Abstract
Background.
Although the linkage between psychological stress and cortisol is believed to mediate the association of stress with health outcomes, several studies have been unable to demonstrate this association. We suggest this inability may be a consequence of limitations in the measurement approach and/or reliance on analytic strategies that focus on associations across, rather than within individuals. The link between psychological stress and cortisol is of particular interest in the context of pregnancy and fetal development. Using an ecological momentary assessment (EMA) design, we examined the association between psychological stress and cortisol at the between- and the within-person level.
Methods.
152 participants completed a 4-day long EMA protocol serially in early, mid and late pregnancy to provide momentary stress appraisals (average of 150 measures/subject) and saliva samples (average of 55 samples/subject) for quantification of cortisol. The association between stress and cortisol was estimated using linear mixed models.
Results.
After accounting for the effects of key determinants of variation in cortisol, momentary stress was significantly and positively associated with cortisol at the within-person level (B = .030, p = .031), but not at the between-person level. No association was evident for traditional retrospective measures of stress with cortisol at either the between- or the within-person level.
Conclusions.
Our study highlights the value of the EMA and linear mixed-modeling approaches in linking maternal psychological and physiological states across pregnancy. These findings may have important implications for the development of personalized risk identification and “just-in-time” intervention strategies to optimize maternal and child health.
Keywords: Ecological-Momentary-Assessment, Stress, Cortisol, Pregnancy, Linear Mixed Modelling, Psychoendocrine Covariance
1. Introduction
The association between psychological stress and disease risk is believed to be mediated, in large part, by stress-responsive physiological systems, notably the hypothalamus-pituitary-adrenal (HPA)-axis and its end product, cortisol (McEwen, 2012). It, therefore, seems reasonable to expect a high degree of correspondence between an individual’s psychological and physiological state (referred to as “response coherence”; Mauss et al., 2005, p. 175). However, and somewhat surprisingly, several studies have been unsuccessful in documenting this link (reviewed in: Mikkelsen et al., 2017). The absence of this association, which has been referred to as “lack of psychoendocrine covariance” (Campbell and Ehlert, 2012; Schlotz et al., 2008), may reflect one of two possibilities: a) there is no covariance, or, b) there are methodological limitations in measurement or analytic approaches that obscure the association. The first possibility seems unlikely given the ample evidence from experimental studies in animals and humans that demonstrate the induction of psychological stress consistently and reliably produces activation of the HPA axis and cortisol (Kirschbaum et al., 1993; Kudielka et al., 2007; McCormick et al., 2010). In terms of the second and more likely possibility, we suggest that three sets of potential issues may be particularly salient: 1) the failure to account for the idiographic (or subjective) nature of the appraisal of psychological stress; 2) the lack of temporal coupling in the assessment of psychological and physiological states; and 3) the reliance on retrospective recall measures to assess psychological stress appraisals.
Studies that examine psychoendocrine covariance have moved away from single (one-time) assessments and now typically employ repeated measurement designs to assess psychological and biological measures. However, even in this second generation of studies, the analytic focus has usually remained on the average association across a group of individuals, i.e., the between-person effect. This between-person approach assumes uniformity across individuals in the magnitude of the association between the appraisal of psychological stress and the physiological state, thereby potentially discounting the idiosyncratic (subjective) nature of such psychological appraisals. Individuals may vary widely in their subjective interpretation of stress level indicators that are commonly used in self-report measures (i.e., the interpretation of descriptors of stress such as “1 = a little,” “2 = a moderate amount” and “3 = a lot”). This subjectivity, or non-differential measurement error, may add “noise” to the ascertainment of stress and thereby attenuate effects. Consequently, the absence of between-person effects in quantifying psychoendocrine covariance may not necessarily be indicative of the absence of within-person effects (Molenaar, 2004, 2013; Molenaar and Campbell, 2009). We note that this limitation can be addressed by conducting a series of repeated measurements and adopting a within-person perspective, wherein the quantification of psychological state (stress) for a given individual at any given measurement moment is expressed relative to her or his own average state across measurements (i.e., mean centering an individual’s stress appraisals; reviewed in: Curran and Bauer, 2011; Molenaar, 2004, 2013; Molenaar and Campbell, 2009; Voelkle et al., 2014; Voelkle et al., 2018). Thus, upon doing so, effects that quantify the statistical association between psychological and endocrine measures are a reflection of both between-person and within-person effects, that then can be further deconstructed (reviewed in: Voelkle et al., 2018).
The second issue potentially contributing to the lack of psychoendocrine covariance may arise from the fact that psychological and endocrine states are often measured along different time scales. In many studies, ratings of prior stress levels (e.g., measures that instruct the respondent to provide a summary measure of her or his stress appraisals over a certain time frame such as during the previous four weeks) are linked to the indicators of endocrine state (e.g., individual’s cortisol concentrations assessed in saliva or blood) that do not reflect or correspond to the same time frame (e.g., cortisol concentrations in saliva reflect adrenal cortisol production over only the previous 20–30 minutes of time; Kirschbaum et al., 1993). And even in the case of studies that do collect multiple biological samples and then collapse them across a day or multiple days into measures that represent the average cortisol output (area under the curve (AUC) of cortisol measurements across a day or multiple days; Hellhammer et al., 2009), the time windows of collection of psychological and cortisol measures are often misaligned in terms of the temporal occurrence of stress and its biological indicator (Weckesser et al., 2019).
Third, the use of retrospective recall measures of stress may represent another potential limitation. Respondents are typically asked to rate how stressed they have felt over a prior period such as the past week, month, or longer. For example, one of the most commonly-used instruments to assess stress appraisals is the Perceived Stress Scale (PSS), a measure that instructs respondents to provide an average (summary) evaluation of the extent to which their life has been unpredictable, uncontrollable, and overwhelming over the past four weeks (Cohen et al., 1983). Such self-report retrospective recall measures are prone to numerous systematic biases that undermine their reliability and validity (Podsakoff et al., 2012), including recall bias (Loaiza et al., 2018), the use of heuristics (Availablility heursitic, Affect heuristic; Bradburn et al., 1987), personal significance, social desirability, and current affective state (Thomas and Diener, 1990). Moreover, such assessments are typically not conducted in the environment in which the stress-related affective states were experienced, limiting their external validity. It is, therefore, not surprising that several previous studies using measures such as the PSS have been unable to provide empirical evidence for a linkage between psychological and physiological states (Engert et al., 2018; reviewed in: Mikkelsen et al., 2017; Weckesser et al., 2019).
