Abstract
Degenerative lumbar spinal stenosis is the most frequent cause of low back pain and/or sciatica in the elderly patient. Epidemiology, pathophysiology, clinical manifestations and testing are reviewed in a wide current bibliographic investigation. The importance of the relationship between clinical presentation and imaging study, especially magnetic resonance imaging (MRI), is emphasized. Prior to treatment indication, it is necessary to identify the precise location of pain, as well as the differential diagnosis between neurological and vascular lameness. Conservative treatment combining medications with various physical therapy techniques solves the problem in most cases, while therapeutic testing with injections, whether epidural, foraminal or facetary, is performed when pain does not subside with conservative treatment and before surgery is indicated. Injections usually perform better results in relieving sciatica symptoms and less in neurological lameness. Equine tail and/or root decompression associated or not with fusion is the gold standard when surgical intervention is required. Fusion after decompression is necessary in cases with segmental instability, such as degenerative spondylolisthesis. When canal stenosis occurs at multiple levels and is accompanied by axis deviation, whether coronal and/or sagittal, correction of axis deviations should be performed in addition to decompression and fusion, especially of the sagittal axis, in which a lumbar lordosis correction is required with techniques that correct the rectified lordosis to values close to the pelvic incidence.
Keywords: arthrodesis, intermittent claudication, decompression, stenosis, low back pain
Introduction
Lumbar spinal stenosis is defined as the narrowing of any vertebral canal, foramina or lateral recess leading to a clinical presentation of low back pain that can radiate to the buttocks and lower limbs and presenting well-defined precipitating and relief causes related to the compression of neurovascular structures within the lumbar canal.
Spinal stenosis causes
A. Congenital conditions, such as short pedicles or joint facets in anomalous orientation
-
B. Acquired conditions:
As a consequence of injuries: vertebra fracture with bone fragment projecting into the spinal cavity, vertebral dislocation;
Bone tumors or metastases from soft tissue neoplasia invading the spinal cavity;
Hematomas from different origins;
Abscess due to an infection originating in the vertebral body or intervertebral disc;
Several bone-metabolic or endocrine diseases such as acromegaly, renal osteodystrophy and hypoparathyroidism;
Other deforming bone diseases such as Paget disease, achondroplasia, rheumatoid arthritis, ankylosing spondylitis and diffuse idiopathic bone hyperostosis;
Iatrogenic conditions: postdecompression or bone resection surgery;
Degenerative disease due to degenerative discopathy and facet arthrosis (the most common cause).
C. Mixed causes: congenital stenosis associated with acquired stenosis: for example, short-pedicled spine cavity stenosis associated with disc arthrosis.
The present review focuses on lumbar spinal stenosis with a degenerative origin.
Epidemiology
Although the degenerative process affects virtually all spines after the 5 th and 6 th decades of life, only ∼6% of adults suffer from symptomatic lumbar spinal stenosis. 1 2
Degenerative spinal cavity stenosis associated with congenital conditions (such as a short pedicle or joint facets in sagittal orientation) may result in clinical manifestations in people aged 30 to 40 years old. 3
Lumbar spinal stenosis is the most common cause of lumbar spine diseases in patients > 65 years old requiring surgical treatment; it is estimated that ∼0.1% of the population will need some procedure to treat degenerative lumbar spine conditions. 4
Vertebral spinal cavity anatomy
The main limits of the vertebral spinal cavity include, anteriorly, the intervertebral disc and the vertebral body; laterally, the two pedicles and the interapophyseal joints along with their capsules; and posteriorly, laminas and ligamentum flavum.
In central stenosis, the spinal cavity narrows anteriorly due to the protrusion of an intervertebral disc and osteophytes in the posterior region of the vertebral bodies and, later, by the indentation of the ligamentum flavum, which is thickened. The spinal cavity space decreases with lumbar spine extension and increases with its flexion, characterizing the dynamic component of the symptomatology of spinal cavity stenosis. 5
Lateral recess stenosis presents with interapophyseal joint capsules hypertrophy; in more advanced states, the projection into the spinal cavity of osteophytes from the upper facets of the lower vertebra is the main cause, resulting in dural sac and adjacent radicular compression.
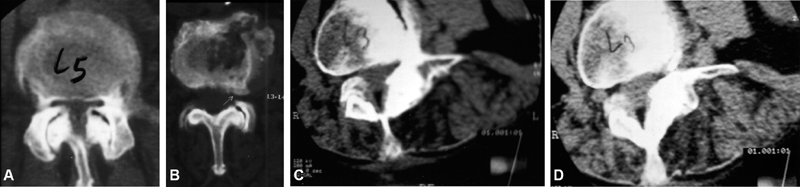
In foraminal stenosis, intervertebral disc protrusion is associated with an osteophyte formed at the upper articular apophysis of the lower vertebra, compressing the emerging nerve root. This is more frequent in the lower lumbar spine, where the foramen diameter is anatomically decreased and the nerve root diameter is increased, rendering the spine more susceptible to compressions even by smaller osteophytes ( Figure 1 ).
Fig. 1.
