Abstract
Goals:
To assess if curcumin improves markers of cholestasis among subjects with primary sclerosing cholangitis (PSC).
Background:
PSC is a chronic cholestatic liver disorder for which there is no established medical therapy. Preclinical data suggest curcumin may have a beneficial effect in PSC.
Study:
Subjects with PSC and a serum alkaline phosphatase (SAP) greater than 1.5 times the upper limit of normal (ULN) received curcumin 750 mg orally twice daily for 12 weeks in an open-label pilot study. The primary composite endpoint was proportion of subjects who had a reduction of SAP to less than 1.5 times ULN or a 40% reduction in SAP between baseline and week 12. Secondary endpoints included changes in serum aspartate aminotransferase, total bilirubin, Mayo PSC risk score and self-reported health questionnaires.
Results:
Two-hundred and fifty-eight patients with PSC were screened and 15 subjects were enrolled and all completed 12 weeks of therapy. The most common reason for subject exclusion was SAP less than 1.5 times the ULN (n = 98). Curcumin did not result in a significant median (interquartile range) change in SAP times the ULN [3.43 (2.10–4.32) to 2.46 (1.89–4.41), p =.36], and only 20% (3/15) subjects achieved the primary endpoint. Similarly, there was no significant change in the secondary endpoints. There were no serious adverse events reported.
Conclusion:
While curcumin was well tolerated, it was not associated with significant improvements in cholestasis or symptoms. Moreover, this study also illustrates that a low SAP is common among those with PSC.
Keywords: Primary sclerosing cholangitis, curcumin, clinical trial, pharmacotherapy, alkaline phosphatase
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder, frequently associated with inflammatory bowel disease (IBD), which can lead to complications from portal hypertension and impart an increased risk of hepatobiliary and colonic malignancies [1]. Individuals with PSC have increased morbidity and mortality compared to the general population and the average transplant free survival ranges from 14 to 21 years [2,3]. Despite prior trials examining over 20 different pharmacologic agents, effective medical therapy for PSC remains lacking.
Curcumin, the principal curcuminoid of the rhizome turmeric (Curcuma longa), is a naturally occurring compound reported to have anti-inflammatory and anti-oxidant effects [4–6]. Indeed, curcumin has been studied in a wide range of chronic conditions including nonalcoholic fatty liver disease, rheumatoid arthritis and IBD and is found to be well tolerated in humans [5,7,8]. In chronic liver diseases, curcumin may act as a free radical scavenger and reduce oxidative damage through several mechanisms including suppressing lipid peroxidation, proinflammatory cytokines, hepatic stellate cell activation, and cellular responses to oxidative stress [9,10]. In the Mdr2 knockout mouse model of sclerosing cholangitis, 4 weeks of oral curcumin was associated with a significant reduction in liver enzymes as well as improvements in liver histology. Specifically, hepatic fibrosis, activated portal myofibroblasts, activated cholangiocytes and recruitment of inflammatory cells were reduced via pleiotropic mechanisms [11]. Similarly, using the alpha-naphthylisothiocyanate induced cholestasis rodent model, curcumin was also found to activate the farsenoid X receptor and reduce markers of cholestasis and inflammation [12].
Based on the promising results of preclinical studies and the urgent need to identify effective medical therapies, we conducted a 12-week open-label pilot study to determine if curcumin could reduce markers of cholestasis among those with PSC.
Materials and methods
Patients
Inclusion criteria included adults (ages 18–75) with large duct PSC diagnosed by standard criteria who also had a serum alkaline phosphatase (SAP) greater than 1.5 times the upper limit of normal (ULN) at study entry [13].
Individuals who met the above criteria were excluded from participating in the study under the following circumstances: use of ursodeoxycholic acid (UDCA), curcumin, corticosteroids, colchicine, methotrexate, cyclosporine, pentoxifylline, tacrolimus, warfarin or changes in IBD therapy within the past 3 months; use of antibiotics within the past month; advanced PSC defined by the presence of decompensated cirrhosis, anticipated need for liver transplantation within 1 year and ascending cholangitis or an untreated dominant stricture within the past 3 months; known or suspected cholangiocarcinoma (CCA); the presence of concomitant liver disease including overlap with autoimmune hepatitis; active alcohol or drug use; pregnancy or lactation. These exclusionary criteria including UDCA use were employed to achieve a homogenous population. There were no patients who stopped UDCA for the purposes of this study.
