Abstract
Cardiac Troponin (hs-TnT) elevation has been reported in unselected patients hospitalized with COVID-19 however the mechanism and relationship with mortality remain unclear. Consecutive patients admitted to a high-volume intensive care unit (ICU) in London with severe COVID-19 pneumonitis were included if hs-TnT concentration at admission was known. Kaplan-Meier survival analysis performed, with cohorts classified a priori by multiples of the upper limit of normal (ULN). 277 patients were admitted during a 7-week period in 2020; 176 were included (90% received invasive ventilation). hs-TnT at admission was 16.5 (9.0 to 49.3) ng/L, 56% had concentrations >ULN. 56 patients (31.8%) died during the index admission. Admission hs-TnT level was lower in survivors (12.0 (8.0-27.8) vs 28.5 (14.0 to 81.0) ng/L, p = 0.001). Univariate predictors of mortality were age, APACHE-II Score and admission hs-TnT (HR 1.73, p = 0.007). By multivariate regression, only age (HR 1.33, CI: 1.16.to 1.51, p < 0.01) and admission hs-TnT (HR 1.94, CI: 1.22 to 3.10, p = 0.006) remained predictive. Survival was significantly lower when admission hs-TnT was >ULN (log-rank p-value<0.001). Peak hs-TnT was higher in those who died but was not predictive of death after adjustment for other factors. In conclusion, in critically ill patients with COVID-19 pneumonitis, the hs-TnT level at admission is a powerful independent predictor of the likelihood of surviving to discharge from ICU. In most cases, hs-TnT elevation does not represent major myocardial injury but acts as a sensitive integrated biomarker of global stress. Whether stratification based on admission Troponin level could be used to guide prognostication and management warrants further evaluation.
Coronavirus disease 2019 (COVID-19) is often associated with increases in cardiac Troponin concentration, particularly in those with advanced organ involvement.1, 2, 3 However, the clinical impact of raised cardiac Troponin levels in COVID-19 remains unclear, with recent studies coming to disparate conclusions regarding whether raised cardiac Troponin levels are an independent predictor of poorer patients outcomes.4 , 5 Furthermore, it is unclear whether the association between cardiac Troponin and mortality is mediated by mechanistically significant myocardial injury as part of the infection and inflammatory response or if the heart is acting as an integrated sensor of global hypoxia and stress. We hypothesized that Troponin elevation does not reflect major myocardial injury but is a marker of global stress and addressed this by examining the prevalence and extent of Troponin elevation and the ability to predict survival in a well-characterized cohort of patients admitted to our intensive care unit (ICU) with advanced COVID-19 pneumonitis, in comparison to existing risk scores and established markers of risk.
Methods
Consecutive patients with COVID-19 pneumonitis, diagnosed according to the interim guidance of the World Health Organization6, admitted to the ICU at Guy's and St Thomas’ Hospital, London, United Kingdom, between 3rd March 2020 and 21st April 2020 were included in the study if high-sensitivity Troponin-T (hs-TnT) concentration at admission was known.
All biochemistry analyses were performed onsite at dedicated institutional laboratory and Troponin T concentration was measured at admission and serially thereafter, using the Roche Elecsys assay; the limit of blank is 3ng/L, detection 5ng/L; and the coefficient of variation 10% at the limit of quantification; the 99th percentile for the normal population is 13ng/L.
Data were retrospectively collected from electronic medical records and validated through review of all source documentation. The following data were collected: patients’ demographics, medical history, laboratory and echocardiography investigations (outlined below). The APACHE (acute physiologic assessment and chronic health evaluation) II7 and SOFA (sequential organ failure assessment)8 scores were calculated on day of admission to ICU. Hyperinflammation was diagnosed by ICU team as per previously published criteria.9 Duration of ICU admission was calculated using date of death or discharge from ICU. Outcome data were verified from hospital records. Six-month mortality was censored using electronic patient records linked to national health service database. The primary outcome was all-cause mortality. The study was approved by institutional review board for use of de-identified data for COVID-19 related research.
A modified British Society of Echocardiography (BSE) Level 1 transthoracic echocardiogram (TTE) was performed during the index admission using GE E9, E95, and S70 ultrasound machines with a M5SC-D probe (GE Healthcare, Amersham, United Kingdom) or Philips CX50, Affinity and CVx ultrasound machines with an S5-1 or X5-1 probe (Philips Healthcare, Andover, Massachusetts). Retrospective analyses of the echocardiograms for this study were performed by BSE or EACVI accredited echocardiographers. The analyses included visual assessment of left ventricular (LV) and right ventricular (RV) systolic function, linear dimension measurements of the LV and RV, and Doppler analysis and measurements. Echocardiographic analysis was performed according to the joint American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guidance.10
Normality of data was assessed by the histogram, normal Q-Q plot and Shapiro-Wilk test. Continuous normal data are expressed as mean ± standard deviation (SD) and compared using paired Student t-tests. Non-normal data are expressed as median (interquartile range) and compared using Mann-Whitney test. Categorical variables are presented as frequency (percentages) and compared using chi-square test. Cox regression analysis was performed to identify potential predictors of all-cause mortality. A multivariate logistic regression analysis model with backward stepwise selection of variables (p-entry=0.05, p-exit=0.10) was constructed to identify potential predictors of all-cause mortality. The model included variables with univariate significance of p ≤ 0.15. Receiver operator characteristic (ROC) analysis was performed and areas under the curve (AUC) calculated to determine the relative ability to predict survival; Youden's index was used to identify the optimal threshold.
