Abstract
Background
Connexin 43 (Cx43) plays a central role in the inflammatory response and wound healing. Targeting Cx43 expression reduces inflammation in a variety of injuries. The expression pattern of Cx43 has not been described for many inflammatory skin diseases.
Objectives
To describe the expression patterns of Cx43 in eczema, psoriasis, Steven‐Johnson syndrome/toxic epidermal necrolysis.
Methods
Archival skin biopsies from patients with eczema, psoriasis, and Steven‐Johnson syndrome/toxic epidermal necrosis were identified and examined, with sister sections stained for Cx43 and imaged by confocal microscopy. All samples were compared to age and site‐matched normal skin controls.
Results
Epidermal Cx43 is reduced in acute eczema, absent in regions of spongiosis, and is highly elevated in subacute and chronic eczema. In plaque psoriasis, Cx43 is overexpressed in areas with psoriasiform hyperplasia with a fish‐scale‐like appearance but is lost in regions surrounding neutrophil microabscesses. Cx43 staining is strong in the neutrophils within these microabscesses. In SJS/TEN, Cx43 expression is elevated in areas bordering normal tissue but is rapidly lost in areas of keratinocyte necrosis.
Conclusions
Dynamic changes in Cx43 levels are seen in inflammatory skin diseases and may represent future potential therapeutic targets.
Keywords: dermatitis, epidermal necrosis, expression patterns, gap junction, psoriasiform
Connexin 43 (Cx43) plays a central role in the inflammatory response and wound healing. Targeting Cx43 expression reduces inflammation in a variety of injuries. Here we examine the changes in Cx43 protein levels in the epidermis in eczema, psoriasis, Steven‐Johnson Syndrome/Toxic Epidermal necrolysis.
1. INTRODUCTION
Gap junctions are dynamic hexameric protein channels formed by ubiquitously expressed proteins called connexins. They allow the passage of small molecules and ions between neighboring cells and have important roles in cell‐cell communication and propagation of cell death signals. 1 , 2 , 3 They are found in many tissues in the body including skin, neural system, heart, muscle, activated leukocytes, and platelets. 1
Connexin 43 (Cx43) is the most widely expressed gap junction protein in the human skin, with epidermal expression mainly located in the stratum spinosum. 3 Studies have shown that expression patterns of connexins alter dynamically during development and differentiation of the epidermis and dermis. 3 Loss‐of function mutations of skin‐expressed connexins result in well‐characterized genodermatoses such as keratitis‐ichthyosis deafness syndrome (KID) and oculodentodigital dysplasia (ODDD). 4 , 5
In addition to its role in skin development, connexins have been shown to mediate epidermal healing in cutaneous wounds and cellular apoptosis in the skin and neural tissue. 6 , 7 , 8 , 9 , 10 , 11 In acute skin injury, Cx43 is initially downregulated at wound edges, but levels increase during wound closure, particularly in areas where there is cell differentiation. 6 Wound closure is shown to be significantly faster in Cx43 knockout mice 8 as well as in murine wound‐healing models using a single topical application of Cx43 antisense gel. 6 , 8
Gap junctions allow the passage of death signals from injured cells to healthy neighboring cells. 12 , 13 In skin burns and spinal cord damage, elevated Cx43 expression leads to exacerbation of the initial injury. 6 , 7
While connexin 26 (Cx26) and Cx43 were found to be upregulated in psoriasis, the expression and role of Cx43 in other skin diseases have not been described. 14 We hypothesize that there is a role for Cx43 in the pathogenesis of inflammatory skin diseases. If proven so, Cx43 can be a novel target for therapeutic intervention for these conditions. In order to determine if Cx43 expression is altered in inflammatory skin conditions and skin conditions characterized by marked epidermal apoptosis, we conducted an exploratory pilot study to describe the expression of Cx43 in eczema, psoriasis, Steven‐Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN).
2. PATIENTS AND METHODS
2.1. Tissue selection
Ten archival skin biopsies on paraffin blocks for the three conditions (eczema, psoriasis, SJS (defined as body surface area with skin denudation <10% on clinical assessment), SJS/TEN overlap syndrome (body surface area 10‐30%), and TEN (body surface area skin denudation >30%) were retrospectively identified through histological diagnostic codes over a 10‐year period (2006‐2016). The pathological diagnosis for each skin biopsy was confirmed by a dermatopathologist (SSJL). Skin biopsies were not included in the study if the clinic‐pathological diagnosis was not established or if the tissue sample was unsuitable for Cx43 immunostaining due to degradation. All specimens were matched 1:1 for age, skin type, and biopsy site with a normal skin control. Immunostaining for Cx43 expression was performed on all samples. A retrospective medical record review of the selected subjects was undertaken for demographic data including age, gender, duration and severity of disease, treatment of disease, and mortality. Ethical approval for this study was obtained from the National Health Group Domain Specific Review Board (NHG DSRB) in September 2016 (DSRB Reference Number: 2016/00954).