All subjects were seen and recruited at Mayo Clinic in Rochester, MN. Informed consent was obtained from each participant and the study protocol adheres to the 1975 Declaration of Helsinki. The study was approved by the institutional review board of the study site and is registered in clinicaltrails.gov (NCT 02978339).
Study design & assessments
This was an investigator initiated, single-site, open-label clinical trial with only 1 study arm. Participants received curcumin 750 mg (CuraMed® BCM-95 curcumin softgel, EuroPharma, Inc.) by mouth twice daily for 12 weeks. The study drug was supplied by EuroPharma, Inc. BCM-95 Curcumin, the patented form of curcumin found in CuraMed, is a preparation of standardized curcuminoids that is blended with the essential oil of turmeric. It contains 95% curcuminoid complex composed of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. The bioavailability of this formulation in humans has previously been described [14]. A 12-week supply of the study drug was assigned and dispensed to each study participant. Drug reconciliation checks and compliance assessments were performed by the study team.
Subjects with PSC were screened from an outpatient hepatology clinic. Screening assessments were completed within 28 days of the baseline visit (Day 1 visit). This included a complete medical history and examination, review of medications and laboratory assessments [complete blood count, c-reactive protein (CRP), SAP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, international normalized ratio (INR), sodium, creatinine, albumin and serum pregnancy for females of childbearing potential] and patient questionnaires [5-D pruritus scale and the fatigue impact scale (FIS) questionnaires]. Subjects were allowed to have blood draws at their local facility after the baseline visit. The same laboratory tests and questionnaires were also collected at week 12. Participants were called by study personnel on week 3, 8, 12 and 2 weeks after completion of the study (week 14) to assess for compliance and for adverse events (AE).
Notably, the reference range or ULN for SAP can vary across laboratory tests and can differ even among the same patient at different points in time. Hence, direct comparison of the SAP value can be misleading when different reference ranges are used. Therefore, we standardized the SAP reporting by dividing the SAP value by the corresponding ULN value for that lab to determine the SAP times the ULN.
The scores of the five domains in the 5-D pruritus scale were summed together to achieve a total 5-D score with higher scores reflecting more severe pruritus [15]. The 40 items of the 3 domains of FIS (physical, cognitive and psychosocial functioning) were summed as previously described [16]. The total score ranges from 0–160, with higher scores indicating greater limitations in functioning.
AE were classified according to severity: mild (transient and easily tolerated by the participant), moderate (causes the participant discomfort and interrupts the participant’s usual activities), or severe (causes considerable interference with the participant’s usual activities and may be incapacitating or life-threatening). The following definitions were used to assess the relationship between any AE and use of the study drug: probably related (AE has a strong temporal relationship to study drug or recurs on re-challenge and another etiology is unlikely or significantly less likely); possibly related (AE has a strong temporal relationship to the study drug and an alternative etiology is equally or less likely compared to the potential relationship to study drug); probably not related (AE has little or no temporal relationship to study drug and/or a more likely alternative etiology exists); and not related (AE due to an underlying or concurrent illness or effect of another drug and is not related to the study drug).
Study endpoints
The primary composite endpoint was the proportion of PSC patients who achieved a clinically significant reduction defined by a 40% reduction in SAP times the ULN or a decrease in SAP to less than 1.5 times the ULN. This surrogate composite endpoint was selected for several reasons. First, multiple studies have shown that reduction of SAP has been associated with improved outcomes in PSC [17–20]. Second, both a SAP threshold of less than 1.5 times the ULN and a 40% SAP reduction have been associated with improved outcomes [17,18,20]. Third, it is not plausible to conduct pilot studies powered to detect rare patient events such as transplantation-free survival or hepatic decompensation [21].
