Abstract
Objective
To report a unique case and literature review of post COVID-19 associated transverse myelitis and dysautonomia with abnormal MRI and CSF findings.
Background
Coronavirus disease have been reported to be associated with several neurological manifestations such as stroke, Guillain-Barré syndrome, meningoencephalitis amongst others. There are only few reported cases of transverse myelitis with the novel coronavirus (n-CoV-2) and only one reported case identifying dysautonomia in COVID-19 patient. Here, we identify a COVID-19 patient diagnosed with acute transverse myelitis in addition to dysautonomia following with complete resolution of symptoms.
Method
A retrospective chart review of a patient diagnosed with post SARS-CoV-2 infection acute transverse myelitis and dysautonomia, and a review of literature of all the reported cases of transverse myelitis and COVID-19, from December 1st, 2019 till December 25th, 2020, was performed.
Conclusion
To our knowledge, this is the first reported case of transverse myelitis and dysautonomia in a patient with SARS-CoV-2 infection, who responded to intravenous methyl prednisone and bromocriptine. Follow-up imaging of the spine showed complete resolution of the lesion. Further studies would be recommended to identify the underlying correlation between COVID-19 and transverse myelitis.
Keywords: COVID-19, Coronavirus disease 2019, SARS-CoV-2, Transverse myelitis, MRI spine, Autoimmune disorder, Dysautonomia
Abbreviation: SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; RT PCR, Reverse Transcription Polymerase Chain; ATM, Acute Transverse Myelitis; CLOCC, Cytotoxic Lesion of Corpus Callosum; ADEM, Acute Disseminated Encephalomyelitis; MERS, Mild Encephalitis/Encephalopathy with Reversible Splenial Lesion; CIS, Clinical Isolated Syndrome; AHNE, Acute Hemorrhagic Necrotizing Encephalopathy; GBS, Guillain-Barré syndrome; CSF, Cerebrospinal fluid; CNS, Central Nervous System; PNS, Peripheral Nervous System; MS, Multiple Sclerosis; NMO, Neuromyelitis Optica
1. Introduction
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which originated in Wuhan, China, is now a worldwide pandemic (Huang et al., 2020). Coronavirus disease 2019 (COVID-19) associated neurological complications and presentations have been reported in one-third of the hospitalized patients (Munhoz et al., 2020). However, there is sparsity in available literature on complications following recovery from SARS-CoV-2 infection (Chowdhary et al., 2020; Sriwastava et al., 2021; Berger, 2020).
Reported neurological manifestations of SARS-CoV-2 infection includes stroke, particularly acute ischemic stroke, seizures, meningoencephalitis, Guillain-Barré syndrome (GBS) myasthenia gravis (MG) and even pseudo-relapse of neurological disorders such as multiple sclerosis (MS) (Chowdhary et al., 2020; Sriwastava et al., 2021; Berger, 2020; Mao et al., 2020; Kataria et al., n.d.; Sriwastava et al., 2020). However, there are only a few reported cases of neurological complications following complete recovery from SARS-CoV-2 infection (Scheidl et al., 2020).
A very rare neurological complication of SARS-CoV-2 infection includes transverse myelitis. Till date, a total of ten cases of transverse myelitis from acute SARS-CoV-2 infection have been reported (Sarma and Bilello, 2020; Zhao et al., 2020; Chow et al., 2020; Chakraborty et al., 2020; Valiuddin et al., 2020; AlKetbi et al., 2020; Durrani et al., 2020; Munz et al., 2020; Zachariadis et al., 2020; Abdelhady et al., 2020). Moreover, transverse myelitis with dysautonomia as a manifestation of COVID-19 hasn't been addressed as of yet in the literature.
Here we present a case of acute transverse myelitis and dysautonomia involving the cervical and thoracic spinal cord following SARS-CoV-2 infection, with complete recovery following treatment with intravenous steroid.