2.2. Eczema
A total of 10 subjects with a histological diagnosis of spongiotic dermatitis and clinical diagnosis of eczema were identified for this study. Age range was 22 to 82 years old (median age = 51.5 years). Six subjects were female. Three subjects had acute eczema (based on clinical history), two subjects had subacute eczema, while five subjects had chronic eczema. Biopsy sites included the arms (five subjects), abdomen (two subjects), and lower limbs (three subjects). Three patients received oral corticosteroids, one patient received phototherapy and topical corticosteroids. The remaining patients received only topical treatment. The subjects' demographic and clinical data are presented in Table 1.
TABLE 1.
Clinical characteristics of subjects with eczema
| Patient code | Gender | Age | Diagnosis | Clinical findings and history | Treatment |
|---|---|---|---|---|---|
| E1 | F | 25 | Acute eczema | Urticated, eczematous plaques on hands, arms, legs, trunk of 1 week duration | Oral prednisolone |
| E2 | M | 22 | Chronic eczema | Eczematous lichenified plaque on arms, legs, trunk, TBSA 15% | Oral prednisolone, topical steroids |
| E3 | F | 53 | Chronic eczema | 5‐6 years history of eczematous plaques and papules on trunk and limbs | Phototherapy, topical steroids |
| E4 | M | 22 | Chronic eczema | 1 month history of eczematous plaques on trunk, limbs, TBSA 15% | Topical steroids |
| E5 | F | 50 | Acute eczema | Nil available | Nil available |
| E6 | M | 71 | Subacute eczema | Eczematous plaques on bilateral lower limbs for 1 month | Topical steroids |
| E7 | F | 53 | Chronic eczema | Eczematous plaques and papules on trunk and limbs for more than 10 years | Topical steroids |
| E8 | F | 39 | Subacute eczema | Eczematous plaques on trunk for several months | Topical steroids |
| E9 | F | 82 | Chronic eczema | 2 months history of generalized itchy eczematous plaques, erythrodermic | Oral prednisolone |
| E10 | M | 60 | Acute eczema | Several week history of annular erythematous plaques on arms, trunk, and limbs | Topical steroids |
| P1 | 62 | M | Plaque psoriasis | 2 months history of erythematous plaques with pustules on trunk and limbs | Started on MTX, defaulted follow‐up |
| P2 | 74 | F | Pustular psoriasis | 8‐9 months history of erythematous plaques on arms | Topical steroids, Vitamin D analogue |
| P3 | 87 | M | Plaque psoriasis | 6 months history of worsening generalized erythematous scaly plaques, TBSA 80% | Started on MTX, defaulted follow‐up |
| P4 | 23 | M | Plaque psoriasis | 5 years history of erythematous plaques on ears, with flare to lower legs | Topical steroids |
| P5 | 22 | F | Guttate psoriasis | 3 weeks history of small discrete erythematous scaly plaques, TBSA 20% | Topical steroids, phototherapy |
| P6 | 52 | M | Plaque psoriasis | 1‐year history of erythematous plaques on elbows and knees, with nail changes | Topical steroids |
| P7 | 39 | F | Guttate psoriasis | 2 weeks history of discrete erythematous plaques with scales on trunk and thighs following a cough | Topical steroids |
| P8 | 63 | M | Plaque psoriasis | 3‐4 months history of erythematous scaly plaques on trunk and thighs, TBSA 5‐10% | Topical steroids followed by phototherapy |
| P9 | 63 | M | Guttate psoriasis | 2 months history of generalized itchy eczematous plaques, erythrodermic | Topical steroids |
| P10 | 27 | F | Plaque psoriasis | 3 years history of itchy, psoriasiform plaques on knees, legs, and forearms | Topical steroids |
| S1 | F | 53 | SJS | TBSA involved <10%, likely due to levetiracetam; survived | Oral Prednisolone + topical corticosteroids |
| S2 | F | 30 | SJS | TBSA involved <10%, likely due to herpes simplex infection; survived | Topical corticosteroids |
| S3 | F | 34 | SJS | TBSA involved <10%, likely due to Viral infection, unable to exclude co‐amoxiclav and ibuprofen; survived | IV hydrocortisone + topical corticosteroids |
| S4 | F | 38 | SJS | TBSA involved 10%, likely due to Paracetamol and amoxicillin; survived | IV hydrocortisone + topical corticosteroids |
| S5 | F | 80 | SJS/TEN overlap | TBSA involved 25%, likely due to allopurinol and omeprazole; died | Intravenous immunoglobulin |
| S6 | M | 53 | SJS/TEN overlap | TBSA involved 23%, likely due to allopurinol and omeprazole; survived | Intravenous immunoglobulin + IV hydrocortisone |
| S7 | F | 51 | SJS | TBSA involved 7‐8%, likely due to mycoplasma infection; survived | Clarithromycin + topical corticosteroids |
| S8 | F | 51 | SJS | TBSA involved <10%, likely due to tolterodine, mirtazapine, duloxetine, alprazolam; survived | IV hydrocortisone |
| S9 | M | 17 | SJS | TBSA involved <10%, likely due to viral infection, lamotrigine; survived | Oral Prednisolone + topical corticosteroids |
| S10 | F | 92 | SJS/TEN overlap | TBSA involved 20‐30%, likely due to piperacillin‐tazobactam, co‐amoxiclav and clarithromycin; survived | Oral Prednisolone + topical corticosteroids |
Abbreviations: F, female; M, male; MTX, methotrexate; SJS, Steven‐Johnson syndrome; TBSA, total body surface area; TEN, toxic epidermal necrolysis.
