Abstract
Introduction
Adverse birth outcomes have important consequences for future lung health. We evaluated patterns of respiratory health services utilization in early childhood among children born preterm (PTB), small and large for gestational age at term (SGA and LGA, respectively), and appropriate-for-gestational age at term.
Materials and methods
We conducted a population-based retrospective cohort study using administrative health data of all singleton live births in Alberta, Canada between 2005–2010. Data on hospitalizations and emergency department (ED) visits from birth to 5 years were collected for asthma, bronchitis, bronchiolitis, croup, influenza, pneumonia, and other acute upper and lower respiratory tract infections (other URTI and other LRTI, respectively). Adjusted rate ratios were estimated for respiratory ED visits and hospitalizations for adverse birth outcomes using the appropriate-for-gestational age at term group as reference. Age-specific trajectories of total respiratory health services utilization rates for each group were estimated in Poisson models.
Results
A total of 293,764 episodes of respiratory care from 206,994 children were analyzed. Very PTB children had the highest rates of health services use for all respiratory conditions, particularly for asthma, pneumonia, and bronchiolitis hospitalizations. Moderate/late PTB children also had elevated ED visits and hospitalizations for all respiratory conditions. Children born SGA showed high rates of ED visits for other LRTI, and of hospitalizations for bronchitis, bronchiolitis, and other URTI. Children born LGA had high rates of croup and other URTI ED visits, and of bronchiolitis and bronchiolitis hospitalizations. Age-specific trajectories showed a decreasing trend in the rates of total respiratory health service utilization from birth to five years of age for all groups studied. Children born PTB and LGA at term significantly required more respiratory health services over time compared to the reference group.
Conclusion
Patterns of paediatric respiratory health services utilization vary according to gestational age and fetal growth.
Introduction
Alterations in fetal growth and duration of gestation are adverse birth outcomes that increase the risk of respiratory diseases both in childhood and adult life [1–6]. Much of the evidence to date about the relationship between adverse birth outcomes and lung problems in childhood has focused on the associations between preterm birth (PTB) or low birth weight and a high risk of asthma and asthma-like symptoms [5–9]. The increased susceptibility to respiratory diseases among children born PTB has been linked to the immaturity of both respiratory and adaptive immune systems at birth [10].
There is limited evidence about how other adverse birth outcomes (i.e., small and large for gestational age [SGA, LGA]) impact respiratory diseases other than asthma or asthma-like symptoms in early childhood. Physiological mechanisms linking SGA and LGA to future respiratory diseases are unclear and conflicting evidence has been reported for the association between SGA and LGA and respiratory health in childhood. For SGA, insufficient input of oxygen and metabolites linked to fetal growth restrictions could negatively impact the lung development of the fetus [2]. For LGA, both the higher risk of experiencing respiratory distress syndrome [11] and the reduced lung functional capacity observed in obese infants may alter the risk of suffering respiratory problems [12].
Some studies have reported that children born SGA have an elevated risk of asthma at ages 3 to 18 years [13], and an increased number of respiratory hospitalizations before the age of five [14]. Other studies have reported inconclusive evidence of associations between being born SGA at term or preterm and asthma or bronchitis/pneumonia symptoms at 5 years of age [15], or with respiratory viral infections during the first 6 months of life [16].
Paediatric respiratory diseases are major causes of morbidity and mortality worldwide [17]. In Canada, approximately 15% of children aged 4 to 11 years are diagnosed with asthma every year [18] while croup affects about 6% of children under six years of age [19]. Along with the high prevalence of pediatric respiratory diseases in Canada, the prevalence of PTB is 8% and fluctuates around 10% for SGA and LGA [20]. The health care costs during the first ten years of life for children who experienced PTB is high in Canada [21]. Knowledge gaps remain about the burden imposed by SGA and LGA on health care systems in relation to paediatric respiratory morbidity, especially for diseases other than asthma. Previous studies evaluating health care services utilization for respiratory diseases in children experiencing adverse birth outcomes have primarily focused on hospitalizations [13–15,22–24], whereas patterns of emergency department visits (ED visits) have seldom been explored [25].
To our knowledge, there are no previous population-based cohort studies examining and comparing patterns of respiratory health services utilization (ED visits and hospitalizations) among children who experienced alterations in fetal growth and duration of gestation. The objectives of this study were: (1) to evaluate patterns of ED visits and hospitalizations up to 5 years of age for a broad range of respiratory diseases among children who experienced adverse birth outcomes; and (2) to compare age-specific trajectories of total respiratory health services utilization across adverse birth outcomes. Results from this study may improve our understanding of the relative importance of alterations in fetal growth and duration of gestation in early childhood healthcare pathways.
Materials and methods
Study design
This population-based retrospective birth cohort study used provincial health data from Alberta, a culturally diverse province located in Western Canada with a population of ~ 4 million people [26] and a universal single-payer health care system. The study is reported following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [27].
Study population
The birth cohort consisted of all singleton live births from deliveries (≥22 weeks of gestation) that occurred between April 1, 2005 and March 31, 2010 in hospitals or attended by registered midwives at home in Alberta. Stillbirths, births that occurred outside of Alberta and multiple births were excluded. Multiple births, representing a small proportion of the total births (approximately 2.6%), were excluded as their neonatal and childhood outcomes are known to differ significantly as compared to the larger proportion of the singleton live births.
Data sources
The birth cohort was identified from the Alberta Perinatal Health Program (APHP), a validated clinical perinatal registry that collects information on maternal demographic and delivery characteristics, pregnancy outcomes, and newborn’s health status for all births occurring in hospitals or attended by registered midwives at home in Alberta [28]. Deaths and stillbirths were identified from Alberta Vital Statistics. Data on health services utilization from birth to five years of age were obtained from the National Ambulatory Care Reporting System (NACRS) and the Discharge Abstracts Database (DAD) for ED visits and hospitalizations, respectively. Both NACRS and DAD record the episodes of care using the International Classification of Diseases, 10th Revision, enhanced Canadian version (ICD-10-CA) [29]. The data were extracted by Alberta Health Services using unique identifiers to link data across health administrative datasets. All final patient records were de-identified before data files were accessed by the authors for data analysis.
Exposures and outcome measures
Preterm birth, SGA at term and LGA at term
Birth cohort members were classified into three exposure groups: (1) PTB (both spontaneous and induced), defined as a live birth with a gestation period < 37 weeks and sub-classified as moderate/late PTB (32–36 complete weeks of gestation) and very PTB (< 32 weeks of gestation) [30]; (2) SGA at term, defined as a live birth with ≥ 37 weeks of gestational age and a weight below the 10th percentile for gestational age and sex as per the 2001 Canadian population growth charts [31]; and (3) LGA at term, defined as a live birth with ≥ 37 weeks of gestational age and weight above the 90th percentile for gestational age and sex as per the 2001 Canadian population growth charts [31]. We focused on SGA and LGA at term to differentiate from PTB, a condition in which lung development immaturity has been well documented [10]. Members of the birth cohort that did not experience any of the study exposures were the reference group (i.e., appropriate-for-gestational age infants born at term).
