Abstract
Background:
Low myocardial cGMP-PKG activity has been associated with increased cardiomyocyte diastolic stiffness in Heart Failure with preserved Ejection Fraction (HFpEF). cGMP is mainly hydrolyzed by phosphodiesterases (PDE) 5a and 9a. Importantly, PDE9a expression has been reported to be upregulated in human HFpEF myocardium and chronic administration of a PDE9a inhibitor reverses pre-established cardiac hypertrophy and systolic dysfunction in mice subjected to Transverse Aortic Constriction (TAC). We hypothesized that inhibiting PDE9a activity ameliorates diastolic dysfunction.
Methods:
To examine the effect of chronic PDE9a inhibition, two diastolic dysfunction mouse models were studied: 1) TAC-DOCA (Deoxycorticosterone acetate) and 2) Leprdb/db. PDE9a inhibitor (5 and 8 mg/kg/day) was administered to the mice via subcutaneously implanted osmotic mini-pumps for 28 days. The effect of acute PDE9a inhibition was investigated in intact cardiomyocytes isolated from TAC-DOCA mice. Atrial natriuretic peptide together with PDE9a inhibitor were administered to the isolated intact cardiomyocytes through the cell perfusate.
Results:
For acute inhibition, no cellular stiffness reduction was found, whereas chronic PDE9a inhibition resulted in reduced LV chamber stiffness in TAC-DOCA, but not in Leprdb/db mice. Passive cardiomyocyte stiffness was reduced by chronic PDE9a inhibition, with no differences in myocardial fibrosis or cardiac morphometry. PDE9a inhibition increased the ventricular-arterial (VA) coupling ratio, reflecting impaired systolic function.
Conclusions:
Chronic PDE9a inhibition lowers LV chamber stiffness in TAC-DOCA mice. However, the usefulness of PDE9a inhibition to treat high diastolic stiffness may be limited as the required PDE9a inhibitor dose also impairs systolic function, observed as a decline in VA coordination, in this model.
Keywords: diastole, diastolic dysfunction, HFpEF, PDE9a, cardiomyocyte, stiffness
Heart Failure with preserved Ejection Fraction (HFpEF) is a clinical syndrome comprising heart failure symptoms with a pathophysiology dominated by impairment of the diastolic phase of the cardiac cycle1, 2. Diastolic dysfunction in HFpEF results from impaired LV relaxation and/or myocardial stiffening, and has been reported to be associated with low myocardial cGMP-PKG (cyclic Guanosine Monophosphate –Protein Kinase G) activity3–5. cGMP serves as an intracellular second-messenger that regulates multiple physiological processes. cGMP is synthesized by Nitric Oxide (NO) coupled soluble Guanylate Cyclase (sGC) in the cytoplasm and Natriuretic Peptide (NP) coupled particulate Guanylate Cyclase (pGC) at the cell membrane6, 7. NO-mediated cGMP is preferentially hydrolyzed by phosphodiesterase (PDE)5a, while NP-mediated cGMP is preferentially hydrolyzed by PDE9a8. Enhancing cGMP through inhibition of PDE5a has been reported to restore LV relaxation kinetics in a pressure overloaded mouse model9 and improve diastolic function in a hypertensive dog model.10 However, a clinical trial using PDE5a inhibition in HFpEF patients had a neutral outcome, possibly due to low myocardial cGMP levels, attributable to a reduction in NO bioavailability.8, 11–14
While the NO-sGC-cGMP pathway is inactivated in HFpEF15, the NP-pGC-cGMP pathway and PDE9a are upregulated8, 16. PDE9a is significantly upregulated in pathological heart conditions, importantly in human HFpEF8. Pde9a−/− mice subjected to pressure overload induced by TAC (transverse aortic constriction), exhibit less severe cardiac dysfunction, hypertrophy and myocardial fibrosis8. Chronic administration of a PDE9a inhibitor (PF-4449613) reversed pre-established cardiac hypertrophy and systolic dysfunction in wild-type mice subjected to TAC, in a NO independent manner.8 This crucial finding raises the important question of whether inhibiting PDE9a is beneficial in reducing diastolic dysfunction.
Here we investigated the effects of chronic and acute PDE9a inhibition on diastolic dysfunction. PDE9a was inhibited by PF-4449613, a selective cGMP-competitive PDE9A inhibitor17. To study chronic PDE9a inhibition, we utilized 2 mouse models of diastolic dysfunction: 1) TAC-DOCA (transverse aortic constriction (TAC) with deoxycorticosterone acetate (DOCA) supplementation) and 2) Leprdb/db, a metabolically induced diastolic dysfunction model. To study acute PDE9a inhibition, a newly developed cellular work loop force measurement system was used to measure systolic and diastolic performance of single intact cardiomyocytes under physiological conditions. We hypothesized that inhibiting PDE9a activity ameliorates diastolic dysfunction.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. To examine the effect of chronic PDE9a inhibition, PF-4449613 (Pfizer Inc) was administered to the mice via subcutaneously implanted osmotic mini-pumps (Alzet osmotic pump, model 2004) for 28 days. The control animals received vehicle at an equivalent volume. The mice were used for exercise testing, hemodynamic study, cardiomyocyte mechanics, etc. To examine the effect of acute PDE9a inhibition, Atrial natriuretic peptide (ANP, Sigma 8208) together with PF-4449613 were administered to the isolated intact cardiomyocytes through the cell perfusate. Intact cardiomyocyte diastolic stiffness and contractility before and after treatment were used for comparison. All experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health Using Animals in Intramural Research guidelines for animal use.
Statistical analysis was performed in Graphpad Prism (GraphPad Software, Inc). Data are shown as mean±SEM. Statistical significance was set at p<0.05. Normality of data was tested with the D’Agostino & Pearson and Shapiro-Wilk tests. Homogeneity of variance was tested with Brown-Forsythe and Bartlett’s test or F-test. For data that were normally distributed and had homogeneity of variance, the unpaired t test, the one-way ANOVA followed by the Tukey multiple comparison, and the two-way ANOVA followed by a Dunnett or a Tukey test were used as appropriate. For data that were not normally distributed, the Mann-Whitney U test, the Kruskal–Wallis followed by a Dunn test, and logarithmic transformation followed by two-way ANOVA and a Dunnett or a Tukey test were used. Two-way ANOVA with repeated measures with a Sidak test was used to analyze the effect of acute PDE9a inhibition before and after treatment within individual cell. Spearman’s rank correlation was used for correlation analysis. * p≤0.05 ** p≤0.01 ***p≤0.001 ****p≤0.0001. Number of animals used are shown in each figure legend and table. Detailed methods and statistics are available in supplemental materials.
Results
Chronic PDE9a inhibition in TAC-DOCA mice
To test the effect of chronic PDE9a inhibition, diastolic dysfunction was induced in adult male C57BL/6 mice at 3 months of age using a previously published TAC-DOCA procedure which promotes cardiac remodeling and left ventricular (LV) chamber stiffening through pressure overload and mineralocorticoid excess18–20. At 1 week after sham or TAC-DOCA surgery, animals were subjected to subcutaneous implantation with osmotic minipumps containing either vehicle or PDE9a inhibitor (PF-4449613) at a concentration of 1, 5, or 8 mg/kg/day. These mice are referred to as sham-veh, sham-inh8, TAC-DOCA-veh, TAC-DOCA-inh1, TAC-DOCA-inh5 and TAC-DOCA-inh8, respectively. After 4 weeks of chronic PDE9a inhibition (or 5 weeks after TAC-DOCA or sham surgery), the animals were studied. To validate successful drug delivery, the concentration of PF-4449613 in mouse plasma was measured. The mean plasma level of PF-4449613 was 15 nM, 305 nM and 391 nM in the C57BL/6 mice that were treated with PF-4449613 at concentrations of 1, 5 and 8 mg/kg/day respectively.
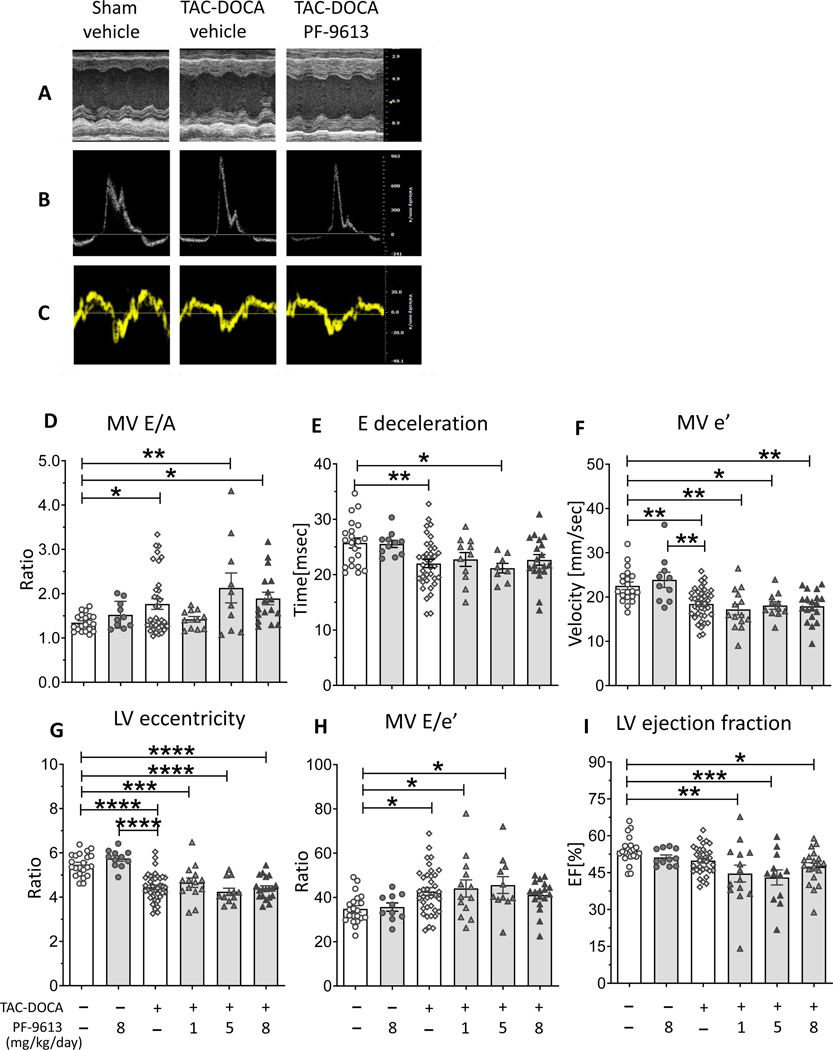
Echocardiography of TAC-DOCA mice.
