Abstract
Purpose:
T-cells engineered to express a chimeric antigen receptor (CAR T-cells) are a promising cancer immunotherapy. Such targeted therapies have shown long-term relapse-free survival in patients with B-cell leukemia and lymphoma. However, cytokine release syndrome (CRS) represents a serious, potentially life-threatening side effect often associated with CAR T-cell therapy. CRS manifests as a rapid (hyper)immune reaction driven by excessive inflammatory cytokine release, including interferon-γ and interleukin-6.
Experimental Design:
Many cytokines implicated in CRS are known to signal through the Janus kinase–signal transducers and activators of transcription (JAK-STAT) pathway. Here we study the effect of blocking JAK pathway signaling on CAR T-cell proliferation, anti-tumor activity and cytokine levels in in vitro and in vivo models.
Results:
We report that itacitinib, a potent, selective JAK1 inhibitor, was able to significantly and dose-dependently reduce levels of multiple cytokines implicated in CRS in several in vitro and in vivo models. Importantly, we also report that at clinically relevant doses that mimic human JAK1 pharmacologic inhibition, itacitinib did not significantly inhibit proliferation or antitumor killing capacity of three different human CAR T-cell constructs (GD2, EGFR, and CD19). Finally, in an in vivo model, antitumor activity of CD19-CAR T-cells adoptively transferred into CD19+ tumor bearing immuno-deficient animals was unabated by oral itacitinib treatment.
Conclusion:
Together, these data suggest that itacitinib has potential as a prophylactic agent for the prevention of CAR T-cell–induced CRS, and a phase II clinical trial of itacitinib for prevention of CRS induced by CAR T-cell therapy has been initiated (NCT04071366).
Introduction
T-cells engineered to express a chimeric antigen receptor (CAR T-cells) have recently been approved by the US Food and Drug Administration, and commercially available CAR T-cell therapies (axicabtagene ciloleucel and tisagenlecleucel) have resulted in an overall response rate of almost 80% (1–4), including long-term relapse-free survival in patients with B-cell leukemia and lymphoma (5). However, an exaggerated immune response by the adoptively transferred CAR T-cells, as well as the host immune system, can lead to a severe cytokine release syndrome (CRS) in up to 40% of treated patients (6, 7). CRS manifests as a rapid immune reaction driven by a massive release of inflammatory cytokines. While individual cytokine levels differs between patients, severe CRS is generally very similar to that seen in hemophagocytic lymphohistiocytosis (HLH) or macrophages activation syndrome (MAS)(8), with elevation of both canonical T-cell cytokines (i.e. IFN-γ) as well as cytokines compatible with HLH/MAS (i.e. IL-6 and IL-10)(9). Typified by chills and fevers, CRS can also progress to a more severe, even life-threatening reaction (10, 11).
To date, low-grade CRS is treated symptomatically with antipyretics and fluids (7). Grade > 2 CRS can be treated with tocilizumab (an anti–IL-6 receptor), recently approved by the US Food and Drug Administration and the European Medicines Agency for treatment of severe and life-threatening CRS (12, 13). Importantly, therapeutic administration of tocilizumab does not influence peak CAR T-cell numbers nor clinical outcomes (14). However, treatment with corticosteroids is also needed in the case of tocilizumab-refractory patients, and concerns remain over the effect of high dose corticosteroids on CAR-T cell expansion and anti-tumor activity (14). To date there are no preventative strategies, and we rely on therapeutic treatments. Thus, the ability to prophylactically inhibit hyper-inflammatory states and the incidence of CRS would represent a new clinical management strategy for patients receiving CAR T therapy.
JAKs play an important role in signal transduction following cytokine and growth factor binding to their cognate receptors. Once activated, JAKs phosphorylate signal transducer and activator of transcription proteins (STATs), which results in dimerization and translocation of STATs to the nucleus in order to activate or suppress gene transcription (15). Aberrant production of JAK-mediated cytokines, such as IL-6 and growth factors, has been associated with a number of chronic inflammatory conditions (16, 17). Thus, we reasoned that the blockade of the JAK signaling pathway would attenuate the excessive cytokine response that occurs during CRS without negatively impacting CAR T-cell function. Itacitinib (INCB039110) is a small-molecule inhibitor of the Janus kinase (JAK) family of protein tyrosine kinases (18). As opposed to other JAK inhibitors developed by Incyte (i.e. ruxolitinib) as well as others (i.e. tofacitanib), itacitinib is a JAK1 selective inhibitor, and is therefore expected to minimize the risk of long-term infection or other complications associated with pan-JAK inhibitors (19), while reducing the levels of cytokines that signal trough JAK1, such as IFN-γ, IL-6, IL-12 or TNF-α. In this study, we show that in both in vitro and in vivo preclinical models, itacitinib doses relevant to cellular half maximal inhibitory concentration (IC50; 50–100 nM) were more effective than tocilizumab in reducing CRS-related cytokines produced by CD19-CAR T-cells. Moreover, itacitinib did not significantly inhibit in vitro proliferation or antitumor killing capacity of CAR T-cells, nor, in a lymphoma model, did it affect the antitumor activity of CAR T-cells in a prophylactic setting. Together, these data suggest that itacitinib has potential as a prophylactic agent for the prevention of CAR T-cell–induced CRS, and a phase II clinical trial of itacitinib for the prevention of CRS induced by CAR T-cells is ongoing (NCT04071366).
Methods
Animals
C57BL/6 and BALB/c animals were purchased from Taconic Biosciences (Rensselaer, New York) and were approximately 8 weeks old at the time experiments were initiated. OT-1 TCR-transgenic mice and severely immunodeficient NOD-scid IL2R gamma null (NSG) animals were purchased from the Jackson Laboratory (Bar Harbor, Maine). All mice were used in protocols approved by the Institutional Animal Care and Use Committee.
T-cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were prepared from human whole blood samples using a Ficoll-Hypaque separation method, and T-cells were then obtained from the PBMCs by centrifugal elutriation. T-cells were maintained in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, Massachusetts) supplemented with 10% fetal bovine serum, 1% HEPES, 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol and 100 μg/mL streptomycin, and 100 units/mL penicillin (complete RPMI or CM). T-cells were activated with Dynabeads (Thermo Fisher Scientific; immobilized agonist antibodies against CD3/CD28) at a 3:1 ratio, resuspended at a density of 0.5 × 106 cells/mL in 24-well plates and treated with itacitinib at various concentrations (from 50 to 1000 nM). The plates were incubated at 37°C in 5% CO2 atmosphere for 10 days, and the proliferation was determined every other day by bead-based methods (CountBright™ Absolute Counting Beads, Molecular Probes, ThermoFisher). Cultures were replenished every other day with fresh CM.
