Abstract
To date, some genetic studies offer medical benefits but lack a clear pathway to benefit for people from underrepresented backgrounds. Historically, Indigenous people, including the Diné (Navajo people), have raised concerns about the lack of benefits, misuse of DNA samples, lack of consultation, and ignoring of cultural and traditional ways of knowing. Shortly after the Navajo Nation Human Research Review Board was established in 1996, the Navajo Nation recognized growing concerns about genetic research, and in 2002 they established a moratorium on human genetic research studies. The moratorium effectively has protected their citizens from potential genetic research harms. Despite the placement of the moratorium, some genetic research studies have continued using blood and DNA samples from Navajo people. To understand the history of genetic research involving Navajo people, the authors conducted a literature review of genetic or genetics-related research publications that involved Navajo people, identifying 79 articles from the years 1926 to 2018. To their knowledge, no known literature review has comprehensively examined the history of genetic research in the Navajo community. This review divides the genetic research articles into the following general classifications: bacteria or virus genetics, blood and human leukocyte antigens, complex diseases, forensics, hereditary diseases, and population genetics and migration. The authors evaluated the methods reported in each article, described the number of Navajo individuals reported, recorded the academic and tribal approval statements, and noted whether the study considered Diné cultural values. Several studies focused on severe combined immunodeficiency disease, population history, neuropathy, albinism, and eye and skin disorders that affect Navajo people. The authors contextualize Diné ways of knowing related to genetics and health with Western scientific concepts to acknowledge the complex philosophy and belief system that guides Diné people and recognizes Indigenous science. They also encourage researchers to consider cultural perspectives and traditional knowledge that has the potential to create stronger conclusions and better-informed, ethical, and respectful science.
Keywords: NAVAJO NATION, DINÉ, GENOMICS, GENETIC RESEARCH, INDIGENOUS SCIENCE
While Indigenous people have been subjects of genetic research for decades, the number of research studies involving them has remained relatively low. Since the first human genome was sequenced in 2003, the field of genetics has rapidly improved its sequencing technology and increased access, allowing more researchers and clinicians to address fundamental questions that may lead to individualized patient health care (National Institutes of Health 2015; Schmutz et al. 2004; Wetterstrand 2019). In genomic medicine, scientists and clinicians are able to use these improved tools to study the role of multiple genetic factors, acting together with the environment, to understand various hereditary and complex diseases. However, most genetic studies have largely been conducted in people of European descent, thus potentially benefiting them and excluding other populations, such as Indigenous people (Mills and Rahal 2019; Popejoy and Fullerton 2016). Now, Western-trained Indigenous genomic scientists are emerging and beginning to propose important research questions to address inequities in genetic research while integrating traditional knowledge.
Indigenous people, the original caretakers of a given land, possess a wealth of inherent knowledge of heredity and genetics. Some Indigenous groups, such as the Navajo, have used such knowledge from time immemorial to recognize kinship and familial relationships, to interact with their environment, and to use complex breeding practices with livestock (e.g., sheep) and crops to yield favorable physical characteristics (Bousselot et al. 2017; Sponenberg and Taylor 2009). While these concepts are known collectively as “genetics” in contemporary Western science, among the Navajo people many concepts involving iiná bitł’ool (DNA; lit. “strands of life”) have been shared through lived experiences, conveyed orally through storytelling, and ultimately woven into the cultural and traditional epistemology for generations. The Navajo Nation is the second largest tribe in the United States with over 350,000 enrolled citizens and has the largest tribal land base, spanning the states of Arizona, Utah, and New Mexico, covering over 27,000 square miles. Diné (Navajo; lit. the People), as they refer to themselves, have lived on their homelands for generations and many still live within the boundaries of the four sacred mountains located in Arizona, New Mexico, and Colorado.
Indigenous populations have been reticent to participate in many types of research studies for various reasons related to historical research harms, such as misuse of DNA samples, disrespecting Indigenous cultural/traditional ways of knowing, and other ethical concerns (American Journal of Medical Genetics 2010; Arbour and Cook 2006; Beans et al. 2019; Claw et al. 2018; Drabiak-Syed 2010; Garrison et al. 2019). To further protect their tribal citizens, the Navajo Nation established nationally recognized research regulations in 1996 requiring all human subject research studies conducted within the jurisdiction of the Navajo Nation be reviewed and approved by the Navajo Nation Human Research Review Board (NNHRRB), while simultaneously abiding by US research regulations, including the US Department of Health, Education, and Welfare regulations for the protection of human subjects in 1974 and the “Common Rule” in 1991, with its recent updates (Brugge and Missaghian 2006; Office for Human Research Protections 2018; NNHRRB 2009; National Commission 1978). Prior to the Navajo Research Act of 1996, medical research on Navajo lands was reviewed and approved by the Indian Health Services Navajo Area Institutional Review Board; thereafter, their functions were assumed by the NNHRRB (Brugge and Missaghian 2006). The purpose of the NNHRRB has been to enhance and promote ethical and beneficial research for the Diné people and to nurture a culturally respectful relationship between Diné and Western scientific knowledge in approved research (NNHRRB 2009).
In April 2002, the Navajo Nation asserted its sovereign authority to limit genetic research when the Navajo Nation Health and Human Services Committee (NNHHSC) approved a “moratorium on genetic research studies conducted within the jurisdiction of the Navajo Nation until such time that a Navajo Nation Human Research Code has been amended by the Navajo Nation Council” (NNHHSC 2002). This suspension of genetic research applies to all studies involving tribal members who live on the Navajo Nation. The moratorium came from a collective decision resulting from lengthy consultations with the NNHHSC, tribal leaders, traditional healers, and Navajo people with medical and scientific training (Brown 2002). A common concern among these groups was that the Navajo Nation lacked an appropriate policy or set of guidelines to regulate genetic research protocols that might be culturally discordant with traditional Diné values (Brown 2002; National Congress of American Indians 2012). For over 18 years, the moratorium has in fact deterred genetic research projects from being conducted on the Navajo Nation and also served to protect the Navajo people from potential genetic research harms.
To our knowledge, no known comprehensive reviews have examined the history of genetic research in the Navajo community to date. We conducted a literature review of genetic research articles that include Navajo people as research subjects, identifying 79 articles that we divide into six general classifications. We further discuss Western scientific concepts in concert with Navajo cultural and traditional examples of genetic knowledge that reflect Diné ways of knowing (epistemology), a complex philosophy and belief system that guides Diné people and acknowledges Indigenous science.
Methods
We conducted an online literature search using PubMed and Google Scholar applying the keywords “Navajo,” “Navaho,” “Diné,” “Dine,” “Dene,” “Athabaskan,” “Athapaskan,” “Southwest tribe,” “Arizona tribe,” “American Indian,” “genetics,” “genomics,” “disease,” and “research.” The word “Athabaskan” (also known as “Dene” or “Na-Dene”) refers to a large language family that includes Northern, Pacific Coast, and Southern Indigenous groups and was included in the search because Navajo people are sometimes referred to as “Southern Athabaskans or Athapaskans” in the literature. In addition to our online search, we also accessed a data set of 300 articles related to Navajo genetic, health, and public health published from 1862 to 1969, curated by Bonnie Duran for the Native Health Database retained at the University of New Mexico.
Figure 1 depicts a flowchart of the literature search, with the exclusion criteria. Our three inclusion criteria were (1) original research published in English before December 2018 (date of search) that (2) involved the use of genetics and family pedigrees, and (3) studied human and nonhuman DNA derived from Navajo biospecimens. News, comments, clinical reports, and reviews were excluded from the results but are cited here as additional references when appropriate. We were not able to confirm tribal enrollment status of individuals; therefore, we report the number of Navajo individuals only if the authors clearly labeled samples or data as “Navajo” or “Diné” and any other spelling versions mentioned above. Finally, we included original articles that use secondary data or shared Navajo samples.
FIGURE 1.
