Abstract
Traffic-related airborne particles are associated with asthma morbidity. The aim of this study was to assess the impact of a high-efficiency particulate air (HEPA) filtration on the concentrations of traffic particles and the resultant effect on children with asthma. Forty-three children with asthma were enrolled in this double-blind, placebo-controlled crossover design. A HEPA air cleaner or a placebo “dummy” was placed in participants’ homes for four weeks, interrupted by a one-month washout period, before crossing over to the other treatment arm for four weeks. Air sampling and health outcomes, including asthma control (ACQ) and quality of life (AQLQ) measures, were completed prior to and at the end of each treatment arm. Indoor concentrations of traffic particles were significantly reduced with the HEPA treatment but not with the “dummy” treatment. In participants with poorly controlled asthma and lower quality of life at baseline, ACQ and AQLQ scores were significantly improved (1.3 to 0.9, p = 0.003 and 4.9 to 5.5, p = 0.02, respectively) following the HEPA treatment. In this study, HEPA filtration is associated with improved clinical outcomes and quality of life measures in children with uncontrolled asthma.
Keywords: PM2.5, Black carbon, high-efficiency particulate air filter, Asthma Control Questionnaire, Asthma Quality of Life Questionnaire
1. Introduction
Despite advances in diagnosis and treatment, asthma remains the leading cause of chronic illness among the pediatric population and affects approximately 1 in 10 children in the United States.1 Asthma is a significant burden on healthcare expenditures and is the leading cause of hospitalizations, emergency department visits, and missed school days.2 The burden of asthma is also disproportionately borne by African-American children, who, between the ages of 0–17, continue to have higher rates of asthma prevalence and mortality than their Caucasian counterparts.3
The indoor environment where children and infants spend eighty percent of their time,4 is contaminated by particulate matter from both indoor and outdoor sources. Traffic is a major source of the latter particularly in buildings in close proximity to major roads where particles below 2.5 μm in size (PM2.5) may efficiently infiltrate the indoor environment.5, 6 Approximately 11.3 million people in the United States live within 150 meters of a highway, where high exposure to traffic-related pollutants may increase their risk of adverse health outcomes, including childhood asthma, wheezing, reduced lung function, and all-cause mortality.7
Exposure to traffic-related airborne pollutants (TRAP), including PM2.5, NOx, and diesel exhaust particles, has been associated with increased incidence of asthma overall, including among young and adolescent children.5, 6, 8 The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) showed significant associations between TRAP exposure and adverse respiratory outcomes in children, including early childhood wheezing, persistent wheezing through age 7, and the development of asthma by age 7.9 Epstein et al. reported that in adults, a reduction in outdoor traffic particles by as little as 25% had a clinically important impact on asthma control.10
In addition to outdoor sources, smoking, cooking, heating, and other indoor activities also contribute to pollutant concentrations. Smoking is a major source of fine and ultrafine particles and cooking with combustible fuels can produce particles such as black carbon. Other types of indoor particles include pet allergens and mold spores in poorly maintained homes. Therefore, reducing indoor contaminants and particulate exposures is a major public health goal.
Previous studies have shown that air purifiers with high efficiency particulate air (HEPA) filtration characteristics significantly reduce the concentration of indoor particles in homes11–23 and schools.24 However, studies reporting on the impact of HEPA filtration on health outcomes have had varied results.25–33 While there were statistically significant benefits of particle filtration on health outcomes for those with asthma and allergic rhinitis, these improvements were defined as “modest”.34, 35 It should be noted, however, that a recent study by Jia-Ying et al. reported improvements in allergic rhinitis symptoms by using HEPA filtration.36 Although HEPA interventions were calculated to produce an economic benefit by reducing premature mortality in general,37, 38, the health benefit of using HEPA air cleaners against traffic related airborne particles is much less studied.
Previously we reported that concentrations of particulate matter less than 2.5 μm (PM2.5), black carbon, second-hand smoke, and mold spores were significantly reduced by placing a HEPA-filtration air purifier in the bedrooms of children with asthma.39 In this report, we examined whether treatment with HEPA filtration, in comparison to placebo-control, was associated with improved asthma control and quality of life measures in children.
2. Methods
2.1. Study Population and Design
Children aged 10 to 16 with a physician diagnosis of asthma were recruited from the CCAAPS cohort, a prospective birth cohort residing in the greater Cincinnati area,40 and through public advertisements at Cincinnati Children’s Hospital Medical Center (CCHMC). Participants were screened for elevated residential TRAP exposure through a previously developed and validated land-use regression model, a method that yielded a quantitative estimate of air pollution exposure based on a group of geographic variables (distance to the roadway, traffic density, elevation, etc.).41 Eligibility to participate in this study required residing in a home with elevated TRAP exposure, defined as a distance less than 500 meters from a major roadway or an estimated concentration of elemental carbon attributable to traffic (ECAT) ≥ 0.33 μg/m3.41
This double-blind, placebo-controlled crossover study was conducted over three months with 43 participants from October 2015 to August 2017. The recruitment included 44 participants of which two were twins residing in the same home. We randomly selected one of the twins to these data analyses. Participants were randomly assigned to the HEPA filter (Whirlpool Whispure; Model AP51030K, Austin, TX) or placebo “dummy” air purifier (the same air cleaner with the HEPA filter removed) group for one month, followed by a one-month washout period, before crossing over to the alternate one-month treatment arm. The one-month washout period was chosen in order to minimize the impact of treatment order on asthma outcomes, some of which reflect asthma control for the previous month.42, 43 The research team completed a home walkthrough for each participant to ascertain home characteristics. Air cleaners were placed in the bedroom of each participant. Participants were also given questionnaires that assessed clinical symptoms over the course of the study. The study was approved by the Institutional Review Board of the University of Cincinnati. All children and their parents gave assent and written informed consent for participation.
2.2. Assessment of Environmental Exposures
Samples of airborne particles were collected in each child’s bedroom for 48 hours before and after both HEPA and “dummy” periods using Personal Modular PM2.5 impactors (SKC Inc., Eight-four, PA) with pre-weighed 37-mm polytetrafluoroethylene (PTFE) filters (Pall, Port Washington, NY). The samples were then analyzed for PM2.5 mass, black carbon (BC), and ultraviolet-absorbing particulate matter (UVPM).44 Inhalable fungal spores were also collected onto 25-mm diameter, PTFE filters (Merck Millipore, Billerica, MA) using Button™ samplers (SKC, Inc., Eighty-four, PA). Button samples were analyzed using a mold-specific quantitative PCR (MSQPCR). An Environmental Relative Moldiness Index-like (ERMI-like) value was calculated from the MSQPCR results.45 Temperature and humidity were recorded (HOBO Humidity Data Logger, Onset, Bourne, MA) for the entire 1-month duration of the HEPA treatment and “dummy” period, including the baseline and treatment sampling periods.