Based on these conceptual and methodological challenges, we suggest that study designs that incorporate repeated and concurrent measures of psychological and endocrine states over time may be better equipped to address the question of psychoendocrine covariance and to parse within- and between-person effects. The ecological momentary assessment (EMA) approach is an example of one such design (Conner and Barrett, 2012; Shiffman et al., 2008). In contrast to retrospective recall summary assessments, EMA incorporates real-time, repeated measurements of respondents’ momentary conditions, states, behaviors and physiology in real time and in the everyday life (ecologically-relevant) context (Mehl and Conner, 2012; Stone et al., 1998). In addition to minimizing the potential limitations discussed above, the availability of serial (repeated) EMA measures within individuals enables the computation and use of measures of deviations of an individual’s momentary psychological state from her/his average psychological state, thereby re-scaling each measure to her or his average stress level and facilitating disaggregation of between-person effects and within-person effects. We note that only a relatively small number of studies have used EMA in the context of assessing psychoendocrine covariance. These studies have more consistently uncovered significant associations between psychological stress and cortisol concentrations, notably at the within-person level (Adam et al., 2006; Jacobs et al., 2007; Smyth et al., 1998; Stawski et al., 2013), but even these investigations have not simultaneously examined both between- and within-person associations.
The relationship between psychological stress and cortisol concentrations is of particular interest in the context of pregnancy and fetal development because maternal stress during pregnancy has been linked to many critical pregnancy-, birth- and offspring-related outcomes (Buss et al., 2017; Entringer et al., 2018; Heim et al., 2019; Van den Bergh et al., 2017; Wadhwa et al., 2011). The stress hormone cortisol is postulated as a key potential mediator of these observed associations. Moreover, it may be important to examine this association across different stages of pregnancy because the responsiveness of the maternal HPA axis to psychological stress may vary as a function of pregnancy stage (the state of pregnancy produces a progressive two- to three-fold increase in maternal cortisol production over the course of gestation (Mastorakos and Ilias, 2003; Wadhwa et al., 2011) that we and others have suggested may attenuate the responsiveness of the system (Entringer et al., 2010; Wadhwa, 2005; Wadhwa et al., 2009; Wadhwa et al., 2011).
In light of these considerations, the aim of the current study was to examine the association between maternal psychological stress and cortisol during pregnancy. We conducted a longitudinal investigation in a population of healthy pregnant women and employed a repeated measures EMA design to examine within- and between-person effects at the momentary, day, and stage-of-pregnancy (early, mid, late) level. Our EMA protocol incorporated the collection of real-time psychological, behavioral and biological data over a consecutive four-day period, and it was serially administered at three windows in early, mid and late pregnancy. We used linear mixed models to account for the nested data structure and to evaluate between-person and within-person associations at the three time scales (moments, days, and stages of pregnancy; Snijders and Bosker, 2012). Analyses were adjusted for the effects of previously-established covariates of cortisol, including time of day, exact gestational age at assessment (GA), weekend/weekday, meal intake, and pre-pregnancy body-mass-index (BMI; Bleker et al., 2017; Gibson et al., 1999; Kudielka et al., 2012; Kunz-Ebrecht et al., 2004; Schlotz, 2018).
2. Materials and Methods
2.1. Participants
Women with a singleton, intrauterine pregnancy were recruited in early pregnancy for a prospective, longitudinal EMA-based study at the University of California, Irvine’s Development, Health and Disease Research Program. The study population (N = 152) was relatively healthy and consisted predominately of non-Hispanic white and Hispanic women. The study protocol included three study visits during early, mid, and late pregnancy (for sample characteristics, see Table 1). The attrition rate for the three serial pregnancy assessments from early to late gestation was 8%. Exclusion criteria included twin pregnancies, uterine, placental/cord anomalies, fetal congenital malformations, and systemic corticosteroid intake. The UCI Institutional Review Board approved the study, and all participants provided written consent.
Table 1.
Maternal sociodemographic and obstetric characteristics.
| Maternal characteristics | N = 152 |
|---|---|
| Gestational age (GA) at assessment (weeks gestation, M ± SD) | |
| T0 – early gestation | 12.3 ± 1.7 |
| T1 – mid gestation | 19.3 ± 1.3 |
| T2 – late gestation | 28.7 ± 1.3 |
| Maternal age (years, M ± SD) | 27.8 ± 5.5 |
| Education (n, %) | |
| Some high school or less | 31 (21.7%) |
| Technical or vocational school or certificate | 16 (11.2%) |
| Some college or associate degree | 54 (37.8%) |
| Bachelor’s degree or higher | 42 (29.4%) |
| Income (USD, n, %) | |
| <15k | 14 (10.4%) |
| 15–50k | 54 (40.3%) |
| 50–100k | 49 (36.6%) |
| >100k | 17 (12.7%) |
| Race/ethnicity (n, %) | |
| Non-Hispanic white | 54 (38.8%) |
| Hispanic | 53 (38.1%) |
| Others | 32 (23.1%) |
| Pre-pregnancy BMI (M ± SD) | 27.2 ± 6.4 |
| Normal weight (< 25) | 70 (47.3%) |
| Overweight (25 – 30) | 40 (27.0%) |
| Obese (> 35) | 38 (25.7%) |
| Obstetric characteristics | |
| Obstetric risk factors (n, %) | 16 (6.7%) |
| Severe infection | 4 (2.7%) |
| Preeclampsia | 3 (2.0%) |
| Diabetes | 9 (2.0%) |
| Parity (n, %) | |
| 0 | 57 (40.1%) |
| 1 | 38 (26.8%) |
| 2 | 31 (21.8%) |
| >2 | 16 (11.3%) |
Note. Due to rounding, some totals may not correspond with the sum of the separate figures.
2.2. Measures
2.2.1. Maternal characteristics.
A structured sociodemographic interview was conducted by trained study personnel to obtain information about maternal age, education, household income, race/ethnicity, pre-pregnancy weight, height, and parity. Estimated date of conception and GA was computed using standard obstetric criteria (combination of LMP and fetal biometry ultrasound). Obstetric data were abstracted from the medical record.
2.2.2. Perceived stress.
At each visit, we assessed maternal perceived stress along three different time scales (momentary, daily, and “last four weeks”/monthly level). We assessed momentary perceived stress using an EMA protocol for ambulatory, real-time measurement (see Figures 1). The 4-day EMA-protocol spanned two consecutive weekdays and a weekend (i.e., Thursday - Sunday, or Saturday - Tuesday). Participants were provided a smart phone containing electronic diary measures, an actigraphy device, and a saliva collection kit. Throughout the EMA period, participants were prompted an average of one time per hour (i.e., every 60 ± 10 mins) during waking hours to evaluate their momentary perceived stress level using a short validated version of the perceived stress scale (PSS-EMA; Cohen et al., 1983; Cohen and Williamson, 1988). At each prompt subjects answered additional questions about contextual factors such as previous meal intake. Following the 4-day EMA protocol, subjects returned to the research office, where they were then administered the PSS-10 questionnaire regarding their perceived stress during the last four days (EMA period, PSS-past-4-days). In addition, perceived stress over the last four weeks was assessed using the validated PSS-10 (retrospective self-report instrument; (PSS-past-4-weeks; Cohen et al., 1983; Cohen and Williamson, 1988).
Figure 1.
