Abstract
This study investigated the underlying nature of apraxia of speech (AOS) by testing two competing hypotheses. The Reduced Buffer Capacity Hypothesis argues that people with AOS can plan speech only one syllable at a time (Rogers & Storkel, 1999). The Program Retrieval Deficit Hypothesis states that selecting a motor program is difficult in face of competition from other simultaneously activated programs (Mailend & Maas, 2013). Speakers with AOS and aphasia, aphasia without AOS, and unimpaired controls were asked to prepare and hold a two-word utterance until a go-signal prompted a spoken response. Phonetic similarity between target words was manipulated. Speakers with AOS had longer reaction times in conditions with two similar words compared to two identical words. The Control and the Aphasia group did not show this effect. These results suggest that speakers with AOS need additional processing time to retrieve target words when multiple motor programs are simultaneously activated.
Keywords: apraxia of speech, aphasia, language production, speech motor planning, reaction time
Apraxia of speech (AOS) is a motor speech disorder that can significantly impede successful communication by reducing speech intelligibility and naturalness (Duffy, 2005; Haley et al., 1998). Considerable progress in AOS research has been made over the last decades in terms of AOS diagnosis (Ballard et al., 2016; Haley et al., 2012; McNeil et al., 2009; Romani et al., 2017; Strand et al., 2014) and treatment (for overview, see Ballard et al., 2015; Wambaugh et al., 2006). However, not all speakers with AOS respond equally to the existing treatments (e.g., Bailey et al., 2015; Wambaugh et al., 2012) and it is typically difficult to predict for whom a certain therapy will be effective. Even experts with substantial experience with AOS and neurogenic communication disorders in general do not always agree on the diagnosis of a specific case (Haley et al., 2012; Maas et al., 2014; Mailend & Maas, 2013). Finally, although there is general agreement that AOS affects the speech motor planning stage of speech production (Duffy, 2005; McNeil et al., 2009), the specific nature of the speech motor planning impairment remains vague and poorly understood (Ziegler et al., 2012).
This article reports an experiment that focuses on two empirically supported hypotheses about the underlying mechanism in AOS: the Reduced Buffer Capacity Hypothesis (Rogers & Storkel, 1999) and the Program Retrieval Deficit Hypothesis (Mailend & Maas, 2013; Mailend et al., 2019; McNeil et al., 2004). These hypotheses are considered in the context of a current model of typical speech production and tested within a reaction time paradigm that relates this work to the literature on speech motor planning processes in unimpaired speakers.
Theoretical background
Although there are several detailed and well-supported speech production models to form a theoretical basis for this investigation, this study is based on the DIVA model (Directions Into the Velocities of Articulators; Guenther, 2016; Guenther et al., 2006) and its extension GODIVA (Gradient Order DIVA; Bohland et al., 2010). The DIVA/GODIVA model is grounded in extensive empirical research (Guenther, 2016; Perkell, 2012), and it is more detailed than other models (e.g., Dell, 1986; Hickok, 2012; Levelt et al., 1999) at the level of speech motor planning and its interface with phonological planning. The DIVA/GODIVA model allows us to draw specific predictions for the hypotheses tested in this study and explicate the assumptions on which these predictions stand. The following review outlines aspects of this detailed neuro-computational model that are critical to the logic and rationale for this study. The reader is referred to Mailend et al. (2019) for a more comprehensive review of the theoretical background, the tested hypotheses within this framework, and the supporting evidence for these accounts.
The organization and control of speech production in the DIVA model begins with the activation of a Speech Sound Map representation for the intended target. The representations at this level may correspond to various linguistic entities, such as phonemes, syllables, words, or even whole phrases for frequently used utterances, however, syllables are thought to be the most typical representation unit at this stage. Thus, the Speech Sound Map is much like the Syllabary in the Nijmegen model (Levelt et al., 1999; Guenther et al., 2006). Input to Speech Sound Map representations comes from the preceding phase of phonological encoding. As the segments in the phonological word become available, their activation starts spreading to the matching Speech Sound Map representations. According to the model, both perfect and partial matches are activated to a degree and compete for selection, but typically only the best match reaches the selection threshold. In addition, the upcoming speech units in the utterance are activated in parallel. Once the unit with the highest level of activation gets selected, it is actively inhibited to avoid reselection allowing the next unit with the highest activation level to reach the selection threshold. Basal ganglia-ventral premotor cortex loops help resolve the competition through parallel inhibitory and excitatory connections (Civier et al., 2013) assuring that the intended progrm gets selected in the intended timing and order.
Apraxia of speech in the context of the DIVA/GODIVA model
According to previous research, there are several specific mechanisms by which speech motor planning operations could be impaired in AOS. The current study focuses on two empirically supported hypotheses about the underlying mechanism in AOS: the Reduced Buffer Capacity Hypothesis (Rogers & Storkel, 1999) and the Program Retrieval Deficit Hypothesis (Bohland et al., 2010; Mailend & Maas, 2013; McNeil et al., 2004).
The first account, termed the Reduced Buffer Capacity Hypothesis (Rogers and Storkel, 1999), suggests that the ability to plan upcoming speech units for production is greatly reduced in people with AOS, spanning no more than a single syllable at a time. In the DIVA and GODIVA models, the ability to plan several upcoming speech units simultaneously is achieved via parallel activation of those units. The selection order is represented in the activity gradient such that the units to be selected first are activated more compared to the units needed later in the sequence. The Reduced Buffer Capacity Hypothesis thus suggests that the parallel activation mechanism at the level of the Speech Sound Map is impaired in AOS (see also Miller & Guenther, 2020).
The alternative hypothesis, termed the Program Retrieval Deficit Hypothesis, argues that people with AOS are able to prepare and maintain more than one syllable just like typical speakers, but they have difficulty retrieving programs in the intended order when more than one Speech Sound Map cell is activated simultaneously to buffer upcoming units in a longer utterance. This difficulty is predicted to be greatest if the motor programs in the planned sequence are also phonetically similar, because the competing programs are partially activated by the same input at the phonological level.
Pitting these hypotheses against one another represents an effort to replicate and extend the findings of a recent study (Mailend et al., 2019) within a different experimental paradigm. In Mailend et al., participants with AOS and/or aphasia produced single words after a written prime. In most trials, the prime was identical to the target encouraging the participants to prepare the prime for production. In 25% of trials, the go-signal prompted the production of a different word, requiring the participants to abandon the prepared response and rapidly activate the motor plan that matched the prompted response. Speakers with AOS required more time to switch to a different response compared to people with aphasia without AOS and unimpaired speakers. This finding aligned with the predictions of the Program Retrieval Deficit Hypothesis, suggesting that speakers with AOS either have difficulties resolving the competition between different programs (i.e., the programs activated by the prime and the target) or accruing the necessary activation levels for selection threshold due to inadequate connections with the phonological plan.
According to the latter possibility, the exaggerated condition effect in the AOS group resulted from facilitation of the identical prime which would allow more time for activation to accumulate, rather than interference from a mismatching prime which would increase competition for selection. The paradigm in the present study provides an opportunity to examine the two conceptualizations of the Program Retrieval Deficit Hypothesis which the previous study by Mailend et al. could not address. Specifically, participants in the present study had advance knowledge of all targets in all conditions. Thus, if reaction time differences between conditions are observed, then this supports the competition account of the Program Retrieval Deficit Hypothesis over the account which argues for generally slowed activation accrual.
The approach of the present study
A primary obstacle for studying the speech motor planning impairment in AOS is identifying a task that would allow the manipulation of the speech motor planning level independently form other stages of speech production. Typically, increasing the speech motor planning load (e.g., by adding syllables or consonant clusters to the speech targets) increases also the difficulty for phonological planning and articulation. The approach for this study combines several different phenomena in an attempt to isolate the observed effects to the speech motor planning stage in speech production.
First, instead of examining the speech output itself, which is the final product of all the speech production levels combined (e.g., speech errors), reaction times (RT) are used as the primary measure of interest. This means measuring aspects of speech planning itself, without including the effects of speech execution in the final output (Maas & Mailend, 2012). Second, in the delayed naming task used in this study, participants have the opportunity to prepare the utterance before the go-signal is presented (e.g., Klapp, 2003; Laganaro & Alario, 2006; Maas et al., 2008; Mooshammer et al., 2009). Hence, the stages of speech production that precede speech motor planning in reading out loud, e.g., grapheme-to-phoneme encoding and phonological planning, are complete before the go-signal is presented (Bohland & Guenther, 2006; Laganaro & Alario, 2006; Sternberg et al., 1978). Finally, in order to influence the planning load at the level of speech motor planning, the phonetic similarity between the words planned for sequential production is manipulated (Meyer & Gordon, 1985; Rogers & Storkel, 1998; Yaniv etal., 1990). According to the DIVA/GODIVA model, phonetic similarity between consecutively produced syllables should increase competition between the Speech Sound Map representations that are competing for selection as described above. The phonetic similarity effect has been established in numerous studies (Meyer & Gordon, 1985; Rogers & Storkel, 1998; Sevald & Dell, 1994; Yaniv et al., 1990) although not all research designs of the above-mentioned studies have made it possible to isolate the phonetic similarity effect to the stages of speech motor planning.