2.3. Assessment of Respiratory Health Outcomes
Respiratory health outcomes were assessed by questionnaires and spirometry by “treatment-blinded” research team members. Baseline surveys addressing participants’ overall health status and medication use for asthma and allergic rhinitis were completed prior to spirometry at each visit. Asthma control and quality of life were evaluated using the Asthma Control Questionnaire (ACQ) and the Mini Asthma Quality of Life Questionnaire (AQLQ), respectively, before and after each arm of the treatments.46, 47 Spirometry was conducted using a portable spirometer (KoKo diagnostic spirometer, Model: Sx 1000, nSpire Health, Longmont, CO) following ATS/ERS recommendations.48 Because age, height, gender, and race explain >65% of the variability in lung function,49 percent predicted spirometry values were used (100 * observed value/predicted value) and were based on Wang et al. (1993) (standards for 6–18-year olds).50 Patients with ACQ scores < 0.75 have an 85% probability of having well-controlled asthma and therefore we defined participants with ACQ scores < 0.75 as being ‘well-controlled’.51 Although this group also suggested 1.50 as the optimal cut-off for poorly controlled asthma, a recent study evaluating the reliability, validity, and responsiveness to change of the ACQ determined a different threshold in children.51 The diagnostic test accuracy of ACQ thresholds ranging between 0.25 and 2.00 was analyzed for a receiver operating characteristic (ROC) curve. A score of >1.25 based on the ROC curve analysis was optimal for identifying poorly controlled asthma status and was corroborated in studies of adults with asthma.42, 52 As such, the median threshold for ‘not well-controlled’ asthma was defined in this study as an ACQ score of >1.25.
The cutoff point between a ‘non-impaired’ quality of life and ‘impaired’ quality of life was placed at 5.40, as indicated in other clinical studies performed in adults.53, 54 A change in score of 0.5 in ACQ and mini-AQLQ scores was considered clinically meaningful, as has been previously established and validated.42, 51
2.4. Statistical Analysis
Indoor concentrations of aerosol particles, ACQ and mini-AQLQ scores were compared using the Wilcoxon-signed rank test. The paired values before and after HEPA as well as before and after “dummy” treatment were separately compared. Further analysis of health outcomes was conducted via stratification of participants by asthma control and quality of life at baseline.
The baseline and treatment in ACQ and AQLQ scores of participants who had ‘not well controlled asthma’ and ‘impaired’ quality of life, respectively, were compared utilizing both the whole dataset and stratified subsets of the data. Data were additionally stratified by smoking in the home, gender, inhaled corticosteroids and allergy medicine. ACQ and AQLQ scores were also compared independently for differences between seasons using Kruskal-Wallis test. Seasonality was tested for the whole dataset and for the dataset including only participants who had ‘not well controlled asthma’ and ‘impaired’ quality of life at baseline. Spring was considered April through June, summer was considered July through September, fall was considered October through December and winter was considered January through March. The difference or change between of the health outcome scores during the HEPA treatment was compared with the change during the “dummy” treatment utilizing Mann-Whitney U test.
Bonferroni adjustment was applied to account for multiple comparisons. Since changes in four indoor air quality measures were being considered, a significance threshold of 0.05/4 = 0.0125 was used for the environmental data. Since two health outcomes were being considered using a single data set, a significance threshold of 0.05/2 = 0.025 was used for the health outcome data. The Bonferroni adjustment allowed for multiple comparisons while still assuring that an overall significance threshold of 0.05 was maintained. Statistical analysis was performed using R (version 3.1.1).
3. Results
The average age (± standard deviation) of the study participants was 12.9 ± 1.9 years Demographic characteristics of the study participants are summarized in Table 1. Participants included 30 males and 13 females. About half of the children were Caucasian while the rest were African-American or biracial. Nine homes reported at least one smoker in the home and 27 homes had pets. The median percent-predicted FEV1 for all participants was 101.5 at baseline and remained within normal range following both treatment arms.
Table 1.
Clinical characteristics of the study population
| Characteristics | Number of homes (%) |
|---|---|
| Gender | |
| Males | 30 (70%) |
| Females | 13 (30%) |
| Race | |
| African-American | 15 (35%) |
| Caucasian | 21 (49%) |
| Biracial | 7 (16%) |
| Medications | |
| Inhaled corticosteroids | 19 (44%) |
| Allergic rhinitis medication (nasal spray, oral) | 14 (32%) |
| Pets* | |
| Dogs | 24 (56%) |
| Cats | 11 (26%) |
| Treatment first | |
| HEPA | 23 (53%) |
| Dummy | 20 (47%) |
| Smoking exposure | 9 (21%) |
| Health Questionnaire | Baseline Values |
| ACQ | 0.8 |
| AQLQ | 5.9 |
8 homes with both types of pets
3.1. Environmental Data
As previously reported by Cox et al., median indoor concentrations of PM2.5, BC, UVPM, fungal spores, and ERMI-like values were significantly decreased following HEPA treatment but not after the “dummy” air cleaner period.39 In addition, the median outdoor concentrations of PM2.5, BC, UVPM, and fungal spores were not significantly different from baseline (10.8, 1.1, 2.4, μg/m3 and 1818 spores/m3, respectively) to HEPA treatment (9.1, 0.9, 2.2 μg/m3, and 2128 spores/m3, respectively) or from baseline (10.4, 1.1, 2.5 μg/m3, and 2653 spores/m3, respectively) to “dummy” treatment (11, 1.0, 2.5 μg/m3, and 1872 spores/m3, respectively).39 Indoor concentrations of PM2.5, BC, UVPM, and fungal spores decreased in the 20 homes of the participants with ‘not well-controlled’ asthma (Table 2) and the 12 homes of participants with ‘impaired’ quality of life at baseline following HEPA treatment (Table 3). This reduction was statistically significant for PM2.5, BC, and UVPM but not for fungal spores and ERMI-like values in the ‘not well-controlled’ homes. The change in median indoor concentrations of the four particle types was not statistically significant following “dummy” treatment in the ‘not well- controlled’ homes nor following the HEPA or “dummy” treatments in the ‘impaired’ quality of life homes. The medians of indoor temperature and humidity during the HEPA and “dummy” months were similar (Table 4).
Table 2.
Median indoor concentrations of the four particle types before and after HEPA and placebo “dummy” treatments for ‘not well-controlled’ asthma group at the beginning of treatment (n=20 HEPA; n=17 “dummy” for PM2.5, BC, UVPM, n=20 “dummy” for fungal)
| Particle type | Pre-HEPA | Post-HEPA | Pre-dummy | Post-dummy |
|---|---|---|---|---|
| PM2.5 (μg/m3) | 12.0 (0.3–80.9) | 4.5** (1.1–18.0) | 10.4 (0.6–53.2) | 7.8 (<LOD-37.9) |
| Black Carbon (μg/m3) | 0.5 (<LOD-4.2) | 0.1** (<LOD-2.3) | 0.6 (<LOD-2.3) | 0.5 (<LOD-2.3) |
| UVPM (μg/m3) | 2.4 (<LOD-54.2) | 0.6** (<LOD-13.8) | 4.3 (0.6–55.1) | 1.3 (<LOD-51.8) |
| Fungal spores (spores/m3) | 141 (12–1159) | 106 (1–396) | 346 (17–2978) | 155 (5–2115) |
post vs.pre: p-value <0.0125; PM2.5 - Particulate matter less than 2.5 μm; UVPM - Ultraviolet absorbing particulate matter; S.E. - Spore Equivalents; Limit of Detection (LOD) for BC = 0.12 μg/m3, LOD for UVPM = 0.18 μg/m3, LOD for PM2.5 = 0.3 μg/m3, < LOD = LOD/2.
