Abstract
Poor patient adherence to antiretroviral medication represents a major obstacle for managing disease and reducing rates of new HIV infections. The measurement of patient drug levels is the most objective method of determining adherence. Tenofovir and tenofovir diphosphate are metabolites of some of the most common HIV medications for treatment and prevention and can be quantified by mass spectrometry. Here, we report the development of a competitive enzyme linked immunoassay as a simplified approach for detecting tenofovir and tenofovir diphosphate. Monoclonal antibodies were produced by two tenofovir-hapten conjugates and screened for binding to immobilized tenofovir, and then for competition by tenofovir and tenofovir diphosphate. Antibody specificity was evaluated against adenosine phosphates, which are close structural analogs. We performed numerical simulations of reaction equilibrium to guide assay optimization. When used to evaluate spiked tenofovir in plasma and spiked tenofovir diphosphate in red blood cell lysate, the optimized assay had high sensitivity and specificity.
Keywords: HIV, adherence, PrEP, antiretroviral therapy (ART), tenofovir, immunoassay
Graphical Abstract
Human immunodeficiency virus (HIV) remains a leading cause of mortality worldwide.1 Oral antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) are highly effective for suppressing viral replication and reducing the risk of HIV acquisition, respectively. Tenofovir disoproxil fumarate (TDF) is common to both ART and PrEP regimens: the World Health Organization recommends ART regimens containing tenofovir (TFV) for first-line therapy, and TDF is the most commonly used HIV drug worldwide.2,3 Tenofovir alafenamide is an alternative formulation of TFV which was recently approved for PrEP. Oral PrEP regimens containing TFV have been shown to be safe and effective at reducing the risk of HIV acquisition in adults.4,5 Altogether, more than 16 million people are currently taking regimens containing TFV for ART, and more than 350 thousand people have been initiated on PrEP.6
Adequate adherence to the once-a-day drug regimen is critically important for ART and PrEP efficacy.7,8 Rates of adherence can vary widely between populations, but roughly one-quarter of people on ART and perhaps more than one-half of people on PrEP do not maintain drug adherence.9,10 Although many adherence-promoting interventions are known to be effective, universal deployment is not feasible in most populations.11,12 In addition to increased risk of poor outcomes, low adherence also affects treatment and prevention initiatives. Viral rebound due to lack of adherence may be clinically indistinguishable from drug resistance, in which case costly second-line regimens must be prescribed.13 Furthermore, rates of drug-resistant HIV mutations are maximized by low or irregular drug dosing.14
Direct measurement of patient drug levels is the most objective and accurate metric of adherence as compared to indirect methods such as self-reporting, pill counting, and electronic medication monitoring systems.15–17 TFV and tenofovir diphosphate (TFV-DP) are two metabolites of TDF which exhibit different pharmacokinetics (Figure 1A). TFV in the plasma is cleared by the kidneys with a half-life of about 10 to 20 h but can also diffuse into cells, where it is phosphorylated to form TFV-DP.18,19 TFV-DP that has accumulated in red blood cells (RBCs) has a half-life of 17 days, and in peripheral blood mononuclear cells, of 4 days.20 Immunoassays for urine TFV have recently been developed as alternatives to liquid chromatography/mass spectrometry for clinical adherence monitoring by drug level measurement.21,22 Although analytically accurate, these tests are hampered by the quick washout period of TFV from the urine. A urine TFV adherence monitoring test can identify those with nonzero TFV exposure in the past 1 to 2 days, but this may have limited correlation with long-term adherence.23 In contrast to plasma or urine TFV, the TFV-DP concentration in red blood cells is highly correlated with average dosing.20,24 Competitive immunoassays are a mature and proven approach in rapid diagnostic test development compared with biosensor alternatives.25 Therefore, we sought to develop immunoassays to measure both TFV and TFV-DP in plasma and RBCs toward the long-term goal of developing a rapid diagnostic test.
Figure 1.

(A) Adherence to Tenofovir disoproxil fumarate can be determined from concentrations of TFV (in plasma, half-life ~15 h) and/or TFV-DP (in red blood cells, half-life ~17 days). TFV-DP is cleared more slowly, so the concentration is better correlated with the long-term average dosing rate. (B) Tenofovir conjugates used in this study.
Monoclonal antibodies were produced against TFV-conjugated proteins by mouse immunization. Two TFV-protein conjugation schemes were developed, denoted as TFV-PO and TFV-NH (Figure 1B). Two different orientations of the TFV molecule were used, one with a linker and one without, to maximize the diversity of produced antibodies and improve the likelihood of generating a highly specific hit. TFV-PO was conjugated via the phosphonate to lysine residues on the carrier proteins, while TFV-NH was conjugated via the primary amine to available carboxylic acids. TFV-PO contained a three-carbon linker, while TFV-NH had no linker. For mouse immunization, conjugates were linked to keyhole limpet hemocyanin (KLH) as an antigen. The resulting monoclonal antibodies were screened by a indirect enzyme-linked immunosorbent assay (ELISA) for (a) sensitivity for both immobilized TFV conjugates, (b) specificity against immobilized adenosine monophosphate (AMP) and adenosine triphosphate (ATP), which are close structural analogs of TFV and TFV-DP, (c) specificity against human transferrin antigen, (d) antibody isotype determination. Altogether, development yielded eight antibody candidates from TFV-NH and four candidates from TFV-PO.