2.3. Psoriasis
Ten subjects with a clinicopathological diagnosis of psoriasis were included in this study, including one subject with pustular psoriasis, and three with guttate psoriasis. Age range was 22 to 87 years old (median age = 57 years), with equal numbers of female and male subjects. Two biopsies were performed on the upper limbs, two were performed on the abdomen, and the remainder from the lower limbs. All patients only received topical treatment at time of biopsy. The subjects' demographic and clinical data are presented in Table 1.
2.4. SJS and TEN
Seven subjects with a clinicopathological diagnosis of SJS and three with SJS/TEN overlap syndrome were included in this study. Age range was 17 to 92 years old (median age = 51 years), eight subjects were female. Two biopsies were performed on the upper limbs, five on the abdomen, and three were performed on the lower limbs. The subjects' demographic and clinical data are presented in Table 1.
2.5. Haematoxylin and eosin staining
All hematoxylin and eosin reagents were procured from Leica (Leica, Germany). Slides were dewaxed in clearene and rehydrated through a graded series of ethanol to water. Slides were then immersed in Haematoxylin stain for 30 to 45 seconds. To remove the excess stain, slides were rinsed in acid alcohol followed by an immediate rinse in Scott's water for 5 minutes. Slides were then immersed in Eosin for 1 minute. After a quick dip into Scott's water, the slides underwent a dehydration process through a series of ethanol, and then followed by a further 5 minutes in fresh clearene. Slides were mounted and cover‐slipped using Limonene mounting medium (Sigma‐Aldrich, United States). When dry, bright‐field images were acquired using the Zeiss Axio Scan microscope at 20× magnification (Zeiss, Germany).
2.6. Immunostaining
Slides were dewaxed in clearene and rehydrated through a graded series of ethanol to water. This was followed by 3 × 5‐minute incubations in phosphate‐buffered saline (PBS, 1X PBS). Tissue samples were circled using a wax pen and covered with permeabilizing solution (0.05% Triton X‐100 in 1X PBS, Sigma‐Aldrich, United States) and incubated for 60 minutes. This was followed by blocking with 1% BSA (Sigma‐Aldrich, United States) and PBS for 45 minutes. The primary antibody against Cx43 (Sigma C6289; Sigma‐Aldrich, United States) was applied at 1:1000 dilution and incubated overnight. Negative controls were incubated overnight in blocking solution containing no primary antibody. Slides were washed 3 × 5 minutes in PBST (0.05% Tween 20 in 1X PBS, Sigma‐Aldrich, United States) before incubation for 1 hour at room temperature with the secondary antibody (Alexa555 goat antirabbit 1:500; ThermoFisher, United States). A counterstain of DAPI (1:10 000 in PBS, ThermoFisher, United States) was applied as a nuclear marker. After a further 3 × 5 minutes PBS washes, the slides were coverslipped using Citiflour (Citifluor Ltd, London, UK) mounting medium and imaged on a confocal microscope (Leica SP8; Leica, Germany) at 40× magnification. All images were acquired using identical parameters and settings to allow for direct comparison of staining intensity.
3. RESULTS
3.1. Eczema
Staining of Cx43 was visibly reduced in areas of epidermal spongiosis in acute eczema specimens (Figure 1G,H; from subject E10). In biopsies from patients with subacute eczema, Cx43 staining was more intense throughout the entire sample and formed larger continuous gap junction plaques (Figure 1I; from subject E6).
FIGURE 1.
