Abstract
Traditional Chinese medicines played an important role in the treatment of COVID-19 in 2020. Ephedra sinica, one of the major constituent herbs of multi-component herbal formula, has been widely used to treat COVID-19 in China. However, its active components are still unclear. The objectives of this study are to screen and evaluate active components from the traditional Chinese medicine Ephedra sinica for the treatment of COVID-19. In our study, we established an ACE2/CMC bioaffinity chromatography model, and then developed an ACE2/CMC-HPLC-IT-TOF-MS system for the active compounds screening and identification from Ephedra sinica extract. We performed molecular docking and surface plasmon resonance (SPR) assays to assess the binding characteristics (binding mode and KD value). We used CCK-8 staining to assess the toxicity of screened compounds, and also used SARS-CoV-2 pseudovirus to observe the viropexis effect of screened compounds in ACE2h cells. In this current work, one fraction was fished out, separated and identified as ephedrine (EP), pseudoephedrine (PEP), and methylephedrine (MEP). Binding assays showed that the three compounds could bind with ACE2 in a special way to some amino acid residues, similar to the way SARS-CoV-2 bound with ACE2. Additionally, the three compounds, especially EP, can inhibit the entrance of SARS-CoV-2 spike pseudovirus into ACE2h cells because they can reduce the entrance ratio of pseudovirus in the pseudovirus model. Overall, the ACE2/CMC-HPLC-IT-TOF-MS system was established and verified to be suitable for ACE2-targeted bioactive compound screening. EP, PEP, and MEP with ACE2-binding features were screened out from Ephedra sinica, and acted as blockers inhibiting SARS-CoV-2 spike pseudovirus entering ACE2h cells.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00216-021-03233-7.
Keywords: ACE2 receptor, Cell membrane chromatography, Ephedra sinica, COVID-19
Introduction
In December 2019, a new type of coronavirus pneumonia began to appear in Wuhan, Hubei [1]. On February 11, 2020, the WHO released the official name of the disease caused by the new coronavirus infection as “COVID-19” (coronavirus disease 19). On the same day, the International Committee on Taxonomy of Viruses (ICTV) named the new coronavirus SARS-CoV-2. Until now, the virus is still raging around the world, especially in some western countries, which is still spreading, threatening human health. Since the pandemic, Chinese medicine has been widely used and played an important role in the prevention and treatment of COVID-19 [2]. In the multiple versions of the “New Coronavirus Infection Pneumonia Diagnosis and Treatment Strategies (Trial)” promulgated by the National Health Commission of the People’s Republic of China, traditional Chinese medicines (TCMs) have been recommended for the treatment of COVID-19 [3, 4].
Since the first discovery of penicillin in 1929, more and more antibiotics have been discovered and applied to the treatment of clinical diseases, which has played a major role in human history. However, due to the particularity of the virus structure, there is still a lack of specific antiviral drugs. In the face of constantly emerging new types of viruses that threaten human life and health, we urgently need to develop new drugs with high efficacy, specificity, and low toxicity. TCM has a unique curative effect on viral infectious diseases in historical epidemics. In recent years, TCM has had remarkable curative effects in anti-influenza virus [5], hepatitis B virus [6], respiratory syncytial virus [7], adenovirus [8], etc., and has made great contributions to national health. Since the outbreak of COVID-19 pneumonia, TCM formulae, such as Qing-Fei-Pai-Du-Tang, have significant effects on the treatment of COVID-19 [9–11].
Drug research and development is a multidisciplinary engineering, and the discovery of a lead compound with novel structure, significant activity, and clear mechanism is a key point in the process of drug research and development [12, 13]. Research on the material basis of TCMs is an important source for the discovery of new drugs. Finding a research method suitable for the material basis of the complex system of TCMs has always been a key issue in TCM research [14]. There are generally two approaches: one is to isolate and screen the crude extracts to find the active part, then to separate the active part and identify the structure of the compound to screen the activity. The second is to start with the absorption, distribution, metabolism, and excretion process of the compound in vivo, and use chromatography or other methods to analyze, separate, and identify complex samples such as urine, plasma, and tissue homogenate, and find out the active compounds [15]. In the process of screening the active ingredients of TCMs, the continuous development of chromatography technology, especially bioaffinity chromatography technology, has played an important role [16].
Coronavirus is irregular in shape, and the outside is wrapped by a membrane containing three glycoproteins on the surface. Among them, a certain receptor-binding domain (RBD) in the spike protein can specifically recognize angiotensin-converting enzyme 2 (ACE2), which makes the ACE2 receptor an important target for drug design and development. Cell membrane chromatography (CMC) technology is a type of bioaffinity chromatography technology invented by professor Langchong He and his team in 1996. Besides its separation ability, CMC also has biological activity. It has been proved to be an effective technology for screening target components from complex systems [17–19].
Ephedra sinica (“Ma-huang” in Chinese Name Pinyin System) has been widely used in traditional Chinese medicine for the treatment of common cold, coughs, asthma, and edema for thousands of years [20, 21]. It was also included in several classical Chinese medicine formulae that have been reported to be efficacious on COVID-19 [11]. No specific analytical method with screening and pharmacological evaluation for this herb has been established; hence, it is of utmost importance to develop more approaches to meet the urgent need. In this work, we established an ACE2/CMC model and combine it with the high-resolution mass spectrometry LCMS-IT-TOF to form a two-dimensional online system for “Screening-analyzing-identifying” effective antiviral components from Ephedra sinica methanol extract (EME), then evaluate the potential antiviral activity of screened constituents both at the molecular level and at the cell level, finally provide ACE2-targeted candidate drugs for the prevention and treatment of COVID-19.