Representative results of M-Mode, mitral inflow pattern and mitral annular velocity are shown in Figures 1A-C. TAC-DOCA-veh mice showed an increase in E/A ratio (Figure 1D) and shortening of E wave deceleration time (Figure 1E), suggesting a restrictive pattern of LV diastolic filling. The reduction of MV e’ indicates abnormal diastolic relaxation (Figure 1F), while the reduction of LV eccentricity indicates a concentric remodeling (Figure 1G). The E/e’, a reliable predictor of LV filling pressure, was increased suggesting elevated LV filling pressure (Figure 1H), whereas LV ejection fraction was preserved (Figure 1I). Together these parameters revealed diastolic dysfunction with preserved ejection fraction in TAC-DOCA-veh. However, the inhibition experiments showed no differences in diastolic parameters or concentric remodeling between TAC-DOCA-veh and TAC-DOCA-inh (Figures 1D-H), except that the ejection fraction was reduced in all 3 groups of TAC-DOCA-inh (Figure 1I).
Figure 1. Echocardiography of TAC-DOCA mice.
Representative figures of M-Mode (A), mitral inflow pattern(B) and mitral annular velocity(C). TAC-DOCA-veh mice exhibit a HFpEF-like condition observed as increased mitral E/A ratio(D), shortened E deceleration time(E), reduced mitral annular e’ velocity(F), LV concentric remodeling (G), increased MV E/e’ (H), but preserved ejection fraction (I). No difference in diastolic parameter is observed between TAC-DOCA-veh and TAC-DOCA-inh. However there is a significant reduction in LV ejection fraction in all 3 groups of TAC-DOCA-inh (I). (n= 22, 11, 40, 14, 12, 19 mice for sham-veh, sham PF-4449613, TAC-DOCA-veh and TAC-DOCA with PF-4449613 at 1mg,5mg and 8 mg/kg/day respectively) * p≤0.05 ** p≤0.01 ***p≤0.001 ****p≤0.0001. Two-way ANOVA, without repeated measures, with a Dunnett test for multiple comparisons of mean of each group with mean of the control group (sham-vehicle or TAC-DOCA-vehicle) was used.
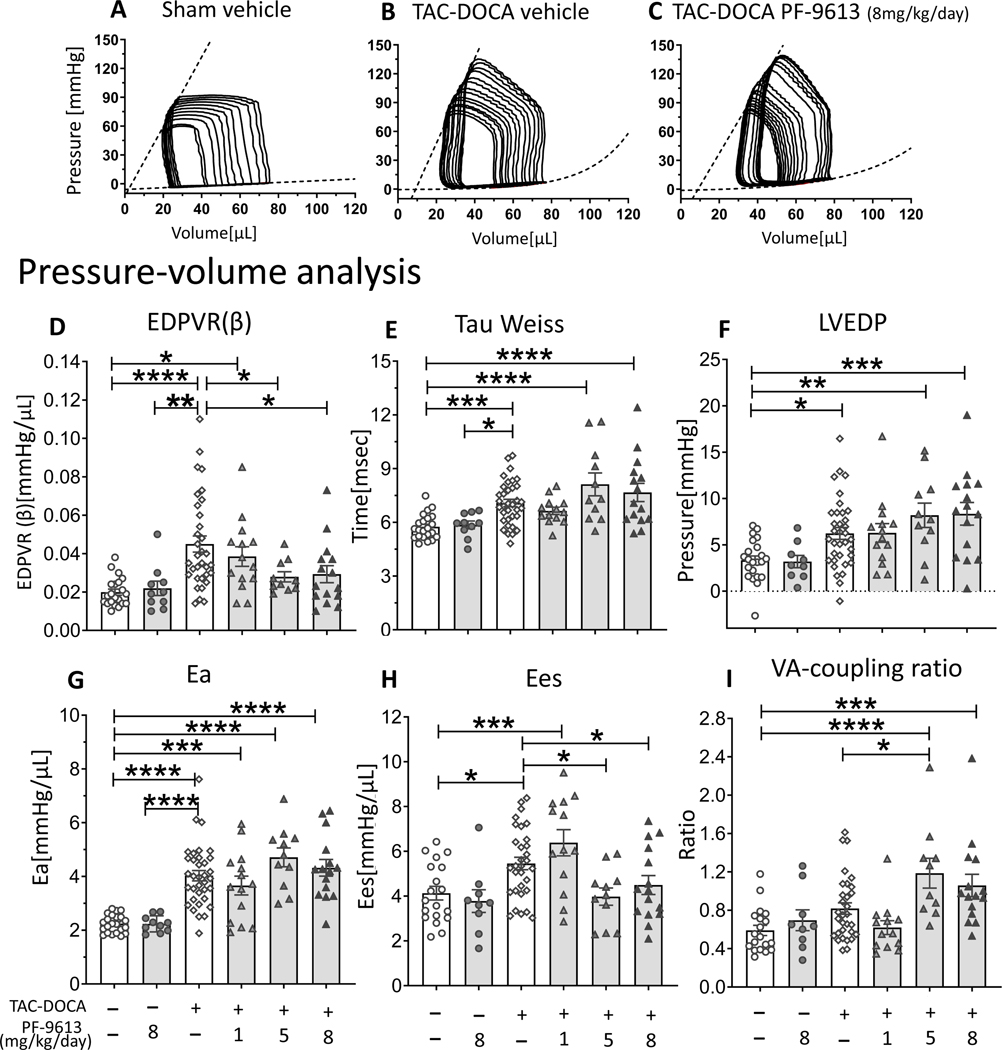
Pressure-volume analysis of TAC-DOCA mice.
Representative PV results of sham-veh, TAC-DOCA-veh and TAC-DOCA-inh8 are in Figures 2A-C. In the TAC-DOCA-veh, the diastolic stiffness coefficient (β) of EDPVR (end diastolic pressure volume relation) which reflects LV chamber diastolic stiffness (Figure 2D), the LV relaxation time constant (Tau) which reflects the time of isovolumetric LV relaxation (Figure 2E), and the LVEDP (LV end diastolic pressure) were increased (Figure 2F), indicating diastolic dysfunction. The Ea (Effective arterial elastance) was elevated as a result of TAC surgery (Figure 2G) and the Ees (End-systolic elastance) was increased (Figure 2H) as a compensation to maintain a normal cardiac output in the face of increased arterial load (increased Ea). Together these parameters further support the echocardiographic data indicating that TAC-DOCA-veh mice exhibited diastolic dysfunction with preserved ejection fraction.
Figure 2. Pressure-Volume analysis of TAC-DOCA mice.
Representative PV analysis of sham-veh (A), TAC-DOCA-veh (B) and TAC-DOCA-inh at PF-4449613 concentration of 8 mg/kg/day (C). TAC-DOCA-veh mice exhibit increased LV chamber stiffness (EDPVR (β)) (D), prolonged relaxation (Tau) (E), elevated LV diastolic filling pressure (LVEDP) (F), increased effective arterial elastance (Ea)(G) and increased LV contractility (Ees) (H). The EDPVR (β) of TAC-DOCA-inh1 is not different from TAC-DOCA-veh, but the EDPVR (β) of TAC-DOCA-inh5 and TAC-DOCA-inh8 are lower compared to TAC-DOCA-veh (D). However LVEDP of TAC-DOCA-inh5 and TAC-DOCA-inh8 remains elevated (F) and the VA coupling ratio increases, suggesting a decline in VA co-ordination (I). EDPVR indicates End diastolic pressure volume relation; Tau, relaxation time constant; Ees, End systolic elastance; LVEDP, left ventricular end diastolic pressure; VA co-ordination, ventricular-arterial coordination. (n= 22, 10, 33, 14, 11, 15 mice) * p≤0.05 ** p≤0.01 ***p≤0.001 ****p≤0.0001. Two-way ANOVA, without repeated measures, with a Dunnett test was used.
The TAC-DOCA mice that were treated with PDE9a inhibitor at concentration of 5 and 8 mg/kg/day had lower LV chamber stiffness (EDPVR(β)) compared to TAC-DOCA-veh (Figure 2D). However, relaxation (Tau) was not improved (Figure 2E) and the LVEDP remained elevated (Figure 2F), despite improvement in LV chamber stiffness (Figure 2D). The ventricular-arterial (VA) coordination, described by the coupling ratio (Ea/Ees), which indicates the coordination between ventricular contractility and arterial elastance, was maintained in TAC-DOCA-veh, but declined (increased VA coupling ratio) in TAC-DOCA-inh5 and inh8 (Figure 2I). The VA coordination revealed that although the Ees was preserved, the TAC-DOCA-inh5 and inh8 could barely maintain optimal pumping function, suggesting that the persistent LVEDP elevation despite a lower LV chamber stiffness in TAC-DOCA-inh5 and TAC-DOCA-inh8 was due to impairment of systolic function. Neither a reduction in LV chamber stiffness nor a decline in VA coordination was observed in TAC-DOCA-inh1 relative to TAC-DOCA-veh. Thus PDE9a inhibition at a sufficiently high dose to reduce LV chamber stiffness in TAC-DOCA hearts also causes a deterioration in VA-interaction, leading to systolic dysfunction. No changes in cardiac function were observed in sham mice after 4 weeks of PDE9a inhibition (at 8 mg/kg/day). Additional hemodynamic parameters are in Suppl Table S1.