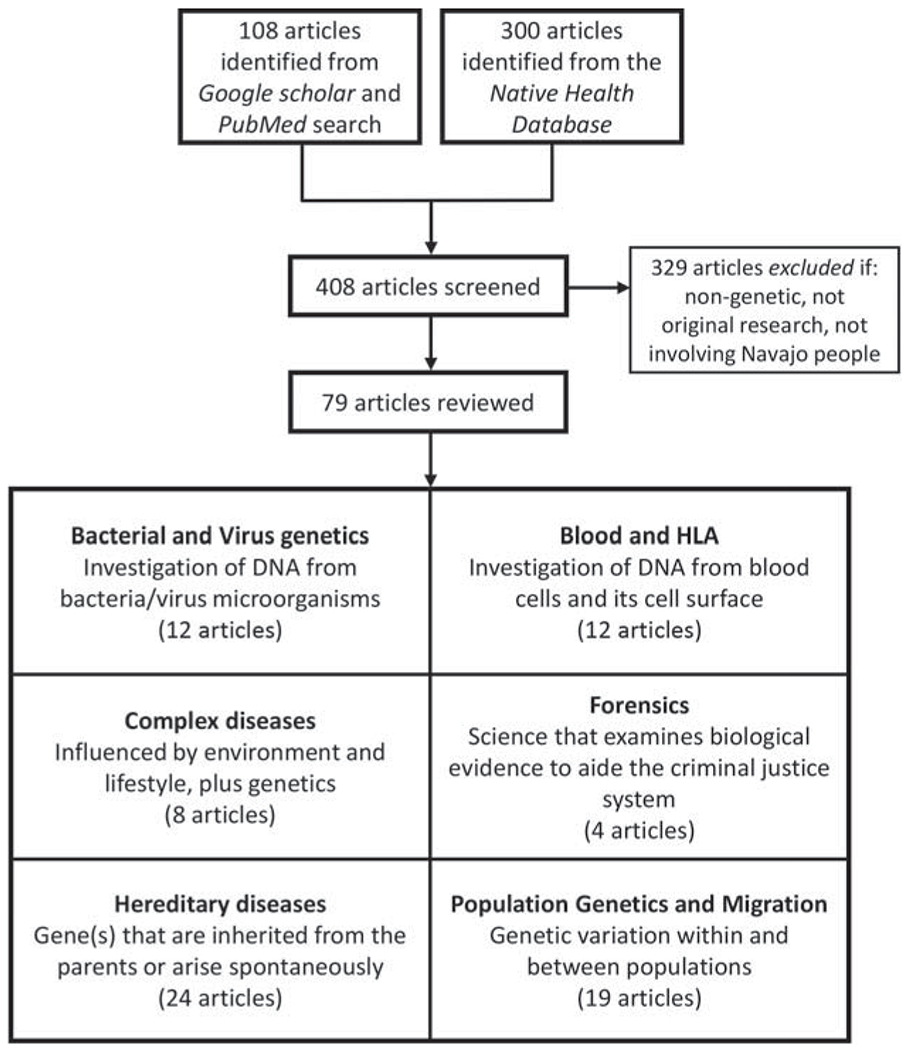
Literature search flowchart showing the number of included and excluded genetic research studies and the number of studies for each classification.
During our analysis of the full-text articles, we extracted the following information from each publication: publication year and journal, disease associated gene(s) or chromosomal region sequenced, mode of inheritance, sequencing technology or methods used, number of Navajo people included, type of institutional review board (IRB) oversight, and secondary use of data or samples. We categorized all articles by general fields of study. Our review also discusses cultural and traditional knowledge of genetics or genetics-related diseases that was informed by our elders or own experiences living in the Navajo community.
We are involved in health research and/or research regulation on the Navajo Nation and most of us are enrolled members of the Navajo Nation. Due to the sensitive nature of our review article topic, we submitted our manuscript to the NNHRRB for prereview and obtained permission on 23 October 2019 to proceed with publication.
Results
Of the 408 articles identified via online search or the Native Health Database, 79 articles of genetic or genetics-related research met our inclusion criteria, which we broadly classified into six general fields (see Figure 1), which we discuss individually below. Table 1 lists these six general fields and specific subfields, with the total number of articles and total number of Navajo individuals reported in each group. In total, 13,355 Navajo people were included in published research articles related to genetics over the past 93 years. Most individuals (n = 8,540) were involved in studies in the hereditary disease category, and the fewest (n = 69) individuals were involved in the complex disease studies.
Table 1.
Summary of 79 Articles Reporting Genetic Studies Involving Navajo Individuals
| Classification | Number of Studies | Number of Navajo Individuals |
|---|---|---|
| Bacteria or virus geneticsc | 6 | 15,142a |
| 6 | 70b | |
| Blood and human leukocyte antigen (HLA)c | 12 | 1,761 |
| Blood typing | 4 | 1,104 |
| HLA typing | 5 | 388 |
| Blood protein/enzyme testing | 3 | 269 |
| Complex diseases | 7 | 69 |
| Alcoholism | 1 | 15 |
| Epilepsy | 1 | 2 |
| Bone-related disorder | 1 | 2 |
| Microvillus inclusion disease | 3 | 22 |
| Influenza | 1 | 28 |
| Forensics studies | 4 | 263 |
| Hereditary diseases | 25 | 8,540 |
| Immune system | 12 | 8,039 |
| Cancer | 4 | 230 |
| Eye-related disorders | 4 | 248 |
| Skin-related disorders | 3 | 7 |
| Metabolic disorder | 1 | 15 |
| Cystic fibrosis | 1 | 1 |
| Population genetics and migration | 19 | 2,652 |
| Inbreeding or mating patterns | 4 | 1,202 |
| Migration/population substructure | 15 | 1,450 |
| Total | 79 | 13,355 |
Six related studies used samples collected from Navajo people and White Mountain Apache children spanning from 1994 to 2009. Some studies did not report the number of Navajo individuals and simply reported aggregate data from multiple tribes, meaning the number does not distinguish between different tribal groups. We report the highest total number reported because it was unclear which samples were shared or unique to the studies.
Six studies included a total of 70 Navajo samples.
Studies in these categories did not sequence human DNA.
Figure 2 depicts a timeline (1926–2018) by publication year for the 79 articles included in our analysis. Interestingly, bacteria and virus articles were more prevalent after the moratorium was established. Before the advent of sequencing technology, most genetic studies used phenotypical observations, such as blood or human leukocyte antigen (HLA) typing, and this trend is reflected in our data, with these articles primarily occurring before the 1990s. Most of the articles (48, in hereditary disease, complex disease, forensics, and population genetics and migration categories) were published from 1990 to 2000, when sequencing technology improved tremendously and just before the Navajo Nation enacted the genetic research moratorium.
FIGURE 2.
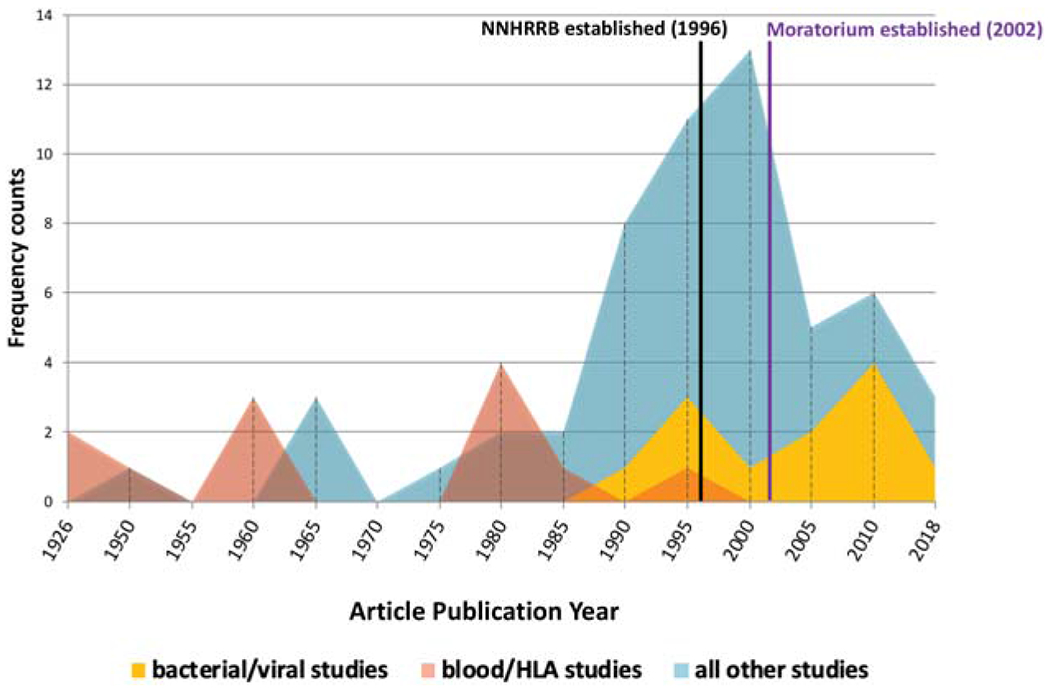
Publication timeline of articles reporting genetic studies involving Navajo individuals, spanning 1926–2018 in five-year intervals. Blood/HLA studies and bacteria/virus studies were separated into different groups since these studies did not use genetic technology or sequence human DNA. All other studies sequenced or analyzed human DNA from Navajo individuals.
Overall, of the 79 articles, 45 (57%) did not cite review board approval, 10 (13%) mentioned both tribal and academic IRB approval, 17 (22%) mentioned only academic IRB approval, and 7 (9%) only acknowledged tribal entities and did not mention other review board approvals (see Supplementary Table S1). Publications before 1991 are distinctive because ethical, journal, and reporting standards drastically changed after 1991 with the establishment of the Common Rule. Before 1991, human subject approval was not routinely sought or reported in publications. We found 21 articles published in or before 1991; of those, 3 thanked tribal entities and 4 mentioned academic IRB approval. Thirty-six (46%) of 79 articles were published in or before 1996, when the NNHRRB was established; of those 36 articles, 5 stated that they obtained academic IRB approval only. Twenty-five (32%) of 79 articles were published after the 2002 moratorium, of which 7 involved bacterial/viral genetics and 18 involved human genetics. Of these 25 articles, 8 did not cite review board approval, 7 mentioned both tribal and academic IRB approval, and 10 obtained only academic IRB approval. Regarding sample collection, 29 (37%) of 79 articles collected samples on the Navajo Nation, 17 (21%) collected samples off the Navajo Nation (e.g., urban areas), 14 (18%) collected samples both on and off the Navajo Nation, and 19 (24%) did not mention where samples were collected. This brings into question the jurisdictional reach of the moratorium and the effects of researchers conducting research away from tribal oversight. A total of 19 articles indicated the study used secondary data from previously published data sets or sample collections.