In this open-label pilot study, our primary objective is to determine the proportion of patients who experience curcumin treatment success, as defined by the above composite endpoint. Approximately 5% of patients with PSC and chronically (>6 months) elevated SAP will spontaneously experience either a clinically significant reduction in SAP to less 1.5 times ULN or a reduction of more than 40% of baseline value [17,18,20]. Therefore, if treatment success is achieved in at least 30% of curcumin treated patients (i.e. a rate that is at least 25% more than the conservative estimate in historical controls) this would encourage further study of curcumin in larger, randomized clinical trials in patients with PSC.
The secondary endpoints examined included changes in AST, total bilirubin, CRP, Mayo PSC risk score, PSC risk estimate tool (PREsTo) and self-reported change in pruritus, fatigue and overall health status. The Mayo PSC risk score has been shown to predict patient survival [22]. Similarly, PREsTo is a validated score created using artificial intelligence and has been found to be highly predictive of hepatic decompensation in PSC [23].
Statistical methods
By enrolling 15 patients with PSC in our study, an exact binomial test with a nominal two-sided significance level of α = 0.05 will have approximately 90% power to detect a 25% difference in clinically significant SAP improvement between the null hypothesis of 5% (historical control) and the alternative hypothesis of 30%.
Statistical analysis was performed with JMP 13.0.0 software (SAS Institute; Cary, NC). All tests were 2-sided with a level of significance of p < .05. Continuous variables were reported as medians [interquartile range (IQR)] and categorical variables were expressed as percentage (proportion). The relative percent change in SAP times the ULN was determined for each patient as follows:
Values at baseline and at 12 weeks were compared using the Wilcoxon signed rank test for comparing medians. The exact binomial test was used to determine the p value when comparing the proportion of individuals who did and did not reach the composite endpoint. Spearman’s test was used to determine the correlation coefficient. All authors had access to the study data and all reviewed and approved the final manuscript.
Results
Patients