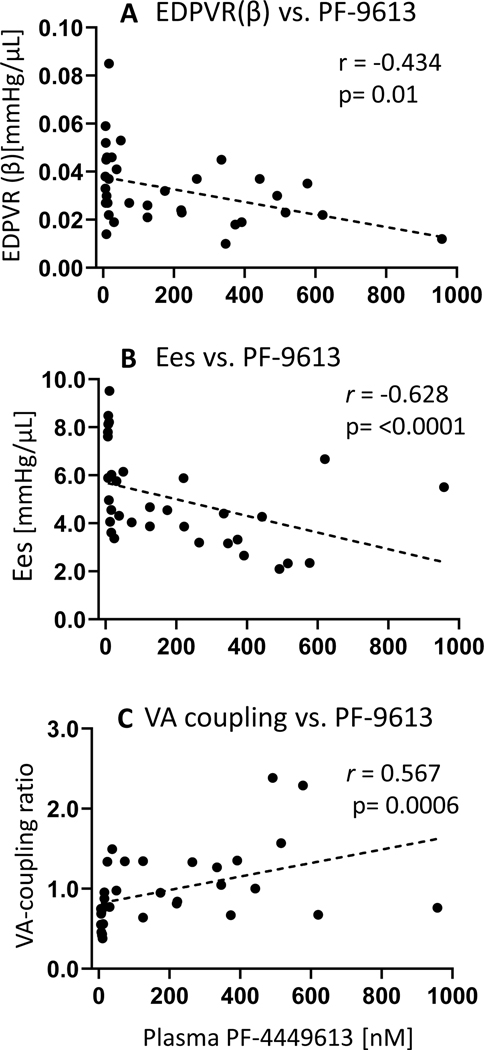
Correlation analysis revealed that the plasma level of PF-4449613 negatively correlated with LV chamber stiffness (EDPVR (β)) (r =− 0.434, p=0.01) (Figure 3A) and LV contractility (Ees) (r =−0.628, p<0.0001) (Figure 3B), but positively correlated with the VA coupling ratio (r = 0.567, p=0.0006) (Figure 3C). Thus a higher PF-4449613 level was associated with reduced LV chamber stiffness but a decline in contractility and VA-coordination.
Figure 3. Relationship between plasma concentration of PF-4449613 and PV analysis-derived assessment of LV function.
Significant correlations between LV chamber stiffness (EDPVR (β))(A), contractility (Ees) (B) and VA coupling ratio (C) vs. plasma PF-4449613 are demonstrated, such that a higher plasma PF-4449613 level is associated with reduced LV chamber stiffness, but attenuated contractile function and deterioration of VA coordination (n=34). Spearman’s rank correlation analysis was used.
Exercise performance of TAC-DOCA mice.
Exercise was studied by treadmill with indirect calorimetry. TAC-DOCA-veh and TAC-DOCA-inh1 exhibited a reduction in maxVO2 (maximal oxygen consumption), an important determinant of cardiovascular fitness (Suppl Figure S2A). This exercise intolerance was accordant with the upregulation of fetal genes (Nppa, Nppb and MyH7), the biomarkers of heart failure progression21 (Suppl Figures S3). There was no improvement in exercise performance or a down regulation of fetal gene markers in TAC-DOCA mice with PDE9a inhibitor treatment (Suppl Figures S2A-C, S3), suggesting no functional improvement in response to PDE9a inhibition.
LV hypertrophic remodeling of TAC-DOCA mice.
All TAC-DOCA mice (with and without PDE9a inhibition) developed LV hypertrophy (Suppl Figure S2D) with concentric remodeling (Figure 1G), and enlargement of left atrium (LA) (Suppl Figure S2E). No attenuations of LV or LA hypertrophic responses were observed after chronic PDE9a inhibition. For more detail, please refer to Suppl Table S1.
Cardiomyocyte passive stiffness of TAC-DOCA mice after chronic PDE9a inhibition.
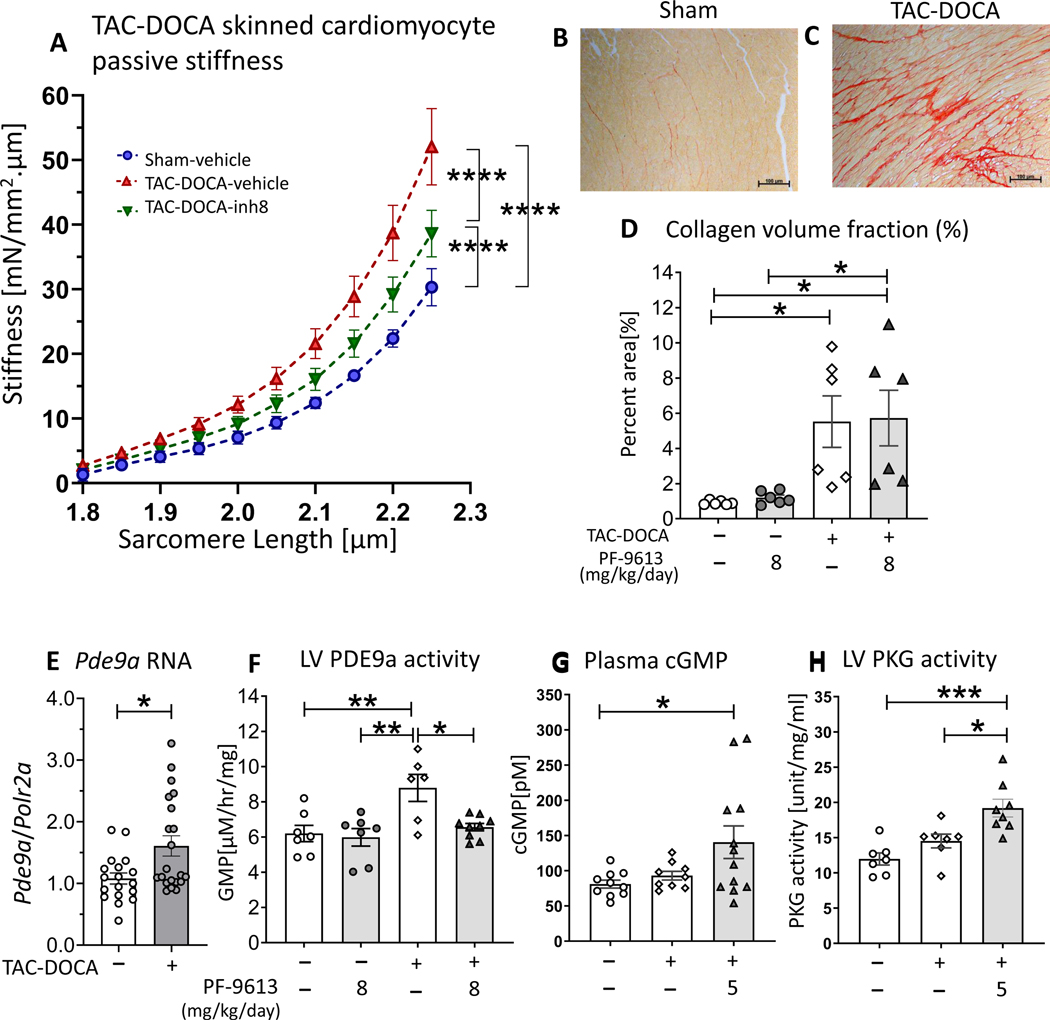
To address the mechanistic basis of LV chamber stiffness reduction, cardiomyocyte passive stiffness was studied in skinned (demembraned) LV cardiomyocytes of sham-veh, TAC-DOCA-veh and TAC-DOCA-inh8. The cardiomyocytes were stretched from baseline sarcomere length (SL ~1.85–1.90 μm) to a targeted SL of 2.3 μm. Passive stress (force normalized by cross sectional area) measured during the stretch was converted to stiffness (slope of stress-SL relationship) (Figure 4A). The result showed an increased cellular stiffness in both TAC-DOCA-veh and TAC-DOCA-inh8 relative to sham-veh and a lower stiffness in TAC-DOCA-inh8 compared to TAC-DOCA-veh (Figure 4A).
Figures 4. Cardiomyocyte passive stiffness, LV collagen content, LV Pdea9 RNA expression, LV PDE9a activity, plasma cGMP and LV PKG activity of TAC-DOCA mice.
Cardiomyocyte passive stiffness after chronic PDE9a inhibition, measured in demembraned (skinned) LV cardiomyocytes (A). Cellular stiffness is increased in both groups of TAC-DOCA mice, however, the stiffness of TAC-DOCA-inh8 is lower than TAC-DOCA-veh. (n= 7,15,14 cells from 4,8,7 mice) (A). Representative Picrosirius Red staining for collagen of LV myocardium (B&C). Quantitative analysis shows a significant increase in percent area of myocardial collagen in both groups of TAC-DOCA mice (D). No difference in percent area of collagen is observed between TAC-DOCA-veh and TAC-DOCA-inh8 (n=7, 6, 6 and 7 mice). Quantitative PCR was used to assess Pde9a RNA expression in LV myocardium. There is a significant upregulation of Pde9a mRNA at 5 weeks after TAC-DOCA surgery (E) (n=18,21 mice). High performance liquid chromatography was used to assess PDE9a activity in LV myocardium. PDE9a activity is increased in TAC-DOCA-veh but is normalized in TAC-DOCA-inh8 (n=7,7,6,9 mice) (F). Plasma concentration of cGMP (G) and LV myocardial PKG activity (H) are increased in TAC-DOCA mice with PDE9a inhibition (n=10,9,12 mice for plasma cGMP and n=7,7,8 mice for PKG activity). * p≤0.05 ** p≤0.01 ***p≤0.001 ****p≤0.0001. Statistical analyses consisted of: (A) Nonlinear regression analysis with a least squares fitting, (D&F) Two-way ANOVA, without repeated measures, with a Tukey test, (E) Mann-Whitney U test, (G) Kruskal-Wallis with a Dunn test,(H) One-way ANOVA without repeated measures with a Tukey test.