The relative ELISA signals of the candidate antibody binding to TFV-PO and TFV-NH were compared to identify suitable antibody-conjugate pairs for competitive ELISA (Figure 2). Seven of the eight antibodies produced by TFV-NH-KLH were bound to immobilized TFV-NH-BSA, but none of these were bound to immobilized TFV-PO-biotin. All four antibodies produced by TFV-PO-KLH were bound to immobilized TFV-PO-biotin, and two of them were also bound to immobilized TFV-NH-BSA as well.
Figure 2.

Screening of antibodies by indirect ELISA for binding to immobilized TFV-NH-BSA and TFV-PO-biotin. None of the antibodies produced by TFV-NH were bound to immobilized TFV-PO-biotin. Two of the four antibodies produced by TFV-PO were bound to TFV-NH-BSA.
The resulting 13 antibody-conjugate hits were next evaluated by competitive ELISA for inhibition by either free TFV or free TFV-DP. Antibodies were premixed with either free TFV or TFV-DP and then transferred to well plates containing immobilized TFV conjugates (Figures S2–S4). We found that none of the antibody-conjugate hits using antibodies raised against TFV-NH were inhibited by even the highest concentrations of free TFV or TFV-DP, indicating that the antibodies were specific for the TFV-NH moiety and therefore unsuitable for use in a competitive immunoassay. Conversely, all six hits containing antibodies produced by TFV-PO-KLH were inhibited by both TFV and TFV-DP (Figures S2 and S3). The inhibition standard curves for the four TFV-PO antibody hits were repeated in triplicate, and the half-maximum inhibitory concentration (IC50) was measured in each case by a four-parameter Hill equation (Figure S5, summarized in Table 1). Overall, antibodies 1H11, 2B5, and 8B7 were found to have the best sensitivity for TFV, and antibodies 2B5 and 8B7 were found to have the highest sensitivity for TFV-DP.
Table 1.
Half-Maximum Inhibitory Concentrations of the Four Best Antibodies for TFV, TFV-DP, AMP, ADP, and ATP
| IC50, μM | |||||
|---|---|---|---|---|---|
| antibody | TFV | TFV-DP | AMP | ADP | ATP |
| 1H11 | 0.48 | 5.0 | >10 000 | >10 000 | >10 000 |
| 2B5 | 0.53 | 1.7 | 65 | 110 | 80 |
| 8B7 | 0.33 | 1.0 | 430 | 940 | 680 |
| 10A7 | 2.8 | 9.6 | 990 | >10 000 | >10 000 |
Next, we evaluated nonspecific inhibition of the four leading candidates by free adenosine monophosphate (AMP), adenosine diphosphate (ADP), and adenosine triphosphate (ATP). Assays were conducted identically as with TFV and TFV-DP but with much higher concentrations of up to 10 mM. 1H11, 8B7, and 10A7 exhibited very good specificity against all three free molecules (Figure S5). Altogether, antibody 1H11 was found to have the greatest specificity for TFV and TFV-DP relative to adenosine phosphates (Figure 3).
Figure 3.

(A) Standard curves of antibody 1H11 for TFV (IC50 = 0.48 μM) and TFV-DP (IC50 = 1.4 μM). (B) Standard curves of 1H11 for adenosine monophosphate (AMP, IC50 > 2 mM) and adenosine triphosphate (ATP, IC50 > 10 mM).
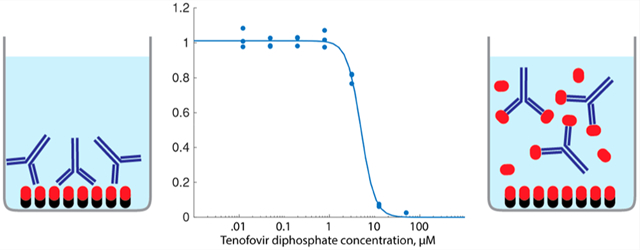
As summarized in Table 1, the IC50 values for TFV and TFV-DP in the screening studies were in the range of 0.5–10 μM. However, the clinical concentration range for these metabolites is 15–170 nM for TFV in plasma and 75–850 nM for TFV-DP in whole blood.20,26 We developed a numerical model to solve for antibody binding at equilibrium to explore the effects of concentration and affinity on the operating range and blank signal of the competitive immunoassay (Figure S6). Results of simulations showed that the IC50 can be decreased by decreasing the amount of immobilized antigen or by reducing the relative affinity of the antibody for the immobilized antigen with respect to the free analyte. Also, the optimal antibody concentration is equal to the effective concentration of immobilized antigen. Finally, the operating range cannot be decreased below the dissociation constant (Kd) of the antibody-free analyte complex. At analyte concentrations below Kd, a majority of antibody binding sites remain unfilled regardless of antibody concentration, so free analyte will not compete with the immobilized antigen for binding sites. On the basis of these findings, we lowered the operating range of the 1H11 immunoassay by switching from TF-PO-biotin to TFV-NH-BSA as the immobilized antigen and immobilizing less antigen on the microwell plates. Together, these modifications reduced the IC50 in buffer for TFV by about 80-fold and that for TFV-DP by about 30-fold (no buffer and no lysate conditions, Figure 4). This corresponded to a 4-fold lower maximum blank signal, indicating that the rest of this improvement was due to 1H11 having a lower affinity for TFV-NH-BSA than for TFV-PO-biotin.