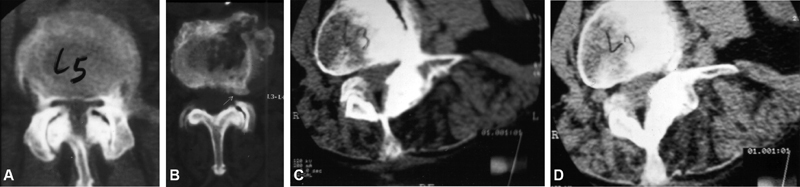
Axial computed tomography images showing: Central stenosis from (A) lateral recess and (B) foramen; (C and D) Degenerative scoliosis with vertebral rotation and laterolysthesis with foraminal stenosis.
Clinical presentation and natural history
Most stenosis cases become symptomatic after the 6 th decade of life; symptoms are usually insidious and related to L3-L4 and L4-L5 degeneration. In early stages, the majority of cases report recurrent low back pain that, over time, becomes permanent. This symptom is generally related to disc degeneration, in its various stages, and to the onset of facet arthrosis, characterized by synovitis.
In its evolution, low back pain can radiate to the flanks and gluteal region and, eventually, to the nerve root path, characterizing the probable association with a herniated disc or even foraminal or lateral recess stenosis.
In central stenosis, the classic symptom is neurogenic claudication, with lower limb pain, paresthesia and decreased strength in an insidious, slow progression. These symptoms are associated with walking or standing up and are relieved when the patient sits down, leans forward or lies down. 6
Symptoms can be better understood using the degenerative cascade reported by Kirkaldy-Willis et al, 7 which describes the evolution of the degenerative process both at intervertebral discs, joint facets and vertebral bodies levels.
A possible cause of degenerative lumbar stenosis is the sagittal orientation of joint facets, which can also result in congenital stenosis. 8
The final stage of the degenerative process consists in ankylosis of the compromised segment(s), which can result in worse clinical symptoms, with pain even during rest, and more severe neurological alterations, such as cauda equina syndrome or neurogenic bladder.
Physical exam
Physical examination findings in central spinal stenosis are often poor and not characteristic.
Lameness can manifest itself when the examiner asks the patient to walk or to extend the spine. In advanced stages, there is often lumbar lordosis rectification and sagittal balance loss, in which the patient bents forward.
Eventually, the patient may show radicular symptoms and signs in cases of foraminal stenosis, or a herniated disc associated with radiological signs of foraminal or lateral recess stenosis. Most of the time, there is no sign of radicular irritation or a positive Laségue sign.
Potential strength decreases in certain muscle groups, and corresponding changes in sensitivity and reflex responses, in a metameric distribution, are rarely present, depending on the location and degree of lumbar spinal stenosis.
In an international consensus, a group of 279 specialists from 29 countries concluded that 7 clinical signs and symptoms are required to be 80% sure of the presence of lumbar spinal stenosis based on history and physical examination, namely: gluteal region or lower limbs pain when walking; symptoms relief at anterior flexion; relief when using a supermarket cart or riding a bicycle; sensory or motor disturbances when walking; normal or symmetrical peripheral pulses; lower extremities weakness; and low back pain. 9
Occasionally, lumbar spinal stenosis is concomitant with cervical or dorsal spinal stenosis; signs of cervical radiculopathy or myelopathy predominate in such cases are characterized by spasticity, hyperreflexia, clonus and loss of balance.
Differential diagnosis between neurogenic and vascular claudication
The differential diagnosis between neurogenic and vascular claudication must be defined at the time of the physical examination of the patient. Treatment must only start after this differentiation.
Most vascular (ischemic) lameness cases present with calf pain with potential proximal extension. The arterial pulses of the lower limbs are often diminished, while aortic, femoral, popliteal, posterior tibial and dorsalis pedis arteries must be searched by palpation or auscultation.
The patient usually reports that symptoms are aggravated and relieved by activities in any position. Relief has nothing to do with flexion or extension. Riding a bicycle worsens symptoms of vascular stenosis, which does not happen in cases of neurogenic stenosis due to trunk flexion.
If vascular alterations are suspected, specific tests to investigate arterial and venous peripheral circulation must be requested.
In elderly patients, it is also important to exclude peripheral neuropathies, especially in diabetics. These neuropathies are mainly characterized by feet hypoesthesia, reduced vibratory sensitivity, nocturnal “burning” and lack of correlation with physical activity. In such cases, an electroneuromyography exam must be requested to establish the differential diagnosis.
Delamarter et al 10 and Rydevik et al 11 described the electrophysiological changes in nerve roots resulting from vascular congestion by extrinsic compression, as occurs in spinal stenosis. These authors attempted to demonstrate that most stenosis signs and symptoms are caused by a vascularization disturbance of the nervous structures, associated with inflammatory alterations, rather than compression itself. Thus, it can be concluded that the signs and symptoms from the result of degenerative spinal cavity stenosis are caused by a sum of mechanical, vascular and neurological changes.
Lumbar stenosis imaging
Lumbar stenosis is a clinical and radiological syndrome; the following information must be provided to radiologists in case of suspicion of lumbar stenosis: first, whether or not the patient meets stenosis criteria; second, in case there is a stenosis, detailed information about its location and the factor(s) causing it. This information is required to recommend appropriate treatment options.
The North American Spine Society guidelines state that imaging is the main noninvasive test for lumbar spinal stenosis diagnosis but does not provide radiological criteria for the condition. Most specialists in musculoskeletal radiology use qualitative criteria for lumbar spinal stenosis diagnosis. According to Genevay et al, 12 there are several criteria to describe lumbar spinal stenosis; however, they are not always clearly defined, potentially hindering a reliable diagnosis.
