1067. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh K, Trivedi R, Goyal S, Kaur P, Singh N, Bhatia T, Deshpande SN, & Khushu S (2016). Microstructural abnormalities of uncinate fasciculus as a function of impaired cognition in schizophrenia: A DTI study. Journal of Biosciences, 41, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TE (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, & Neufeld AH (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20, 1714–1722. [DOI] [PubMed] [Google Scholar]
- Steinman KJ, Spence SJ, Ramocki MB, Proud MB, Kessler SK, Marco EJ, Green Snyder L, D’Angelo D, Chen Q, Chung WK, Sherr EH, & Simons VIPC (2016). 16p11.2 deletion and duplication: Characterizing neurologic phenotypes in a large clinically ascertained cohort. American Journal of Medical Genetics. Part A, 170, 2943–2955. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda AM, & Grant PE (2012). Emerging cerebral connectivity in the human fetal brain: An MR tractography study. Cerebral Cortex, 22, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Hayashi E, Schmahmann JD, & Grant PE (2014). Development of cerebellar connectivity in human fetal brains revealed by high angular resolution diffusion tractography. NeuroImage, 96, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell’Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DG, & Catani M (2011). Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage, 54, 49–59. [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, & Alexander AL (2012). Diffusion tensor imaging in autism spectrum disorder: A review. Autism Research, 5, 289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, & Dronkers NF (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Van Oudenhove L, Lagae L, Sunaert S, & Rommel N (2014). Structural and functional underconnectivity as a negative predictor for language in autism. Human Brain Mapping, 35, 3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vydrova R, Komarek V, Sanda J, Sterbova K, Jahodova A, Maulisova A, Zackova J, Reissigova J, Krsek P, & Kyncl M (2015). Structural alterations of the language connectome in children with specific language impairment. Brain and Language, 151, 35–41. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. 1999. Comprehensive Test of Phonological Processing. Austin, TX: Pro: Ed. [Google Scholar]
- Wan CY, Marchina S, Norton A, & Schlaug G (2012). Atypical hemispheric asymmetry in the arcuate fasciculus of completely non-verbal children with autism. Annals of the New York Academy of Sciences, 1252, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen A, & Wedeen V (2007). Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proceedings on International Society for Magnetic Resonance in Medicine, 15, 3720. [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, & de Crespigny AJ (2008). Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage, 41, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, & Fischl B (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikou AK, Xydis VG, Astrakas LG, Nakou I, Tzarouchi LC, Tzoufi M, & Argyropoulou MI (2016). Diffusion tensor imaging in children with tuberous sclerosis complex: Tract-based spatial statistics assessment of brain microstructural changes. Pediatric Radiology, 46, 1158–1164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