Table 3.
Median indoor concentrations of the four particle types before and after HEPA and placebo “dummy” treatments for ‘impaired’ quality of life asthma group at the beginning of treatment (n=12 HEPA, n=10 “dummy” for PM2.5, BC, UVPM; n=13 HEPA, n=11 “dummy” for fungal)
| Particle type | Pre-HEPA | Post-HEPA | Pre-dummy | Post-dummy |
|---|---|---|---|---|
| PM2.5 (μg/m3) | 8.0 (0.3–24.6) | 4.8 (0.2–18.0) | 12.4 (0.6–45.7) | 9.3 (2.6–33.0) |
| Black Carbon (μg/m3) | 0.3 (<LOD-1.2) | 0.05 (<LOD-0.6) | 1.7 (<LOD-2.3) | 0.9 (<LOD-1.2) |
| UVPM (μg/m3) | 2.3 (0.3–23.9) | 0.7 (<LOD-7.5) | 13.2 (0.6–21.7) | 3.2 (0.3–8.3) |
| Fungal spores (spores/m3) | 149 (14–958) | 132 (5–396) | 346 (17–1715) | 230 (5–648) |
post vs.pre: p-value <0.0125; PM2.5 - Particulate matter less than 2.5 μm; UVPM - Ultraviolet absorbing particulate matter; S.E. - Spore Equivalents; Limit of Detection (LOD) for BC = 0.12 mg/m3, LOD for UVPM = 0.18 mg/m3, LOD for PM2.5 = 0.3 mg/m3, < LOD = LOD/2.
Table 4.
Median and range of temperature and relative humidity during HEPA and “dummy” treatments and Wilcoxon signed rank test
| HEPA All (n=42) | Dummy All (n=42) | p-value | |
|---|---|---|---|
| Indoor Temperature (°C) Average (Range) | 23.0 (0.0–44.4) | 23.1 (8.4–36.1) | 0.70 |
| Indoor Relative Humidity (%) Average (Range) | 48.1 (15.0–98.7) | 44.1 (15.0–98.1) | 0.40 |
p-value<0.0125
3.2. Changes in ACQ Scores
The median ACQ score for all participants was 0.58 prior to HEPA treatment and increased to 0.67 after the HEPA treatment (Figure 1), while the median ACQ decreased from 0.50 to 0.33 following the “dummy” treatment. These changes were neither statistically significant or clinically meaningful; therefore, neither treatment was considered medically beneficial in improving asthma control in the group overall.
Figure 1. ACQ (asthma controlled questionnaire) score by intervention for all participants (n=42).
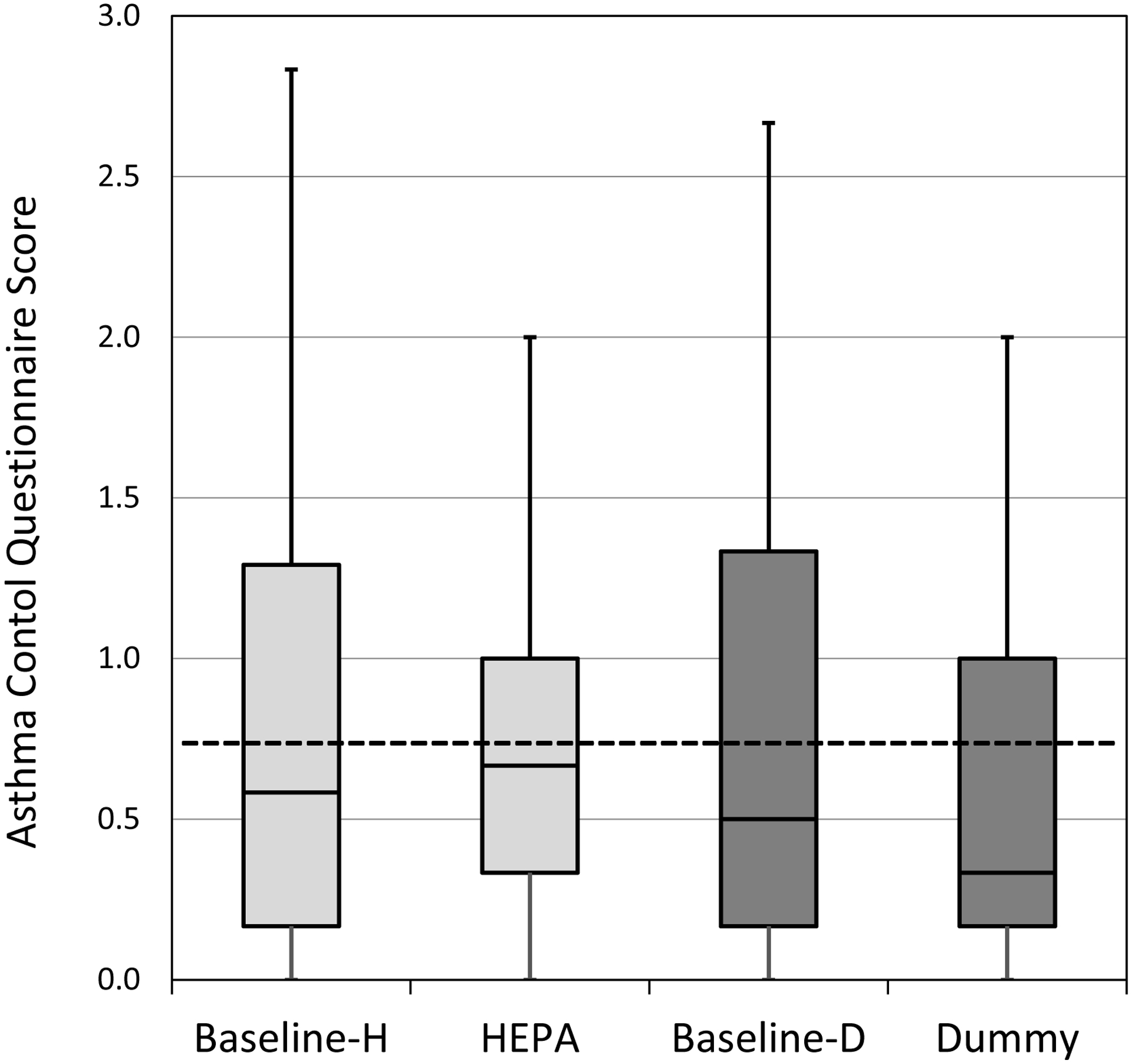
Horizontal lines in the box plots represent the minimum, 25%, 50%, 75% percentiles, and maximum. The cut-off point for ‘well-controlled’ asthma is ≤0.75 (dotted lined). H-HEPA. D-Dummy.