Study outcomes
Respiratory health services utilization
Respiratory health services utilization was defined as all ED visits and hospitalizations that occurred from birth to 5 years of age with an ICD-10-CA primary diagnostic code indicative of acute bronchitis (J20), bronchiolitis (J21), asthma (J45), croup (J05), influenza (J09-J11), pneumonia (J12-J18), other acute lower respiratory tract infections (other LRTI) (J22), and other acute upper respiratory tract infections (other URTI) (J00-J06, except J05). Recurrent wheezing (R06.2) events were merged with asthma or bronchiolitis based on the most prevalent condition after the first wheezing episode. This merge was made because early life diagnosis of asthma and/or bronchiolitis is particularly difficult in acute settings, and recurrent wheezing has been associated with the development of asthma and respiratory viral infections [32–34]. Data were censored at date of death or end of the follow-up period (i.e., 5 years of age).
Other variables
Clinical and sociodemographic characteristics related to pediatric respiratory problems were considered for statistical analysis. They included bronchopulmonary dysplasia (BPD; yes/no), Apgar score at 5 minutes (classified as low [0–3], moderately abnormal [4–6], and reassuring [7–10]) [35], sex recorded at birth (female, male), the use (yes/no) of significant resuscitative measures at birth (bag/mask, cardiopulmonary resuscitation, endotracheal tube or epinephrine), and maternal socioeconomic status (SES) at delivery.
We used the Pampalon Material and Social Deprivation Index as a proxy measure of SES. The Pampalon Index is a nationwide area-level composite indicator that integrates 2006 Canadian census data by dissemination area (the smallest standard geographic area for which census data is disseminated) regarding income, education, employment (for the material component), marital status, one-person household, and single-parent families (for the social component) for the population aged 15 and over [36]. The Pampalon Index has been used in previous Canadian studies as a valid measure of area-level SES [37]. The material and social deprivation components of the Index are reported in quintiles, where Q1 and Q5 correspond to the least and most deprived groups, respectively. The six-character maternal postal codes at delivery were geographically linked to the dissemination areas (which are a conglomerate of postal codes), as reported elsewhere [38].
The previous clinical and sociodemographic characteristics were treated as risk factors associated with respiratory problems during early childhood stages regardless of their role in causal pathways (i.e., confounders, moderators, or effect-modifiers). Our comparative analysis was not aimed to formally test causal pathways, but in generating measures of association balanced for other known risk factors. Moreover, the essential role of socioeconomic in producing respiratory health differences for each respiratory condition included in this study has been previously evaluated [39].
Statistical analysis
Baseline demographic and clinical characteristics were described using frequencies and percentages. Counts of ED visits and hospitalizations were tabulated. Crude rates of ED visits and hospitalizations were calculated for each respiratory condition by adverse birth outcome. The total number of episodes of respiratory care was used as the numerator, and the total number of singleton live births in each group as denominator. Rates were expressed as episodes of respiratory care per 1,000 singleton live births up to five years of age.
We used random intercept coefficient Poisson regression models to evaluate patterns of ED visits and hospitalizations up to 5 years of age for each respiratory condition. Crude and adjusted rate ratios (RR and aRR, respectively) with 95% confidence intervals (CI) were calculated in relation to the reference group. Separate models for ED visits and hospitalizations were estimated for each respiratory disease. The dependent variable was the number of respiratory events from birth to five years of age. Independent variables were the adverse birth groups adjusted by relevant clinical and sociodemographic factors (i.e., sex, 5-minute Apgar score, bronchopulmonary dysplasia, use of significant resuscitation methods, and material and social deprivation). The random-intercept coefficients accounted for area-level variations in the DA for which material and social deprivation indexes were reported.
Using a hierarchical longitudinal Poisson model, we estimated age-specific trajectories of total respiratory health services utilization up to five years of age for the combined respiratory conditions by each birth group. ED visits and hospitalizations occurring on the same day were counted as one event in the trajectories analysis. The dependent variable was the total number of respiratory episodes of care (both hospitalizations and ED visits combined) per year of life. Independent variables were the adverse birth group, year of age (1 to 5), and an interaction term for adverse birth group and year of age. The model was adjusted for baseline factors (i.e., sex, bronchopulmonary dysplasia, Apgar 5-score, use of significant resuscitative measures, and material and social deprivation). Random intercepts were also considered at the DA level. After calculating incidence RR from the models, we estimated marginal respiratory care utilization rates with 95% CI per adverse birth group for the ages one to five. We graphically displayed the longitudinal trajectories of health services utilization rates as number of episodes of respiratory health services utilization every year of life per 1,000 singleton live births. Finally, for each year of life, we estimated the difference in marginal rates between the adverse birth group and the reference group using contrast tests with Bonferroni’s correction to adjust the 95% CIs. We used the log number of years of follow-up as offset in all Poisson models to correct for unequal lengths of follow-up due to censoring before the age of five. Missing data were not replaced in the analyses. Age-specific trajectories of respiratory health services utilization for single respiratory conditions by birth groups were estimated and reported in the supplementary material (Tables A to H in S1 Appendix). Statistical analyses were conducted using Stata 15.1 [40].
Ethics statement
We used de-identified data from Alberta Health Services, the sole provider of health services in the province and legal custodian of the data. Alberta Health Services’ policies and acts protect the security, privacy, and confidentiality of patient data collected. The anonymity of cases was guaranteed using the dissemination areas as the geographic reference instead of the maternal postal code at delivery. Therefore, informed consent was not necessary. The research project was approved by the University of Alberta’s Health Research Ethics Board (Pro00081365).
Results
Demographics and clinical characteristics
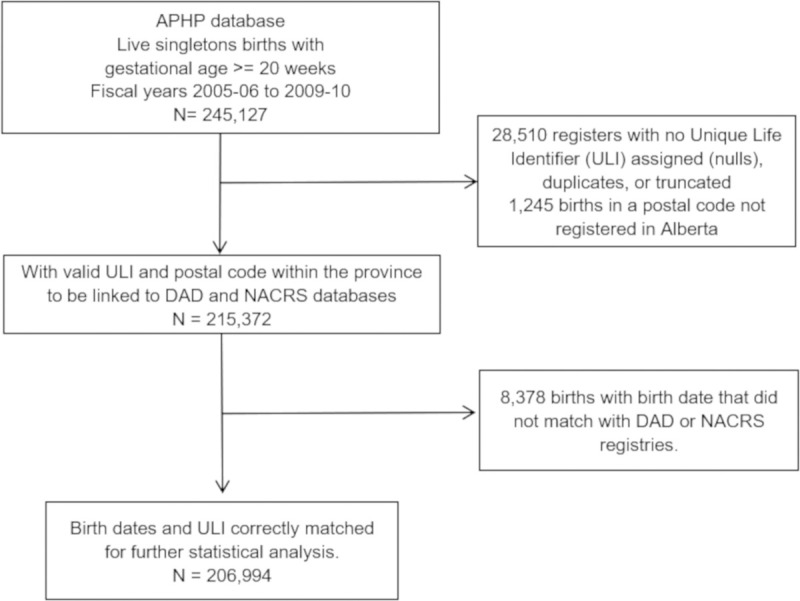
The birth cohort consisted of 206,994 live singleton births (Fig 1), which represent 96.6% of the total births registered in the province during the study period.