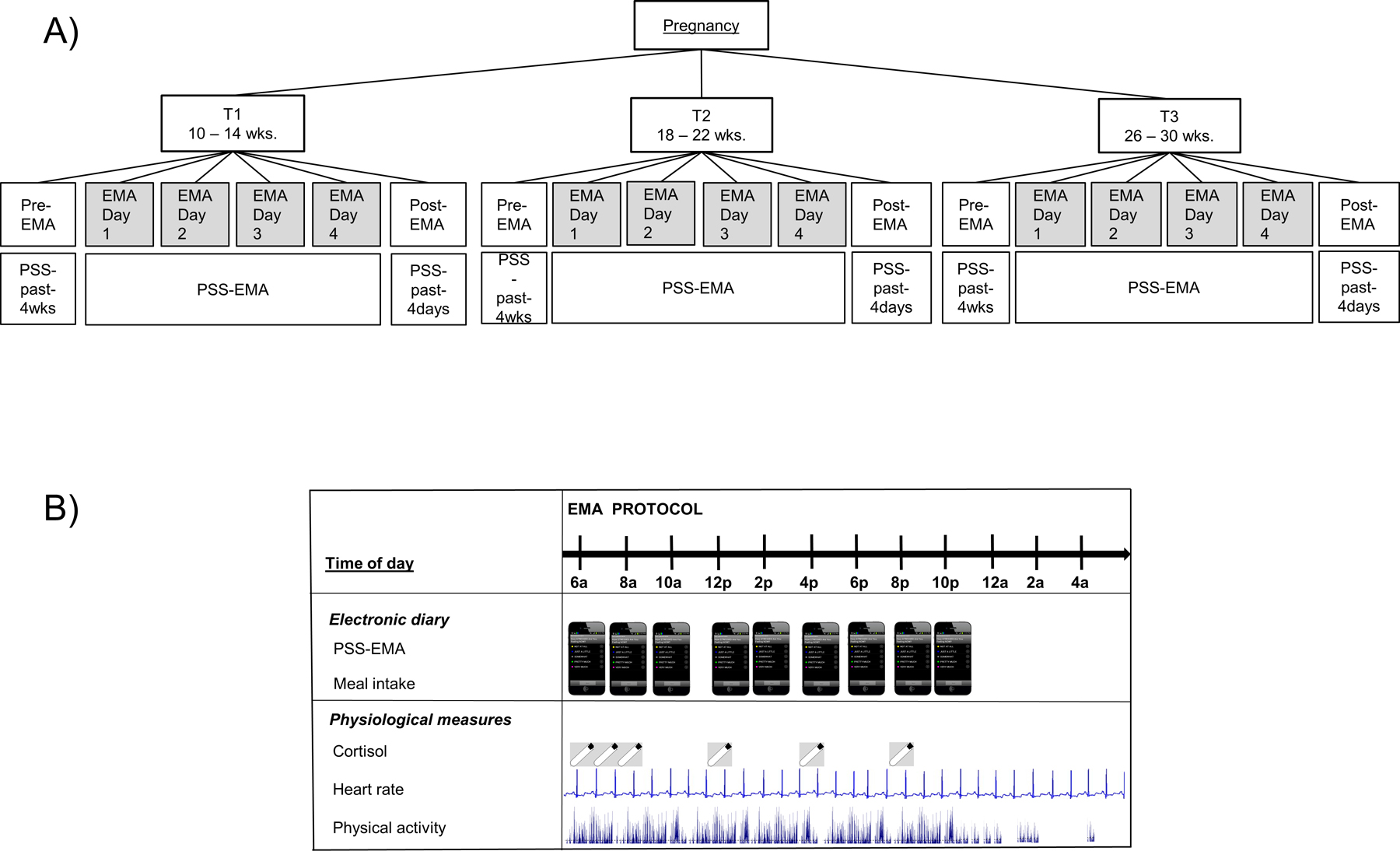
Study design; A) Ecological-Momentary-Assessment (EMA) sampling scheme across pregnancy: The pre-EMA visit included assessment of stress during the past 4 weeks (PSS-past-4-weeks), followed by the 4-day EMA-period assessing momentary stress via PSS-EMA, and followed by a post-EMA visit assessing stress over the past 4 days (PSS-past-4-days); B) Ecological-Momentary-Assessment (EMA) protocol within a 4-day EMA-period.
2.2.3. Cortisol assessments.
During each EMA-period participants provided five daily saliva samples in salivettes immediately upon, and 30 mins after awakening, and at 12pm, 4pm, and 8pm. The electronic diary prompted the participants to provide samples. Participants stored the salivettes in a refrigerator until the post-EMA visit. Electronic monitors (MEMS®, Aardex group, Union City, CA, USA) were used to date-time-stamp the sampling. Participants were fitted with an Actiheart monitoring device to continuously record their EKG and physical activity. To verify compliance with the immediately-upon-awakening saliva sampling each participant’s EKG and actigraphy was examined by two independent raters to establish time of awakening indicated by a substantial change in heart rate and physical activity (Steinberg et al., 2017). Salivary cortisol concentrations were analyzed using ELISA (Salimetrics, Carlsbad, CA). 10% of the samples were run in duplicates. The assay had a lower limit of sensitivity of 0.007 μg/dl, standard curve range from 0.012 μg/dl to 3.0 μg/dl, an average intra-assay coefficient of variation (CV) of 5.42%, and an average inter-assay CV < 10%. At the day and stage-at-pregnancy level, momentary cortisol concentrations were aggregated across the day and across the entire 4-day EMA-periods, respectively, using the area-under-the-curve with respect to ground (AUC.g; Khoury et al., 2015).
2.3. Statistical Analysis
We performed all statistical analyses in R version 3.5.1 (R Development Core Team, 2018). The R-package nlme version 3.1–137 was used for linear mixed model analyses (Pinheiro et al., 2018) .
2.3.1. Variance decomposition of momentary measures.
We used linear mixed(-effect) models (LMMs; cf. multilevel models) to identify the sources of variance in momentary PSS-EMA and cortisol across the different levels of the data (Snijders and Bosker, 2012). Momentary cortisol was log-transformed due to its skewed distribution (Table S1, Supplement). This paper focuses on the association between perceived stress and cortisol during the everyday life of individuals. In contrast to controlled experimental settings with discrete time points for stressor onset and end of stress exposure, in which cortisol peaks within 20–30 mins after the beginning of the stressor (Hellhammer and Schubert, 2012; Kirschbaum et al., 1993; Kudielka et al., 2007), perceived stress in everyday life might extend across longer periods. We therefore decided a priori to use the closest perceived stress ratings to the cortisol sample within a window of ± 60 min around cortisol sampling. In case of more than one response, the perceived stress rating closest to the cortisol sampling was chosen. In two separate 4-level LMMs, random intercepts for momentary measurements (level 1), days (level 2), stages of pregnancy (level 3), and participants (level 4) were estimated and used to calculate the percentage of total variance in PSS-EMA, and cortisol. To account for the unequal spacing of auto-correlated measurements across a day a continuous autoregressive covariance structure was specified. Restricted maximum likelihood was used for parameter estimation.
