Summary/abstract
The liver is unique in its remarkable regenerative capacity, which enables use of liver resection as a treatment for specific liver diseases, including removal of neoplastic liver disease. After resection, the remaining liver tissue (i.e, liver remnant) regenerates to maintain normal hepatic function. In experimental settings as well as patients, removal of up to 2/3rd of the liver mass stimulates a rapid and highly coordinated process resulting in regeneration of the remaining liver. Mechanisms controlling the initiation and termination of regeneration continue to be discovered, and many of the fundamental signaling pathways controlling proliferation of liver parenchymal cells (i.e., hepatocytes) have been uncovered. Interestingly, while hemostatic complications (i.e., bleeding and thrombosis) are primarily thought of as a complication of surgery itself, strong evidence suggests that components of the hemostatic system are in fact, powerful drivers of liver regeneration. This review focuses on clinical and translational evidence supporting a link between the hemostatic system and liver regeneration, and the mechanisms whereby the hemostatic system directs liver regeneration discovered using experimental settings.
Liver regeneration: Clinical application and current needs
Liver resection is considered the only curative treatment option for several neoplastic entities of the liver1, 2. Approximately 50% of all patients suffering from colorectal cancer will develop metastatic disease within the liver3 and liver failure caused by extensive tumor burden is one of the leading causes of death in these patients. For patients with metastatic disease limited to the liver, surgical resection of liver metastasis can result in long-term survival and even cure, as approximately 15% of these patients will never relapse4. Hepatectomies are performed for eligible patients based on the resection of all radiologically and macroscopically detectable tumor, while preserving at least 20–25% of healthy total liver volume5, 6. In most patients, the liver remnant regenerates to its original size to restore normal hepatic function. Importantly, despite substantial improvements in surgical techniques and peri-operative care, postoperative morbidity and mortality remain an important concern after liver resection7, 8. In fact, postoperative death after liver resection is reported to occur in up to 13% of high-risk patients9, 10.
Failed regeneration of the liver remnant can ultimately contribute to postoperative hepatic dysfunction and liver failure. Although extensive research has been performed on molecular mechanisms of liver regeneration, very limited translational research has been performed to document the relevance of these findings in the human setting, thereby impeding the development of new therapeutic compounds and strategies. Indeed, there is no treatment option available to promote liver regeneration for patients developing postoperative liver failure after liver resection. New surgical strategies to accelerate (postoperative) liver regeneration have been developed. Portal vein embolization (PVE) and portal vein ligation (PVL) have been widely used to increase the volume of the future liver remnant. Both techniques involve occlusion of the portal branches that provide blood to the liver fragments that will undergo resection, leading to hypertrophy of the future liver remnant11. In fact, PVL is one of the first steps in the two-stage surgical hepatectomy model called ALPPS (Associating Liver Partition and Portal vein ligation for Staged hepatectomy), where rapid liver regeneration is initiated by a combination of PVL and parenchymal transection. The more rapid liver regeneration observed after ALPPS has been attributed to a reduction in portal collateral formation12. A recently proposed method to increase the future liver remnant is the Liver Venous Deprivation (LVD) technique. It combines embolization of both the portal and hepatic vein in order to increase the damage of the embolized liver leading to rapid hypertrophy of the future liver remnant13. The underlying mechanisms triggering liver regeneration in these models are not completely understood. Previous studies suggest that one mechanism is an alteration in portal blood flow, which may stimulate liver regeneration via increased shear stress, increased accessibility of pro-regenerative factors, or reduced oxygen pressure14. However, even with these surgical advances, postoperative liver failure remains a frequent and critical issue without any available pharmacologic strategies for prophylaxis or treatment9, 10. As postoperative liver dysfunction and failure remains the main cause of death after liver resection, identifying strategies to promote liver regeneration is critical. Such therapies ideally should reduce post-operative complications caused by failed liver regeneration, potentially accelerate patient recovery after liver resection and most importantly, reduce the incidence of postoperative mortality.
Hemostatic factors in liver regeneration: experimental and translational evidence.
The fundamental mechanisms driving liver regeneration have been discovered using the well-characterized experimental setting of partial hepatectomy (PHx) in rodents. The standard PHx model most commonly involves surgical removal of 2/3rd of the rodent liver15. PHx is followed rapidly by a series of cell signaling events coordinating proliferation of the remaining liver parenchymal cells (i.e., hepatocytes) and reconstitution of hepatic non-parenchymal cells, ultimately restoring liver mass and function within 7–10 days15–17. Strong experimental evidence indicates this process is mediated by a complex interplay of pathways. Hepatocyte proliferation, for example, involves a number of growth factors including hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), thrombospondin-1 (TSP-1), serotonin and transforming growth factor β (TGF-β)18–20. Additional factors contributing to hepatocyte proliferation include non-mitogenic cytokines, bile acids, hormones, and other small molecules. The reader is referred to excellent reviews in which these processes have been detailed21, 22. There is considerable redundancy in the various pathways to ensure maximal regeneration. Indeed, there are numerous examples of deficiencies in a single pathway delaying liver regeneration, but few if any examples where a single deficit fully blocks the regenerative response. It seems as if the inhibition of multiple critical pathways is needed to block liver regeneration completely23.
Platelets and liver regeneration
Liver regeneration is initiated very quickly after surgical resection. Strong evidence suggests that components of the hemostatic system are among the earliest triggers of liver regeneration after PHx. Indeed since the first description that platelets can promote hepatocyte proliferation in 198224, significant evidence has been generated supporting a relevant role of platelets in liver regeneration. In particular, the significance of platelets during liver regeneration is supported by 1) their rapid accumulation (i.e., within 5 min) in the liver sinusoids after PHx25, 26 and translocation into the space of Disse 26 and 2) evidence that platelet number is directly associated with successful regeneration after PHx. Administration of platelet inhibitors or platelet depletion each significantly reduced hepatocyte proliferation and delayed liver regeneration after PHx25, 27. On the other hand, increasing circulating blood platelet count significantly promoted liver regeneration after 70% PHx28 and counteracted liver failure induced by extended (90%) PHx29, 30. Collectively, these studies suggest that platelets play a critical role as one of the earliest triggers of regeneration after PHx.