Materials and methods
Chemicals and reagents
Acetonitrile (ACN) and methanol (MeOH) were HPLC grade and purchased from Fisher Scientific (Pittsburgh, PA). Ultrapure water was prepared on a MK-459 Millipore Milli-Q Plus ultrapure water system (Darmstadt, Germany). Ephedrine hydrochloride (purity up to 100%), pseudoephedrine hydrochloride (purity up to 99.8%), and methylephedrine hydrochloride (purity up to 99.6%) were kindly provided by the national institutes for food and drug control. Hydroxychloroquine (HCQ, purity of 98%) was provided by Energy Chemical (Shanghai, China). Ephedra sinica was obtained from the traditional Chinese medicine store (Xi’an, China). The receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 (SARS-CoV-2 RBD, purity >95% as determined by SDS-PAGE) and human ACE2 protein (purity >95% as determined by SDS-PAGE) were supplied by Sino Biological Inc. (Beijing, China). All reagents were used without further purification.
ACE2h cell culture
ACE2 over-expressed HEK293T cell line (ACE2h cell line) was constructed and verified by Genomeditech (Shanghai, China) and was cultured in DMEM with high glucose containing 10% PBS, 1% penicillin-streptomycin, and 4 μg/mL puromycin; cells were seeded in a flask and kept at 37 °C in a 5% CO2 incubator. Cells from the exponentially growing culture were used in all experiments.
System validation of the ACE2/CMC online HPLC-IT-TOF-MS system
Diagram of ACE2/CMC model and ACE2/CMC online HPLC-IT-TOF-MS system development are shown in Supplementary Information (ESM) Fig. S1. Preparation procedure of ACE2/CMC is shown in ESM; briefly, ACE2h cells (1 × 107 cells) were collected, washed, and broken, and cell membrane was separated, mixed with pre-activated silica to generate cell membrane stationary phase, which was then packed into standard column (10 × 2.0 mm I.D.) to prepare ACE2/CMC column; HPLC system equipped with ACE2/CMC column was used as the first dimension for compound screening, HPLC-IT-TOF-MS system was used as the second dimension for fraction separation and identification. The two dimensions were combined by a two-position 10-port switching valve (VICI Valco Instrument Co. Inc., Houston, TX, USA) and two C18 enrichment columns (RP-18C, 10 × 4.6 mm, Merck, Germany). The coupled online system was verified by analyzing HCQ through the two dimensions [22]. HCQ was first injected into the ACE2/CMC column, then enriched on the pre-column, and finally switched to the HPLC-IT-TOF-MS for identification. Chromatography features and mass spectrometry characteristics were recorded for further assessment.
Instrument configuration and chromatographic conditions
Chromatographic conditions for the 1st dimension were as follows: HPLC (Shimadzu Corporation, Kyoto, Japan) equipped with ACE2/CMC column, ultrapure water as the mobile phase with flow rate at 0.2 mL/min, column was kept at 37 °C in a CTO-20A column oven, the detector wavelength for HCQ was set at 254 nm, injection volume was 5 μL.
The second dimension included an HPLC system coupled with an ion trap-time of flight mass spectrometer (Shimadzu Corporation, Kyoto, Japan) equipped with an electrospray ionization source. Chromatographic conditions for the 2nd dimension: Inertsil ODS-3 (150 × 4.6 mm, 3 μm, Shimadzu) was used as the analytical column for separation; 0.1% formic acid water solution (A)-acetonitrile (B) mixed mobile phase was used with gradient elution set as follows: 0–40 min, 5%–100% B; 40-50 min, 100% B; 50–50.01 min, 100%–5% B; 50.01–70 min, 5% B. Flow rate was 1 mL/min; column temperature was 30 °C; the detector wavelength was set at 254 nm. The optimal parameters for mass spectrometry were as follows: ESI source, nebulizer gas (N2, purity >99.999%) flow rate, 3 L/min; drying gas (N2, purity >99.999%) pressure, 109 kPa. Both curve desolvation line and heat block temperatures were 200 °C. Interface voltage was 4.5 kV; detector voltage, 1.57 kV; positive ionization scan mode with scan range at m/z 100–1000.
Application of the coupled two-dimensional system
In this study, the validated two-dimensional ACE2/CMC-HPLC-IT-TOF-MS system was then used to screen ligands of ACE2 from EME. After EME sample analysis, the corresponding standard compound mixture of the retained fractions identified by the online system was analyzed again for further verification. Both standards and EME sample were repeatedly analyzed five times on the same column, and also on three batches of columns, the retention time was recorded for further assessment.
Surface plasmon resonance experiments
Surface plasmon resonance (SPR) test was carried out to assess interaction strength between the bioactive compounds and human ACE2 (His-tag)/SARS-CoV-2 RBD (His-tag). Target protein was immobilized on a carboxyl sensor chip (Nicoya Lifesciences, Canada) to a level of around 500 response units using Open SPR™ (Nicoya Lifesciences, Canada) and a running buffer of PBS with flow rate of 20 μL/s. Serial dilutions of the three small molecules were flowed through with a concentration ranging from 12.5 to 100 μM, and the chip was regenerated with hydrochloric acid (pH 2.0). The resulting data were fit to a 1:1 binding model and analyzed by TraceDrawer software.
Molecular docking assay
To determine the binding mode of the screened bioactive compounds with ACE2 protein, the molecular docking assay was performed by the Surflex-dock module in SYBYL-X 2.0 version. Prior to docking, the structure file of human ACE2 was acquired from PDB.org website (PDB ID: 6M0J). The 3D structures of targets were constructed with Sybyl/Sketch module. Tripos force field with convergence criterion of 0.005 kcal/(Åmol), Gasteiger-Hückel charges, and a maximum of 1000 iterations were used for energy minimization. A nonbonded cutoff distance of 5.0 Å was utilized to consider the intramolecular interaction.