In sum, the primary aims of this project were to test competing hypotheses regarding the underlying speech motor planning impairment in AOS and to examine whether the motor planning impairment is specific to AOS or whether speakers with aphasia without AOS also exhibit signs of impairment at this processing level. With corresponding aims, this study seeks to extend the results of Mailend et al. (2019) with a different experimental paradigm to find converging evidence from divergent sources. Specifically, all targets in Mailend et al. were monosyllabic, and thus, did not tax the planning buffer beyond its hypothesized limit within the Reduced Buffer Capacity Hypothesis. The present study requires simultaneous planning of two monosyllabic words. In addition, our present task provides an opportunity to further specify the mechanism of impairment under the Program Retrieval Deficit Hypothesis.
Method
Participants
Study participants included eight speakers with AOS (six of whom also had aphasia according to the Western Aphasia Battery, WAB; Kertesz, 1982), ten speakers with aphasia without AOS, and 24 neurologically healthy control participants. These participants are the same as in Mailend et al. (2019), except that this study has one additional person in the aphasia group (APH 010). The groups did not differ in age (F=2.89, p>.05) or years of education (F=0.13, p>.05). All participants were native English speakers; five also spoke another language before the age of five based on self-report (three control, two with AOS). Ten additional participants were initially recruited for this study but were excluded from the analysis because they did not meet the inclusionary criteria (no aphasia according to WAB for two participants initially recruited to the aphasia group) or because they met the exclusionary criteria (history of dyslexia according to self-report for one control participant; significant reading difficulties for three speakers with AOS and/or aphasia; additional neurological or speech diagnosis for three speakers with AOS and/or aphasia). Additionally, reading difficulties were evaluated via a reading task where experimental stimuli were presented one word at a time. Three speakers with AOS and/or aphasia did not meet criterion of 80% accuracy or higher to be included. Data from one control speaker was discarded due to a recording error. All study procedures were approved by the Institutional Review Boards of the University of Arizona and Temple University. Participants signed a consent form before taking part in the study and were compensated for their time at the rate of $10 per hour.
AOS was initially diagnosed by the first author who holds a clinical Master’s degree in Speech Therapy. Following the example of Haley et al. (2012) the diagnosis was made on a three-point scale (1=no AOS, 2=possible AOS, and 3=AOS) with one modification that allowed 1.5 and 2.5 values to indicate “inclination towards no AOS” or “inclination towards AOS” judgments respectively. The following criteria served as the basis for AOS diagnosis: (1) slow speech rate, (2) sound distortions and distorted substitutions, and (3) impaired prosody, in particular syllable segmentation and equal stress across syllables (Ballard et al., 2015). These characteristics were assessed from several different speaking tasks, including conversation, picture description, repetition of words and phrases and diadochokinetic tasks administered in the context of standardized tests (Apraxia Battery for Adults, 2nd Edition; Dabul, 2000; Western Aphasia Battery; WAB; Kertesz, 1982). Considering the well-known challenges with agreement with clinical diagnosis of AOS (e.g., Galluzzi et al., 2015; Haley et al., 2012, 2019; Maas, Gutiérrez, & Ballard, 2014; Mailend & Maas, 2013), the diagnosis was subsequently confirmed by an ASHA-certified speech language pathologist who evaluated each participant independently from video and audio recordings, following the same procedure and criteria. Unanimous agreement between raters was achieved for 14 participants; for 2 participants the disagreement was within 0.5 point, and for 2 participants the disagreement was 1 and 1.5 points respectively. For these latter two speakers, a third ASHA-certified speech-language pathologist independently evaluated the speech samples based on the same criteria and procedures. The final group assignment was based on the average score of the two (or three) raters: participants with a score of 2 or more were assigned to the AOS group (Mailend & Maas, 2013), others to the aphasia group. The mean AOS rating for each participant is indicated in Table 1.
Table1.
Participant information
| AOS 001 |
AOS 002 |
AOS 003 |
AOS 004 |
AOS 005 |
AOS 006 |
AOS 007 |
AOS 008 |
APH 001 |
APH 002 |
APH 003 |
APH 004 |
APH 005 |
APH 006 |
APH 007 |
APH 008 |
APH 009 |
APH 010 |
Control (N=25) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 79 | 32 | 65 | 57 | 63 | 64 | 56 | 45 | 77 | 72 | 43 | 65 | 63 | 58 | 54 | 72 | 54 | 73 | 69(8) |
| Sex | M | F | M | M | F | M | M | F | M | M | M | M | M | M | M | M | M | F | 20F, 5M |
| Education | 16 | 23 | 14 | 17 | 14 | 12 | 13 | 18 | 25 | 16 | 14 | 18 | 16 | 14 | 14 | 19 | 17 | 12 | 18(3) |
| Hand | R | R | R | R | R | R | R | R | R | R | R | R | L/R | R | L/R | R | L/R | R | |
| Language | AE | AE | SE | AE | AE | AE | AE | AE | BE | BE | AE | AE | AE | AE | AE | AE | AE | AE | |
| TPO (y;m) | 5;6 | 0;10 | 7;1 | 1;4 | 11;0 | 9;6 | 5;8 | 0;6 | 1;0 | 10;9 | 6;8 | 5;7 | 1;1 | 7;0 | 3;11 | 1:9 | 3;7 | 3;4 | |
| Aphasia type1 | Anm | Broc | Cnd | wnl | Cnd | wnl | Anm | Anm | Anm | Cnd | Anm | Anm | Anm | Anm | Anm | Wer | Cnd | Anm | |
| WAB AQ1 | 81.1 | 61.0 | 82.3 | 96.8 | 73.6 | 96.0 | 92.1 | 91.4 | 80.4 | 66.8 | 91.6 | 85.9 | 92.3 | 82.5 | 89.1 | 59.8 | 74.2 | 85.0 | |
| Repetition1 | 7.4 | 4.6 | 6.4 | 5.5 | 9.6 | 9.6 | 9.9 | 7 | 8.4 | 5.8 | 8 | 8.6 | 10 | 7.5 | 7.8 | 2.3 | 3.5 | 7.6 | |
| ASRS2 | 31 | 30 | 19 | 13 | 18 | 20 | 9 | 22 | 2 | 5 | 4 | 4 | 0 | 6 | 1 | 4 | 8 | 1 | |
| AOS rating3 | 3 | 3 | 3 | 3 | 3 | 3 | 2.5 | 3 | 1 | 1.83 | 1 | 1.25 | 1 | 1.75 | 1 | 1 | 1.83 | 1 | |
| Oral apraxia4 | mild | none | mild | none | mild | none | mod | none | mild | mild | none | mod | none | none | none | none | none | mild | |
| Limb apraxia4 | none | none | none | none | none | none | none | none | none | none | none | sev | none | none | none | none | none | mild | |
| Dysarthria5 | mild | none | mod | none | mild/mod | none | none | none | none | none | none | none | none | none | none | none | none | none | |
| Picture Span FW6 | n/a | 3 | 2 | 5 | 3 | 4 | 2 | 3 | 3 | 2 | 3 | 4 | 3 | 2 | 3 | 2 | 2 | n/a | 4.9 (0.7) |
| Picture Span BW6 | n/a | 2 | 0 | 4 | 2 | 3.5 | 2 | 3 | 2.5 | 2.5 | 3.5 | 4 | 2.5 | 2 | 2 | 2 | 2 | n/a | 4.2 (0.9) |
AE – American English; SE – Spanish-English bilingual; BE – British English; Anm – Anomic; Broc – Broca’s; Cnd – Conduction, wnl – within normal limits; Wer – Wericke’s; n/a – not available
based on WAB (Kertesz, 1982)
sum of all ratings on the Revised Apraxia of Speech Rating Scale (Clark et al., 2016)
mean rating across three diagnosticians (1 = no AOS, 2 = possible AOS, 3 = AOS)
based on ABA-2 (Dabul, 2000) and clinical judgment
dysarthrias were diagnosed perceptually based on a motor speech exam (Duffy, 2005) and were all of the unilateral upper motor neuron type
forward and backward version of picture span (DeDe et al., 2014
To further characterize speech and AOS severity, the Revised Apraxia of Speech Rating Scale (Clark et al., 2016) was used to judge speech samples of all participants with AOS and/or aphasia. Judgments were based on reviewing video recordings of the same speech samples used for AOS diagnosis. Results from the rating scale are presented in Table 1. Note that the suggested cut-score for AOS on the scale (8 or more points; Strand et al., 2014) confirms the clinical diagnosis of AOS, except for APH 009 who scored just in the range of AOS (total score = 8) despite the clinical judgement that AOS was not present in this speaker.