Myocardial fibrosis of TAC-DOCA mice.
Myocardial fibrosis was quantified by Picrosirius red staining of LV section. Quantitative analysis showed an increase in percent area of myocardial collagen (Figure 4D) as well as upregulation of fibrosis associated genes (Col1a2, Col3a1, Mmp2, Loxl2 and Sparc) in both groups of TAC-DOCA mice (Suppl Figure S3). However, neither a difference in severity of myocardial fibrosis nor a difference in expression of fibrosis associated genes was observed between TAC-DOCA-veh and TAC-DOCA-inh8, suggesting no effect of PDE9a inhibition on fibrotic phenotype.
Pde9a RNA expression and PDE9a activity in LV myocardium of TAC-DOCA mice.
Quantitative PCR was used to assess Pde9a RNA expression in the LV myocardium. There was a significant upregulation of Pde9a (~1.6 fold) at 5 weeks after TAC-DOCA surgery (Figure 4E). We also performed a western blot study to measure PDE9a expression using commercial antibodies and antibodies that we raised against the catalytic domain of human PDE9a (amino acid 181–508). Mouse brain of WT and LV of Pde9a−/−mice (kindly provided by Drs. David A. Kass and Dong I. Lee, at Johns Hopkins University) functioned as positive and negative controls, respectively. However, the antibody labeled multiple protein bands which were all present in Pde9a−/− LV samples, thus we deemed results as unreliable (data not shown). As an alternative, we measured PDE9a activity in LV myocardial lysates, which revealed that PDE9a activity was increased in TAC-DOCA-vehicle compared to sham mice but is normalized to sham levels in TAC-DOCA mice that were treated with PF-4449613 (Figure 4F).
cGMP concentration and PKG activity of TAC-DOCA mice.
cGMP concentration was determined in plasma (Figure 4G) and PKG activity was assessed in LV myocardial samples (Figure 4H). The results revealed that the plasma concentration of cGMP and PKG activity in LV myocardium are increased in TAC-DOCA mice that were treated with PF-4449613. These results provide supportive evidence that PF-4449613 was efficacious in reducing PDE9a activity (Figure 4F), thereby increasing the cGMP levels (Figure 4G) globally as well as increasing PKG activity in the LV (Figure 4H) in the TAC-DOCA model.
Acute PDE9a inhibition in intact cardiomyocytes.
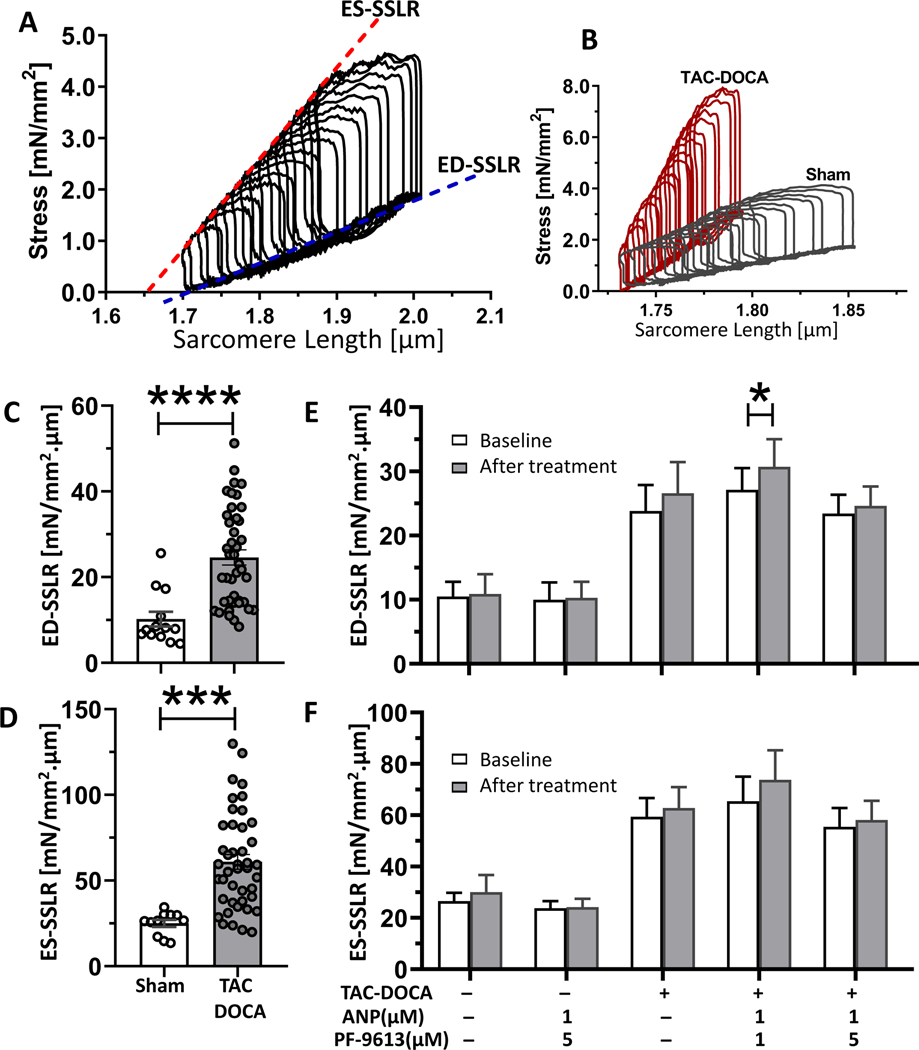
Intact LV cardiomyocytes were isolated from TAC-DOCA or sham mice. Intact cell force measurement was performed using a cellular work loop protocol, which is the cellular equivalent of PV analysis 22. The slopes of ED-SSLR (end diastolic stress-sarcomere length relation) and ES-SSLR (end systolic stress-sarcomere length relation) reflect the cellular diastolic stiffness and systolic contractility, respectively (Figure 5A). Representative cellular work loops of intact cardiomyocytes of TAC-DOCA and sham hearts are shown in Figure 5B. Intact cardiomyocytes from TAC-DOCA hearts had increased slopes of ED-SSLR and ES-SSLR compared to myocytes from sham hearts (Figures 5C and 5D). The increased ED-SSLR and ES-SSLR slopes were correlated with increased EDPVR(β) and Ees at the LV chamber level (Figures 2D and 2H). Note that the cellular stiffness of intact cardiomyocytes (slope of ED-SSLR) was higher than the stiffness measured in skinned cardiomyocytes (Figure 4A) (24.6±1.8 vs. 17.7±1.9 mN/mm2.μm, respectively), which might be explained by stiffness contributors that only exist in the intact cardiomyocytes but not in the skinned preparation, such as residual crossbridges23, 24 and a microtubular network25.
Figure 5. Testing the effect of acute PDE9a inhibition in intact cardiomyocytes.
The cellular stress-sarcomere length relation mimics the pressure-volume relation of the LV chamber (A). The end diastolic stress-SL relation (ED-SSLR) and the end systolic stress-SL relation (ES-SSLR) represent diastolic stiffness and systolic contractility of the single cardiomyocytes respectively. Representative cellular work loops of intact cardiomyocytes of sham in comparison with TAC-DOCA (B). Intact cardiomyocytes from TAC-DOCA hearts have increased slopes of ED-SSLR (C) and ES-SSLR (D) suggesting increased in both cellular diastolic stiffness and contractility compared to myocytes from sham hearts. To investigate the acute effect of PF-4449613, cellular work loops of myocytes were obtained before and 15 mins after administration of PF-4449613 combined with ANP (Atrial Natriuretic Peptide). There are no significant alterations in slopes of ED-SSLR (E) or ES-SSLR (F) after PF-4449613 treatment except the increase in slope of ED-SSLR of TAC-DOCA cells that were treated with PF-4449613 at concentration of 1 μM, which is not observed at concentration of 5 μM. Figures C&D, n=13 and 42 cells from 6,13 mice, Figures E&F, n= 6,7,10,10,13 cells from 3,3,8,5,6 mice. * p≤0.05 significant vs. baseline. Statistical analyses consisted of: (C&D) unpaired t test, (E-F) Two-way ANOVA with repeated measures with a Sidak test.
To test the acute effect, PF-4449613 at a concentration of 1 or 5 μM, together with 1 μM ANP was administered through the cell perfusate (addition of ANP ensures a sufficient level of NP-pGC-cGMP to reveal the effect of PDE inhibition, see Methods for details). Cellular work loops were conducted before and 15 mins after treatment. The result showed that there was no significant reduction in slope of ED-SSLR (Figure 5E) or alteration in ES-SSLR (Figure 5F) except an increase in slope of ED-SSLR of TAC-DOCA cells at PF-4449613 concentration of 1 μM, which was not observed at concentration of 5 μM (Figure 5E, Suppl Table S2).
Chronic PDE9a inhibition in Leprdb/db mice with diastolic dysfunction
Considering the unclear beneficial effects in the TAC-DOCA model, a second mouse model was used to study the effect of PDE9a inhibition. The B6.BKS(D)-Leprdb/J mouse, a model of metabolic syndrome induced diastolic dysfunction26, 27 with a pathophysiology based on cGMP-PKG deficiency26, was selected. Male Leprdb/db mice at ~ 3.5 months of age were subjected to subcutaneous implantation with osmotic minipumps containing either vehicle or PDE9a inhibitor at concentration of 8 mg/kg/day, for 4 weeks. Mice heterozygous for leptin receptor, Leprdb/+, were used as controls. These mice are referred to as Leprdb/+veh, Leprdb/+inh, Leprdb/dbveh, and Leprdb/dbinh. Mass spectrometry revealed that the plasma level of PF-4449613 in the Leprdb/db mice treated with PF-4449613 at 8 mg/kg /day was 191 nM.