Figure 4.

(A) Detection of TFV in plasma using antibody 1H11 and immobilized TFV-NH. (B) Detection of TFV-DP in blood cell lysate using the same. Ht 2.5% corresponds to a 40:1 final dilution of the cell pellet.
We tested the assay with antibody 1H11 for the detection of spiked TFV in plasma and spiked TFV-DP in RBC lysate. For TFV detection, plasma was added to the primary incubation volume in amounts of up to 50% v/v (Figure 4a). Increasing amounts of plasma blocked 1H11 binding to immobilized TFV-NH-BSA in the absence of TFV and also increased the nonspecific signal. We anticipate that the nonspecific signal was caused by the nonspecific adsorption of human immunoglobulins to the plate since the antimouse secondary antibody was not cross-adsorbed against human immunoglobulins. Nonspecific binding and decreased signals were both rescued by diluting the plasma in water at least 20-fold. Dilution by 1:20 and 1:200 both resulted in an IC50 value similar to that of the blank sample. The effective IC50 for a 1:100 plasma dilution was estimated to be 600 nM, which was at least 4-fold higher than the clinical range of TFV in plasma (15–170 nM).
For TFV-DP detection, blood cells were pelleted and lysed by surfactant and then added to the primary incubation volume in quantities of up to 2.5% v/v (i.e., 40-fold final dilution of the cell pellet, Figure 4b). Antibody 1H11 binding was inhibited by increasing the amounts of lysate to up to 2.5% effective hematocrit (Ht), where binding was reduced by about half. The IC50 values of 0.625% Ht and 1.25% Ht dilutions (197 nM and 183 nM, respectively) were comparable to the no lysate control (150 nM). For the 1.25% Ht case, this corresponds to a concentration in 40% Ht blood of 5.9 μM. This is at least 7-fold higher than the clinical range of 75–850 nM.
Tenofovir monophosphate (TFV-MP) is a metabolic intermediate formed by the phosphorylation of TFV, which is then itself phosphorylated to yield TFV-DP. In vitro studies of TFV metabolism in peripheral blood mononuclear cells led to the conclusion that intracellular TFV-MP is rapidly converted to TFV-DP by endogenous nucleotide diphosphate kinases.27,28 These candidate antibodies may also bind TFVMP. However, antibody binding to TFV-MP was not tested since TFV-DP is the most abundant intracellular compound and will therefore contribute the most to the signal in either case.
Adenosine phosphates are present over a wide range of concentrations in bodily fluids and are highly concentrated within cells. In RBCs, ATP and ADP concentrations are usually about 1500 and 800 μM, respectively.29,30 AMP is less prevalent, with concentrations about 20–100 μM in RBCs.30 Likewise, deoxyadenosine phosphates are only about 1 μM in blood.31 In contrast, the total concentration of adenosine phosphates in the plasma is 10–20 μM.32 On the basis of these numbers, the total concentration of adenosine phosphates in RBCs is typically 2 to 3 mM. Competition by these compounds may have contributed to the reduced ELISA signal observed at increasing concentrations of RBC lysate.
Because the antibodies are not specific for TFV or TFV-DP, RBCs must be separated from the plasma before lysis to quantify intracellular TFV-DP independently of plasma TFV. Plasma separation membranes which filter blood cells are currently used in commercial rapid diagnostic tests, and we envision that this technology could be adapted to perform the sample preparation required for this assay. This approach may allow simultaneous tests on each compartment, in which both plasma TFV and intracellular TFV-DP are measured from the same sample to provide information on both short- and long-term adherence.
In summary, we have developed antibodies which have high specificity for TFV and TFV-DP to meet the expanding clinical need for monitoring adherence to HIV treatment and prevention. The leading antibody candidate was used to develop reproducible competitive immunoassays for TFV and TFV-DP. On the basis of insights from a qualitative numerical model of the assay, we increased the sensitivity to the necessary operating ranges for both analytes. Matrix effects from plasma and RBC lysate significantly reduced assay performance. Dilutions of both matrixes by approximately 1:100 were required to fully recover assay function. As a result, the effective operating ranges of both assays remained at least 5- to 10-fold higher than the desired clinical range. For this reason, we did not evaluate the assay on patient samples.
Assay performance can likely be improved through the optimization of reagents and sample preparation methods. Assay-specific optimization of reaction conditions such as pH, ionic content, and surfactants is expected to improve sensitivity and reproducibility in less-dilute samples. Likewise, the candidate antibodies can be directly conjugated to reporter enzymes, particles, or fluorophores to improve sensitivity and reduce nonspecific background signal. The optimized assay can then be tested with PrEP patient samples to assess the utility of the test. We envision that this optimized assay could then be translated to a lateral flow format to enable the rapid point-of-care assessment of both short- and long-term drug adherence.