Cell viability assay
Cell viability was determined followed by the manufacturer’s instructions. Briefly, ACE2h cells were seeded into 96-well plates at a concentration of 5 × 103 cells/well, treated with different concentrations of screened compounds, and then incubated for 24 h. A volume of 10 μL Cell Counting Kit solution was added to each well followed by 2 h’s incubation. The optical density (OD) of each well was determined by a microplate reader (Bio-Rad Instruments, USA) under 450 nm and expressed as absorbance values. Relative cell viability was calculated using the following equation:
Intracellular Ca2+ mobilization assay
ACE2h cells were seeded into 96-well plates (5 × 103 cells/well) for incubation. Cells were washed twice with CIB after 24 h. The incubation buffer was then added and incubated for staining; 40 min later, the dyed cells were washed with CIB and then immediately imaged under the blue light of a fluorescence microscope. The analyte solutions (200 μL, dissolved in CIB) were added into each well at 10 s after initial imaging. Responses were monitored at 1-s intervals for a total of 120 s.
Detection of SARS-CoV-2 spike pseudovirus entry into ACE2h cells
In this assay, ACE2h cells (1 × 106 cells/mL) were seeded into 96-well plates, 50 μL per well, and cultured with DMEM in an incubator set at 37 °C and containing 5% CO2 for 2 h. A volume of 25 μL medium was removed carefully and the same volume of tested compound dissolved in medium was added and incubated for 2 h. Five microliters of SARS-CoV-2 spike pseudovirus (Sino Biological, PSV001) was added followed by 4 h’s incubation, and then, 100 μL of complemented DMEM was added. After 6–8 h’s further infection, all the culture medium containing virus was removed and cultured in 200 μL of fresh medium for 48 h. Luciferase Assay System (Promega™ E1500) was used to assess the entrance of virus, briefly, cell culture lysis reagent was added to each well after the medium was removed, then with the luminescence solution added, chemiluminescence was detected by a microplate reader at 560 nm.
Statistical analysis
All the acquired data was first analyzed for normality by the Shapiro-Wilk test. The statistical comparisons were performed with Student’s t test or one-way ANOVA with 2-sided Dunnett’s test, using SPSS PASW Statistics 18.0 software. Data were presented as the mean ± SD. Statistical significance value was set at P < 0.05.
Results and discussion
Three components (EP, PEP, and MEP) were screened out from Ephedra sinica
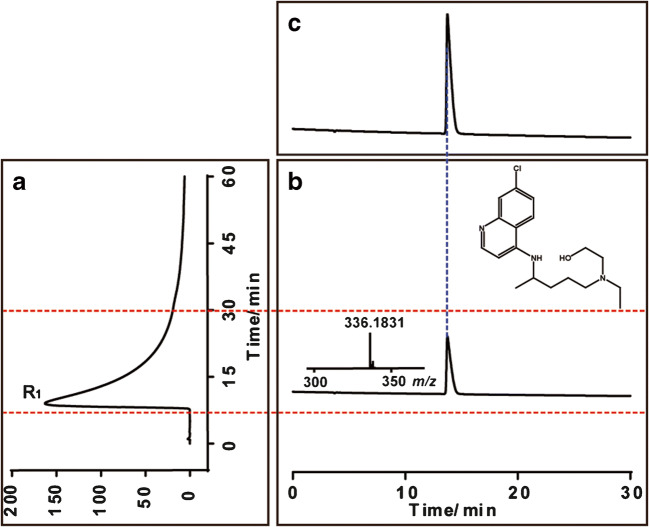
A new two-dimensional combination method was established and was applied for screening active constituents from TCMs. In this work, an ACE2/CMC-HPLC-IT-TOF-MS system was developed and verified. First, the 1st dimension ACE2/CMC was constructed and then coupled with HPLC-IT-TOF-MS system; after this, the whole two-dimensional system was verified by HCQ; results are shown in Fig. 1; here, HCQ can be well retained by ACE2/CMC (Fig. 1a), and subsequently identified by the 2nd dimension (Fig. 1b), also showed same chromatographic and MS features as the results acquired from direct analysis by HPLC-IT-TOF-MS alone (Fig. 1c). This provided a good verification that the established method is capable of screening ACE2-targeted active components.
Fig. 1.