Qualitative criteria used for lumbar spinal stenosis diagnosis include:
Disc protrusion
Perineural fat fading
Joint facet degeneration and hypertrophy
Lack of fluid around the cauda equina
Ligamentum flavum hypertrophy
Cauda equina roots redundancy and serpentine shape
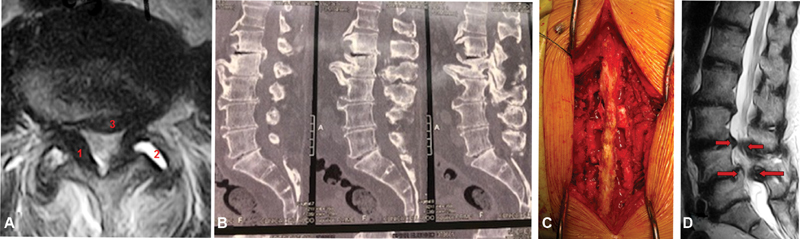
Fig. 2.
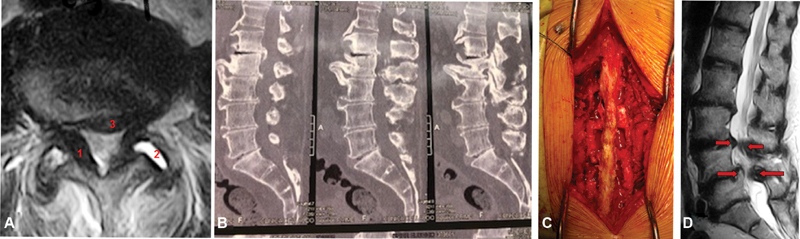
(A) Axial magnetic resonance imaging (MRI) showing spinal stenosis with ligamentum flavum thickening (1), facet arthrosis with synovitis (2) and disc protrusion (3). (B) Sagittal computed tomography (CT) scan showing L1-L2-L3-L4 spinal stenosis. (C) Surgical photography: three-level decompression using the conventional laminectomy technique plus lateral recess resection and bilateral foraminotomy. (D) Sagittal MRI showing degenerative L3-L4 and L4-L5 stenosis with degenerative spondylolisthesis in L4-L5.
Plain radiography
Spinal stenosis can be strongly suspected on plain radiographs of patients with back pain. The anteroposterior (AP) diameter of the vertebral canal increases caudally and it must be considered abnormal if it is < 12 mm at the lumbar spine 14 and < 10 mm at the cervical spine. 15 16
Magnetic resonance imaging and computed tomography
Magnetic resonance imaging (MRI) is suggested as the most appropriate noninvasive test to confirm the presence of anatomical narrowing of the vertebral canal or radicular impingement in patients with clinical suspicion of lumbar spinal stenosis. Lumbar spinal stenosis can be diagnosed based on the AP diameter of the spinal canal or the cross-sectional area of the dural sac.
The cross-sectional area of the dural sac is considered normal if it is > 100 mm 2 at its narrowest point; it is stenotic if it measures between 76 and 100 mm 2 and severely stenotic if it is < 76 mm 2 . Magnetic resonance imaging and computed tomography (CT) allow the direct visualization of central and lateral canals. Magnetic resonance imaging has the added benefit of soft tissue visualization. 17
Evaluation of the vertebral canal – diagnostic criteria
Bony canal AP diameter < 10 mm at the cervical spine or < 12 mm at the lumbar spine;
The cross-sectional area of the dural sac at its narrowest point is considered stenotic if it measures between 76 and 100 mm 2 and severely stenotic if it is < 76 mm 2 .
Evaluation of the neuroforamen and lateral recess – diagnostic criteria
Foraminal AP diameter < 3 mm in sagittal imaging is considered a diagnostic factor for stenosis;
Lateral recess height < 3 mm or a lateral recess angle < 30° are also evidence of spinal stenosis.
Functional spine stenosis
Functional spine stenosis is more important than bony spine stenosis in clinical presentation. 14 Functional spine stenosis is produced by several soft tissue components, such as disc protrusion; ligament hypertrophy; synovial cysts; and instabilities, which determine neurological symptoms and manifestations, often not demonstrated in imaging studies. Orthostatic scans (orthostatic MRI) and functional studies (dynamic radiographs) can help to confirm the stenosis diagnosis at imaging.
More recent neurography exams (specialized nerve resonance) can provide specific quantitative information about physiological root and ganglia changes through water diffusion from axons, contributing to a better understanding of symptoms and clinical correlation.
Lumbar spinal stenosis: conservative treatment
In 1993, Onel et al 17 published their experience with conservative treatment in a prospective study including 145 patients with lumbar spinal stenosis
Conservative treatment consisted of physical therapy with thermal analgesia plus exercises and calcitonin. The patients showed statistically significant improvement, except for deep reflex changes. The authors concluded that conservative treatment may be the method of choice in older patients and those without clinical conditions for decompression surgery.