Fig 1. Flow diagram of assembly of the study cohort.
Demographic and clinical characteristics of the study population are shown in Table 1. The prevalence of PTB in the birth cohort was 9.2% (moderate/late PTB 7.7%, and very PTB 1.5%), while 7.9% and 8.8% were SGA and LGA at term, respectively. There were 183 (0.1%) deaths of birth cohort members at follow-up.
Table 1. Characteristics of the study population: Singleton live births in Alberta from April 1, 2005 to March 31, 2010.
| Characteristic | N | % |
|---|---|---|
| Live singletons | 206,994 | 100.0 |
| Sex | ||
| Female | 100,795 | 48.7 |
| Male | 105,967 | 51.2 |
| Missing data | 232 | 0.1 |
| Adverse birth group | ||
| Reference group | 149,743 | 72.3 |
| Moderate/late PTB (32–26) | 15,861 | 7.7 |
| Very PTB (<32) | 3,107 | 1.5 |
| SGA at term | 16,241 | 7.9 |
| LGA at term | 18,134 | 8.8 |
| Missing data | 3,908 | 1.9 |
| 5-min Apgar | ||
| Reassuring (7–10) | 194,943 | 94.2 |
| Moderately abnormal (4–6) | 3,552 | 1.7 |
| Low (0–3) | 832 | 0.4 |
| Missing data | 7,667 | 3.7 |
| Significant resuscitation measures | 18,942 | 9.2 |
| Bronchopulmonary dysplasia | 128 | 0.1 |
| Material deprivation (quintiles) | ||
| Q1 (least deprived) | 39,806 | 19.2 |
| Q2 | 38,931 | 18.8 |
| Q3 | 39,007 | 18.8 |
| Q4 | 37,635 | 18.2 |
| Q5 (most deprived) | 43,193 | 20.9 |
| Missing data | 8,422 | 4.1 |
| Social Deprivation (quintiles) | ||
| Q1 (least deprived) | 27,353 | 13.2 |
| Q2 | 39,990 | 19.3 |
| Q3 | 43,927 | 21.2 |
| Q4 | 46,120 | 22.3 |
| Q5 (most deprived) | 41,182 | 19.9 |
| Missing data | 8,422 | 4.1 |
| Deaths at follow-up | 183 | 0.1 |
Reference group = appropriate-for-gestational age infants born at term; LGA = large for gestational age. PTB = preterm birth. SGA = small for gestational age.
Rates of respiratory health services utilization
A total of 293,764 episodes of respiratory care (276,293 ED visits and 17,471 hospitalizations) was made by the 206,994 members of the birth cohort during the 5-year follow-up period. Ninety-four percent of the episodes of care were registered as ED visits while 6% were hospitalizations. Fifty-eight percent of children had two or more ED visits, while 33% had two or more hospitalizations during the study period. The number of events per child until they turned five years of age are shown in the supplementary S1 and S2 Figs.
Overall, the highest ED visit rates in all study groups were attributed to other URTI, followed by croup, asthma, and pneumonia (Table 2). Hospitalizations in all study groups were mostly for bronchiolitis and pneumonia. The least frequent reasons for ED visits were influenza and other LRTI, whereas influenza and bronchitis were the least frequent reasons for hospitalizations, compared to all other respiratory diseases.
Table 2. Crude rates of ED visits and hospitalizations per 1,000 singleton live birth for respiratory health services from birth to 5 years in Alberta.
| Total | Reference group | Moderate/late PTB | Very/extremely PTB | SGA at term | LGA at term | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Rate | Events | Rate | Events | Rate | Events | Rate | Events | Rate | Events | Rate | |
| ED visits | ||||||||||||
| URTI | 150,551 | 741.3 | 109,076 | 728.4 | 12,935 | 815.5 | 2,261 | 727.7 | 11,264 | 693.6 | 15,015 | 828.0 |
| Croup | 33,072 | 162.8 | 23,819 | 159.1 | 3,031 | 191.1 | 588 | 189.3 | 2,195 | 135.2 | 3,439 | 189.6 |
| Asthma | 25,593 | 126.0 | 17,718 | 118.3 | 2,841 | 179.1 | 829 | 266.8 | 2,025 | 124.7 | 2,180 | 120.2 |
| Pneumonia | 22,342 | 110.0 | 15,419 | 103.0 | 2,417 | 152.4 | 831 | 267.5 | 1,651 | 101.7 | 2,024 | 111.6 |
| Bronchiolitis | 19,302 | 95.0 | 13,075 | 87.3 | 2,396 | 151.1 | 618 | 198.9 | 1,414 | 87.1 | 1,799 | 99.2 |
| Bronchitis | 14,015 | 69.0 | 10,098 | 67.4 | 1,264 | 79.7 | 311 | 100.1 | 923 | 56.8 | 1,419 | 78.3 |
| Influenza | 6,858 | 33.8 | 4,956 | 33.1 | 655 | 41.3 | 98 | 31.5 | 517 | 31.8 | 632 | 34.9 |
| LRTI | 4,560 | 22.5 | 3,171 | 21.2 | 475 | 29.9 | 93 | 29.9 | 371 | 22.8 | 450 | 24.8 |
| Hospitalizations | ||||||||||||
| Bronchiolitis | 5,109 | 25.2 | 3188 | 21.3 | 754 | 47.5 | 303 | 97.5 | 387 | 23.8 | 477 | 26.3 |
| Pneumonia | 4,906 | 24.2 | 3175 | 21.2 | 612 | 38.6 | 336 | 108.1 | 358 | 22.0 | 425 | 23.4 |
| Asthma | 2,984 | 14.7 | 1962 | 13.1 | 385 | 24.3 | 181 | 58.3 | 232 | 14.3 | 224 | 12.4 |
| URTI | 2,212 | 10.9 | 1451 | 9.7 | 292 | 18.4 | 108 | 34.8 | 198 | 12.2 | 163 | 9.0 |
| Croup | 993 | 4.9 | 677 | 4.5 | 100 | 6.3 | 49 | 15.8 | 63 | 3.9 | 104 | 5.7 |
| LRTI | 493 | 2.4 | 288 | 1.9 | 71 | 4.5 | 53 | 17.1 | 40 | 2.5 | 41 | 2.3 |
| Influenza | 478 | 2.4 | 292 | 2.0 | 60 | 3.8 | 39 | 12.6 | 42 | 2.6 | 45 | 2.5 |
| Bronchitis | 296 | 1.5 | 181 | 1.2 | 34 | 2.1 | 10 | 3.2 | 25 | 1.5 | 46 | 2.5 |
Reference group = appropriate-for-gestational age infants born at term; LGA = large for gestational age; LRTI = lower respiratory tract infections; URTI = upper respiratory tract infections; PTB = preterm birth; SGA small for gestational age.