Aphasia diagnosis was based on the WAB (Kertesz, 1982). All participants with AOS and/or aphasia were administered a Mini Mental State Examination (MMSE; Folstein et al., 1975) to assess their general cognitive state. For short-term memory and working memory assessment, the forward and backward version of picture span (DeDe et al., 2014) was used because the validity of this instrument has been demonstrated for people with aphasia. The two patient groups did not differ from one another on any of these measures (WAB AQ: t=0.70, p>.05; Repetition Section of WAB: t=0.48, p>.05; Picture Span Forward: t=1.19, p>.05; Picture Span Backward: t=−0.43, p>.05; MMSE: t=1.59, p>.05; Time Post Onset: t=0.68, p>.05). Finally, an oral mechanism exam (Duffy, 2005) was administered when dysarthria was suspected (Table 1).
Task and Procedure
This study used a simple reaction time paradigm, in which participants prepared two similar words in advance and produced these words after a go-signal. The task required participants to select the intended words for articulation in the specified order and to quickly switch between words with varying degrees of similarity based upon the condition. Since the materials were blocked by the specific target word pairs, each block started with the presentation of the target words in the upcoming block. Participants were asked to read the target words out loud before proceeding to the experimental trials. If the participant struggled with the correct production of the target words, he or she had the opportunity to practice saying the target words with the experimenter before the first trial in the block. The experimenter provided visual and articulatory cues as needed until the participant correctly produced the word pair.
The timeline of a trial (see Figure 1) was as follows: A red traffic light together with two written words appeared indicating to the participant to get ready to produce the words. Once the traffic light turned green (after a variable delay, varying randomly between 700 and 2400 ms) the participant was asked to immediately say the words that they had prepared. Following each response, the experimenter made an accuracy judgment which also initiated the next trial.
Figure 1.
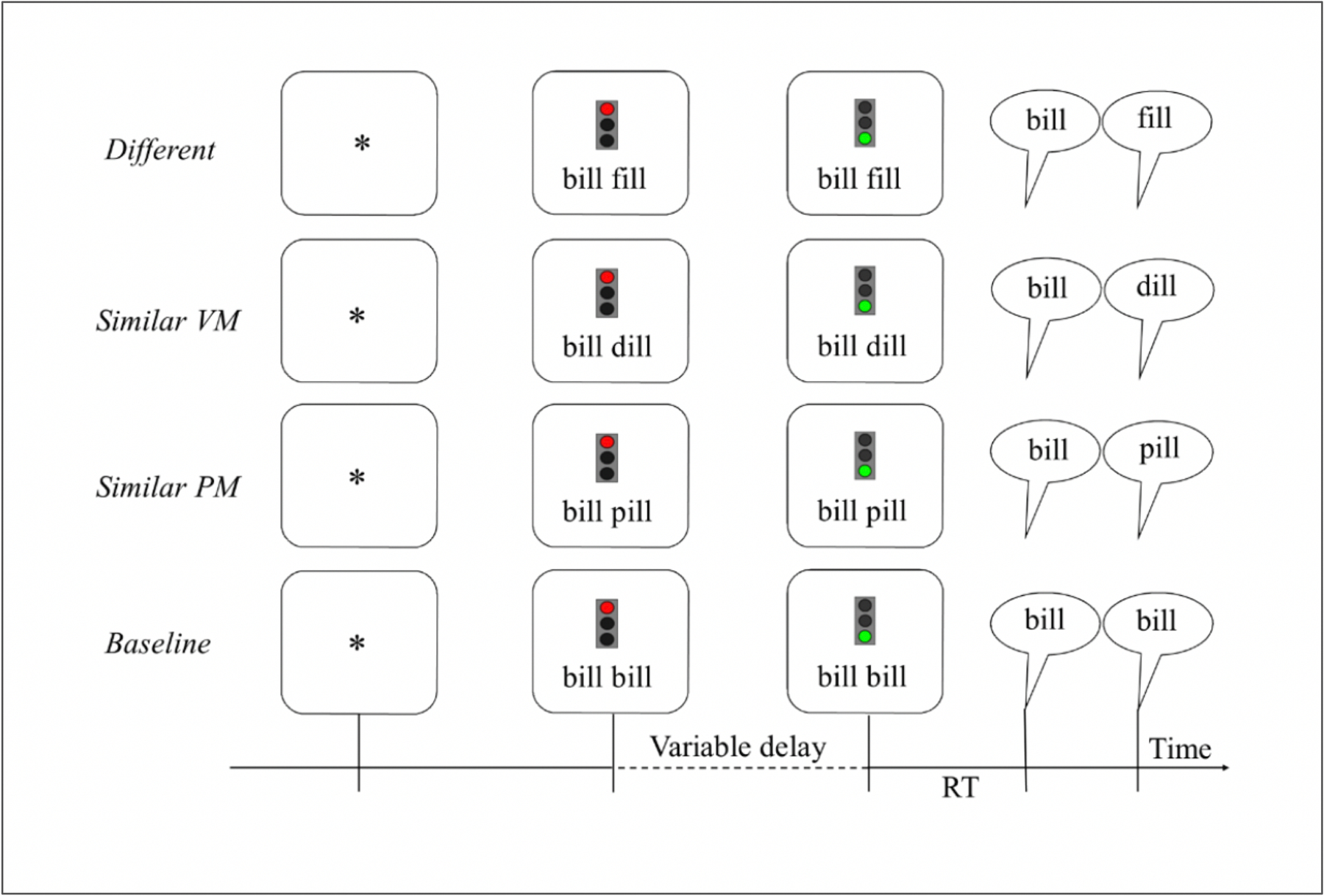
The timeline of the Experimental task with an example from all four conditions (VM=voicing and manner; PM=place and manner; RT=reaction time).
The word pairs were presented in blocked manner with seven repetitions of the same targets in each block. Blocked presentation was chosen for consistency with previous research (Deger & Ziegler, 2002; Rogers & Storkel, 1999) and to keep phonological planning load as small as possible. Incorrect trials were rerun in the end of each block for up to three times, to minimize data loss due to errors. Each block concluded with a summary slide that reported the average reaction time of the completed block to encourage maximally fast response times. Participants were offered an opportunity to take a break between blocks. The presentation order of the 24 blocks was randomized for each person. The experiment started with a practice block of five trials that were not included in the analysis.
This experiment was conducted as part of a larger study which included two experiments, one of them reported here, the other one elsewhere (Mailend et al., 2019). Both experiments used the same set of materials. Hence, to control for the familiarity with the materials, the order of experiments was counterbalanced between participants within each group. The experiments were conducted on separate days, 4–5 days apart on average. Speakers with AOS and/or aphasia also attended a third session for the evaluation of speech, language, and cognitive function.
Control speakers completed the experiment within 15–20 minutes. For people with AOS and/or aphasia the completion time was up to 60 minutes depending on the time they requested for breaks or for practicing the next word pair before the next block. With 24 blocks of seven to ten trials per block, there were 168 to 240 trials in total in this experiment and a minimum of 42 trials presented in each condition.
Materials
The materials consisted of 24 words arranged into 24 word pairs with identical rimes (see Table 2). All words were monosyllabic with a CVC syllable structure. Speech motor planning processes were experimentally manipulated by varying the phonetic similarity between the word onsets. More specifically, the critical manipulation was in the featural overlap of the onset consonant, which determined the four conditions of the experiment. In the Identical condition, the word pair was formed by presenting the same word twice (e.g., bill bill); in the Similar Place-Manner condition, the onsets shared all phonetic features except for the feature of voicing (e.g., bill pill); in the Similar Voicing-Manner condition, the onsets shared all but the place of articulation feature (e.g., bill dill). Finally, in the Different condition, the onsets did not share any phonetic features (e.g., bill fill). The three conditions where the word pair consisted of two different words are collectively called the switch conditions. Note that the first sound of each pair was a stop consonant in all conditions and that the first word of the pair was the same across all four conditions. Both levels of voicing with all possible places of articulation for the stops in the English language were represented (see Table 2).
Table 2.
Materials for each condition, where the similarity of the onset condition is manipulated to create sounds that are identical, similar in place and manner (PM) or voicing and manner (VM), or different in all three features of place, manner, and voicing.
| Identical | Similar PM | Similar VM | Different |
|---|---|---|---|
| teal teal | teal deal | teal peal | teal meal |
| pail pail | pail bail | pail tail | pail rail |
| cane cane | cane gain | cane pane | cane rain |
| bill bill | bill pill | bill dill | bill fill |
| dame dame | dame tame | dame game | dame fame |
| gall gall | gall call | gall ball | gall fall |
The second word, which differed between conditions, was controlled for relevant psycholinguistic variables, such as syllable frequency, orthographic neighborhood density, phonologic neighborhood density, average frequency of the neighbors, mean log bigram frequency, and mean log biphone frequency (Table 3).
Table 3.