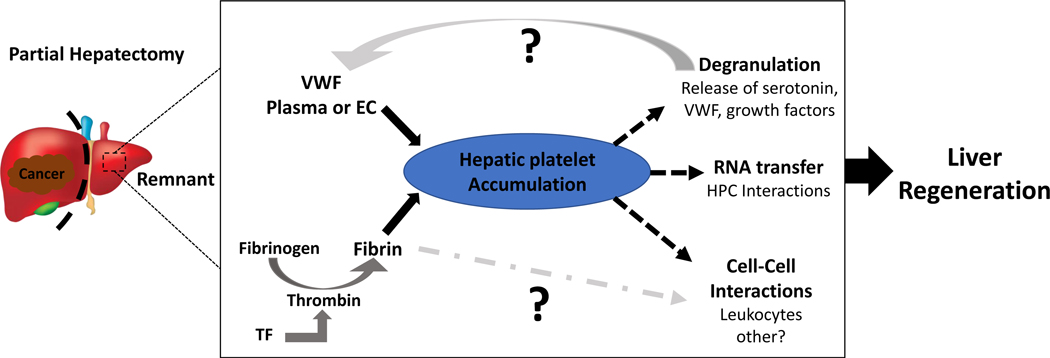
The exact molecular mechanisms whereby platelets contribute to liver regeneration need to be clarified (Figure 1). It has been proposed that local release of platelet granule contents in response to liver resection is responsible for platelet-mediated liver regeneration31–34. Indeed, in vitro studies have shown that platelets induce hepatocyte proliferation by release of growth factors35, 36. In this context, it is important to differentiate alpha and dense granules, which store different types of growth factor/molecules that may have divergent effects on liver regeneration.
Figure 1:
Mechanisms linking hemostatic factors to liver regeneration: Platelets rapidly accumulate in the liver remnant after partial hepatectomy. Accumulation of platelets in the liver appears to depend on multiple factors including von Willebrand factor (VWF), and on fibrin deposits formed as a result of intrahepatic coagulation. Experimental evidence suggests that platelets contribute to liver regeneration through multiple mechanisms including 1) degranulation and release of serotonin and other growth factors, 2) transfer of RNA by direct interactions with hepatocytes and 3) potentially through cell-cell interactions with leukocytes or other non-parenchymal cells. Among the gaps in knowledge include the source of VWF responsible for driving platelet accumulation (plasma, endothelial cells or platelets themselves), and whether fibrin drives regeneration through mechanisms independent of its role in platelet accumulation.
TF = Tissue factor
VWF = von Willebrand factor
EC = Endothelial Cell
HPC = Hepatocyte
Dense granules:
Multiple experimental and clinical studies suggest that platelet-derived serotonin could form a key component of platelet-mediated liver regeneration27, 37–39. However, there is also conflicting data on the role of serotonin in liver regeneration after liver resection. Mice lacking platelet serotonin (TPH1−/− mice) show a clear delay in liver regeneration after PHx27. However, TPH1−/− mice also have reduced platelet adhesion in vivo40, most likely due to defects in both von Willebrand factor (VWF)-mediated platelet adhesion and secondary platelet activation40, 41. As both platelet activation27 and VWF-dependent platelet accumulation25 are required for platelet-mediated liver regeneration, the delayed regenerative capacity in TPH1−/− mice may also be explained by these defects in hemostasis. It is also worth noting that liver regeneration after PHx is unaffected in plasma membrane serotonin transporter (SERT) deficient rats, despite having a marked reduction in platelet serotonin levels42. While experimental and translational evidence is growing, the precise contribution of serotonin to liver regeneration deserves further study.
α-granules:
Platelets store a wide variety of growth factors, including insulin-like growth factor (IGF), VEGF, HGF, in their α-granules which are released upon platelet activation. Most of these are potent inducers of liver regeneration. However, thrombospondin-1 (TSP-1), an inhibitor of regeneration, is also stored in this granule subtype. Clear evidence supports a pro-regenerative role for platelets, and yet the cargo of α-granules would be anticipated to have dichotomous effects. One potential explanation for this is the distinct packaging and agonist-dependent α-granule subset release, hypothesized by Italiano et al.43, which would allow for distinct platelet responses to specific stimuli43–48. Further studies are required to evaluate this possibility after liver resection, although our recent studies suggest a connection between levels of pro- and anti-proliferative growth factors and outcome in patients after liver resection33. Indeed, platelets from patients with postoperative liver dysfunction lacked capacity to secrete VEGF stored in their α-granules49. It should be noted that not all studies align with this hypothesis. Kirschbaum et al. found no evidence of release and consumption of platelet-derived growth factors after partial hepatectomy in humans50. One possible difference between these studies is differences in sample collection strategies51, highlighting both the need for standardized collection techniques and for more studies to evaluate the contribution of α-granule content to liver regeneration. Similarly, it is also important to consider that there may be general effects of major abdominal surgery on growth factor release and/or expression50.
In addition to release of platelet growth factors, another mechanism whereby platelets could stimulate liver regeneration is via transfer of platelet RNA to hepatocytes31. In vitro studies have shown that platelets stimulate proliferation of hepatocytes, which required direct contact between platelets and these cells52. Not only was direct contact required, but platelets were also internalized by hepatocytes53. Although platelets do not contain a nucleus, they do contain a wide selection of circulating RNAs54. In recent years it has been demonstrated that platelets are capable of RNA transfer to recipient cells, including monocytic and endothelial cells55. The Lisman group has demonstrated that RNA transfer from platelets to hepatocytes partly drives hepatocyte proliferation in vitro53. However, whether functional RNA transfer also occurs in vivo during liver regeneration has yet to be demonstrated.
