Abstract
Fine particulate matter (PM2.5) with an average aerodynamic diameter of <2.5 µm can cause severe lung injury. Oxidative stress and inflammation are considered the main outcomes of PM2.5 exposure. Curcumin is a well-known antioxidant; however, its effect on PM2.5-induced oxidative injury in airway epithelial cells remains unclear. In the present study, it was demonstrated that pre-treatment with curcumin significantly reduced the PM2.5-induced apoptosis of BEAS-2B human bronchial epithelial cells by decreasing the level of intercellular reactive oxygen species. Western blot analysis revealed that curcumin increased the expression of nuclear factor erythroid 2-related factor 2 (NRF2) and regulated the transcription of downstream genes, particularly those encoding antioxidant enzymes. Moreover, curcumin reduced the PM2.5-induced expression and production of inflammatory factors, and induced the expression of the anti-inflammatory factors, interleukin (IL)-5 and IL-13. Taken together, the present study demonstrates that curcumin protects BEAS-2B cells against PM2.5-induced oxidative damage and inflammation, and prevents cell apoptosis by increasing the activation of NRF2-related pathways. It is thus suggested that curcumin may be a potential compound for use in the prevention of PM2.5-induced tissue injury.
Keywords: fine particulate matter, curcumin, human bronchial epithelial cell, nuclear factor erythroid 2-related factor 2/antioxidant response element pathway, antioxidant, inflammation
Introduction
Air pollution is one of the most severe threats to human health worldwide, particularly lung health (1). Fine particulate matter (PM2.5) with an average aerodynamic diameter of <2.5 µm can directly access the alveoli of the lungs and induce airway inflammation (2). The airway epithelium acts as a mechanical and immunologic barrier and is the first point of contact for air pollution in the lungs (3). Recent studies have suggested that the pro-inflammatory effects of PM2.5 on airway epithelium are associated with the disruption of oxidation reduction homeostasis, such as by inducing reactive oxygen species (ROS) generation (4-6).
Exposure to particulate matter allows ROS to accumulate in airway epithelial cells, and excess ROS triggers mitogen-activated protein kinase signaling cascades and the activation of redox-sensitive nuclear factor erythroid 2-related factor 2 (NRF2) and nuclear factor-κB. Under normal conditions, NRF2 is anchored in the cytoplasm through its interaction with Kelch-like ECH-associated protein 1 (KEAP1). Oxidants interfere with this interaction, resulting in the nuclear localization of NRF2, which then promotes the transcription of several antioxidant and detoxifying enzymes, such as heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase l (NQO-1) (7). Previous studies reported that the exposure of cells to a low concentration of PM2.5 (2 µg/cm2) induced the activity of NRF2 and the transcription of target genes. However, the induction of NRF2 genes was reduced in cells exposed to a high concentration of PM2.5 (10 µg/cm2) (2,5). In the early stages of inflammation-mediated tissue injury, the activation of NRF2/antioxidant response element (ARE) inhibits the production of inflammatory factors, including cytokines, chemokines and cellular adhesion molecules (8). Thus, NRF2 plays important roles in particulate matter-induced cell and tissue injury by regulating the expression of target genes. However, the mechanisms of NRF2 in PM2.5-induced tissue injury remain unknown.
Curcumin, a natural polyphenolic compound derived from the rhizomes of Curcuma longa (turmeric), has demonstrated anti-inflammatory and antioxidant properties in a number of diseases (9). In cancer, curcumin reduces cisplatin-related ototoxic adverse effects by targeting the p-STAT3 and NRF2 signaling pathways in vivo (10). Furthermore, curcumin induces the translocation of NRF2 and promotes the expression of ARE-related genes to mediate the antioxidant response (10). Thus, it was hypothesized that curcumin may prevent PM2.5-induced oxidative stress-related injury by upregulating the NRF2/ARE pathways.
To validate this hypothesis, the present study evaluated the potential mechanisms of action of NRF2 in PM2.5-induced tissue injury by investigating the proliferation and apoptosis of BEAS-2B cells exposed to PM2.5. In addition, the expression of NRF2 and inflammatory factors was analyzed. Furthermore, cells exposed to PM2.5 were treated with curcumin to determine whether curcumin could alleviate PM2.5-induced oxidative stress. The findings of the present study provide new insight into the development of treatments against tissue injury caused by air pollution-derived PM2.5.
Materials and methods
Reagents
The BEAS-2B cell line was purchased from the China Center for Type Culture Collection. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco; Thermo Fisher Scientific, Inc. PM2.5 was purchased from Wuxi NEST Biotechnology Co., Ltd. Curcumin was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. and dissolved in dimethyl sulfoxide (DMSO). Trypsin-EDTA (0.25%), the cell counting kit-8 (CCK-8), Hoechst 33258, kits to measure the levels of ROS, the Annexin V-FITC/PI apoptosis kit, BCA protein quantification kit, goat anti-rabbit IgG, and enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-25, IL-33, IL-9, interferon (IFN)-γ, IL-6, tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF)-A, IL-5 and IL-13 were purchased from Bioswamp Biotechnology Co., Ltd. Lipofectamine 2000 and TRIzol reagent were purchased from Invitrogen; Thermo Fisher Scientific, Inc. The SYBR Green PCR kit was purchased from KAPA Biosystems. The reverse transcription reagent kit was purchased from Takara Biotechnology Co., Ltd. All primary antibodies were purchased from Abcam. Polyvinylidene difluoride (PVDF) membranes and enhanced chemiluminescence (ECL) reagents were obtained from EMD Millipore.
Cells, cell culture, PM2.5 and curcumin treatment
BEAS-2B cells were maintained in DMEM with 10% FBS at 37°C in a humidified atmosphere containing 5% CO2. Cells were treated with PM2.5 at concentrations of 0, 12.5, 25, 50 and 100 mg/ml for 24 h to analyze the intracellular response. Cells were treated with curcumin at concentrations of 0, 1, 2.5, 5, 7.5, 10, 20 and 50 µM for 48 h for CCK-8 assay (11,12). To evaluate the effects of curcumin, the cells were incubated in DMEM without or with curcumin (10 µM) for 1 h (10) and treated with PM2.5 at 25 and 50 µg/ml for 24 h.