Participants were stratified as defined a priori by baseline asthma control. Among participants with ‘not well-controlled’ asthma at baseline, there was a significant but not clinically meaningful reduction in the median ACQ score following HEPA treatment compared to “dummy” (Figure 2). Among ‘not well-controlled’ asthmatic participants, the median ACQ was 1.33 before HEPA treatment and decreased to 0.92 (p = 0.0025) after HEPA treatment. In comparison, the median score decreased from 1.33 to 1.00 with the “dummy” treatment. This decrease was statistically significant (p = 0.028) but it was not clinically meaningful. The difference from baseline to treatment in the ACQ scores in the ‘not well-controlled’ group was not significantly different between HEPA and “dummy” treatments (Figure S1). Further analysis showed that 45% (9/20) of the ‘not well-controlled’ asthma group reported decreases in ACQ scores to below 0.75 (threshold for ‘well-controlled’) following HEPA treatment compared to 21% (4/19) of participants in the “dummy” treatment group. The improvements in ACQ were most clearly seen in males and in participants who did not have smoking in the home, used inhaled corticosteroids or did not use allergy medicine (Table S1). The ACQ scores among participants with ‘not well-controlled’ asthma were not significantly different between seasons (Table S2).
Figure 2. ACQ scores by intervention for ‘not well-controlled’ asthma group (n=20 HEPA; n=19 Dummy).
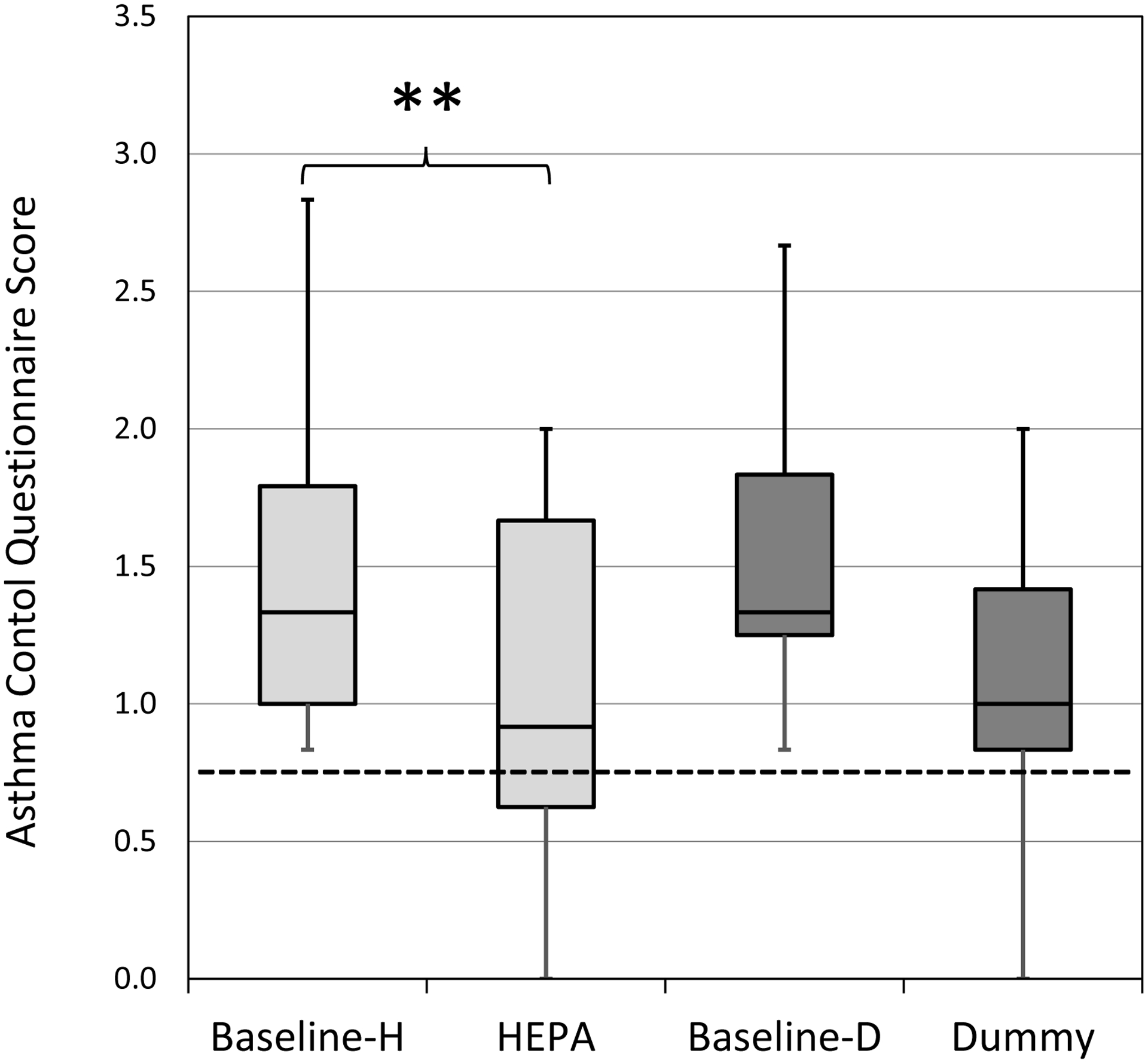
Horizontal lines in the box plots represent the minimum, 25%, 50%, 75% percentiles, and maximum. The cut-off point for ‘well-controlled’ asthma is ≤0.75 (dotted lined). H-HEPA. D-Dummy. **Due to Bonferroni adjustment, p-value <0.025 was considered significant.
3.3. Changes in mini-AQLQ Scores
The median mini-AQLQ scores for all participants together indicated a ‘non-impaired’ quality of life prior to both HEPA and “dummy” treatments (6.03 and 6.23, respectively). Change in median AQLQ scores following HEPA (6.27) and “dummy” (6.40) treatments was statistically significant, but not clinically meaningful (Figure 3).
Figure 3. AQLQ (asthma quality of life questionnaire) scores by intervention for all participants (n=42).