Bacteria or Virus Genetics
Articles reporting bacteria and virus studies were included in this review because of their symbiotic relationship with the human body. Genetic material from bacteria and viruses is distinct from human DNA, yet bacteria inhabit almost every tissue of the human body, and microbes outnumber human cells by 10:1. Twelve microbiology articles reported studies that used genetic techniques to assess the epidemiology of the diseases or the diversity of the bacteria or virus strains that are known to affect humans. These studies extracted and studied bacterial or viral DNA from samples acquired from human subjects.
Of the 12 articles, 3 focused on T-cell lymphotropic/leukemia virus type 2 (HTLV-2) (Biggar et al. 1996; Hjelle et al. 1993; Switzer et al. 1995), 2 on pneumococcal isolates (Scott et al. 2012a, 2012b), 2 on Streptococcus pneumoniae (Azarian et al. 2018; Lipsitch et al. 2007), 2 on rotavirus (Grant et al. 2011, 2012), 2 on the Human polyomavirus 2, formerly called the JC virus (Agostini et al. 1997; Fernandez-Cobo et al. 2002), and 1 on Haemophilus influenzae types A and B (Millar et al. 2005) (see Table 1).
Most of these infectious microbes have high prevalence on the Navajo Nation. For example, H. influenzae is a leading cause of bacterial meningitis in children, and HTLV-2 can be spread by blood transfusions, sexual contact, and sharing of needles. In particular, we highlight the studies that analyzed H. influenzae and S. pneumoniae strains because of the researchers’ inclusive and collaborative nature (Lipsitch et al. 2007; Millar et al. 2005). Both articles mention obtaining NNHRRB and academic IRB approval and also include Navajo researchers as coauthors.
Blood and HLA
Blood samples provide clinicians and researchers with metabolic, immunologic, and genetic (DNA, RNA, and proteins) information that is easily obtainable and has a long storage life. Four articles related to blood type frequencies were published in the first half of the 20th century. Two of the earliest studies used blood type frequencies to understand why populations differ from one another (Corcoran et al. 1962; Nigg 1926). These studies examined blood types in 457 Navajo children from two communities in Arizona (Fort Defiance and St. Michaels; Nigg 1926) and 237 children from Pinon, Arizona (Corcoran et al. 1962), investigating similarities to other American Indian tribes and Asian “races” that supported their hypothesis that “American Indian[s] [were] of Mongolian origin” (Nigg 1926). Both studies found the O blood type at “unusually high” frequencies in Navajo people, about twice as high as other ethnic populations. Nigg (1926) excluded the “doubtful full bloods” to maintain purity for analysis. The other two articles (Boyd 1951; Boyd and Boyd 1949) reported studies that used blood collected from 410 Navajo people living near Ramah, New Mexico, to examine the relationship of Rh factor to Asian blood type frequencies, concluding that Navajo individuals mostly have Rh negative factors.
Similar to research on blood types, scientists used HLA typing to examine population and migration patterns and how certain diseases manifest in humans. HLA genes provide instructions to make surface proteins on cells that react to foreign substances in the blood to fight viral and bacterial infections in the human body. In our search, five articles described HLA typing in relation to migration and described the similarity of Navajo people to other North American Indigenous groups (Garber et al. 1996; Troup et al. 1982; Williams et al. 1981; Morse et al. 1980; Kuberski et al. 1981). The HLA-A, -B, -C, and -DR antigens were analyzed by Troup et al. (1982), who found specific HLA types (n = 139 Navajo samples) associated with cancer and diabetes. Using HLA-B antigen typing, Garber et al. (1996) and Troup et al. (1982) found no variability between North American Indigenous groups but high variability among South American Indigenous groups. Three articles assessed HLA typing in 100 Navajo and Hopi individuals. Williams et al. (1981) concluded that Navajo and Hopi people were similar genetically but culturally diverse. Morse et al. (1980) found that Reiter’s syndrome (found in 13 of 18 Navajo individuals), or reactive arthritis, was associated with HLA-B27 frequency. Kuberski et al. (1981) found sacroiliitis disease (inflammation in one or both of the sacroiliac joints causing pain in the legs) to be associated with HLA-B27 as well.
Proteins and enzymes found in the blood were also analyzed in three studies (Parker and Bearn 1961; Balsamo et al. 1964; Crist et al. 1985). Parker and Bearn 1961 analyzed 263 Navajo blood samples and found a high frequency of a new transferrin variant (iron-binding globulins in serum); they commented on how unique the variant frequencies are among the Navajo people compared to other ethnic populations. Balsamo et al. (1964) described methemoglobinemia, a distinct blood disorder related to an overproduction of methemoglobin in erythrocytes, in three related Navajo individuals. Crist et al. (1985) identified a null (silent) allele in the GPT (glutamate pyruvate transaminase) gene in three members of a Navajo family. In summary, studies of blood and HLA typing in Navajo people were used to make inferences about population similarity, disease associations, and distinction from other Indigenous populations.
Complex Diseases
Seven articles were related to complex or multifactorial diseases that are influenced by the environment and lifestyles. A total of 69 Navajo people were included in the complex disease studies spanning from 1994 to 2018.
Three articles (Erickson et al. 2008; Knowles et al. 2014; Schlegel et al. 2018) discussed microvillus inclusion disease (MVID), which causes severe diarrhea in infants (Pohl et al. 1999) and has been associated with the gene encoding MYO5B (myosin Vb; Erickson et al. 2008). Combined, the three studies collected blood from 22 Navajo individuals to examine the genetic variants associated with MVID. The researchers’ findings provide examples that MVID affects Navajo people at an early age, but the incidence rate remained unknown (Erickson et al. 2008; see also Pohl et al., 1999).
Three studies reported on specific complex diseases: Paget (“bone”) disease (n = 2; Whyte et al. 2002), epilepsy (n = 2; Appavu et al. 2016), and the risk of alcoholic liver disease (n = 15; Raucy et al. 1999). These studies found unique genetic variations in Navajo patients, in particular a homozygous deletion in the TNFRSF11B gene associated with bone abnormalities (Whyte et al. 2002; see also Kraft 2018) and a unique genetic variant c.121C>T in the TBC1D24 gene associated with developmental delays and seizures (Appavu et al. 2016). In contrast, polymorphisms related to CYP2E1 activity and alcoholic liver disease were uninformative and not linked to a predisposition of an alcoholic liver (Raucy et al. 1999).
Lastly, Feeney et al. (1996) estimated that Navajo, Apache, and Eskimo children have a greater (5- to 10-fold) incidence of contracting Haemophilus influenzae type B (Hib) compared to the Caucasian population. The gene most likely associated with anti-Hib response is Vk II. In particular, the A2 variant in the gene can trigger a detrimental anti-Hib response, resulting in a unique A2b variant in the Vk II gene that showed in 15 of 28 Navajo individuals tested (43% [n = 12] were heterozygous and 11% [n = 3] homozygous for the A2b variant), suggesting the variant may lead to infection with Hib.
Forensics
We identified four studies involving forensic genetics (Budowle and Moretti 1998; Gallo et al. 1997; Scholl et al. 1996; Rohlfs et al. 2012). Forensics is the application of science to examine biological evidence from a crime scene to assist the criminal justice system and the law.
In the 1990s, the Federal Bureau of Investigation’s Combined Index System (CODIS) sponsored a project to create and validate DNA profiles (Budowle and Moretti 1998; Federal Bureau of Investigation n.d.). Over 21 laboratories across the United States and Canada contributed samples to one study of 50 populations, including 187 Navajo people (Budowle and Moretti 1998). This database was made publicly available and was used by Gallo et al. (1997) to look at the effects of population structure on probability calculations (the likelihood of identifying a person based on DNA profile) in forensic analyses and by Scholl et al. (1996) to verify that the databases were sufficient to identify Native American (including Navajo) individuals using the DNA profiles. The probability calculations (or chance of a random match) suggested that researchers were able to identify Navajo individuals based on their DNA profile.
While these studies were used to validate the CODIS system, the data are also accessible to the public. Rohlfs et al. (2012) used this database to study familial identification across populations, including Navajo individuals. This article concluded that, when searching for a profile match in a database that lacks an appropriate reference database (inclusive of diverse populations), relatives and unrelated individuals are more difficult to distinguish. Rohlfs et al. (2012) concluded that Navajo individuals look more similar to each other at some of the CODIS loci than to other populations, and this has the potential to falsely lead police to investigate more Navajo family members compared to other populations.