Cell viability assessment
Cell viability was evaluated by CCK-8 assay according to the manufacturer's protocol. BEAS-2B cells were seeded into 96-well plates (5×103 cells/well) and maintained in regular growth medium overnight. The cells were then treated with PM2.5 or curcumin as described above. CCK-8 solution (10 µl) was added to each well, and after 4 h, the absorbance was measured at 450 nm using a Multiskan FC microplate photometer (Thermo Fisher Scientific, Inc.). The experiment was performed in triplicate.
Apoptosis assessment
The apoptosis of BEAS-2B cells was quantified by flow cytometric analysis using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit. Treated cells were washed with cold phosphate-buffered saline (PBS), trypsinized and harvested following centrifugation at 1,000 × g for 5 min at 4°C. The cells were resuspended in cold PBS, washed, diluted with cold PBS (1×105 cells/ml), and harvested following centrifugation at 1,000 × g for 5 min at 4°C. The cells were re-suspended in 100 µl of binding buffer and stained with 5 µl of Annexin V-FITC and 5 µl of PI for 30 min at 4°C in the dark. The fluorescence intensity of the stained cells was measured using an FC500 MCL flow cytometer (Beckman Coulter, Inc.). The data were analyzed using CXP analysis software (CXP Analysis 2.0, Beckman Coulter, Inc.). The experiment was performed in triplicate.
Hoechst staining
After treatment, the cells were fixed in 4% formaldehyde for 10 min. After 2 washes in PBS, Hoechst 33258 solution was dropped onto the cells and incubated for 5 min in the dark at 4°C. The cells were then observed and photographed under a TS100-F microscope (Nikon Corporation).
Measurement of intracellular ROS production
Intracellular ROS production was measured using the fluorogenic dye 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA). Following treatment, the cells were washed and incubated with DCFH2-DA (10 µmol/l) for 20 min at 37°C in the dark. The fluorescence intensity of the stained cells was analyzed using an FC500 MCL flow cytometer, and the data were analyzed using CXP analysis software (CXP Analysis 2.0). The experiment was performed in triplicate.
Luciferase reporter assay
pmirGLO (Addgene, Inc.) containing ARE (5′-TCA CAG TGA CTC AGC AAA ATT-3′) reporter plasmids were constructed as previously described (13). BEAS-2B cells were seeded into 24-well plates and incubated for 24 h. The pmirGLO/ARE plasmids were transfected into the cells using Lipofectamine 2000 according to the manufacturer's instructions. pmirGLO plasmids (without ARE) transferred into BEAS-2B cells were considered as the control group. Following 24 h of transfection, the transfection efficiency was validated by RT-qPCR, and cells were treated with PM2.5 or curcumin for 24 h. The activities of Firefly and Renilla luciferase were detected using the Dual-luciferase Reporter Assay System (GeneCopoeia) and evaluated using a SynergyH multiscan spectrum spectrophotometer (BioTek Instruments, Inc.).
Western blot analysis
Following treatment, the cells were lysed in radioimmunoprecipitation assay lysis buffer supplemented with a protease and phosphatase inhibitor cocktail. Protein concentrations were determined using a bicinchoninic acid protein quantification kit. The proteins (20 µg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were blocked in 5% dry milk for 2 h and incubated overnight at 4°C with primary antibodies (Table I). Following 3 washes with PBS/Tween (PBST), the membranes were incubated with goat anti-rabbit IgG secondary antibodies (1:10,000, PAB150011, Bioswamp Biotechnology Co., Ltd.) for 1 h at 4°C. The blots were washed 3 times with PBST. The resulting immunoreactive protein complexes were detected using an ECL reagent according to the manufacturer's instructions and imaged using a Tanon-5200 auto chemiluminescence analyzer (Tanon Science & Technology Co., Ltd.) and analyzed using Tanon Gis image analysis software (Version 4.2, Tanon Science & Technology Co., Ltd.).
Table I.
Information of primary antibodies used for western blot analysis.
| Name | Species | Dilution rate | Size (kDa) | Catalogue nos. | Supplier |
|---|---|---|---|---|---|
| NRF2 | Rabbit | 1:2,000 | 68 | ab62352 | Abcam |
| HO-1 | Rabbit | 1:2,000 | 33 | ab13248 | Abcam |
| NQO-1 | Rabbit | 1:1,000 | 31 | ab34173 | Abcam |
| KEAP1 | Rabbit | 1:1,000 | 70 | ab139729 | Abcam |
| GAPDH | Rabbit | 1:1,000 | 37 | 2118 | Cell Signaling Technology |
| β-actin | Rabbit | 1:1,000 | 42 | ab8227 | Abcam |
| Histone H3 | Rabbit | 1:1,000 | 17 | ab8580 | Abcam |
NRF2, NRF2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; NQO-1, NAD(P)H quinone dehydrogenase 1; KEAP1, Kelch-like ECH-associated protein.
ELISA
BEAS-2B cells were seeded into 24-well plates and cultured for 24 h. Following 24 h of treatment, the supernatants were collected and the release of cytokines (IL-25, IL-33, IL-9, IFN-γ, IL-6, TNF-α, VEGF-A, IL-5, IL-10 and IL-13) was analyzed using ELISA kits according to the manufacturer's instructions. The absorbance of the wells was measured using a Multiskan MS apparatus (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using Trizol reagent, and 1 µg of total RNA was reverse transcribed using the PrimeScript™ RT reagent kit (KM4101, KAPA Biosystems) with gDNA Eraser according to the manufacturer's instructions (KAPA Biosystems). qPCR was conducted using the SYBR-Green PCR kit in a real-time system (Bio-Rad Laboratories, Inc.). The primers used are listed in Table II. Amplification was conducted at 95°C for 3 min, followed by 40 cycles at 95°C for 5 sec, 56°C for 10 sec, and 72°C for 25 sec; and 65°C for 5 sec, 95°C for 50 sec, and 40°C for 60 sec. The data were analyzed using the 2−ΔΔCq method (14). GADPH was used as an internal reference. The experiment was performed in triplicate.