If neurogenic claudication is not severe and there are no symptoms of motor deficit, the initial treatment must attempt to relieve pain with rest and changes in daily activities. Initially, analgesics and nonsteroidal anti-inflammatory drugs are recommended, possibly associated with muscle relaxants. In this phase, physical therapy with thermal analgesia, transcutaneous nerve stimulation (TENS) and light mobilization exercises with stretching and progressive muscle strength for postural correction can be performed for pain relief. Acupuncture, chiropractic procedures and McKenzie exercises can also be used for pain relief. None of these methods has proven superiority over another, and none provides significant neurogenic claudication improvement. 18 19
If there is a history of lower limb chronic pain, paresthesia, dysesthesia, or neuropathic pain, this treatment can be associated with tricyclic antidepressants and/or anticonvulsants such as gabapentin or pregabalin. Pregabalin is the drug of choice in neuropathic pain, with ∼ 40% of patients reporting relief. 20
In addition, analgesics, anti-inflammatories and anticonvulsants do not demonstrate great efficacy in neurogenic claudication improvement. Steroids may be indicated in case of radicular irritation worsening, always for a brief period of time to reduce the risk of side effects; narcotic analgesics can also be used.
Epidural block with steroid injection into the epidural space relieves spinal stenosis symptoms; this treatment is more efficient in radiculopathies than in neurogenic claudication, although there are no studies demonstrating its long-term effectiveness. Riew et al reported that 71% of patients who initially agreed with surgery gave up on it after being subjected to a selective injection of betamethasone and bupivacaine into nerve roots. 21
These blocks can be performed via interlaminar or caudal approaches and, when radicular pain predominates, a selective transforaminal injection into the nerve root with steroids and bupivacaine performed under fluoroscopic guidance is indicated.
Conservative or surgical treatment?
A study by Johnsson et al 22 reported the conservative treatment outcomes in a group of 49 patients, revealing that only 18% of them required surgical treatment.
Zaina et al 23 evaluated the effectiveness of the different surgical techniques and compared them with different types of conservative treatment for lumbar spinal stenosis; these authors concluded that it is impossible to say which therapy (surgical or conservative) is best due to the wide variety of performed approaches.
Patients usually opt for surgical treatment when the clinical presentation is aggravated by symptoms of radicular involvement, as in lateral recess stenosis. 24
Outcomes from surgical decompression associated or not with arthrodesis are superior in the first postoperative years and usually converge after 8 years of surgery. 25 26
Based on the various studies, it can be concluded that conservative treatment is the method of choice at an early stage for both radicular symptoms and neurogenic claudication, as long as there is no neurological impairment with motor deficit and progressive worsening for the former, or lameness at a short walking test for the latter. In such cases, surgical treatment is recommended. 27
Surgical treatment
Surgical treatment for lumbar spinal stenosis is indicated in cases of conservative treatment failure. It is also indicated in cases with very acute symptoms and radicular involvement associated with dermatome sensorial and motor changes and progressive worsening of severe neurogenic claudication. In these circumstances, symptoms must be related to imaging findings, which will guide the type of surgery to be performed regarding the segment and area requiring decompression.
It is also important to assess the need for decompression and arthrodesis in a situation of clinical and/or radiological instability, especially when imaging studies reveal a diagnosis of degenerative spondylolisthesis. The need for decompression associated with arthrodesis and deformity correction must be determined in both coronal and sagittal axis at the time of strategic planning for surgical intervention. The goal of surgical treatment is to improve function, relieve pain and reduce or prevent neurological deficit. To do so, neural structures decompression is required, and its extension will be determined by signs, symptoms and imaging findings from each case. Even when low back pain is important compared to radicular symptoms, pain relief is achieved in most cases undergoing decompression. 28
Vertebral canal decompression can be performed with several techniques. The gold standard is the open technique with laminectomy or laminotomy, in which laminae are resected or opened; next, the ligamentum flavum, usually thickened, is resected, exposing the nervous structures under compression. Laminotomy can be unilateral, bilateral or divide the spinous process. The latter decreases paravertebral musculature injury, reducing postoperative complications related to hematomas, seromas and infections and trunk extensor musculature atrophy. 29 30 The dural sac is decompressed after its exposure and removed to allow the resection of the lateral recess and foramen opening to decompress an adjacent and/or emerging nerve root ( Figures 2B and 2C ). Such decompression can also be achieved with minimally invasive techniques. 31
A bilateral foraminal decompression, if required, can be performed using several approaches: bilateral opening; unilateral opening to reach both lateral recesses and foramina; or unilateral approach, reaching the contralateral side using the endoscopic over-the-top technique ( Figure 2 ). 32
Today, there is a lot of discussion about outcomes from open techniques compared to minimally invasive techniques. Evidence from systematic reviews and meta-analyzes suggest that the unilateral laminectomy technique for minimally invasive bilateral decompression is associated with less blood loss and shorter hospital stay, with similar complications and long-term results compared with the open technique. 33
Pure foraminal stenosis can be resolved surgically with an open technique using laminectomy or a percutaneous endoscopic technique with osteophytes resection or discectomy. 34
Decompression surgery and arthrodesis
The need for arthrodesis after decompression due to lumbar spinal stenosis depends basically on the presence of clinical and/or radiological segmental instability
Radiological parameters must be considered when accompanied by an indicative clinical presentation of spine stenosis, remembering that variations occasionally exist with no significant symptoms. 35 36
Arthrodesis surgery associated with decompression is also indicated when a bilateral resection of > 50% of the facets is required to decompress neurological structures, leading to the risk of iatrogenic instability, or in the presence of progressive degenerative scoliosis with coronal and sagittal axis deviation and spinal stenosis at several levels. 37
Degenerative spondylolisthesis
Many symptomatic cases of lumbar spinal stenosis present with degenerative spondylolisthesis at imaging studies. The degree of facet and disc degeneration leading to listhesis varies significantly in each case and there are different degrees of upper vertebra slipping over lower vertebra in the AP or lateral direction. This translation can lead to nerve roots compression at the foramen or the lateral recess; in addition, it may decrease the diameter of the vertebral canal, leading to central stenosis ( Figure 2D ).