Cytotoxicity assays
Luciferase expressing SY5Y neuroblastoma cells (GD-2+) were plated in a 96-well plate at 50,000 cells/well. Twenty-four hours later, 150,000 CAR T-cells were added to corresponding wells in a final volume of 200 μL. Target cells alone were seeded in parallel to quantify the maximum luciferase expression (relative luminescent units; RLUmax). Seventeen hours later 100 μL of luciferin substrate (Bright-Glo™ Luciferase Assay System, Promega, Madison, Wisconsin) was added to the co-culture. Luminescence was measured after a 10-minute incubation using an EnVision (PerkinElmer, Waltham, Massachusetts) plate reader. The percent cell lysis was obtained using the following calculation: [1 − (RLUexperimental)/(RLUmax)] × 100. The experiment was performed in triplicates.
Itacitinib modulation of mouse CRS models
CRS was induced in BALB/c animals by intravenous injection (IV) of Concanavalin-A (ConA; 20 mg/kg) or 100 μg of an anti-CD3ε antibody (clone 145–2C11). Corresponding animals were orally dosed with 60 or 120 mg/kg of itacitinib 60 minutes prior CRS induction (prophylactic) or 30 minutes after (therapeutic) CRS induction. Two hours after ConA or anti-CD3 injection, mice were sacrificed and blood was collected into K2EDTA tubes for cytokine measurement.
Activated macrophage models
Spleens were collected from 6- to 8-week-old C57BL-6 female mice and mechanically dissociated. Splenocytes were cultured in RPMI medium supplemented with 10% fetal bovine serum and macrophage colony-stimulating factor. On day 6, cells were treated with itacitinib and then treated with 5 ng/mL lipopolysaccharide (LPS) on day 7. The following day, supernatant was collected and cytokines were measured as described below. Six- to 8-week-old female C57BL/6 mice were prophylactically orally dosed with vehicle, or 60 or 120 mg/kg of itacitinib twice a day (b.i.d.) for 3 days. Mice then received intraperitoneal injections of LPS (5 μg per animal). Two hours after injection, mice were euthanized and 3 mL of sterile saline was injected into the peritoneum to perform a peritoneal lavage. Subsequent cytokine measurements were performed as described below.
Cytokine measurement
Plasma cytokines were measured using multi-spot assay system, pro-inflammatory panel 1 (for IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-1p70, and TNFα; Meso Scale Discovery, Meso Scale Diagnostics [MSD], Rockville, Maryland), following manufacturer’s instructions. Briefly, samples, standards, and controls were added at 25 μL per well. The plate was sealed and incubated for 2 hours at room temperature on an orbital shaker (600 rpm). At the end of the incubation, the wells were washed three times using 200 μL phosphate-buffered saline + 0.05% Tween 20, soaking for 30 seconds and then discarding. Detection antibody was added at 25 μL per well, and the plate sealed and incubated for 1 hour at room temperature on an orbital shaker (600 rpm). At the end of the incubation, the plate was washed three times as done previously. 150 μL of the MSD Read Buffer was added to each well, and the MSD plates were measured on a MSD Sector S 600 plate reader. The raw data were measured as an electrochemiluminescence signal detected by photodetectors and analyzed using the MSD Discovery Workbench software. A four-parameter logistic fit curve was generated for each analyte using the standards, and the concentration of each sample was calculated.
OT-1 T-cell expansion and antitumor activity
The OT-1 strain is transgenic for the TCR VαVβ5 specific for the OVA257–264 peptide (SIINFEKL) and restricted to H2-Kb. Naïve OT-1 CD8 cells were isolated from OT-1 spleens by negative selection using Naïve CD8a+ T Cell Isolation Kit (Miltenyi Biotec, Auburn, California).
To measure OT-1 CD8+ cell expansion, cells were CFSE-labeled (2 μM) and resuspended at 5 × 106 cells/mL in complete RPMI, 20 IU IL-2, 2 μg/mL of SIINFEKL peptide. and increasing itacitinib concentrations, from 0 to 1000 nM. Three to five days later, OT-1 cells were collected, stained with an APC-anti-CD8 antibody, and the number of divisions was calculated by measuring the relative carboxyfluorescein succinimidyl ester (CFSE) fluorescence intensity of the different conditions by flow cytometry (20).
To study the effect of itacitinib on the antitumor activity of CD8 OT-1 cells, C57BL/6 animals received subcutaneous injections of 0.5 × 106 OVA-expressing EG7 tumor cells into the shaved right flank. At day 7, corresponding animals were orally dosed with itacitinib b.i.d. at 60 or 120 mg/kg, and at day 10, corresponding animals received an intravenous (tail vein) injection of 5 × 106 naïve OT-1 CD8 cells. Tumor sizes were measured on two perpendicular axes using a digital caliper at least three times a week. Tumor volumes were calculated with the formula:
Where V is tumor volume, W is tumor width, and L is tumor length.
Cell Viability Assay
Cell proliferation was measured using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI), following manufacturer’s instructions. Briefly, 1000 EG7 cells were seeded in a total volume of 200 μl of RPMI + 10% FBS per well in a 96-well plate. The next day, compound plates were reconstituted in complete media with a 10 point dose curve. Cell media of the plate was aspirated and compound with a total volume of 100 μl was incubated with the cells for 72 hours. 100 μl of CellTiter-Glow reagent was added to each well and protected from the light with a plate seal. Plates were shaken at room temperature for 2 minutes to induce cell lysis and then allowed to sit for approximately 10 minutes. The raw data was measured on a TopCount Luminescence Counter (Perkin Elmer, Waltham, MA) and analyzed in Prism using an [inhibitor] vs. response – variable slope (four parameters) equation.
Generation of CAR constructs and CAR T-cell preparation
The CD19-BBζ CAR consisting of a CD8 hinge, 4–1BB costimulatory domain, and CD3ζ signaling domain was generated as previously described (21). PBMCs were stimulated with Dynabeads (3:1 ratio) and then plated in 24-well plates in X-Vivo medium (Lonza, Morristown, New Jersey) containing IL-2 (200 IU/mL), IL-7 (10 ng/mL), IL-15 (5 ng/mL), and IL-21 (5 ng/mL) at 0.5 × 106 cells/mL. Three days later, T-cells were transduced using a lentiviral vector expressing the CAR construct, as described elsewhere (22).
Generation of the GD2-E101K CAR lentiviral plasmid was previously described (23). The CAR contains the 14G2a scFv, as well as an EF1α promoter, CD8 hinge, 4–1BB costimulatory domain, and CD3ζ signaling domain. The anti-EGFR CAR, with 3C10 scFv, was previously described (24). The CAR contains the 3C10 scFV, as well as an EF1α promoter, CD8 hinge, 4–1BB costimulatory domain, and CD3ζ signaling domain.