Although platelets seem to be able to stimulate liver regeneration directly, other mechanisms involve their interaction with liver cell types to indirectly support liver regeneration. Platelets have been shown to stimulate liver sinusoidal endothelial cells (LSECs) to release potent hepatocyte growth factors such as HGF, VEGF and IL-652. This is of importance as immediately after induction of liver regeneration, LSECs are likely to contact platelets56. Indeed, 2 hours after induction of liver regeneration in humans, abundant interaction between LSECs and platelets has been documented56. Moreover, there is evidence to suggest a role for Kupffer cells in platelet-dependent liver regeneration, although thrombocytosis also promotes liver regeneration even after Kupffer cell depletion57, 58. The possibility that platelets could stimulate liver regeneration via non-liver cell dependent mechanisms has been largely unexplored. However, one alternative hypothesis involves platelet-dependent facilitation of the inflammatory response. Leukocytes accumulate in the liver remnant after PHx and liver regeneration is delayed in leukocytopenic mice59. Platelets are well known to recruit inflammatory cells60, and thus, it seems plausible that platelets could promote liver regeneration by facilitating leukocyte recruitment to the remnant liver. The interaction between platelets and the inflammatory response in the setting of PHx has not been investigated extensively, but recent studies in other models have demonstrated that platelets play an important role in neutrophil accumulation to sites of inflammation and tissue repair61, 62.
Platelet accumulation within the liver
Regardless of the potential mechanism(s) whereby platelets stimulate liver regeneration, accumulation of platelets within the liver remnant is likely a critical first step in platelet-directed liver regeneration (Figure 1). As mentioned before, hepatic platelet accumulation is very rapid after PHx, peaking within 30 minutes25, 63, and platelet accumulation in the liver shortly after PHx is most impactful with respect to liver regeneration. Indeed, timing seems to be of critical relevance as platelet depletion prior to PHx significantly reduced hepatocyte proliferation, whereas antibody-mediated thrombocytopenia initiated 2 hours after PHx had much less impact25. These studies imply that hepatic platelet accumulation in the first several hours after PHx is critical for liver regeneration. The mechanism driving platelet accumulation in the liver after PHx is likely multifactorial. Kirschbaum et al. found that administration of an anti-VWF antibody reduced hepatic platelet accumulation 30 min after PHx, and that hepatocyte proliferation was reduced in VWF-deficient mice after PHx25. Besides VWF, components of the coagulation cascade may attract platelets to the liver after PHx. For example, we have shown that depletion of plasma fibrinogen with ancrod also prevented hepatic platelet accumulation63. This suggests that in addition to adhesive proteins like VWF, coagulation factors are critical drivers of platelet accumulation in the liver after PHx, a potential basis for prior studies connecting blood coagulation to liver regeneration63, 64.
Coagulation factors in liver regeneration
Activation of coagulation has been shown to promote liver regeneration. Indeed, hepatic fibrin(ogen) deposition is already evident in the liver remnant of mice 30 min after PHx, and this corresponded to an increase in activation of blood coagulation, indicated by increased plasma thrombin-antithrombin (TAT) levels compared to sham-operated animals63. The source of intrahepatic coagulation activity after PHx appears to be activation of the extrinsic pathway. We found that liver-specific tissue factor (TF) deficiency attenuated plasma TAT levels and reduced hepatic fibrin(ogen) deposition after PHx63. Furthermore, Beier et. al. reported that hepatic fibrin(ogen) deposition was increased in mice even 48 hours after PHx64, suggesting a persistent response. In agreement with observations in liver TF-deficient mice63, administration of the thrombin inhibitor lepirudin reduced hepatic fibrin(ogen) deposition64, providing further evidence that thrombin drives fibrin(ogen) deposition in this setting. Beier et al. also discovered that plasminogen activator inhibitor-1 deficiency, anticipated to increase plasmin activity, reduced hepatic fibrinogen deposition after PHx64. Indeed, prior studies show that activation of fibrinolysis follows shortly after fibrin deposition in mice after PHx65. Notably, interpretation of these studies is complex as prior studies suggest a pro-regenerative role for plasmin(ogen) in liver regeneration66–68, although remodeling of the liver may be fibrin(ogen)-independent69. Collectively, there is strong evidence to suggest that hepatic fibrin(ogen) deposition after PHx is rapid and controlled by both procoagulant and fibrinolytic pathways. Importantly, inhibition of hepatic fibrin(ogen) deposition by liver TF-deficiency reduced hepatocyte proliferation, a result consistent with prior studies suggesting a reduction in hepatocyte proliferation after treatment with a direct thrombin inhibitor64. Furthermore, depletion of plasma fibrinogen with ancrod prior to PHx prevented hepatic fibrin(ogen) deposition and subsequently reduced hepatocyte proliferation after PHx63. Collectively, these studies suggest that coagulation activation and fibrin(ogen) deposition contributes to liver regeneration after PHx.