Two-hundred and fifty-eight patients with PSC were screened for the purpose of this study. Approximately, 38% of subjects had a SAP less than 1.5 times the ULN and were excluded from participating (Figure 1). Ultimately, 15 patients were enrolled. Baseline features are shown in Table 1.
Figure 1.
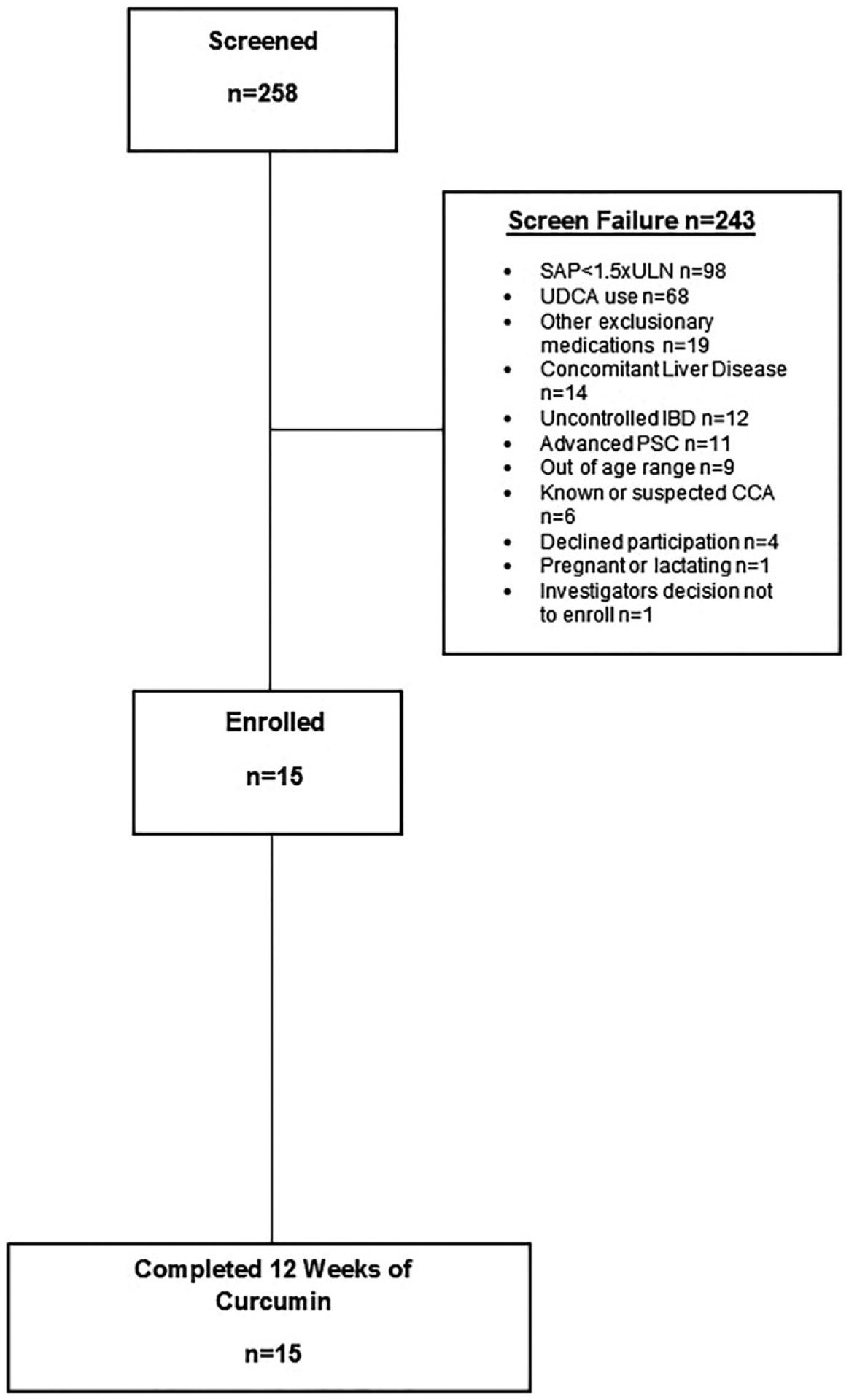
Patients enrolled.
Table 1.
Baseline features of study population.
| Feature | Study Subjects (n = 15) |
|---|---|
| Age (years) | 46.00 (39.00–57.00) |
| Male gender | 47% (7/15) |
| PSC duration (years) | 9.00 (4.00–13.00) |
| Inflammatory Bowel Disease Present | 67% (10/15) |
| • Ulcerative Colitis | 70% (7/10) |
| • Crohn’s disease | 10% (1/10) |
| • Indeterminate colitis | 20% (2/10) |
| Platelets (×109/L) | 272.00 (210.00–307.00) |
| SAP times the ULN | 3.43 (2.10–4.32) |
| Total Bilirubin (mg/dL) | 0.80 (0.40–1.40) |
| Mayo PSC Risk Scorea | 0.28 (−0.23–0.69) |
PSC: primary sclerosing cholangitis; SAP: serum alkaline phosphatase; ULN: upper limit of normal.
Continuous variables expressed as median (interquartile range).
Available for 14/15 subjects.
Endpoints & safety
All 15 subjects completed the study. Only 20% (3/15) achieved the primary composite endpoint (SAP reduction by 40% n = 2; SAP decreased to less than 1.5 times the ULN n = 1), p = .03 (Figure 2(a)). While this change was statistically significant, we did not reach our a priori threshold of a 30% response rate. There was no significant difference among baseline clinical features or SAP values among those who did and did not achieve the primary endpoint (data not shown). There was no significant difference between the median SAP times the ULN at baseline and at 12 weeks (Figure 2(b)). Indeed, the median (IQR) percent change in SAP times the ULN was only 1.57% (−31.00% to 8.09%). Assuming a best case scenario and excluding the one outlier who had a 33% increase in SAP times the ULN, the median reduction for the entire cohort was 6.6% (−32.78% to 7.83%). Of the 15 participants, 8 (53.33%) had an increase in SAP while 7 (46.67%) had a reduction in SAP (Figure 2(c)). The median (IQR) percent increase of SAP among those 8 participants was 7.92%(2.72%–15.26%). The median (IQR) percent reduction of SAP among those 7 subjects was 32.00% (16.24%–43.96%) with three individuals achieving greater than a 30% reduction. The single subject who reduced their SAP to less than 1.5 times the ULN had a SAP at baseline and week 12 that was1.76 and 1.47 times the ULN, respectively. None of the participants normalized their SAP.