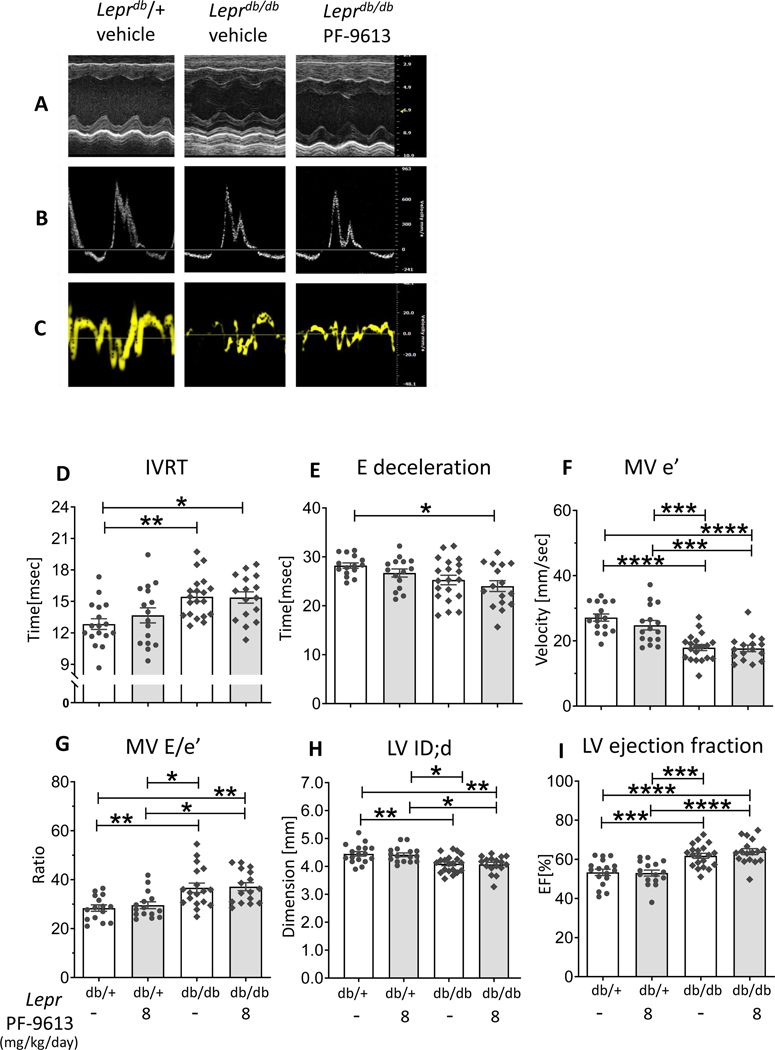
Representative results of M-Mode, mitral inflow pattern and mitral annular velocity are in Figures 6A-C. Leprdb/db mice at ~4.5 months of age exhibited prolonged IVRT (isovolumic relaxation time) (Figure 6D), shortening of E wave deceleration time (Figure 6E), reduced MV e’ velocity(Figure 6F), increased MV E/e’ ratio (Figure 6G), reduced LV internal dimension in diastole (LVID;d) (Figure 6H), and enhanced ejection fraction (Figure 6I), suggesting diastolic dysfunction with preserved ejection fraction. However, no improvement in echocardiographic indices were observed in Leprdb/db mice after PDE9a inhibition (Figures 6D-I).
Figure 6. Echocardiography of Leprdb/db mice.
Representative figures of M-Mode(A), mitral inflow pattern(B) and mitral annular velocity(C). Leprdb/db mice exhibit diastolic dysfunction observed as prolonged isovolumic relaxation time (IVRT)(D), shorten E wave deceleration time(E), reduced mitral annular e’ velocity(F), elevated LV diastolic filling pressure (E/e’ ratio) (G), reduced LV internal dimension in diastole (LVID;d) (H), but preserved ejection fraction(I). No improvement in diastolic parameters is observed in Leprdb/db mice with PDE9a inhibition. (n= 17, 16, 20, 17 mice), * p≤0.05 ** p≤0.01 ***p≤0.001 ****p≤0.0001. Two-way ANOVA, without repeated measures with a Tukey test was performed.
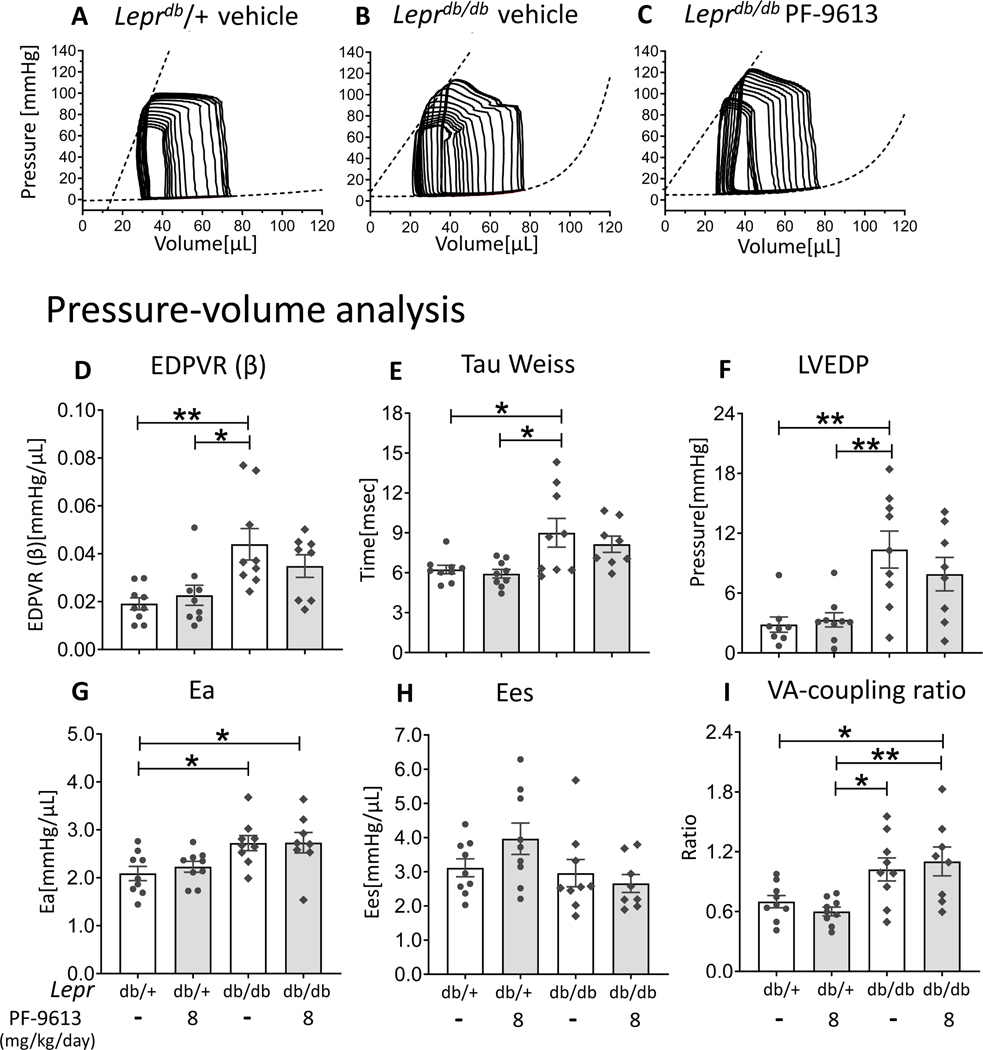
Pressure-volume analysis confirmed the diastolic dysfunction as Leprdb/dbveh exhibited a significant increase in LV chamber stiffness (EDPVR (β)) (Figure 7D), prolonged LV relaxation time constant (Tau) (Figure 7E) and increased LVEDP (Figure 7F) relative to Leprdb/+, suggesting diastolic dysfunction with increased LV filling pressure. Representative PV results of Lepr mice are shown in Figures 7A-C. The Ea was increased in both groups of Leprdb/db mice (Figure 7G), explained by systemic hypertension which is one of the metabolic comorbidities of this mouse model. Despite exhibiting an increase in LV ejection fraction (Figure 6I), the Ees which reflects a load-independent LV contractility was not increased in Leprdb/db mice (Figure 7H). The VA coupling ratio (Ea/Ees) revealed a significant VA decoupling response in Leprdb/dbinh (Figure 7I). Overall, in Leprdb/dbinh, there was only a trend of LV diastolic improvement (as observed in multiple parameters i.e. EDPVR (β), Tau and LVEDP), but a VA coupling ratio became significantly elevated compared to Leprdb/+veh. Additional hemodynamic parameters are displayed in Suppl Table S3.
Figure 7. Pressure-Volume analysis of Leprdb/db mice.
Representative PV analysis of Leprdb/+ veh (A), Leprdb/dbveh (B) and Leprdb/db inh (C). Leprdb/dbveh exhibit increased LV chamber stiffness (EDPVR (β)) (D), prolonged relaxation (Tau) (E), elevated LV diastolic filling pressure (LVEDP) (F) and increased effective arterial elastance (Ea) (G), without significant change in contractility (Ees) (H). No improvement in cardiac parameters is observed in Leprdb/dbinh compared with Leprdb/db veh, whereas the deterioration of VA co-ordination becomes significant in the Leprdb/db after chronic PDE9a inhibition (I). (n= 9, 9, 9, 8 mice), * p≤0.05 ** p≤0.01. Two-way ANOVA without repeated measures with a Tukey test was performed.