Hereditary Diseases
Human hereditary diseases can be linked to single or multiple genetic variants that are inherited from parents or arise spontaneously. A total of 25 articles related to hereditary disease were included in our results. Twelve were related to the immune system, which includes severe combined immunodeficiency disease (SCID) (Jones et al. 1991; Kwan et al. 2014, 2015; Li et al. 1998, 2002a, 2002b; O’Marcaigh et al. 1997) and mitochondrial DNA depletion syndrome (MDS; also known as neurohepatopathy or neuropathy) (Ortiz et al. 2002; Spinazzola et al. 2008; Vu et al. 2001; El-Hattab et al. 2010; Karadimas et al. 2006). Four articles reported studies investigating nonpolyposis colorectal cancer, also known as Lynch syndrome (Lynch et al. 1985, 1992, 1994, 1996). Three articles were related to skin disorders, such as poikiloderma (Chantorn and Shwayder 2012; Clericuzio et al. 2011; Wang et al. 2003). Further, four articles were related to the eye, describing such diseases as retinitis, retinoblastoma, and oculocutaneous albinism (Berkow and Fleshman 1983; Heckenlively et al. 1981; Woolf 1965; Yi et al. 2003). Finally, one study characterized metachromatic leukodystrophy disease (Pastor-Soler et al. 1994) and another described cystic fibrosis in one Navajo patient (Grebe et al. 1992).
We highlight seven articles about SCID that involved Navajo patients spanning 1991–2015 (Jones et al. 1991; Kwan et al. 2014, 2015; Li et al. 1998, 2002a, 2002b; O’Marcaigh et al. 1997). SCID is a rare genetic disease that affects the development of T-cells, which are necessary for combating infections. Newborns are especially vulnerable and are highly susceptible to severe infections that can lead to death (Kwan et al. 2014). SCID cases were first phenotypically characterized in 1980 when four cases were reported in “Athabaskan Indians” (Murphy et al. 1980). A clinical chart review from 1969 to 1982 by Jones et al. (1991) estimated a gene frequency of 2.1% from detailed family genealogies; they estimated that the highest rate of death due to SCIDs occurred in 1976, affecting an estimated 10% of all infants under 24 months of age. For the first time, O’Marcaigh et al. (1997) located a genetic variant in the IL2RG gene encoding the interleukin-2 receptor γ chain, linked to a X-linked maternal inheritance pattern of SCIDs absent of T- and B-cells in two Navajo patients. An additional 15 cases from “Athabaskan”-speaking parents (1 in 2,000 live births) were determined to have “SCID-A” (A for Athabaskan) localized to chromosome 10p (Li et al. 1998). A nonsense variant was found in the Artemis protein encoded by the DCLRE1C gene in six at-risk Navajo people (Li et al. 2002b). Later that year, the same research group published on another type of SCID in the Navajo people (n = 18) that exhibited an absence of T- and B-cells but normal natural killer cells (Li et al. 2002a). Li et al. (2002a) studied the Artemis gene and found it was a nuclear protein and that many genetic variants in the exons of the gene were associated with SCID. This same research team developed and tested the effectiveness of a newborn screening test to detect SCID by analyzing gene by-products in newborns across the Navajo reservation (Kwan et al. 2014, 2015), which has since become a part of routine newborn screening for early diagnosis in infants across the United States. While these newborn screening tests occurred after the moratorium was established, the investigators in all these studies delicately worked to ensure they were not in violation.
Five hereditary disease studies focused on MDS from 2001 to 2010 (Ortiz et al. 2002; Spinazzola et al. 2008; Vu et al. 2001; El-Hattab et al. 2010; Karadimas et al. 2006). Patients with MDS have a deficiency in mitochondria production, which can lead to critical organ failure (e.g., in the liver, brain, or kidneys). The first study on MDS (Vu et al. 2001) examined liver biopsies of two Navajo patients, suggesting a defect in a nuclear-DNA-encoded protein associated with replication of mitochondria. Shortly after, another Navajo patient with liver damage was diagnosed with MDS and Ortiz et al. (2002) proposed that the MDR3 protein (encoded by the MDR3 gene) may be associated with a nontranscriptional variant. In a study of eight previously studied Navajo samples, Karadimas et al. (2006) found that the genetic variant R50Q (c.149G>A) in the MVP17 gene was associated with the MDS phenotype (see also Singleton et al. 1990). Interestingly, MDS was labeled “Navajo neurohepatopathy,” implying that this disease is specific to Navajo people (Karadimas et al. 2006; Ortiz et al. 2002; Vu et al. 2001). However, MDS was later found in Italian populations (Spinazzola et al. 2008). Spinazzola et al. (2008) analyzed the MPV17 and CAD genes in Navajo and Italian patients and concluded that the mutation c.149G>A does not appear to have shared origins between the Italian and Navajo populations. A final study (El-Hattab et al. 2010) detected the same c.149G>A variant in seven Navajo patients and compared those findings to results from other ethnic groups, which revealed many genetic variants clustered in the region of protein kinase C phosphorylation located on the MPV17 protein, providing further evidence the MPV17 protein is associated with MDS.
Four studies examined Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) in Navajo people (Lynch et al. 1985, 1992, 1994, 1996). In the 1980s, physicians on the Navajo Nation began to notice a pattern of multiple colorectal cancers in a single family. A pedigree of over 100 extended family members displayed an autosomal dominant inheritance pattern with a high frequency of HNPCC in affected individuals, despite colorectal cancers being rare in the Navajo population (Lynch et al. 1985). In the late 1990s, the family was revisited, and three additional family members presented with HNPCC, leading researchers to investigate the possibility of a genetic link (Lynch et al. 1992). Genetic analysis of two affected family members identified a 4-base-pair deletion in the MLH1 gene, which may be associated with HNPCC (Lynch et al. 1994). Following the detection of the variant in MLH1, 51 Navajo patient DNA samples were sequenced, confirming that each affected member had the associated genetic variant (Lynch et al. 1996). Genetic counseling was provided but was challenging due to the differences in cultural views about genetics and medicine between counselors and Navajo patients (Lynch et al. 1996).
Three articles published from 2002 to 2012 reported studies investigating poikiloderma (a skin disease manifesting with hyperpigmentation, rashes, nail abnormalities, recurrent infections, and growth deficiency) that affected seven Navajo people, mostly children (Chantorn and Shwayder 2012; Clericuzio et al. 2011; Wang et al. 2003). These articles suggested a possible association with genetic variants in the RECQL4 and C16orf57 genes.
Four articles described studies related to eye disorders reported in the Navajo population (Heckenlively et al. 1981; Berkow and Fleshman 1983; Woolf 1965; Yi et al. 2003). Heckenlively et al. (1981) identified 42 Navajo individuals with the autosomal recessive and 33 with autosomal dominant forms of retinitis pigmentosa. Retinoblastoma, a malignant cancer of the eye, was reported with a 90% penetrance in affected families with the autosomal dominant form (Berkow and Fleshman 1983). Albinism, which affects pigmentation and visual acuity, was detected in the Navajo population with a prevalence of 1 in 3,750 (Woolf 1965). This was later confirmed as oculocutaneous albinism type 2 in a study of 142 Navajo individuals to determine the carrier frequency based on allele frequencies of the OCA2 gene. The disease-associated variant was a 1.2-kb deletion in the P gene, which resulted in a different prevalence of 1 in 1,500–2,000 (Yi et al. 2003).
One study examined metachromatic leukodystrophy, an autosomal recessive disorder that phenotypically presents with a deficiency in the lysosomal enzyme arylsulfatase (Pastor-Soler et al. 1994). Six Navajo people with metachromatic leukodystrophy from five different families were found to have a single splice variant in the ARSA gene, but further investigation was needed to find a direct pathophysiological link. Finally, one Navajo patient was diagnosed with a spontaneous form of cystic fibrosis associated with an AA haplotype, and no other cases had been reported before 1992 (Grebe et al. 1992).
Population Genetics and Migration
One of the fundamental goals of population genetics is to reconstruct the history of modern humans and determine the migration routes and dispersal of global human populations. Researchers study genetic variation within populations to trace changes in gene variant frequency over time and space, to infer migration patterns. We report 19 articles involving Navajo individuals, reporting on mating patterns (4 studies from 1953 to 1998) and population migration (15 studies from 1953 to 2008).
The studies that relate to mating patterns and inbreeding in the Navajo derive from the extremely detailed genealogical and demographic records collected by anthropologist C. Kluckhohn and colleagues. Data were collected from Navajo people residing in Ramah, New Mexico (Spuhler and Kluckhohn 1953), a noncontiguous Navajo community southeast of the Navajo Nation. The detailed genealogy dates back to 1870, when the community was founded by 31 individuals (see Hornsby and Mcpherson 2009), and continues to 1948, when the community consisted of about 614 individuals. The records span seven generations over 78 years and include 1,105 individuals (Spuhler and Kluckhohn 1953). These detailed pedigrees allowed researchers to study the relatedness of individuals, including measurements of the degree of inbreeding, and to make inferences about small, isolated human populations (Allen 1965; Morgan and Spuhler 1965; Spuhler 1989; Spuhler and Kluckhohn 1953). These data were supplemented with an additional collection of blood samples from 34 Navajo individuals between 1991 and 1993 for sequencing of 13 dinucleotide repeat loci on chromosomes 9, 10, 11, and 20 (Long et al. 1998). It is not known what type of consent was obtained, if any, although the sensitive nature of the topics should have required community input. These studies concluded that this Navajo community was not highly inbred, based on calculations using a coefficient of inbreeding (the probability of inheriting two copies of the same allele from an ancestor) and Wright’s F-statistic.