Decompression is sufficient in most cases requiring surgical treatment for degenerative spondylolisthesis. For broader facet resection or if there is evidence of clinical or radiological instability, decompression is associated with arthrodesis. 38
A meta-analysis from Martdjetko et al 39 on studies published from 1970 to 1993 about degenerative spondylolisthesis surgery showed better outcomes in patients undergoing decompression and arthrodesis, either in situ or instrumented, compared to those submitted only to decompression. 40
Arthrodesis associated with decompression for degenerative spondylolisthesis can be performed using an open posterolateral route or a minimally invasive technique – transforaminal lumbar interbody fusion (TLIF) or minimally invasive (MIS) TLIF. Published reports did not find major differences in outcomes from both techniques, except for the shortest hospitalization time, the least amount of bleeding and the least degree of pain in patients undergoing MIS TLIF. 41
A mini-open arthrodesis using the transmuscular posterolateral approach (Wiltse technique), which is less expensive and has the same outcomes as MIS TLIF, is the preference of the authors. 42 43
Interspinous devices
These devices intend to promote, through minimally invasive (mini-open) techniques, distraction between spinous processes to restore foraminal height and stabilize the affected segment. Several works were published when these devices were first introduced, showing promising results, superior to those obtained with the simple decompression technique. 44 45 46
However, in recent years, systematic reviews and meta-analyzes have questioned these outcomes, and the North American Spine Society stated that there is insufficient evidence to indicate the use of interspinous devices, which are considered an investigational technique. 47 48 49 50
Degenerative scoliosis
Degenerative scoliosis is the most advanced stage of the Kirkaldy-Willis degenerative cascade.
Coronal axis deformity is not usually accentuated in these circumstances and, in general, the Cobb angle is not superior to 30°. Sagittal axis deformity develops due to a progressive loss of lumbar lordosis, with consequent axial imbalance.
The spinopelvic relationship must be studied not only to assess the degree of sagittal axis imbalance, but also the degree of pelvic version, which may have increased to compensate such imbalance. It is also important to determine the lumbar lordosis discrepancy to the degree of pelvic incidence to plan the surgery that will eventually be required in case of worsening symptoms of pain, muscle fatigue, loss of strength and progressive walking difficulty. Restoring lumbar lordosis is the main goal when correcting degenerative scoliosis, associated with neural elements decompression and arthrodesis ( Figure 3A–F ). 51 52 53 54 55
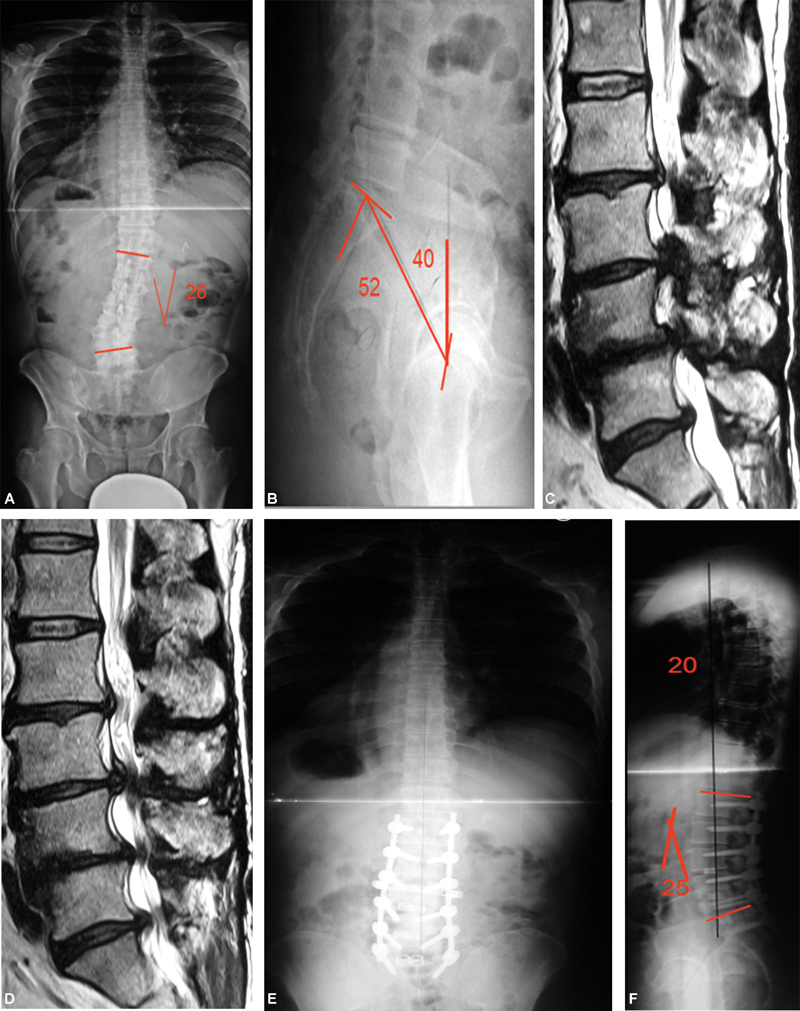
Fig. 3.