Figure 2.
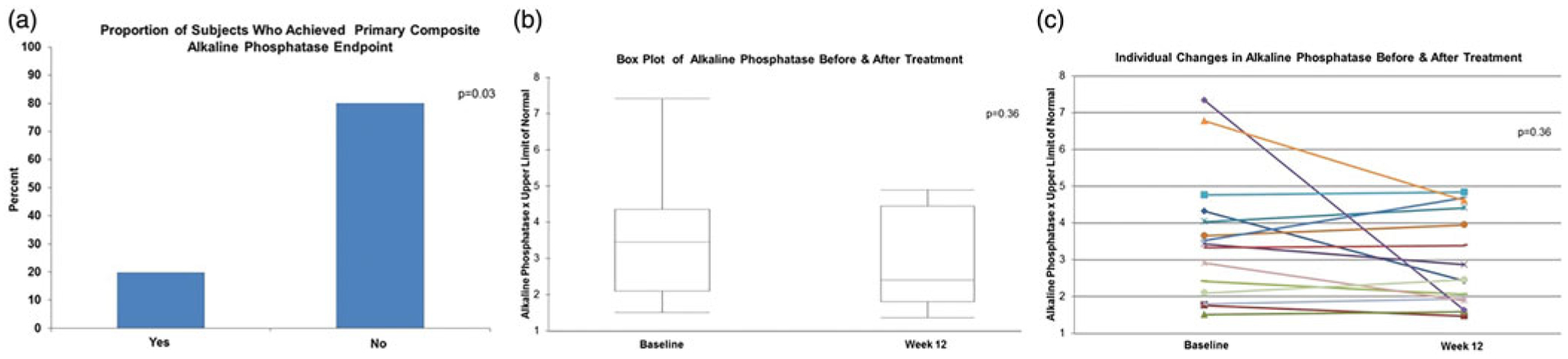
Effect of curcumin on alkaline phosphatase. Box plot of alkaline phosphatase before and after treatment: Peripheral lines indicated maximum and minimum range while box outline reflects interquartile range and horizontal line in box denotes the median.
Regarding the secondary endpoints, there was no significant change in the AST, total bilirubin, CRP (Figure 3(a–c)). Compared to baseline, the week 12 median (IQR) Mayo PSC risk score [0.28 (−0.23–0.69) versus 0.54 (−0.17–0.63), p =.15] and PREsTo score [10.20% (4.15%–19.08%) versus 8.55% (3.55%–18.03%), p = .79] were similar. Twelve of the 15 subjects completed questionnaires. As measured by the 5-D itch and FIS questionnaires, neither itching [10.00(8.00–13.00) versus 8.00 (7.50–14.50), p =.93] or fatigue [6.00(1.00–22.50) versus 8.00 (4.00–23.00), p= .06] improved after 12 weeks of curcumin. There was not a significant correlation between the change in SAP and either the change in 5-D itch score (Spearman correlation −0.39, p=.19) or FIS (spearman correlation −0.45, p=.13).
Figure 3.