2. Case report
A 41-year-old man with no significant past medical history presents to the emergency department (ED) with new onset paresthesia (numbness and tingling) in bilateral upper and lower extremities along with urinary and fecal retention which stared two days prior to his presentation. Of note, he had visited his primary care clinic few weeks ago for headache, nausea and low-grade fever; and was tested for COVID-19 which turned out to be positive. He had an exposure to his friend who had mild COVID like symptoms. He did not require any intervention at that time and was asked to self-quarantine for a period of two weeks. During this span, he experienced mild symptoms of fatigue, myalgia, and low-grade fever. He presented at nearest emergency department with difficulty in voiding urine, and vomiting a week after his diagnosis; for which he underwent straight catherization after which he was discharged.
He was doing fine until the second week after diagnosis; when he was brought to the ED second time with headache, acute onset low-grade fever, extreme fatigue, retention of urine, neck and mid-thoracic back pain with generalized weakness. In the ED, he had foley catherization and was admitted for further management. He also complained of no bowel movement for more than a week, and new onset of numbness since few days which he described as patchy in distribution; involving right side of his face along with right side involvement of upper and lower extremities. He also mentioned tingling in right lower extremity and difficulty in ambulating requiring assistance for walking. He denied any vision changes, difficulty in swallowing or speech, shortness of breath, chest pain, and loss of smell or taste sensation. There was also no history of preceding diarrhea, recent vaccinations or any trauma.
On arrival, he was lethargic, oriented x3, afebrile and vitals were stable. Physical examination demonstrated cranial nerves were intact, however motor strength showed MRC grade of 4/5 throughout right lower extremity. Upper motor neuron signs were present in bilateral lower extremities with Grade 3+ reflex, positive bilateral Babinski reflex and decreased sensation in right lower extremity with sensory level at T6. Proprioception and vibration sensation were intact.
Initial workup revealed elevated C-reactive protein (78.1 mg/l), serum ferritin (592 ng/ml), lactate dehydrogenase (41 U/l), D-dimer (208 ng/ml). Nasopharyngeal RT-PCR was negative, but serum SARS-CoV-2 IgG turned out to be positive. Chest radiograph was unremarkable.
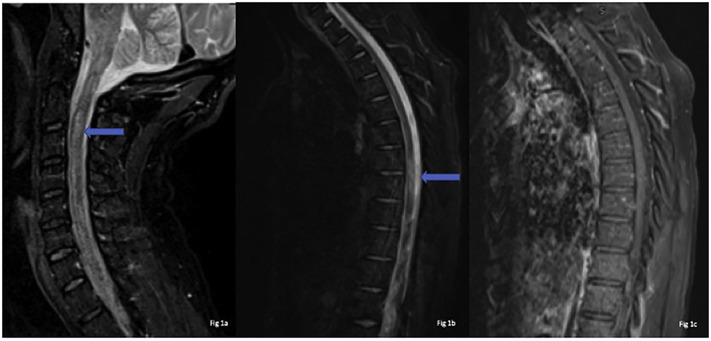
For etiological workup of fatigue, myalgia and upper motor neuron deficits, MRI of the brain, cervical spine, thoracic spine and lumbar spine were obtained with intravenous contrast administration. MRI cervical and thoracic spine demonstrated abnormal patchy T2 hyperintense signals involving the spinal cord longitudinally from C2 through C6 levels (Fig. 1a) and from T3 through T5 levels (Fig. 1b) without any abnormal enhancement (Fig. 1c). Differentials included meningoencephalomyelitis, multiple sclerosis (MS), neuromyelitis Optica (NMO), myelin oligodendrocyte glycoprotein (MOG) and post SARS-CoV-2 transverse myelitis.
Fig. 1.
MRI sagittal STIR weighted image of Cervical spine – Fig. 1(a), sagittal Thoracic spine 1(b) & sagittal T1 post-contrest 1(c) reveals ill defined long segment signal alteration with mild cord expansion (blue arrow) 1(a) & (b) and no abnormal enhancement or the post-contrast study 1(c).
Serum panel was unremarkable for antinuclear antibodies (ANA), neutrophil cytoplasmic antibodies (ANCA), ENA screen, SS-A/Ro and SS-B/La antibodies, Anti-Smith antibodies, RNP antibodies, Anti-Scl-70 antibodies, Anti-double stranded DNA antibodies, Anti-chromatin antibodies, Anti-centromere antibodies, antimitochondrial antibodies, and TB QuantiFERON test.