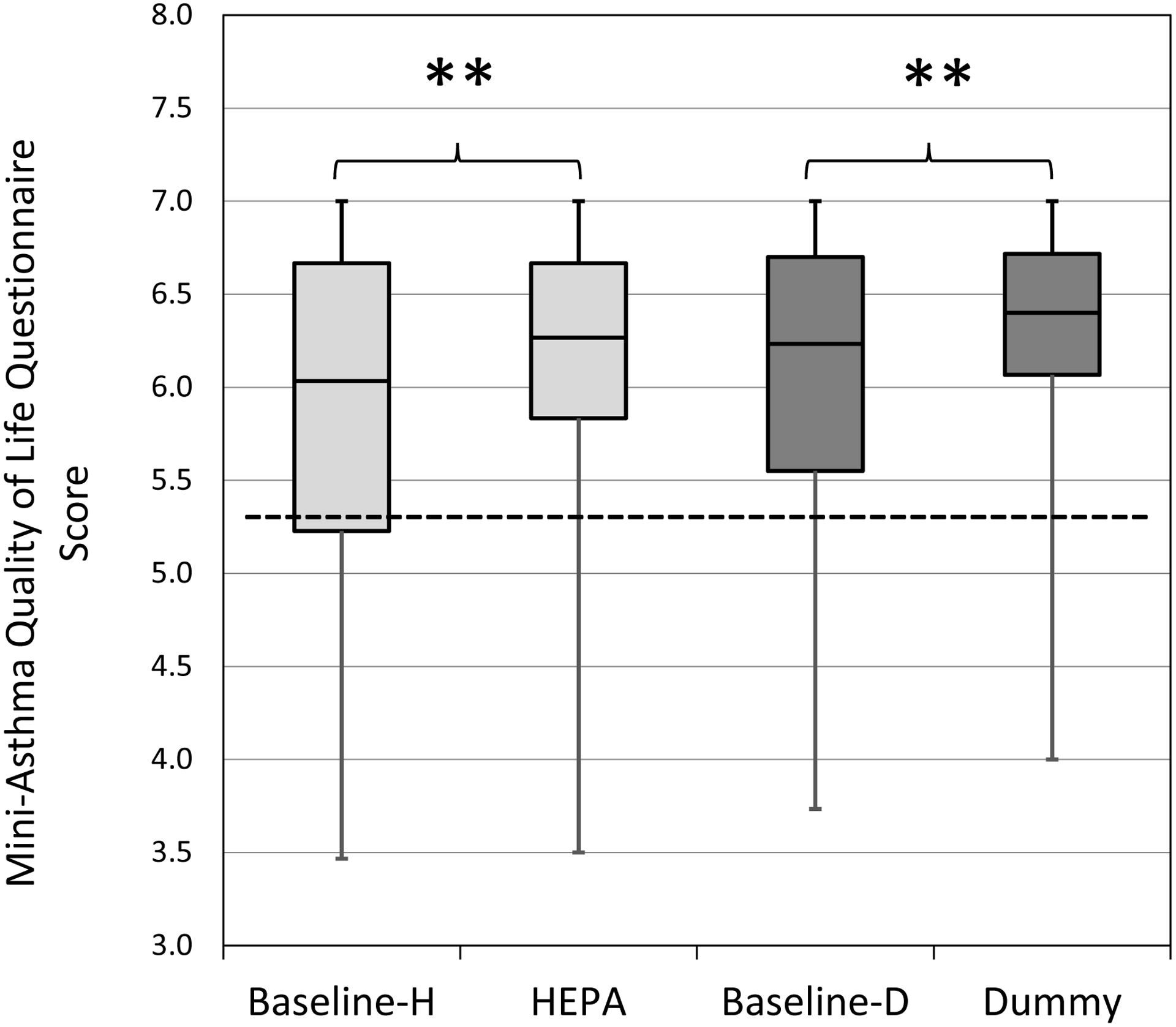
Horizontal lines in the box plots represent the minimum, 25%, 50%, 75% percentiles, and maximum. The cut-off point for ‘adequate’ quality of life is ≥5.40 (dotted lined). H-HEPA. D-Dummy. **Due to Bonferroni adjustment, p-value <0.025 was considered significant.
Among the subgroup of participants with ‘impaired’ quality of life at baseline, the median score statistically and clinically increased from 4.93 to 5.47 following HEPA treatment (p <0.01) (Figure 4). Among this same subgroup, AQLQ scores increased from 4.97 to 5.23 following “dummy” treatment, but this increase was not clinically or statistically significant. The difference from baseline to treatment with the ‘impaired’ AQLQ scores was not significantly different between HEPA and “dummy” treatments (Figure S1). Among the participants with ‘impaired’ quality of life at baseline, 62% (8/13) had an increase in AQLQ scoring above 5.40 after HEPA treatment compared to 36% (4/10) following “dummy” treatment. The improvements in AQLQ were most clearly seen in males and in participants who used inhaled corticosteroids (Table S1). The AQLQ scores among participants with ‘impaired’ quality of life scores were not significantly different between seasons (Table S2).
Figure 4. AQLQ scores by intervention for ‘impaired’ quality of life. (HEPA n=13, Dummy n=10).
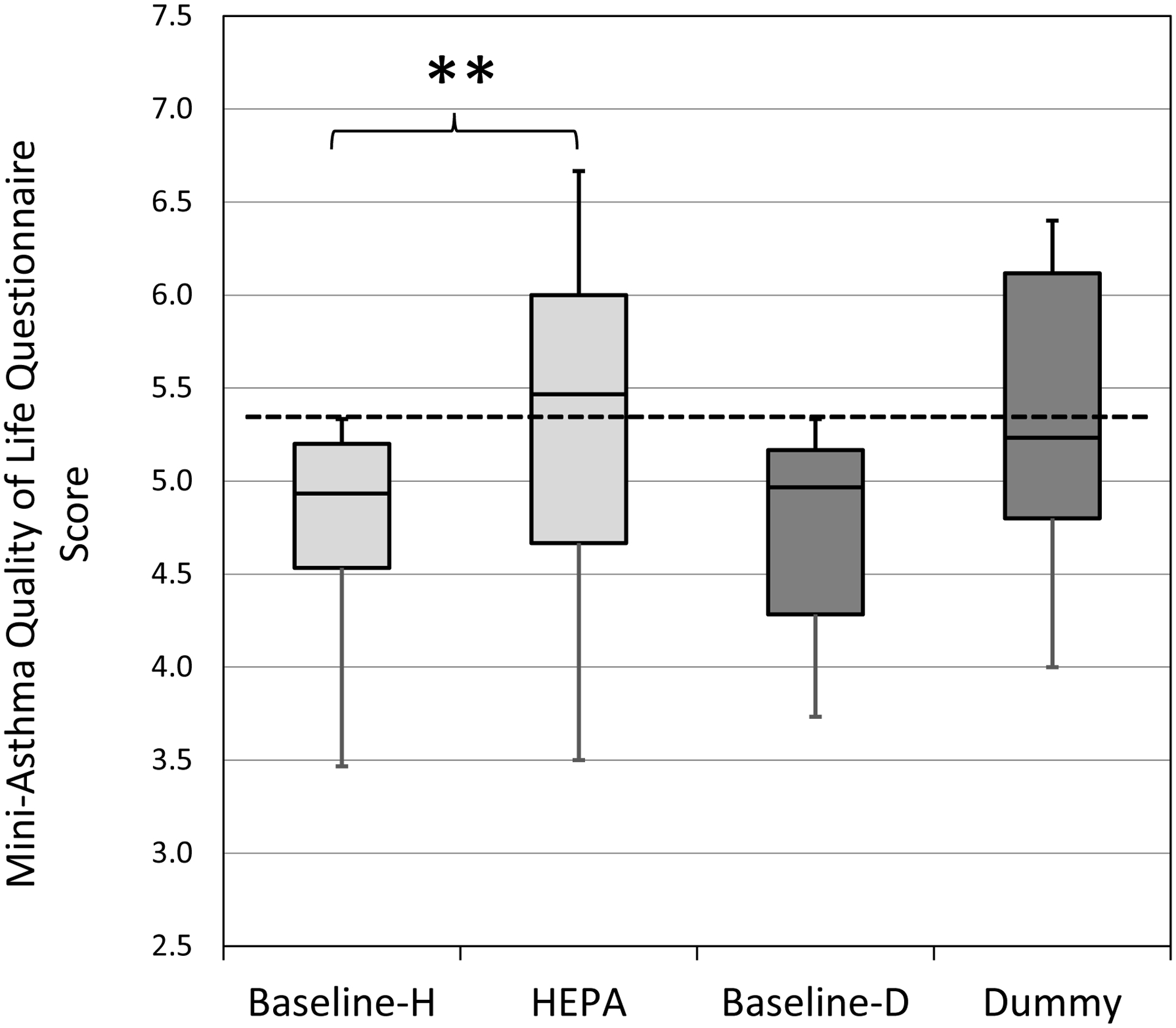
Horizontal lines in the box plots represent the minimum, 25%, 50%, 75% percentiles, and maximum. The cut-off point for adequate quality of life is ≥5.40 (dotted line). H-HEPA. D-Dummy. **Due to Bonferroni adjustment, p-value <0.025 was considered significant.