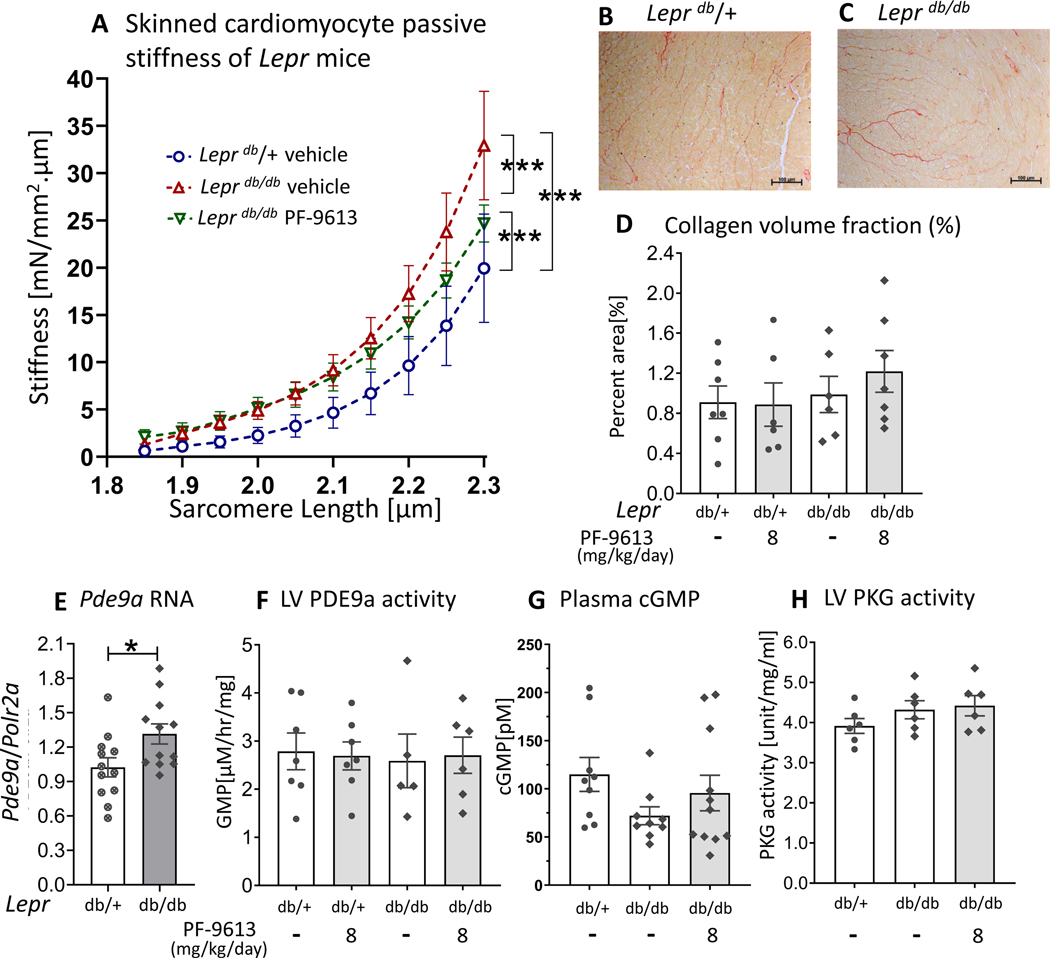
Cardiomyocyte passive stiffness of Leprdb/db mice was higher than Leprdb/+ and was minimally reduced with chronic PDE9a inhibition (Figure 8A). Interestingly, myocardial fibrosis quantified by Picrosirius red staining of LV section (Figure 8D) and mRNA expression of fibrosis associated genes (Suppl Figure S4) did not show any differences among groups.
Figure 8. Cardiomyocyte passive stiffness, LV collagen content, LV Pde9a RNA expression, LV PDE9a activity, plasma cGMP and LV PKG activity of Leprdb/db mice.
Cardiomyocyte passive stiffness after chronic PDE9a inhibition, measured in demembraned (skinned) LV cardiomyocytes (A). Cardiomyocyte stiffness is increased in both groups of Leprdb/db mice, however, the stiffness is slightly reduced in the Leprdb/db mice that were treated with PF-4449613 compared to vehicle (A) (n= 3,16,15 cells from 2,6,7 mice). Representative Picrosirius Red staining for collagen of LV myocardium (B&C). Quantitative analysis does not show a significant difference in percent area of collagen among groups (n=7, 6, 6 and 7 mice) (D). There is a significant upregulation of Pde9a mRNA (n=12,12 mice) (E), however, there is no increase in PDE9a activity in LV myocardium of Leprdb/db mice (F) (n= 7,7,5,6 mice). There is no increase in plasma cGMP concentration (G) or LV PKG activity (H) in Leprdb/db mice with PDE9a inhibition (n =9,9,11 mice for plasma cGMP and n = 6,6,6 mice for LV PKG activity), * p≤0.05** p≤0.01 ***p≤0.001 ****p≤0.0001. Statistical analyses consisted of: (A) Nonlinear regression analysis with a least squares fitting, (D&F) Two-way ANOVA, without repeated measures, with a Tukey test, (E) unpaired t test, (G) Kruskal-Wallis with Dunn test, (H) One-way ANOVA without repeated measures with a Tukey test.
RNA expression analysis revealed a significant upregulation of Pde9a mRNA in Leprdb/db mice (Figure 8E). However, there was no increase in PDE9a activity in the LV myocardium of Leprdb/db mice compared to Leprdb/+ (Figure 8F), which explains the lack of an increase in cGMP- PKG activity (Figures 8G-H) and lack of diastolic improvement (Figure 7D)in Leprdb/db mice after PDE9a inhibition.
Discussion
cGMP-PKG deficiency has been focused on as an important pathological mechanism and potential therapeutic target of HFpEF1, 5, 28, 29. cGMP-PKG modulates cardiac systolic and diastolic function30, 31, and confers long term cardioprotective effects through anti-hypertrophic, anti-fibrotic, and anti-apoptotic pathways32, 33. To study whether inhibition of PDE9a alleviates pre-established diastolic dysfunction, we utilized two mouse models: TAC-DOCA18–20 and Leprdb/db mice26, 27. From our studies, chronic PDE9a inhibition showed a reduction in cardiomyocyte stiffness in both models, but improved LV diastolic stiffness only in TAC-DOCA mice. However, chronic PDE9a inhibition at doses required to achieve diastolic benefit suppress systolic function as evidenced by a decline in VA coordination. Below we discuss these findings in detail.
Effect of chronic PDE9a inhibition on in-vivo cardiac function
Based on previous work8, the mean plasma concentration of PF-4449613 that effectively reverses the LV hypertrophy and systolic dysfunction in TAC mice is 77 nM (with peaks of 1.5 uM), for twice a day oral administration. In our study, we administered PF-4449613 through continuous perfusion via subcutaneous osmotic mini-pumps. The plasma levels of PF-4449613 of TAC-DOCA-inh mice that were treated with PF-4449613 at concentration of 1, 5 and 8 mg/kg/day were 15 nM, 305 nM and 391 nM respectively. Thus, the level of PF-4449613 of TAC-DOCA-inh5 and TAC-DOCA-inh8 is likely within an effective range, which is supported by the observed reduction in EDPVR (Figure 2D). Conversely, the 1 mg/kg was likely inadequate, explaining the lack of diastolic stiffness reduction and a preserved VA coupling response in TAC-DOCA-inh1.
The higher doses of inhibitor demonstrated a reduction in diastolic stiffness in the TAC-DOCA model; however, a decline in VA coordination was observed. Coordination of the heart and arteries determines the effective transfer of blood from ventricle to the arterial system34, 35. The VA coupling ratio, indexed by Ea/Ees, is strongly associated with clinical outcomes in heart failure34, 35. When contractility cannot keep up with increased arterial elastance, the VA coordination declines resulting in insufficient cardiac output. In such circumstance, VA coordination can be maintained by lowering arterial elastance34 to accommodate reduced contractility. However, in our experimental models, the arterial elastance (Ea) was persistently elevated, in the TAC-DOCA model from permanent aortic constriction and in the Leprdb/db model from the full-blown untreated metabolic co-morbidities. Without a lowering of the arterial elastance, the relative decline in contractility (Figures 2H,7H) as a consequence of PDE9a inhibition resulted in VA-discoordination in these animals (Figures 2I,7I). The suppression of contractility is likely explained by PKG negatively mediating inotropy by reducing myofilament Ca2+ sensitivity and counteracting the signaling effect of PKA (Protein Kinase A) stimulation since PKG phosphorylates many Ca2+ handling and sarcomeric proteins targeted by PKA 33, 36, 37. In addition to the negative ionotropic effects induced by cGMP-PKG activity, increased cGMP level could also attenuate cyclic Adenosine Monophosphate (cAMP)-mediated signaling through interactions with the regulatory subunit of PDE2, and potentiating its cAMP hydrolytic activity. Thus activation of PDE2 by cGMP could lead to a reduction of cAMP levels, a reduced PKA-mediated response, and negative inotropy 38. However, in our study, measurement of cAMP-targeted-PDE activity in LV myocardium of the mice that were treated with PF-4449613 (Suppl Figure S5) showed no alteration in cAMP-targeted-PDE activity. Thus, it is unlikely that the effect is due to increased cAMP hydrolysis. Nevertheless, due to the complexity of heart failure pathomechanisms, it cannot be excluded that other signaling pathways are also involved.
It is important to note that PDE9a inhibition did not affect contractility in the sham animals (Figure 2H) and that while contractility of TAC-DOCA-inh5 and inh8 was reduced compared to TAC-DOCA-veh, it was normalized (not reduced) when compared to sham-veh (Figure 2H). Thus, PDE9a inhibition might be beneficial for treating diastolic stiffening if this inhibition is combined with a therapeutic that lowers arterial elastance, as a means to avoid VA discoordination.