Validation of the ACE2/CMC-HPLC-IT-TOF-MS system by hydroxychloroquine. (a) Chromatogram of hydroxychloroquine retained on the ACE2/CMC column; (b) HPLC-IT-TOF-MS chromatogram of the retained fraction (R1); (c) Direct HPLC-IT-TOF-MS chromatogram of hydroxychloroquine
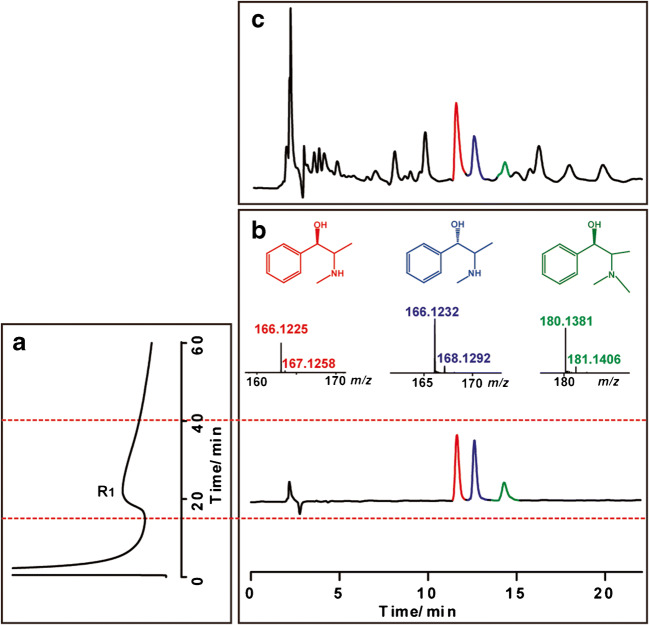
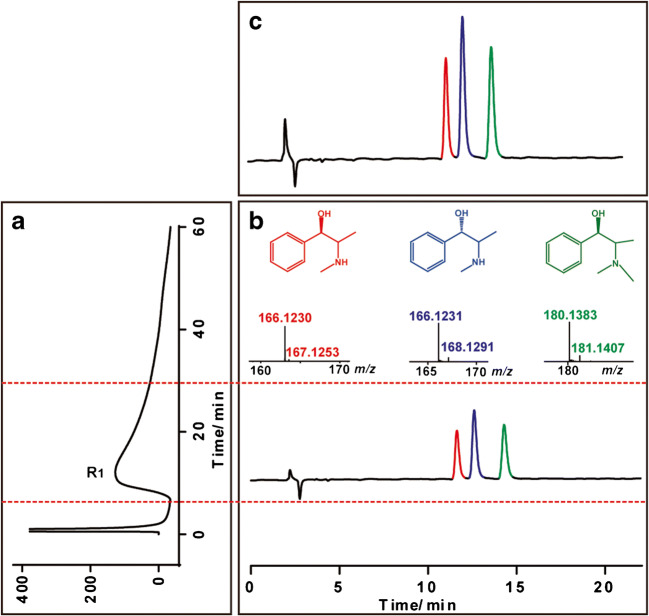
Then, we applied this method to discover the effective components in the EME that act on the ACE2 receptor; as shown in Fig. 2, we observed an active fraction binding to the ACE2 receptor was screened from the EME and further identified as three effective compounds, namely EP, PEP, and MEP (hereinafter EPs). For further verification of the screened-and-identified results, we prepared a standard mixture of EPs, then perform all the procedure using this method, and get similar results including CMC features, HPLC retention time, and MS characteristics (Fig. 3). All of these provided strong evidence that EPs from EME are active on ACE2 receptor.
Fig. 2.
Application of the coupled two-dimensional system. (a) Chromatogram of EME retained on the CMC column. (b) Total ion current chromatogram of the retained fraction (R1) on the HPLC-IT-TOF-MS system. (c) Direct total ion current chromatogram of EME on the HPLC-IT-TOF-MS system
Fig. 3.
Chromatograms of the standard solutions of EP, PEP and MEP analyzed by ACE2/CMC coupled with HPLC-IT-TOF-MS system. (a) Chromatogram of standard solutions retained on the ACE2/CMC column. (b) HPLC-IT-TOF-MS chromatogram of the retained fraction (R1). (c) Direct HPLC-IT-TOF-MS chromatogram of the standard solutions
We searched literatures and books for the extraction method of Ephedra sinica. Xin et al. pipetted methanol containing 0.1% formic acid onto the tip surface for DI-MS (direct ionization-mass spectrometry)-based metabolomics analysis of three species of medicinal Ephedrae Herba [23]. Lee et al. tested the aqueous and methanol extracts of EH, and found methanol extract shown to be effective and safe to hyperlipidaemic mice [24]; Lv et al. extracted Ephedra sinica with methanol/water (80:20, v/v) for 30 min at room temperature for component analysis [25]. The Chinese Pharmacopoeia also presented a standard method for several alkaloids quantification as follows: Ephedra sinica were extracted by 1.44% aqueous phosphate solution, and alkaloids were quantified by HPLC method with a mixture solution of methanol, aqueous phosphate, and other adding reagents as the mobile phase. Additionally, methanol extraction combined with TLC separation method was used for the identification of Ephedrae Radix et Rhizoma (the root of Ephedra sinica) [26]. In our study, EP, PEP, and MEP were confirmed to have antiviral effect; in our preliminary study for extraction solution for Ephedra sinica (see ESM Fig. S2), we adopted both methanol and aqueous phosphate according to literatures and the Chinese Pharmacopoeia; HPLC results of methanol extract showed little difference with aqueous phosphate extract, but a slightly high intensity of the three main components (peaks 1, 2, and 3). Hence, we selected methanol as the extraction solvent for the sample preparation.
Binding characteristics of EPs with ACE2 and SARS-CoV-2 RBD protein
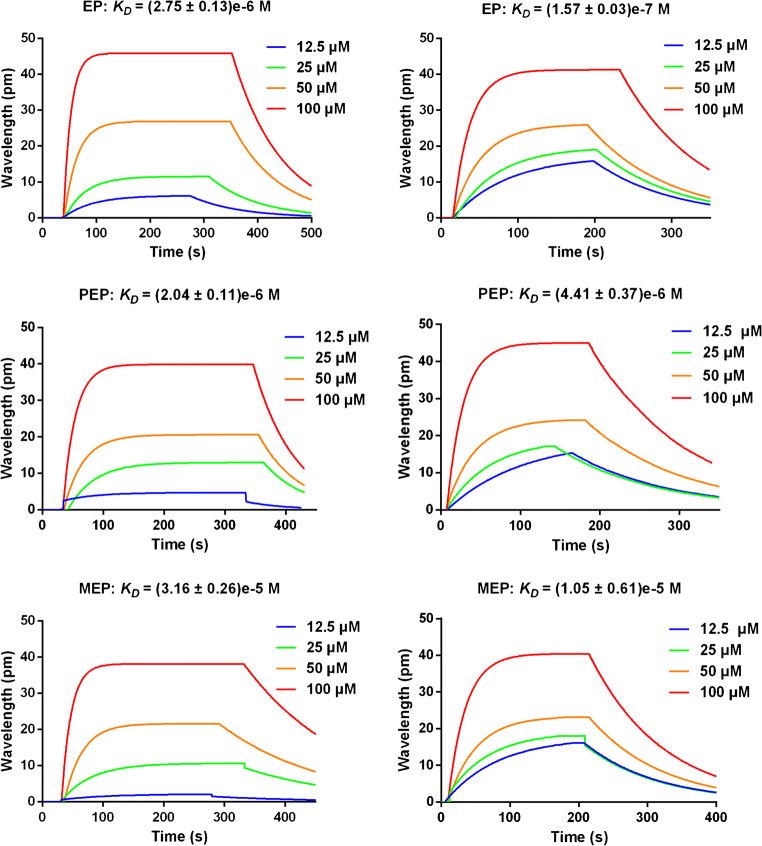
Since EPs can bind to ACE2, and have potential for the prevention and treatment of COVID-19, how strong is the binding force? We tested KD values between EPs and human ACE2 or SARS-CoV-2 RBD with SPR assays (Fig. 4). From the binding curves of EPs on the left, we observed a binding capacity between EPs and human ACE2 with the KD values in 10−6 M level, results also confirmed the co-elution of EPs from CMC column. From the binding curves of EPs on the right column, we noticed that EP showed a stronger binding affinity with SARS-CoV-2 RBD than PEP and MEP behaved; this revealed a potential double interaction of EPs both on ACE2 and on SARS-CoV-2 RBD protein.