4. Discussion
This study demonstrated that HEPA treatment was effective in improving asthma control and quality of life scores among participants with poor control and impaired quality of life. The results also showed that in the ‘not well-controlled’ homes indoor TRAP, specifically BC and PM2.5, and other aerosol particles (UVPM) were significantly reduced with HEPA air filtration. Out of 184 metropolitan areas in the USA, Cincinnati is ranked 20th for annual particle pollution (annual PM2.5)55, and one of the major sources of PM2.5 includes motor vehicle traffic.56 Infiltration of outdoor particles into indoor air is of particular concern, as these exposures have been associated with adverse respiratory outcomes such as increased asthma prevalence, wheezing, and recurrent dry cough.7 This study was completed in homes that were estimated to have high TRAP exposures, based on distance to highway and ECAT value, in order to determine the effects of HEPA air purifiers on indoor TRAP concentration and the potential clinical effects on asthma symptoms.
Previous studies have shown varied results on the impact of HEPA filtration on health outcomes. In recent years, intervention studies have been performed in adult populations living in homes near major roadways or in highly polluted cities.23, 26, 57–60 While HEPA filtration successfully reduced PM2.5 concentrations in these studies, effects on health outcomes were mixed. In elderly patients, HEPA filtration was thought to be able to reduce cardiovascular disease,58 though microvascular function only improved under very limited conditions.26 Two studies demonstrated improvement in cardiorespiratory health,23, 59 but others found little benefit with HEPA filtration.60, 61 In a more recent study on cardiovascular disease, the lack of expected health benefits from HEPA filtration led researchers to suggest that improved study designs were needed for more definitive conclusions.61 Besides one study that looked at patients with asthma,59 other studies did not target participants with asthma or allergic rhinitis, and therefore, effects of HEPA intervention on respiratory health outcomes were less definitive.
Unlike the aforementioned studies, others considered the effect of HEPA filtration specifically on those with allergic diseases related to indoor aeroallergens. However, the impact of reducing ambient aeroallergen concentration with HEPA on asthma outcomes in children was uncertain. Wood et al. showed that HEPA air filters could reduce airborne cat allergen (Fel d 1) levels, but this reduction did not impact symptoms in cat-allergic patients.15 This seems to be consistent with findings by van der Heide et al., who showed that HEPA interventions were associated with reduced bronchial hyperresponsiveness, but symptom scores and medication use remained unchanged in pet-allergic children with asthma.16 In a study by Sulser et al., HEPA intervention in the homes of children with asthma with cat and/or dog sensitization did not significantly lower pet allergen concentrations in dust samples, nor did it have a significant impact on lung function or medication use.18 Although studies were negative in children, HEPA intervention improved a ‘combined asthma outcome’ in pet-allergic adults with asthma.14 This was defined as an improvement in bronchial hyperreactivity to histamine and/or reduction in treatment by at least one step according to asthma treatment guidelines.
In our study, when analyzing the data from all controlled and uncontrolled asthmatics together, HEPA treatment did impact respiratory health outcomes. This is consistent with the experience of Park et al., who utilized HEPA intervention in the homes of 16 children with asthma and/or allergic rhinitis in a highly polluted city.25 Eight participants were given a HEPA air purifier, while the participants in the control group did not have an air purifier. Their results demonstrated a trend towards improvement in Asthma Control Test (ACT) scores and mean peak flow in the HEPA filter group, but these trends did not reach statistical significance, possibly due to the small size of the study population. The study may have been further underpowered by the parallel study design. As our data suggest in this crossover study, negative studies may also result from including too many well-controlled participants which could ultimately reduce effect size.
However, participants with poor asthma control and impaired quality of life at baseline were found to have clinically meaningful and statistically significant improvement in their AQLQ scores after the HEPA treatment. The change in score with the “dummy” treatment was not statistically significant or clinically meaningful (absolute change in score >0.5). When looking at ACQ scores, HEPA filtration resulted in a statistically significant improvement although not clinically meaningful for those with ‘not well-controlled’ asthma at baseline. The change in score with the “dummy” treatment was not clinically meaningful or statistically significant. Since stratification was not used in earlier studies, this may be one reason that the role of HEPA filtration on health outcomes was indiscernible.
In all of the study homes39 and in the ‘not well-controlled’ homes, there was a significant decrease in BC, PM2.5, and UVPM. These findings seem to correlate with that of McCreanor et al., in which increased exposure to fine particles (<2.5μm), ultrafine particles, and elemental carbon led to consistent reductions in FEV1 and FVC and increases in biomarkers of neutrophilic inflammation in adults with mild to moderate asthma; however, it should be noted that the participants in the aforementioned study did not note any symptomatic changes.62
To understand if the particle concentrations were high or low we compared to other studies. In our previous study assessing the effect of green renovation on indoor air quality in Cincinnati, the median PM2.5 (41 μg/m3) and BC (0.9 μg/m3) concentrations were higher than the baseline concentrations in the current study.63 Higher baseline concentrations (28.5 μg/m3) were also reported in an air cleaner study by Batterman et al.12 In several other air cleaner studies, the baseline concentrations were lower or at the same level as in the current study.22, 23, 64
We cannot rule out that having the HEPA or “dummy” unit in the home could potentially lead to a placebo effect as the participants may change their behaviors, such as smoke outdoors or close their windows. We would expect this effect to be similar between either treatment arm. While no statistically significant difference was seen between the changes in scores for HEPA treatment when compared to the changes in scores for the “dummy” treatment, the analysis still demonstrated that the ‘not well-controlled’ participants significantly improved and the ‘impaired’ participants significantly and clinically improved during HEPA filtration and did not during “dummy” treatment. In addition, while the Hawthorne effect from potential increased compliance with asthma medication might be a contributor, children were consistently asked throughout the study about their medication use, and alterations in behavior in this regard were not observed with either treatment arm. Overall, our data suggest that the use of a HEPA air cleaner could be an effective intervention for children with uncontrolled asthma living in proximity to sources of traffic pollution.