Importantly, not all proteolytic targets of thrombin contribute to regeneration. For example, hepatocyte proliferation was unaffected after PHx in mice lacking protease activated receptor-4 (PAR-4)63, the primary receptor for thrombin on mouse platelets70. It is also important to note that fibrin(ogen) may contribute to liver regeneration through multiple mechanisms, as it engages not only platelet integrins but also β2 integrins expressed by leukocytes, another cell type known to play a key role in regeneration after PHx59, 71, 72. Further studies are needed to identify specific mechanisms underlying fibrin(ogen)-directed platelet accumulation in the liver after PHx. Notably, platelets themselves amplified hepatic fibrin(ogen) deposition after PHx63, highlighting clear cross-talk contributing to amplification of intrahepatic coagulation after PHx. Similarly, additional studies are required to determine how fibrin(ogen) and VWF collectively promote hepatic platelet accumulation after PHx. Analogous to PHx in mice, an increase in hepatic fibrin(ogen) deposition was also evident in liver biopsies from liver resection patients taken 2 hours after ligation of the portal vein, perceived as the start of regeneration, compared to baseline levels63. Interestingly, increased hepatic fibrin(ogen) deposition was not observed in patients that went on to develop liver dysfunction63. A limitation of this study was the relatively small cohort of patients examined, and further analysis is required. However, this association suggests rapid hepatic fibrin(ogen) deposition is an essential early trigger for liver regeneration, a hypothesis supported by the aforementioned observation in mice wherein preventing hepatic fibrin(ogen) deposition reduced hepatocyte proliferation after PHx63, 64. There is also evidence to suggest that changes in plasma fibrinogen shortly after surgery are associated with outcome. Although pre-operative plasma fibrinogen concentration was similar between patients that had a complication-free recovery and patients that developed liver dysfunction, two independent studies have shown that postoperative plasma fibrinogen levels can be used to distinguish patients at risk of developing post-hepatectomy liver dysfunction63, 73. Overall, there is emerging evidence to suggest fibrin(ogen) has an important function in liver regeneration in mice and patients and highlight the importance of various components of the coagulation system in liver regeneration. This may lead to new opportunities to identify novel mechanisms driving intrahepatic coagulation after liver surgery.
Hemodynamic changes and hemostasis-mediated liver regeneration.
One of the earliest changes occurring after partial hepatectomy is an increase in hemodynamic forces imposed on the liver remnant. While effects on arterial blood flow seem to be minimal, the portal blood flow increases since a significant amount of blood from the extrahepatic splanchnic organs now needs to pass through a liver that is significantly reduced in size 18. Several studies have shown that the portal hyperdynamic state and the resulting increased intrahepatic shear stress is critical for liver regeneration, and may even serve as the starting point 72, 74–76. However, while an initial increase in portal venous flow seems to be required for adequate liver regeneration, excessive portal venous flow (termed “portal hyperperfusion”) may also contribute to liver dysfunction and failure after extended liver resection and segmented liver transplantation14.
The sudden increase in portal pressure after resection exposes the endothelial cells to excessive shear forces. Indeed, immediate (i.e. within 10 minutes) ultrastructural changes in the endothelial lining have been observed following PHx77. It is reasonable to speculate that platelets respond to these changes as they are known to adhere to LSECs78. In this context, potential VWF release from activated endothelial cells in response to increased shear stress may play a role in recruiting platelets. Indeed, VWF plasma levels increase rapidly after partial hepatectomy56, 79. Although these changes may be a general effect of major abdominal surgery79, 80, VWF plasma levels increased within two hours after induction of liver regeneration in the liver vein of patients undergoing partial hepatectomy56. Furthermore, this rapid VWF increase after induction of liver regeneration seemed to be required for platelet accumulation and liver regeneration in patients56.
Although our previous studies identified that the rapid procoagulant response after PHx is initiated by hepatic TF, the trigger for hepatocyte TF activation after PHx has yet to be discovered. Upon vascular injury, subendothelial TF is exposed to its ligand factor VIIa (FVIIa), initiating coagulation. However, hepatocytes expresses a TF:FVIIa complex that is an encrypted state and requires activation via decryption to activate coagulation81. Phosphatidylserine (PS) externalization triggered by apoptosis is one potential mechanism of TF decryption in the injured/diseased liver82–84. However, apoptosis after partial hepatectomy is minimal due to activation of the anti-apoptotic Akt pathway85. It seems plausible that the immediate change in portal blood flow could activate intrahepatic coagulation. Indeed, in other experimental settings, it has been shown that shear stress increased TF expression and activity86, 87. Whether hemodynamic changes after partial hepatectomy activates coagulation remains to be determined.
Targeting the hemostatic system to promote regeneration?
Strong evidence from experimental studies links changes in the hemostatic system with liver regeneration. Moreover, clinical studies have provided corresponding evidence in patients, wherein fibrinogen or platelet-centered measurements associate with development of liver dysfunction and outcome after liver resection. Thus, with further study it may be possible to identify strategies to accelerate liver regeneration in patients after liver resection.
Although many of the pro-regenerative factors released by platelets have very short half-lives when circulating free in blood, storage in platelets serves to protect these factors51. In this way, platelets represent very sensitive sentinels that rapidly respond to injury by releasing their cargo. Platelet activation and aggregation leads to site-specific release of granule content, which produces locally high concentrations of platelet-derived factors important for liver regeneration. Rapid availability of these growths factors is a prerequisite for functional liver regeneration processes. Thrombopoietin (TPO) has been shown to promote liver regeneration in mice, presumably by increasing platelet number88. Therefore, TPO might represent a potential therapeutic target as TPO mimetics are approved and in clinical use. However, elevating platelet number might increase the risk for (portal vein) thrombosis, mortality, and cancer recurrence89–91. In addition to modulation of platelet counts, pharmacological modulation of perioperative hepatic hemodynamics, via its impact on intrahepatic platelet accumulation and perhaps coagulation activation, might represent a tool to modulate liver regeneration. Indeed, pharmacologic manipulation of portal pressure using terlipressin has been shown to promote liver regeneration in mice92. However, the prospective randomized trial of the same group that followed this experimental trial did not show a significant improvement in patients’ outcome93. Although an effect might have been missed by trial design and power, this illustrates the complexity of translating observations from experimental PHx to liver resection in patients. The heterogeneity of underlying liver disease within patients is only one of several potential reasons why translation of treatment strategies to the real-life clinical setting might fail. This, however, also demonstrates the importance of documenting the clinical relevance of experimental findings. Ultimately, more detailed analyses in this area is needed and studies examining platelet activation markers or degranulation in clinical studies measuring or modifying portal pressure could be very revealing.