2.3.2. Linear mixed models.
(a) To examine the relationship between PSS-EMA and cortisol at the momentary level, a 4-level LMM was fitted to the hierarchical data. We used a continuous autoregressive covariance structure of order 1. To account for the diurnal shape of cortisol (Adam and Kumari, 2009), time since awakening on each day was modeled using B-spline functions (Eilers and Marx, 1996). The derived estimates (t1- t5) were used as predictors in the LMM, interpolating the time course on to the momentary cortisol measures. The complete model specification is provided in the Supplement A1. (b) To examine the coupling at the day level, aggregated PSS-EMA on a day and the AUC.g on that day were associated in a similar 4-level LMM (for model specifications, see Supplement A2). (c) To compare traditional recall questionnaires and EMA-based stress with respect to their association with cortisol, PSS-EMA was aggregated across each EMA-period (PSS-EMA.4days). At each stage-of-pregnancy level, the aggregated PSS-EMA-4-days (Supplement A3), the PSS-past-4-days (Supplement A4), and the PSS-past-4-weekss (Supplement A5) were used to predict the AUC.g aggregated across each EMA-period in three separate 3-level LMMs.
2.3.3. Centering.
To differentiate the within-person and between-person effects of stress, all PSS scores were centered (Enders and Tofighi, 2007). The individual PSS-EMA scores were centered at the individual’s mean PSS-EMA across all EMA responses (PSS-EMA.cwc, cwc = “centered within cluster”). Therefore, the PSS-EMA.cwc represents the extent to which an individual deviates at each given momentary assessment from their average stress level (i.e., the within-person effect). Both, PSS-EMA.cwc and the individual’s mean PSS-EMA (PSS-EMA.pm, i.e., between-person effect) were included as predictors in the LMMs. At the day level, PSS-EMA was aggregated across each day and centered at the person mean as described (PSS-EMA.day.cwc). The PSS-EMA.day.cwc represents the deviation of a person’s average PSS-EMA on a given day from their average stress level. At the stage-of-pregnancy level, PSS-EMA was aggregated across each EMA-period within the stages of pregnancy and centered as described (PSS_EMA.4day.cwc). The same was done for PSS-past-4-weeks (PSS-past4weeks.cwc) and PSS-past-4-days (PSS-past4days.cwc).
2.3.4. Covariates.
The following covariates were included as fixed effects in the LMMs (for formal model specifications, see Supplement A1–A5): at the momentary level (A1), time since awakening (t1-t5), meal intake (yes = 1, no = 0), GA, weekend/weekday (weekend = 1, weekday = 0), and pre-pregnancy BMI; at the day level (A2), GA, weekend/weekday, and pre-pregnancy BMI; at the stage-of-pregnancy level (A3-A5), GA, and pre-pregnancy BMI.
3. Results
3.1. Descriptive statistics of cortisol and perceived stress
3.1.1. Salivary cortisol.
As expected, momentary cortisol concentrations exhibited a diurnal pattern, with a steep morning increase and a progressive decrease across the rest of the day. Also as expected, maternal cortisol increased with advancing gestation, and were positively skewed (see Supplement Table S1). Compliance for saliva collection was high (M = 93.8 % ± 10.4 % (SD) of samples were collected). Participants collected on average 56 out of 60 possible samples on average across gestation. Reasons for missing data included attrition over the course of the study, and non-compliant EMA or saliva collection.
3.1.2. Perceived Stress Scale (PSS).
The sample had a relatively low prevalence of highly stressed individuals (see Supplement Table S1). Compliance with the EMA-protocol was very high (M = 90.3 % ± 9.0 % (SD) of prompts were answered). Participants answered on average 143 prompts out of the 157 possible prompts within the stipulated 5-min time window over the 4-day period (three times across gestation).The different PSS measures were significantly correlated (for bivariate correlations, see Supplement Table S2 and S3). PSS-EMA increased slightly across gestation, whereas the PSS-4-weeks and PSS-4-days measures were not associated with GA (detailed results displayed in the Supplement, Table S4).
3.2. Decomposition of variance of momentary cortisol and momentary perceived stress
3.2.1. Salivary cortisol.
The variance decomposition indicates how much of the total variation in cortisol is derived from different data levels (i.e., variation between individuals, within an individual, across the stages of pregnancy, across a day, and across moments; de Haan-Rietdijk et al., 2016; Schmiedek et al., 2013). Based on the LMM, cortisol varied primarily at the momentary level (79.6%), and there was a relatively small degree of variance between individuals (7.0%) and across the stages of pregnancy (12.9%; for detailed results, see Supplement, Table S5).
3.2.2. PSS-EMA.
The decomposition of variance of PSS-EMA based on the LMM suggested that momentary stress varied considerably from moment-to-moment (39.5%) and between individuals (44.5%). The variations due to stage of pregnancy and day-to-day changes accounted for only small proportions of variance (stage-of-pregnancy: 10.8%; day-to-day: 8.1%; Supplement, Table S6).
Taken together, these findings suggest that psychoendocrine covariance is more likely to be observed within an individual, rather than across individuals, and that this covariation potentially occurs at the momentary level.
3.3. Momentary-level associations between PSS-EMA and cortisol
At the within-person level, momentary stress was positively associated with momentary cortisol, indicating that in moments when a participant reported higher momentary stress (PSS-EMA.cwc) relative to her average stress level (PSS-EMA.pm), her momentary cortisol was higher than in those moments when she reported momentary stress that was lower than her average stress level (log-transformed cortisol, B = 0.030, p = .031, Table 2). At the between-person level, the average stress level across participants based on momentary EMA assessment (PSS-EMA.pm) was not associated with momentary cortisol (B = 0.023, p = .583). In addition, significant effects on cortisol were evident for GA (increase in cortisol across the course of pregnancy (B = 0.116, p<.001), the diurnal course of cortisol (t1-t5, Table 2), weekend/weekday (B = −0.058, p = <.001), and preceding meal intake (B = 0.104, p = <.001). Momentary cortisol increased as pregnancy progressed, was higher after a meal, and was lower on weekends compared to weekdays. Pre-pregnancy BMI was negatively associated with cortisol (B = −0.008, p = .022).
Table 2.