Fig. 4.
Measurement of binding affinities between the three compounds (EP, PEP and MEP) and human ACE2 (left column) or SARS-CoV-2 RBD (right column) by surface plasmon resonance assay. Purified recombinant SARS-CoV-2 RBD or recombinant human ACE2 were covalently immobilized to the sensor chip via their amine groups, and EPs flowed by. Here EPs was diluted to different concentrations (from 12.5 to 100 μM) before being injected. Data are shown as different colored lines
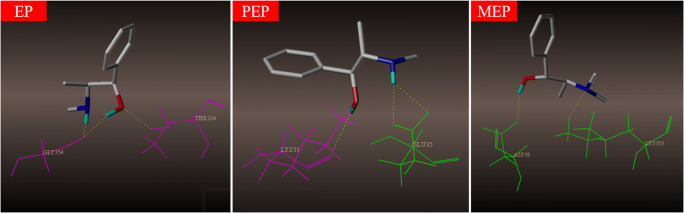
Binding mode of EPs with human ACE2
Results of binding characteristics demonstrated interactions between EPs and human ACE2, which sites does they interact with each other is not clear, hence, we explored the binding mode between the three small molecules and human ACE2 by performing molecular docking assay, as shown in Fig. 5, amino acid residues in green (GLU35 in PEP, ASP38, and LYS353 in MEP) presented that the ACE2-binding sites of EPs are identical to that of SARS-CoV-2 RBD reported [27, 28], and amino acid residues in purple (GLY354 and THR 324 in EP, LYS31 in MEP) revealed some new ACE2-binding sites. From the docking results of EPs, we noticed that MEP showed two amino acid residues binding sites similar to SARS-CoV-2 RBD on ACE2; as for EP and PEP, some new binding sites were observed; this indicated a potential different interaction mode with SARS-CoV-2 RBD protein on ACE2.
Fig. 5.
Structural details at the interface between the three compounds (EP, PEP, and MEP) and human ACE2 (PDB ID: 6M0J). Amino acid residues in green present the same ACE2-binding sites with SARS-CoV-2 RBD reported. Amino acid residues in purple present the different ACE2-binding sites with SARS-CoV-2 RBD
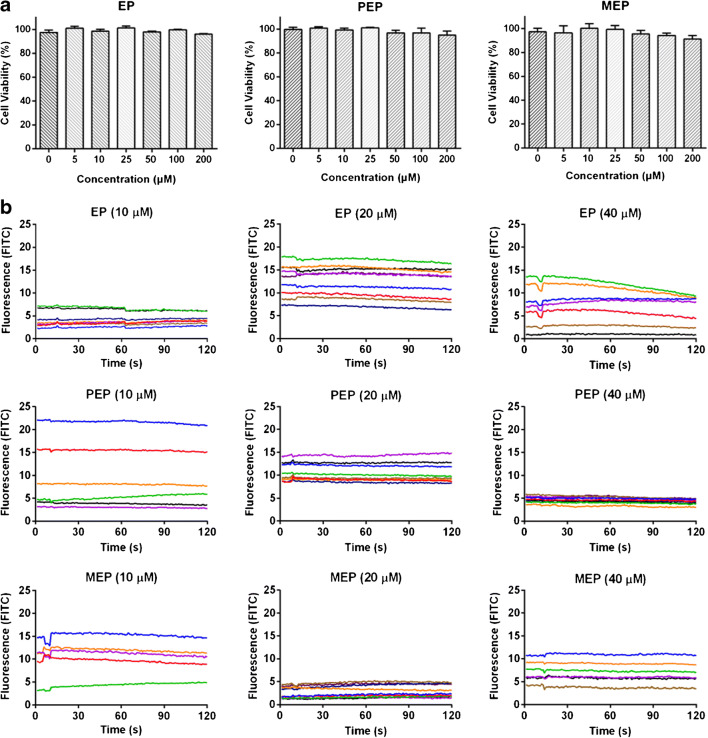
Effect of EPs on ACE2h cell viability
We then investigated the effect of EPs on the viability of ACE2h cells, as shown in Fig. 6a. EP, PEP, and MEP had no significant effect on the activity of ACE2h cells even under a high concentration of 200 μM, which indicated that EPs showing little cytotoxicity towards ACE2h cells under the selected concentration. In several cell signaling pathways, Ca2+ acts as an essential second messenger; therefore, we assessed the calcium flux change in ACE2h cells; as shown in Fig. 6b, we noticed EPs rarely cause calcium flux change in ACE2h cells.
Fig. 6.