Summary of the psycholinguistic properties of the second word in each condition (Identical, Similar Place-Manner (PM), Similar Voicing-Manner (VM), and Different; the first word is identical across all conditions.
| Identical |
Similar PM |
Similar VM |
Different |
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Syll freqa | 1.23 | 0.75 | 1.59 | 0.70 | 1.85 | 0.80 | 1.84 | 0.44 |
| CELEX logb | 0.86 | 0.65 | 1.54 | 0.67 | 1.22 | 0.81 | 1.61 | 0.36 |
| SLBFc | 9.69 | 0.31 | 9.73 | 0.47 | 9.57 | 0.68 | 9.73 | 0.66 |
| MLBFd | 3.23 | 0.10 | 3.24 | 0.16 | 3.19 | 0.23 | 3.24 | 0.22 |
| SLBPFe | 5.41 | 0.77 | 5.56 | 0.52 | 5.69 | 0.47 | 5.86 | 0.58 |
| MLBPFf | 2.70 | 0.39 | 2.78 | 0.26 | 2.84 | 0.24 | 2.93 | 0.29 |
| Ng | 15.66 | 1.97 | 12.66 | 2.73 | 13.66 | 3.61 | 12.33 | 1.63 |
| PNh | 27.66 | 5.85 | 26.50 | 7.23 | 27.00 | 4.81 | 29.33 | 5.20 |
| Freq Ni | 87.73 | 49.36 | 120.00 | 134.76 | 78.76 | 72.66 | 103.61 | 74.10 |
Log Syllable frequency regardless of word position and
log total CELEX word frequency (Baayen, Piepenbrock, & Gulikers, 1995)
summed log bigram frequency and
mean log bigram frequency
summed log biphone frequency and
mean log biphone frequency
orthographic neighborhood size
phonological neighborhood
frequency of orthographic neighbors (Davis, 2005)
Equipment
The experiment was programmed in E-Prime Software (Version 2; Psychology Software Tools, Inc., Sharpsburg, PA) and run on a Dell Inspiron 530 computer with a 21.5 inch LCD screen. The experimenter made on-line accuracy judgments and advanced the experiment via a button box (Serial Response Box; Psychology Software Tools, Inc.). An M-Audio Aries condenser microphone (M-Audio, Cumberland, RI) placed on a desk stand approximately 30 cm from the participants’ mouth recorded the speech responses on one channel at 44.1 kHz onto a Marantz CDR-420 CD recorder (Marantz America LLC, Mahwah, NJ). Beeps associated with the onset of the go-signal were recorded directly onto the other channel. These beeps were only used for reaction time measurement; they were not audible to the participant.
Analysis
The primary dependent variables for this study was reaction time (the duration between the go-signal and speech onset) based on correct responses only. Errors were excluded and analyzed separately, except for responses with a single speech sound distortion where the target sound remained clearly identifiable (65 errors; 1% of trials). The inclusion of these trials was deemed justified because single distortion errors likely involve speech execution difficulties and thus reflect activating and selecting the intended motor program. This analysis is substantiated by the fact that 63% of single distortion errors were committed by the three participants who also had dysarthria. The remaining 24 distortions were distributed equally between the rest of the AOS group (11 distortions) and Aphasia group (13 distortions).
Reaction times.
As mentioned above, the timing measures were obtained from correct responses (with the exception for including single distortions). Only the onset of the first word was considered because the predictions for the onset of the second word are not clear in light of the tested hypotheses. After the removal of errors, one person (AOS 003) had only two trials left per Different condition and therefore his data was not included in the reaction time analysis. Next, two customized MATLAB scripts (MATLAB, 2016) were created. The first script was used to splice the trials in each experiment into separate analysis windows of pre-specified lengths (see below) starting at the midpoint of the 60-ms beep presented simultaneously with the go-signal. The second script was used to mark the onset consonant of the first word. Reaction time (RT) was defined as the duration from the beginning of the analysis window to the release burst of the onset consonant of the first word. Release bursts were identified by simultaneous inspection of the waveform (sudden increase in amplitude) and the spectrogram (sudden increase in amplitude that spans the visible frequencies of 0–6000 Hz and is continuous with the signal for the rest of the word).
Some speakers needed more time to initiate their response. The majority of these speakers were in the AOS group. Therefore, the duration of the analysis window was set according to the diagnostic group: 1200 ms for the control group, 1500 ms for the aphasia group, and 2500 ms for the AOS group. Additionally, 64 trials were reanalyzed with the analysis window between 4000 and 5500 ms because the response, or part of the response, occurred outside of the initial analysis window. To establish the reliability of the RT measures obtained with different analysis window sizes, the data from 4 participants (10% of all data) initially analyzed with 1200 ms or 1500 ms were remeasured with a 2500 ms window. The reliability was considered excellent (Hallgren, 2012): the ICC (1,1) = .99 (Shrout & Fleiss, 1979), mean difference between measures was 1.15 ms.
Errors.
Three different types of errors were identified: (a) speech errors, (b) timing errors, and (c) other errors. Only the first type, speech errors, were included in the error analysis. The class of speech errors included all verbal responses where the participant attempted to say the target words but said something different instead. Among these were responses with distortions of multiple segments, distorted substitutions, substitutions, deletions, omissions, restarts, self-corrections, disfluencies, saying a different word from the experiment, mixed errors (i.e., semantic substitutions that were phonologically similar to the target), neologisms, etc. Timing errors included responses that were produced prior to the go-signal (AOS group: 4.32%; Aphasia group: 5.12%; Control group: 1.66%). Other errors included everything else from inattention, laughter, background noise and trials that were discarded due to technical failures (AOS group: 2.73%; Aphasia group: 1.95%; Control group: 1.92%). Inter-rater reliability of error coding was established by a second rater who re-evaluated all trials of three randomly selected blocks for each participant with AOS and/or aphasia. The agreement between raters was 98% for presence of error and 93 – 99% for type of error.
Statistical analysis.
Statistical analysis was performed in R, version 3.3.2 “Sincere Pumpkin Patch” (R Core Team, 2016) with packages lme4, version 1.1–12 (Bates et al., 2016) and lmerTest, version 2.0–33 (Kuznetsova et al., 2016). Errors were analyzed with Generalized Linear Mixed Models for binomially distributed data (Jaeger, 2008); RT were analyzed with Linear Mixed Effects Models for continuous normally distributed data (Baayen et al., 2008). Mixed methods were preferred over the traditional by-subject and by-items analysis with ANOVA because (a) this method allows by-subject and by-items random effects to be modeled simultaneously within a single model and (b) this analysis is based on trial-level data and is therefore more robust to unequal group sizes and missing data points (Baayen et al., 2008). Finally, individual data were analyzed with Bayesian Standardized Difference Test (Crawford et al., 2010).
Predictions.
The Reduced Buffer Capacity Hypothesis predicts that there is no Condition effect in the AOS group in reaction time measures because the first word (and by hypothesis, the only word that can be loaded to the buffer for correct responses) is identical across conditions.
The Program Retrieval Deficit Hypothesis predicts that speakers with AOS, unlike other groups, will show longer reaction times in conditions that involve the activation of two different motor programs rather than two copies of the same program. Longer reaction times are predicted because the different programs compete for selection of the first word. This hypothesis also predicts larger error rates in Similar and Different conditions compared to the Identical condition.
Results
Group-level analysis
RT.
The average reaction time values by Group and Condition are presented in Figure 2. Speakers with AOS were slower overall, but more importantly, the reaction times appeared longer in the switch conditions compared to the Identical condition in the AOS group but were within the same range across conditions for the other groups. These differences were tested statistically with a linear mixed effects model that included the main predictor variables (Group and Condition) and their interaction as fixed effects. Furthermore, to control for the effect of the variable delay between the target presentation and the go-signal as well as possible fatigue or practice effects, Variable Delay values and Trial Number were also included as fixed effects. The intercepts for subjects and items were included as random effects, random slopes for Subjects were added only if their inclusion significantly improved model fit. The formula for the final model was the following: Reaction Time ~ Condition * Group + Variable Delay + Trial Number (1 + Condition | Subject) + (1|Target). The model was first run with the Control group and the Identical condition as reference categories.
Figure 2.