Due to reported gender-related differences in HFpEF characteristics and outcomes39, 40, we have conducted a study in female mice undergoing the same regimen of TAC-DOCA followed by PDE9a inhibition (Suppl Figures S6 and Table S4). Similar trends of diastolic improvement along with systolic impairment were observed, in line with the result of adult male mice (Figures 2D-I). These findings suggest that PDE9a inhibition may be effective in LV stiffness reduction in both genders, although systolic dysfunction is still a concern. Additionally, the potential benefit of a longer duration of PDE9a inhibition along with 1 week pretreatment was studied in a cohort of male mice that received treatment with PDE9a inhibitor (8mg/kg/day) beginning 1 week prior to TAC-DOCA surgery and continuing for the duration of 5 weeks post-surgery (6 weeks total PDE9a inhibition) (Suppl Figures S7 and Table S5). Several diastolic parameters were trending towards improvement similar to the observed effects with treatment beginning 1 week post-TAC-DOCA (Figures 2D-I and Table S1). Interestingly, initiation of PDE9a inhibition prior to surgery tends to preserve LV contractility (Ees) and VA interaction, in contrast to the impaired contractility and a decline in VA coupling observed in TAC-DOCA mice that had PDE9a inhibition initiated after diastolic dysfunction had already developed. This finding raises the possibility that early initiation of PDE9a inhibition may be another strategy to avoid systolic consequences of PDE9a inhibition. However, more extensive studies are required.
Effect of PDE9a inhibition at the cardiomyocyte level
Consistent with diastolic stiffness measurement on the whole LV level, passive stiffness was increased in cardiomyocytes from the TAC-DOCA model and chronic PDE9a inhibition lowered cellular stiffness (Figures 4A). Because the passive stiffness of demembraned cardiomyocyte is mainly due to titin41, 42, the reduced cellular passive stiffness that was found is likely due to an effect of PDE9a inhibition on titin. In addition, another important sarcomeric protein that presents in the permeabilized preparation, and also contributes to LV stiffness is Cardiac Myosin-Binding Protein-C (cMyBP-C)43. cMyBP-C is a substrate of PKG44, and its phosphorylated state reduces stiffness of the LV chamber43. Thus PKG phosphorylation of cMyBP-C is another potential mechanism through which PDE9a inhibition reduces stiffness of cardiomyocytes and LV chamber.
Titin phosphorylation studies with currently available antibodies for PKG phosphosites in the mouse did not reveal changes due to PDE9a inhibition (results not shown). Owing to the massive size of titin, studying titin phosphorylation using phospho-specific antibodies is insufficient to cover the full spectrum of possible titin phosphorylation. Future whole proteome studies that focus on phosphorylation of titin and other possible substrates, including cMyBP-C, are required and the present lack of such data is a study limitation.
In contrast to chronic administration, acute PDE9a inhibition failed to show any benefit at the cardiomyocyte level assessed by the intact cellular work loop protocol. Cellular work loops offer several advantages over PV analysis in that this novel technology makes it possible to measure the cardiomyocyte-specific effects of pharmacological compounds without confounding effects of neurohormonal reflex, preload-afterload status and extracellular matrix. Nevertheless, there are some limitations. For example, the time window for continuous measurement on a single cell is limited and the effect of compounds with slow onset of action may go unnoticeable. The fact that we did not observe a cellular stiffness change within 15 mins in PDE9a inhibitor, whereas chronic effects are seen, indicates that a longer measurement interval than is practical may be required.
Effect of chronic PDE9a inhibition on LV structure
In addition to the effects on diastolic function, PDE9a inhibition may offer further potential benefit in HFpEF pathology as the cGMP-PKG1α pathway has been shown to confer cardioprotective effects through suppressing pathological hypertrophy and myocardial fibrosis32. Previous work has shown that Pde9a−/− mice are resistant to TAC-induced cardiac fibrosis8 and PDE9a inhibition reverses pre-established cardiac hypertrophy in wild-type mice subjected to TAC8, 45; however, in the TAC-DOCA model we did not observe an attenuated cardiac hypertrophy after PDE9a inhibition (Suppl Figures S2D-E and Table S1). This difference between TAC vs. TAC-DOCA model might be explained by the mineralocorticoid excess in TAC-DOCA which led to a more complex hypertrophic response18. Additionally, the TAC model was characterized by LV dilation and systolic failure8, while the TAC-DOCA model exhibits concentric hypertrophy and preserved systolic function. Analysis of myocardial collagen content (Figure 4D) as well as expression of fibrosis associated genes ( Suppl Figure S3) showed no difference in severity of myocardial fibrosis between TAC-DOCA mice with or without PDE9a inhibition.
In our study, PDE9a inhibition was started 1 week after TAC-DOCA surgery, a time at which the inflammatory process, fibrogenesis and hypertrophic response had already taken place, plus the duration of inhibition was limited to 4 weeks. These factors might explain the lack of an effect on myocardial fibrosis and LV morphology. Whether PDE9a inhibition can be therapeutic in pathological fibrosis or hypertrophic remodeling requires further investigation where inhibition is started earlier or the duration of treatment is extended longer than in our work.
Effect of chronic PDE9a inhibition in the Leprdb/db mice
In contrast to the TAC-DOCA model, PDE9a inhibition showed little efficacy in the Leprdb/db mouse model. The Leprdb/db mice exhibited diastolic dysfunction and increased diastolic stiffness at both the chamber and cardiomyocyte levels. Although no improvement in diastolic function of LV chamber stiffness was observed following PDE9a inhibition, there was a reduction in cardiomyocyte passive stiffness. However, the cellular stiffness reduction was subtle, particularly within the physiological SL range (SL 1.8–2.2 um)42, and the effect was clearly not sufficient to translate to a beneficial effect at the LV chamber level in this model. This is likely explained by the observation that PDE9a activity was only increased in TAC-DOCA but not the Leprdb/db model (Figures 4F,8F).
Variation of PDE9a expression patterns.
The RNA expression study showed a significant upregulation of Pde9a RNA in LV myocardium of TAC-DOCA and Leprdb/db mice. We measured PDE9a activity and confirmed that the activity was increased in TAC-DOCA mice (Figure 4F). In addition, Kokkonen et al. studied RNA expression profiles in response to hemodynamic stresses and reported beneficial changes at the transcript level in TAC mice after PDE9a inhibition46. Together, these results support the activity and the role of PDE9a in cardiac pathology in mice. Interestingly, in TAC-DOCA mice, there was considerable variation in the Pde9a RNA expression level with the appearance of two subpopulations (Figure 4E). Studying PDE9a expression in human samples also revealed variable results with some studies showing a high expression level8 and others not detecting PDE9a expression in human failing myocardium47. These findings suggest considerable variation in PDE9a expression among individuals which could affect the efficacy of PDE9a inhibition. Although the result of chronic PDE9a inhibition on diastolic function is encouraging, the fact that Pde9a RNA expression was only slightly upregulated in our models and observations that PDE9a expression studies in human was variable8, 47 imply that targeting PDE9a as a potential therapeutic intervention for diastolic dysfunction might be beneficial only in a subgroup of the patients that have significantly increased PDE9a expression. Further studies in larger mammals and in both genders are necessary to confirm the potential benefit of PDE9a targeted therapies.
Other possible pathomechanisms of HFpEF and potential therapeutic targets
The variable response to PDE9a inhibition in the two diastolic dysfunction models presented here demonstrate that potential therapeutics are not a one size fits all remedy. HFpEF is a syndrome of phenotypic heterogeneity and compelling evidence supports the importance of comorbidities that drive systemic inflammatory responses in HFpEF pathogenesis28. Oxidative stress induced cGMP-PKG deficiency plays a significant role as an important pathological pathway28 in HFpEF, but other protein kinases including PKC and PKA are also reported to be involved in the HFpEF pathogenesis3, 20, 48. Although a deranged phosphorylation status has been regarded as crucial, emerging evidence suggests the importance of other types of post-translational modification, e.g., disulfide bond formation, S-gluthathionylation, sulfenylation, acetylation, carbonylation, and S-nitrosylation, which also play a role in the diastolic properties of the heart, and some of which are irreversible49. Recently, a study by Schiattarella et al., revealed that increased iNOS (inducible nitric oxide synthase) activity and nitrosative stress promote S-nitrosylation of IRE1α (the endonuclease inositol-requiring protein 1α), which plays roles in the UPR (unfolded protein response) system, leading to diastolic dysfunction, and inhibition of iNOS activity ameliorates HFpEF phenotype50. The discovery of enhanced iNOS activity as a factor in HFpEF pathogenesis explains the unsuccessful outcome of the NO-inducing HFpEF therapy50. Nevertheless, how S-nitrosylation of IRE1α of the UPR system translates to mechanical stiffness of LV chamber and whether the enhanced iNOS activity is associated with low NO-cGMP-PKG activity in human HFpEF myocardium, reported by Heerebreek et al.20125 needs further investigation. In addition to targeting post-translational modification, upregulation of compliant titin isoforms through inhibition of the splicing factor RBM20 is another promising alternative in diastolic dysfunction treatment19. Better understanding of the complex signaling pathways and post-translational modifications underlying HFpEF pathogenesis provides hope for targeted therapeutics to fill an important and unmet need in HFpEF treatment.
Conclusions
PDE9a activity was upregulated in the TAC-DOCA diastolic dysfunction mouse model. Chronic PDE9a inhibition in TAC-DOCA mice showed a reduction in LV diastolic stiffness, which likely can be explained by cardiomyocyte stiffness reduction. However, chronic PDE9a inhibition also suppressed the compensatory enhancement of contractile function associated with TAC. Without any relief of the elevated arterial elastance this resulted in a decline in VA coordination, which offsets the beneficial effect on diastole. A potential role of PDE9a inhibition in treating diastolic dysfunction might be possible if this inhibition is combined with drugs that reduce effective arterial elastance to prevent VA discoordination.
Supplementary Material
WHAT IS NEW?