Effect of EP, PEP, or MEP on the viability of ACE2h cells. (a) Viability of ACE2h cells treated with EPs for 24 h. (b) Calcium flux change in ACE2h cells treated with EPs
The screened EPs suppressed the entrance of SARS-CoV-2 spike pseudovirus into ACE2h cells
The pseudovirus relies on the spike protein to infect the target cell, and simulate the virus infection process; therefore, we used pseudovirus model as a standard to evaluate the antiviral activity of compounds in vitro. In this part, ACE2h cells infected only with SARS-CoV-2 spike pseudovirus were set as the control group, and the luciferase luminescence value was defined as 100%. After treatment with EP, PEP, and MEP at the same dosage of 20 μM, the entrance ratio of pseudovirus was reduced to 71% ± 14.28%, 69% ± 18.29%, and 86% ±11.84%, respectively (Fig. 7). While for EP, the ratio was reduced to 53% ± 2.47% at the concentration of 100 μM. Pseudovirus results indicated that all the three compounds can block SARS-CoV-2 from entering the ACE2 receptor because they can reduce the entrance ratio of pseudovirus into the pseudovirus model, showing antiviral effect.
Fig. 7.
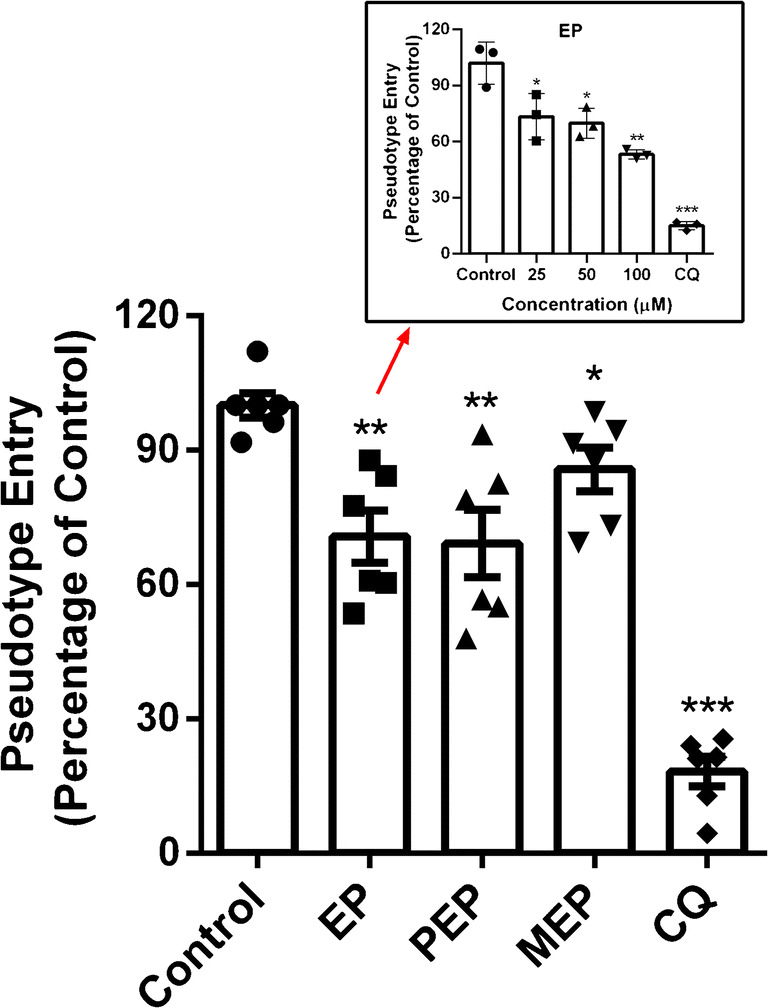
Effect of EP, PEP and MEP on the entrance of SARS-CoV-2 spike pseudovirus into ACE2h cells. Inset is the graph of EP. Data are presented as mean ± SD. *P ˂ 0.05, **P ˂ 0.01, ***P ˂ 0.001 compared with group Control
Following the emergence of the virus in late 2019, the COVID-19 pandemic was announced on 11 March 2020 [29]. At the time of writing, it has caused more than 2.26 million deaths among the 100 million infected worldwide. Although many small molecule drugs are in clinical trials, there is still no specific drug for COVID-19. Therefore, it is imperative to find a treatment for COVID-19 infection. SARS-COV-2 is thought to enter host cells by binding to ACE2 on host cell membranes and infect host cells [30, 31]. Therefore, ACE2 is the key point and main target for drug screening.
For the drug screening strategies, virtual screening was widely used to find the anti-SARS-CoV-2 drugs in the early stage of the pandemic. Niu et al. [32] established a rapid screening model based on clinical experience and molecular docking technology, 4 of 23 compounds from Ephedra sinica were screened out, and could block S protein from binding to ACE2 according to the molecular docking result and the lowest binding energy ΔG<−6.7 kcal·mol−1; however, the specific components were not listed. Wang et al. [33] established a novel screening strategy via blocking binding between SARS-CoV-2 S RBD and hACE2, and concluded the key functional groups among the highest active compounds and SARS-CoV-2 Spike were nitrogen-containing groups (–NH2), oxygen-containing groups (–OH, –O–, –CO–, –CONH–, and –COO–), and conjugate rings (phenyl group, nitrogen-containing conjugate rings). In our work, the screened ephedrine compounds all have phenyl group, –OH, and nitrogen-containing groups, which is consistent with the above literature.
With the normal operation of most laboratories, experimental screening methods contributed a lot for anti-SARS-CoV-2 drug development. Yang et al. [34] provided an integration method of in silico and experimental study for TCM formula screening; they verified the major contribution of Ma Xing Shi Gan Decoction (MXSG) to the overall efficacy of Qingfei Paidu Decoction (QFPD). We also screened Ephedra sinica, Semen armeniacae amarum, and Radix Glycyrrhizae herbs consisted in MXSG with ACE2/CMC model, and found several ACE2-targeted constituents. In addition to the ephedrine compounds described in this work, other active components showed relatively weak activity, and the assistant effect on Ephedra sinica will be further studied.