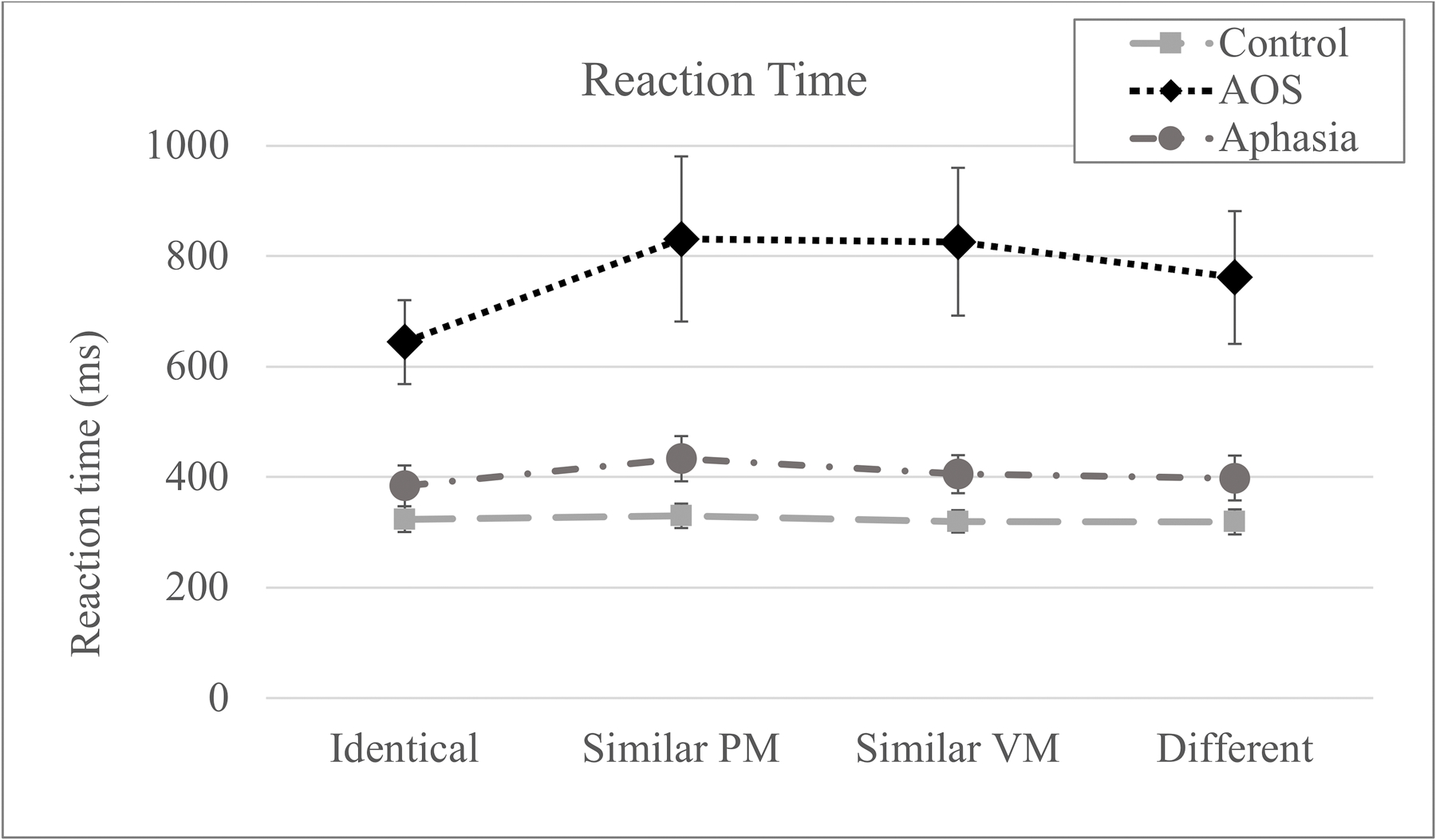
Mean reaction time by group and condition. Error bars represent standard error (PM = place and manner; VM = voicing and manner).
The linear mixed effects model output suggested that the estimated correlations for the random slopes were close to 1 in the non-identical conditions leading to a near singular model fit. Thus, all non-identical conditions (Similar VM, Similar PM, and Different) were collapsed such that the Condition effect reflected the contrast between the Identical condition and all other conditions combined, which is the primary contrast of theoretical interest in light of the hypotheses considered here. Results were the following1. First, the AOS group had longer RT compared to controls (t = 6.34, p < .001). Second, significant effects of Variable Delay and Trial Number suggest that reaction times were longer for trials with longer delays (t = −15.31, p < .001) and for trials that occurred later in the experiment (t = −8.03, p < .001). Finally, the model revealed a significant interaction between Group and Condition indicating a larger difference between all switch conditions and the Identical condition for the AOS group compared to the Control group (t = 3.44, p = .001). None of the other effects were significant.
Differences between the AOS group and the Aphasia group were tested by rerunning the model with the AOS group as the reference category. In addition to the effects reported above, the model identified a significant difference in reaction time between the AOS and Aphasia groups (t = −4.53, p < .001). In addition, there was a significant Condition effect in the AOS group, namely all switch conditions had longer reaction times compared to the Identical condition (t = 3.78, p < .001); this effect, in turn, was greater in the AOS group compared to the Aphasia group as identified by the significant interaction term (t = −2.42, p = .021). Rerunning the model with Aphasia group as the reference category did not yield a significant Condition effect.
Speech errors.
The error rate for all groups and conditions is presented in Figure 3. Speech errors were analyzed statistically with Generalized Linear Mixed Models (Jaeger, 2008). Group effects (Error~Group+(1|Subject)+(1|Target), family=binomial) and Condition effects for each patient group were tested separately (Error~Condition+(1|Subject)+(1|Target), family=binomial) because the model including group-by-condition interaction did not converge and neither did the model testing for condition effects for the Control group. The nonconvergence was likely due to the fact that the overall probability of an error on a single trial was very close to zero in the Control group in both cases. By subject and by-target intercepts were included as random effects.
Figure 3.
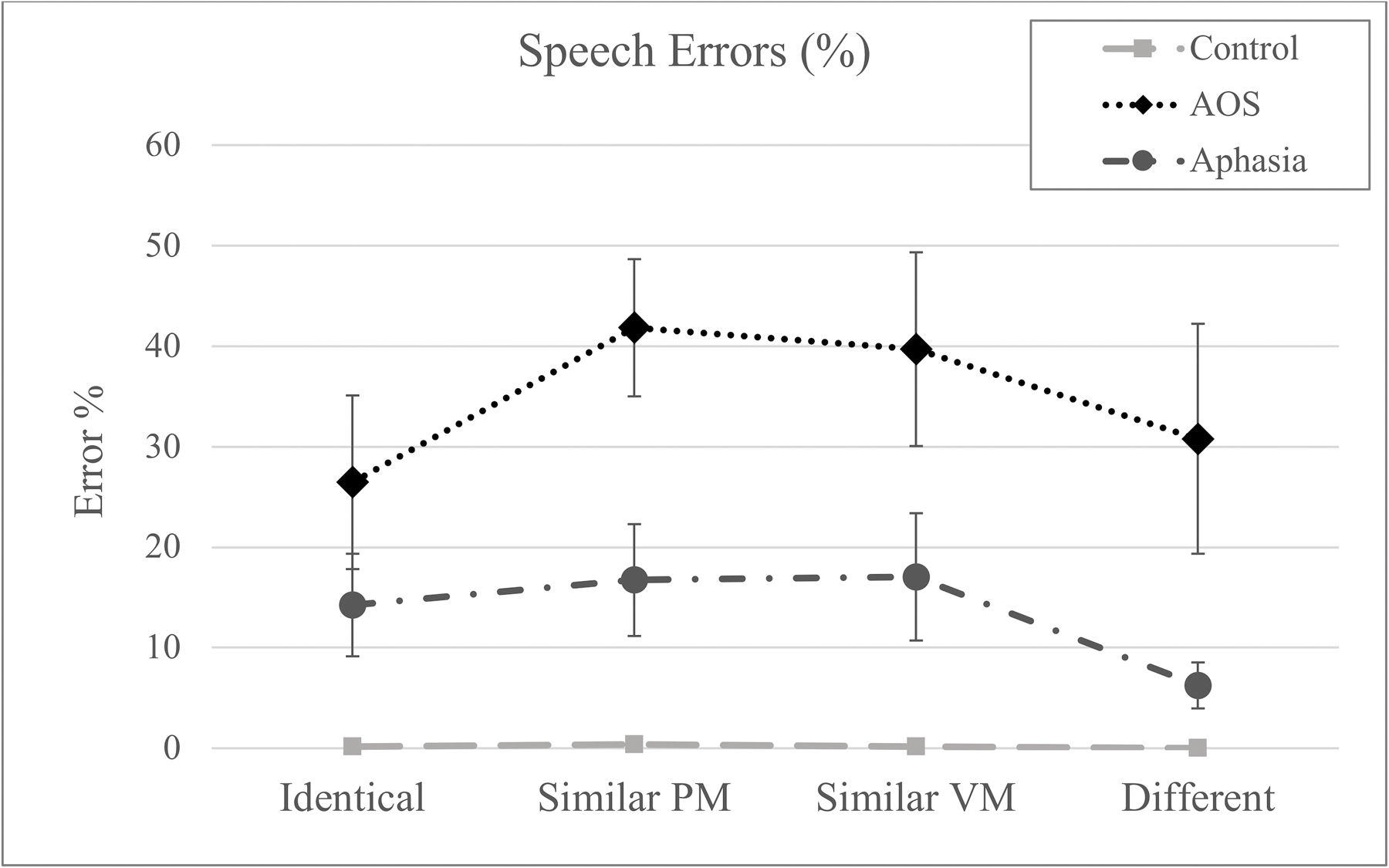
The proportion of trials with speech errors across conditions. Error bars represent the standard error. (PM = place and manner; VM = voicing and manner)
The error rate was significantly larger for both patient groups compared to the controls (AOS: z=9.13, p<.001; Aphasia: z=7.04, p<.001) and significantly smaller in the Aphasia group compared to the AOS group (z=−2.76, p=.006). As to the condition effects in the AOS group, the error rate was significantly higher in the Similar Place-Manner condition compared to the Identical condition (z=2.38, p=.018); Similar Voicing-Manner revealed a trend for statistical significance in the same direction (z=1.95, p=.052). Rerunning the model with the Different condition as the reference category did not reveal any significant effects. A very different error pattern was observed for the Aphasia group. While neither of the Similar conditions differed significantly from the Identical condition, this group made fewer errors in the Different condition compared to all other conditions (Identical (z=2.14, p=.032; Similar Place-Manner: z=2.86, p=.004; Similar Voicing-Manner: z=2.71, p=.007).