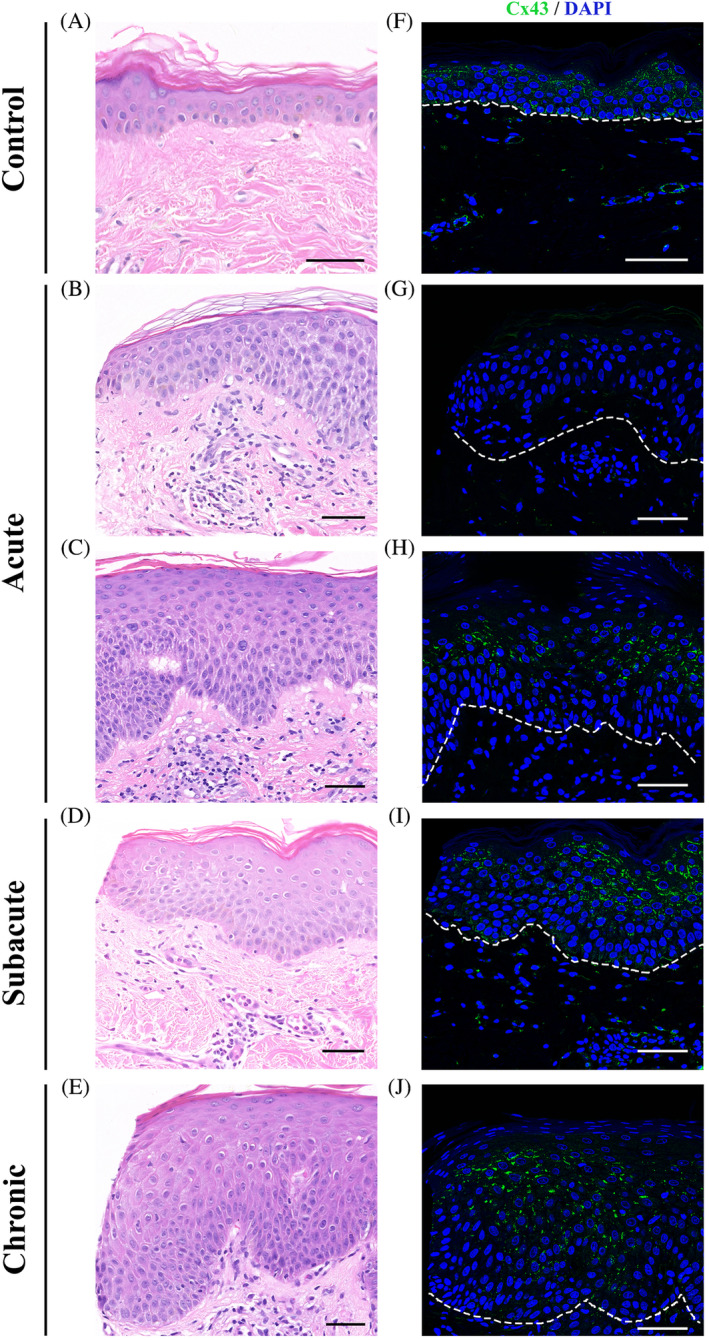
Downregulation of Cx43 expression in the spongiotic epidermis of eczema patients. Representative images of Cx43 staining of skin biopsy samples from normal skin (F) as well as patients with acute eczema (G, H; subject E10), subacute eczema (I; subject E6), and chronic eczema (J, subject E9). Corresponding H&E images of Cx43 staining (A‐E). Scale bars = 50 μm, 40× images. Dotted line marks the outline between the epidermis and dermis. Cx43 staining was visibly reduced in spongiotic areas, which are more commonly found in acute eczema samples. In subacute and chronic eczema samples Cx43 staining was closer to normal Cx43 expression shown in control skin except in regions where spongiosis could be observed
In chronic eczema specimens, where epidermal psoriasiform hyperplasia predominates over spongiosis, Cx43 expression levels were still elevated compared with normal. Cx43 levels were lower in a few areas with spongiosis (Figure 1J; from subject E9).
3.2. Psoriasis
In plaque psoriasis specimens, Cx43 levels were highly elevated in the spinous layers to granular layers, forming strong linear arrays of gap junction plaques (Figure 2E; from subject P6) compared to the punctate staining typically seen in normal skin controls (Figure 2D). In contrast, in areas of the epidermis where pockets of neutrophilic microabscesses were present, the linear plaque staining of Cx43 was lost from the cell membrane and was replaced with a diffuse cytoplasmic Cx43 stain. However, Cx43 was highly expressed within the neutrophils themselves within the microabscesses (Figure 2F, from subject P4). Cx43 also appeared to be elevated in the blood vessels and neutrophils in the dermis and was particularly high in the blood vessels between the rete pegs.
FIGURE 2.