METHODS
Small-Molecule Conjugates.
TFV-PO-biotin (chemical structure in Figure S1) was synthesized by New England Discovery Partners (Branford, CT). A conjugate purity of >99% was confirmed by high-performance liquid chromatography (Figure S7). TFV-PO-KLH and TFV-PO-BSA were synthesized by first linking the phosphonate to a succinimidyl ester via a three-carbon linker (chemical structure Figure S8), followed by EDC coupling to primary amines on the protein. TFV-NH-BSA and TFV-NH-KLH were synthesized by Rockland Immunochemicals (Limerick, PA) by direct EDC coupling of the primary amine on the tenofovir adenine to carboxylic acids on the protein. AMP-biotin (5′-Biotin-AMP no. N-6004, TriLink Biotechnologies, San Diego, CA) and ATP-biotin (bio-17-ATP no. ENZ-32817, Enzo Life Sciences, Framingdale, NY) were purchased commercially.
Antibody Production.
Mouse monoclonal antibody production and primary screening were performed by ImmunoPrecise Antibodies LTD (Victoria, BC, Canada) and Ponoco Rabbit Farm & Laboratory (Canadensis, PA). Animal handling protocols were conducted under protocols approved by the Institutional Review Board of Massachusetts General Hospital. Four BALB/c mice were immunized with TFV-NH-KLH, and four were immunized with TFV-PO-KLH following the ImmunoPrecise RapidPrime method over 19 days. Lymphocytes were isolated and fused to SP2/0 myeloma partner cells by poly(ethylene glycol) fusion. Successful hybridomas were isolated following incubation in semisolid HAT selection medium. This resulted in 1632 (17 × 96 well plates) successful hybridomas from TFV-NH-KLH and 384 (4 plates) from TFV-PO-KLH. Hybridomas were screened by indirect ELISA on their antigens, yielding 49 hits on TFV-NH-KLH and 51 hits on TFV-PO-KLH. Hits were screened on respective BSA-conjugated TFV-NH and TFV-PO, yielding the eight candidates produced by TFV-NH and the four produced by TFV-PO which were selected for further testing.
Indirect ELISA Screening.
To immobilize TFV-NH-BSA, 96-well plates (Nunc MaxiSorp, ThermoFisher Scientific) were incubated with 50 μL of 5 μg/mL TF-NH-BSA in 100 mM carbonate buffer at pH 9.5, overnight at 4 C. Later, the amount of immobilized TFV was reduced by instead immobilizing 50 μL of 1 μg/mL TFV-NH-BSA for 2 h at room temperature. Plates were washed twice with PBS with 0.05% Tween-20 (wash buffer) and blocked with 200 μL of PBS containing 1% BSA (blocking buffer) for 2 h at room temperature. TFV-PO-biotin was similarly immobilized on preblocked streptavidin-coated high-capacity 96-well plates (Pierce no. 15500, ThermoFisher Scientific): 50 μL of 1 μg/mL TFV-PO-biotin was incubated overnight in blocking buffer at 4 C. Plates were then incubated with antibodies at 1 μg/mL in blocking buffer for 30 min with gentle agitation at room temperature. Plates were then washed four times and incubated with 50 μL of 0.5 μg/mL horseradish peroxidase conjugated goat antimouse IgG/IgM polyclonal secondary antibody (ThermoFisher Scientific no. A10677) for 30 min in blocking buffer and likewise with agitation at room temperature. This concentration of secondary antibody was found to be the highest concentration which did not result in a detectable amount of nonspecific binding following a 30 min incubation in blocking buffer (data not shown). The relative reactivity of the HRP-conjugated polyclonal secondary antibody with respect to each candidate antibody was measured by directly adsorbing antibodies onto microwell plates following the same protocol as used for the adsorption of TFV-NH-BSA described above, followed by 0.5 μg/mL secondary antibody incubation (Figure S9). HRP activity was measured by the addition of 50 μL of tetramethylbenzidine solution (Ultra TMB, ThermoFisher Scientific no. 34028) and measuring absorbance at 405 nm every 20 s for up to 30 min. Absorbance traces were fitted with an exponential decay function using MATLAB (Mathworks, Natick MA) to determine the initial reaction rate (slope) at t = 0. Differences in secondary antibody affinity for the different candidate antibodies were measured by direct ELISA: candidate antibodies were nonspecifically adsorbed at 5 μg/mL for 2 h on MaxiSorp plates, followed by blocking and secondary incubation as described above (Figure S4). Indirect ELISA signals were normalized by these values to control for these differences.
Competitive ELISA.