Individual analysis
As a next step, individual analyses were conducted to test whether data from individual patients follows the group pattern. Differences between two conditions in an individual patient were compared to the same difference in the control group with the Bayesian Standardized Difference Test (Crawford et al., 2010). The individual reaction time patterns are presented in Table 4. All participants in the AOS group showed significantly greater differences in at least one switch condition compared to the Identical condition, except for participant AOS 007 who exhibited a significant effect but in the opposite direction: his reaction times were shorter in switch conditions compared to the Identical condition. In contrast to the group results however, four speakers with APH (APH 003, APH 004, APH 009, and APH 010) also showed longer reaction times in at least one of the switch conditions compared to the identical condition.
Table 4.
Reaction time means (in milliseconds) for individual patients in all experimental conditions and differences between conditions. Condition names are abbreviated: Identical (Ident), Similar Place-Manner (Sim PM), Similar Voicing-Manner (Sim VM), Different (Diff). Significant differences in the predicted direction (p<.05 in Bayesian Standardized Difference Test) are indicated in bold print, significant differences in the opposite direction are bolded and italicized.
| RT Means |
Differences in RT Means |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Partic. ID | Ident | Sim PM | Sim VM | Diff | Sim PM - Ident | Sim VM - Ident | Diff - Ident | Sim PM - Diff | SimVM - Diff |
| AOS 001 | 1072 | 1098 | 1141 | 1005 | 26 | 69 | −67 | 93 | 136 |
| AOS 002 | 695 | 1615 | 1487 | 1380 | 920 | 792 | 685 | 235 | 107 |
| AOS 004 | 414 | 450 | 484 | 433 | 36 | 70 | 19 | 16 | 51 |
| AOS 005 | 658 | 833 | 765 | 727 | 175 | 107 | 69 | 106 | 38 |
| AOS 006 | 610 | 679 | 665 | 695 | 69 | 55 | 86 | −17 | −31 |
| AOS 007 | 454 | 366 | 406 | 395 | −88 | −48 | −59 | −29 | 10 |
| AOS 008 | 609 | 779 | 837 | 696 | 171 | 229 | 88 | 83 | 141 |
| APH 001 | 381 | 355 | 337 | 353 | −26 | −44 | −28 | 2 | −16 |
| APH 002 | 573 | 630 | 530 | 580 | 57 | −43 | 7 | 50 | −50 |
| APH 003 | 222 | 297 | 263 | 242 | 75 | 41 | 20 | 55 | 21 |
| APH 004 | 367 | 487 | 499 | 440 | 121 | 132 | 73 | 48 | 59 |
| APH 005 | 293 | 278 | 291 | 305 | −15 | −2 | 12 | −27 | −14 |
| APH 006 | 295 | 346 | 331 | 275 | 51 | 36 | −20 | 71 | 56 |
| APH 007 | 373 | 425 | 400 | 401 | 52 | 27 | 28 | 24 | −1 |
| APH 008 | 384 | 418 | 402 | 367 | 34 | 18 | −17 | 51 | 35 |
| APH 009 | 593 | 662 | 602 | 646 | 69 | 9 | 52 | 16 | −44 |
| APH 010 | 361 | 437 | 399 | 376 | 76 | 38 | 15 | 61 | 23 |
Discussion
This study aimed to replicate and extend the findings in Mailend et al. (2019) in two ways: (a) to further specify the underlying speech motor planning impairment in AOS, and (b) to determine whether the observed data pattern is specific to AOS. The hypotheses were framed within a model of typical speech production and tested in a reaction time paradigm that has been successfully used to study speech production in typical speakers (e.g., Mooshammer et al., 2009). This continuity in methodology with the literature on typical speech production provides a common ground for interpreting the observed effects in speakers with apraxia of speech and/or aphasia (Maas & Mailend, 2012).
The experiment used a delayed reaction time paradigm where participants are expected to prepare and maintain the utterance until prompted to speak (Laganaro & Alario, 2006; Sternberg et al., 1978). Under such conditions, speech onset latencies are not expected to vary based on the specific phonemic make-up of the word as long as the utterance length (in terms of the number of chunks represented by separate motor programs) is held constant (Sternberg et al., 1978; Klapp, 2003). In terms of the DIVA/GODIVA model, typical speakers are able to simultaneously activate programs corresponding to both words within the Speech Sound Map with a well-defined activation gradient between the programs, which assures the smooth sequential selection of programs once speech is initiated. This is precisely the pattern that was observed in the control speakers who showed no reaction time differences between the condition that required the same word to be spoken twice (the Identical condition) and the conditions where two different words had to be planned, maintained, and produced (the switch conditions).
Results from the Aphasia group resemble the control group: no RT differences between conditions. The similarity in the RT measure between the control group and the aphasia group replicates previous findings (Deger & Ziegler, 2002; Rogers & Storkel, 1999) and provides further evidence for the interpretation that the task in this experiment taps into the speech motor planning operations that are presumed to be intact in speakers with aphasia without AOS.
The findings in the AOS group differed considerably from the control group and the aphasia group. The AOS group exhibited a significant Condition effect for reaction time. Namely, speakers with AOS had longer reaction times in the switch conditions compared to the Identical condition. This finding is consistent with, and predicted by, the Program Retrieval Deficit Hypothesis, which suggests that retrieving the intended motor program is more difficult in the context where other motor programs are simultaneously activated. These findings add to an emerging picture of the underlying nature of AOS. Converging evidence from three studies with diverging experimental paradigms points to a deficit in retrieving speech motor programs (Mailend & Maas, 2013; Mailend et al., 2019). In addition to replication within a different paradigm, these data extend the findings of Mailend et al. (2019) by further specifying the mechanism of the retrieval deficit. In this paradigm, participants had the opportunity to prepare the entire response in each condition ahead of the go-signal and they indicated readiness to respond before the go-signal. In this regard, the condition effect observed in the AOS group is not easily explained by an overall slowness of activation accumulation and a difficulty with reaching the selection threshold; instead it supports the interpretation that selecting the intended motor program over the competing ones is difficult for people with AOS. However, most people with AOS also exhibited longer RTs in the Identical condition which is consistent with the idea of slower activation accrual overall. Therefore, it is possible that both reaching the necessary activation levels for selection as well as resolving the competition between activated representations is difficult for some but not all speakers with AOS. It is also worth noting that some participants with AOS had a concomitant diagnosis of dysarthria. However, rerunning the analyses without these individuals did not change the observed pattern, suggesting that the results were not driven by those individuals with impairments also at the level of speech execution.
The present findings do not support the Reduced Buffer Capacity Hypothesis in reaction time or the error analyses. If people with AOS were only able to plan the first syllable by the time of the go-signal, then there should be no differences in RT between conditions because the first word was identical across conditions (see also Deger & Ziegler [2002] for discussion and similar findings). It is worth noting that these findings are also incompatible with a more relaxed version of a Reduced Buffer Capacity Hypothesis than what was originally proposed by Rogers and Storkel (1999). If the buffer is limited to two or more motor programs, then all utterances within this experiment would have been within the capacity of the buffer, leaving the observed differences between conditions unexplained. Other post-hoc explanations compatible with the observed findings are certainly possible, particularly as our understanding of the speech motor planning operations expands. The conception and subsequent experimental tests of such hypotheses would be an important future direction for advancing understanding of the speech motor planning impairment in AOS.
In terms of error rates, it is interesting to note the parallel findings with the RT patterns across conditions, easily appreciated by comparing Figures 2 and 3. The group with the longest reaction times (AOS) also demonstrated the highest overall error rate, suggesting that the task was taxing for the speech motor planning operations although the specific mechanism is not elucidated by the overall increased error rate. More relevant in terms of the goals of this study is the finding that the AOS group showed higher error rates in the Similar conditions compared to the Identical condition while other groups did not demonstrate any condition effects in the error rates. This provides further evidence for the idea that retrieving the intended motor program is more challenging for people with AOS in the context where multiple partially matching motor programs are activated simultaneously. In addition to corroborating the story in the temporal measure, these error patterns also rule out the possibility that the increased reaction times in switch conditions for the AOS group reflect a speed-accuracy tradeoff rather than increased competition from the simultaneous activation of multiple speech motor programs. Beyond the effect of condition, the significantly greater overall error rate in AOS group raises the possibility that a factor other than speech motor planning impairment was driving this result. This possibility is mitigated by the fact that the groups did not differ in general measures like working memory deficit, aphasia severity, and aphasic speech production difficulty, in particular. For example, the fact that working memory does not explain the pattern indicates that the deficit is not one of a general verbal working memory buffer but rather more specific to speech motor planning. While the Aphasia group serves as a control group for general effects of brain damage and concomitant aphasia, it can be argued that the most relevant comparison for associating the observed effects with a speech motor planning impairment involves differentiation from phonological planning deficits. In that light it is important to note that speech errors involving substitution, addition, or omission of a single phoneme in the experimental responses were observed in both patient groups at comparable rates (t=1.67; p>.05).