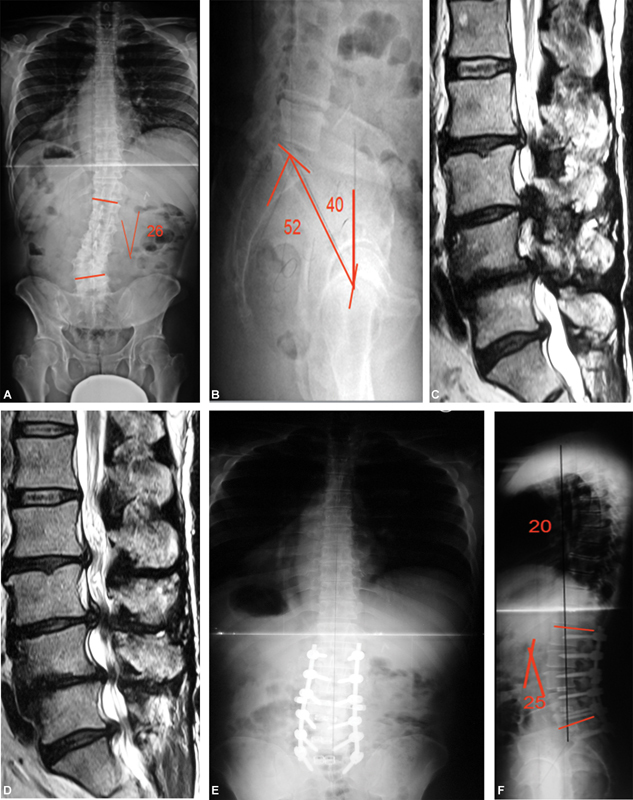
Degenerative scoliosis: (A and B) Radiography: degenerative scoliosis with decreased lumbar lordosis and increased pelvic version. (C and D) Sagittal magnetic resonance imaging: L2 to S1 spinal stenosis. (E and F) Postoperative radiography: deformities correction with increased lumbar lordosis and decreased pelvic version.
Today, to decrease the risk of complications, the association of a minimally invasive anterior (anterior lumbar interbody fusion [ALIF]), lateral (extreme lateral interbody fusion,[X-LIF]) or oblique (oblique lateral interbody fusion [OLIF]) arthrodesis 56 57 58 59 with posterior fixation is recommended when a large increase in lumbar lordosis is required, instead of posterior subtraction pedicular osteotomy.
Final considerations
It is crucial to know the pathophysiology of spinal stenosis and to determine the precise location of the cause of pain using clinical and imaging findings to indicate a conservative or surgical treatment. As for surgical treatment, knowing when to indicate only decompression or to associate it to arthrodesis is essential to obtain the best outcome.
Footnotes
Conflito de Interesses Os autores declaram não haver conflito de interesses.
Referências
- 1.Eisenstein S. The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br. 1977;59(02):173–180. doi: 10.1302/0301-620X.59B2.873978. [DOI] [PubMed] [Google Scholar]
- 2.Paine K W. Clinical features of lumbar spinal stenosis. Clin Orthop Relat Res. 1976;(115):77–82. [PubMed] [Google Scholar]
- 3.Grobler L J, Robertson P A, Novotny J E, Ahern J W. Decompression for degenerative spondylolisthesis and spinal stenosis at L4-5. The effects on facet joint morphology. Spine (Phila Pa 1976) 1993;18(11):1475–1482. [PubMed] [Google Scholar]
- 4.Turner J A, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992;17(01):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989;7(01):115–121. doi: 10.1002/jor.1100070116. [DOI] [PubMed] [Google Scholar]
- 6.Amundsen T, Weber H, Lilleås F, Nordal H J, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976) 1995;20(10):1178–1186. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Kirkaldy-Willis W H, Wedge J H, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine (Phila Pa 1976) 1978;3(04):319–328. doi: 10.1097/00007632-197812000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Zhao X, Long Y, Huang K, Xie D, Wang F. Facet Sagittal Orientation: Possible Role in the Pathology of Degenerative Lumbar Spinal Stenosis. Spine (Phila Pa 1976) 2018;43(14):955–958. doi: 10.1097/BRS.0000000000002493. [DOI] [PubMed] [Google Scholar]
- 9.Tomkins-Lane C, Melloh M, Lurie J. ISSLS Prize Winner: Consensus on the Clinical Diagnosis of Lumbar Spinal Stenosis: Results of an International Delphi Study. Spine (Phila Pa 1976) 2016;41(15):1239–1246. doi: 10.1097/BRS.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delamarter R B, Bohlman H H, Dodge L D, Biro C. Experimental lumbar spinal stenosis. Analysis of the cortical evoked potentials, microvasculature, and histopathology. J Bone Joint Surg Am. 1990;72(01):110–120. [PubMed] [Google Scholar]
- 11.Rydevik B, Brown M D, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine (Phila Pa 1976) 1984;9(01):7–15. doi: 10.1097/00007632-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Genevay S, Atlas S J, Katz J N. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine (Phila Pa 1976) 2010;35(07):803–811. doi: 10.1097/BRS.0b013e3181bc9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamisch N, Brumann M, Hodler J, Held U, Brunner F, Steurer J. Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology. 2012;264(01):174–179. doi: 10.1148/radiol.12111930. [DOI] [PubMed] [Google Scholar]
- 14.Gopinathan P. Lumbar spinal canal stenosis-special features. J Orthop. 2015;12(03):123–125. doi: 10.1016/j.jor.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo M, Keith Smith J, Mukherji Suresh K. Philadelphia: Lippincott-Raven; 1998. The spine; pp. 1451–1454. [Google Scholar]
- 16.Verbiest H.Neurogenic Intermittent Claudication with Special Reference to Stenosis of the Lumbar Vertebral Canal in Hand Book of Clinical Radiology Amsterdam: North Holand pub co.1976;611:e807 [Google Scholar]
- 17.Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine (Phila Pa 1976) 1993;18(02):291–298. [PubMed] [Google Scholar]
- 18.Tomkins C C, Dimoff K H, Forman H S. Physical therapy treatment options for lumbar spinal stenosis. J Back Musculoskeletal Rehabil. 2010;23(01):31–37. doi: 10.3233/BMR-2010-0245. [DOI] [PubMed] [Google Scholar]