In a mouse model of diastolic dysfunction, chronic PDE9a inhibition increases cGMP-PKG activity in LV myocardium and reduces LV diastolic stiffness through reduction of cardiomyocyte stiffness.
Considerable variation in the Pde9a expression levels was observed.
Chronic PDE9a inhibition suppresses the compensatory enhancement of contractile function resulting in systolic dysfunction and ventricular-arterial (VA) discoordination, which may limit the beneficial effect on diastole.
WHAT ARE THE CLINICAL IMPLICATIONS?
The prevalence of HFpEF continues to increase, but no specific treatment is available. PDE9a inhibition offers potential benefit in lowering diastolic stiffness. However, due to the negative impact of PDE9a inhibition on systolic function, combination treatment with a strategy to reduce effective arterial elastance would be required.
Due to variability in PDE9a expression in the studied models of diastolic dysfunction, as well as variability previously reported in HFpEF patients, targeting PDE9a as a potential therapeutic intervention for diastolic dysfunction may be beneficial in a subgroup of patients that have significantly increased PDE9a activity.
Acknowledgments
We thank Pfizer for providing PF-4449613. We acknowledge our lab members and the University of Arizona Cancer Center supported by the NCI of the NIH under award number P30 CA023074. Finally we thank the animals that have been used for our research.
Funding
National Institutes of Health R35HL144998, Foundation Leducq (TNE-13CVD04) (H.L.), and American Heart Association 19CDA34660099 (M.M.).
Non-standard Abbreviations and Acronyms
- ANP
Atrial Natriuretic Peptide
- cGMP
cyclic Guanosine Monophosphate
- cMyBP-C
Cardiac Myosin-Binding Protein-C
- COL1a2
Collagen type I alpha 2 chain
- COL3a1
Collagen type III alpha 1 chain
- DOCA
Deoxycorticosterone Acetate
- Ea
Effective arterial elastance
- EDPVR
End-Diastolic Pressure-Volume Relation
- ED-SSLR
End-Diastolic - Stress Sarcomere Length Relation
- Ees
End-systolic elastance
- ES-SSLR
End-Systolic - Stress Sarcomere Length Relation
- HFpEF
Heart Failure with preserved Ejection Fraction
- IRE1α
Endonuclease Inositol-Requiring protein 1α
- IVRT
Isovolumic Relaxation Time
- (LA)
Left Atrium
- Lepr
Leptin receptor
- LV
Left Ventricle
- LVEDP
Left Ventricular End Diastolic Pressure
- LVID;d
Left Ventricular Internal Dimension in diastole
- LOXL2
Lysyl Oxidase Like 2
- maxVO2
maximal oxygen consumption
- MMP2
Matrix Metallopeptidase 2
- MyH7
Myosin Heavy Chain 7
- MV
Mitral Valve
- NO
Nitric Oxide
- NP
Natriuretic Peptide
- NPPA
Natriuretic Peptide A
- NPPB
Natriuretic Peptide B
- PDE
Phosphodiesterase
- pGC
particulate Guanylate Cyclase
- PKA
Protein Kinase A
- PKG
Protein Kinase G
- sGC
soluble Guanylate Cyclase
- SPARC
Secreted Protein Acidic and Cysteine Rich
- TAC
Transverse Aortic Constriction
- Tau
Relaxation time constant
- UPR
Unfolded Protein Response
- VA
Ventricular-Arterial
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Pfeffer MA, Shah AM and Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circulation research. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016;375:1868–1877. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG and Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circulation research. 2009;104:87–94. [DOI] [PubMed] [Google Scholar]
- 5.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–9. [DOI] [PubMed] [Google Scholar]
- 6.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J and Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circulation research. 2006;99:816–28. [DOI] [PubMed] [Google Scholar]
- 7.Castro LR, Verde I, Cooper DM and Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y and Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nature medicine. 2005;11:214–22. [DOI] [PubMed] [Google Scholar]
- 10.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC Jr., Linke WA and Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–24. [DOI] [PubMed] [Google Scholar]
- 13.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degen CV, Bishu K, Zakeri R, Ogut O, Redfield MM and Brozovich FV. The emperor’s new clothes: PDE5 and the heart. PLoS One. 2015;10:e0118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E and Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circulation research. 2012;110:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. [ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012]. Turk Kardiyol Dern Ars. 2012;40 Suppl 3:77–137. [PubMed] [Google Scholar]
- 17.Kleiman RJ, Chapin DS, Christoffersen C, Freeman J, Fonseca KR, Geoghegan KF, Grimwood S, Guanowsky V, Hajos M, Harms JF, et al. Phosphodiesterase 9A regulates central cGMP and modulates responses to cholinergic and monoaminergic perturbation in vivo. J Pharmacol Exp Ther. 2012;341:396–409. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed SF, Ohtani T, Korinek J, Lam CS, Larsen K, Simari RD, Valencik ML, Burnett JC Jr., and Redfield MM. Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via “nongenomic effects”. Circulation. 2010;122:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Methawasin M, Strom JG, Slater RE, Fernandez V, Saripalli C and Granzier H. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation. 2016;134:1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slater RE, Strom JG, Methawasin M, Liss M, Gotthardt M, Sweitzer N and Granzier HL. Metformin improves diastolic function in an HFpEF-like mouse model by increasing titin compliance. The Journal of general physiology. 2019;151:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshijima M and Chien KR. Mixed signals in heart failure: cancer rules. The Journal of clinical investigation. 2002;109:849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmes M, Najafi A, Palmer BM, Breel E, Rijnveld N, Iannuzzi D and van der Velden J. Mimicking the cardiac cycle in intact cardiomyocytes using diastolic and systolic force clamps; measuring power output. Cardiovascular research. 2016;111:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King NM, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M and Granzier H. Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. The Journal of general physiology. 2011;137:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sequeira V, Najafi A, McConnell M, Fowler ED, Bollen IA, Wust RC, dos Remedios C, Helmes M, White E, Stienen GJ, et al. Synergistic role of ADP and Ca(2+) in diastolic myocardial stiffness. The Journal of physiology. 2015;593:3899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB and Prosser BL. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. 2016;352:aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamdani N, Hervent AS, Vandekerckhove L, Matheeussen V, Demolder M, Baerts L, De Meester I, Linke WA, Paulus WJ and De Keulenaer GW. Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovascular research. 2014;104:423–31. [DOI] [PubMed] [Google Scholar]
- 27.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD and Oudit GY. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circulation Heart failure. 2014;7:327–39. [DOI] [PubMed] [Google Scholar]
- 28.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA and Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–15. [DOI] [PubMed] [Google Scholar]
- 30.Frantz S, Klaiber M, Baba HA, Oberwinkler H, Volker K, Gabetaner B, Bayer B, Abebetaer M, Schuh K, Feil R, et al. Stress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. European heart journal. 2013;34:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanton RM, Takimoto E, Lane AM, Aronovitz M, Piotrowski R, Karas RH, Kass DA and Mendelsohn ME. Protein kinase g ialpha inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. Journal of the American Heart Association. 2012;1:e003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirk JA, Holewinski RJ, Crowgey EL and Van Eyk JE. Protein kinase G signaling in cardiac pathophysiology: Impact of proteomics on clinical trials. Proteomics. 2016;16:894–905. [DOI] [PubMed] [Google Scholar]
- 33.Kim GE and Kass DA. Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease. Handb Exp Pharmacol. 2017;243:249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. Journal of the American College of Cardiology. 2013;62:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlaug BA and Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin. 2011;29:447–59. [DOI] [PubMed] [Google Scholar]
- 36.Layland J, Li JM and Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F and Feil R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90:18–20. [DOI] [PubMed] [Google Scholar]
- 38.Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G et al. cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circulation research. 2011;108:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, et al. Effects of Sacubitril-Valsartan, versus Valsartan, in Women Compared to Men with Heart Failure and Preserved Ejection Fraction: Insights from PARAGON-HF. Circulation. 2019;141:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewan P, Rorth R, Raparelli V, Campbell RT, Shen L, Jhund PS, Petrie MC, Anand IS, Carson PE, Desai AS, et al. Sex-Related Differences in Heart Failure With Preserved Ejection Fraction. Circulation Heart failure. 2019;12:e006539. [DOI] [PubMed] [Google Scholar]
- 41.Granzier HL and Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophysical journal. 1995;68:1027–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung CS and Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. Journal of molecular and cellular cardiology. 2011;50:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosas PC, Liu Y, Abdalla MI, Thomas CM, Kidwell DT, Dusio GF, Mukhopadhyay D, Kumar R, Baker KM, Mitchell BM, et al. Phosphorylation of cardiac Myosin-binding protein-C is a critical mediator of diastolic function. Circ Heart Fail. 2015;8:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoonen R, Giovanni S, Govindan S, Lee DI, Wang GR, Calamaras TD, Takimoto E, Kass DA, Sadayappan S and Blanton RM. Molecular Screen Identifies Cardiac Myosin-Binding Protein-C as a Protein Kinase G-Ialpha Substrate. Circulation Heart failure. 2015;8:1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang PX, Li ZM, Cai SD, Li JY, He P, Huang Y, Feng GS, Luo HB, Chen SR and Liu PQ. C33(S), a novel PDE9A inhibitor, protects against rat cardiac hypertrophy through upregulating cGMP signaling. Acta pharmacologica Sinica. 2017;38:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokkonen-Simon KM, Saberi A, Nakamura T, Ranek MJ, Zhu G, Bedja D, Kuhn M, Halushka MK, Lee DI and Kass DA. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li EA, Xi W, Han YS and Brozovich FV. Phosphodiesterase expression in the normal and failing heart. Arch Biochem Biophys. 2019;662:160–168. [DOI] [PubMed] [Google Scholar]
- 48.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circulation research. 2009;104:780–6. [DOI] [PubMed] [Google Scholar]
- 49.Beckendorf L and Linke WA. Emerging importance of oxidative stress in regulating striated muscle elasticity. Journal of muscle research and cell motility. 2015;36:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.