The group-level reaction time patterns were largely reflected in the individual data of the speakers in the clinical groups. All speakers in the AOS group had a significantly larger difference between the Identical condition and at least one switch condition compared to the same difference in the Control group, except for one individual (AOS 007) who showed the opposite pattern: his reaction times were significantly shorter in the Similar conditions compared to the Identical condition. It is hard to pinpoint the reasons for the different reaction time pattern for AOS 007 but one possibility is that the task used in this study did not capture his actual reaction times as reliably as for other participants because AOS 007 had a high rate of too-early responses (10% of trials, more than twice the group average of 4.3%). These responses suggest that the participant did not interact with the go-signal reliably, and even though responses given before the go-signal or up to 150 ms after it were discarded, it is possible that many of his reaction times reflect a reasonably good guess about the upcoming go-signal which may make these reaction times hard to interpret.
As expected, most speakers in the aphasia group did not differ from the control group in cross-condition comparisons. However, four speakers resembled the AOS group and showed a significantly greater difference between the Identical condition and at least one of the switch conditions, with longer reaction time in the switch condition. Although not detected at the group level, this finding suggests that some speakers with aphasia may have also had deficits to the speech motor planning operations even though this was not evident in the clinical presentation of their disorder. Alternatively, it is possible that speech motor planning difficulties detected in these speakers were secondary to the phonological impairment. Since the phonological stage serves as input to the speech motor planning stage, it is likely that unstable or poorly specified representations at the phonological level would affect speech motor planning to a degree even though the experimental design aimed to keep the phonological planning demands to the minimum. Finally, these individual data, that dissociate from the group pattern, stress the importance of reporting individual analyses. Divergent cases may help identify potential discrepancies between clinical diagnosis and mechanistic explanations or invite consideration of possible subtypes within a clinical group.
Clinical Implications
RT methods, like the one employed in this study, may themselves not be practical for clinical use because they are time-consuming to administer and analyze (at least as implemented here). Furthermore, in addition to being sensitive to the speech planning operations of interest, they are also sensitive to a variety of other variables, such as comorbid speech or neurological diagnosis, inhibition problems and attention difficulties, severity of the communication impairment, etc. Nevertheless, the cumulative evidence in support of the Program Retrieval Deficit Hypothesis invites the consideration of how this knowledge could be used in the development of diagnostic tools and treatment approaches in the clinical setting.
One consideration suggested by the results of this study is the idea of a process-oriented approach to the diagnosis and remediation of communication disorders (Terband et al., 2019) rather than a syndrome-based approach. At present, the prevalent method for selecting a treatment approach for AOS is largely dictated by the diagnosis at the syndrome level (e.g., AOS versus conduction aphasia etc.), but this approach overlooks the possibility that the syndromes identified at the gestalt level may tell us more about which impairments of specific speech production operations commonly co-occur in stroke patients rather than which operations are impaired in a specific individual. It stands to reason that the latter may provide better treatment outcomes if the impaired operations can be identified and targeted specifically in a treatment program. Participant APH 009 illustrates the point. In the current experiment, and in Mailend et al. (2019), this participant’s data appears to pattern with the AOS group. Specifically, his reaction times indicated difficulty with planning and switching between phonetically similar words even though the clinical diagnosis was inconsistent with AOS, primarily due to fluent speech within a normal rate and lack of sound distortions. One possibility is that this participant had a program retrieval deficit, but it occurred in the context of different additional speech production impairments in comparison to what is typically observed in speakers with AOS. Alternatively, this finding may suggest that the criteria for AOS diagnosis may need to be revised (see also Galluzzi et al., 2015; Haley et al., 2019 for converging conclusions based on error analysis).
Finally, most AOS treatment approaches fall under the category of articulatory-kinematic treatments where the focus appears to be on rebuilding damaged speech motor programs via guided and repeated practice (Ballard et al., 2015; Wambaugh et al., 2006). The Program Retrieval Deficit Hypothesis appears largely unexplored in the treatment literature but it is easy to imagine how the interference from similar motor programs could be manipulated in a treatment context. For example, if the core problem in AOS is the program retrieval deficit and a difficulty with resolving the competition between simultaneously activated motor programs, then the most effective treatments may occur in the context of phonetically similar words that specifically manipulate the conflict at the level of speech motor planning. Needless to say, this suggestion is merely a speculation at this point but the results of this study together with the results of Mailend and Maas (2013) and Mailend et al. (2019) suggest that it is worth-while investigating further.
Conclusion
The findings of the current study provide convergent evidence in support of the Program Retrieval Deficit Hypothesis from group-level and individual-level chronometric data and from the error patterns. Furthermore, this study reports findings from the third experiment in the series of experiments that have systematically tested the Program Retrieval Deficit Hypothesis against other detailed hypotheses of the underlying mechanism of AOS (e.g., the Damaged Program Hypothesis in Mailend & Maas (2013); the Reduced Buffer Capacity Hypothesis in Mailend et al. (2019). All three studies have provided consistent evidence in support of the Program Retrieval Deficit Hypothesis. In this vein, it is interesting to note that a similar mechanism – difficulty resolving competition between different response patterns – has also been observed in patients with ideomotor limb apraxia (Jax & Buxbaum, 2013). Observations of a similar mechanism in a different domain of motor control strengthens the interpretation that apraxia is a disorder of high-level organization of motor control where the mechanism of selecting the intended motor program over its competitors is impaired. Finally, the results of this study strongly suggest that carefully designed reaction time experiments are useful in the study of AOS and aphasia: most speakers with these communication impairments can complete the task and the results are interpretable and informative in terms of the processes they are designed to capture.
Acknowledgements
Research reported in this publication was supported by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number F31DC014375. We would like to thank Fabiane Hirsch, Lisa Jackson, Kindle Rising, Chelsea Bayley, Janet Hawley, and Andrew DeMarco, Cailey Busker, and Patrice Moritz. Finally, we thank our participants for offering their time and cooperation in support of this research.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
Two participants with AOS (AOS 001 and AOS 005) also had a diagnosis of dysarthria (AOS 003 had to be excluded due to lack of correct responses as explained above). To evaluate dysarthria as a possible confounding factor, the linear mixed effects models were also run without these two people. Results remained unchanged in terms of the reported significant effects.
References
- Baayen RH, Davidson DJ, & Bates DM (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59(4), 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Baayen RH, Piepenbrock R, & Gulikers L (1995). The Celex lexical database (Version 2)[CD-ROM]. Philadelphia: University of Pennsylvania, Linguistic Data Consortium.