- 19.Donelson R, Silva G, Murphy K. Centralization phenomenon. Its usefulness in evaluating and treating referred pain. Spine (Phila Pa 1976) 1990;15(03):211–213. [PubMed] [Google Scholar]
- 20.Moore R A, Straube S, Wiffen P J, Derry S, McQuay H J. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(03):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riew K D, Yin Y, Gilula L. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82(11):1589–1593. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Johnsson K E, Udén A, Rosén I. The effect of decompression on the natural course of spinal stenosis. A comparison of surgically treated and untreated patients. Spine (Phila Pa 1976) 1991;16(06):615–619. doi: 10.1097/00007632-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical Versus Nonsurgical Treatment for Lumbar Spinal Stenosis. Spine (Phila Pa 1976) 2016;41(14):E857–E868. doi: 10.1097/BRS.0000000000001635. [DOI] [PubMed] [Google Scholar]
- 24.Kurd M F, Lurie J D, Zhao W. Predictors of treatment choice in lumbar spinal stenosis: a spine patient outcomes research trial study. Spine (Phila Pa 1976) 2012;37(19):1702–1707. doi: 10.1097/BRS.0b013e3182541955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atlas S J, Keller R B, Wu Y A, Deyo R A, Singer D E. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30(08):936–943. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 26.Malmivaara A, Slätis P, Heliövaara M. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;32(01):1–8. doi: 10.1097/01.brs.0000251014.81875.6d. [DOI] [PubMed] [Google Scholar]
- 27.Amundsen T, Weber H, Nordal H J, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine (Phila Pa 1976) 2000;25(11):1424–1435. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 28.Srinivas S, Paquet J, Bailey C. Effect of spinal decompression on back pain in lumbar spinal stenosis: a Canadian Spine Outcomes Research Network (CSORN) study. Spine J. 2019;19(06):1001–1008. doi: 10.1016/j.spinee.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Rajasekaran S, Thomas A, Kanna R M, Prasad Shetty A. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: a prospective, randomized controlled study of 51 patients. Spine (Phila Pa 1976) 2013;38(20):1737–1743. doi: 10.1097/BRS.0b013e3182a056c1. [DOI] [PubMed] [Google Scholar]
- 30.Cho D Y, Lin H L, Lee W Y, Lee H C. Split-spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: a preliminary report. J Neurosurg Spine. 2007;6(03):229–239. doi: 10.3171/spi.2007.6.3.229. [DOI] [PubMed] [Google Scholar]
- 31.Lee C H, Choi M, Ryu D S. Efficacy and Safety of Full-endoscopic Decompression via Interlaminar Approach for Central or Lateral Recess Spinal Stenosis of the Lumbar Spine: A Meta-analysis. Spine (Phila Pa 1976) 2018;43(24):1756–1764. doi: 10.1097/BRS.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 32.Siepe C J, Sauer D, Michael Mayer H. Full endoscopic, bilateral over-the-top decompression for lumbar spinal stenosis. Eur Spine J. 2018;27 04:563–565. doi: 10.1007/s00586-018-5656-3. [DOI] [PubMed] [Google Scholar]
- 33.Rosen D S, O'Toole J E, Eichholz K M. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(03):503–509. doi: 10.1227/01.NEU.0000255332.87909.58. [DOI] [PubMed] [Google Scholar]
- 34.Woo Y H, Jung H T, Kim I B, Sun W S, Jung D W. Percutaneous Transforaminal Endoscopic Decompression for Lumbar Foraminal Stenosis. J Clin Exp Orthop. 3:42. [Google Scholar]
- 35.Leone A, Guglielmi G, Cassar-Pullicino V N, Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245(01):62–77. doi: 10.1148/radiol.2451051359. [DOI] [PubMed] [Google Scholar]
- 36.da Costa L M, Hennemann A S, de Abreu M R, Antoneli P H. Correlação entre instabilidade radiográfica e presença do sinal de modic. Coluna/Columna. 2011;10(02):132–135. [Google Scholar]
- 37.Hansraj K K, O'Leary P F, Cammisa F P., Jr Decompression, fusion, and instrumentation surgery for complex lumbar spinal stenosis. Clin Orthop Relat Res. 2001;200(384):18–25. doi: 10.1097/00003086-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder G D, Kepler C K, Kurd M F. Rationale for the Surgical Treatment of Lumbar Degenerative Spondylolisthesis. Spine (Phila Pa 1976) 2015;40(21):E1161–E1166. doi: 10.1097/BRS.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 39.Mardjetko S M, Connolly P J, Shott S.Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970-1993 Spine (Phila Pa 1976) 199419(20, Suppl)2256S–2265S. [PubMed] [Google Scholar]