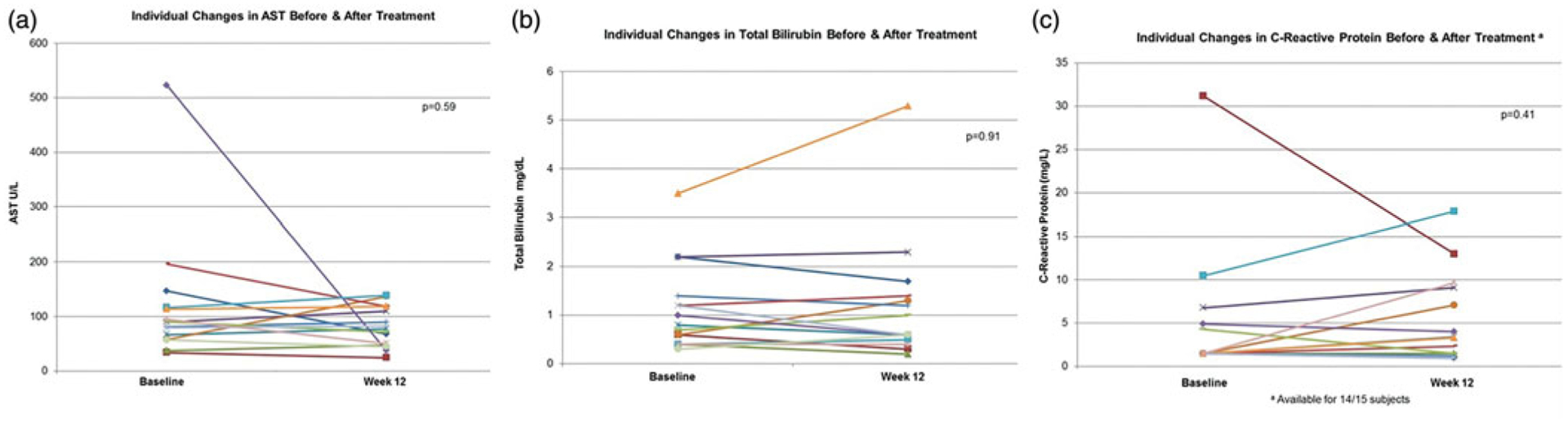
Individual changes of secondary biochemical endpoints.
Curcumin was well tolerated and there were no serious AE. None were in the probably related category and only two subjects reported symptoms that were possibly related to curcumin (nausea and headache). One subject’s bilirubin increased from 3.6 mg/dL at baseline to 5.3 mg/dL at week 12 (Figure 3(b)) due to a recurrent benign biliary stricture and required an ERCP at the end of the study. Otherwise there were no PSC related clinical events that occurred during the 12-week study.
Discussion
This is the first clinical trial to explore the use of curcumin in patients with chronic cholestatic liver disease. In this open label pilot study, curcumin was well tolerated, but we did not achieve the desired 30% response rate as assessed by changes in SAP after 12 weeks of therapy using 750 mg twice daily. There are several lines of preclinical and clinical evidence which have suggested that curcumin could hold promise for the treatment of PSC. In two different cholestasis mouse models, curcumin was associated with improvement in liver enzymes and histology [11,12]. In addition, curcumin was previously found to have anti-proliferative and pro-apoptotic effects in human CCA cell lines [24]. This is relevant in PSC as CCA represents the leading cause of mortality [25]. Despite these preclinical data, we did not find substantive improvement in liver biochemistries. IBD is frequently associated with PSC and curcumin has also been associated with improvement in IBD activity when studied in humans and was well tolerated [7]. There is limited human data concerning the use of curcumin among those with chronic liver disease. However, a prior randomized, placebo-controlled clinical trial examined the use of curcumin 1000 mg/day over an 8-week period among 87 subjects with nonalcoholic fatty liver disease. This study found that curcumin was well tolerated among patients with liver disease and was associated with reductions in serum transaminase levels [8]. Despite using a higher daily dose of curcumin, we did not observe substantive improvements in liver biochemistries.
Twenty percent of participants, rather than our target of 30%, met the composite endpoint. While this was statistically significant, it may not be clinically significant as the median percent change in SAP times the ULN for the entire cohort was less than 2%. By comparison, this is similar to the change in SAP in the placebo group in the recent nor-ursodeoxycholic acid trial [26]. In that same study, 12 weeks of nor-ursodeoxycholic acid trial was associated with a 26% SAP reduction [26]. This suggests that the impact of curcumin on cholestasis is minimal in the majority of patients. If a more lenient percent change in SAP was selected (example, 30% reduction rather than 40%) as part of the composite endpoint, this would have improved our response rate to 27%. However, a 30% threshold for SAP reduction alone has not been associated with clinically significant benefit. Indeed, even the clinical value of 40% SAP reduction has been called into question with a recent study that failed to replicate its prognostic value [17].