Lumbar puncture was performed which revealed CSF pleocytosis (230 nucleated cells) with 56% lymphocytic predominance, 32% neutrophils, and a CSF protein of 62 mg/dl with CSF glucose of 44 mg/dl. CSF oligoclonal bands and NMO panel was negative. CSF culture and PCR were negative for Borrelia burgdorferi, Varicella-Zoster virus, enterovirus, Herpes simplex virus1/2, Cryptococcal Antigen, Cytomegalovirus, and VDRL. CSF RT-PCR and CSF IgM, IgG Antibody tests were not performed for SARS-CoV-2.
Serum NMO/AQP4, MOG antibodies (Ab), HTLV I/II Ab, MTB PCR, lymphocytic choriomeningitis virus (titer <1:10), listeria Ab, west nile virus IgM, IgG and PCR, were all negative. Myelin basic protein, serum and CSF autoimmune panel were negative including anti-NMDA-receptor, anti-CASPR2, anti-LGl1, anti-GABAB-receptor, anti-AMPA. Overall, findings were compatible with inflammatory central nervous system disorder and the presentation was thought to be from post viral transverse myelitis.
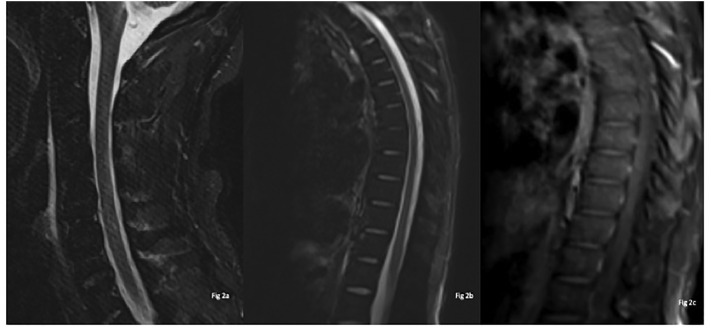
The patient was initiated on intravenous methyl prednisone and was managed with foley catheter placement for neurogenic bladder. Patient reported gradual improvement in symptoms at day 6 of admission, with improved mentation, increase in muscle strength and decrease in paresthesia. Repeat MRI of the cervical and thoracic spine was performed on day 10 of admission, which showed interval resolution of previously identified signal abnormalities in the cervical and thoracic spine (Fig. 2 ). Following 5 days of intravenous methyl prednisone, he was transitioned to oral prednisone taper. He was categorized as mild COVID-19 based on IDSA/ATS guidelines (Metlay et al., 2019).
Fig. 2.
Follow-up scan after 10 days from the first scan: MRI sagittal STIR weighted sagittal of Cervical spine Fig. 2(a), sagittal Thoracic spine 2(b) & sagittal T1 post-contrast 2(c) reveals no abnormal hyperintense cord signal changes and no abnormal enhancement on post contrast study 2(c).
Of note, during the course of admission on day 4, patient had unstable blood pressure and episodes of tachyarrhythmias concerning for dysautonomia. Patient had frequent episodes of transient elevations in blood pressure ranging in 160 s to 180 s, and asymptomatic sinus tachycardia in 120 s. He was started on IV labetalol 10 mg push and was switched to IV enalapril 1.25 mg q6 hourly and as needed. He was also started on oral amlodipine 10 mg daily and oral bromocriptine 2.5 mg q8 hourly. Dysautonomia eventually resolved by the time of discharge, and amlodipine as well as bromocriptine were discontinued.
Patient was discharged to skilled nursing facility on day 15 with oral prednisone for 3 weeks. He continued to show improvement in sensory and motor deficits. However, urinary retention persisted, which required indwelling foley catheter on discharge. Patient was followed-up with his urologist within a week of discharge and his foley catheter was removed. The patient will be followed up in neurology clinics in 6 weeks. Though NMO antibody tests were negative, NMOSD can still be considered in the differentials and so a repeat MRI spine including cervical and thoracic will be performed; along with repeat serum NMO antibody test on the patient's follow-up visit.