Table II.
Sequences of primers used for RT-qPCR.
| Name | Sequence (5′-3′) | Size (bp) |
|---|---|---|
| IL-25-F | GGAGATATGAGTTGGA | 144 |
| IL-25-R | TCTGGTTGTGGTAGAG | |
| IL-33-F | AGAGAAACCACCAAAA | 114 |
| IL-33-R | TGATATACCAAAGGCA | |
| IL-9-F | AGTTGTCTCTGTTTGGGC | 104 |
| IL-9-R | AGTGGGTATCTTGTTTGC | |
| IFN-γ-F | CTCTTTTCTTAGGCATTT | 192 |
| IFN-γ-R | CATCTCGTTTCTTTTTGT | |
| IL-6-F | TGGTCTTTTGGAGTTTGA | 130 |
| IL-6-R | ATTCTTTGCCTTTTTCTG | |
| TNF-α-F | TACTCCTCACCCACACCA | 152 |
| TNF-α-R | GAAGACCCCTCCCAGATA | |
| VEGFA-F | AAGACAAGAAAATCCCTG | 134 |
| VEGFA-R | GTTCGTTTAACTCAAGCT | |
| IL-5-R | CATAAAAATCACCAACTG | 160 |
| IL-5-R | GTCTTTCTTCTCCACACT | |
| IL-13-F | CCACGGTCATTGCTCTCA | 150 |
| IL-13-R | TGCTCCATACCATGCTGC | |
| GAPDH-F | CCACTCCTCCACCTTTG | 106 |
| GAPDH-R | CACCACCCTGTTGCTGT |
IL, interferon; IFN-γ, interferon γ; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Statistical analysis
The data are presented as the means ± standard deviation (SD). The significant difference between 2 groups was analyzed by an unpaired Student's t-test and those between multiple groups by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
PM2.5 exposure-induced oxidative damage inhibits the viability of BEAS-2B cells
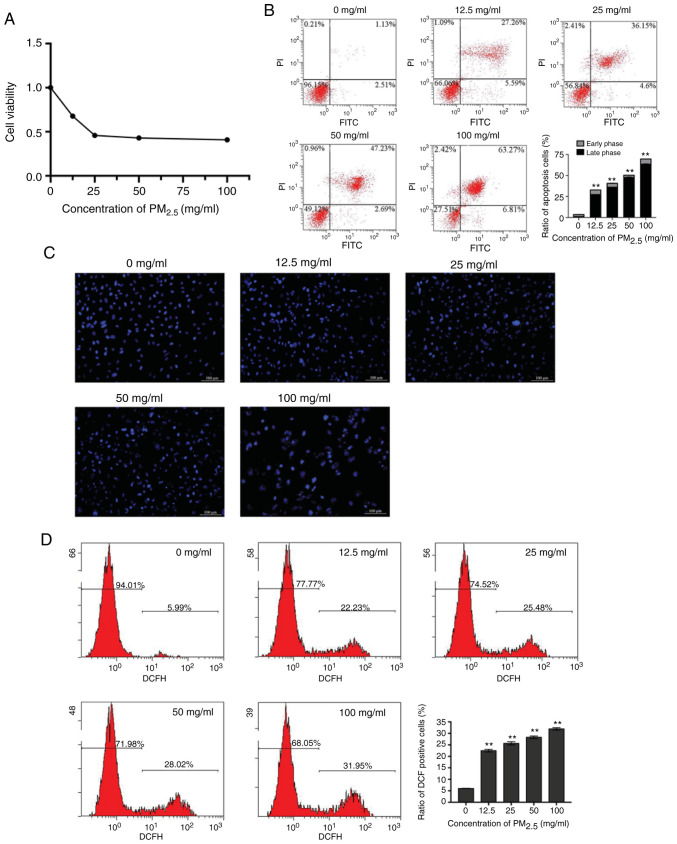
To determine the effects of PM2.5 on the viability of lung epithelial cells, BEAS-2B cells were exposed to PM2.5 at 0, 12.5, 25, 50 and 100 mg/ml. The results of CCK-8 assay revealed that the proliferation of the BEAS-2B cells was inhibited by PM2.5 in a concentration-dependent manner (Fig. 1A). The apoptosis of the BEAS-2B cells was examined by flow cytometry and Hoechst 33258 staining. The percentage of apoptotic cells was significantly elevated by PM2.5 (Fig. 1B). Microscopic observations revealed that chromatin condensation and marginalization appeared in the PM2.5-exposed cells in a concentration-dependent manner (Fig. 1C). The results suggested that PM2.5 exposure induced the apoptosis of lung epithelial cells, and these effects were more evident at a higher concentration of PM2.5 compared with the lower concentrations. To determine the association between the cellular redox level and the inhibitory effects of PM2.5 on the growth of BEAS-2B cells, the levels of intracellular ROS were measured. The results revealed that intracellular ROS production was increased by PM2.5 in a concentration-dependent manner (Fig. 1D).
Figure 1.
PM2.5 exposure-induced oxidative damage inhibits the viability and induces the apoptosis of BEAS-2B cells. BEAS-2B cells were exposed to various concentrations of PM2.5 for 24 h. (A) Cell viability was analyzed by CCK-8 assay. Cell apoptosis was evaluated by (B) flow cytometry and (C) Hoechst 33258 staining. (D) Intracellular ROS production was examined using the fluorogenic dye, DCFH-DA. Data are presented as the means ± SD; n=3, **P<0.01 compared with the control (0 mg/ml). PM2.5, fine particulate matter.