Multivariable analysis of respiratory health services utilization
Figs 2 and 3 display adjusted RRs for respiratory hospitalizations and ED visits during the first five years of life for adverse birth groups (all unadjusted and adjusted RRs are presented in S1 Table). Compared to the reference group, children born PTB had the highest rates of respiratory hospitalizations and ED visits, followed by LGA at term and SGA at term.
Fig 2. Adjusted rate ratios of respiratory hospitalizations for adverse birth groups.
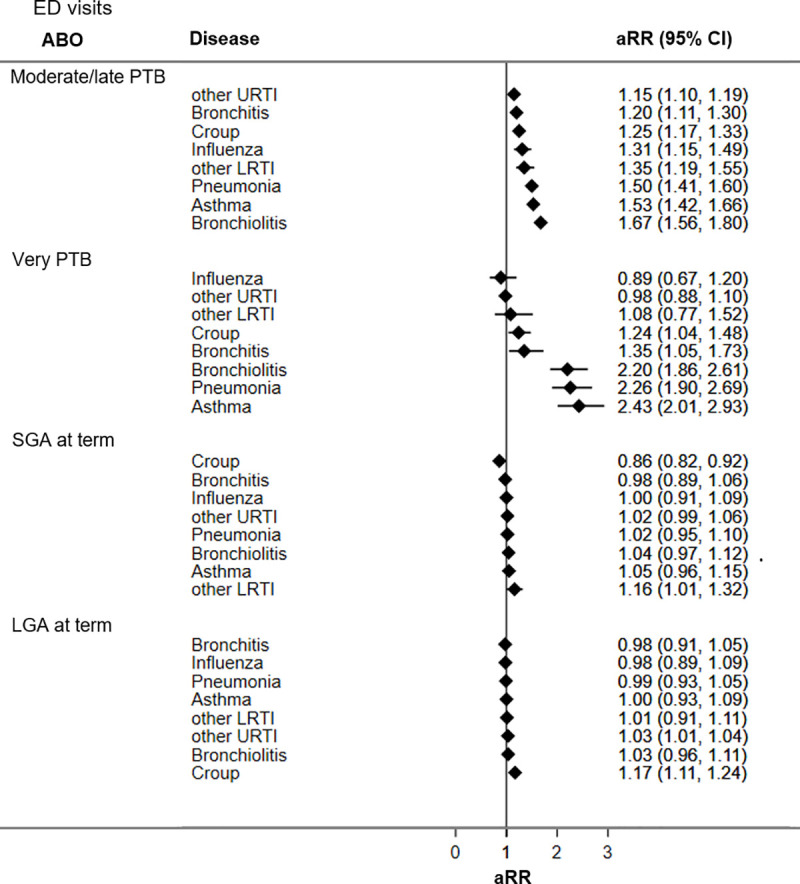
CI = confidence interval; LGA = large for gestational age; LRTI = lower respiratory tract infections; URTI = upper respiratory tract infections; PTB = preterm birth; SGA = small for gestational age. Reference group = appropriate-for-gestational age infants born at term; aRR = rate ratio adjusted for sex, bronchopulmonary dysplasia, Apgar 5, score, use of significant resuscitative measures, and material and social deprivation.
Fig 3. Adjusted rate ratios of respiratory ED visits for adverse birth groups.
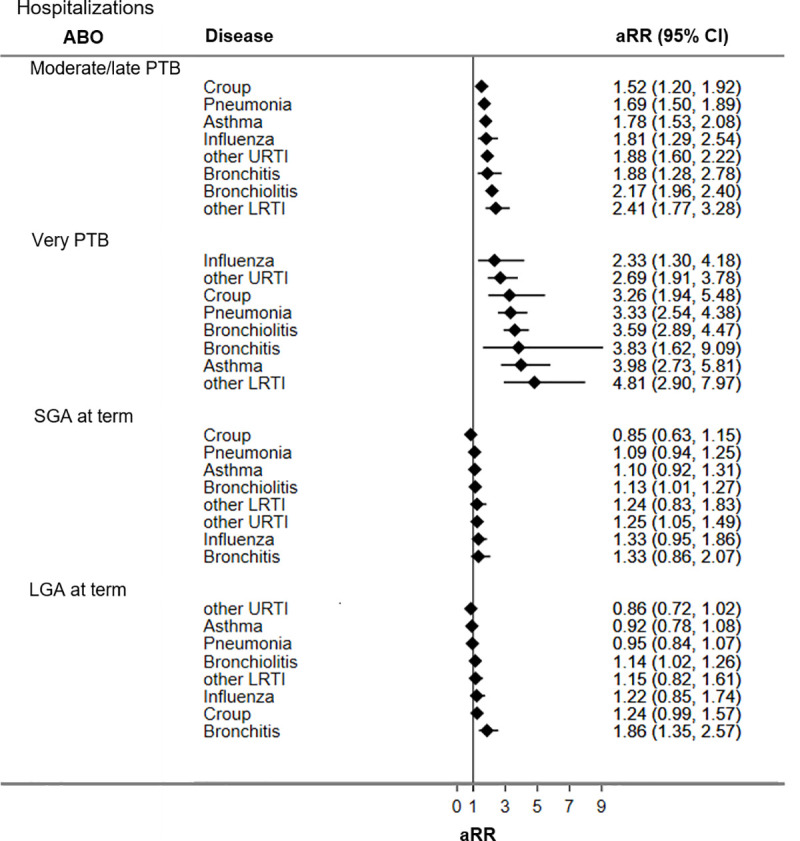
CI = confidence interval; LGA = large for gestational age; LRTI = lower respiratory tract infections; URTI = upper respiratory tract infections; PTB = preterm birth; SGA = small for gestational age. Reference group = appropriate-for-gestational age infants born at term; aRR = rate ratio adjusted for sex, bronchopulmonary dysplasia, Apgar 5, score, use of significant resuscitative measures, material and social deprivation.
Compared to the reference group, children born moderate/late PTB had significantly higher ED visits and hospitalization rates for all seven respiratory diseases studied. Increases in ED visit rates ranged from 1.2 times for other URTI (aRR 1.15; 95% CI 1.10, 1.19) to 1.7 times for bronchiolitis (aRR 1.67; CI 1.56, 1.80). Increased hospitalization rates among children born moderate/late PTB ranged from being 1.5 times higher for croup (aRR 1.52; 95% CI 1.20, 1.92) to 2.4 times higher for other LRTI (aRR 2.41; CI 1.77, 3.28) relative to the reference group.