Antitumor activity (Nalm6 model)
To study whether JAK1 inhibition affects the antitumor effect of CAR T-cells, a xenograft model was used as previously reported (25). Briefly, 6- to 10-week-old immunodeficient NOD-SCID γc−/− (NSG) mice were injected intravenously via tail vein with 2.5 × 106 luciferase expressing Nalm6 acute lymphoblastic leukemia cells. Corresponding animals received 120 mg/kg of itacitinib per 10 days (orally, b.i.d.), starting 1 day after the tumor challenge. Mice were then randomized into groups for adoptive transfer of 3 × 106 CAR T-cells, non-transduced cells, or vehicle intravenously at day 4. Anesthetized mice were imaged using a Xenogen IVIS Spectrum system (Caliper Life Sciences, Hopkinton, Massachusetts) once a week. Mice were given an intraperitoneal injection of D-luciferin (150 mg/kg; Caliper Life Sciences). Total flux was quantified using Living Image 4.4 (PerkinElmer) by drawing rectangles of identical area around mice, reaching from head to 50% of the tail length. Background bioluminescence was subtracted for each image individually. Animals were monitored daily, and survival rates were compared between groups.
Antitumor activity (NAMALWA model)
Six- to 8-week-old male NSG mice were subcutaneously inoculated with luciferase-expressing CD19+ NAMALWA lymphoma cells (5 × 106). Mice were weighed and monitored two times a week, and bioluminescence signals were measured every 5 days. At day 5 after tumor challenge, animals started a b.i.d. oral treatment of vehicle or INCB039110 (120 mg/kg) for 8 days. At day 8 after tumor challenge, animals received an adoptive transfer of 5 × 106 CD19-CAR T-cells. Bioluminiscence was measured as done previously. Animals were monitored daily, and survival rates were compared between groups.
Data and statistical analysis
Data are reported as mean ± SD or mean ± SEM in the relevant figures. Differences between two groups were analyzed by nonparametric Mann-Whitney test. Statistical analysis for multiple groups was performed by Kruskal-Wallis with Dunn’s post hoc test for nonparametric data sets, or ANOVA with Holm-Sidak’s test for parametric results. All tests were performed using GraphPad Prism (GraphPad Software Inc, San Diego, California).
Results
Itacitinib reduces cytokine levels in murine models of acute hyperinflammation
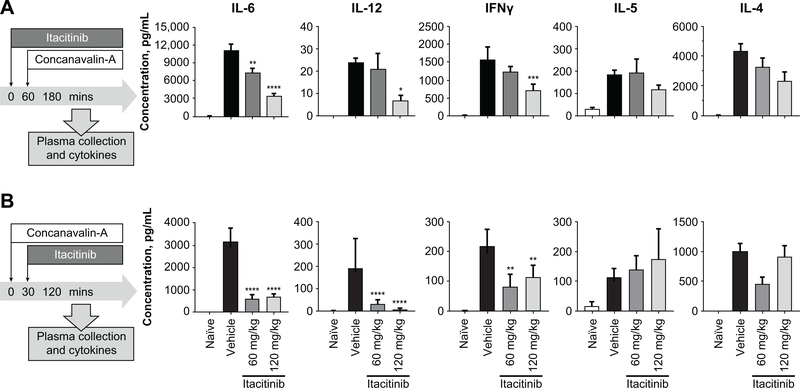
As CRS is the most common side effect associated with CAR T-cell treatment, we investigated whether itacitinib is able to reduce the levels of cytokines associated with acute hyperactivation leading to CRS. Therefore, we conducted experiments in which naïve animals were challenged with ConA, a potent T-cell mitogen capable of inducing broad inflammatory cytokine releases and proliferation (26). Similar to individuals experiencing CRS, animals receiving ConA have elevated serum levels of multiple inflammatory cytokines as well as behavioral changes such as fever, malaise, hypotension, hypoxia, capillary leak, multi-organ toxicity, and potentially death. To study the effect of itacitinib in this model, corresponding animals were prophylactically dosed with 60 or 120 mg/kg of itacitinib to achieve JAK1 inhibition coverage equivalent to that observed in clinical trials (27–29). When compared with vehicle-dosed animals, itacitinib was able to significantly reduce serum levels of many of the cytokines implicated in CRS (i.e., IL-6, IL-12, and IFN-γ) in a dose-dependent manner (Fig. 1A). As expected, itacitinib did not have a significant effect on cytokines independent of the JAK1 pathway (i.e., IL-5, Fig. 1A). However, not all JAK-mediated cytokines were significantly decreased (i.e. IL-4, Fig 1A). Additionally, itacitinib was also able to dose-dependently reduce CRS-implicated cytokines in a therapeutic mode, where animals were dosed with itacitinib 30 minutes after ConA challenge (Fig. 1B).
Figure 1. Itacitinib reduces cytokine levels in murine models of acute inflammation.
(A) BALB/c mice were orally dosed with vehicle control, or 60 or 120 mg/kg of itacitinib. One hour later, animals were challenged with Concanavalin-A (ConA), and 120 minutes later sacrificed and plasma collected (prophylactic). (B) BALB/c mice were challenged with ConA, 30 minutes later were orally dosed with vehicle control, or 60 or 120 mg/kg of itacitinib and 2 hours after ConA challenge sacrificed (therapeutic). N = 5 animals per group. MSD analysis was performed to detect the levels of pro-inflammatory cytokines. Data represent mean ± SEM, and P values were calculated by two-way ANOVA. *P < 0.05, ** P < 0.01, ***P < 0.001, **** P < 0.0001. Data are representative of four independent experiments.
To confirm the capacity of itacitinib to reduce hyperinflammation, naïve animals were challenged with anti-CD3 to induce nonspecific T-cell activation and cytokine response. Once again, corresponding animals were either prophylactically or therapeutically dosed with 120 mg/kg of itacitinib. Compared with vehicle-treated mice, itacitinib was able to significantly reduce serum levels of many of the cytokines implicated in CRS, but have no effect on cytokines independent of the JAK1 pathway (Supplementary Fig. S1A).