3. Discussion
Several studies describing the neurological manifestations of the novel coronavirus disease have been published recently showing its potential to invade both the central nervous system (CNS) as well as the peripheral nervous system (PNS). In one of the most comprehensive reviews on neurological imaging findings and COVID-19 according to our knowledge, we found that more than one third of hospitalized patients with COVID-19 developed some form of neurologic symptoms (Munhoz et al., 2020; Chowdhary et al., 2020; Berger, 2020).
The manifestations include: stroke (both ischemic and hemorrhagic), encephalitis, CNS inflammatory disorder such as acute disseminated encephalomyelitis (ADEM), clinical isolated syndrome (CIS), vasculitis, cytotoxic lesion of corpus callosum (CLOCC) or mild encephalitis/encephalopathy with reversible splenial lesion (MERS), transverse myelitis, acute hemorrhagic necrotizing encephalopathy (AHNE), multiple sclerosis (MS) and PNS disorder such as Guillain-Barré syndrome (GBS), myasthenia gravis (Chowdhary et al., 2020; Sriwastava et al., 2021; Berger, 2020; Kataria et al., n.d.; Sriwastava et al., 2020; Poyiadji et al., 2020). Only a few case reports of COVID-19 related spinal cord disorders are described in the literature, which include transverse myelitis, and acute disseminated encephalomyelitis (Chow et al., 2020; Chakraborty et al., 2020; Zhang et al., 2020). Additionally, literature on correlation of SARS-CoV-2 and dysautonomia is very scarce. To our knowledge, a study by Eshak N et.al is the only report which identifies dysautonomia in a COVID-19 patient (Eshak et al., 2020). We present a case of acute transverse myelitis with dysautonomia, possibly due to COVID-19 infection, and review the available literatures on transverse myelitis associated with COVID-19.
Transverse myelitis has been attributed to infectious, parainfectious, systemic autoimmune diseases, paraneoplastic, and ischemic diseases (Joshi et al., 2020; Kincaid and Lipton, 2020; Lycklama et al., 2020; Borchers and Gershwin, 2020). Acute infectious transverse myelitis is usually caused by herpes simplex virus type 2, varicella zoster virus (VZV), Epstein-Barr virus (EBV) or cytomegalovirus (CMV), flaviviruses and enteroviruses (Joshi et al., 2020; Kincaid and Lipton, 2020). Our case report points towards COVID-19 as a possible cause of acute transverse myelitis.
Acute transverse myelitis basically presents with a varied signs and symptoms of sensory, motor and autonomic dysfunction. Pathological changes in the spinal cord tract are seen on spinal imaging, as a result of focal inflammation. As this disorder can lead to debilitating effects and permanent disability, it is quintessential to recognize it early and distinguish it from other neurological entities. The diagnosis of transverse myelitis involves characteristic clinical presentation of bilateral signs and symptoms with a clearly defined sensory level, in addition to evidence on neuroimaging, CSF and serologic studies (Proposed diagnostic criteria and nosology of acute transverse myelitis, 2002). The inclusion criteria of transverse myelitis include the non-specific hyperintense lesion of spinal cord and isolated grey matter involvement which makes the differential of demyelinating diseases less likely as MS affects short segments usually and concerns white matter (Lycklama et al., 2020). Nevertheless, neuromyelitis optica spectrum of disorders (NMOSD) stand as a significant differential due to longitudinally extensive spinal cord involvement but since it is usually seen surrounding the central canal of spinal cord, it can be ruled out in MRI findings to make transverse myelitis the definitive choice of diagnosis (Kim et al., 2020).
Table 2 includes Transverse Myelitis Consortium Working Group criteria which is used for diagnosis of cases of transverse myelitis (Proposed diagnostic criteria and nosology of acute transverse myelitis, 2002). However, on a comprehensive literature review, we found that amongst the ten reported cases of COVID-19 associated transverse myelitis, only 9 could fulfill the criteria. A case report by Zhao et al., did not have adequate investigations or their investigations findings lacked essential data to fulfill all the inclusion criteria for diagnosis of transverse myelitis (Zhao et al., 2020) (refer Table 1, Table 2 ). Majority (6 out of 10) of the cases reported a high protein value in CSF, some reported pleocytosis (4 out of 10), while the WBC counts were inconsistent in the majority of the cases. The time elapsed between development of the symptoms suspicious of transverse myelitis and COVID-19 RT-PCR positivity varied between 2 and 12, with an average of ~7 days (refer Table 1).