The studies of population migration and population substructure involved specific genetic loci located on mitochondria (hypervariable regions 1 and 2), the Y chromosome, and other repeats present in the genome. Navajo individuals were included in eight studies of mitochondrial DNA (mtDNA) (Brown et al. 1998; Budowle et al. 2002; Malhi 2001; Malhi et al. 2002, 2003; Smith et al. 2000; Torroni et al. 1992, 1993), three studies of the Y chromosome (Budowle et al. 2005; Malhi et al. 2008; Zegura et al. 2004), and four studies of other loci, such as variable number tandem repeats, human immunoglobulin GM allotypes, cholinesterase, and D1S80 loci (Balazs 1993; Duncan et al. 1996; Garry 1977; Williams et al. 1985). Some of these studies collected samples from Navajo individuals (Brown et al. 1998; Budowle et al. 2002, 2005; Duncan et al. 1996; Garry 1977; Smith et al. 2000; Torroni et al. 1992; Zegura et al. 2004), and others relied on publicly available secondary data or samples (Budowle et al. 2002; Malhi 2001; Malhi et al. 2002, 2003, 2008; Torroni et al. 1993). Some studies may have used modern methods to analyze “orphaned” samples: blood or serum samples collected decades ago and stored in freezers for subsequent studies. These studies made inferences from the data about the migration of Navajo people into the Southwest and how they related to other tribes and populations. Four studies recognized tribal groups in the acknowledgment section with such statements such as, “We are indebted to numerous personnel of Indian Health Service Facilities, where most of the samples studied were obtained, as well as to individuals who provided samples used in this analysis and to the Native Americans who authorized their use” (Malhi et al. 2002).
Discussion
Genetic research involving Navajo people has been published over the past 93 years. As genomic technology and methods rapidly advance, there is a need for more robust ethical research practices that address tangible benefits. Important aspects of conducting ethical genetic research include acknowledging Indigenous traditional views about DNA, as well as learning about the culture, history, and language. Allowing for other worldviews and engaging the community can help promote unbiased research. With this in mind, we discuss the articles in our review and incorporate Navajo traditional knowledge informed by cultural experts and our own cultural teachings.
Diné Heredity and Genetic Knowledge
Navajo people possess a fundamental sense of place, which is rooted in hajíínéí (their origin story) that establishes a strong relationship with Diné Bikéyah (lands of the Navajo people) and their hooghan (traditional homes) within the four sacred mountains that protect the people. In the Navajo worldview, humans are not separate from the land, animals, water, and air that make up our world. We coexist in a state of hózhǬ (beauty and harmony) to maintain balance emotionally, physically, mentally, and spiritually. Individuals who are not in balance increase their risk of acquiring diseases or sickness. Therefore, it is important to consider holistically the entire human body and environment to understand Navajo relationality and its role in health (Kahn-John 2010; Kahn-John and Koithan 2015).
Navajo people monitor familial relationships through k’é, a complex genealogical system that takes into account one’s clanship, family roles, relationships with different families, and geographic region. A Navajo person has four clans that are inherited from the mother, father’s mother, paternal grandfather’s mother, and maternal grandfather’s mother, respectively. The Navajo clan system guides whom one can have relations with (e.g., marriage, partnership, sexual relations) and is in essence Navajo peoples’ form of genetic inheritance knowledge. This knowledge has been passed down for generations, yet many of the genetic studies involving mtDNA and Y-chromosome patterns of inheritance did not consider the clan system.
The environment, diet, and lifestyles of Navajo people have changed dramatically in the last 200 years of colonization and modernization. First, typical lifestyles have shifted in just a few generations from a nomadic herding and gathering way of life to sedentary lifestyles and westernized diets. Second, Hwéeldi (“Long Walk”; in Navajo called Land of Suffering) was a forced relocation march of Navajo people over 350 miles from Navajo lands to Bosque Redondo, New Mexico, with subsequent detainment from 1864 to 1868 (Johnson 1973). Although written and oral history accounts suggest that many Navajo people fled capture, the population was greatly reduced to 8,000 to 10,000 people (Kluckhohn and Leighton 1962; Williams 1992), resulting in a population bottleneck (Erickson 1999, 2009; Yi et al. 2003). When Navajo people were allowed to return to their homelands, the population size eventually increased to over 350,000 enrolled members today. It is possible that certain genetic variants may have increased in frequency since Hweeldi, leading to a founder effect or genetic drift, a natural process (Kunitz 1983), in certain communities and an increase in rare conditions and diseases compared to the general population (Li et al. 1998; Lynch et al. 1992; Yi et al. 2003). Third, the US government employed hundreds of Navajo miners to extract uranium from mines constructed on the Navajo Nation to develop nuclear weapons. Many mines remain exposed, which has led to widespread contamination and disease (Brugge et al. 2007; Pasternak 2010). Finally, infectious diseases have significantly impacted Navajo people, leading to widespread death or altered population demographics. These and other elements of Navajo knowledge and oral histories should be considered in understanding historical interpretations of genetic research studies.
Impact of Genetic Research on Navajo People
Some studies have positively impacted the health of Navajo people. One helpful program emerging from the SCID studies was the implementation of a successful newborn screening program (Kwan et al. 2014, 2015; Li et al. 2002b) that has since been adopted by most states across the United States. Another positive program is the development of vaccines based on studies of viruses and bacteria (e.g., H. influenzae, S. pneumoniae) that are now being used worldwide (Lipsitch et al. 2007; Millar et al. 2005). Of note, some of these articles included Navajo people as coauthors.
Conversely, some publications on population and migration genetic studies raised concerns about the descriptions used and conclusions made about Navajo people. Some articles describe Navajo individuals or families as “doubtful full-bloods” (Nigg 1926) or “pure stock,” in reference to admixture (Lynch et al. 1985), and others describe the ancestors of Navajo people as having a “comparatively meager material and social culture” compared to Puebloan peoples (Malhi et al. 2008). Had input from the Navajo community been sought, these types of biased language could have been avoided. Studies that measured degrees of inbreeding (Allen 1965; Long et al. 1998; Morgan and Spuhler 1965; Spuhler 1989; Spuhler and Kluckhohn 1953) also raise concerns about exacerbating stereotypes that are viewed as untrue, especially since the Navajo clan system forbids intermarriages. Further, with a legacy of exclusion and displacement from their original lands by colonizers, concerns about using genetics to determine ancestral origins (Eveleth 2015) can potentially challenge a tribe’s claims to their lands. Finally, the names associated with some of these diseases are stigmatizing, such as “Navajo neuropathy” or “severe combined immunodeficiency, Athabascan type.” Since the publication of these articles, both diseases have been found in other populations, including non-Indigenous ones, so researchers should be cognizant to not label these diseases as Navajo specific or with community identifiers.
A striking assumption made in some blood and HLA typing articles was that DNA from Navajo people represented other North American Indigenous groups. However, tribes in the United States have distinct languages, cultures, histories, and genetic makeup (National Congress of American Indians 2020). In addition, the publication of the detailed pedigrees of Navajo families from Ramah, New Mexico, and in the Lynch syndrome articles could affect the privacy of individuals living in these small communities. Studies about alcoholism and migration should be approached delicately, as these topics have numerous social, ethical, and political implications for tribal communities. For example, studying alcoholism in tribal communities has the potential to perpetuate stereotypes and negatively impact the health and well-being of Navajo people. Further, in many population genetic and migration studies researchers used only a specific region of the genome and a certain time point to infer their results; we know that group movements and interactions were dynamic in the past and still continue today. These issues have numerous cultural, social, ethical, and political implications for tribal communities.
There are also significant ethical issues with both small sample sizes and continuing to use data that may not have been ethically collected and without the permission of the community and individual. The sample sizes reported in the articles reviewed here ranged from one to hundreds of Navajo individuals, which raises questions about whether the studies were statistically meaningful for clinical treatment or if they were underpowered and not statistically significant. Such underpowered studies may offer little to no benefit, thus exacerbating concerns about potential secondary uses of samples and data. The practice of sharing samples and data among collaborators, also referred to as “freezer diving,” may continue and presents ethical concerns (Kowal 2013; Merriwether 1999).
Reach of the Moratorium on Genetic Research
Our results show a general increasing trend in genetic studies in the late 1980s, peaking in 2002 and then decreasing from 2000 to 2005 (Figure 2). After 2000, the studies decline dramatically in number and mostly comprise genetic studies relating to bacteria or virus genetics, complex diseases, and hereditary disease research. This suggests that the moratorium deterred researchers from conducting genetic studies but not completely.
Studies that are conducted outside of the Navajo Nation raise questions about the jurisdictional reach of the Navajo Nation. The Navajo Nation Human Research Code (13 N.N.C. 3253 C.F.R. 1994) states that “research information and data generated by and about Navajo individuals, communities, culture represent inalienable intellectual properties of Navajo people and over which the Navajo Nation will provide oversight.” However, publicly available data and samples may effectively sidestep the Navajo Nation’s oversight. For example, the use of CODIS to identify criminal suspects raises concerns about privacy, security, and the biases made when employing these methods across historically underrepresented minority populations. In addition, Navajo patients with rare diseases requiring specialized treatment are often referred off the reservation for treatment, where they may be enrolled in genetic research studies. Today, nearly 36% of tribal members live off the Navajo Nation in border towns or metropolitan areas (Navajo Division of Health and Navajo Epidemiology Center 2013), which can further complicate the role of tribal research oversight. These are very real concerns that tribes are facing in light of large-scale cohort studies.