Itacitinib reduces IL-6 production by macrophages
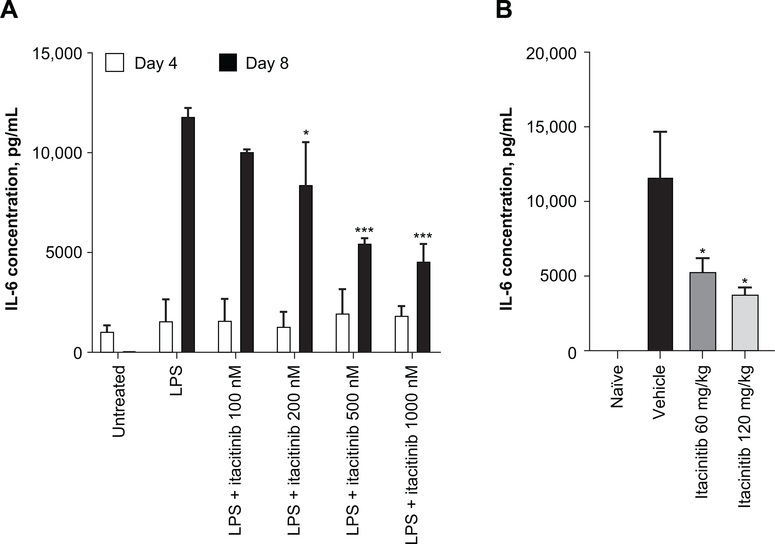
Recent evidence shows that host macrophages are the major producers of IL-6 after CAR T-cell treatment (30). Therefore, we queried whether itacitinib would prevent IL-6 production by host macrophages. First, murine bone marrow–derived macrophages were expanded in vitro with granulocyte-colony stimulating factor, and itacitinib was added to the cultures at day 6. LPS was added to the culture at day 7 to activate the macrophages (31). Prophylactic treatment with itacitinib reduced IL-6 production in a dose-dependent manner, indicating that the activity of itacitinib in reducing production of inflammatory cytokines is not exclusive to T-cells (Fig. 2A). We also observed a non-significant trend towards reduction on several other cytokines (i.e. IL-10, IL-12p70, KC/GRO, Supplementary Fig. S2)
Figure 2. Itacitinib reduces IL-6 production by macrophages.
(A) Macrophages harvested from C57BL-6 mice were treated with increasing doses of itacitinib 1 day in advance of being activated with 5 ng/mL LPS. Twenty-four hours after LPS treatment, supernatant was harvested and cytokine levels measured. (B) C57BL/6 mice were prophylactically orally dosed with vehicle, or 60 or 120 mg/kg of itacitinib b.i.d. for 3 days. Mice then received intraperitoneal injections of LPS (5 μg per animal). Two hours after injection, mice were euthanized and IL-6 levels were measured in the peritoneal lavage. Data represent mean ± SEM, and P values were calculated by two-way ANOVA. *P < 0.05, ***P < 0.001. Data are representative of two independent experiments.
Having determined that itacitinib is able to reduce IL-6 production in vitro, we next expanded our studies to an in vivo context to assess the effect of itacitinib on activated macrophages in mice. Mice were prophylactically treated with itacitinib or vehicle for 3 days to achieve steady state before receiving an intraperitoneal injection of LPS. Two hours after injection, cytokines were measured from intraperitoneal lavages. As seen in Fig. 2B, doses of itacitinib that mimic the JAK1 inhibition coverage achieved in clinical trials significantly reduced IL-6 production by activated macrophages in vivo. Thus, data from both in vitro and in vivo systems indicate that itacitinib is capable of downregulating the major cellular source of inflammatory IL-6 during an experimentally induced model of CRS.
Itacitinib reduces CAR T-cell cytokine production
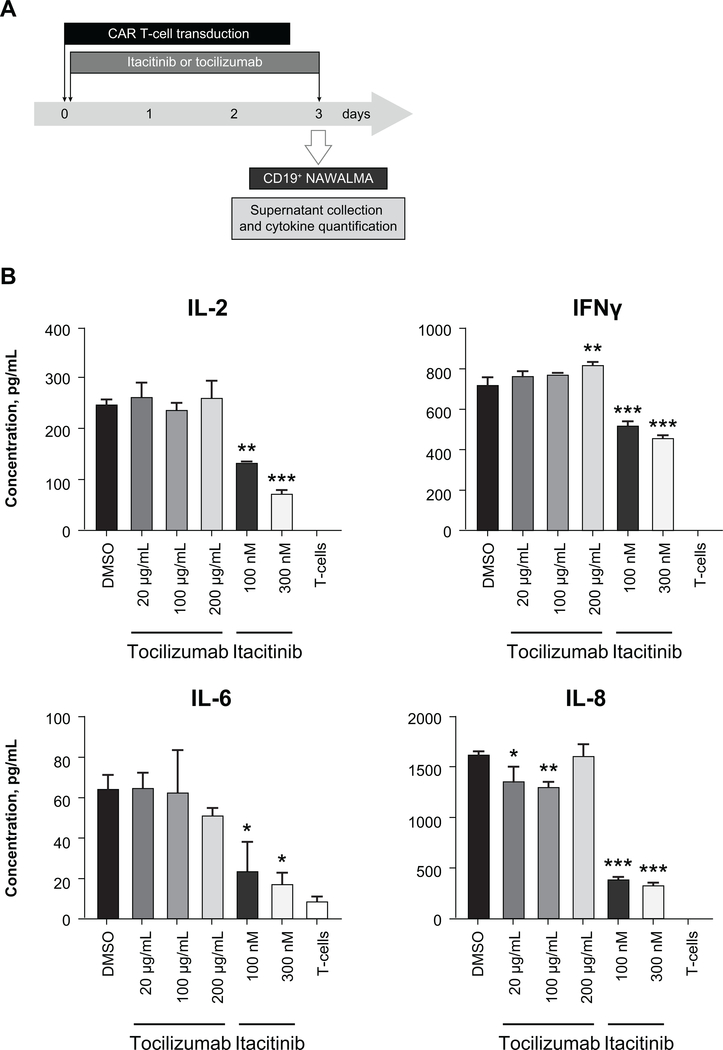
Whereas cytokine production by the host immune system is a primary cause of CRS, production of inflammatory cytokines by CAR T-cells is also a major contributor (32). Having shown that itacitinib is capable of reducing inflammatory cytokine production by the host immune system, we next wanted to examine whether itacitinib reduces inflammatory cytokine production by CAR T-cells. Human CD19-CAR T-cells were expanded under concentrations of tocilizumab or itacitinib equivalent to human doses achieved in clinical trials (33). After a 3-day expansion, CAR T-cells were co-cultured with CD19 expressing NAMALWA target cells, and 6 hours later supernatants were collected to quantify cytokines. Itacitinib, but not tocilizumab, was able to significantly reduce the levels of many inflammatory cytokines (i.e., IL-2, IFN-γ, IL-6, and IL-8) (Fig. 3). As an internal control (background), we also measured cytokines produced by non-transduced T-cells (Fig. 3).
Figure 3. Itacitinib reduces CAR T-cell cytokine production.