Curcumin promotes the survival and reduces the oxidative stress of PM2.5-exposed BEAS-2B cells
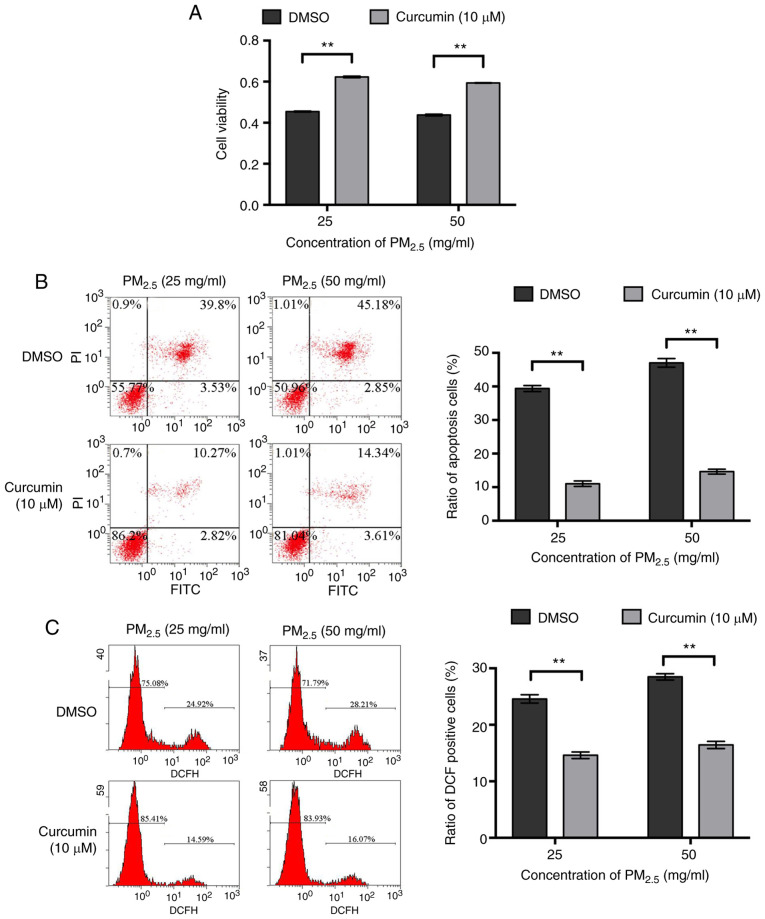
The effects of curcumin on the viability of BEAS-2B cells were then detected and it was found that within a certain concentration range (0-10 µM), curcumin promoted cell proliferation in a concentration-dependent manner (Fig. S1A). Hoechst 33258 staining revealed that curcumin did not affect cell apoptosis (Fig. S1B). The viability of the BEAS-2B cells pre-treated with curcumin was significantly higher than that of the cells pre-treated with DMSO following exposure to the same concentration of PM2.5 (Fig. 2A). Flow cytometry also revealed that following exposure to the same concentration of PM2.5, the percentage of apoptosis was lower when the cells were pre-treated with curcumin (Fig. 2B). In addition, following exposure to the same concentration of PM2.5, the levels of intracellular ROS in the cells pre-treated with curcumin were significantly lower than those in the cells pre-treated with DMSO (Fig. 2C). These results suggest that pre-treatment with curcumin protected the BEAS-2B cells from undergoing apoptosis induced by PM2.5 and promoted cell survival by attenuating oxidative stress in PM2.5-exposed cells.
Figure 2.
Effect of curcumin on the PM2.5-exposed BEAS-2B cells. BEAS-2B cells were treated with 10 µM curcumin or DMSO prior to PM2.5 exposure. (A) Cell viability was analyzed by CCK-8 assay. (B) Cell apoptosis was evaluated by flow cytometry. (C) Intracellular ROS was examined using the fluorogenic dye, DCFH-DA. Data are presented as the means ± SD; n=3, **P<0.01 compared with the control (DMSO). PM2.5, fine particulate matter; ROS, reactive oxygen species.
Curcumin activates NRF2/ARE pathways
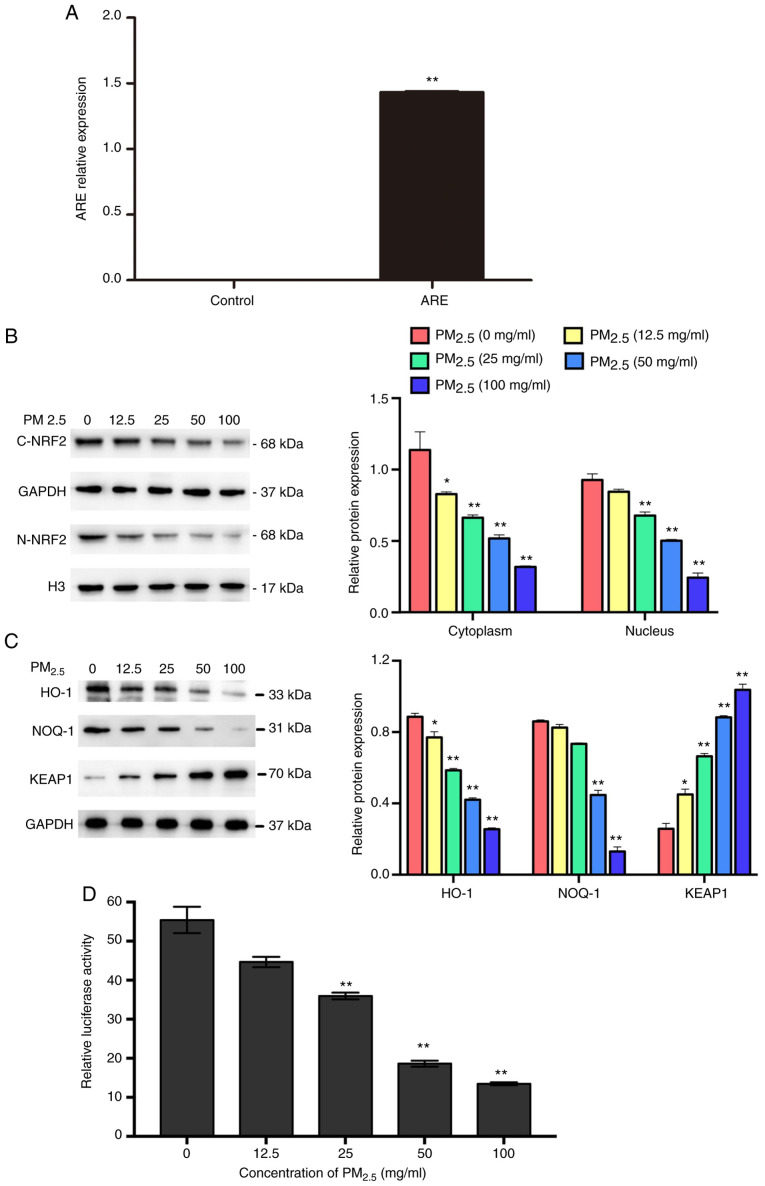
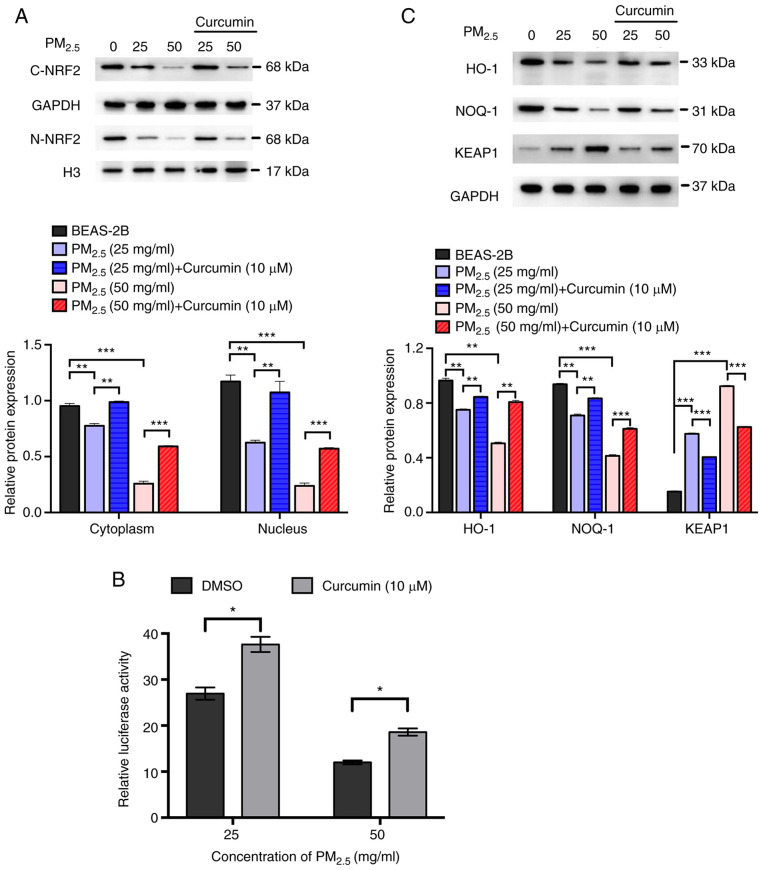