Our study also illustrates that a SAP less than 1.5 times the ULN is common among those with PSC. Indeed, 38% of those screened were ultimately excluded on the basis of having a low SAP. This reinforces prior estimates which have suggested that approximately 40% of patients with PSC may have a normal SAP at some point in their disease course [19]. However, the true long-term natural history of SAP fluctuations in PSC remains poorly described and warrants further study. In future studies, the limitation of SAP fluctuations could be attenuated by requiring a maximum threshold for SAP variation at enrollment. This could be quantified by obtaining at least 2 SAP measurements over a predefined period of time. Additional study would be required to determine an acceptable threshold for SAP variation. Multiple studies have suggested a lower SAP is associated with improved outcomes [17–20]. However, there are also several lines of evidence to suggest SAP as a prognostic marker is suboptimal. For example, a proportion of individuals with a normal SAP may still develop cirrhosis and liver-related complications [27]. In addition, the treatment group in the high-dose UDCA trial had an increase in adverse liver-related endpoints despite a reduction in SAP [28]. Conversely, many patients with an elevated SAP will not develop an endpoint over prolonged follow up. For example, 60% of patients with PSC and a SAP greater than 1.5 times the ULN did not develop liver related endpoints despite 10 years of follow up [18]. Whilst SAP is regularly used as a method to stratify PSC patients and serve as a surrogate endpoint in clinical trials, the above observations highlight the shortcomings of SAP for these indications and reinforce the need for improved biomarkers [21]. This is particularly important for drug development, as outcomes of interest such as death, liver transplantation, and hepatic decompensation occur infrequently except over a prolonged time period.
In the future, routinely incorporating exploratory biomarkers in early phase randomized controlled clinical trials will be important to improve our understanding of the biology of the study drug, natural fluctuations in candidate biomarkers during the disease course and their prognostic significance. Similarly, a more refined and sophisticated strategy toward surrogate endpoint selection based on the mechanism of action of the study drug is rationale and pragmatic. For example, an anti-fibrotic therapeutic trial could enroll PSC patients with a normal SAP level and examine changes in hepatic collagen content, enhanced liver fibrosis (ELF) score, liver stiffness or histology.
This study has several limitations. First, it was an open-label study without a placebo group. Consequently, it is unclear if the changes in the SAP among the subset of individuals who had a SAP reduction were actually related to the curcumin or simply related to the natural fluctuations of the disease. Second, the length of the pilot study was only 12 weeks (similar to most PSC pilot studies) and it is unclear if a more prolonged course of curcumin would have resulted in SAP improvements among those who had some SAP reduction. Third, we were only able to study one dose of curcumin at 750 mg orally twice a day and are unable to determine if a higher dose may have enhanced efficacy. Fourth, we did not objectively assess for clinical or endoscopic changes in IBD after curcumin exposure. Therefore, we are unable to comment about the potential efficacy of curcumin at this dose among those with IBD. Last, we were not able to examine the impact of curcumin on other exploratory biomarkers. The exploration of new biomarkers that have potential to serve as surrogate endpoints in future studies is important in larger randomized control trials. However, the impact of doing so in small, single arm studies such as this is limited and challenging to interpret in the absence of a control group. Indeed, our understanding of the natural fluctuations in biomarkers such as ELF, liver stiffness, bile acids or cytokine levels and their prognostic significance is limited. Consequently, it would be difficult to know if the present study was adequately powered to detect a meaningful change in a particular biomarker.
In conclusion, the use of curcumin 750 mg twice daily is well tolerated among those with PSC but does not appear to be effective at improving biochemical markers of cholestasis or symptoms. These results suggest it would be low yield to move forward with a larger randomized placebo-controlled trial using curcumin at this dose. Individuals with PSC often have a low SAP and improved methods of risk stratifying subjects with PSC and enhanced biomarkers beyond SAP are needed.