Momentary-level associations between PSS-EMA (Perceived Stress Scale – Ecological-Momentary-Assessment) and cortisol: Results from linear mixed models predicting momentary log-transformed cortisol by PSS-EMA (within-/between-person effect), adjusted for time of day, meal intake, gestational age (GA), weekend/-day, pre-pregnancy body-mass-index (BMI).
| Fixed effects | ||||
|---|---|---|---|---|
| B (SE) | beta | 95% CI for B | p | |
| Intercept | −2.290 (0.164) | −2.613 – −1.968 | <.001*** | |
| Level 1 | ||||
| PSS-EMA.cwc | 0.030 (0.014) | .021 | 0.003 – 0.058 | .031* |
| t1 | 1.625 (0.159) | .496 | 1.312 – 1.938 | <.001*** |
| t2 | 0.801 (0.108) | .226 | 0.588 – 1.013 | <.001*** |
| t3 | 0.367 (0.124) | .135 | 0.124 – 0.610 | .003** |
| t4 | −0.143 (0.119) | −.042 | −0.376 – 0.090 | .230 |
| t5 | −0.846 (0.131) | −.187 | −1.103 – −0.589 | <.001*** |
| Meal intake | 0.104 (0.019) | .052 | 0.067 – 0.141 | <.001*** |
| GA | 0.116 (0.007) | .250 | 0.102 – 0.129 | <.001*** |
| Level 2 | ||||
| Weekend | −0.058 (0.016) | −.037 | −0.091 – −0.026 | <.001*** |
| Level 3 | ||||
| Level 4 | ||||
| PSS-EMA.pm | 0.023 (0.046) | .017 | −0.066 – 0.117 | .583 |
| Pre-pregnancy BMI | −0.008 (0.003) | −.007 | −0.014 – −0.001 | .022* |
| Random effects | ||||
| Variance | SD | |||
| ID | 0.067 | 0.258 | ||
| Stage of pregnancy | 0.027 | 0.164 | ||
| Day | 0.015 | 0.123 | ||
| Residual | 0.185 | 0.430 | ||
Note. Significance codes:
p > .01 ‘ ‘
p < .10 ‘.’
p < .05 ‘*’
p < 0.01 ‘**’
p < .001 ‘***’.
Results displayed for log-transformed cortisol. Transformation did not change magnitude, direction nor significance level of the reported effects. For fit indices, see Supplement Table S7.
3.4. Day-level associations between PSS-EMA and cortisol
At the within-person day level, cortisol output (log-transformed AUC.g of cortisol) was greater on days when the subject reported higher EMA-based day-level stress (PSS-EMA.day.cwc) than her average stress level (PSS-EMA.pm) (B = 0.038, p = .035; Table 3). At the between-person level there was no significant association between average stress level (PSS-EMA.pm) and overall cortisol output. Similar to the momentary level, GA (B = 0.104, p < .001), weekend/weekday (B = −0.088, p = <.001), and pre-pregnancy BMI (B = −0.009, p = .018, Table 3) were significantly associated with day-level cortisol output.
Table 3.
Day-level associations between PSS-EMA (Perceived Stress Scale – Ecological-Momentary-Assessment) and cortisol: Results from linear mixed model predicting log-transformed AUC.g (area under curve) of cortisol by PSS-EMA aggregated across a day (within-/between-person effect) adjusted for gestational age (GA), weekend/weekday, pre-pregnancy body-mass-index (BMI).
| Fixed effects | ||||
|---|---|---|---|---|
| B (SE) | beta | 95% CI for B | p | |
| Intercept | 0.841 (0.116) | 0.619 – 1.070 | <.001*** | |
| Level 1 | ||||
| PSS-EMA.day.cwc | 0.038 (0.016) | . 037 | 0.006 – 0.069 | .035* |
| GA | 0.104 (0.005) | .431 | 0.093 – 0.114 | <.001*** |
| Level 2 | ||||
| Weekend | −0.088 (0.012) | −.107 | −0.111 – −0.065 | <.001*** |
| Level 3 | ||||
| Level 4 | ||||
| PSS-EMA.pm | 0.019 (0.043) | .024 | −0.066 – 0.104 | .320 |
| Pre-pregnancy BMI | −0.009 (0.004) | −.132 | −0.016 – −0.001 | .018* |
| Random effects | ||||
| Variance | SD | |||
| ID | 0.065 | 0.256 | ||
| Stage of pregnancy | 0.020 | 0.143 | ||
| Day | 0.046 | 0.215 | ||
| Residual | 0.009 | 0.094 | ||
Note. Significance codes:
p > .01 ‘ ‘
p < .10 ‘.’
p < .05 ‘*’
p < 0.01 ‘**’
p < .001 ‘***’.
Results displayed for log-transformed AUC.g. Transformation did not change magnitude, direction nor significance level of the reported effects. Default covariance structure corresponding to no within-group correlations was chosen based on non-significant likelihood ratio test comparing different covariance structures. For fit indices, see Supplement Table S7.
3.5. Stage-of-pregnancy (early-, mid and late-pregnancy averages) associations between PSS and cortisol
As shown in Table 4 Model A, there was no association between average EMA-stress and the average cortisol output across the corresponding 4-day EMA-period (AUC.g.4days) at either the between-person (PSS-EMA.4days.pm) or the within-person level (PSS-EMA.4days.cwc). With regard to retrospective recall questionnaires, neither of the PSS measures (based on the recall over the last four days or the traditional PSS recalling the past four weeks) were associated with cortisol output across four days at either the within-person level (PSS.4weeks.cwc, PSS.4days.cwc) or the between-person level (PSS.4weeks.pm, PSS.4days.pm, Table 4, Model B and C). At the stage-of-pregnancy level, the effects of GA and pre-pregnancy BMI were similar in direction, magnitude, and significance level to the day level and momentary level results. In summary, the results of these analyses do not provide evidence for psychoendocrine covariance at the stage-of-pregnancy level when stress was assessed retrospectively, and when momentary stress was aggregated across multiple days.
Table 4.
Stage-of-pregnancy associations between PSS (Perceived Stress Scale) and cortisol: Results from linear mixed models predicting log-transformed AUC.g (area under the curve) averaged across four days by averaged PSS-EMA (Model A), PSS-past-4-days (Model B), and PSS-past-4-weeks (Model C) adjusted for gestation age (GA) and pre-pregnancy body-mass-index (BMI).