Although manipulating platelet aggregation is an attractive option to drive liver regeneration, targeting platelet contents might also be an effective therapeutic approach to modulate liver regeneration. As an example, we were able to recently demonstrate that selective serotonin reuptake inhibitor (SSRI) therapy reduced intraplatelet serotonin content prior to surgery in patients undergoing liver resection39. In line with our existing translational result, we observed that patients taking SSRIs displayed worse postoperative outcome after liver resection when platelet serotonin levels were compromised. Identifying creative strategies to modify granule content could represent an attractive therapeutic option to promote liver regeneration. However, as with other suggestions herein, all these potential therapeutic options require exploration and validation in clinical trials.
Identifying mechanisms driving hepatic platelet accumulation seems likely to reveal novel strategies to promote platelet-directed liver regeneration. Because platelets accumulate in the liver very rapidly after liver resection in both mice and humans, and this rapid accumulation seems of the utmost importance, interventions that boost this early platelet response may be most effective. Mechanistic studies suggest that both VWF and fibrinogen contribute to platelet accumulation after PHx in mice. Stimulating the release of VWF from endothelial cells using drugs like DDAVP (desmopressin) would be one possibility. However, as VWF is stored in endothelial cells, endothelial exhaustion may limit the efficiency of the drug. Indeed, DDAVP has limited efficacy in patients with underlying liver disease94. Furthermore, our results indicate that the increase in VWF after liver resection is limited in patients with underlying liver disease56. As an alternative, administration of these proteins to augment the early hepatic platelet response after liver resection seems plausible and would circumvent possible endothelial exhaustion. Both VWF and fibrinogen are available as concentrates and used clinically (i.e., Humate-P/Haemate-P, RiaSTAP/Haemocomplettan P). It seems plausible that in patients where fibrinogen or VWF levels are deemed too low to support regeneration, this defect could easily be corrected intraoperatively, or even modestly increased to further promote regeneration. It is worth noting that liver resection patients commonly receive anticoagulants approximately 6 hours after surgery to reduce the risk of thrombosis. Hepatic platelet and fibrin(ogen) deposits responsible for driving liver regeneration occur prior to this63, making it unlikely that postoperative anticoagulation interferes with this mechanism of regeneration. This also keeps with the plausibility of using intraoperative interventions to promote platelet-directed liver regeneration while limiting the risk of thrombosis after surgery.
Summary and Conclusions:
In summary, strong experimental evidence links components of the hemostatic system (e.g., VWF, platelets, coagulation factors) to liver regeneration after PHx in mice. Clinical results supporting translation of these results to liver resection patients are compelling and additional details continue to emerge. Indeed, the combined strength of mechanisms discovered in experimental PHx and clinical association studies may reveal 1) novel strategies to predict post-hepatectomy liver dysfunction and 2) new therapies using components of the hemostatic system to promote liver regeneration.
Acknowledgments
Research support: This research was supported by grants from the National Institutes of Health (NIH) to JPL (R01 ES017537, DK120289), support from the USDA National Institute of Food and Agriculture, and an EHA Research Grant from the European Hematology Association (EHA) to DG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
References:
- [1].Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M: Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. British journal of cancer 2006, 94:982–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, Ychou M, Rougier P: Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. European journal of cancer (Oxford, England : 1990) 2006, 42:2212–21. [DOI] [PubMed] [Google Scholar]
- [3].Bramhall SR, Gur U, Coldham C, Gunson BK, Mayer AD, McMaster P, Candinas D, Buckels JA, Mirza DF: Liver resection for colorectal metastases. Annals of the Royal College of Surgeons of England 2003, 85:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D: Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008, 26:3672–80. [DOI] [PubMed] [Google Scholar]
- [5].Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR: Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009, 27:3677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D: Improving resectability of hepatic colorectal metastases: expert consensus statement. Annals of surgical oncology 2006, 13:1271–80. [DOI] [PubMed] [Google Scholar]
- [7].Wei AC, Tung-Ping Poon R, Fan ST, Wong J: Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. The British journal of surgery 2003, 90:33–41. [DOI] [PubMed] [Google Scholar]
- [8].Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN: Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. Journal of the American College of Surgeons 2007, 204:854–62; discussion 62–4. [DOI] [PubMed] [Google Scholar]
- [9].Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V, Laurent A, Gauzolino R, Castro Benitez C, Pequignot A, Donckier V, Lim C, Blanleuil ML, Brustia R, Le Treut YP, Soubrane O, Azoulay D, Farges O, Adam R, Pruvot FR, e HSGftAdCH-BedT: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2015, 41:674–82. [DOI] [PubMed] [Google Scholar]
- [10].Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA: Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015, 22:3109–20. [DOI] [PubMed] [Google Scholar]
- [11].Szijarto A, Fulop A: Triggered liver regeneration: from experimental model to clinical implications. Eur Surg Res 2015, 54:148–61. [DOI] [PubMed] [Google Scholar]