As shown in Fig. 3A, the expression of ARE was significantly higher in the ARE-transfected group compared with the control group, suggesting that the ARE luciferase reporter vector was successfully transfected into the BEAS-2B cells. The results of western blot analysis revealed that PM2.5 exposure downregulated the expression of NRF2 (Fig. 3B), suppressed the activation of ARE and other downstream proteins (HO-1 and NQO-1), and increased the expression of KEAP1 (Fig. 3C and D). To further examine whether curcumin reduces oxidative stress by regulating the activation of NRF2/ARE pathways, the expression of NRF2 and downstream proteins in cells pre-treated with curcumin was analyzed. The results of western blot analysis revealed that curcumin pre-treatment significantly increased the expression of NRF2 in the cell cytoplasm and nucleus (Fig. 4A). The luciferase activity assay revealed that ARE activation was significantly increased by curcumin pre-treatment (Fig. 4B). Furthermore, the expression of HO-1 and NQO-1 was significantly elevated, whereas that of KEAP1 was reduced by curcumin pre-treatment (Fig. 4C). These results suggested that curcumin attenuated oxidative stress in PM2.5-exposed cells by activating the NRF2/ARE pathways.
Figure 3.
Effect of PM2.5 on the expression and activation of NRF2/ARE pathways. (A) Relative expression of ARE was measured by RT-qPCR. (B) Expression of NRF2 in the cytoplasm (C-NRF2) and nucleus (N-NRF2). The C-NRF2 and GAPDH proteins come from the one gel, and N-NRF2 and GAPDH blots originate from the same gel. (C) Expression of KEAP1, HO-1 and NOQ-1. (D) ARE activation was evaluated by luciferase assay. Data are presented as the means ± SD; n=3, *P<0.05 and **P<0.01 compared with the control (0 mg/ml). PM2.5, fine particulate matter; NRF2, nuclear factor erythroid 2-related factor 2; ARE, antioxidant response element.
Figure 4.
Effect of curcumin on the expression and activation of NRF2/ARE pathways in PM2.5-exposed cells. (A) Expression of NRF2 in the cytoplasm and nucleus. (B) Expression of KEAP1, HO-1, and NOQ-1. (C) Activation of ARE was evaluated by luciferase assay. Data are presented as the means ± SD; n=3, *P<0.05, **P<0.01 and ***P<0.001. PM2.5, fine particulate matter; NRF2, nuclear factor erythroid 2-related factor 2; ARE, antioxidant response element.