- 40.McAnany S J, Baird E O, Qureshi S A, Hecht A C, Heller J G, Anderson P A. Posterolateral Fusion Versus Interbody Fusion for Degenerative Spondylolisthesis: A Systematic Review and Meta-Analysis. Spine (Phila Pa 1976) 2016;41(23):E1408–E1414. doi: 10.1097/BRS.0000000000001638. [DOI] [PubMed] [Google Scholar]
- 41.Hammad A, Wirries A, Ardeshiri A, Nikiforov O, Geiger F. Open versus minimally invasive TLIF: literature review and meta-analysis. J Orthop Surg Res. 2019;14(01):229. doi: 10.1186/s13018-019-1266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pakzaban P. Modified Mini-open Transforaminal Lumbar Interbody Fusion: Description of Surgical Technique and Assessment of Free-hand Pedicle Screw Insertion. Spine (Phila Pa 1976) 2016;41(18):E1124–E1130. doi: 10.1097/BRS.0000000000001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge D H, Stekas N D, Varlotta C G. Comparative Analysis of Two Transforaminal Lumbar Interbody Fusion Techniques: Open TLIF Versus Wiltse MIS TLIF. Spine (Phila Pa 1976) 2019;44(09):E555–E560. doi: 10.1097/BRS.0000000000002903. [DOI] [PubMed] [Google Scholar]
- 44.Zucherman J F, Hsu K Y, Hartjen C A. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine (Phila Pa 1976) 2005;30(12):1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 45.Kuchta J, Sobottke R, Eysel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18(06):823–829. doi: 10.1007/s00586-009-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter A, Schütz C, Hauck M, Halm H. Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients. Eur Spine J. 2010;19(02):283–289. doi: 10.1007/s00586-009-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strömqvist B H, Berg S, Gerdhem P. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: randomized controlled trial with 2-year follow-up. Spine (Phila Pa 1976) 2013;38(17):1436–1442. doi: 10.1097/BRS.0b013e31828ba413. [DOI] [PubMed] [Google Scholar]
- 48.Poetscher A W, Gentil A F, Ferretti M, Lenza M. Interspinous process devices for treatment of degenerative lumbar spine stenosis: A systematic review and meta-analysis. PLoS One. 2018;13(07):e0199623. doi: 10.1371/journal.pone.0199623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gazzeri R, Galarza M, Alfieri A. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: past, present, and future. BioMed Res Int. 2014;2014:975052. doi: 10.1155/2014/975052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gazzeri R, Galarza M, Neroni M. Failure rates and complications of interspinous process decompression devices: a European multicenter study. Neurosurg Focus. 2015;39(04):E14. doi: 10.3171/2015.7.FOCUS15244. [DOI] [PubMed] [Google Scholar]
- 51.Yong-Hing K, Kirkaldy-Willis W H. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14(03):491–504. [PubMed] [Google Scholar]
- 52.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976) 2005;30(03):346–353. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 53.Glassman S D, Berven S, Bridwell K, Horton W, Dimar J R. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30(06):682–688. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y J, Bridwell K H, Lenke L G, Rhim S, Cheh G. An analysis of sagittal spinal alignment following long adult lumbar instrumentation and fusion to L5 or S1: can we predict ideal lumbar lordosis? Spine (Phila Pa 1976) 2006;31(20):2343–2352. doi: 10.1097/01.brs.0000238970.67552.f5. [DOI] [PubMed] [Google Scholar]
- 55.Rose P S, Bridwell K H, Lenke L G. Role of pelvic incidence, thoracic kyphosis, and patient factors on sagittal plane correction following pedicle subtraction osteotomy. Spine (Phila Pa 1976) 2009;34(08):785–791. doi: 10.1097/BRS.0b013e31819d0c86. [DOI] [PubMed] [Google Scholar]
- 56.Mundis G M, Akbarnia B A, Phillips F M.Adult deformity correction through minimally invasive lateral approach techniques Spine (Phila Pa 1976) 201035(26, Suppl)S312–S321. [DOI] [PubMed] [Google Scholar]
- 57.Phillips F M, Isaacs R E, Rodgers W B. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38(21):1853–1861. doi: 10.1097/BRS.0b013e3182a43f0b. [DOI] [PubMed] [Google Scholar]
- 58.Amaral R, Marchi L, Oliveira L. Opção minimamente invasiva lateral para artrodese intersomática tóraco-lombar. Coluna/Columna. 2011;10(03):239–243. [Google Scholar]
- 59.Abbasi H, Miller L, Abbasi A, Orandi V, Khaghany K. Minimally invasive scoliosis surgery with oblique lateral lumbar interbody fusion: single surgeon feasibility. Cureus. 2017;9(06):e1389. doi: 10.7759/cureus.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]