- Bailey DJ, Eatchel K, & Wambaugh J (2015). Sound production treatment: Synthesis and quantification of outcomes. American Journal of Speech-Language Pathology, 24(4), S798–S814. 10.1044/2015_AJSLP-14-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard KJ, Wambaugh JL, Duffy JR, Layfield C, Maas E, Mauszycki S, & McNeil MR (2015). Treatment for acquired apraxia of speech: A systematic review of intervention research between 2004 and 2012. American Journal of Speech-Language Pathology, 24(2), 316–337. 10.1044/2015_AJSLP-14-0118 [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Azizi L, Duffy JR, McNeil MR, Halaki M, O’Dwyer N, … & Robin DA (2016). A predictive model for diagnosing stroke-related apraxia of speech. Neuropsychologia, 81, 129–139. [DOI] [PubMed] [Google Scholar]
- Bates DM, Maechler M, Bolker B, & Walker S (2016). lme4: Linear Mixed-Effects Models using “Eigen” and S4 (Version R package version 1.1–12). Retrieved from https://github.com/lme4/lme4/http://lme4.r-forge.r-project.org/
- Bohland JW, Bullock D, & Guenther FH (2010). Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience, 22(7), 1504–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, & Guenther FH (2006). An fMRI investigation of syllable sequence production. Neuroimage, 32(2), 821–841. [DOI] [PubMed] [Google Scholar]
- Civier O, Bullock D, Max L, & Guenther FH (2013). Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain and Language, 126(3), 263–278. 10.1016/j.bandl.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, Duffy JR, Strand EA, & Josephs KA (2016, March). Revision of the Apraxia of Speech Rating Scale. Presented at the Motor Speech Conference. [Google Scholar]
- Crawford JR, Garthwaite PH, & Porter S (2010). Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology, 27(3), 245–260. 10.1080/02643294.2010.513967 [DOI] [PubMed] [Google Scholar]
- Dabul B (2000). Apraxia Battery for Adults: Examiner’s Manual. Austin,TX: Pro-ed. [Google Scholar]
- Davis CJ (2005). N-Watch: A program for deriving neighborhood size and other psycholinguistic statistics. Behavior Research Methods, 37(1), 65–70. 10.3758/BF03206399 [DOI] [PubMed] [Google Scholar]
- DeDe G, Ricca M, Knilans J, & Trubl B (2014). Construct validity and reliability of working memory tasks for people with aphasia. Aphasiology, 28(6), 692–712. 10.1080/02687038.2014.895973 [DOI] [Google Scholar]
- Deger K, & Ziegler W (2002). Speech motor programming in apraxia of speech. Journal of Phonetics, 30(3), 321–335. [Google Scholar]
- Dell GS (1986). A spreading-activation theory of retrieval in sentence production. Psychological Review, 93(3), 283. [PubMed] [Google Scholar]
- Duffy JR (2005). Motor Speech Disorders: Substrates Differential Diagnosis, and Management. St Louis, MO: Elsevier Mosby. [Google Scholar]
- Folstein M, Folstein SE, & McHugh PR (1975). “Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Galluzzi C, Bureca I, Guariglia C, & Romani C (2015). Phonological simplifications, apraxia of speech and the interaction between phonological and phonetic processing. Neuropsychologia, 71, 64–83. [DOI] [PubMed] [Google Scholar]
- Guenther FH (2016). Neural Control of Speech. Gambrifge, MA: Mit Press. [Google Scholar]
- Guenther FH, Ghosh SS, & Tourville JA (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96(3), 280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley KL, Jacks A, de Riesthal M, Abou-Khalil R, & Roth HL (2012). Toward a quantitative basis for assessment and diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55(5), S1502–S1517. [DOI] [PubMed] [Google Scholar]
- Haley KL, Ohde RN, & Wertz RT (2001). Vowel quality in aphasia and apraxia of speech: Phonetic transcription and formant analyses. Aphasiology, 15(12), 1107–1123. [Google Scholar]
- Haley KL, Smith M, & Wambaugh JL (2019). Sound distortion errors in aphasia with apraxia of speech. American Journal of Speech-Language-Pathology, 28(1), 121–135. [DOI] [PubMed] [Google Scholar]
- Haley KL, Wertz RT, & Ohde RN (1998). Single word intelligibility in aphasia and apraxia of speech. Aphasiology, 12(7–8), 715–730. [Google Scholar]
- Hallgren KA (2012). Computing inter-rater reliability for observational data: an overview and tutorial. Tutorials in quantitative methods for psychology, 8(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G (2012). Computational neuroanatomy of speech production. Nature Reviews Neuroscience, 13(2), 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger TF (2008). Categorical data analysis: Away from ANOVAs (transformation or not) and towards logit mixed models. Journal of Memory and Language, 59(4), 434–446. 10.1016/j.jml.2007.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, & Buxbaum LJ (2013). Response interference between functional and structural object-related actions is increased in patients with ideomotor apraxia. Journal of Neuropsychology, 7(1), 12–18. 10.1111/j.1748-6653.2012.02031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (1982). Western aphasia battery test manual. Psychological Corp. [Google Scholar]
- Klapp ST (2003). Reaction time analysis of two types of motor preparation for speech articulation: Action as a sequence of chunks. Journal of Motor Behavior, 35(2), 135–150. 10.1080/00222890309602129 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2016). lmerTest: Tests in Linear Mixed Effects Models (Version R package version 2.0–33). Retrieved from https://cran.r-project.org/package=lmerTest
- Laganaro M, & Alario F (2006). On the locus of the syllable frequency effect in speech production. Journal of Memory and Language, 55(2), 178–196. [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Maas E, Gutiérrez K, & Ballard KJ (2014). Phonological encoding in apraxia of speech and aphasia. Aphasiology, 28(1), 25–48. 10.1080/02687038.2013.850651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas E, & Mailend M-L (2012). Speech planning happens before speech execution: Online reaction time methods in the study of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55(5), S1523–S1534. 10.1044/1092-4388(2012/11-0311) [DOI] [PubMed] [Google Scholar]
- Maas E, Robin DA, Wright DL, & Ballard KJ (2008). Motor programming in apraxia of speech. Brain and Language, 106, 107–118. [DOI] [PubMed] [Google Scholar]
- Mailend M-L, & Maas E (2013). Speech motor programming in apraxia of speech: Evidence from a delayed picture-word interference task. American Journal of Speech-Language Pathology, 22(2), S380–S396. 10.1044/1058-0360(2013/12-0101) [DOI] [PubMed] [Google Scholar]
- Mailend ML, Maas E, Beeson PM, Story BH, & Forster KI (2019). Speech motor planning in the context of phonetically similar words: Evidence from apraxia of speech and aphasia. Neuropsychologia, 127, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATLAB. (2016). 9.1.0, The MathWorks Inc. [Google Scholar]
- McNeil MR, Pratt SR, & Fossett TRD (2004). The differential diagnosis of apraxia of speech In van Lieshout P, Maassen B, & Terband H (Eds.), Speech Motor Control in Normal and Disordered Speech: Future Developments in Theory and Methodology (pp. 389–413). Rockville, MD: American Speech-Language-Hearing Association. [Google Scholar]
- McNeil MR, Robin D, & Schmidt R (2009). Apraxia of speech: Definition, differentiation, and treatment In. McNeil MR(Ed.), Clinical management of sensorimotor speech disorders (pp. 249–268). Ney York, NY: Thieme. [Google Scholar]
- Meyer DE, & Gordon PC (1985). Speech production: Motor programming of phonetic features. Journal of Memory and Language, 24(1), 3–26. 10.1016/0749-596X(85)90013-0 [DOI] [Google Scholar]
- Miller HE, & Guenther FH (in press). Modelling speech motor programming and apraxia of speech in the DIVA/GODIVA neurocomputational framework. Aphasiology. [DOI] [PMC free article] [PubMed]
- Mooshammer C, Goldstein L, Tiede M, Kulshreshtha M, McClure S, & Katsika A (2009). Planning time effects of phonological competition: Articulatory and acoustic data. The Journal of the Acoustical Society of America, 125(4), 2657–2657. 10.1121/1.4784180 [DOI] [Google Scholar]
- Perkell JS (2012). Movement goals and feedback and feedforward control mechanisms in speech production. Journal of Neurolinguistics, 25(5), 382–407. 10.1016/j.jneuroling.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Rogers MA, & Storkel HL (1998). Reprogramming phonologically similar utterances: The role of phonetic features in pre-motor encoding. Journal of Speech, Language, and Hearing Research, 41(2), 258–274. [DOI] [PubMed] [Google Scholar]
- Rogers MA, & Storkel HL (1999). Planning speech one syllable at a time: the reduced buffer capacity hypothesis in apraxia of speech. Aphasiology, 13(9–11), 793–805. 10.1080/026870399401885 [DOI] [Google Scholar]
- Romani C, Galuzzi C, Guariglia C, & Goslin J (2017). Comparing phoneme frequency, age of acquisition, and loss in aphasia: Implications for phonological universals. Cognitive neuropsychology, 34(7–8), 449–471. [DOI] [PubMed] [Google Scholar]
- Sevald CA, & Dell GS (1994). The sequential cuing effect in speech production. Cognition, 53(2), 91–127. 10.1016/0010-0277(94)90067-1 [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin, 86(2), 420. [DOI] [PubMed] [Google Scholar]
- Sternberg S, Monsell S, Knoll RL, & Wright CE (1978). The latency and duration of rapid movement sequences: Comparisons of speech and typewriting. Information Processing in Motor Control and Learning, 117–152.
- Strand EA, Duffy JR, Clark HM, & Josephs K (2014). The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terband H, Maassen B, & Maas E (2019). A model of speech development and disorders for diagnosis and treatment planning. Folia Phoniatrica et Logopaedica, 71, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JL, Duffy JR, McNeil MR, Robin DA, & Rogers MA (2006). Treatment guidelines for acquired apraxia of speech: A synthesis and evaluation of the evidence. Journal of Medical Speech-Language Pathology, 14(2), xv–xv. [Google Scholar]
- Wambaugh JL, Nessler C, Cameron R, & Mauszycki SC (2012). Acquired apraxia of speech: The effects of repeated practice and rate/rhythm control treatments on sound production accuracy. American Journal of Speech-Language Pathology, 21(2), S5–S27. 10.1044/1058-0360(2011/11-0102) [DOI] [PubMed] [Google Scholar]
- Yaniv I, Meyer DE, Gordon PC, Huff CA, & Sevald CA (1990). Vowel similarity, connectionist models, and syllable structure in motor programming of speech. Journal of Memory and Language, 29(1), 1–26. 10.1016/0749-596X(90)90007-M [DOI] [Google Scholar]
- Ziegler W, Aichert I, & Staiger A (2012). Apraxia of Speech: Concepts and Controversies. Journal of Speech, Language, and Hearing Research, 55(5), S1485–S1501. 10.1044/1092-4388(2012/12-0128) [DOI] [PubMed] [Google Scholar]