| MODEL A | ||||
|---|---|---|---|---|
| Fixed effects | ||||
| B (SE) | beta | 95% CI for B | p | |
| Intercept | 0.834 (0.120) | 0.598 –1.071 | <.001*** | |
| Level 1 | ||||
| PSS-EMA-4-day.cwc | 0.040 (0.041) | .026 | −0.041 – 0.122 | .330 |
| GA | 0.102 (0.006) | .459 | 0.090 – 0.114 | <.001*** |
| Level 2 | ||||
| Level 3 | ||||
| PSS-EMA.pm | 0.014 (0.044) | .019 | −0.073 – 0.101 | .758 |
| Pre-pregnancy BMI | −0.009 (0.004) | −.144 | −0.016 – −0.001 | .021* |
| Random effects | ||||
| Variance | SD | |||
| ID | 0.067 | 0.259 | ||
| Stage of pregnancy | 0.035 | 0.187 | ||
| Residual | 0.007 | 0.088 | ||
| MODEL B | ||||
| Fixed effects | ||||
| B (SE) | beta | 95% CI for B | p | |
| Intercept | 0.887 (0.122) | 0.647 – 1.127 | <.001*** | |
| Level 1 | ||||
| PSS-past-4-days.cwc | 0.032 (0.032) | .028 | −0.031 – 0.095 | .316 |
| GA | 0.104 (0.006) | .469 | 0.091 – 0.117 | <.001*** |
| Level 2 | ||||
| Level 3 | ||||
| PSS-past-4-days.pm | −0.034 (0.044) | −.049 | −0.122 – 0.053 | .439 |
| Pre-pregnancy BMI | −0.009 (0.004) | −.148 | −0.016 – −0.002 | .018* |
| Random effects | ||||
| Variance | SD | |||
| ID | 0.066 | 0.257 | ||
| Stage of pregnancy | 0.037 | 0.191 | ||
| Residual | 0.008 | 0.089 | ||
| MODEL C | ||||
| Fixed effects | ||||
| B (SE) | beta | 95% CI for B | p | |
| Intercept | 0.943 (0.127) | 0.694 – 1.192 | <.001*** | |
| Level 1 | ||||
| PSS-past-4-weeks.cwc | 0.013 (0.034) | .011 | −0.053 – 0.080 | .693 |
| GA | 0.103 (0.006) | .465 | 0.091 – 0.115 | <.001*** |
| Level 2 | ||||
| Level 3 | ||||
| PSS-past-4-weeks.pm | −0.060 (0.045) | −.083 | −0.149 – 0.028 | .180 |
| Pre-pregnancy BMI | −0.009 (0.004) | −.149 | −0.016 – −0.002 | .016* |
| Random effects | ||||
| Variance | SD | |||
| ID | 0.066 | 0.256 | ||
| Stage of pregnancy | 0.036 | 0.190 | ||
| Residual | 0.007 | 0.088 | ||
Note. Significance codes:
p > .01 ‘ ‘
p < .10 ‘.’
p < .05 ‘*’
p < 0.01 ‘**’
p < .001 ‘***’.
Results displayed for log-transformed AUC.g averaged across four days. Transformation did not change magnitude, direction nor significance level of the reported effects. Default covariance structure was chosen based on non-significant likelihood ratio test comparing different covariance structures. For fit indices, see Supplement Table S7.
4. Discussion and Conclusions
Our study addressed the issue of the association across pregnancy between maternal psychological (stress) and endocrine (cortisol) states using EMA-based and traditional retrospective recall-based questionnaires. To the best of our knowledge, this is the first study to examine the question of whether this psychoendocrine covariance is evident at the between- or within-person level during pregnancy. To summarize, our key findings suggested, firstly, that variation in stress as well as in cortisol occurred primarily within individuals from moment to moment; and secondly, that after accounting for the effects of key covariates maternal stress was significantly and independently associated with cortisol, and this association was evident only within (but not across) individuals, and only when stress and cortisol were assessed concurrently and analyzed at the momentary or at the day level. This association was not evident using measures aggregated across individuals or psychological measures based on retrospective recall approaches.
Although the overall (average) level of perceived stress in our study population was relatively low (which is consistent with expectations for a normative, low risk population), there was considerable variation within individuals across time, and also across individuals. Thus, these findings support the importance of accounting for subjectivity across individuals in psychological stress appraisals, temporal alignment in the assessment of psychological and physiological states, and the reliance on momentary (EMA) as opposed to retrospective recall measures to assess psychological stress appraisals. With respect to the nature and magnitude of the maternal stress and cortisol relationship, our findings suggest that after accounting for the effect on cortisol of time of day, prior meal intake, GA, weekday/weekend, and pre-pregnancy BMI, each 1 SD difference in an individual’s stress at a given moment from her own average stress level was associated with a 3% difference in her cortisol concentration. And at the day level, a difference of 1 SD stress relative to the average stress level was associated with an approximately 4% difference in cortisol output (AUC.g) over the course of the day. To place the magnitude of this effect in context, we suggest it may be important to appreciate that the major determinants of variation in cortisol production are time of day (cortisol production was approximately double in the mornings compared to evenings), meal intake (10% higher cortisol following a meal), and stage of pregnancy (11% higher cortisol with each 1 SD advance in gestation). Against the backdrop of these major drivers of cortisol production (i.e., over and above these influences), we suggest that an effect of 3% change in cortisol of each SD unit relative (within subject) change in psychological stress is meaningful in magnitude.
Our study has several notable strengths. First, as previously mentioned, this investigation represents the first study to examine the within-person and between-person association of psychological stress and stress physiology (cortisol) during pregnancy at different time scales. Second, in the context of EMA-based studies, the size of our study population is considerable, and subject compliance with the data collection protocol was very high compared to that reported in other studies (Jones et al., 2019; Wen et al., 2017). Previous studies in this context have used smaller sample sizes and were based on fewer assessment days and fewer assessment windows during gestation (Giesbrecht et al., 2012). Third, we employed a state-of-the-art approach to model cortisol over the course of each day using non-linear spline functions to capture different aspects of cortisol’s diurnal variation. Moreover, we validated the exact time of awakening using EKG and actigraphy data, thereby ensuring greater reliability in the assessment of the awakening and post-awakening cortisol measures. By using a continuous autoregressive covariance structure, our models also accounted for the unequal spacing of time between cortisol measurements - a limitation that has often been noted in previous studies.
There are some limitations of our study. Because our study participants represented a normative, low risk population of pregnant women in terms of sociodemographic or obstetric risk, the range of variation in stress appraisals was relatively constricted, thereby potentially limiting the generalizability of our findings to other high risk populations (Lasikiewicz et al., 2008; Simpson et al., 2008). We note, however, that for this very reason the magnitude of our observed effects is likely to be a conservative estimate of that which would be expected in more heterogeneous high-risk populations. Second, the psychological stress measures in our study captured only the stress appraisal component, but not other components such as exposure and responses to discrete stressful events (Linz et al., 2018; Smyth et al., 2018) .