We recognize that there are limitations in a study such as this one. One inherent limitation is the inability to determine which specific particulate matter component was responsible for the observed improvement in asthma measures. HEPA filtration has been shown to improve asthma outcomes when smoking was investigated as the main exposure,17 which is similar to the decrease in UVPM in our study. However, as concentrations of particulate matter associated with TRAP also decreased, this suggests that there could be an important effect on respiratory health outside of smoke exposure alone. HEPA filtration was also limited to the bedroom, and particulate levels have been demonstrated to increase in areas of the home with activity during the day.65 In addition, the “dummy” treatment included a carbon pre-filter that removed large particles. It should be considered, however, that the particles during the “dummy” intervention arm were not significantly reduced. It is possible that the carbon filter reduced pollutants that we did not measure, such as volatile organic compounds. This could have caused the slight improvements in ACQ and AQLQ that we observed.
We did not look at the allergen sensitization of our participants, but our study showed that HEPA filtration had an important impact on respiratory health outcomes, despite possible confounding effects of pet allergens on sensitized participants. The length of the treatment periods was limited to four weeks. Although the sample size was small and was also affected by occasional incomplete questionnaires, this was overcome through the crossover design in this study, and statistical analysis still demonstrated significance.
Future larger parallel placebo-controlled trials in uncontrolled asthmatic participants will be needed to confirm these results. Such studies should evaluate underlying indoor aeroallergen sensitization and pet-keeping as possible confounding variables. Much effort and cost have been directed at developing new biologic drugs for poorly controlled asthma with less consideration and evaluation of environmental inventions. Our study suggests that significant public health benefits could be derived from reducing personal TRAP exposure. Combined with the mitigation of other environmental triggers associated with worsening asthma outcomes, interventions such as HEPA filters could provide more cost-effective strategies in improving the respiratory health of people with asthma.
Families with children who have asthma are seeking practical ways to help improve their children’s asthma control and quality of life. Our study shows that the indoor concentration of traffic-related airborne particles can be reduced with the use of HEPA filtration, and this decline could improve clinical outcomes in children with asthma poor control and quality of life.
Supplementary Material
Practical Implications.
Traffic-related air pollution represents an important public health concern worldwide, especially for children with asthma, as a large portion of the population is moving to major metropolitan areas and reside near major roadways. Families with children who have asthma are seeking practical ways to help improve their children’s asthma control and quality of life. Our study shows that the indoor concentration of traffic-related airborne particles can be reduced with the use of HEPA filtration, and this decline could improve clinical outcomes in children with poor control and quality of life.
Acknowledgements:
This study was funded by the U.S. Department of House and Urban Development (Grant OHHHU0027-14). Dr. Christine James was supported by the National Institute of Environmental Health Sciences (Grant 2T32ES010957). The authors declare no conflicts of interest.
References
- 1.Centers for Disease Control And Prevention. Asthma prevalence, disease characteristics, and self-management education Vital signs May 2011. Centers for Disease Control and Prevention: Atlanta, GA; 2011. [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, et al. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009; 123: S131–S145. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Simon AERossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016; 137: e20152354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich J Influence of indoor factors in dwellings on the development of childhood asthma. International journal of hygiene and environmental health. 2011; 214: 1–25. [DOI] [PubMed] [Google Scholar]
- 5.Brunekreef B, Janssen NA, De Hartog J, et al. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997; 8: 298–303. [DOI] [PubMed] [Google Scholar]
- 6.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. New Engl J Med. 2004; 351: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 7.Health Effects Institute. Panel on the Health Effects of Traffic-Related Air Pollution Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Health Effects Institute; 2010. [Google Scholar]
- 8.Centers for Disease Control And Prevention. Conclusion and future directions: CDC health disparities and inequalities report—United States. Morbidity and Mortality Weekly Report. 2013. November 22, 2013; 62: 189.23486384 [Google Scholar]
- 9.Ryan PH, Bernstein DI, Lockey J, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. American journal of respiratory and critical care medicine. 2009; 180: 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein TG, Ryan PH, LeMasters GK, et al. Poor asthma control and exposure to traffic pollutants and obesity in older adults. Ann Allerg Asthma Im. 2012; 108: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barn P, Larson T, Noullett M, et al. Infiltration of forest fire and residential wood smoke: an evaluation of air cleaner effectiveness. J Expo Sci Env Epid. 2008; 18: 503. [DOI] [PubMed] [Google Scholar]
- 12.Batterman S, Du L, Mentz G, et al. Particulate matter concentrations in residences: an intervention study evaluating stand-alone filters and air conditioners. Indoor Air. 2012; 22: 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacker D, Sparrow EM. Use of air-cleaning devices to create airborne particle-free spaces intended to alleviate allergic rhinitis and asthma during sleep. Indoor Air. 2005; 15: 420–431. [DOI] [PubMed] [Google Scholar]
- 14.Francis H, Fletcher G, Anthony C, et al. Clinical effects of air filters in homes of asthmatic adults sensitized and exposed to pet allergens. Clin Exp Allergy. 2003; 33: 101–105. [DOI] [PubMed] [Google Scholar]
- 15.Wood RA, Johnson EF, Van Natta ML, et al. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Resp Crit Care. 1998; 158: 115–120. [DOI] [PubMed] [Google Scholar]
- 16.van der Heide S, van Aalderen WM, Kauffman HF, et al. Clinical effects of air cleaners in homes of asthmatic children sensitized to pet allergens. J Allergy Clin Immun. 1999; 104: 447–451. [DOI] [PubMed] [Google Scholar]
- 17.Butz AM, Matsui EC, Breysse P, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediat Adol Med. 2011; 165: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulser C, Schulz G, Wagner P, et al. Can the use of HEPA cleaners in homes of asthmatic children and adolescents sensitized to cat and dog allergens decrease bronchial hyperresponsiveness and allergen contents in solid dust? Int Arch Allergy Imm. 2009; 148: 23–30. [DOI] [PubMed] [Google Scholar]
- 19.Lanphear BP, Hornung RW, Khoury J, et al. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011; 127: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allerg Asthma Im. 2005; 95: 518–524. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Environmental Protection Agency. Residential Air Cleaners (Third Edition): A Summary of Available Information. 2018.