Acknowledgements
The authors would like to thank the patients who participated in this study.
Funding
Funding for study provided by EuroPharma. EuroPharma was not involved in data collection/analysis or drafting of the manuscript.
ABBREVIATIONS
- PSC
Primary sclerosing cholangitis
- IBD
inflammatory bowel disease
- CCA
cholangiocarcinoma
- SAP
serum alkaline phosphatase
- ULN
upper limit of normal
- UDCA
ursodeoxycholic acid
- CRP
c-reactive protein
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- INR
international normalized ratio
- FIS
fatigue impact scale
- AE
adverse events
- PREsTo
PSC risk estimate tool
- IQR
interquartile range
- ELF
enhanced liver fibrosis
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Trial registration number: NCT 02978339
References
- [1].Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. [DOI] [PubMed] [Google Scholar]
- [3].Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. [DOI] [PubMed] [Google Scholar]
- [4].Aggarwal BB, Sundaram C, Malani N, et al. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. [DOI] [PubMed] [Google Scholar]
- [5].Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. [DOI] [PubMed] [Google Scholar]
- [6].Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. [DOI] [PubMed] [Google Scholar]
- [7].Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444–1449 e1. [DOI] [PubMed] [Google Scholar]
- [8].Panahi Y, Kianpour P, Mohtashami R, et al. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg). 2017;67:(4): 244–251. [DOI] [PubMed] [Google Scholar]
- [9].Hu RW, Carey EJ, Lindor KD, et al. Curcumin in hepatobiliary disease: pharmacotherapeutic properties and emerging potential clinical applications. Ann Hepatol. 2017;16:835–841. [DOI] [PubMed] [Google Scholar]
- [10].Farzaei MH, Zobeiri M, Parvizi F, et al. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. 2018;10:pii: E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baghdasaryan A, Claudel T, Kosters A, et al. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang F, Tang X, Ding L, et al. Curcumin protects ANIT-induced cholestasis through signaling pathway of FXR-regulated bile acid and inflammation. Sci Rep. 2016;6:33052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010; 51:660–678. [DOI] [PubMed] [Google Scholar]
- [14].Antony B, Merina B, Iyer VS, et al. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Elman S, Hynan LS, Gabriel V, et al. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lundgren-Nilsson A, Tennant A, Jakobsson S, et al. Validation of Fatigue Impact Scale with various item sets - a Rasch analysis. Disabil Rehabil. 2017;41:1–7. [DOI] [PubMed] [Google Scholar]
- [17].Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–1301. [DOI] [PubMed] [Google Scholar]
- [18].Al Mamari S, Djordjevic J, Halliday JS, et al. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2012;58:329–334. [DOI] [PubMed] [Google Scholar]
- [19].Stanich PP, Bjornsson E, Gossard AA, et al. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lindstrom L, Hultcrantz R, Boberg KM, et al. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841–846. [DOI] [PubMed] [Google Scholar]
- [21].Ponsioen CY, Chapman RW, Chazouilleres O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–1367. [DOI] [PubMed] [Google Scholar]
- [22].Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75: 688–694. [DOI] [PubMed] [Google Scholar]
- [23].Eaton JE, Vesterhus M, McCauley BM, et al. Primary sclerosing cholangitis risk estimate tool (PREsTo) predicts outcomes in PSC: a derivation & validation study using machine learning. Hepatology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prakobwong S, Gupta SC, Kim JH, et al. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32:1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. [DOI] [PubMed] [Google Scholar]
- [26].Fickert P, Hirschfield GM, Denk G, et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. [DOI] [PubMed] [Google Scholar]
- [27].Balasubramaniam K, Wiesner RH, LaRusso NF. Primary sclerosing cholangitis with normal serum alkaline phosphatase activity. Gastroenterology. 1988;95:1395–1398. [DOI] [PubMed] [Google Scholar]
- [28].Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]