Children born very PTB had significantly increased ED visit rates for croup (aRR 1.24; 95% CI 1.04, 1.48), bronchitis (aRR 1.35; 95% CI 1.05, 1.73), bronchiolitis (aRR 2.20; 95% CI 1.86, 2.61), pneumonia (aRR 2.26; 95% CI 1.90, 2.69), and asthma (aRR 2.43; 95% CI 2.01, 2.93). Children born very PTB had increased hospitalization rates for all seven respiratory conditions evaluated, ranging from 2.3 times for influenza (aRR 2.33; 95% CI 1.30, 4.18) to near 5 times for other LRTI (aRR 4.81; 95% CI 2.90, 7.97).
Children born SGA at term had significantly higher ED visit rates for other LRTI (aRR 1.16; 95% CI 1.01, 1.32) but lower ED visit rates for croup (aRR 0.86; 95% CI 0.82, 0.92) compared to the reference group. Hospitalization rates for bronchiolitis (aRR 1.13; 95% CI 1.01, 1.27) and other URTI (aRR 1.25; 95% CI 1.05, 1.49) were significantly higher among children born SGA relative to the reference group.
Children born LGA at term, had increased ED visit rates for croup (aRR 1.17; CI 1.11, 1.24) and other URTI (aRR 1.03; 95% CI 1.01–1.04), and higher hospitalization rates for bronchiolitis (aRR 1.14; 95% CI 1.02, 1.26) and bronchitis (aRR 1.86; 95% CI 1.35, 2.57) relative to the reference group.
Age-specific trajectories of total respiratory health service utilization rates
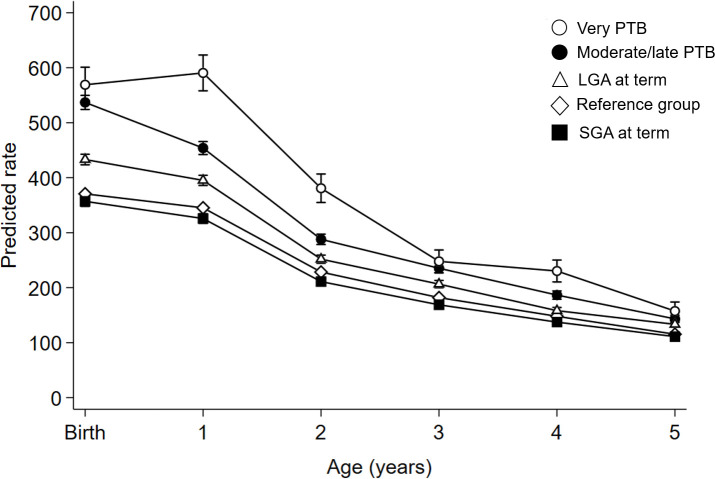
Age-specific trajectories of total respiratory health service utilization rates from birth to five years of age among the different adverse birth groups are presented in Table 3 and Fig 4. All trajectories followed a decreasing trend in the rates of respiratory health service utilization from birth to five years of age.
Table 3. Adjusted rates and rate differences for age-specific trajectories of respiratory health service utilization for birth groups.
| Age (in years) | Birth group | Adjusted Rate [95% CI] | Rate difference [95% CI] | Percentage of change (% [95% CI]) |
|---|---|---|---|---|
| Birth to < 1 | Reference | 370.7 [367.6, 373.8] | Reference | |
| Moderate/late PTB | 536.9 [524.1, 549.8] | 166.2 [147.3, 185.2]* | 45 [41, 47] | |
| Very PTB | 569.1 [537.1, 601.0] | 198.4 [152.4, 244.3]* | 54 [45, 61] | |
| SGA at term | 357.0 [347.9, 366.2] | -13.7 [-27.5, 0.1] | -4 [–6, 0] | |
| LGA at term | 433.1 [423.4, 442.7] | 62.4 [47.9, 76.9]* | 17 [14, 18] | |
| 1 to < 2 | Reference | 345.3 [342.3, 348.3] | Reference | |
| Moderate/late PTB | 454.0 [442.2, 465.8] | 108.7 [91.3, 126.2]* | 31 [29, 34] | |
| Very PTB | 590.6 [558.0, 623.1] | 245.3 [198.4, 292.2]* | 71 [63, 79] | |
| SGA at term | 325.8 [317.1, 334.6] | -19.5 [-32.7, -6.2]* | - 6 [–7, –4] | |
| LGA at term | 395.2 [386.0, 404.4] | 49.9 [36.0, 63.8]* | 14 [13, 16] | |
| 2 to < 3 | Reference | 228.5 [226.0, 230.9] | Reference | |
| Moderate/late PTB | 288.0 [278.6, 297.4] | 59.5 [45.7, 73.4]* | 26 [23, 29] | |
| Very PTB | 380.8 [355.0, 406.7] | 152.3 [115.2, 189.6]* | 67 [57, 76] | |
| SGA at term | 211.0 [204.0, 218.1] | -17.5 [-28.1, -6.8]* | - 8 [–10, –6] | |
| LGA at term | 251.9 [244.6, 259.3] | 23.4 [12.3, 34.5]* | 10 [8, 12] | |
| 3 to < 4 | Reference | 181.9 [179.8, 184.1] | Reference | |
| Moderate/late PTB | 235.4 [226.9, 243.9] | 53.5 [40.9, 66.0]* | 29 [26, 33] | |
| Very PTB | 247.9 [227.2, 268.6] | 66 [36.1, 95.8]* | 36 [26, 46] | |
| SGA at term | 168.9 [162.6, 175.2] | -13 [-22.6, -3.5]* | - 7 [–9, –5] | |
| LGA at term | 206.5 [199.9, 213.2] | 24.6 [14.6, 34.6]* | 14 [11, 16] | |
| 4 to < 5 | Reference | 147.9 [146.0, 149.9] | Reference | |
| Moderate/late PTB | 186.6 [179.0, 194.2] | 38.7 [27.4, 49.8]* | 26 [23, 29] | |
| Very PTB | 230.3 [210.4, 250.3] | 82.4 [53.7, 111.1]* | 56 [44, 67] | |
| SGA at term | 137.4 [131.7, 143.1] | -10.5 [-19.2, -2.0]* | - 7 [–10, –5] | |
| LGA at term | 158.2 [152.4, 164.1] | 10.3 [1.5, 19.1]* | 7 [4, 9] | |
| 5 to < 6 | Reference | 115.4 [113.7, 117.1] | Reference | |
| Moderate/late PTB | 143.3 [136.7, 149.9] | 27.9 [18.1, 37.7]* | 24 [20, 28] | |
| Very PTB | 157.5 [141.1, 173.9] | 42.1 [18.4, 65.8]* | 36 [24, 49] | |
| SGA at term | 111.1 [106.0, 116.2] | -4.3 [-12.0, 3.4] | -4 [–7, 3] | |
| LGA at term | 133.4 [128.0, 138.7] | 18 [9.9, 26.1]* | 16 [12, 19] |
Reference group = appropriate-for-gestational age infants born at term; CI = confidence interval; LGA = large for gestational age; PTB = preterm birth; SGA = small for gestational age.