With rapidly changing technology and potential genomic research harms, it was the sovereign decision of the Navajo Nation to establish the moratorium to protect their tribal citizens and emergent data. The decision to establish the moratorium was heavily influenced by Navajo traditional healers, who uphold that the human being is sacred, including DNA; therefore, DNA should not be utilized in a harmful way or manipulated with no beneficial outcome. Humans are sacred beings created by Diyín Diné’e (the Holy Ones), and it is important for researchers to acknowledge this Navajo worldview. With improved policies and attention to bioethical standards, an increase in the application of community-based participatory research approaches, and emerging Navajo scientists, there could be opportunities for Navajo people to benefit from genetic research.
Limitations
We acknowledge that there are limitations to our review. We could assess only articles with a full-text copy available. We evaluated only those articles that specifically included Navajo individuals according to our search terms; other articles may have used broader definitions such as “American Indians” or “Native Americans” and would have been excluded based on our criteria. Our summary of the total number of Navajo individuals involved in the research articles is a conservative estimate and we could only infer where the samples were collected from the available text, because some publications were unclear. Table 1 includes the number of individuals or samples that were collected in the first publication of these data and excludes secondarily collected data in the count. Many of the older articles may also have excluded mentioning IRB approval, which may be consistent with manuscript publication guidelines or scientific reporting trends of that time. In fact, ethical standards have evolved and changed significantly, and the norms of the past would not be acceptable for researchers today.
Conclusions
In interpreting research results involving Indigenous people, multiple worldviews should be considered to avoid bias and limited viewpoints. We encourage researchers to take into consideration cultural perspectives and traditional knowledge that will result in not only better informed science but also more ethical and respectful science. We hope that more studies will acknowledge and respect Indigenous knowledge by involving more Indigenous researchers to coinvestigate or be included in other aspects of the research process. Many Indigenous researchers are currently being trained in genetic and genomic research methods, many of whom are Navajo (Wade 2018; Yates 2019). With stronger collaborations, it will be possible to bridge the gap between Indigenous and Western science.
Supplementary Material
ACKNOWLEDGMENTS
T’éé íiyisíí ahé’hee’, nitsaago. We are extremely thankful for the large database of literature provided and manually curated by Dr. Bonnie Duran. We thank Bret Colby for his contributions to the literature search. We thank Drs. Jani Ingram, Gilbert John, and William Freeman, as well as the reviewers and editor, for providing edits and feedback on the manuscript. We also thank the Navajo Nation Human Research Review Board, the Navajo Nation Genetics Policy Development Working Group, and Diné cultural leaders for their insights and feedback. Finally, we thank our elders and ancestors for passing down important cultural knowledge that informs this work. This work was partially supported by National Institutes of Health grants NHGRI K01 HG008818, RM1 HG009042-03S2, RM1 HG009042-04S1, and F32 GM119237.
LITERATURE CITED
- Agostini HT, Yanagihara R, Davis V et al. 1997. Asian genotypes of JC virus in Native Americans and in a Pacific Island population: Markers of viral evolution and human migration. Proc. Natl. Acad. Sci. U. S. A 94:14,542–14,546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G 1965. Random and nonrandom inbreeding. Eugen. Q 12:181–198. [DOI] [PubMed] [Google Scholar]
- American Journal of Medical Genetics. 2010. After Havasupai litigation, Native Americans wary of genetic research. Am. J. Med. Genet. A 152A:ix. [DOI] [PubMed] [Google Scholar]
- Appavu B, Guido-Estrada N, Lindstrom K et al. 2016. Electroclinical phenotypes and outcomes in TBCID24-related epilepsy. Epileptic Disord. 18:324–328. [DOI] [PubMed] [Google Scholar]
- Arbour L, and Cook D. 2006. DNA on loan: Issues to consider when carrying out genetic research with aboriginal families and communities. Community Genet. 9:153–160. [DOI] [PubMed] [Google Scholar]
- Azarian T, Grant LR, Arnold BJ et al. 2018. The impact of serotype-specific vaccination on phylodynamic parameters of Streptococcus pneumoniae and the pneumococcal pan-genome. PLoS Pathog. 14:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs I 1993. Population genetics of 14 ethnic groups using phenotypic data from VNTR loci. EXS 67:193–210. [DOI] [PubMed] [Google Scholar]
- Balsamo P, Hardy WR, and Scott EM. 1964. Hereditary methemoglobinemia due to diaphorase deficiency in Navajo Indians. J. Pediatr 65:928–931. [DOI] [PubMed] [Google Scholar]
- Beans JA, Saunkeah B, Woodbury RB et al. 2019. Community protections in American Indian and Alaska Native participatory research—A scoping review. Soc. Sci. (Basel) 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow RL, and Fleshman JK. 1983. Retinoblastoma in Navajo Indian children. Am. J. Dis. Child 137:137–138. [DOI] [PubMed] [Google Scholar]
- Biggar RJ, Taylor ME, Neel JV et al. 1996. Genetic variants of human T-lymphotrophic virus type II in American Indian groups. Virology 216:165–173. [DOI] [PubMed] [Google Scholar]
- Bousselot JM, Muenchrath D, Knapp AD et al. 2017. Emergence and seedling characteristics of maize native to the southwestern US. Am. J. Plant Sci 8:1,304–1,318. [Google Scholar]
- Boyd WC 1951. Rh and the races of man. Sci. Am 185:22–25. [Google Scholar]
- Boyd WC, and Boyd LG. 1949. The blood groups and types of the Ramah Navaho. Am. J. Phys. Anthropol 7:569–574. [DOI] [PubMed] [Google Scholar]
- Brown MD, Hosseini SH, Torroni A et al. 1998. mtDNA haplogroup X: An ancient link between Europe/Western Asia and North America? Am. J. Hum. Genet 63:1,852–1,861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NP 2002. A Gift of Life Education and Awareness about Genetic Research on the Navajo Nation. USA: Navajo Nation; Videocassette. [Google Scholar]
- Brugge D, Benally T, Yazzie-Lewis E, eds. 2007. The Navajo People and Uranium Mining. Albuquerque: University of New Mexico Press. [Google Scholar]
- Brugge D, and Missaghian M. 2006. Protecting the Navajo people through tribal regulation of research. Sci. Eng. Ethics 12:491–507. [DOI] [PubMed] [Google Scholar]
- Budowle B, Adamowicz M, Aranda XG et al. 2005. Twelve short tandem repeat loci Y chromosome haplotypes: Genetic analysis on populations residing in North America. Forensic Sci. Int 150:1–15. [DOI] [PubMed] [Google Scholar]
- Budowle B, Allard MW, Fisher CL et al. 2002. HVI and HVII mitochondrial DNA data in Apaches and Navajos. Int. J. Legal Med 116:212–215. [DOI] [PubMed] [Google Scholar]
- Budowle B, and Moretti TR. 1998. Examples of STR population databases for CODIS and for casework. Paper presented at the Ninth International Symposium on Human Identification, Orlando, FL, 8–10 October. [Google Scholar]
- Chantorn R, and Shwayder T. 2012. Poikiloderma with neutropenia: Report of three cases including one with calcinosis cutis. Pediatr. Dermatol 29:463–472. [DOI] [PubMed] [Google Scholar]
- Claw KG, Anderson MZ, Begay RL et al. 2018. A framework for enhancing ethical genomic research with indigenous communities. Nat. Commun 9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clericuzio C, Harutyunyan K, Jin W et al. 2011. Identification of a novel C16orf57 mutation in Athabaskan patients with poikiloderma with neutropenia. Am. J. Med. Genet. A 155A:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran PA, Rabin DL, and Allen FH Jr. 1962. Blood groups of 237 Navajo school children at Pinon Boarding School, Pinon, Arizona (1961). Am. J. Phys. AnthropoL 20:389–390. [DOI] [PubMed] [Google Scholar]
- Crist MC, Heckenlively JR, Field LL et al. 1985. Glutamate pyruvate transaminase null allele (GPT0) in the Navajo. Hum. Hered 35:59–61. [DOI] [PubMed] [Google Scholar]
- Drabiak-Syed K 2010. Lessons from Havasupai Tribe v. Arizona State University Board of Regents: Recognizing group, cultural, and dignitary harms as legitimate risks warranting integration into research practice. J. Health Biomed Law 6:175–225. [Google Scholar]
- Duncan G, Thomas E, Gallo JC et al. 1996. Human phylogenetic relationships according to the D1S80 locus. Genetica 98:277–287. [DOI] [PubMed] [Google Scholar]
- El-Hattab AW, Li F-Y, Schmitt E et al. 2010. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: New patients and novel mutations. Mol. Genet. Metab 99:300–308. [DOI] [PubMed] [Google Scholar]
- Erickson RP 1999. Southwestern Athabaskan (Navajo and Apache) genetic diseases. Genet. Med 1:151–157. [DOI] [PubMed] [Google Scholar]
- Erickson RP 2009. Autosomal recessive diseases among the Athabaskans of the southwestern United States: Recent advances and implications for the future. Am. J. Med. Genet. A 149A:2,602–2,611. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Larson-Thomé K, Valenzuela RK et al. 2008. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am. J. Med. Genet. A 146A:3,117–3,119. [DOI] [PubMed] [Google Scholar]
- Eveleth R 2015. Genetic testing and tribal identity. Atlantic, 26 January https://www.theatlantic.com/technology/archive/2015/01/the-cultural-limitations-of-genetic-testing/384740/.