For competitive ELISA, primary antibodies were first preincubated with antigens (Tenofovir, Sigma-Aldrich no. PHR1592; Tenofovir diphosphate, BOC Sciences, Shirley, NY; adenosine monophosphate, Sigma-Aldrich no. A2252; adenosine diphosphate, Sigma-Aldrich no. A2754; adenosine triphosphate, Sigma-Aldrich no. A26209) in blocking buffer for 10 min in a low-adhesion well plate (Corning Costar, Sigma-Aldrich no. CLS3471). Mixtures were then transferred to the TFV-coated plate prepared as described above. Likewise, secondary antibody incubation and quantification were performed as described above. Standard curves were fitted using MATLAB with a four-parameter Hill equation , where S0, τ, n, and b are the fitted baseline signal, IC50, Hill coefficient, and background signal, respectively.
Simulations of Equilibrium Reactions.
Numerical simulations of binding were performed in MATLAB. The code is provided in the Supporting Information. The model had three molecular species: an immobilized antigen, a free analyte, and a monovalent binding agent (antibody). The resulting system has five equations and five unknowns. The equations are three mass conservation equations (one for each species) and two first-order Hill equilibrium equations of the form (one for the antibody-analyte complex and one for the antibody-immobilized antigen complex). The unknowns are the concentrations of each molecular species and complex.
Plasma and Red Blood Cell Lysate Sample Preparation.
Human blood components from healthy donors were acquired by vendors under protocols approved by the Institutional Review Board of Massachsuetts General Hospital. For TFV detection in plasma, frozen unfiltered pooled human plasma (BioIVT, Westbury, NY) was thawed and used to spike the primary incubation mix directly. For TFV-DP detection in RBC lysate, fresh human whole blood collected in EDTA tubes (Research Blood Components, Watertown, MA) was used the same day. For the ADP binding screen, refrigerated packed RBCs (Valley Biomedical, Winchester, VA) were procured and refrigerated for less than 1 week before use. Whole blood (500 μL) was 6000 rcf for 5 min. The supernatant was discarded, and the cell pellet was gently resuspended in PBS. The wash was repeated a second time. The cell pellet volume was estimated from the resuspended volume and supernatant volume. The second pellet was resuspended 1:1 in lysate buffer (PBS + 1% Triton X-100) and vortex mixed for 10 s, and hemolysis was visually confirmed by the loss of turbidity. This 1:1 dilution was considered to be 50% hematocrit.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ayokunle Olanrewaju, Jane Zhang, and Jonathan Posner for helpful discussions and graciously sharing reagents; Tim Cressey for helpful feedback and advice; Teri Otto for work on antibody generation; Octavio Hurdato for laboratory guidance; and Jenny Nesbitt for providing RBCs. This work was supported by the NIH NIAID (R01AI136648).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00010.
Chemical structures of TFV-PO-biotin and TFV-NH-NHS; HPLC characterization of TFV-PO-biotin; inhibition standard curves of TFV for antibody binding to immobilized TFV-NH and/or TFV-PO; inhibition standard curves of AMP, ADP, ATP, TFV, and TFV-DP for antibodies 2B5, 8B7, and 10A6 binding to immobilized TFV-PO; source code and results of simulations of binding for the reaction equilibrium model of competitive immunoassay; and the reactivity of antimouse/HRP secondary antibody with respect to candidate antibodies (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acsinfecdis.0c00010
Contributor Information
Derin Sevenler, Center for Engineering in Medicine, Massachusetts General Hospital, Boston, Massachusetts 02139, United States.
Ashley Bardon, Departments of Global Health, Schools of Medicine and Public Health and Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington 98195, United States.
Marta Fernandez Suarez, Daktari Diagnostics, Inc, Cambridge, Massachusetts 02140, United States.
Lisa Marshall, Daktari Diagnostics, Inc, Cambridge, Massachusetts 02140, United States.
Mehmet Toner, Center for Engineering in Medicine, Massachusetts General Hospital, Boston, Massachusetts 02139, United States.