Adjusted rates expressed as aggregated ED visits and hospitalizations for all combined respiratory conditions per 1,000 singleton live births.
* statistically significant differences (p-value < 0.05) between the corresponding adverse birth group and the reference group using Bonferroni’s correction of p-value for multiple comparisons.
Fig 4. Age-specific trajectories of respiratory health service utilization rates from birth to five years of age for birth groups.
Reference group = appropriate-for-gestational age infants born at term; LGA = large for gestational age; PTB = preterm birth; SGA = small for gestational age. Rates expressed as number of events per 1,000 singleton live births. Bars express 95% confidence intervals.
Compared to the reference group, children born very PTB, moderate/late PTB, and LGA at term significantly required more respiratory health services over time. Children born SGA at term had similar respiratory health services utilization rates as the reference group at birth and at five years of age, and small reductions of health services utilization rates at ages 1 to 2 (-6%), 2 to 3 (-8%) and 3 to 5 (-7% each year).
Discussion
Using a population-based retrospective cohort design, this study found patterns of respiratory health services utilization in the first five years of life that were distinctive for the group of adverse birth outcomes evaluated. While PTB accounted for the highest rates of ED visits and/or hospitalizations for all respiratory conditions evaluated, SGA at term associated with higher rates of ED visits for other LRTI, higher hospitalizations rates for bronchiolitis and other URTI, but lower ED visit rates for croup. LGA at term resulted in higher rates of ED visits for croup and other URTI and for bronchitis and bronchiolitis hospitalizations. Age-specific trajectories of respiratory health services utilization rates throughout the first five years of life also showed distinct patterns among the adverse birth groups: increased rates among children born PTB, moderately high for children LGA at term, and similar or lower rates for SGA compared to the reference group.
The observed decline in respiratory health service utilization after the first two years of life for all analyzed groups is consistent with prior literature as lung development increases alveolarization during the first 2–4 years of life [41]. Our results showed that children who experienced PTB (moderate and very-PTB) and LGA had higher rates of respiratory health services utilization for the first 5 years compared to the reference group, suggesting that there is room for more preventive management to help families keep these children healthy at home. Targeted public health interventions are required to promote health and minimize respiratory illnesses in these babies to improve morbidity and decrease health care costs. As well, knowing that a patient is in a higher risk group such as LGA will help to direct strategies for parent/caregiver counseling.
Our study results about the large impact of PTB on lung health in early childhood are consistent with a growing body of evidence summarized in meta-analyses of observational studies about the strong association between PTB and asthma [8,42–44]. Children born PTB in our study had higher hospitalization rates for pneumonia and bronchiolitis, conditions that are frequently caused by respiratory viral infections. Importantly, our study adds evidence of the strong association between PTB and respiratory infections in early childhood. Compared to the reference group, very PTB children had a 4-fold increase in the hospitalization rate for other LRTI and a 3-fold increase in the rate of hospital admissions for croup. These results are consistent with other reports of increased risk of respiratory infections among PTB children [14] due to impaired lung development and defects in the immune system [45] that are accentuated by decreasing gestational age [46].
The high rates of other LRTI and bronchiolitis ED visits and other URTI hospitalizations among children born SGA at term align with other population-based studies showing a high risk of respiratory hospitalizations in SGA children (including those born prematurely) during early childhood [14]. Our results also align with those reported by Yoshimoto et al. [15], who did not find a significant association between SGA at term and an increased risk of bronchitis, pneumonia, and asthma hospitalizations at five years of age. However, we found high rates of hospitalizations for bronchiolitis, suggesting that SGA at term could be associated with inflammation of the small airways. Our results and those of earlier studies suggest a vulnerability in respiratory function likely associated with impaired immune responses to infection in children with low weight at birth [45], either because of nutritional deprivation [47] or lung abnormalities that predispose to airway inflammation [48]. We did not observe increased rates for asthma among children born SGA at term compared to the reference group. It has been previously reported that SGA preterm children have an increased risk of developing asthma when low birth weight (< 2500 g) is used as an indicator of intrauterine growth restriction [49]. Our results align with those of a population-based study that did not find associations between SGA and asthma in children aged less than 10 years of age [50]. Additionally, the similar and even lower age-trajectories rates for the combined respiratory conditions for SGA at term compared to the reference group may be explained by the inclusion of exclusively SGA at term in the case definition. Most of the studies reporting SGA include SGA preterm cases, which limits comparability with our results. Another potential explanation for the small differences between rates may be related to the population growth chart that was used to classify SGA cases. The 2001 Canadian population growth chart likely classify small babies of certain immigrant populations as SGA cases. It is known that immigration in Alberta has been growing considerably since 2000 [51], and Heaman et al. [52] reported an odds ratio of 1.7 (95%CI: 1.1–2.4) for SGA related to recent immigrants to Canada.
Previous research suggests that children born LGA are at a high risk of developing respiratory distress at birth [11]. We found higher rates of respiratory health service utilization among LGA children for upper tract infections and bronchiolitis, especially during the first two years of life. Finally, children born LGA at term in our study did not have higher rates of asthma health services utilization. This result is consistent with findings from a recent meta-analysis of 90,000 children and adults reporting no association between birth weight > 4 kg and subsequent risk of asthma [49]. Physiological pathways leading to respiratory problems in LGA babies remain unclear. It has been observed that LGA neonates have an increased risk of respiratory distress at birth [11], but it has also been reported that infants born large for gestational age are more likely to be obese [53]. A high prevalence of childhood obesity (around 30%) has been reported in Canada [54]. Obese children have several complications including breathing difficulties [12,53]. This can be a potential explanation for the respiratory trajectories among LGA cases. Lack of longitudinal weight gain data in our research precluded exploration of the extent of respiratory morbidity among LGA cases that is associated with overweight.
Strengths of this study include the use of a validated perinatal clinical registry to identify the adverse birth exposure groups. The linkage of population-based administrative health data allowed us to assemble a large cohort of children born in Alberta over a 5-year period (2005–2010) and follow up their history of respiratory health services utilization over the first five years of their life.
Study limitations are related to potential misclassification of respiratory outcomes. We used ICD diagnostic codes recorded in administrative databases to estimate rates of respiratory health services utilization. The impact of potential misclassification in outcome assessment is expected to be minimal because the healthcare databases used in this study follow high-quality data protocols [55]. A set of quality control measures to ensure high-quality hospitalization data are applied by the Canadian Institute for Health Information such as examining relationships between data elements and element edits to each abstract [55]. However, limitations of using single code diagnoses data for some conditions like asthma exist, which is difficult to diagnose using single coding administrative data [56] especially during early childhood. There is a potential overestimation of both hospitalizations and ED visit rates as we were not able to adjust the denominators for the number of children who moved out of the province during the follow-up period due to lack of migration data. However, as a low annual average rate of emigration during the study period has been estimated (around 0.18% [57]), the impact of migration on rate calculations is likely low. Residual confounding is expected to some extent since the datasets do not register clinical information about other important factors related to respiratory health (e.g., ventilation therapies or improvements in subsequent health care for neonates experiencing adverse birth outcomes, and breastfeeding). The use of population-level data and hierarchical models at the DA level accounted for possible differential geographical access to health services and climate conditions that may modulate the frequency of some respiratory diseases, helping to reduce bias in RR estimations.