CD19-CAR T-cells were expanded with either itacitinib or the anti–IL-6 receptor tocilizumab. Three days later, they were co-cultured with CD19+ lymphoma cells (E:T ratio = 2.5:1) for 6 hours and cytokines were measured in the supernatant by MSD. (A) Experiment scheme. (B) Data represent mean ± SEM, and P values were calculated by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001. Data are representative of two independent experiments.
Itacitinib at clinically relevant concentrations does not affect PBMC proliferation
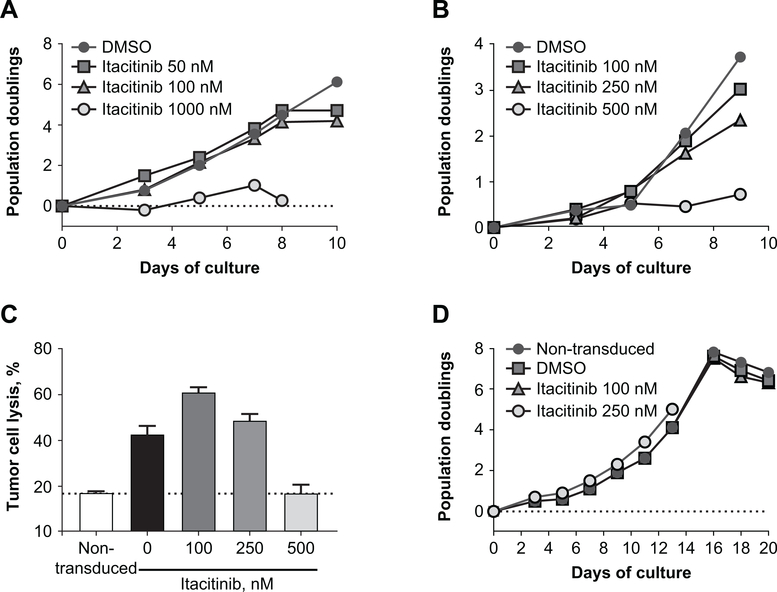
As T-cells play an important antitumor role, the effect of itacitinib on T-cells was studied to determine if treatment may result in broad immunosuppression potentially interfering with normal T-cell proliferation and function. T-cells were obtained from freshly isolated human PBMCs of healthy adults. Following activation with anti-CD3/anti-CD28 coated beads (23), T-cells were expanded in the presence of increasing concentrations of itacitinib, and proliferation was measured by flow cytometry. When compared with the dimethyl sulfoxide (DMSO) control, concentrations of itacitinib relevant to the IC50 (50–100 nM) (34) did not have a significant effect on anti-CD3/anti-CD28–induced expansion of human T-cells (Fig. 4A), demonstrating that itacitinib treatment has no negative impact on the ability of T-cells to proliferate. As an internal control, and consistent with the disruption of the JAK/STAT signaling activity, higher concentrations of itacitinib (1000 nM) blocked T-cell proliferation (Fig. 4A).
Figure 4. Itacitinib does not affect PBMC proliferation nor cytolytic activity.
(A) Flow cytometry quantification of T-cell expansion of human PBMCs expanded with DMSO (positive control) or 50, 150, or 1000 nM of itacitinib. T-cells were expanded using anti-CD3/CD28-coated beads, and proliferation was measured every other day by flow cytometry. (B) GD2-CAR T-cells were expanded in the presence of different itacitinib concentrations and proliferation was measured every other day by flow cytometry. (C) Lytic activity of GD2-CAR T-cells against SY5Y neuroblastoma cells (GD-2+) was determined by measuring luciferase activity. As an internal control, lytic activity of non-transduced T cells was also measured (background dotted line). (D) EGFR-CAR T-cells were expanded with anti-CD3/CD28 beads for 2 weeks and then restimulated with EGFR beads in the presence of itacitinib (100 or 250 nM). Proliferation was measured every other day by flow cytometry.
Itacitinib does not affect CAR T-cell proliferation
To study the effect of itacitinib specifically on CAR T-cell expansion and cytolytic activity, GD2-CAR T-cells (23) were expanded with anti-CD3/anti-CD28 coated beads in the presence of increasing itacitinib concentrations, ranging from 100 to 500 nM. Once again, when compared with the DMSO control, itacitinib at 100–250 nM did not have a significant effect on CD3/CD28-induced expansion of GD2-CAR T-cells, whereas 500 nM was enough to block CAR T-cell proliferation (Fig. 4B). Importantly, GD2-CAR T-cells expanded in the presence of low (100 and 250 nM) itacitinib concentrations were able to efficiently lyse GD2-expressing tumor cells (Fig. 4C). As a negative (background) control, we also measured target cell lysis by non-transduced (nonspecific) T-cells. As expected, GD2-CAR T-cells expanded in the presence of a high dose of itacitinib (500 nM) and were unable to induce the lysis of target cells beyond background levels (Fig. 4C). Taken together, these data indicate that IC50 relevant doses of itacitinib do not interfere with CAR T-cell proliferation or antitumor activity in vitro.
Next, we queried whether itacitinib has an effect on CAR T-cell antigen-specific proliferation. EGFR-CAR T-cells (35) were expanded with anti-CD3/CD28 beads for 2 weeks and then restimulated with EGFR beads in the presence of itacitinib or DMSO controls. Once again, doses of itacitinib relevant to the IC50 (100 or 250 nM) did not affect CAR T-cell antigen-specific proliferation (Fig. 4D).