- Federal Bureau of Investigation. n.d. Frequently Asked Questions on CODIS and NDIS. https://www.fbi.gov/services/laboratory/biometric-analysis/codis/codis-and-ndis-fact-sheet (accessed 2 September 2019).
- Feeney AJ, Atkinson MJ, Cowan MJ et al. 1996. A defective Vkappa A2 allele in Navajos which may play a role in increased susceptibility to Haemophilus influenzae type B disease. J. Clin. Invest 97:2,277–2,282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cobo M, Agostini HT, Britez G et al. 2002. Strains of JC virus in Amerind-speakers of North America (Salish) and South America (Guaraní), Na-Dene-speakers of New Mexico (Navajo), and modern Japanese suggest links through an ancestral Asian population. Am. J. Phys. AnthropoL 118:154–168. [DOI] [PubMed] [Google Scholar]
- Gallo JC, Thomas E, Novick GE et al. 1997. Effects of subpopulation structure on probability calculations of DNA profiles from forensic PCR analysis. Genetica 101:1–12. [DOI] [PubMed] [Google Scholar]
- Garber TL, McAdam SN, Butler LM et al. 1996. HLA-B alleles of the Navajo: No evidence for rapid evolution in the Nadene. Tissue Antigens 47:143–146. [DOI] [PubMed] [Google Scholar]
- Garrison NA, Hudson M, Ballantyne LL et al. 2019. Genomic research through an Indigenous lens: Understanding the expectations. Annu. Rev. Genomics Hum. Genet 20:495–517. [DOI] [PubMed] [Google Scholar]
- Garry PJ 1977. Atypical (Ea1) and fluoride-resistant (Ef1) cholinesterase genes: Absent in a Native American Indian population. Hum. Hered 27:433–436. [DOI] [PubMed] [Google Scholar]
- Grant L, Esona M, Gentsch J et al. 2011. Detection of G3P[3] and G3P[9] rotavirus strains in American Indian children with evidence of gene reassortment between human and animal rotaviruses. J. Med. Virol 83:1,288–1,299. [DOI] [PubMed] [Google Scholar]
- Grant LR, Watt JP, Weatherholtz RC et al. 2012. Efficacy of a pentavalent human-bovine reassortant rotavirus vaccine against rotavirus gastroenteritis among American Indian children. Pediatr. Infect. Dis. J 31:184–188. [DOI] [PubMed] [Google Scholar]
- Grebe TA, Doane WW, Richter SF et al. 1992. Mutation analysis of the cystic fibrosis transmembrane regulator gene in Native American populations of the Southwest. Am. J. Hum. Genet 51:736–740. [PMC free article] [PubMed] [Google Scholar]
- Heckenlively J, Friederich R, Farson C et al. 1981. Retinitis pigmentosa in the Navajo. Metab. Pediatr. Ophthalmol 5:201–206. [PubMed] [Google Scholar]
- Hjelle B, Zhu SW, Takahashi H et al. 1993. Endemic human T cell leukemia virus type II infection in southwestern US Indians involves two prototype variants of virus. J. Infect. Dis 168:737–740. [DOI] [PubMed] [Google Scholar]
- Hornsby S, and Mcpherson RS. 2009. “Enemies like a road covered with ice”: The Utah Navajos’ experience during the Long Walk period 1858–1868. Am. Indian Cult. Res. J 33:1–22. [Google Scholar]
- Johnson BH 1973. Navajo Stories of the Long Walk Period. Tsaile, AZ: Navajo Community College Press. [Google Scholar]
- Jones JF, Ritenbaugh CK, Spence MA et al. 1991. Severe combined immunodeficiency among the Navajo. I. Characterization of phenotypes, epidemiology, and population genetics. Hum. Biol 63:669–682. [PubMed] [Google Scholar]
- Kahn-John M 2010. Concept analysis of Diné Hózhó: A Diné wellness philosophy. ANS Adv. Nurs. Sci 33:113–125. [DOI] [PubMed] [Google Scholar]
- Kahn-John M, and Koithan M. 2015. Living in health, harmony, and beauty: The Diné (Navajo) Hózhó wellness philosophy. Glob. Adv. Health Med 4:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadimas CL, Vu TH, Holve SA et al. 2006. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am. J. Hum. Genet 79:544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluckhohn C, and Leighton D. 1962. The Navaho. New York: Doubleday Press. [Google Scholar]
- Knowles BC, Roland JT, Krishnan M et al. 2014. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J. Clin. Invest 124:2,947–2,962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal E 2013. Orphan DNA: Indigenous samples, ethical biovalue and postcolonial science. Soc. Stud. Sci 43:577–597. [Google Scholar]
- Kraft S 2018. What is Paget’s disease of bone? Medical News Today, 30 November https://www.medicalnewstoday.com/articles/177668. [Google Scholar]
- Kuberski TT, Morse HG, Rate RG et al. 1981. A hospital-based survey of radiological sacroiliitis and HLA-B27 and Cw2 in Navajo and Hopi Indians. Hum. Immunol 3:77–83. [DOI] [PubMed] [Google Scholar]
- Kunitz SJ 1983. Disease Change and the Role of Medicine: The Navajo Experience. Berkeley: University of California Press. [Google Scholar]
- Kwan A, Abraham RS, Currier R et al. 2014. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 312:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A, Hu D, Song M et al. 2015. Successful newborn screening for SCID in the Navajo Nation. Clin. Immunol 158:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Drayna D, Hu D et al. 1998. The gene for severe combined immunodeficiency disease in Athabascan-speaking Native Americans is located on chromosome 10p. Am. J. Hum. Genet 62:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Moshous D, Zhou Y et al. 2002a. A founder mutation in Artemis, an SNM1-like protein, causes SCID in Athabascan-speaking Native Americans. J. Immunol 168:6,323–6,329. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou Y, Wang J et al. 2002b. Prenatal diagnosis and carrier detection for Athabascan severe combined immunodeficiency disease. Prenat. Diagn 22:763–768. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, O’Neill K, Cordy D et al. 2007. Strain characteristics of Streptococcus pneumoniae carriage and invasive disease isolates during a cluster-randomized clinical trial of the 7-valent pneumococcal conjugate vaccine. J. Infect. Dis 196:1,221–1,227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Romero FC, Urbanek M et al. 1998. Mating patterns and gene dynamics of a population isolate of Native Americans. J. Mammal 79:681–691. [Google Scholar]
- Lynch HT, Drouhard T, Lanspa SJ et al. 1992. Lynch syndrome II in a Navajo family: A revisit. Am. Indian Cult. Res. J 16:65–76. [Google Scholar]
- Lynch HT, Drouhard T, Lanspa S et al. 1994. Mutation of an mutL homologue in a Navajo family with hereditary nonpolyposis colorectal cancer. J. Natl. Cancer Inst 86:1,417–1,419. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Drouhard TJ, Schuelke GS et al. 1985. Hereditary nonpolyposis colorectal cancer in a Navajo Indian family. Cancer Genet. Cytogenet 15:209–213. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Drouhard T, Vasen HF et al. 1996. Genetic counseling in a Navajo hereditary nonpolyposis colorectal cancer kindred. Cancer 77:30–35. [DOI] [PubMed] [Google Scholar]
- Malhi RS 2001. Investigating prehistoric population movements in North America with ancient and modern mtDNA. PhD diss., University of California, Davis. [Google Scholar]
- Malhi RS, Eshleman JA, Greenberg JA et al. 2002. The structure of diversity within New World mitochondrial DNA haplogroups: Implications for the prehistory of North America. Am. J. Hum. Genet 70:905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi RS, Gonzalez-Oliver A, Schroeder KB et al. 2008. Distribution of Y chromosomes among native North Americans: A study of Athapaskan population history. Am. J. Phys. Anthropol 137:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi RS, Mortensen HM, Eshleman JA et al. 2003. Native American mtDNA prehistory in the American Southwest. Am. J. Phys. Anthropol 120:108–124. [DOI] [PubMed] [Google Scholar]
- Merriwether DA 1999. Freezer anthropology: New uses for old blood. Philos. Trans. R. Soc. Lond. B Biol. Sci 354:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar EV, O’Brien KL, Watt JP et al. 2005. Epidemiology of invasive Haemophilus influenzae type A disease among Navajo and White Mountain Apache children 1988–2003. Clin. Infect. Dis 40:823–830. [DOI] [PubMed] [Google Scholar]
- Mills MC, and Rahal C. 2019. A scientometric review of genome-wide association studies. Commun. Biol 2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, and Spuhler JN. 1965. Inbreeding in small human populations. Eugen. Q 12:204–208. [DOI] [PubMed] [Google Scholar]
- Morse HG, Rate RG, Bonnell MD, et al. 1980. High frequency of HLA-B27 and Reiter’s syndrome in Navajo Indians. J. Rheumatol 7:900–902. [PubMed] [Google Scholar]
- Murphy S, Troup G, Hayward A et al. 1980. Gene enrichment in an American Indian population: An excess of severe combined immunodeficiency disease. Lancet 316:502–505. [DOI] [PubMed] [Google Scholar]
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research 1978. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Appendix vol. 2 Washington, DC: US Government Printing Office. [PubMed] [Google Scholar]
- National Congress of American Indians. 2012. Genetics Research and American Indian and Alaska Native Communities Washington, DC: National Congress of American Indians; http://www.ncai.org/policy-research-center/initiatives/projects/genetics-resource-center. [Google Scholar]
- National Congress of American Indians. 2020. Tribal Nations and the United States: An Introduction. Washington, DC: National Congress of American Indians. [Google Scholar]
- National Institutes of Health. 2015. What is precision medicine? In Help Me Understand Genetics. https://ghr.nlm.nih.gov/primer/precisionmedicine/definition.