Patients were classified by hs-TnT concentration at admission according to multiples of the upper limit of normal (ULN), as <ULN, 1-2 xULN or >2 xULN. Major myocardial injury was defined as peak hs-TnT >20 xULN. Kaplan-Meier curves were used to examine cumulative death rate and differences between groups, tested using a log-rank test. For all analyses, a P value of 0.05 was considered significant, and all p values were 2-sided. All statistical analyses were performed using SPSS version 26.0 (SPSS Inc. Chicago, Illinois) and GraphPad Prism version 9.0 (GraphPad Software, La Jolla, California).
Results
During the study period, 277 consecutive patients with confirmed COVID-19 were admitted to ICU, of whom 176 patients had hs-TnT measured at admission to the unit and comprised the study population (aged 55.1 ± 13.9 years; 71% male). 101 patients were excluded from the study as they had no recorded Troponin levels at admission – 58 were transferred from another ICU for advanced care and 43 did not have hs-TnT performed at admission to our ICU (Figure 1 ). Patients excluded from the study had a higher rate of venovenous extracorporeal membrane oxygenation (VV-ECMO) (38% vs 3%, p < 0.001; these were largely tertiary/quaternary referrals from other ICUs). Demographic, biochemistry and echocardiographic characteristics of the included (study population) and excluded groups are detailed in Table 1 . Characteristics of the study population are summarized in Table 2 . 90% of the cohort received invasive ventilation and a third of all patients required renal replacement therapy. 56 (31.8%) patients died during ICU admission (“non-survivors” group) and 120 (68.2%) survived (“survivors” group). Those who survived were discharged from ICU 20.5 ± 18.1 days after admission. There was no difference between ventilated and non-ventilated patient in baseline characteristics, hs-TnT level or outcomes. At 6-months from admission, overall mortality was 33% in the entire study population.
Figure 1.
Study flow chart.
Table 1.
Characteristics of whole population
| Variable | Overall (n=277) | Included (n=176) | Excluded (n=101) | p Value |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Age (years) | 55.1 ± 13.9 | 55.1 ± 12.9 | 52.3 ± 15.6 | 0.14 |
| Men | 196 (71%) | 124 (71%) | 72 (71%) | 0.88 |
| Body mass index (kg/m2) | 28.5 (26.3) | 28.5 ± 6.6 | 28.7 ± 5.8 | 0.82 |
| Ethnicity | ||||
| White | 114 (41%) | 74 (42%) | 40 (40%) | |
| Black | 80 (29%) | 50 (27%) | 30 (30%) | |
| Asian | 40 (15%) | 26 (15%) | 14 (14%) | 0.38 |
| Mixed | 6 (2%) | 5 (3%) | 1 (1%) | |
| Other | 15 (5%) | 13 (7%) | 2 (2%) | |
| Not stated | 22 (8%) | 8 (5%) | 14 (14%) | |
| Diabetes Mellitus | 89 (32%) | 60 (34%) | 29 (29%) | 0.36 |
| Hypertension | 126 (46%) | 82 (47%) | 44 (44%) | 0.63 |
| SOFA Score | 6.0 (4.0 – 7.0) | 6.0 (4.0-7.3) | 5.0 (3.0 – 7.0) | 0.11 |
| APACHE II Score | 14.0 ± 5.0 | 14.0 ± 5.0 | 14 ± 5.1 | 0.95 |
| Hyperinflammation | 84 (30%) | 57 (32%) | 27 (27%) | 0.32 |
| Biochemistry characteristics | ||||
| Admission Hs-TnT (ng/L) | 16.5 (9.0-49.3) | 16.5 (9.0-49.3) | - | - |
| Peak Hs-TnT (ng/L) | 49.5 (16.5 – 115.5) | 53.00 (16.0 – 124.0) | 44.0 (24.5 – 111.0) | 0.57 |
| Admission CRP (mg/L) | 160.0 (84.3 – 278.5) | 162.0 (85.0-269.0) | 152.0 (77.0 – 281.5) | 0.51 |