Many other drugs and compounds, such as monoclonal antibodies, TCMs [35], and antiviral peptides [36], were screened. Our group experimentally screened ACE2 binding components from antipsychotics [37], histamine H1 receptor antagonists [38], and glucocorticoid drugs [39] with ACE2/CMC model. In the present study, the ACE2/CMC model was combined with HPLC-IT-TOF-MS to develop a two-dimensional analysis method for TCMs screening, making the screening work more efficient, fast, and convenient. Chen et al. [40] also synthesized a novel ACE2 biochromatographic stationary phase for the screening of potential ACE2-targeted components from extract of Lianhuaqingwen capsule. Results confirmed the suitability and efficiency of ACE2/CMC model and its coupled system.
Conclusion
This study presents a simple two-dimensional ACE2/CMC-HPLC-IT-TOF-MS approach. The developed approach was verified and applied to screen the active components that can bind to ACE2 from traditional Chinese medicine Ephedra sinica. Three active constituents with ACE2-binding activity were fished out and identified as EP, PEP, and MEP. It has been distinctly proved from the molecular level and pharmacological experiments that the screened compounds can bind to SARS-CoV-2 RBD and prevent SARS-CoV-2 from entering ACE2h cells, showing antiviral effect. The established method can be used to study the material basis of drugs for the treatment of COVID-19, screen ACE2-targeted active components, and provide technical reference for the research of antiviral drugs.
Supplementary information
(PDF 335 kb)
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- ACE2h cell line
ACE2 over-expressed HEK293T cell line
- CMC
Cell membrane chromatography
- EME
Ephedra sinica methanol extract
- EP
Ephedrine
- EPs
EP, PEP and MEP
- HCQ
Hydroxychloroquine
- MEP
Methylephedrine
- PEP
Pseudoephedrine
- SARS-CoV-2 RBD
The receptor-binding domain (RBD) of the spike protein of SARS-CoV-2
- SPR
Surface plasmon resonance
- TCMs
Traditional Chinese medicines
Funding
This work was supported by the National Natural Science Foundation of China (81973278 and 81930096), China Postdoctoral Science Foundation (2019T120923 and 2018M641003), and National Science Foundation of Shaanxi Province (2020SF-309).
Declarations
The authors declare that they have no conflict of interest. Ethical approval is not applicable. The presented research did not involve humans or animals. Informed consent is not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu RJ, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui HT, Li YT, Guo LY, Liu XG, Wang LS, Jia JW, Liao JB, Miao J, Zhang ZY, Wang L, Wang HW, Wen WB. Traditional Chinese medicine for treatment of coronavirus disease 2019: a review. Traditional Med Res. 2020;5(2):65–73. [Google Scholar]
- 3.Chen J, Wang YK, Gao Y, Hu LS, Yang JW, Wang JR, Sun WJ, Liang ZQ, Cao YM, Cao YB. Protection against COVID-19 injury by qingfei paidu decoction via antiviral, anti-inflammatory activity and metabolic programming. Biomed Pharmacother. 2020;129:110281. doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu DY, Xu YL, Wang ZW, Lu YL, Zhu HL, Song T. Mechanism of Qingfeipaidu decoction on COVID-19 based on network pharmacology. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(1):26–32. [Google Scholar]
- 5.Wang XY, Jia W, Zhao AH, Wang XR. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhu J. Anti-HBV effect of individual traditional Chinese herbal medicine in vitro and in vivo: an analytic review. J Viral Hepatitis. 2013;20(7):445–452. doi: 10.1111/jvh.12112. [DOI] [PubMed] [Google Scholar]
- 7.Ma SC, Du J, But PPH, Deng XL, Zhang YW, Ooi VEC, Xu HX, Lee SHS, Lee SF. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol. 2002;79(2):205–211. doi: 10.1016/S0378-8741(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 8.Qu LX, Zhang ZX, Dong TX, Wang YC, Qu ZY. Research progress on anti-adenovirus effect of traditional Chinese medicine. China journal of traditional Chinese medicine and pharmacy. 2019;34(7):3143–3146. [Google Scholar]
- 9.Zheng SC, Baak JP, Li S, Xiao WK, Ren H, Yang H, Gan YX, Wen CB. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine. 2020;79:153336. doi: 10.1016/j.phymed.2020.153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Zhang LN, Huang JM, Nandakumar KS, Liu SW, Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem. 2020;205:112687. doi: 10.1016/j.ejmech.2020.112687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong LDLD, Lam WC, Yang W, Chan KW, Sze SCW, Miao JX, Yung KKL, Bian ZX, Wong VT. Potential targets for treatment of coronavirus disease 2019 (COVID-19): a review of Qing-Fei-Pai-Du-Tang and its major herbs. Am J Chin Med. 2020;48(5):1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- 12.Itokawa H, Morris-Natschke SL, Akiyama T, et al. Plant-derived natural product research aimed at new drug discovery. J Nat Med. 2008;62(3):263–280. doi: 10.1007/s11418-008-0246-z. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wong YK, Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20:1–9. doi: 10.1017/erm.2018.3. [DOI] [PubMed] [Google Scholar]