Itacitinib does not impair T-cell antitumor activity in vivo
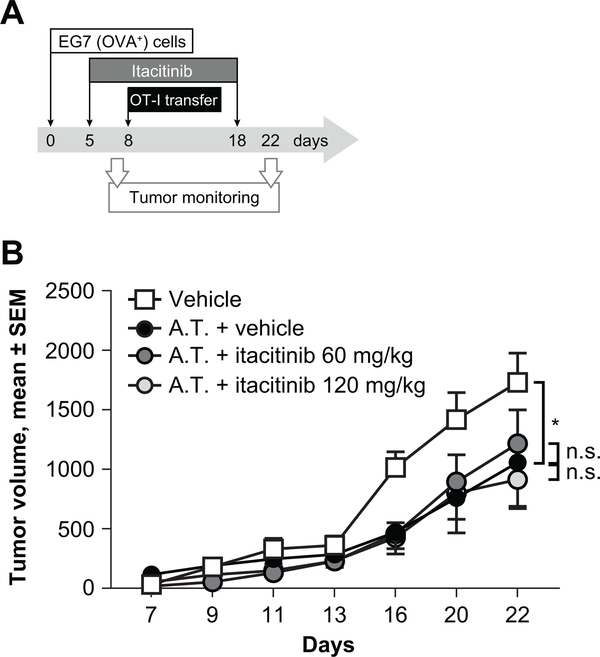
To further test the effect of itacitinib on antigen-specific T-cell proliferation and in vivo antitumor activity, we isolated splenocytes from OT-1 mice, transgenic for the TCR Vα2Vβ5 specific for the peptide OVA257–264 (SIINFEKL) (36). OT-1 T-cells were expanded with the SIINFEKL peptide in the presence of increasing concentrations of itacitinib, and their expansion was measured by flow cytometry. As expected, itacitinib concentrations relevant to the IC50 have a minimal effect on the expansion rate (Supplementary Fig. S3A). Itacitinib concentrations relevant to maximum free concentrations (189 and 244 nM, in the absence or presence of potent CYP3A4 inhibitors, respectively) induced a modest reduction on T-cell proliferation (Supplementary Fig. S3A). To evaluate the effect of itacitinib on the in vivo antitumor efficacy of OT-1 CD8, we conducted experiments involving adoptive transfer of OVA-specific CD8 cells into C57BL/6 mice previously transplanted with an OVA-expressing tumor cell line (EG7). Five days after tumor challenge, corresponding animals were orally dosed b.i.d. with vehicle or 60 or 120 mg/kg of itacitinib for 2 weeks. Eight days after tumor inoculation, animals received an adoptive transfer of OVA-specific OT-1 naïve CD8 cells. When compared with the control group, adoptive transfer of OT-1 CD8 was able to significantly reduce tumor growth (Fig. 4). Importantly, itacitinib doses equivalent to the JAK1 coverage target doses do not affect the antitumor efficacy of OT-1 cells (Fig. 5).
Figure 5. Itacitinib does not impair T-cell antitumor activity in vivo.
C57BL/6 animals were subcutaneously inoculated with an OVA-expressing EG-7 tumor cell line. Starting at day 5, corresponding groups received oral doses of itacitinib or vehicle for 2 weeks, and at day 8 they received an adoptive transfer (A.T.) of naïve OT-1 CD8 T-cells. (A) Experiment scheme. (B) Tumor growth was monitored and compared between groups. N = 10 animals per group. Data represent mean ± SEM, and P values were calculated by two-way ANOVA. * P < 0.05, n.s., not significant. Data are representative of two independent experiments.
To rule out a direct effect of itacitinib on EG7 tumor cell growth, we expanded EG7 cells in the presence of increasing itacitinib concentration. Even very high concentrations of itacitinib (25 μM) were unable to inhibit tumor proliferation (Supplementary Fig. S3B).
Itacitinib does not affect CD19-CAR T-cell cytolytic activity in vitro
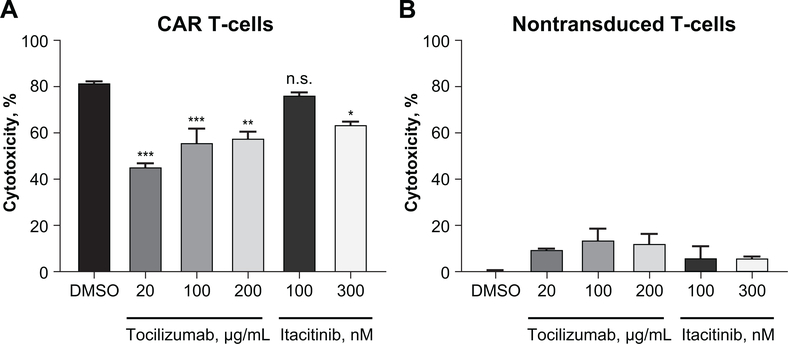
To study the effect of itacitinib on human CAR T-cells targeting the CD19 antigen, we treated CD19-CAR T-cells with either itacitinib or anti–IL-6 receptor (tocilizumab) for 3 days and then measured their cytolytic activity against CD19-expressing target cells. Whereas concentrations of tocilizumab that mimic human pharmacologic activity significantly reduced the cytolytic activity of CAR T-cells (37), 100 nM of itacitinib showed no significant effect when compared with control CAR T-cells (Fig. 6A). CAR T-cells expanded with a higher dose of itacitinib (300 nM, approximately five-fold higher that the cellular IC50) showed a modest but statistically significant inhibition on the antitumor activity (Fig. 6A). As internal controls, the antitumor activity of non-transfected PBMCs was also tested. Non-transduced T-cells were unable to specifically lyse the target cells and were unaffected by neither itacitinib nor tocilizumab treatment beyond background levels (Fig. 6B).
Figure 6. Itacitinib does not affect CD19-CAR T-cell cytolytic activity in vitro.
(A) CD19-CAR T-cells or (B) nontransduced PBMC were expanded with either itacitinib or anti–IL-6 receptor (tocilizumab) at the indicated concentrations. Three days later, they were co-cultured with luciferin expressing CD19+ lymphoma cells (E:T ratio = 2.5:1) for 6 hours, luciferase substrate was added and luminescence was recorded by a luminometer. Data represent mean ± SEM, and P values were calculated by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
Itacitinib does not affect CD19-CAR T-cell antitumor activity in vivo
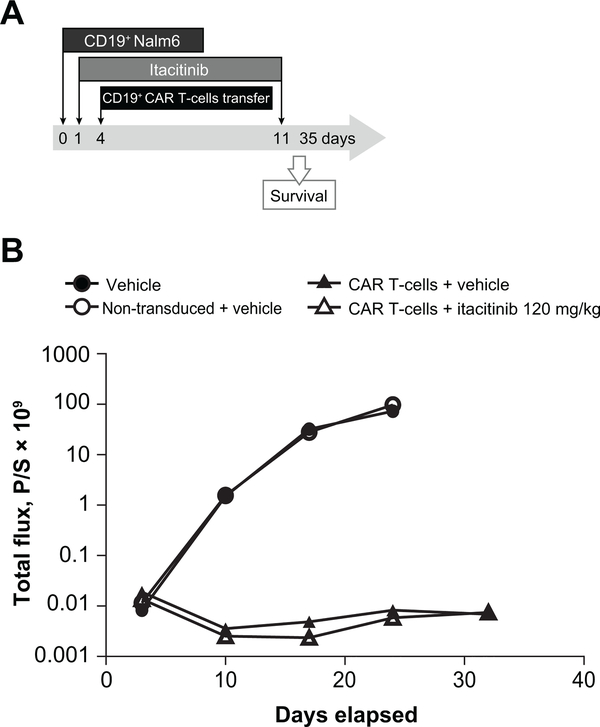
To test the effect of itacitinib in vivo, we studied the effect of oral itacitinib on the antitumor efficacy of CD19-CAR T-cells. Immunodeficient mice (NSG) were challenged with CD19+ nalm6-luciferase expressing human lymphoma cells. Once the tumor was engrafted (day 4), the mice received an adoptive transfer of 3 × 106 human CD19-CAR T-cells. In this model, CAR T-cells were able to control tumor growth, measured by luciferin expression (Fig. 7). Importantly, daily oral itacitinib dosing (120 mg/kg) of the animals did not affect the antitumor activity of adoptively transferred CAR T-cells, thus indicating that, at targeted doses, itacitinib does not have a significant effect on the antitumor activity of CAR T-cells.