Conclusion
This study supports evidence about increased ED visits and hospitalizations for respiratory problems in the first five years of life in children born PTB. Additionally, the study showed that alterations in fetal growth, especially in LGA at term cases, increased the use of respiratory health services utilization during early childhood, particularly for respiratory infections. The population-level patterns of age-specific trajectories in health service utilization have the potential to inform the development of targeted paediatric strategies supportive of healthy lung development in children who experienced adverse birth outcomes. Future research using life-course and environmental approaches are needed to improve our knowledge of the relationships between adverse birth outcomes and lung function in early childhood.
Supporting information
(DOC)
(TIFF)
(TIFF)
(PDF)
(PDF)
Data Availability
Data cannot be shared publicly because it is held securely in coded form at Alberta Health Services. Alberta Health Services is the legal custodian of the original data. Alberta Health Services’ policies and acts (e.g., Health Information Act of Alberta) guarantee the security, privacy and confidentiality of the patient data. Data agreement with Alberta Health Services prohibits researchers from making the dataset publicly available. Access to data may be granted to those who meet pre-specified criteria for confidential access. Data are available from Alberta Health Services Provincial Research Data Services for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from Alberta Health Services’ (AHS) Health System Access (HSA): https://www.albertahealthservices.ca/research/page8579.aspx. More information at: research.administration@ahs.ca.
Funding Statement
This research was funded by the Lois Hole Hospital for Women through a Women and Children’s Health Research Institute Recruitment Award (MBO) (https://www.wchri.org), and the Lung Association Alberta & NWT through the 2017-2018 National Grant Review program (https://www.ab.lung.ca/what-we-do/research/grant-opportunities/national-grant-review). MBO’s research is supported by the Canada Research Chair Program (Government of Canada; Ottawa, Canada). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–675. 10.1136/bmj.303.6804.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt RD Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med. 2013;7(5):515–531. 10.1586/17476348.2013.838020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27(1):5–14. 10.1007/s10654-012-9657-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards CA, Osman LM, Godden DJ, Campbell DM, Douglas JG. Relationship between birth weight and adult lung function: controlling for maternal factors. Thorax. 2003;58(12):1061–1065. 10.1136/thorax.58.12.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner S, Prabhu N, Danielan P, McNeill G, Craig L, Allan K, et al. First, and second, trimester fetal size and asthma outcomes at age 10 years. Am J Respir Crit Care Med. 2011;184(4):407–413. 10.1164/rccm.201012-2075OC [DOI] [PubMed] [Google Scholar]
- 6.Patelarou E, Chochlidaki M, Vivilaki V, Brokalaki H. Is there a link between wheezing in early childhood and adverse birth outcomes? A systematic review. Int J Environ Res Public Health. 2009;6(11):2752–2761. 10.3390/ijerph6112752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brew BK, Marks GB, CAPS [Childhood Asthma Prevention Study] Investigators. Perinatal factors and respiratory health in children. Clin Exp Allergy. 2012;42(11):1621–1629. 10.1111/j.1365-2222.2012.04056.x [DOI] [PubMed] [Google Scholar]
- 8.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta, analysis. J Allergy Clin Immunol. 2006;118(4):823–830. 10.1016/j.jaci.2006.06.043 [DOI] [PubMed] [Google Scholar]
- 9.Henderson AJ, Warner JO. Fetal origins of asthma. Semin Fetal Neonatal Med. 2012;17(2):82–91. 10.1016/j.siny.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Pike KC, Lucas JSA. Respiratory consequences of late preterm birth. Paediatr Resp Rev. 2015;16(3):182–188. 10.1016/j.prrv.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes?. World J Diabetes. 2015;6(5):734–743. 10.4239/wjd.v6.i5.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorino EK, Brooks LJ. Obesity and respiratory diseases in childhood. Clin Chest Med. 2009;30(3):601–608. 10.1016/j.ccm.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Olsen J, Agerbo E, Yuan W, Cnattingius S, Gissler M, Li J. Birth weight, gestational age, fetal growth and childhood asthma hospitalization. Allergy Asthma Clin Immunol. 2014;10(1):13 10.1186/1710-1492-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paranjothy S, Dunstan F, Watkins WJ, Hyatt M, Demmler JC, Lyons RA, et al. Gestational age, birth weight, and risk of respiratory hospital admission in childhood. Pediatrics. 2013;132(6):1562–1569. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto J, Yorifuji T, Washio Y, Okamura T, Watanabe H, Doi H, et al. Population, based longitudinal study showed that children born small for gestational age faced a higher risk of hospitalisation during early childhood. Acta Paediatr. 2019;108(3):473–478. 10.1111/apa.14507 [DOI] [PubMed] [Google Scholar]
- 16.Boonyaratanakornkit J, Englund JA, Magaret AS, Bu Y, Tielsch JM, Khatry SK, et al. Primary and repeated respiratory viral infections among infants in rural Nepal. J Pediatric Infect Dis Soc. 2020;9(1):21–29. 10.1093/jpids/piy107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zar HJ, Ferkol TW. The global burden of respiratory disease, impact on child health. Pediatr Pulmonol. 2014;49(5):430–434. 10.1002/ppul.23030 [DOI] [PubMed] [Google Scholar]
- 18.Canadian Institute for Health Information. Asthma Hospitalizations Among Children and Youth in Canada: Trends and Inequalities. 2018 [cited 2019 March 22]. Available from: https://www.cihi.ca/sites/default/files/document/asthma, hospitalization, children, 2018, chartbook, en, web.pdf.
- 19.Johnson DW, Williamson J. Croup in children. Can Med Assoc J. 2013;185(15):1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics Canada. Table 13-10-0745-01. Birth, related indicators (low and high birth weight, small and large for gestational age, pre, term births), by sex, three, year period, health regions and peer groups. [Cited 2019 March 22]. Data repository [Internet]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310074501.
- 21.Johnston KM, Gooch K, Korol E, Vo P, Eyawo O, Bradt P. et al. The economic burden of prematurity in Canada. Pediatrics. 2014;14:93 10.1186/1471-2431-14-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Algert CS, Bowen JR, Lain SL, Allen HD, Vivian-Taylor JM, Roberts CL. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol. 2011;22(8):836–842. 10.1111/j.1399-3038.2011.01206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. 2010;10:14 10.1186/1471-2466-10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haataja P, Korhonen P, Ojala R, Hirvonen M, Korppi M, Gissler M, et al. Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Pediatr Pulmonol. 2018;53(2):209–217. 10.1002/ppul.23908 [DOI] [PubMed] [Google Scholar]
- 25.Johnson J, Ryan T, Aherrera AD, McGrath-Morrow SA, Collaco JM. The influence of small for gestational age status on outpatient bronchopulmonary dysplasia outcomes. J Perinatol. 2015;35(1):72–76. 10.1038/jp.2014.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics Canada. Census Profile, 2016 Census. 2017 [cited 2020 May 20]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E.