- [12].Deal R, Frederiks C, Williams L, Olthof PB, Dirscherl K, Keutgen X, Chan E, Deziel D, Hertl M, Schadde E: Rapid Liver Hypertrophy After Portal Vein Occlusion Correlates with the Degree of Collateralization Between Lobes-a Study in Pigs. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2018, 22:203–13. [DOI] [PubMed] [Google Scholar]
- [13].Panaro F, Giannone F, Riviere B, Sgarbura O, Cusumano C, Deshayes E, Navarro F, Guiu B, Quenet F: Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. Hepatobiliary Surg Nutr 2019, 8:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abshagen K, Eipel C, Vollmar B: A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg 2012, 397:579–90. [DOI] [PubMed] [Google Scholar]
- [15].Mitchell C, Willenbring H: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature protocols 2008, 3:1167–70. [DOI] [PubMed] [Google Scholar]
- [16].Michalopoulos GK: Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. The American journal of pathology 2010, 176:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nevzorova YA, Tolba R, Trautwein C, Liedtke C: Partial hepatectomy in mice. Laboratory animals 2015, 49:81–8. [DOI] [PubMed] [Google Scholar]
- [18].Michalopoulos GK: Liver regeneration. J Cell Physiol 2007, 213:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clavien PA: Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss Med Wkly 2008, 138:361–70. [DOI] [PubMed] [Google Scholar]
- [20].Clavien PA, Petrowsky H, DeOliveira ML, Graf R: Strategies for safer liver surgery and partial liver transplantation. The New England journal of medicine 2007, 356:1545–59. [DOI] [PubMed] [Google Scholar]
- [21].Michalopoulos GK: Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65:1384–92. [DOI] [PubMed] [Google Scholar]
- [22].Michalopoulos GK: Advances in liver regeneration. Expert review of gastroenterology & hepatology 2014, 8:897–907. [DOI] [PubMed] [Google Scholar]
- [23].Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, Liu S, Tseng GC, Tsagianni A, Michalopoulos GK: Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology 2016, 64:1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Strain AJ, McGowan JA, Bucher NL: Stimulation of DNA synthesis in primary cultures of adult rat hepatocytes by rat platelet-associated substance(s). In Vitro 1982, 18:108–16. [DOI] [PubMed] [Google Scholar]
- [25].Kirschbaum M, Jenne CN, Veldhuis ZJ, Sjollema KA, Lenting PJ, Giepmans BNG, Porte RJ, Kubes P, Denis CV, Lisman T: Transient von Willebrand factor-mediated platelet influx stimulates liver regeneration after partial hepatectomy in mice. Liver international : official journal of the International Association for the Study of the Liver 2017, 37:1731–7. [DOI] [PubMed] [Google Scholar]
- [26].Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R: Platelets promote liver regeneration in early period after hepatectomy in mice. World journal of surgery 2007, 31:808–16. [DOI] [PubMed] [Google Scholar]
- [27].Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA: Platelet-derived serotonin mediates liver regeneration. Science (New York, NY) 2006, 312:104–7. [DOI] [PubMed] [Google Scholar]
- [28].Matsuo R, Nakano Y, Ohkohchi N: Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Annals of surgery 2011, 253:759–63. [DOI] [PubMed] [Google Scholar]
- [29].Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T, Hashimoto I, Shibasaki Y, Yasue H, Ohkohchi N: Role of platelets on liver regeneration after 90% hepatectomy in mice. Journal of hepatology 2008, 49:363–72. [DOI] [PubMed] [Google Scholar]
- [30].Lopez ML, Kieling CO, Uribe Cruz C, Osvaldt A, Ochs de Munoz G, Meurer L, Silla L, Matte U: Platelet increases survival in a model of 90% hepatectomy in rats. Liver international : official journal of the International Association for the Study of the Liver 2014, 34:1049–56. [DOI] [PubMed] [Google Scholar]
- [31].Lisman T, Porte RJ: Mechanisms of platelet-mediated liver regeneration. Blood 2016, 128:625–9. [DOI] [PubMed] [Google Scholar]
- [32].Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi F, Kohler A, Offensperger F, Reiberger T, Ferlitsch A, Messner B, Beldi G, Staettner S, Brostjan C, Gruenberger T: Perioperative Von Willebrand Factor Dynamics are Associated with Liver Regeneration and Predict Outcome after Liver Resection. Hepatology (Baltimore, Md) 2017. [DOI] [PubMed] [Google Scholar]
- [33].Starlinger P, Haegele S, Offensperger F, Oehlberger L, Pereyra D, Kral JB, Schrottmaier WC, Badrnya S, Reiberger T, Ferlitsch A, Stift J, Luf F, Brostjan C, Gruenberger T, Assinger A: The profile of platelet alpha-granule released molecules affects postoperative liver regeneration. Hepatology 2016, 63:1675–88. [DOI] [PubMed] [Google Scholar]
- [34].Starlinger P, Assinger A: Importance of platelet-derived growth factors in liver regeneration. Expert review of gastroenterology & hepatology 2016:1–3. [DOI] [PubMed] [Google Scholar]
- [35].Balasubramanian S, Paulose CS: Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology 1998, 27:62–6. [DOI] [PubMed] [Google Scholar]
- [36].Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H, Ozaki M: Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. The Journal of surgical research 2008, 145:279–86. [DOI] [PubMed] [Google Scholar]
- [37].Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, Papadopoulou-Daifoti Z, Mykoniatis MG: Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver international : official journal of the International Association for the Study of the Liver 2006, 26:352–61. [DOI] [PubMed] [Google Scholar]
- [38].Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D, Birner P, Fleischmann E, Gruenberger B, Brostjan C, Gruenberger T: Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology 2014, 60:257–66. [DOI] [PubMed] [Google Scholar]
- [39].Padickakudy R, Pereyra D, Offensperger F, Jonas P, Oehlberger L, Schwarz C, Haegele S, Assinger A, Brostjan C, Gruenberger T, Starlinger P: Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. Journal of hepatology 2017, 67:1243–52. [DOI] [PubMed] [Google Scholar]