Curcumin inhibits inflammatory factor production induced by PM2.5 exposure in BEAS-2B cells
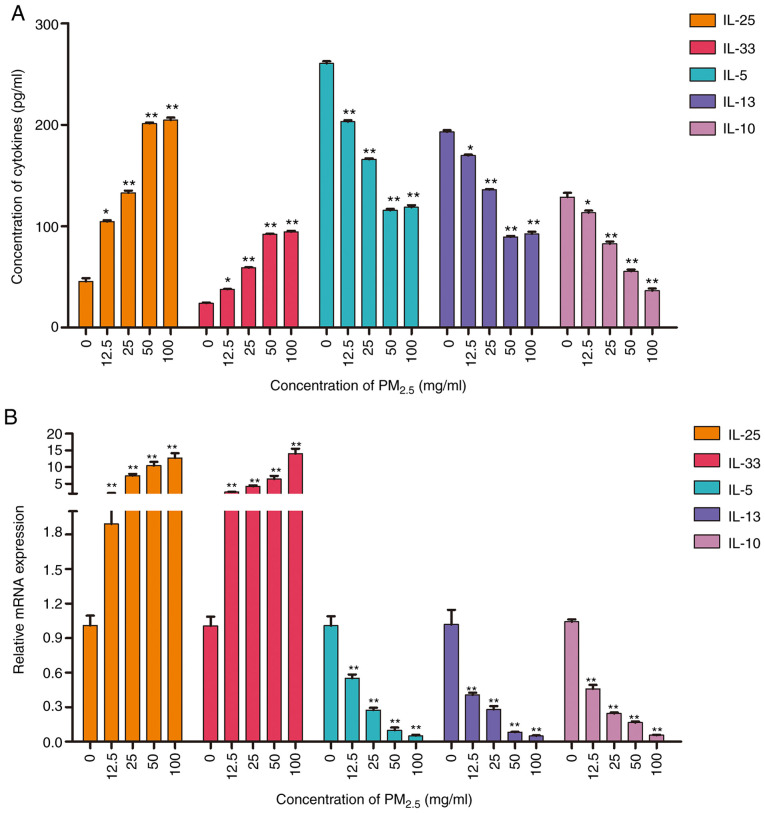
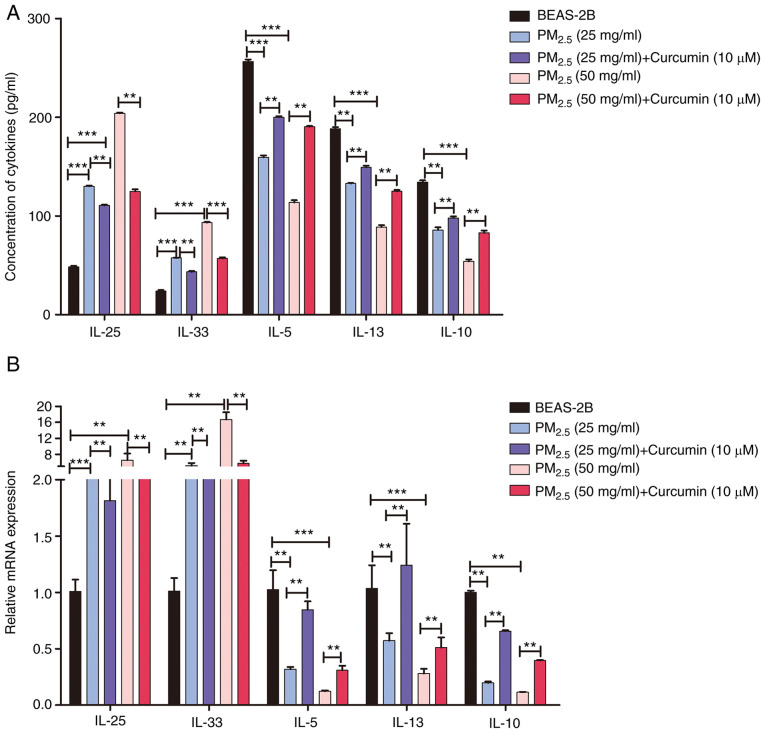
To assess the pro-inflammatory effects of PM2.5, the production and expression of inflammatory factors in PM2.5-exposed BEAS-2B cells was evaluated by ELISA and RT-qPCR, respectively. Both assays revealed that PM2.5 exposure induced the upregulation of the pro-inflammatory factors IL-25, IL-33, IL-9, IFN-γ, IL-6, TNF-α, and VEGF-A (Figs. 5 and S2). Moreover, the expression of the anti-inflammatory factors, IL-5, IL-10 and IL-13, was decreased by PM2.5 exposure (Fig. 5). The results suggested that long-term exposure to PM2.5 (for >24 h) induced a type II inflammatory reaction in lung epithelial cells. The production and expression of inflammatory factors in cells pre-treated with curcumin were also analyzed. The results of RT-qPCR and ELISA revealed that curcumin pre-treatment inhibited the expression and production of pro-inflammatory factors (Figs. 6 and S3), and increased the levels of anti-inflammatory factors (Fig. 6). The results suggested that curcumin inhibited the type II inflammatory reaction induced by PM2.5.
Figure 5.
Effect of PM2.5 on the production and expression of inflammatory factors. The production and mRNA expression of IL-25, IL-33, IL-5, IL-10 and IL-13 were assessed by (A) ELISA and (B) RT-qPCR, respectively. Data are presented as the means ± SD; n=3, *P<0.05 and **P<0.01 compared with the control (0 mg/ml). PM2.5, fine particulate matter; IL, interleukin.
Figure 6.
Effect of curcumin on the production and expression of inflammatory factors in PM2.5-exposed cells. The production and mRNA expression of IL-25, IL-33, IL-5, IL-10 and IL-13 were assessed by (A) ELISA and (B) RT-qPCR, respectively. Data are presented as the means ± SD; n=3, **P<0.01 and ***P<0.001. PM2.5, fine particulate matter; IL, interleukin.
Discussion
Air pollution, particularly from PM2.5, has severely threatened human health in recent decades. Previous studies have demonstrated that oxidative stress is an important factor in PM2.5-induced cell apoptosis (6,15). Thus, it is necessary to identify appropriate antioxidants which may be used to protect human bronchial epithelial cells from PM2.5 exposure. In the present study, it was found that curcumin promoted the survival and reduced the oxidative stress of PM2.5-exposed BEAS-2B cells by activating NRF2/ARE pathways, subsequently inhibiting the type II inflammatory reaction.
Mitochondria are the main target of ROS, the over-production of which, together with cell internalization, induces mitochondrial permeability transition pore opening and results in mitochondrial dysfunction (7). Previous studies have indicated that oxidative stress induced by PM2.5 disrupts the antioxidant system and subsequently triggers mitochondrial-dependent apoptosis by activating caspase-3 (3,16). The present study demonstrated that PM2.5 exposure significantly increased the levels of intracellular ROS and promoted BEAS-2B cell apoptosis, suggesting that PM2.5-suppressed BEAS-2B cell viability was associated with oxidative stress.