Table 2.
Transverse myelitis consortium working group criteria's (Proposed diagnostic criteria and nosology of acute transverse myelitis, 2002)
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Development of sensory, motor, or autonomic dysfunction attributable to the spinal cord | History of previous radiation to the spine within the last 10 y |
| Bilateral signs and/or symptoms (though not necessarily symmetric) | Clear arterial distribution clinical deficit consistent with thrombosis of the anterior spinal artery |
| Clearly defined sensory level | Abnormal flow voids on the surface of the spinal cord c/w AVM |
| Exclusion of extra-axial compressive etiology by neuroimaging (MRI or myelography; CT of spine not adequate) | Serologic or clinical evidence of connective tissue disease (sarcoidosis, Behcet's disease, Sjogren's syndrome, SLE, mixed connective tissue disorder, etc.)* |
| Inflammation within the spinal cord demonstrated by CSF pleocytosis or elevated IgG index or gadolinium enhancement. If none of the inflammatory criteria is met at symptom onset, repeat MRI and lumbar puncture evaluation between 2 and 7 d following symptom onset meet criteria | CNS manifestations of syphilis, Lyme disease, HIV, HTLV-1, Mycoplasma, other viral infection (e.g. HSV1, HSV-2, VZV, EBV, CMV, HHV-6, enteroviruses)* |
| Progression to nadir between 4 h and 21 d following the onset of symptoms (if patient awakens with symptoms, symptoms must become more pronounced from point of awakening) | Brain MRI abnormalities suggestive of MS* |
| History of clinically apparent optic neuritis* |
Table 1.
Review of published cases of COVID-19 and Transverse myelitis.
| Author/country | Patient age/gender | Time duration from COVID−19 to neurological symptom onset | Clinical presentation | Lab work, CSF, serological and immunologic markers | MRI findings | Management | Outcomes | Severitya based on IDSA/ATS |
|---|---|---|---|---|---|---|---|---|
| Sarma D et al. /USA | 28y/F | 7 days | Paresthesia in all extremities, as well as numbness to the tip of her tongue and urinary retention | Lumbar puncture showed 125/per microliter (/μl) mononuclear cells,60 mg/dl protein, normal glucose negative antibodies; and gram stain and culture negative for infection. No serum immunological markers available. |
Magnetic resonance imaging (MRI) with and without contrast of the cervical, thoracic, and lumbar spine showed elongated signal changes throughout the spinal cord to the conus medullaris. Had reported abnormal enhancement Not mentioned about level. |
The patient was started on prednisolone and received two plasma exchange treatments. | Rapid improvement in symptoms, discharged on a steroid taper | Non-severe |
| Kang Zhao et al. /China | 66y/M | 5 days | Developed weakness in both lower limbs with urinary and bowel incontinence. | CSF testing was not performed for pandemic-related reasons during hospitalization. | MRI of spinal cord was not performed for pandemic during hospitalization. He was diagnosed with transverse myelitis based on clinical suspicion. | Treated with ganciclovir for 14 days, lopinavir/ritonavir for 5 days, broad spectrum antibiotics and dexamethasone for 10 days; human immunoglobulin (15 g once daily) for 7 days. | The muscle strength partial improvement. Discharged to rehabilitation therapy. |
Severe |
| Chow CCN et.al/Australia | 60y/M | 10 days | Bilateral lower limb weakness, urinary retention and constipation | CSF findings glucose of 58 mg/dl, protein 79 mg/dl, WBC <5/μl. Serum NMO, MOG AB negative. |
MRI scan of thoracic spine showed T2 hyperintense signal from T7 to T10, without abnormal enhancement. | IV methyl prednisone 1 g per day for 3 days. | Neurological symptoms improved shortly after completion of corticosteroid therapy. | Non-severe |
| Chakraborty U et al. / India | 59y/F | 4 days? | Ascending flaccid paraplegia along with retention of urine and constipation. | CSF findings glucose 75 mg/dl, protein 72 mg/dl, WBC 5/μl all lymphocytes No serum immunological markers available. |