Paul K. Drain, Departments of Global Health, Schools of Medicine and Public Health, Department of Epidemiology, School of Public Health, and Department of Medicine, School of Medicine, University of Washington, Seattle, Washington 98195, United States
Rebecca D. Sandlin, Center for Engineering in Medicine, Massachusetts General Hospital, Boston, Massachusetts 02139, United States
REFERENCES
- (1).Frank TD, Carter A, Jahagirdar D, Biehl MH, Douwes-Schultz D, Larson SL, Arora M, Dwyer-Lindgren L, Steuben KM, Abbastabar H, Abu-Raddad LJ, Abyu DM, Adabi M, Adebayo OM, Adekanmbi V, Adetokunboh OO, Ahmadi A, Ahmadi K, Ahmadian E, Ahmadpour E, Ahmed MB, Akal CG, Alahdab F, Alam N, Albertson SB, Alemnew BTT, Alene KA, Alipour V, Alvis-Guzman N, Amini S, Anbari Z, Anber NH, Anjomshoa M, Antonio CAT, Arabloo J, Aremu O, Areri HA, Asfaw ET, Ashagre AF, Asmelash D, Asrat AA, Avokpaho EFGA, Awasthi A, Awoke N, Ayanore MA, Azari S, Badawi A, Bagherzadeh M, Banach M, Barac A, Bärnighausen TW, Basu S, Bedi N, Behzadifar M, Bekele BB, Belay SA, Belay YB, Belayneh YM, Berhane A, Bhat AG, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Bitew H, Blinov A, Bogale KA, Bojia HA, Burugina Nagaraja SBN, Butt ZA, Cahuana-Hurtado L, Campuzano Rincon JC, Carvalho F, Chattu VK, Christopher DJ, Chu D-T, Crider R, Dahiru T, Dandona L, Dandona R, Daryani A, das Neves J, De Neve J-W, Degenhardt L, Demeke FM, Demis AB, Demissie DB, Demoz GT, Deribe K, Des Jarlais D, Dhungana GP, Diaz D, Djalalinia S, Do HP, Doan LP, Duber H, Dubey M, Dubljanin E, Duken EE, Duko Adema B, Effiong A, Eftekhari A, El Sayed Zaki M, El-Jaafary SI, El-Khatib Z, Elsharkawy A, Endries AY, Eskandarieh S, Eyawo O, Farzadfar F, Fatima B, Fentahun N, Fernandes E, Filip I, Fischer F, Folayan MO, Foroutan M, Fukumoto T, Fullman N, Garcia-Basteiro AL, Gayesa RT, Gebremedhin KB, Gebremeskel GGG, Gebreyohannes KK, Gedefaw GA, Gelaw BK, Gesesew HA, Geta B, Gezae KE, Ghadiri K, Ghashghaee A, Ginindza TTG, Gugnani HC, Guimarães RA, Haile MT, Hailu GB, Haj-Mirzaian A, Haj-Mirzaian A, Hamidi S, Handanagic S, Handiso DW, Hanfore LK, Hasanzadeh A, Hassankhani H, Hassen HY, Hay SI, Henok A, Hoang CL, Hosgood HD, Hosseinzadeh M, Hsairi M, Ibitoye SE, Idrisov B, Ikuta KS, Ilesanmi OS, Irvani SSN, Iwu CJ, Jacobsen KH, James SL, Jenabi E, Jha RP, Jonas JB, Jorjoran Shushtari Z, Kabir A, Kabir Z, Kadel R, Kasaeian A, Kassa B, Kassa GM, Kassa TD, Kayode GA, Kebede MM, Kefale AT, Kengne AP, Khader YS, Khafaie MA, Khalid N, Khan EA, Khan G, Khan J, Khang Y-H, Khatab K, Khazaei S, Khoja AT, Kiadaliri AA, Kim YJ, Kisa A, Kisa S, Kochhar S, Komaki H, Koul PA, Koyanagi A, Kuate Defo B, Kumar GA, Kumar M, Kuupiel D, Lal DK, Lee JJ-H, Lenjebo TL, Leshargie CT, Macarayan ERK, Maddison ER, Magdy Abd El Razek H, Magis-Rodriguez C, Mahasha PW, Majdan M, Majeed A, Malekzadeh R, Manafi N, Mapoma CC, Martins-Melo FR, Masaka A, Mayenga ENL, Mehta V, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Memish ZA, Mena AT, Mendoza W, Mengistu DT, Mengistu G, Meretoja TJ, Mestrovic T, Miller TR, Moazen B, Mohajer B, Mohamadi-Bolbanabad A, Mohammad KA, Mohammad Y, Mohammad Darwesh A, Mohammad Gholi Mezerji N, Mohammadi M, Mohammadibakhsh R, Mohammadoo-Khorasani M, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Moodley Y, Moossavi M, Moradi G, Moradi-Lakeh M, Moschos MM, Mossie TB, Mousavi SM, Muchie KF, Muluneh AG, Muriithi MK, Mustafa G, Muthupandian S, Nagarajan AJ, Naik G, Najafi F, Nazari J, Ndwandwe DE, Nguyen CT, Nguyen HLT, Nguyen SH, Nguyen TH, Ningrum DNA, Nixon MR, Nnaji CA, Noroozi M, Noubiap JJ, Nourollahpour Shiadeh M, Obsa MS, Odame EA, Ofori-Asenso R, Ogbo FA, Okoro A, Oladimeji O, Olagunju AT, Olagunju TO, Olum S, Oppong Asante KOA, Oren E, Otstavnov SS, Pa M, Padubidri JR, Pakhale S, Pakpour AH, Patel SK, Paulos K, Pepito VCF, Peprah EK, Piroozi B, Pourshams A, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafay A, Rafiei A, Rahim F, Rahimi-Movaghar A, Rahimi-Movaghar V, ur Rahman S, Ranabhat CL, Rawaf S, Reis C, Renjith V, Reta MA, Rezai MS, Rios González CM, Roro EM, Rostami A, Rubino S, Saeedi Moghaddam S, Safari S, Sagar R, Sahraian MA, Salem MRR, Salimi Y, Salomon JA, Sambala EZ, Samy AM, Sartorius B, Satpathy M, Sawhney M, Sayyah M, Schutte AE, Sepanlou SG, Seyedmousavi S, Shabaninejad H, Shaheen AA, Shaikh MA, Shallo SA, Shamsizadeh M, Sharifi H, Shibuya K, Shin JI, Shirkoohi R, Silva DAS, Silveira DGA, Singh JA, Sisay MMM, Sisay M, Sisay S, Smith AE, Sokhan A, Somayaji R, Soshnikov S, Stein DJ, Sufiyan MB, Sunguya BF, Sykes BL, Tadesse BT, Tadesse DB, Tamirat KS, Taveira N, Tekelemedhin SW, Temesgen HD, Tesfay FH, Teshale MY, Thapa S, Tlaye KG, Topp SM, Tovani-Palone MR, Tran BX, Tran KB, Ullah I, Unnikrishnan B, Uthman OA, Veisani Y, Vladimirov SK, Wada FW, Waheed Y, Weldegwergs KG, Weldesamuel GTT, Westerman R, Wijeratne T, Wolde HF, Wondafrash DZ, Wonde TE, Wondmagegn BY, Yeshanew AG, Yilma MT, Yimer EM, Yonemoto N, Yotebieng M, Youm Y, Yu C, Zaidi Z, Zarghi A, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Naghavi M, Vollset SE, Wang H, Lim SS, Kyu HH, and Murray CJL (2019) Global, Regional, and National Incidence, Prevalence, and Mortality of HIV, 1980–2017, and Forecasts to 2030, for 195 Countries and Territories: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 6, e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).WHO | Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Oragnization, 2016. Accessed 10/15/2019. [PubMed] [Google Scholar]
- (3).WHO | Update of Recommendations on First- and Second-Line Antiretroviral Regimens; 2019. Accessed 10/15/2019.