- Navajo Division of Health and Navajo Epidemiology Center. 2013. Navajo Population Profile: 2010 U.S. Census. https://www.nec.navajo-nsn.gov/Portals/0/Reports/NN2010PopulationProfile.pdf.
- Nigg C 1926. A study of the blood groups among the American Indians. J. Immunol 11:319–322. [Google Scholar]
- NNHHSC (Navajo Nation Health and Human Services Committee). 2002. Resolution of the Health and Social Services Committee of the Navajo Nation Council HSS-CAP-20-02. Window Rock, AZ: Navajo Nation Council. [Google Scholar]
- NNHRRB (Navajo Nation Human Research Review Board). 2009. Navajo Nation Human Research Review Board. Window Rock, AZ: Navajo Nation and the Center for Native American Health. [Google Scholar]
- Office for Human Research Protections. 2018. 45 CFR 46: Protection of Human Subjects. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html.
- O’Marcaigh AS, Puck JM, Pepper AE et al. 1997. Maternal mosaicism for a novel interleukin-2 receptor gamma-chain mutation causing X-linked severe combined immunodeficiency in a Navajo kindred. J. Clin. Immunol 17:29–33. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Arias IM, McKellar L et al. 2002. Navajo neuropathy: Relation to MDR3 mRNA deficiency. Hepatology 35:1,548. [DOI] [PubMed] [Google Scholar]
- Parker WC, and Bearn AG. 1961. Haptoglobin and transferrin gene frequencies in a Navajo population: A new transferrin variant. Science 134:106–108. [DOI] [PubMed] [Google Scholar]
- Pasternak J 2010. Yellow Dirt: A Poisoned Land and the Betrayal of the Navajos. New York: Free Press. [Google Scholar]
- Pastor-Soler NM, Rafi MA, Hoffman JD et al. 1994. Metachromatic leukodystrophy in the Navajo Indian population: A splice site mutation in intron 4 of the arylsulfatase A gene. Hum. Mutat 4:199–207. [DOI] [PubMed] [Google Scholar]
- Pohl JF, Shub MD, Trevelline EE et al. 1999. A cluster of microvillous inclusion disease in the Navajo population. J. Pediatr 134:103–106. [DOI] [PubMed] [Google Scholar]
- Popejoy AB, and Fullerton SM. 2016. Genomics is failing on diversity. Nature 538:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucy JL, Schultz ED, Kearins MC et al. 1999. CYP2E1 expression in human lymphocytes from various ethnic populations. Alcohol Clin. Exp. Res 23:1,868–1,874. [PubMed] [Google Scholar]
- Rohlfs RV, Fullerton SM, and Weir BS. 2012. Familial identification: Population structure and relationship distinguishability. PLoS Genet. 8:e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel C, Weis VG, Knowles BC et al. 2018. Apical membrane alterations in nonintestinal organs in microvillus inclusion disease. Dig. Dis. Sci 63:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Wheeler J, Grimwood J et al. 2004. Quality assessment of the human genome sequence. Nature 429:365–368. [DOI] [PubMed] [Google Scholar]
- Scholl S, Budowle B, Radecki K et al. 1996. Navajo, Pueblo, and Sioux population data on the loci HLA-DQA1, LDLR, GYPA, HBGG, D7S8, Gc, and D1S80. J. Forensic Sci 41:47–51. [PubMed] [Google Scholar]
- Scott JR, Hanage WP, Lipsitch M et al. 2012a. Pneumococcal sequence type replacement among American Indian children: A comparison of pre- and routine-PCV7 eras. Vaccine 30:2,376–2,381. [DOI] [PubMed] [Google Scholar]
- Scott JR, Hinds J, Gould KA et al. 2012b. Nontypeable pneumococcal isolates among Navajo and White Mountain Apache communities: Are these really a cause of invasive disease? J. Infect. Dis 206:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R, Helgerson SD, Snyder RD et al. 1990. Neuropathy in Navajo children: Clinical and epidemiologic features. Neurology 40:363–367. [DOI] [PubMed] [Google Scholar]
- Smith DG, Lorenz J, Rolfs BK et al. 2000. Implications of the distribution of Albumin Naskapi and Albumin Mexico for New World prehistory. Am. J. Phys. Anthropol 111:557–572. [DOI] [PubMed] [Google Scholar]
- Spinazzola A, Massa V, Hirano M et al. 2008. Lack of founder effect for an identical mtDNA depletion syndrome (MDS)-associated MPV17 mutation shared by Navajos and Italians. Neuromuscul. Disord 18:315–318. [DOI] [PubMed] [Google Scholar]
- Sponenberg DP, and Taylor C. 2009. Navajo-Churro sheep and wool in the United States. Anim. Genet. Resour. Inf 45:99–105. [Google Scholar]
- Spuhler JN 1989. Raymond Pearl Memorial Lecture 1988: Evolution of mitochondrial DNA in human and other organisms. Am. J. Hum. Biol 1:509–528. [DOI] [PubMed] [Google Scholar]
- Spuhler JN, and Kluckhohn C. 1953. Inbreeding coefficients of the Ramah Navaho population. Hum. Biol 25:295–317. [PubMed] [Google Scholar]
- Switzer WM, Pieniazek D, Swanson P et al. 1995. Phylogenetic relationship and geographic distribution of multiple human T-cell lymphotropic virus type II subtypes. J. Virol 69:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Schurr TG, Cabell MF et al. 1993. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am. J. Hum. Genet 53:563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Schurr TG, Yang CC et al. 1992. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics 130:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troup GM, Schanfield MS, Singaraju CH et al. 1982. Study of HLA alloantigens of the Navajo Indians of North America: II. HLA-A, B, C, DR and other genetic markers. Tissue Antigens 20:339–351. [DOI] [PubMed] [Google Scholar]
- Vu TH, Tanji K, Holve SA et al. 2001. Navajo neurohepatopathy: A mitochondrial DNA depletion syndrome? Hepatology 34:116–120. [DOI] [PubMed] [Google Scholar]
- Wade L 2018. To overcome decades of mistrust, a workshop aims to train Indigenous researchers to be their own genome experts. Science, 27 September https://www.sciencemag.org/news/2018/09/overcome-decades-mistrust-workshop-aims-train-indigenous-researchers-be-their-own#.
- Wang LL, Gannavarapu A, Clericuzio CL et al. 2003. Absence of RECQL4 mutations in poikiloderma with neutropenia in Navajo and non-Navajo patients. Am. J. Med. Genet. A 118A:299–301. [DOI] [PubMed] [Google Scholar]
- Wetterstrand KA 2019. DNA sequencing costs: Data from the NHGRI Genome Sequencing Program (GSP). National Human Genome Research Institute; https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data. [Google Scholar]
- Whyte MP, Obrecht SE, Finnegan PM et al. 2002. Osteoprotegerin deficiency and juvenile Paget’s disease. N. Engl. J. Med 347:175–184. [DOI] [PubMed] [Google Scholar]
- Williams J 1992. Trails of Tears: American Indians Driven from Their Lands. Dallas, TX: Hendrick-Long. [Google Scholar]
- Williams RC, Morse HG, Bonnell MD et al. 1981. The HLA loci of the Hopi and Navajo. Am. J. Phys. Anthropol 56:291–296. [DOI] [PubMed] [Google Scholar]
- Williams RC, Steinberg AG, Gershowitz H et al. 1985. GM allotypes in Native Americans: Evidence for three distinct migrations across the Bering land bridge. Am. J. Phys. Anthropol 66:1–19. [DOI] [PubMed] [Google Scholar]
- Woolf CM 1965. Albinism among Indians in Arizona and New Mexico. Am. J. Hum. Genet 17:23–35. [PMC free article] [PubMed] [Google Scholar]
- Yates D 2019. Indigenous scholars confront the power, limitations of genomics. Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana–Champaign; https://phys.org/news/2019-08-indigenous-scholars-power-limitations-genomics.html. [Google Scholar]
- Yi Z, Garrison N, Cohen-Barak O et al. 2003. A 122.5-kilobase deletion of the P gene underlies the high prevalence of oculocutaneous albinism type 2 in the Navajo population. Am. J. Hum. Genet 72:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegura SL, Karafet TM, Zhivotovsky LA et al. 2004. High-resolution SNPs and microsatellite haplotypes point to a single, recent entry of Native American Y chromosomes into the Americas. Mol. Biol. Evol 21:164–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