MRI T2-weighted imaging of thoracic spine revealed hyperintensity signal at T6–T7. Post contrast study not reported. | IV methyl-prednisolone at 1 g/day. | However, developed respiratory failure and cardiac arrest and deceased. | Severe |
| Valiuddin H et al. /USA | 61y/F | 7 days | Paresthesia over hand and feet followed by severe weakness in lower extremities and constipation and difficulty in voiding urine | CSF findings glucose of 73 mg/dl, protein of 87 mg/dl, and WBC 3/μl, negative gram stain and culture. No serum immunological markers available. |
MRI of spine revealed extensive hyperintense signal entire length of the cervical spine without abnormal contrast enhancement. | IV Methylprednisolone for 5 days with no improvement and underwent five sessions of plasmapheresis. | Partial improvement undergoing inpatient physical rehabilitation, continue to be paraplegia, neurogenic bladder. |
Non-severe |
| Alkebti R et al./UAE | 32 y/M | 2 days | Bilateral lower limb weakness, difficulty in passing urine | LP was not done as the patient was started on anticoagulants for pulmonary embolism. Immunological screening was positive for Lupus anticoagulant and in conjunction with the low Protein S. |
MRI of spine revealed extensive hyperintense signal in cervical, thoracic spine without abnormal contrast enhancement. | IV methylprednisolone for 5 days, Acyclovir and Enoxaparin. | Regained partial improvement in motor strength. | Non-severe |
| Durrani M et al./USA | 24 y/M | 9 days | bilateral lower-extremity weakness in addition to developing overflow urinary incontinence | CSF studies lymphocytic pleocytosis, normal glucose and protein levels. CSF-specific oligoclonal bands, aquaporin-4 antibodies were negative. Autoimmune panel negative. | The MRI showed a non-enhancing T2-weighted hyperintense signal T7-T12 level. No abnormal enhancement seen. |
(IV) methylprednisolone | demonstrated clinical improvement. | Non-severe |
| Munz M et al. / Germany | 60 y/M | 8 days | Retention of urine and progressive weakness of the lower limbs | CSF showed lymphocytic pleocytosis (16/μl) and protein level (79 mg/dl). CSF oligoclonal bands were negative. Antineuronal antibody panel, NMO, and MOG AB negative |
MRI of the spine revealed T2 signal hyperintensity of the thoracic spinal cord at T-9 level. Follow-up MRI on day 6 further showed a patchy hyperintensity of the thoracic spine at T9-10 and at T3-5 level. No abnormal enhancement seen. |
IV methylprednisolone was started at a dose of 100 mg/d | The patient improved and was discharged home with a slight spastic paraparesis. | Non-severe |
| Zachariadis A et al./Switzerland | 63 y/M | 12 days | paresthesia over feet, progressive weakness in lower extremities | CSF showed WBC 16/μl, protein 57 mg/dl, glucose 62 mg/dl. NMO and MOG AB negative. Immunological panel were unremarkable. |
Brain and spinal cord MRI did not show any abnormality. A second spine MRI, 7 days after admission was normal. No abnormal enhancement seen. |
IVIG 0.4 g/kg for 5 days. Followed by corticosteroid therapy IV for 5 days. | Partial improvement in motor strength transferred to rehabilitation therapy. | Non-severe |
| Abdelhady M et al. /Qatar | 52 y/M | 3 days | Inability to pass urine for 3 days, bilateral lower limb weakness. | CSF showed lymphocytic pleocytosis and increased proteins. | Brain MRI was normal, spinal cord MRI displayed a continuous long segment hyperintensity signal in the upper and mid-thoracic cord. No abnormal enhancement seen. |
Patient received steroids and acyclovir (antiviral drug) | Two days following MRI, the patient developed cardiac arrest and eventually died. | Severe |
M – Male; F – Female
IV-Intravenous.
IVIG- Intravenous human immunoglobulins.
CSF- cerebrospinal fluid.
Severity based on Infectious Disease Society of America/American Thoracic Society.