Figure 7. Itacitinib does not affect CD19-CAR T-cell antitumor activity in vivo.
Immunodeficient NSG mice were inoculated with 2.5 × 106 CD19+ human lymphoma Nalm6 luciferase-expressing Nalm6 cell line. Starting 1 day later, animals received b.i.d. doses of itacitinib or vehicle for 10 days. At day 4, post-tumor injection, corresponding animals received an adoptive transfer of 3 × 106 CD19-CAR T-cells. (A) Experiment scheme. (B) Anesthetized mice were imaged using a Xenogen IVIS Spectrum system to measure bioluminescence once a week. N = 5–10 animals per group.
Finally, to confirm that itacitinib would not affect CAR T-cell activity on a more aggressive model, immunodeficient mice (NSG) were challenged with 5 × 106 CD19+ NAMALWA-luciferase expressing human lymphoma cells. Once the tumor was engrafted (day 7), the mice received an adoptive transfer of human CD19-CAR T-cells. When compared with animals receiving control cells, animals receiving an adoptive transfer of CAR T-cells had a significant tumor growth delay, measured both by luciferin expression (Supplementary Fig. S4B) and expansion of survival (P = 0.0014) (Supplementary Fig. S4C). Even in this aggressive tumor model, daily oral itacitinib dosing of the animals did not affect the antitumor activity of adoptively transferred CAR T-cells (P = 0.1860), thus confirming the safety of prophylactic itacitinib dosing to prevent CRS.
Discussion
CAR T-cell therapy has shown unprecedented remission rates in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Unfortunately, the high frequency of side effects (mainly CRS and neurotoxicity) that often require extensive critical care support remains a barrier to the further development of CAR T-cell therapy. Current therapeutic options are limited to treating severe cases of CRS with tocilizumab or corticosteroids, but unfortunately severe toxicities and death still occasionally occur (38). Therefore, there is a clear need to develop prophylactic treatments to prevent (rather than treat) CRS without impairing antitumor efficacy.
Despite being a very active research field, only recently clear predictors of CAR T-cell associated toxicities have been identified, and several factors, such as ferritin, C-reactive protein or tumor burden can be reliable predictors of severe CRS (11). A recent clinical trial on pediatric leukemia patients suggests that early therapeutic intervention with tocilizumab is effective at reducing CRS rates without affecting CAR T-cell proliferation or antitumor efficacy (39). However, this data should not be generalized to other conditions such as lymphoma as clinical management strategies are different than leukemia and concerns remain over the potential impact of corticosteroids on CAR T-cell activation and proliferation (14).
Recent advances in our understanding of the JAK/STAT pathway have highlighted its role in T-cell priming, activation, and expansion. Indeed, several small-molecules targeting the JAK/STAT pathway have shown clinical potential in a number of indications (reviewed in (40)). Biochemical and cellular profiling of itacitinib confirmed it to be a potent JAK1 inhibitor with large fold-selectivity margins over the other family members (JAK2, JAK3, and TYK2) or unrelated kinases (41). Importantly, JAK1 either independently or in cooperation with other members of the JAK family, mediate the signaling of a number of inflammatory cytokines implicated in CRS, including GM-CSF, IFN-γ, IL-6 and IL-12 (42, 43). Crucially, specific inhibition of JAK1 may prevent some of the side effects associated with JAK1/JAK2 inhibitors such as lymphopenia (42).
Consistent with the potent disruption of the JAK1-specific STAT signaling activity, here we show that itacitinib inhibited multiple cytokines involved in CRS (including IL-6, IL-12, and IFN-γ) in two different models of systemic inflammation in a dose-dependent manner. Importantly, only cytokines dependent upon JAK1 were affected, as levels of IL-5 and other JAK1-independent cytokines were unaffected by itacitinib treatment. It is also important to note that while the effect on inflammatory cytokine levels was profound and statistically significant, plasma levels of such cytokines were still elevated when compared with naïve animals, thus potentially dampening the hyper-immune activation state, but avoiding broad immunosuppression.
In addition to the effect of itacitinib on the production of cytokines by T-cells and CAR T-cells, our findings show that itacitinib is also able to reduce the secretion of inflammatory cytokines by macrophages, both in vitro and in vivo. Recent studies have shown that IL-1 and IL-6 produced by host monocytes/macrophages play a key role in the pathogenesis of CRS and neurotoxicity (30, 44). Our data demonstrate that itacitinib is able to inhibit IL-6 production by murine macrophages in vivo, demonstrating its potential utility in treating the most prominent source of inflammatory cytokines during CRS. The ability of itacitinib to inhibit the production of CRS-associated cytokines produced by a range of cell types (both of murine and human origin, an important distinction considering the differences between mouse and human biology (45)) is particularly noteworthy, as such a strategy may be necessary for the effective treatment of CRS.
To achieve clinical efficacy while preventing CRS, it is imperative that clinical doses of itacitinib do not interfere with CAR T-cell proliferation and antitumor activity. Our findings confirm that itacitinib concentrations relevant for cellular IC50 (in vitro) and drug pharmacokinetic exposures that mimic JAK1 target coverage in vivo have no significant effect on the proliferation rate of PBMCs or four different CAR T-cell constructs. More importantly, our data show that in preclinical models, where animals receive an oral dose of itacitinib that mimic human JAK1 pharmacologic inhibition, prophylactic treatment with itacitinib does not affect the in vivo antitumor efficacy of CAR T-cells.
In summary, we have demonstrated that itacitinib at clinically relevant doses can prevent CAR T-cell associated CRS in preclinical models without negatively affecting antitumor efficacy. Reducing the incidence and severity of CRS has the potential to benefit clinical practice, including the use of CAR T-cell therapy in patients in previously high-risk populations. Based on these results, a phase II clinical trial of itacitinib for the prevention of CRS induced by CD19-CAR T-cell therapy was initiated (NCT04071366). Results from our preclinical study may have implications in other settings; future studies are required to further investigate the role of JAK inhibition beyond CAR T-cells induced CRS (i.e., bispecific antibodies (46)) or conditions (including HLH (9, 47) and respiratory infections such as SARS (48), MERS (49), or Covid-19 (50–52)) where CSR may play a role.