- 14.Luo JY, Chen GS, Liu DH, Wang Y, Qi Q, Hu HY, Li PY, Bai J, Du SY, Lu Y, Wang YM, Liu C. Study on the material basis of Houpo Wenzhong Decoction by HPLC fingerprint, UHPLC-ESI-LTQ-Orbitrap-MS, and network pharmacology. Molecules. 2019;24(14):2561. doi: 10.3390/molecules24142561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Wu WY, Jiang BH, Yang M, Guo DA. Pharmacological tools for the development of traditional Chinese medicine. Trends Pharmacol Sci. 2013;34(11):620–628. doi: 10.1016/j.tips.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Qu HH, Wang QG. New method for analyzing pharmacodynamic material basis of traditional Chinese medicines. China journal of Chinese materia medica. 2013;38(17):2906–2910. [PubMed] [Google Scholar]
- 17.He LC, Geng XD. Cell membrane chromatography-a new method for studying the drug-receptor interactions. New Prog Biomed Chromatogr. 1996;3:8–9. [Google Scholar]
- 18.He LC, Yang GD, Geng XD. Enzymatic activity and chromatographic characteristics of the cell membrane immobilized on silica surface. Chin Sci Bull. 1999;44:826–831. doi: 10.1007/BF02885029. [DOI] [Google Scholar]
- 19.Hou XF, Zhou MZ, Jiang Q, Wang SC, He LC. A vascular smooth muscle/cell membrane chromatography–offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines. J Chromatogr A. 2009;1216:7081–7087. doi: 10.1016/j.chroma.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Wei P, Huo HL, Ma QH, Li HC, Xing XF, Tan XM, Luo JB. Pharmacokinetic comparisons of five ephedrine alkaloids following oral administration of four different Mahuang-Guizhi herb-pair aqueous extracts ratios in rats. J Ethnopharmacol. 2014;155(1):642–648. doi: 10.1016/j.jep.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 21.Ha SJ, Lee CJ, Jung SK. Preventive effect of Ephedra sinica extract on UVB-induced COX-2 and MMP-1 expression. Food Sci Biotechnol. 2018;27(4):1157–1163. doi: 10.1007/s10068-018-0331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin GZ, Hu B, Shi ZQ, Zheng JY, Wang L, Chang WQ, Li P, Yao Z, Liu LF. A direct ionization mass spectrometry-based approach for differentiation of medicinal Ephedra species. J Pharm Biomed Anal. 2016;117:492–498. doi: 10.1016/j.jpba.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Lee SE, Lim C, Lim S, Lee B, Cho S. Effect of Ephedrae Herba methanol extract on high-fat diet-induced hyperlipidaemic mice. Pharm Biol. 2019;57(1):676–683. doi: 10.1080/13880209.2019.1666883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv MY, Chen JQ, Gao YQ, Sun JB, Zhang QQ, Zhang MH, Xu FG, Zhang ZJ. Metabolomics based on liquid chromatography with mass spectrometry reveals the chemical difference in the stems and roots derived from Ephedra sinica. J Sep Sci. 2015;38(19):3331–3336. doi: 10.1002/jssc.201500529. [DOI] [PubMed] [Google Scholar]
- 26.Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China. 2015;1:320–1.
- 27.Lan J, Ge JW, Yu JF, Shan SS, Zhou H, Fan SL, Zhang Q, Shi XL, Wang QS, Zhang LQ, Wang XQ. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 28.Shang J, Ye G, Shi K, Wan YS, Luo CM, Aihara H, Geng QB, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu JT, Kathy L, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;39(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu M, Wang RL, Wang ZX, Zhang P, Bai ZF, Jing J, Guo YM, Zhao X, Zhan XY, Zhang ZT, Song XA, Qin EQ, Wang JB, Xiao XH. Rapid establishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking. China journal of traditional Chinese medicine and pharmacy. 2020;45(6):1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- 33.Wang XN, Yang CX, Sun YY, Sui X, Zhu T, Wang Q, Wang S, Yang J, Yang WJ, Liu FY, Zhang MM, Wang YG, Luo Y. A novel screening strategy of anti-SARS-CoV-2 drugs via blocking interaction between Spike RBD and ACE2. Environ Int. 2021;147:106361. doi: 10.1016/j.envint.2020.106361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang RC, Liu H, Bai C, Wang YC, Zhang XH, Guo R, Wu SY, Wang JX, Leung E, Chang H, Li P, Liu TG, Wang Y. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res. 2020;157:104820. doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang YP, Zhao XX, Lv HQ, Wen CP. Drug screening and development from the affinity of S protein of new coronavirus with ACE2. Eur J Clin Microbiol Infect Dis. 2020;9:1–9. doi: 10.1007/s10096-020-04048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathod SB, Prajapati PB, Punjabi LB, Prajapati KN, Chauhan N, Mansuri MF. Peptide modelling and screening against human ACE2 and spike glycoprotein RBD of SARS-CoV-2. In Silico Pharmacol. 2020;8(1):3. doi: 10.1007/s40203-020-00055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu JY, Hou YJ, Ge S, Wang XJ, Wang J, Hu T, Lv YX, He HZ, Wang C. Screened antipsychotic drugs inhibit SARS-CoV-2 binding with ACE2 in vitro. Life Sci. 2021;266:118889. doi: 10.1016/j.lfs.2020.118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge S, Wang XJ, Hou YJ, Lv YX, Wang C, He HZ. Repositioning of histamine H1 receptor antagonist: doxepin inhibits viropexis of SARS-CoV-2 spike pseudovirus by blocking ACE2. Eur J Pharmacol. 2021;896:173897. doi: 10.1016/j.ejphar.2021.173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YJ, Hu SL, Wang J, Xue ZY, Wang C, Wang N. Dexamethasone inhibits SARS-CoV-2 spike pseudotyped virus viropexis by binding to ACE2. Virology. 2021;554:83–88. doi: 10.1016/j.virol.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XF, Wu YL, Chen C, Gu YQ, Zhu CY, Wang SP, Chen JY, Zhang L, Lv L, Zhang GQ, Yuan YF, Chai YF, Zhu MS, Wu CS. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B. 2021;11(1):222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 335 kb)