The age of the patients also varied from 24 to 66 years. All of the patients had development of lower extremity weakness or sensory disturbances as one or the only presenting symptom. The most common MRI finding was hyperintensity in two to three consecutive segments of spinal cord. In our review, amongst all reported cases of post infectious COVID-19 transverse myelitis, only one study by Sarma et al. found to have abnormal enhancement on post contrast study, however the remaining cases did not report any abnormal post contrast enhancement in the spinal cord. Eight out of ten cases received standard doses of steroids and showed significant improvement, however the remaining cases succumbed to death. Moreover, the overall course of seven cases were not severe likely due to timely detection and management (Sarma and Bilello, 2020; Zhao et al., 2020; Chow et al., 2020; Chakraborty et al., 2020; Valiuddin et al., 2020; AlKetbi et al., 2020; Durrani et al., 2020; Munz et al., 2020; Zachariadis et al., 2020; Abdelhady et al., 2020) (refer Table 1).
Various theories have been put forward to explain the unknown pathogenesis of transverse myelitis in COVID-19. Inflammatory processes may be triggered by retrograde transmission of virus through axons starting a demyelinating or inflammatory response (Bohmwald et al., 2020) whereas massive cytokine release as a consequence of systemic inflammation may also affect neuronal tissues. Baig A.M. et al. proposed the relation between COVID-19 and ACE-2 as a theoretical cause of this association (Baig et al., 2020). Transverse myelitis is a potentially serious complication which can be seen temporally associated with COVID-19 infection with serious repercussions if not identified and treated vigilantly.
4. Conclusion
We report the very first case of acute transverse myelitis with dysautonomia, post SARS-CoV-2 infection. Neurological manifestations can lead to severe debilitating effects and thus it is important for the emergency physicians and the neurologists to act swiftly in recognizing rare entities such as transverse myelitis early in the course of the novel coronavirus infection. Guidelines should be formulated for early imaging and CSF analysis in patients with COVID-19, in order to provide quick and efficient management to these patients. We believe, this case report will not only add to the existing database of COVID-19 and transverse myelitis, but also would serve as a source for researches to conduct more studies in the future.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Disclosures
Maria M Escobar - Report no disclosure.
Saurabh Kataria - Report no disclosure.
Erum Khan - Reports no disclosure.
Roshan Subedi - Reports no disclosure.
Medha Tandon - Reports no disclosure.
Kirthika Peshwe - Reports no disclosure.
Joshua Kramer - Reports no disclosure.
Faraze Niaze - Reports no disclosure.
Shitiz Sriwastava - Reports no disclosure.
Author contribution statement
Conceptualization: Maria M Escobar, Saurabh Kataria, Erum Khan, Roshan Subedi, Medha Tandon, Shitiz Sriwastava.
Drafting the manuscript: Saurabh Kataria, Erum Khan, Roshan Subedi, Medha Tandon, Kirthika Peshwe, Joshua Kramer, Faraze Niaze, Shitiz Sriwastava.
Editing and Final Draft: Shitiz Sriwastava.
Declarations
None.
Code availability
Not applicable.
Ethics approval and consent to participate
Patient Consent available, IRB approval from West Virginia University protocol Id 2,004,958,561.
IDSA/ATS criteria (Metlay et al., 2019).
Acknowledgments
None.