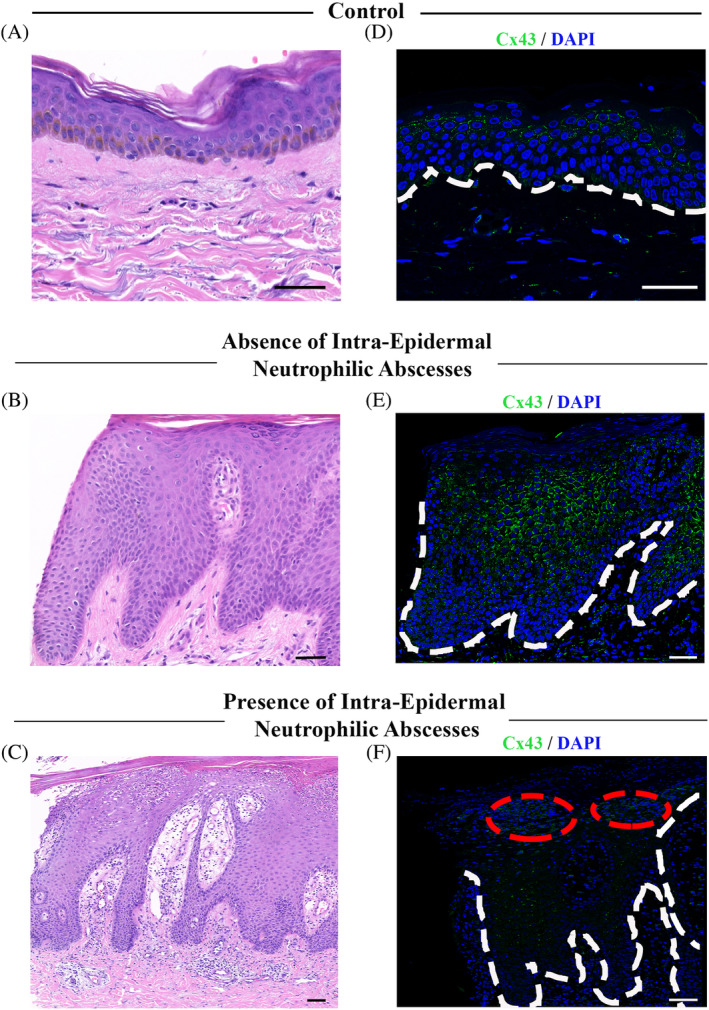
Upregulation of Cx43 in intraepidermal neutrophilic abscesses and downregulation in surrounding epidermis of patients with psoriasis. Representative images of Cx43 staining of skin biopsy samples from normal skin (D) as well as patients with psoriasis either in the absence of neutrophilic abscess (E; subject P6) or in the presence of neutrophilic abscesses (F; subject P4). Corresponding H&E images of Cx43 staining (A‐C). Red circle marks areas of neutrophilic abscess. Dotted line marks the outline between the epidermis and dermis. Scale bars = 50 μm. In the absence of neutrophilic abscesses, Cx43 appears to be upregulated in the spinous to granular layer forming linear arrays of gap junction plaques compared to normal punctate staining shown in control skin. With the appearance of neutrophilic abscesses, Cx43 expression is found in the neutrophils, however Cx43 downregulation was observed in the surrounding epidermis
3.3. Steven‐Johnson syndrome/toxic epidermal necrolysis
Biopsies from SJS/TEN patients demonstrated varying degrees of epidermal injury. In skin specimens showing focal keratinocyte necrosis, there was a hyper‐expression of Cx43 levels at the adjacent normal appearing epidermis, predominantly at the apical surface of the keratinocytes, forming almost continuous fish scale‐like gap junction plaques (Figure 3H,I; from subject S8). In skin samples that showed larger areas of keratinocyte necrosis, the punctate membranous Cx43 staining seen in the epidermis of normal matched controls was lost and Cx43 staining became more cytoplasmic and diffuse (Figure 3J, from subject S1). However, Cx43 remained elevated in the dermis in blood vessels, fibroblasts, and leukocytes. In areas where leucocyte infiltration was observed, Cx43 levels were high in the leukocytes (Figure 3K; from subject S10). In skin specimens showing pan‐epidermal necrolysis, Cx43 expression was completely lost at these areas of necrotic detached epidermis but was strong in dermal leukocytes, fibroblasts, and blood vessels (Figure 3L from subject S5).
FIGURE 3.
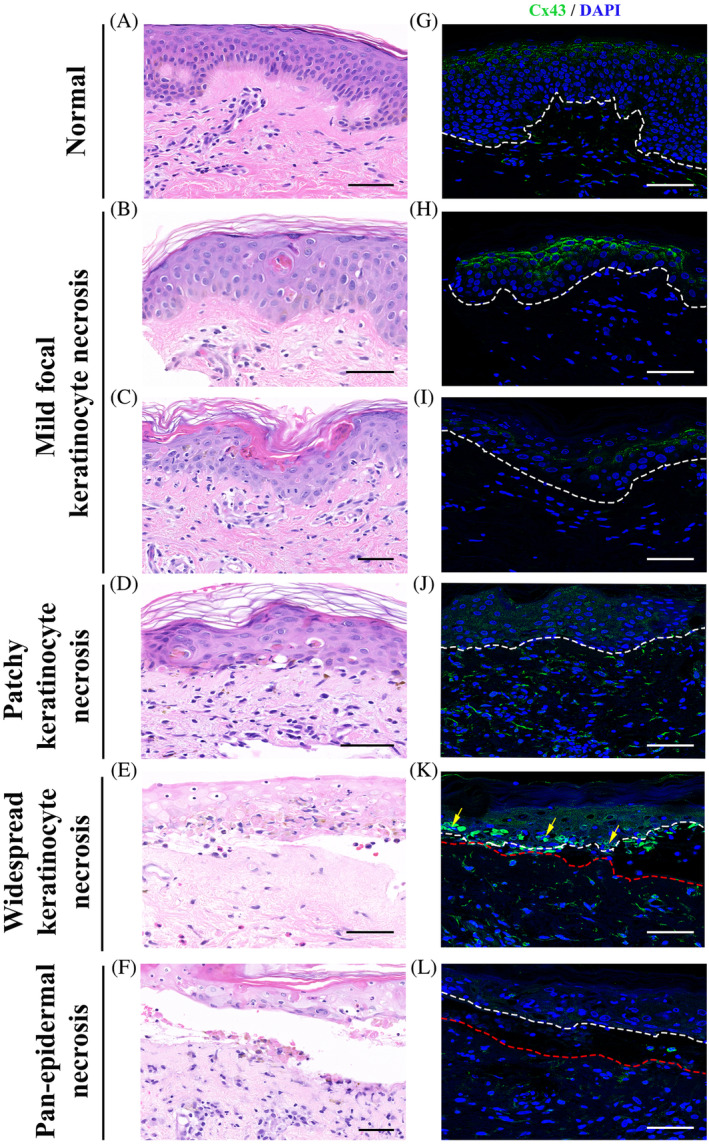