To better understand the mechanisms of ROS accumulation in cells and the related mitochondrial dysfunction following PM2.5 exposure, the activation of NRF2 pathways was investigated. NRF2 is a key regulator of cell redox homeostasis that counterbalances ROS production and maintains redox balance (17). Under normal conditions, NRF2 is located in the cytoplasm by binding to KEAP1. In response to oxidative stress, activated NRF2 is translocated into the nucleus, where it binds to the ARE of the target genes of NRF2, including HO-1 and NQO-1, and induces their expression (18). HO-1 and NQO-1 are important intracellular, antioxidant, anti-inflammatory and anti-apoptotic enzymes. In the present study, it was found that PM2.5 exposure suppressed the expression of NRF2 and increased that of KEAP1, which inhibits the activity of NRF2 pathways. The results also revealed that the activity of ARE and the expression of HO-1 and NQO-1 were inhibited by PM2.5 exposure. These results are in agreement with those of previous studies (3,19). In NRF2-deficient mice, the expression of NQO-1 and HO-1 has been shown to be absent (20). These findings indicate that PM2.5 exposure increases the levels of intracellular ROS by suppressing the activation of NRF2 and decreasing the expression of downstream genes. However, the effects of NRF2 pathways on PM2.5-exposed BEAS-2B cells remain unclear.
Inflammation is the main outcome of PM2.5-induced cell injury (21). Airway epithelial cells secrete various cytokines and participate in immune response after stimulation by allergens or pathogenic microorganisms. IL-33 is expressed in epithelial and smooth muscle cells, and its expression level is positively related to the severity of asthma (22,23). IL-25, also known as IL-17, belongs to the IL-17 cytokine family and induces a Th2-type immune response (24). The overexpression of IL-25 induces eosinophilia and B-lymphocyte hyperplasia, and alters antibody production in mice (25). A previous study demonstrated that PM2.5 exposure induced an innate immune cellular response through the expression of IL-25 and IL-33, which was reduced by Nac, a ROS-quenching agent (26). NRF2 has been reported to be an anti-inflammatory factor as it directly inhibits the transcription of pro-inflammatory cytokine genes (27). In the present study, following PM2.5 exposure, the levels of pro-inflammatory factors were significantly upregulated, whereas those of anti-inflammatory factors were significantly downregulated. Taken together, the results indicated that PM2.5-induced inflammation was associated with a redox imbalance via the suppression of NRF2 pathway activation, which subsequently induced cell apoptosis.
Curcumin has been shown to have antioxidant and anti-inflammatory properties (28,29) and has been used in the treatment of allergies and asthma in Asia for a number of years (30). It has been proven to increase the activity of NRF2/ARE pathways, as well as the expression of downstream genes (HO-1 and NOQ-1) in different types of cells in recent studies (31). In the present study, BEAS-2B cells pre-treated with curcumin exhibited higher levels of NRF2/ARE activation and lower levels of intercellular ROS than untreated cells. This effect rendered BEAS-2B cells resistant to PM2.5-induced oxidative damage. Moreover, curcumin pre-treatment reduced the expression and production of PM2.5-induced inflammatory factors. Thus, the results of the present study suggest that curcumin pre-treatment can protect BEAS-2B cells from apoptosis in response to PM2.5-induced oxidative stress. However, elucidating the mechanisms of NRF2 activation by curcumin in PM2.5-exposed human bronchial epithelial cells requires further experiments.
As mentioned above, curcumin has demonstrated to exhibit anti-inflammatory and antioxidant properties in a number of diseases (9), which is widely used in the treatment of respiratory diseases, such as lung tissue injury (32-34). These previous studies focused on the specific mechanisms of action of curcumin in the treatment of severe lung injury rather than its efficacy. Therefore, the present study did not use a positive control group, which is one of the limitations of the present study; thus, the current experiments may not be optimal, but may be sufficient to draw a conclusion in that curcumin protects BEAS-2B cells against PM2.5-induced oxidative damage and inflammation, and prevents cell apoptosis by increasing the activation of NRF2-related pathways. The effects of curcumin on PM2.5-induced oxidative stress and inflammation were also not detected in in vivo experiments using animals. Thus, the authors aim to verify the present findings in in vivo experiments and using a positive control in future studies.
In conclusion, the present study confirmed that exposure to PM2.5 induced the apoptosis of BEAS-2B human bronchial epithelial cells through redox imbalance and inflammation via the suppression of NRF2 activation. Moreover, curcumin pre-treatment protected the cells against PM2.5-induced oxidative damage and inflammation and prevented cell apoptosis by promoting the activation of NRF2 pathways. These findings provide a potential treatment scheme against diseases induced by PM2.5 exposure.
Supplementary Data
Acknowledgments
Not applicable.
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant no. 81300008) and the Hubei Provincial Natural Science Fund (grant no. 2013CFB372).