- [40].Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M: Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003, 115:851–62. [DOI] [PubMed] [Google Scholar]
- [41].Duerschmied D, Canault M, Lievens D, Brill A, Cifuni SM, Bader M, Wagner DD: Serotonin stimulates platelet receptor shedding by tumor necrosis factor-alpha-converting enzyme (ADAM17). Journal of thrombosis and haemostasis : JTH 2009, 7:1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, Akkerman JW, Cuppen E, de Bruin A: Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol 2009, 296:G963–8. [DOI] [PubMed] [Google Scholar]
- [43].Italiano JE Jr., Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL: Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008, 111:1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sehgal S, Storrie B: Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. Journal of thrombosis and haemostasis : JTH 2007, 5:2009–16. [DOI] [PubMed] [Google Scholar]
- [45].Battinelli EM, Markens BA, Italiano JE Jr., Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011, 118:1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chatterjee M, Huang Z, Zhang W, Jiang L, Hultenby K, Zhu L, Hu H, Nilsson GP, Li N: Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood 2011, 117:3907–11. [DOI] [PubMed] [Google Scholar]
- [47].van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF: The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood 2010, 116:1147–56. [DOI] [PubMed] [Google Scholar]
- [48].Peters CG, Michelson AD, Flaumenhaft R: Granule exocytosis is required for platelet spreading: differential sorting of alpha-granules expressing VAMP-7. Blood 2012, 120:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Starlinger P, Assinger A: Reply. Hepatology (Baltimore, Md) 2016, 64:992–3. [DOI] [PubMed] [Google Scholar]
- [50].Kirschbaum M, Adelmeijer J, Alkozai EM, Porte RJ, Lisman T: Evidence against a role for platelet-derived molecules in liver regeneration after partial hepatectomy in humans. Journal of clinical and translational research 2016, 2:97–106. [PMC free article] [PubMed] [Google Scholar]
- [51].Mussbacher M, Schrottmaier WC, Salzmann M, Brostjan C, Schmid JA, Starlinger P, Assinger A: Optimized plasma preparation is essential to monitor platelet-stored molecules in humans. PloS one 2017, 12:e0188921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kawasaki T, Murata S, Takahashi K, Nozaki R, Ohshiro Y, Ikeda N, Pak S, Myronovych A, Hisakura K, Fukunaga K, Oda T, Sasaki R, Ohkohchi N: Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. Journal of hepatology 2010, 53:648–54. [DOI] [PubMed] [Google Scholar]
- [53].Kirschbaum M, Karimian G, Adelmeijer J, Giepmans BN, Porte RJ, Lisman T: Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood 2015, 126:798–806. [DOI] [PubMed] [Google Scholar]
- [54].Schubert S, Weyrich AS, Rowley JW: A tour through the transcriptional landscape of platelets. Blood 2014, 124:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Risitano A, Beaulieu LM, Vitseva O, Freedman JE: Platelets and platelet-like particles mediate intercellular RNA transfer. Blood 2012, 119:6288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi F, Kohler A, Offensperger F, Reiberger T, Ferlitsch A, Messner B, Beldi G, Staettner S, Brostjan C, Gruenberger T: Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018, 67:1516–30. [DOI] [PubMed] [Google Scholar]
- [57].Takahashi K, Kozuma Y, Suzuki H, Tamura T, Maruyama T, Fukunaga K, Murata S, Ohkohchi N: Human platelets promote liver regeneration with Kupffer cells in SCID mice. The Journal of surgical research 2013, 180:62–72. [DOI] [PubMed] [Google Scholar]
- [58].Murata S, Matsuo R, Ikeda O, Myronovych A, Watanabe M, Hisakura K, Nakano Y, Hashimoto I, Ohkohchi N: Platelets promote liver regeneration under conditions of Kupffer cell depletion after hepatectomy in mice. World J Surg 2008, 32:1088–96. [DOI] [PubMed] [Google Scholar]
- [59].Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA: ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology 2003, 124:692–700. [DOI] [PubMed] [Google Scholar]
- [60].Jenne CN, Kubes P: Platelets in inflammation and infection. Platelets 2015, 26:286–92. [DOI] [PubMed] [Google Scholar]
- [61].Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee WY, Kubes P: Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 2015, 62:1593–605. [DOI] [PubMed] [Google Scholar]
- [62].Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, Wagner DD: Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Groeneveld D, Pereyra D, Veldhuis Z, Adelmeijer J, Ottens P, Kopec AK, Starlinger P, Lisman T, Luyendyk JP: Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood 2019, 133:1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Beier JI, Guo L, Ritzenthaler JD, Joshi-Barve S, Roman J, Arteel GE: Fibrin-mediated integrin signaling plays a critical role in hepatic regeneration after partial hepatectomy in mice. Annals of hepatology 2016, 15:762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK: Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology 1997, 26:896–904. [DOI] [PubMed] [Google Scholar]
- [66].Roselli HT, Su M, Washington K, Kerins DM, Vaughan DE, Russell WE: Liver regeneration is transiently impaired in urokinase-deficient mice. Am J Physiol 1998, 275:G1472–9. [DOI] [PubMed] [Google Scholar]
- [67].Drixler TA, Vogten JM, Gebbink MF, Carmeliet P, Voest EE, Borel Rinkes IH: Plasminogen mediates liver regeneration and angiogenesis after experimental partial hepatectomy. The British journal of surgery 2003, 90:1384–90. [DOI] [PubMed] [Google Scholar]
- [68].Miura A, Ishiguro K, Koizumi K, Yaita Y, Ozaki-Masuzawa Y, Hosono T, Seki T: Effects of pharmacological inhibition of plasminogen binding on liver regeneration in rats. Biosci Biotechnol Biochem 2017, 81:2105–11. [DOI] [PubMed] [Google Scholar]