- 22.Allen RW, Carlsten C, Karlen B, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Resp Crit Care. 2011; 183: 1222–1230. [DOI] [PubMed] [Google Scholar]
- 23.Kajbafzadeh M, Brauer M, Karlen B, et al. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. 2015; 72: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jhun I, Gaffin JM, Coull BA, et al. School environmental intervention to reduce particulate pollutant exposures for children with asthma. The Journal of Allergy and Clinical Immunology: In Practice. 2017; 5: 154–159. e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park H-K, Cheng K-C, Tetteh AO, et al. Effectiveness of air purifier on health outcomes and indoor particles in homes of children with allergic diseases in Fresno, California: A pilot study. Journal of Asthma. 2017; 54: 341–346. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, Zhao A, Chen H, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. Journal of the American College of Cardiology. 2015; 65: 2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day D, Xiang J, Mo J, et al. Combined use of an electrostatic precipitator and a high-efficiency particulate air filter in building ventilation systems: Effects on cardiorespiratory health indicators in healthy adults. Indoor Air. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis TC, Parker EA, Robins TG, et al. In:Bedroom Hepa Air Filters Can Improve Respiratory Health Of Children With Asthma In Detroit. American Thoracic Society, 2016: A3805–A3805. [Google Scholar]
- 29.Shao D, Du Y, Liu S, et al. Cardiorespiratory responses of air filtration: A randomized crossover intervention trial in seniors living in Beijing: Beijing Indoor Air Purifier StudY, BIAPSY. Sci Total Environ. 2017; 603: 541–549. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Li F, Xiang J, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: A double-blind randomized crossover study. Environ Int. 2018; 114: 27–36. [DOI] [PubMed] [Google Scholar]
- 31.Brugge D, Simon MC, Hudda N, et al. Lessons from in-home air filtration intervention trials to reduce urban ultrafine particle number concentrations. Building and environment. 2017; 126: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barn P, Gombojav E, Ochir C, et al. The effect of portable HEPA filter air cleaner use during pregnancy on fetal growth: The UGAAR randomized controlled trial. Environ Int. 2018; 121: 981–989. [DOI] [PubMed] [Google Scholar]
- 33.Morishita M, Adar SD, D’Souza J, et al. Effect of portable air filtration systems on personal exposure to fine particulate matter and blood pressure among residents in a low-income senior facility: a randomized clinical trial. JAMA internal medicine. 2018; 178: 1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisk WJ. Health benefits of particle filtration. Indoor Air. 2013; 23: 357–368. [DOI] [PubMed] [Google Scholar]
- 35.Leas BF, D’Anci KE, Apter AJ, et al. Effectiveness of indoor allergen reduction in asthma management: A systematic review. J Allergy Clin Immun. 2018; 141: 1854–1869. [DOI] [PubMed] [Google Scholar]
- 36.Jia-ying L, Zhao C, Jia-jun G, et al. Efficacy of air purifier therapy in allergic rhinitis. Asian Pacific journal of allergy and immunology. 2018; 36: 217–221. [DOI] [PubMed] [Google Scholar]
- 37.Zhao D, Azimi P, Stephens B. Evaluating the long-term health and economic impacts of central residential air filtration for reducing premature mortality associated with indoor fine particulate matter (PM2.5) of outdoor origin. Int J Env Res Pub He. 2015; 12: 8448–8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisk W, Chan WR. Effectiveness and cost of reducing particle-related mortality with particle filtration. Indoor air. 2017; 27: 909–920. [DOI] [PubMed] [Google Scholar]
- 39.Cox J, Isiugo K, Ryan P, et al. Effectiveness of a portable air cleaner in removing aerosol particles in homes close to highways. Indoor Air. 2018; 28: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. The Journal of Pediatrics. 2006; 149: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan P, LeMasters G, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Science of the Total Environment. 2008; 404: 139–147. [DOI] [PubMed] [Google Scholar]
- 42.Juniper EF, Bousquet J, Abetz L, et al. Identifying ‘well-controlled’and ‘not well-controlled’asthma using the Asthma Control Questionnaire. Respiratory medicine. 2006; 100: 616–621. [DOI] [PubMed] [Google Scholar]
- 43.Stillerman A, Nachtsheim C, Li W, et al. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Ann Allerg Asthma Im. 2010; 104: 440–449. [DOI] [PubMed] [Google Scholar]
- 44.Yan B, Kennedy D, Miller R, et al. Validating a nondestructive optical method for apportioning colored particulate matter into black carbon and additional components. Atmos Environ. 2011; 45: 7478–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vesper S, McKinstry C, Haugland R, et al. Development of an environmental relative moldiness index for US homes. J Occup Environ Med. 2007; 49: 829–833. [DOI] [PubMed] [Google Scholar]
- 46.Juniper E, O′ byrne P, Guyatt G, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999; 14: 902–907. [DOI] [PubMed] [Google Scholar]
- 47.Juniper EF, Guyatt G, Epstein R, et al. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992; 47: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brusasco EV, Crapo R, Viegi G, et al. Series “ATS/ERS task force: standardisation of lung function testing”. Eur Respir J. 2005; 26: 319–338.16055882 [Google Scholar]
- 49.Hankinson JL, Odencrantz JR Fedan KB. Spirometric reference values from a sample of the general US population. Am J Resp Crit Care. 1999; 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am J Resp Crit Care. 1993; 148: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen JM, Holbrook JT, Wei CY, et al. Validation and psychometric properties of the Asthma Control Questionnaire among children. J Allergy Clin Immun. 2014; 133: 91–97. e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sastre J, Olaguibel J, Vega JM, et al. Cut-off points for defining asthma control in three versions of the Asthma Control Questionnaire. Journal of Asthma. 2010; 47: 865–870. [DOI] [PubMed] [Google Scholar]
- 53.de Sousa JC, Pina A, Cruz AM, et al. Asthma control, quality of life, and the role of patient enablement: a cross-sectional observational study. Primary Care Respiratory Journal. 2013; 22: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sá-Sousa A, Amaral R, Morais-Almeida M, et al. Asthma control in the Portuguese National Asthma Survey. Revista Portuguesa de Pneumologia (English Edition). 2015; 21: 209–213. [DOI] [PubMed] [Google Scholar]
- 55.American Lung Association. State of the Air 2017. Chicago, IL; 2017. [Google Scholar]
- 56.Thurston G, Ito KLall R. A source apportionment of US fine particulate matter air pollution. Atmos Environ. 2011; 45: 3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brauner EV, Forchhammer L, Møller P, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Resp Crit Care. 2008; 177: 419–425. [DOI] [PubMed] [Google Scholar]
- 58.Weichenthal S, Mallach G, Kulka R, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013; 23: 175–184. [DOI] [PubMed] [Google Scholar]
- 59.Karottki DG, Spilak M, Frederiksen M, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environmental Health. 2013; 12: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padró-Martínez LT, Owusu E, Reisner E, et al. A randomized cross-over air filtration intervention trial for reducing cardiovascular health risks in residents of public housing near a highway. Int J Env Res Pub He. 2015; 12: 7814–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brugge D, Durant JL, Rioux C. Near-highway pollutants in motor vehicle exhaust: a review of epidemiologic evidence of cardiac and pulmonary health risks. Environmental Health. 2007; 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. New Engl J Med. 2007; 357: 2348–2358. [DOI] [PubMed] [Google Scholar]
- 63.Coombs KC, Chew GL, Schaffer C, et al. Indoor air quality in green-renovated vs. non-green low-income homes of children living in a temperate region of US (Ohio). Sci Total Environ. 2016; 554: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barn P Residential Air Cleaner Use to Improve Indoor Air Quality and Health: A Review of the Evidence. National Collaborating Centre for Environmental Health; 2011. [Google Scholar]
- 65.Chen Q, Hildemann Lynn M. The effects of human activities on exposure to particulate matter and bioaerosols in residential homes. Environ Sci Technol. 2009; 43: 4641–4646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