- (4).Hodges-Mameletzis I, Dalal S, Msimanga-Radebe B, Rodolph M, and Baggaley R (2018) Going Global: The Adoption of the World Health Organization’s Enabling Recommendation on Oral Pre-Exposure Prophylaxis for HIV. Sex Health 15 (6), 489–500. [DOI] [PubMed] [Google Scholar]
- (5).Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, Chiamwongpaet S, Kitisin P, Natrujirote P, Kittimunkong S, Chuachoowong R, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Hendrix CW, and Vanichseni S (2013) Antiretroviral Prophylaxis for HIV Infection in Injecting Drug Users in Bangkok, Thailand (the Bangkok Tenofovir Study): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 381 (9883), 2083–2090. [DOI] [PubMed] [Google Scholar]
- (6).2018 HIV Market Report; Clinton Health Access Initiative, 2018. Accessed 11/18/2019.
- (7).Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapía M, Montoya-Herrera O, Veloso VG, Mayer KH, Chariyalertsak S, Schechter M, Bekker L-G, Kallás EG, Grant RM, and Team F (2012) the iPrEx S. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci. Trans. Med 4 (151), 151ra125–151ra125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, and Celum C (2014) HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. JAIDS, J. Acquired Immune Defic. Syndr 66 (3), 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, and Bangsberg DR (2006) Adherence to Antiretroviral Therapy in Sub-Saharan Africa and North America: A Meta-Analysis. JAMA 296 (6), 679–690. [DOI] [PubMed] [Google Scholar]
- (10).Sidebottom D, Ekström AM, and Strömdahl S (2018) A Systematic Review of Adherence to Oral Pre-Exposure Prophylaxis for HIV - How Can We Improve Uptake and Adherence? BMC Infect. Dis 18 (1), 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Marcus JL, Buisker T, Horvath T, Amico KR, Fuchs JD, Buchbinder SP, Grant RM, and Liu AY (2014) Helping Our Patients Take HIV Pre-Exposure Prophylaxis (PrEP): A Systematic Review of Adherence Interventions. HIV Med 15 (7), 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, and Newell M-L (2011) Interventions to Increase Antiretroviral Adherence in Sub-Saharan Africa: A Systematic Review of Evaluation Studies. Lancet Infect. Dis 11 (12), 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).WHO | Global Action Plan on HIV Drug Resistance 2017–2021; World Health Oragnization, 2017. Accessed 11/13/2019. [Google Scholar]
- (14).Bangsberg DR, Moss AR, and Deeks SG (2004) Paradoxes of Adherence and Drug Resistance to HIV Antiretroviral Therapy. J. Antimicrob. Chemother 53 (5), 696–699. [DOI] [PubMed] [Google Scholar]
- (15).Stekler JD, Scanlan JM, Simoni JM, Crane HM, Fredericksen RJ, Marquard J, and Saver BG (2018) Predictors of Art and PrEP Adherence and Medication-Taking Practices and Preferences to Inform Development of a Wrist-Worn Adherence System. AIDS Education and Prevention 30 (5), 357–368. [DOI] [PubMed] [Google Scholar]
- (16).Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, Aikens JE, Hunter CM, Velligan DI, Huntley K, Ogedegbe G, Rand CS, Schron E, and Nilsen WJ (2015) Self-Report Measures of Medication Adherence Behavior: Recommendations on Optimal Use. Transl Behav Med 5 (4), 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bell KM, and Haberer JE (2018) Actionable Adherence Monitoring: Technological Methods to Monitor and Support Adherence to Antiretroviral Therapy. Curr. HIV/AIDS Rep 15 (5), 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fonsart J, Saragosti S, Taouk M, Peytavin G, Bushman L, Charreau I, Hance A, Goldwirt L, Morel S, Mammano F, Loze B, Capitant C, Clavel F, Mahjoub N, Meyer L, Anderson PL, Delaugerre C, and Molina J-M (2017) Single-Dose Pharmacokinetics and Pharmacodynamics of Oral Tenofovir and Emtricitabine in Blood, Saliva and Rectal Tissue: A Sub-Study of the ANRS IPERGAY Trial. J. Antimicrob. Chemother 72 (2), 478–485. [DOI] [PubMed] [Google Scholar]