- [69].Bezerra JA, Bugge TH, Melin-Aldana H, Sabla G, Kombrinck KW, Witte DP, Degen JL: Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proceedings of the National Academy of Sciences of the United States of America 1999, 96:15143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV Jr., Tam C, Coughlin SR: A dual thrombin receptor system for platelet activation. Nature 1998, 394:690–4. [DOI] [PubMed] [Google Scholar]
- [71].Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, Dijkstra CD, van Leeuwen PA: Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 2000, 20:66–77. [DOI] [PubMed] [Google Scholar]
- [72].Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B: Kupffer cells are mandatory for adequate liver regeneration by mediating hyperperfusion via modulation of vasoactive proteins. Microcirculation 2008, 15:37–47. [DOI] [PubMed] [Google Scholar]
- [73].Giovannini I, Chiarla C, Giuliante F, Vellone M, Nuzzo G: Modulation of plasma fibrinogen levels in acute-phase response after hepatectomy. Clin Chem Lab Med 2004, 42:261–5. [DOI] [PubMed] [Google Scholar]
- [74].Marubashi S, Sakon M, Nagano H, Gotoh K, Hashimoto K, Kubota M, Kobayashi S, Yamamoto S, Miyamoto A, Dono K, Nakamori S, Umeshita K, Monden M: Effect of portal hemodynamics on liver regeneration studied in a novel portohepatic shunt rat model. Surgery 2004, 136:1028–37. [DOI] [PubMed] [Google Scholar]
- [75].Niiya T, Murakami M, Aoki T, Murai N, Shimizu Y, Kusano M: Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg 1999, 6:275–80. [DOI] [PubMed] [Google Scholar]
- [76].Sato Y, Tsukada K, Hatakeyama K: Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today 1999, 29:1–9. [DOI] [PubMed] [Google Scholar]
- [77].Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, Spira G: Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol 2004, 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lalor PF, Herbert J, Bicknell R, Adams DH: Hepatic sinusoidal endothelium avidly binds platelets in an integrin-dependent manner, leading to platelet and endothelial activation and leukocyte recruitment. Am J Physiol Gastrointest Liver Physiol 2013, 304:G469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Baruch Y, Neubauer K, Shenkar L, Sabo E, Ritzel A, Wilfling T, Ramadori G: Von Willebrand factor in plasma and in liver tissue after partial hepatectomy in the rat. Journal of hepatology 2002, 37:471–7. [DOI] [PubMed] [Google Scholar]
- [80].Groeneveld DJ, Alkozai EM, Adelmeijer J, Porte RJ, Lisman T: Balance between von Willebrand factor and ADAMTS13 following major partial hepatectomy. The British journal of surgery 2016, 103:735–43. [DOI] [PubMed] [Google Scholar]
- [81].Kopec AK, Luyendyk JP: Coagulation in liver toxicity and disease: role of hepatocyte tissue factor. Thrombosis research 2014, 133 Suppl 1:S57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lopez M, Kopec AK, Joshi N, Geddings JE, Cline H, Towery KL, Rockwell CE, Mackman N, Luyendyk JP: Fas-induced apoptosis increases hepatocyte tissue factor procoagulant activity in vitro and in vivo. Toxicological sciences : an official journal of the Society of Toxicology 2014, 141:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Weerasinghe SV, Moons DS, Altshuler PJ, Shah YM, Omary MB: Fibrinogen-gamma proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage. Hepatology 2011, 53:1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kopec AK, Spada AP, Contreras PC, Mackman N, Luyendyk JP: Caspase Inhibition Reduces Hepatic Tissue Factor-Driven Coagulation In Vitro and In Vivo. Toxicological sciences : an official journal of the Society of Toxicology 2018, 162:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hong F, Nguyen VA, Shen X, Kunos G, Gao B: Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem Biophys Res Commun 2000, 279:974–9. [DOI] [PubMed] [Google Scholar]
- [86].Lin MC, Almus-Jacobs F, Chen HH, Parry GC, Mackman N, Shyy JY, Chien S: Shear stress induction of the tissue factor gene. The Journal of clinical investigation 1997, 99:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Houston P, Dickson MC, Ludbrook V, White B, Schwachtgen JL, McVey JH, Mackman N, Reese JM, Gorman DG, Campbell C, Braddock M: Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arteriosclerosis, thrombosis, and vascular biology 1999, 19:281–9. [DOI] [PubMed] [Google Scholar]
- [88].Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N: Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Annals of surgery 2008, 248:821–8. [DOI] [PubMed] [Google Scholar]
- [89].Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, Campbell F, Hyde D, Brainsky A, Theodore D, Group ES: Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. The New England journal of medicine 2012, 367:716–24. [DOI] [PubMed] [Google Scholar]
- [90].de Boer MT, Christensen MC, Asmussen M, van der Hilst CS, Hendriks HG, Slooff MJ, Porte RJ: The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg 2008, 106:32–44, table of contents. [DOI] [PubMed] [Google Scholar]
- [91].Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV, Iannacone M, Guidotti LG: Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proceedings of the National Academy of Sciences of the United States of America 2012, 109:E2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fahrner R, Patsenker E, de Gottardi A, Stickel F, Montani M, Stroka D, Candinas D, Beldi G: Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation 2014, 97:892–900. [DOI] [PubMed] [Google Scholar]
- [93].Kohler A, Perrodin S, De Gottardi A, Candinas D, Beldi G: Effectiveness of terlipressin for prevention of complications after major liver resection - A randomized placebo-controlled trial. HPB (Oxford) 2019. [DOI] [PubMed] [Google Scholar]
- [94].Arshad F, Stoof SC, Leebeek FW, Ruitenbeek K, Adelmeijer J, Blokzijl H, van den Berg AP, Porte RJ, Kruip MJ, Lisman T: Infusion of DDAVP does not improve primary hemostasis in patients with cirrhosis. Liver international : official journal of the International Association for the Study of the Liver 2015, 35:1809–15. [DOI] [PubMed] [Google Scholar]