Availability of data and materials
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
SY, XLH and LNM were involved in the conceptualization of the study. JC was involved in the study methodology. LNM provided software. XL was involved in data validation and provided resources. WSY was involved in formal analysis. XJW was involved in the investigative aspects of the study. XLH was involved in data curation. WSY, XL and GWL were involved in the writing and preparation of the original draft and visualization, as well as in project design and administration. SY was involved in the writing, reviewing and editing of the manuscript, and in study supervision, and also in funding acquisition. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Huff RD, Carlsten C, Hirota JA. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J Allergy Clin Immunol. 2019;143:1989–2001. doi: 10.1016/j.jaci.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Alam J, Venkatesan MI, Eiguren FA, Schmitz D, Di SE, Slaughter N, Killeen E, Wang X, Huang A, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: Protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 3.Georas SN, Rezaee F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan L, Rui W, Bai R, Zhang W, Zhang F, Ding W. Effects of size-fractionated particulate matter on cellular oxidant radical generation in human bronchial epithelial BEAS-2B cells. Int J Environ Res Public Health. 2016;13:483. doi: 10.3390/ijerph13050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclercq B, Kluza J, Antherieu S, Sotty J, Alleman LY, Perdrix E, Loyens A, Coddeville P, Lo Guidice JM, Marchetti P, Garçon G. Air pollution-derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ Pollut. 2018;243:1434–1449. doi: 10.1016/j.envpol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Raudoniute J, Stasiulaitiene I, Kulvinskiene I, Bagdonas E, Garbaras A, Krugly E, Martuzevicius D, Bironaite D, Aldonyte R. Pro-inflammatory effects of extracted urban fine particulate matter on human bronchial epithelial cells BEAS-2B. Environ Sci Pollut Res Int. 2018;25:32277–32291. doi: 10.1007/s11356-018-3167-8. [DOI] [PubMed] [Google Scholar]
- 7.Gong P, Hu B, Stewart D, Ellerbe M, Figueroa YG, Blank V, Beckman BS, Alam J. Cobalt induces heme oxygenase-1 expression by a hypoxia-inducible factor-independent mechanism in Chinese hamster ovary cells: Regulation by Nrf2 and MafG transcription factors. J Biol Chem. 2001;276:27018–27025. doi: 10.1074/jbc.M103658200. [DOI] [PubMed] [Google Scholar]
- 8.Xu MX, Zhu YF, Chang HF, Liang Y. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic Biol Med. 2016;99:259–272. doi: 10.1016/j.freeradbiomed.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an anti-oxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 10.Fetoni AR, Paciello F, Mezzogori D, Rolesi R, Eramo SL, Paludetti G, Troiani D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: The role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer. 2015;113:1434–1444. doi: 10.1038/bjc.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi Ni, Zeng SE, Tan N, Huang LZ, Cheng QY, Zhang MY. Effect of curcumin on proliferation of hepatocellular carcinoma Hep1 cells and expression of HIF-1α mRNA in normal oxygen conditions. Liaoning Tra Chin Med. 2010;8:21–23. [Google Scholar]
- 12.Song XJ, Zhou HY, Sun YX, Huang HC. Inhibitory effects of curcumin on H2O2-induced cell damage and APP expression and processing in SH-SY5Y cells transfected with APP gene with Swedish mutation. Mol Biol Rep. 2020;47:2047–2059. doi: 10.1007/s11033-020-05305-w. [DOI] [PubMed] [Google Scholar]
- 13.Woods CG, Fu J, Xue P, Hou Y, Pluta LJ, Yang L, Zhang Q, Thomas RS, Andersen ME, Pi J. Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol Appl Pharmacol. 2009;238:27–36. doi: 10.1016/j.taap.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Suo D, Zeng S, Zhang J, Meng L, Weng L. PM2.5 induces apoptosis, oxidative stress injury and melanin metabolic disorder in human melanocytes. Exp Ther Med. 2020;19:3227–3238. doi: 10.3892/etm.2020.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Ding Z, Zhang C, Zhang X, Meng Q, Wu S, Wang S, Yin L, Pu Y, Chen R. MicroRNA-1228(*) inhibit apoptosis in A549 cells exposed to fine particulate matter. Environ Sci Pollut Res Int. 2016;23:10103–10113. doi: 10.1007/s11356-016-6253-9. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: Implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 18.Wang LF, Su SW, Wang L, Zhang GQ, Zhang R, Niu YJ, Guo YS, Li CY, Jiang WB, Liu Y, Guo HC. Tert-butylhydroquinone ameliorates doxorubicin-induced cardiotoxicity by activating Nrf2 and inducing the expression of its target genes. Am J Transl Res. 2015;7:1724–35. [PMC free article] [PubMed] [Google Scholar]
- 19.Block ML, Calderón-Garcidueñas L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Jiao Y, Li C, Liang X, Jia H, Nie Z, Zhang Y. Dimethyl itaconate alleviates the inflammatory responses of macrophages in sepsis. Inflammation. 2020 Oct 7; doi: 10.1007/s10753-020-01352-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Jia H, Liu Y, Guo D, He W, Zhao L, Xia S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ Toxicol. 2020 Sep 30; doi: 10.1002/tox.23035. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravanetti L, Dijkhuis A, Dekker T, Sabogal Pineros YS, Ravi A, Dierdorp BS, Erjefält JS, Mori M, Pavlidis S, Adcock IM, et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. J Allergy Clin Immunol. 2019;143:1355–1370.e16. doi: 10.1016/j.jaci.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 23.Gorska K, Nejman-Gryz P, Paplinska-Goryca M, Korczynski P, Prochorec-Sobieszek M, Krenke R. Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD. 2018;15:36–45. doi: 10.1080/15412555.2017.1416074. [DOI] [PubMed] [Google Scholar]
- 24.Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35:611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao ZJ, Fan YM, Cui YF, Sheng YF, Zhu M. Effect of PM2.5 mediated oxidative stress on the innate immune cellular response of Der p1 treated human bronchial epithelial cells. Eur Rev Med Pharmacol Sci. 2017;21:2907–2912. [PubMed] [Google Scholar]
- 27.Keleku LN, Suzuki M, Yamamoto M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid Redox Signal. 2018;29:1746–1755. doi: 10.1089/ars.2017.7358. [DOI] [PubMed] [Google Scholar]
- 28.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 29.Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly(epsilon-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin Exp Pharmacol Physiol. 2009;36:1149–1156. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Lv JN, Li H, Jiao B, Zhang QH, Zhang Y, Zhang J, Liu YQ, Zhang M, Shan H, et al. Curcumin reduces lung inflammation via Wnt/β-catenin signaling in mouse model of asthma. J Asthma. 2017;54:335–340. doi: 10.1080/02770903.2016.1218018. [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019;14:e0216711. doi: 10.1371/journal.pone.0216711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng LZ, Chen LW, Hu XY, Lian J, Zhao GJ, Hong GL, Lu ZQ, Qiu QM. Curcumin upregitates mitochondrial fusion protein 2 to relieve acute lung injury in sepsis mice. Chin J Burns. 2020;29:58–64. [Google Scholar]
- 33.Qin K, Guo XJ. Research progress on the mechanism of curcumin in the treatment of chronic obstructive pulmonary disease. Chin J Respirat Cri Care. 2020;19:99–103. [Google Scholar]
- 34.Qin Z, Wang B, Tan ZX, Luo SH. Curcumin inhibits the TLR4/HMGB1 pathway to protect lipopolysaccharide from inducing acute lung injury. Chin J Clin Thor Cardiovasc Sur. 2020;27:89–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.