- (19).Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, Clayton C, Austin G, Palmer BE, Zheng J-H, Klein B, Kerr BJ, Guida LA, Rower C, Rower JE, Kiser JJ, Bushman LR, MaWhinney S, and Anderson PL (2016) Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res. Hum. Retroviruses 32 (10–11), 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Castillo-Mancilla JR, Zheng J-H, Rower JE, Meditz A, Gardner EM, Predhomme J, Fernandez C, Langness J, Kiser JJ, Bushman LR, and Anderson PL (2013) Tenofovir, Emtricitabine, and Tenofovir Diphosphate in Dried Blood Spots for Determining Recent and Cumulative Drug Exposure. AIDS Res. Hum. Retroviruses 29 (2), 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pratt GW, Fan A, Melakeberhan B, and Klapperich CM (2018) A Competitive Lateral Flow Assay for the Detection of Tenofovir. Anal. Chim. Acta 1017, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gandhi M, Bacchetti P, Rodrigues WC, Spinelli M, Koss CA, Drain PK, Baeten JM, Mugo NR, Ngure K, Benet LZ, Okochi H, Wang G, and Vincent M (2018) Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinicalMedicine 2–3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Spinelli MA, Glidden DV, Rodrigues WC, Wang G, Vincent M, Okochi H, Kuncze K, Mehrotra M, Defechereux P, Buchbinder SP, Grant RM, and Gandhi M (2019) Low Tenofovir Level in Urine by a Novel Immunoassay Is Associated with Seroconversion in a Preexposure Prophylaxis Demonstration Project. AIDS 33 (5), 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L, Anderson PL, Flynn R, Lee DP, Bolan RK, Jordan WC, Tseng CH, Dierst-Davies R, Rooney J, and Wohl AR (2017) Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. JAIDS, J. Acquired Immune Defic. Syndr 76 (5), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Olanrewaju AO, Sullivan BP, Zhang JY, Bender AT, Sevenler D, Lo TJ, Fernandez-Suarez M, Drain PK, and Posner JD (2019) An Enzymatic Assay to Measure Long-Term Adherence to Pre-Exposure Prophylaxis and Antiretroviral Therapy. bioRxiv, 832410. [Google Scholar]
- (26).Drain PK, Kubiak RW, Siriprakaisil O, Klinbuayaem V, Quame-Amaglo J, Sukrakanchana P-O, Tanasri S, Punyati P, Sirirungsi W, Cressey R, Bacchetti P, Okochi H, Baeten JM, Gandhi M, and Cressey TR (2020) Urine Tenofovir Concentrations Correlate With Plasma and Relate to Tenofovir Disoproxil Fumarate Adherence: A Randomized, Directly Observed Pharmacokinetic Trial (TARGET Study). Clin. Infect. Dis 70, 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Robbins BL, Srinivas RV, Kim C, Bischofberger N, and Fridland A (1998) Anti-Human Immunodeficiency Virus Activity and Cellular Metabolism of a Potential Prodrug of the Acyclic Nucleoside Phosphonate 9-R-(2-Phosphonomethoxypropyl)Adenine (PMPA), Bis(Isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother 42 (3), 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kearney BP, Flaherty JF, and Shah J (2004) Tenofovir Disoproxil Fumarate. Clin. Pharmacokinet 43 (9), 595–612. [DOI] [PubMed] [Google Scholar]
- (29).Ataullakhanov FI, and Vitvitsky VM (2002) What Determines the Intracellular ATP Concentration. Biosci. Rep 22 (5), 501–511. [DOI] [PubMed] [Google Scholar]
- (30).Traut TW (1994) Physiological Concentrations of Purines and Pyrimidines. Mol. Cell. Biochem 140 (1), 1–22. [DOI] [PubMed] [Google Scholar]
- (31).Chen X, Castillo-Mancilla JR, Seifert SM, McAllister KB, Zheng J-H, Bushman LR, MaWhinney S, and Anderson PL (2016) Analysis of the Endogenous Deoxynucleoside Triphosphate Pool in HIV-Positive and -Negative Individuals Receiving Tenofovir-Emtricitabine. Antimicrob. Agents Chemother 60 (9), 5387–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Harkness RA, Simmonds RJ, and Coade SB (1983) Purine Transport and Metabolism in Man: The Effect of Exercise on Concentrations of Purine Bases, Nucleosides and Nucleotides in Plasma, Urine, Leucocytes and Erythrocytes. Clin. Sci 64 (3), 333–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