Change in Cx43 expression in patients with SJS/TEN. Representative images of Cx43 staining of skin biopsy samples from normal skin (G) and patients with SJS or SJS/TEN overlap showing mild focal keratinocyte necrosis (H, I; from subject S8), patchy areas of keratinocyte necrosis (J; from subject S1), widespread keratinocyte necrosis (K; from subject S10), and pan‐epidermal necrosis (L; from subject S5). Corresponding H&E images of Cx43 staining (A‐F). Arrows mark infiltration of leucocytes. White and red dotted line marks the epidermis and dermis, respectively. Scale bars = 50 μm, 40× images. Cx43 expression is elevated in the epidermis showing focal keratinocyte necrosis. As the keratinocyte necrosis becomes more widespread in the epidermis, Cx43 expression becomes cytoplasmic compared to normal punctate staining in control skin. In the sections showing pan‐epidermal necrosis Cx43 expression is lost at areas of epidermal detachment
4. DISCUSSION
The dynamic expression of Cx43 has previously been shown to affect skin physiology, leading to changes in wound healing and susceptibility to skin disease. Here, we describe the changes in Cx43 in eczema, psoriasis, and SJS/TEN.
Membranous Cx43 expression was elevated in the epidermis in conditions with more psoriasiform hyperplasia and less spongiosis, such as subacute and chronic eczema. This is consistent with previous findings of elevated Cx43 mRNA in the skin of eczema patients and is reminiscent of the elevated Cx43 levels seen at the edges of chronic wounds. 10 , 15 , 16 In contrast, in acute eczema, membranous Cx43 protein levels were generally reduced or completely absent in areas of spongiosis. This may be related to the disruption of connexons that are unable to dock and form gap junctions due to intercellular edema. Loss of gap junctional communication in areas of spongiosis may also impede the transfer of nutrients and signaling molecules between keratinocytes. 17 Alterations in Cx43 may also interfere with the skin's barrier function, given that the cytoplasmic tail of Cx43 binds to and regulates zonula occludin 1 (ZO1), a tight junction protein. 18 , 19 Indeed, genetically modified mice, which have the cytoplasmic tail of Cx43 deleted, suffer from epidermal barrier defects, supporting the notion that normal Cx43 interactions are required to maintain the skins barrier. 20 In these mice, the epidermis was thicker than normal and the level of Cx43 was elevated and expressed in more layers than normal. Along with this, there was an elevation in the levels of ZO‐1, filaggrin, and Cx26.