Supplementary Material
Translational relevance.
The development of chimeric antigen receptor (CAR) technology has dramatically altered the global landscape of cancer cell therapy with unprecedented responses of CAR T-cells targeting CD19 and other B-cell antigens. However, cytokine release syndrome (CRS) and neurotoxicity represent serious, potentially life-threatening side effects often associated with CAR T-cell therapy. In the present study, we demonstrated that JAK1 inhibition with itacitinib reduces levels of cytokines implicated in CRS without affecting CAR T-cell proliferation or cytolytic activity in vitro. Our data show that prophylactic JAK1 inhibition reduces hyperinflammation, and does not affect CAR T-cell antitumor response in preclinical models. This study provides the rationale for a phase II clinical trial of itacitinib for the prevention of CRS induced by CAR T-cell therapy (NCT04071366).
Acknowledgments
This study was funded by Incyte Corporation, and supported in part by an NIH grant RO1CA226983-03 awarded to R.O’C.
We are thankful to April Barbour, Michael Howell, and Lea Burke for useful discussions about pharmacokinetics and translational relevance of the manuscript. Editorial assistance was provided by Envision Pharma Group, Inc. (Philadelphia, PA, USA), and funded by Incyte Corporation.
Footnotes
Disclosure of Potential Conflict of Interests: Eduardo Huarte, Michael T. Peel, Ashish Juvekar, Yan-ou Yang, Lisa Truong, Taisheng Huang, Ahmad Naim and Paul S. Smith are employees and stockholders of Incyte. Michael C. Milone is cofounder and co-chair of Cabaletta Bio and consultant for Novartis.
References
- 1.Jain MD, Bachmeier CA, Phuoc VH, Chavez JC. Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin’s lymphoma. Ther Clin Risk Manag 2018;14:1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 4.Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev 2016;30:157–67. [DOI] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book 2019;39:433–44. [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol 2019;37 Suppl 1:48–52. [DOI] [PubMed] [Google Scholar]
- 8.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014;20:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das R, Guan P, Sprague L, Verbist K, Tedrick P, An QA, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 2016;127:1666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugelski PJ, Achuthanandam R, Capocasale RJ, Treacy G, Bouman-Thio E. Monoclonal antibody-induced cytokine-release syndrome. Expert Rev Clin Immunol 2009;5:499–521. [DOI] [PubMed] [Google Scholar]
- 11.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018;23:943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol 2019;10:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017;77:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford) 2019;58:i43–i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Warren MS, Zhang X, Diamond S, Williams B, Punwani N, et al. Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110. Drug Metab Dispos 2015;43:485–9. [DOI] [PubMed] [Google Scholar]
- 19.Verstovsek S, Mesa RA, Gotlib J, Gupta V, DiPersio JF, Catalano JV, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol 2017;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quah BJC, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc 2007;2:2049–56. [DOI] [PubMed] [Google Scholar]
- 21.Milone M, Batish SD, Daube JR. Myotonic dystrophy type 2 with focal asymmetric muscle weakness and no electrical myotonia. Muscle Nerve 2009;39:383–5. [DOI] [PubMed] [Google Scholar]
- 22.Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 2015;64:817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, et al. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res 2018;6:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015;7:275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghassemi S, Nunez-Cruz S, O’Connor RS, Fraietta JA, Patel PR, Scholler J, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res 2018;6:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 1995;21:190–8. [DOI] [PubMed] [Google Scholar]
- 27.Juvekar A, Ruggeri B, Condon S, Borkowski A, Huber R, Smith P. Itacitinib, a JAK1 Selective Inhibitor Preserves Graft-Versus-Leukemia (GVL), Enhances Survival and Is Highly Efficacious in a MHC-Mismatched Mouse Model of Acute GvHD. Blood 2018;132:4522. [Google Scholar]
- 28.Huarte E, O’Connor RS, Parker M, Huang T, Milone MC, Smith P. Prophylactic Itacitinib (INCB039110) for the Prevention of Cytokine Release Syndrome Induced By Chimeric Antigen Receptor T-Cells (CAR-T-cells) Therapy. Blood 2019;134:1934. [Google Scholar]
- 29.Schroeder MA, Khoury HJ, Jagasia M, Ali H, Schiller GJ, Staser K, et al. A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv 2020;4:1656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24:739–48. [DOI] [PubMed] [Google Scholar]
- 31.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 2012;189:3439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levi M, Grange S, Frey N. Exposure-exposure relationship of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in a large population of patients with rheumatoid arthritis. J Clin Pharmacol 2013. doi: 10.1177/0091270011437585. [DOI] [PubMed] [Google Scholar]
- 34.Barbour AM, Punwani N, Epstein N, Landman R, Cimino E, Yuska B, et al. Effect of itraconazole or rifampin on itacitinib pharmacokinetics when administered orally in healthy subjects. J Clin Pharmacol 2019;59:1641–7. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Huang Y, Jiang DQ, Cui LZ, He Z, Wang C, et al. Antitumor activity of EGFR-specific CAR T cells against non-small-cell lung cancer cells in vitro and in mice. Cell Death Dis 2018;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011;121:4015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol 2010;50:754–66. [DOI] [PubMed] [Google Scholar]
- 38.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17:147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 2019;134:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Covington M, He X, Scuron M, Li J, Collins R, Juvekar A, et al. Preclinical characterization of itacitinib (INCB039110), a novel selective inhibitor of JAK1, for the treatment of inflammatory diseases. Eur J Pharmacol 2020;885:173505. [DOI] [PubMed] [Google Scholar]
- 41.Srinivas N, Barbour AM, Epstein N, Zhou G, Petusky S, Xun Z, et al. The effect of renal impairment on the pharmacokinetics and safety of itacitinib. J Clin Pharmacol 2020. doi: 10.1002/jcph.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007;178:2623–9. [DOI] [PubMed] [Google Scholar]
- 43.Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol 2020;5. [DOI] [PubMed] [Google Scholar]
- 44.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–8. [DOI] [PubMed] [Google Scholar]
- 46.Duell J, Lammers PE, Djuretic I, Chunyk AG, Alekar S, Jacobs I, et al. Bispecific antibodies in the treatment of hematologic malignancies. Clin Pharmacol Ther 2019;106:781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med 2009;10:387–92. [DOI] [PubMed] [Google Scholar]
- 48.Castilletti C, Bordi L, Lalle E, Rozera G, Poccia F, Agrati C, et al. Coordinate induction of IFN-alpha and -gamma by SARS-CoV also in the absence of virus replication. Virology 2005;341:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y, Wang QY. Bioinformatics analysis on molecular mechanism of ribavirin and interferon-alpha in treating MERS-CoV. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:291–3. [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.