- 27.Von Elm E, Altman DG, Egger M, Pocock SJ, Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberta Perinatal Health Program. Alberta Perinatal Health Program. 2016 [cited 2016 October 2]. Available from: http://aphp.dapasoft.com/Lists/HTMLPages/index.aspx.
- 29.Canadian Institute for Health Information. ICD-10-CA. International Statistical Classification of Diseases and Related Health Problems. Ottawa [ON]: CIHI; 2015 [cited 2016 October 2]. Available from: https://www.cihi.ca/sites/default/files/icd_volume_one_2015_en_0.pdf.
- 30.Quinn JA, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047–6056. 10.1016/j.vaccine.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population, based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E135 10.1542/peds.108.2.e35 [DOI] [PubMed] [Google Scholar]
- 32.Ducharme FM, Dell SD, Radhakrishnan D, Grad RM, Watson WTA, Yang CL, et al. Diagnosis and management of asthma in preschoolers: A Canadian Thoracic Society and Canadian Paediatric Society position paper. Can Respir J. 2015;22(3):135–143. 10.1155/2015/101572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douros K, Everard ML. Time to say goodbye to bronchiolitis, viral wheeze, reactive airways disease, wheeze bronchitis and all that. Front Pediatr. 2020;8:218 10.3389/fped.2020.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren CL, Esther CR, Debley JS, Sockrider M, Yilmaz O, Amin N, et al. Official American Thoracic Society Clinical Practice Guidelines: Diagnostic evaluation of infants with recurrent or persistent wheezing. Am J Respir Crit Care Med. 2016;194(3):356–373. 10.1164/rccm.201604-0694ST [DOI] [PubMed] [Google Scholar]
- 35.American College of Obstetricians and Gynecologists. The Apgar score. Committee Opinion No. 644. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;126:e52–e55. 10.1097/AOG.0000000000001108 [DOI] [PubMed] [Google Scholar]
- 36.Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29(4):178–191. [PubMed] [Google Scholar]
- 37.Pampalon R, Hamel D, Gamache P, Philibert MD, Raymond G, Simpson A. An area based material and social deprivation index for public health in Québec and Canada. Can J Public Health. 2012;103(8 Suppl 2):S17–S22. 10.1007/BF03403824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano-Lomelin J, Nielsen CC, Hicks A, Crawford S, Bakal JA, Ospina MB. Geographic inequalities of respiratory health services utilization during childhood in Edmonton and Calgary, Canada: A tale of two cities. Int J Environ Res Public Health. 2020;17(23):8973 10.3390/ijerph17238973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belon AP, Serrano‐Lomelin J, Nykiforuk CIJ, Hicks A, Crawford S, Bakal J, et al. Health gradients in emergency visits and hospitalisations for paediatric respiratory diseases: A population‐based retrospective cohort study. Paediatr Perinat Epidemiol. 2020;34(2):150–160. 10.1111/ppe.12639 [DOI] [PubMed] [Google Scholar]
- 40.StataCorp. Stata Statistical Software: Release 15.1 2017. College Station, TX: StataCorp. [Google Scholar]
- 41.Carraro S, Scheltema N, Bont L, Baraldi E. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. Eur Respir J. 2014;44(6):1682–1696. 10.1183/09031936.00084114 [DOI] [PubMed] [Google Scholar]
- 42.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: A meta, analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133(5):1317–1329. 10.1016/j.jaci.2013.12.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, Anessi, Maesano I, Arshad SH, Barros H, et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta, analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026–1035. 10.1016/j.jaci.2015.08.050 [DOI] [PubMed] [Google Scholar]
- 44.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm Birth and Childhood Wheezing Disorders: A Systematic Review and Meta, Analysis. PLoS Med. 2014;11(1):e1001596 10.1371/journal.pmed.1001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baxter D. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Human Vaccines. 2010;6(6):494–505. 10.4161/hv.6.6.12008 [DOI] [PubMed] [Google Scholar]
- 46.Townsi N, Laing IA, Hall GL, Simpson SJ. The impact of respiratory viruses on lung health after preterm birth. Eur Clin Respir J. 2018;5(1):1487214 10.1080/20018525.2018.1487214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosmi E, Fanelli T, Visentin S, Trevisanuto D, Zanardo V. Consequences in infants that were intrauterine growth restricted. J Pregnancy. 2011;2011:1–6. 10.1155/2011/364381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016;10:67–83. 10.4137/CMPed.S40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu M, Ye S, Bai M-J, Liu G-L, Tong Y, Wang S-F, et al. Birth weight and subsequent risk of asthma: a systematic review and meta, analysis. Heart Lung and Circ. 2014;23(6):511–519. 10.1016/j.hlc.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 50.Gessner BD, Chimonas M-AR. Asthma is associated with preterm birth but not with small for gestational age status among a population, based cohort of Medicaid, enrolled children <10 years of age. Thorax. 2007;62(3):231–236. 10.1136/thx.2005.053363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Government of Alberta. International Migration in Alberta. Demographic Spotlight. 2009 [cited 2019 April 11]. Available from: https://open.alberta.ca/dataset/4c01ba53-2ba2-4ff5-b82c-8868d377c155/resource/c54fa3c7-2bf6-4d84-b7c7-aefabb043dce/download/20091221internationalmigrationspotlightfinal.pdf.
- 52.Heaman M, Kingston D, Chalmers B, Sauve R, Lee L, Young D. Risk factors for preterm birth and small-for-gestational-age births among Canadian women: risk factors for PTB and SGA births. Paediatric Perinat Epidemiol. 2013;27(1):54–61. [DOI] [PubMed] [Google Scholar]
- 53.Weschenfelder F, Lehmann T, Schleussner E, Groten T. Gestational weight gain particularly affects the risk of large for gestational age infants in non-obese mothers. Geburtsh Frauenheilkd. 2019;79(11):1183–1190. 10.1055/a-0891-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can. 2016;36(9):194–198. 10.24095/hpcdp.36.9.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinds A, Lix LM, Smith M, Quan H, Sanmartin C. Quality of administrative health databases in Canada: a scoping review. Can J Public Health. 2016;27;107(1):56 10.17269/cjph.107.5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissen F, Quint J, Wilkinson S, Müllerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol. 2017;9:643–656. 10.2147/CLEP.S143718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Government of Alberta. Alberta Population Estimates, Data Tables. 2020 [cited 2019 March 30]. Data repository [Internet]. Available from: https://open.alberta.ca/opendata/alberta-population-estimates-data-tables.