In plaque psoriasis, Cx43 was overexpressed in areas with psoriasiform hyperplasia and was distributed in a fish‐scale like pattern, particularly in areas without neutrophilic abscesses. This is similar to the pattern seen in hyperplastic edges of chronic wound biopsies from venous leg ulcers and diabetic foot ulcers. 9 , 16 The similarity of expression patterns of Cx26 in psoriasis and chronic wound edges lends credence to our observations in this study. Cx26 has been reported to be overexpressed in chronic plaque psoriasis and chronic wound edges. 9 , 14 , 21 Interestingly, the overexpression of Cx26 in the epidermis of transgenic mice resulted in hyperplasia of the epidermis and a breakdown of barrier function to produce a psoriasis‐like appearance to the skin. 22
In areas with neutrophilic abscesses within psoriatic plaques, Cx43 staining was lost from the membrane of the surrounding keratinocytes and a diffuse cytoplasmic signal can be seen in the keratinocytes instead. However, Cx43 was highly expressed within the neutrophils in these clusters, suggesting that upregulation of Cx43 may play a role in the pathogenesis of psoriasis. Upregulation of Cx43 has been shown to recruit neutrophils and facilitate the release of proinflammatory signals, and ATP Cx43 staining was also strong in blood vessels in the dermis between rete pegs. 23 , 24 Elevation of Cx43 in blood vessels has been shown to promote blood vessel leakiness. 7 , 25
Gap junctions have been shown to mediate the spread of cell death following injury by allowing the passage of death signals from injured cells to healthy neighboring cells. 12 , 13 , 15 The elevated levels of Cx43 seen on normal‐appearing epidermis surrounding focal apoptotic keratinocytes in SJS specimens suggest that Cx43 may play a role in the spread of apoptotic death signals between keratinocytes, resulting in further damage to neighboring healthy keratinocytes and eventual progression to pan‐epidermal necrolysis. Our study suggests a therapeutic opportunity whereby downregulation of Cx43 at the interface between normal and dying keratinocytes may help prevent the spread of apoptotic and inflammatory signals, and eventually halt disease progression. However, the delivery of agents that target Cx43 through the intact epidermis may prove to be challenging as most of these agents that are currently being developed are too large (>500 Da) to penetrate the intact epidermis. Given that leukocytes are also involved in driving inflammation, downregulation of Cx43 within leukocytes may help break the pro‐inflammatory cycle and return homeostasis to the epidermis. 7 , 26 , 27 , 28 , 29 , 30
There are several limitations to our study. The use of archival tissue, which may have degraded over time, may alter the staining for Cx43 and affect the accuracy of our observation. We attempted to minimize this by screening the histology specimens, and visibly degraded specimens on H&E were excluded from this study. As this is an exploratory pilot study, we included only a small number of cases for each condition. Our study involved patients of Asian ancestry, and future studies in different ethnic populations are useful to confirm the generalizability of our findings.
In conclusion, we have demonstrated the changes in Cx43 expression in inflammatory skin conditions such as eczema, psoriasis, and SJS/TEN. In order to study the dynamic changes in Cx43 expression and confirm our observations, prospective studies are required. These should ideally involve analysis of Cx43 expression in sequential lesional and peri‐lesional skin biopsies as the condition (eg, Stevens‐Johnson syndrome) evolves. Our observations identify several instances in which Cx43 may be targeted therapeutically to reduce inflammation in psoriasis or to prevent the spread of death signals in early SJS in an attempt to limit disease progression. More research is required to look at the role of Cx43 in these diseases and to confirm Cx43 as a viable therapeutic target in these conditions.
FUNDING
This work was supported by the NTU SUG, NTU/SCBE pump priming grant and the Ministry of Education Tier 1 (RG38/19). We acknowledge the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund—Pre‐Positioning Programme (IAF‐PP) (Grant number H17/01/a0/0C9) for providing the floor infrastructure and core equipment. BMRC IAFPP for Skin Research Institute Singapore. Tan Li Ling Mandy is supported by IGS's studentship from Nanyang Technological University. The funding sources had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; the decision to submit the report for publication.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Conceptualization: David Laurence Becker, Chia Chun Ang, Joyce Lee
Data Curation: David Laurence Becker, Mandy Li Ling Tan, Hui Li Kwong
Formal Analysis: Mandy Li Ling Tan, Hui Li Kwong
Funding Acquisition: David Laurence Becker, Hong Liang Tey
Investigation: Mandy Li Ling Tan
Project Administration: David Laurence Becker, Hui Li Kwong
Resources: David Laurence Becker, Joyce Lee, Hong Liang Tey
Supervision: David Laurence Becker, Chia Chun Ang, Hong Liang Tey
Validation: David Laurence Becker
Visualization: Mandy Li Ling Tan, Chia Chun Ang, Joyce Lee, Hui Li Kwong
Writing—Original Draft Preparation: David Laurence Becker, Mandy Li Ling Tan, Hui Li Kwong
Writing—Review & Editing: David Laurence Becker, Chia Chun Ang, Joyce Lee, Hong Liang Tey
All authors have read and approved the final version of the manuscript.
David L. Becker had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
David Becker affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Tan MLL, Kwong HL, Ang CC, Tey HL, Lee JSS, Becker DL. Changes in connexin 43 in inflammatory skin disorders: Eczema, psoriasis, and Steven‐Johnson syndrome/toxic epidermal necrolysis. Health Sci Rep. 2021;4:e247 10.1002/hsr2.247
Mandy L. L. Tan and Hui L. Kwong are joint first authors.