As discussed previously, many prior studies of stress and stress physiology make the implicit assumption that the effect of stress on physiology is uniform across individuals. Based, however, on our findings, it appears that the magnitude of the within-person coupling (assessed necessarily at the momentary level) between stress and cortisol may represent a better psychobiological indicator of the pathway from psychological stress to disease risk. We suggest this observation may have important implications for the development and subsequent evaluation of risk identification and intervention strategies. Here, we discuss these implications in the specific context of our interest in the process of fetal/developmental programming of health and disease risk. Briefly, the fetal/developmental programming paradigm refers to the process wherein conditions during critical developmental periods exert a major influence on structural and functional characteristics of cells, tissues and organ systems, with important implications for the individual’s subsequent state of health and susceptibility for a range of common disorders. In this context, maternal exposure to stress has been identified as an important condition of interest, and cortisol has been identified as a key mediator of the programming effects of stress. Higher cortisol concentrations during pregnancy are linked to increased risk for adverse pregnancy and birth outcomes such as spontaneous abortion and preterm birth (Buss et al., 2009; Nepomnaschy et al., 2006), thereby constituting a risk factor for such outcomes. Under physiological conditions, the fetus is protected from higher concentrations of cortisol by 11β-HSD, a placental enzyme transforming cortisol in to its inactive form cortisone (Siebe et al., 1993; Wadhwa et al., 1996; Wadhwa et al., 2011; Wadhwa et al., 2004; Wadhwa et al., 1998; Welberg et al., 2000). However, higher concentrations of maternal cortisol reduce the activity of the enzyme (reviewed in: Chapman et al., 2013; Clarke et al., 2002; Kerzner, 2002) potentially exposing the fetus to higher concentrations of cortisol. In the fetal compartment, cortisol binds to glucocorticoid responsive elements (GREs) in DNA to induce or repress gene expression (Binder, 2009; Reddy et al., 2012), potentially resulting in long lasting changes in the biological characteristics of fetal tissues and organ systems, with important implications for long-term developmental and health outcomes over the life span of the individual (Entringer et al., 2012, 2015; Entringer et al., 2018; Entringer et al., 2011; Segar et al., 1995). Based on the consideration that the effects of stress on various maternal and child health outcomes of interest are expected to vary as a function of individual differences in the responsivity of stress-responsive physiological systems (cortisol), we and others have highlighted the need for the development of measures to better identify vulnerable/susceptible individuals and to develop and test the efficacy of targeted, personalized intervention strategies. Based on our findings, we suggest, firstly, that subject-specific indicators of the strength of the association between stress and cortisol could be computed and used as an individual difference measure in predicting the index individual’s risk of developing stress-related disorders. This concept is akin to that which relies on the use of markers of individual differences in stress reactivity that typically are obtained by characterizing individual differences in evoked physiological changes after experimental (in-laboratory) induction of stress, but with the advantage of inferring this analogous measure in the context of the individual’s day-to-day natural (ecologically-relevant) setting. And secondly, we suggest that this approach could be used to develop and test the efficacy of individualized (personalized) “just-in-time” interventions, i.e., those that are tailored to the identification and delivery in real time and natural environment of any given index individual’s need based on their stress appraisal and/or psychophysiological coupling (e.g.: Nahum-Shani et al., 2018; Spruijt-Metz and Nilsen, 2014).
In conclusion, our study highlights the value of the ecological momentary assessment and multilevel modeling approach in addressing prior limitations and establishing the nature and magnitude of the linkage between maternal psychological (stress) and physiological (cortisol) states across human pregnancy. These findings may, in turn, have important implications for the development and subsequent evaluation of personalized risk identification and “just-in-time” intervention strategies to optimize maternal and child health.
Supplementary Material
Funding:
This work was supported in part by US PHS (NIH) grants R01 HD-060628, R01 AG-050455, R01 HD-065825, UH3 OD-O23349, and European Research Council grant ERC-Stg 678073.
Footnotes
Declarations of Interest: None.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT, 2006. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A 103, 17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M, 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Binder EB, 2009. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34 Suppl 1, S186–195. [DOI] [PubMed] [Google Scholar]
- Bleker LS, Roseboom TJ, Vrijkotte TG, Reynolds RM, de Rooij SR, 2017. Determinants of cortisol during pregnancy - The ABCD cohort. Psychoneuroendocrinology 83, 172–181. [DOI] [PubMed] [Google Scholar]
- Bradburn NM, Rips LJ, Shevell SK, 1987. Answering autobiographical questions: the impact of memory and inference on surveys. Science 236, 157–161. [DOI] [PubMed] [Google Scholar]
- Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, Heim CM, Wadhwa PD, 2017. Intergenerational Transmission of Maternal Childhood Maltreatment Exposure: Implications for Fetal Brain Development. J Am Acad Child Adolesc Psychiatry 56, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, Wadhwa PD, 2009. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol 201, 398 e391–398. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U, 2012. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 37, 1111–1134. [DOI] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J, 2013. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93, 1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke KA, Ward JW, Forhead AJ, Giussani DA, Fowden AL, 2002. Regulation of 11 beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endocrinol 172, 527–534. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G, 1988. Perceived stress in a probability sample of the United States., in: Spacapan S, Oskamp S (Eds.), The Social Psychology of Health. Sage, Newbury Park, CA. [Google Scholar]
- Conner TS, Barrett LF, 2012. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom Med 74, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ, 2011. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol 62, 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan-Rietdijk S, Kuppens P, Hamaker EL, 2016. What’s in a Day? A Guide to Decomposing the Variance in Intensive Longitudinal Data. Front Psychol 7, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers PHC, Marx BD, 1996. Flexible Smoothing with $B$-splines and Penalties. Statistical Science 11, 89–102. [Google Scholar]
- Enders CK, Tofighi D, 2007. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods 12, 121–138. [DOI] [PubMed] [Google Scholar]
- Engert V, Kok BE, Puhlmann LMC, Stalder T, Kirschbaum C, Apostolakou F, Papanastasopoulou C, Papassotiriou I, Pervanidou P, Chrousos GP, Singer T, 2018. Exploring the multidimensional complex systems structure of the stress response and its relation to health and sleep outcomes. Brain Behav Immun 73, 390–402. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD, 2010. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress 13, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2012. Prenatal Stress, Telomere Biology, and Fetal Programming of Health and Disease Risk. Science Signaling. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2015. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD, 2018. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD, 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A 108, E513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J, 1999. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med 61, 214–224. [DOI] [PubMed] [Google Scholar]
- Giesbrecht GF, Campbell T, Letourneau N, Kooistra L, Kaplan B, Team APS, 2012. Psychological distress and salivary cortisol covary within persons during pregnancy. Psychoneuroendocrinology 37, 270–279. [DOI] [PubMed] [Google Scholar]
- Heim CM, Entringer S, Buss C, 2019. Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology 105, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM, 2009. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34, 163–171. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Schubert M, 2012. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology 37, 119–124. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA, 2007. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology 74, 60–66. [DOI] [PubMed] [Google Scholar]
- Jones A, Remmerswaal D, Verveer I, Robinson E, Franken IHA, Wen CKF, Field M, 2019. Compliance with ecological momentary assessment protocols in substance users: a meta-analysis. Addiction 114, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzner LS, 2002. Antenatal Dexamethasone: Effect on Ovine Placental 11beta-Hydroxysteroid Dehydrogenase Type 2 Expression and Fetal Growth. Pediatric Research 52, 706–712. [DOI] [PubMed] [Google Scholar]
- Khoury JE, Gonzalez A, Levitan RD, Pruessner JC, Chopra K, Basile VS, Masellis M, Goodwill A, Atkinson L, 2015. Summary cortisol reactivity indicators: Interrelations and meaning. Neurobiol Stress 2, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Gierens A, Hellhammer DH, Wust S, Schlotz W, 2012. Salivary cortisol in ambulatory assessment--some dos, some don’ts, and some open questions. Psychosom Med 74, 418–431. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, 2007. Ten years of research with the Trier Social Stress Test (TSST) – revisited, in: Harmon-Jones E, Winkielman P (Eds.), Social Neuroscience. Guilford Press, New York. [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A, 2004. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 29, 516–528. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L, 2008. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters. Psychoneuroendocrinology 33, 143–151. [DOI] [PubMed] [Google Scholar]
- Linz R, Singer T, Engert V, 2018. Interactions of momentary thought content and subjective stress predict cortisol fluctuations in a daily life experience sampling study. Sci Rep 8, 15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza VM, Walentynowicz M, Schneider S, Stone AA, 2018. The effects of time frames on self-report. Plos One 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I, 2003. Maternal and Fetal Hypothalamic-Pituitary-Adrenal Axes During Pregnancy and Postpartum. Annals of the New York Academy of Sciences 997, 136–149. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ, 2005. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion 5, 175–190. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P, 2010. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn 72, 73–85. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2012. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A 109 Suppl 2, 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl MR, Conner TS, 2012. Handbook of research methods for studying daily life. The Guilford Press, New York, NY, US. [Google Scholar]
- Mikkelsen S, Forman JL, Fink S, Vammen MA, Thomsen JF, Grynderup MB, Hansen AM, Kaerlev L, Kolstad HA, Rugulies R, Bonde JP, 2017. Prolonged perceived stress and saliva cortisol in a large cohort of Danish public service employees: cross-sectional and longitudinal associations. Int Arch Occup Environ Health 90, 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar PCM, 2004. A Manifesto on Psychology as Idiographic Science: Bringing the Person Back Into Scientific Psychology, This Time Forever. Measurement: Interdisciplinary Research & Perspective 2, 201–218. [Google Scholar]
- Molenaar PCM, 2013. On the necessity to use person-specific data analysis approaches in psychology. European Journal of Developmental Psychology 10, 29–39. [Google Scholar]
- Molenaar PCM, Campbell CG, 2009. The New Person-Specific Paradigm in Psychology. Current Directions in Psychological Science 18, 112–117. [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA, 2018. Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann Behav Med 52, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG, 2006. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A 103, 3938–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, 2018. _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1–137.