References
- Abdelhady M., Elsotouhy A., Vattoth S., Vattoth S. 2020. Acute Flaccid Myelitis in COVID-19. (2055–7159 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlKetbi R., AlNuaimi D., AlMulla M., AlTalai N., Samir M., Kumar N., et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol. Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.A.-O.X., Khaleeq A., Ali U., Syeda H. 2020. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. (1948–7193 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Berger J.R. COVID-19 and the nervous system. J. Neuro-Oncol. 2020;1 doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. 2020. Neurologic Alterations Due to Respiratory Virus Infections. (1662–5102 (Print)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A.T., Gershwin M.E. 2020. Transverse Myelitis. (1873–0183 (Electronic)) [Google Scholar]
- Chakraborty U., Chandra A.A.-O., Ray A.K., Biswas P. 2020. COVID-19-Associated Acute Transverse Myelitis: a Rare Entity. (LID - 10.1136/bcr-2020-238668 [doi] LID - e238668. (1757-790X (Electronic))) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.A.-O., Magnussen J., Ip J., Su Y.A.-O. 2020. Acute Transverse Myelitis in COVID-19 Infection. (LID - 10.1136/bcr-2020-236720 [doi] LID - e236720. (1757-790X (Electronic))) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A., Subedi R., Tandon M., Wen S., Patel J., Kataria S., et al. Relevance and clinical significance of magnetic resonance imaging of neurological manifestations in COVID-19: a systematic review of case reports and case series. Brain Sci. 2020;10(12):1017. doi: 10.3390/brainsci10121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrani M., Kucharski K., Smith Z., Fien S. 2020. Acute Transverse Myelitis Secondary to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Case Report. (2474-252X (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshak N., Abdelnabi M., Ball S., Elgwairi E., Creed K., Test V., et al. 2020. Dysautonomia: An Overlooked Neurological Manifestation in a Critically ill COVID-19 Patient. (1538–2990 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi U.A.-O., Subedi R., Gajurel B.P. 2020. Hepatitis B Virus Induced Cytoplasmic Antineutrophil Cytoplasmic Antibody-Mediated Vasculitis Causing Subarachnoid Hemorrhage, Acute Transverse Myelitis, and Nephropathy: a Case Report. (1752–1947 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria S., Tandon M., Melnic V., Sriwastava S. 2020. A Case Series and Literature Review of Multiple Sclerosis and COVID-19: Clinical Characteristics, Outcomes and a Brief Review of Immunotherapies. (2405–6502 (Electronic)) (Epub 2020 Nov 2. PMID: 33163634; PMCID: PMC7605741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Paul F., Lana-Peixoto M.A., Tenembaum S., Asgari N., Palace J., et al. 2020. MRI Characteristics of Neuromyelitis Optica Spectrum Disorder: an International Update. (1526-632X (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid O., Lipton H.L. 2020. Viral Myelitis: an Update. (1528–4042 (Print)) [DOI] [PubMed] [Google Scholar]
- Lycklama G., Thompson A., Filippi M., Filippi M., Miller D., Miller D., Polman C., Polman C., Fazekas F., Fazekas F., Barkhof F., et al. 2020. Spinal-cord MRI in Multiple Sclerosis. (1474–4422 (Print)) [Google Scholar]
- Mao L., Wang M., Chen S., et al. Neurological manifestations of hospitalised patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz R.P., Pedroso J.L., Nascimento F.A., SMD Almeida, OGP Barsottini, FEC Cardoso, et al. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq. Neuropsiquiatr. 2020;78(5):290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- Munz M., Wessendorf S., Koretsis G., Tewald F., Baegi R., Krämer S., et al. 2020. Acute Transverse Myelitis after COVID-19 Pneumonia. (1432–1459 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proposed Diagnostic Criteria and Nosology of Acute Transverse Myelitis. 2002. (0028–3878 (Print)) [DOI] [PubMed] [Google Scholar]
- Sarma D., Bilello L.A. 2020. A Case Report of Acute Transverse Myelitis Following Novel Coronavirus Infection. (2474-252X (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J. Peripher. Nerv. Syst. 2020;25(2):204–207. doi: 10.1111/jns.12382. (Epub 2020 May 26. PMID: 32388880; PMCID: PMC7273104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwastava S., Tandon M., Kataria S., Daimee M., Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J. Neurol. 2020:1–7. doi: 10.1007/s00415-020-10263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwastava S., Kataria S., Tandon M., Patel J., Patel R., Jowkar A., et al. 2021. Guillain Barré Syndrome and Its Variants as a Manifestation of COVID-19: A Systematic Review of Case Reports and Case Series. (1878–5883 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiuddin H., Skwirsk B., Paz-Arabo P. 2020. Acute Transverse Myelitis Associated with SARS-CoV-2: A Case-Report. (2666–3546 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariadis A., Tulbu A., Strambo D., Dumoulin A., Di Virgilio G.A.-O. 2020. Transverse Myelitis Related to COVID-19 Infection. (1432–1459 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Rodricks M.B., Hirsh E. COVID-19-associated acute disseminated encephalomyelitis: a case report. medRxiv. 2020 doi: 10.1007/s12028-020-01119-7. [DOI] [Google Scholar]
- Zhao K., Huang J., Feng Y., Liu L., Nie S. 2020. Acute Myelitis after SARS-CoV-2 Infection: A Case Report. [Google Scholar]