Funding information Agency for Science, Technology and Research (A*STAR), Grant/Award Number: H17/01/a0/0C9; Ministry of Education, Grant/Award Number: RG38/19; NTU SUG, NTU/SCBE pump priming grant
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
REFERENCES
- 1. Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045‐2058. [DOI] [PubMed] [Google Scholar]
- 2. Churko JM, Laird DW. Gap junction remodeling in skin repair following wounding and disease. Physiology (Bethesda). 2013;28:190‐198. [DOI] [PubMed] [Google Scholar]
- 3. Salomon D, Masgrau E, Vischer S. Topography of mammalian connexins in human skin. J Invest Dermatol. 1994;103:240‐247. [DOI] [PubMed] [Google Scholar]
- 4. Van Steensel MA, van Geel M, Nahuys MA, et al. Novel connexin 26 mutation in a patient diagnosed with keratitis‐ichthyosis‐deafness syndrome. J Invest Dermatol. 2002. Apr;118:724‐727. [DOI] [PubMed] [Google Scholar]
- 5. Huang T, Shao Q, MacDonald A, et al. Autosomal recessive GJA1 (Cx43) gene mutations cause oculodentodigital dysplasia by distinct mechanisms. J Cell Sci. 2013. Jul 1;126:2857‐2866. [DOI] [PubMed] [Google Scholar]
- 6. Coutinho P, Qiu C, Frank S, et al. Limiting burn extension by transient inhibition of Connexin43 expression at the site of injury. Br J Plast Surg. 2005;58:658‐667. [DOI] [PubMed] [Google Scholar]
- 7. Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152‐160. [DOI] [PubMed] [Google Scholar]
- 8. Coutinho P, Qiu C, Frank S, Tamber K, Becker DL. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int. 2003;27:525‐541. [DOI] [PubMed] [Google Scholar]
- 9. Sutcliffe JE, Chin KY, Thrasivoulou C, et al. Abnormal connexin expression in human chronic wounds. Br J Dermatol. 2015;173:1205‐1215. [DOI] [PubMed] [Google Scholar]
- 10. Kretz M, Euwens C, Hombach S, et al. Altered connexin expression and wound healing in the epidermis of connexin‐deficient mice. J Cell Sci. 2003;116:3443‐3452. [DOI] [PubMed] [Google Scholar]
- 11. Qiu C, Coutinho P, Frank S, et al. Targeting connexin 43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697‐1703. [DOI] [PubMed] [Google Scholar]
- 12. Edwards GO, Botchway SW, Hirst G, Wharton CW, Chipman JK, Meldrum RA. Gap junction communication dynamics and bystander effects from ultrasoft X‐rays. Br J Cancer. 2004;90:1450‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin JH, Weigel H, Cotrina ML, et al. Gap‐junction‐mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494‐500. [DOI] [PubMed] [Google Scholar]
- 14. Labarthe MP, Bosco D, Saurat JH, Meda P, Salomon D. Upregulation of connexin 26 between keratinocytes of psoriatic lesions. J Invest Dermatol. 1998;111:72‐76. [DOI] [PubMed] [Google Scholar]
- 15. El Tawdy AM, Rashed LM, Alhanafy HM. Gap junction and atopic dermatitis: a study of connexin 43 mRNA expression in atopic skin lesions. J Egypt Women Dermatol Soc. 2011;8:78‐83. [Google Scholar]
- 16. Mendoza‐Naranjo A, Cormie P, Serrano AE, et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol Int. 2012;36:661‐667. [DOI] [PubMed] [Google Scholar]
- 17. Andrew H. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens‐1 protein. Curr Biol. 1998;8:931‐934. [DOI] [PubMed] [Google Scholar]
- 19. Mendoza‐Naranjo A, Cormie P, Serrano AE, et al. Targeting Cx43 and N‐cadherin, which are abnormally upregulated in venous leg ulcers, influences migration, adhesion and activation of Rho GTPases. PLoS One. 2012;7:e37374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maass K, Ghanem A, Kim JS, et al. Defective epidermial barrier in neonatal mice lacking the C‐terminal region of connexin 43. Mol Biol Cell. 2004;15:459704608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310‐1320. [DOI] [PubMed] [Google Scholar]
- 22. Djalilian AR, McGaughey D, Patel S, et al. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006;116:1243‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine‐dependent endothelial cell function. Circ Res. 2006;99:1100‐1108. [DOI] [PubMed] [Google Scholar]
- 24. Sarieddine MZ, Scheckenbach KE, Foglia B, et al. Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med. 2009;13:4560‐4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soon AS, Chua JW, Becker DL. Connexins in endothelial barrier function – novel therapeutic targets countering vascular hyperpermeability. Thromb Haemost. 2016;116:852‐867. [DOI] [PubMed] [Google Scholar]
- 26. Danesh‐Meyer HV, Huang R, Nicholson LF, Green CR. Connexin43 antisense oligodeoxynucleotide treatment down‐regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J Clin Neurosci. 2008;15:1253‐1263. [DOI] [PubMed] [Google Scholar]
- 27. Danesh‐Meyer HV, Kerr NM, Zhang J, et al. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506‐520. [DOI] [PubMed] [Google Scholar]
- 28. Ghatnekar GS, O'Quinn MP, Jourdan LJ, Gurjarpadhye AA, Draughn RL, Gourdie RG. Connexin43 carboxyl‐terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen Med. 2009;4:205‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci. 2006;119:5193‐5203. [DOI] [PubMed] [Google Scholar]
- 30. O'Carroll SJ, Becker DL, Davidson JO, Gunn AJ, Nicholson LF, Green CR. The use of connexin‐based therapeutic approaches to target inflammatory diseases. Methods Mol Biol. 2013;1037:519‐546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