- Podsakoff PM, MacKenzie SB, Podsakoff NP, 2012. Sources of method bias in social science research and recommendations on how to control it. Annu Rev Psychol 63, 539–569. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reddy TE, Gertz J, Crawford GE, Garabedian MJ, Myers RM, 2012. The hypersensitive glucocorticoid response specifically regulates period 1 and expression of circadian genes. Mol Cell Biol 32, 3756–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, 2018. Investigating associations between momentary stress and cortisol in daily life: What have we learned so far? Psychoneuroendocrinology. [DOI] [PubMed]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S, 2008. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: a question of timing. Psychosom Med 70, 787–796. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lovden M, Lindenberger U, 2013. Keeping it steady: older adults perform more consistently on cognitive tasks than younger adults. Psychol Sci 24, 1747–1754. [DOI] [PubMed] [Google Scholar]
- Segar JL, Bedell K, Page WV, Mazursky JE, Nuyt AM, Robillard JE, 1995. Effect of cortisol on gene expression of the renin-angiotensin system in fetal sheep. Pediatr Res 37, 741–746. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR, 2008. Ecological Momentary Assessment. Annual Review of Clinical Psychology 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Siebe H, Baude G, Lichtenstein I, Wang D, Bühler H, Hoyer G-A, Hierholzer K, 1993. Metabolism of dexamethasone: sites and activity in mammalian tissues. Kidney and Blood Pressure Research 16, 79–88. [DOI] [PubMed] [Google Scholar]
- Simpson EEA, McConville C, Rae G, O’Connor JM, Stewart-Knox BJ, Coudray C, Strain JJ, 2008. Salivary cortisol, stress and mood in healthy older adults: The Zenith study. Biological Psychology 78, 1–9. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA, 1998. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology 23, 353–370. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Sliwinski MJ, Zawadzki MJ, Scott SB, Conroy DE, Lanza ST, Marcusson-Clavertz D, Kim J, Stawski RS, Stoney CM, Buxton OM, Sciamanna CN, Green PM, Almeida DM, 2018. Everyday stress response targets in the science of behavior change. Behav Res Ther 101, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ, 2012. Multilevel analysis: an introduction to basic and advanced multilevel modeling, 2 ed. Sage Publishers, London. [Google Scholar]
- Spruijt-Metz D, Nilsen W, 2014. Dynamic Models of Behavior for Just-in-Time Adaptive Interventions. Ieee Pervas Comput 13, 13–17. [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM, 2013. Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology 38, 2654–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, El-Sherif N, Krol J, Kurpesa M, La Rovere MT, Lobodzinski SS, Locati ET, Mittal S, Olshansky B, Piotrowicz E, Saxon L, Stone PH, Tereshchenko L, Turitto G, Wimmer NJ, Verrier RL, Zareba W, Piotrowicz R, 2017. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 14, e55–e96. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Neale JM, Shiffman S, Marco CA, Hickcox M, Paty J, Porter LS, Cruise LJ, 1998. A comparison of coping assessed by ecological momentary assessment and retrospective recall. J Pers Soc Psychol 74, 1670–1680. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Diener E, 1990. Memory Accuracy in the Recall of Emotions. Journal of Personality and Social Psychology 59, 291–297. [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, Schwab M, 2017. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed]
- Voelkle MC, Brose A, Schmiedek F, Lindenberger U, 2014. Toward a Unified Framework for the Study of Between-Person and Within-Person Structures: Building a Bridge Between Two Research Paradigms. Multivariate Behav Res 49, 193–213. [DOI] [PubMed] [Google Scholar]
- Voelkle MC, Gische C, Driver CC, Lindenberger U, 2018. The Role of Time in the Quest for Understanding Psychological Mechanisms. Multivariate Behav Res 53, 782–805. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, 2005. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology 30, 724–743. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM, 2009. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Dunkel Schetter C, Chicz-DeMet A, Porto M, Sandman CA, 1996. Prenatal Psychosocial Factors and the Neuroendocrine Axis in Human Pregnancy. Psychosomatic Medicine 58. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, Lu MC, 2011. The Contribution of Maternal Stress to Preterm Birth: Issues and Considerations. Clinics in Perinatology 38, 351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA, 2004. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol 191, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA, 1998. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol 179, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Weckesser LJ, Dietz F, Schmidt K, Grass J, Kirschbaum C, Miller R, 2019. The psychometric properties and temporal dynamics of subjective stress, retrospectively assessed by different informants and questionnaires, and hair cortisol concentrations. Sci Rep 9, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR, Holmes MC, 2000. Inhibition of 11b-hydroxysteroid dehydrogenase, the foetoplacental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxietylike behaviour in the offspring. European Journal of Neuroscience 12. [DOI] [PubMed] [Google Scholar]
- Wen CKF, Schneider S, Stone AA, Spruijt-Metz D, 2017. Compliance With Mobile Ecological Momentary Assessment Protocols in Children and Adolescents: A Systematic Review and Meta-Analysis. J Med Internet Res 19, e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