| Peak CRP (mg/L) | 334.5 (226.0 – 414.8) | 344.0 (246.0 – 427.0) | 315.0 (196.0 – 395.0) | 0.50 |
| Admission Ferritin (μg/L) | 1164.0 (708.0 – 2182.0) | 1176.5 (717.8-2106.8) | 1118.0 (689.0 – 2459.5) | 0.96 |
| Peak Ferritin (μg/L) | 1919.0 (999.0 – 3379.3) | 1963.0 (10.45.5 – 3428.5) | 1627.0 (999.0 – 3342.5) | 0.45 |
| Echocardiographic characteristics | ||||
| LVEF, % (n=164) | 57.9 ± 11.1 | 56.7 ± 11.5 | 60.3 ± 9.6 | 0.02 |
| LV Impairment (≥Moderate) (n=164) | 19 (11%) | 17 (14%) | 2 (4%) | 0.20 |
| TAPSE (mm) (n=136) | 19.9 ± 4.7 | 20.0 ± 4.8 | 19.7 ± 4.4 | 0.75 |
| RV Impairment (n=136) | 30 (22%) | 21 (22%) | 9 (22%) | 0.93 |
| IVC (mm) (n=117) | 19.39 ± 4.84 | 19.7 ± 4.7 | 18.7 ± 5.1 | 0.35 |
| Pericardial Effusion (n=164) | 31 (19%) | 21 (18%) | 10 (22%) | 0.78 |
| Organ Support | ||||
| Non-invasive ventilation | 35 (13%) | 23 (13%) | 12 (11%) | 0.78 |
| Invasive ventilation | 238 (86%) | 158 (90%) | 80 (79%) | 0.02 |
| VV-ECMO | 25 (14%) | 4 (3%) | 21 (38%) | <0.001 |
| Renal Replacement Therapy | 89 (32%) | 58 (33%) | 31 (31%) | 0.70 |
| Outcomes | ||||
| Survivors | 191 (69%) | 120 (68%) | 71 (70%) | 0.48 |
| Length of stay (days) | 16.8 ± 15.7 | 18.5 ± 16.8 | 14.6 ± 14.1 | 0.06 |
*Where measures are available only in a sub-sample this is shown in parenthesis.
¥ APACHE= Acute physiologic assessment and chronic health evaluation; CRP = C-reactive protein; Hs-TnT = high-sensitive Troponin-T; IVC = inferior vena cava; LV = Left ventricular; LVEF = left ventricular ejection fraction; RV = Right ventricle; SOFA = sequential organ failure assessment TAPSE = Tricuspid Annular Plane Systolic Excursion; VV-ECMO = Venovenous extracorporeal membrane oxygenation.
Table 2.
Characteristics of included population
| Variable | Survivors (n=120) | Non-survivors (n=56) | p Value |
|---|---|---|---|
| Baseline Characteristic | |||
| Age (years) | 52.2 ±12.9 | 61.1 ± 10.7 | <0.001 |
| Men | 82 (68%) | 42 (75%) | 0.37 |
| Body mass index (kg/m2) | 28.3 ± 6.6 | 28.7 ± 6.7 | 0.79 |
| Ethnicity | |||
| White | 46 (39%) | 28 (50%) | |
| Black | 35 (29%) | 15 (27%) | |
| Asian | 18 (15%) | 8 (14%) | 0.39 |
| Mixed | 4 (3%) | 1 (2%) | |
| Other | 12 (10%) | 1 (2%) | |
| Not stated | 5 (4%) | 3 (5%) | |
| Diabetes Mellitus | 39 (33%) | 20 (36%) | 0.30 |
| Hypertension | 51 (43%) | 31 (55%) | 0.11 |
| SOFA Score | 6.0 (4.0-7.0) | 6.5 (4.0-8.0) | 0.18 |
| APACHE II Score | 12.9 ± 4.9 | 16.3 ±4.5 | <0.001 |
| Hyperinflammation | 32 (27%) | 25 (45%) | 0.02 |
| Biochemistry characteristics | |||
| Admission Hs-TnT (ng/L) | 12.0 (8.0 – 27.8) | 28.5 (14.0 – 81.0) | 0.001 |
| Peak Hs-TnT (ng/L) | 34.5 (11.25 – 105.75) | 78.0 (39.0 – 188.0) | <0.001 |
| Admission CRP (mg/L) | 150.0 (68.0 - 241.0) | 206.5 (113.3 - 318.8) | 0.07 |
| Peak CRP (mg/L) | 326.0 (196.0 – 414.0) | 363.0 (327.8 – 444.0) | 0.07 |
| Admission Ferritin (μg/L) | 1091.0 (684.0-1907.0) | 1469.0 (721.0-3170.0) | 0.10 |
| Peak Ferritin (μg/L) | 1833.5 (960.0 – 2794.0) | 2363.0 (1251.0 – 5333.0) | 0.02 |
| Echocardiographic characteristics | |||
| LVEF, % (n=120) | 57.7 ± 9.3 | 54.9 ± 14.3 | 0.24 |
| LV Impairment (≥Moderate) (n=120) | 7 (10%) | 10 (22%) | 0.051 |
| TAPSE (mm) (n=96) | 20.4 ± 4.9 | 19.2 ± 4.6 | 0.25 |
| RV Impairment (n=96) | 12 (20%) | 9 (25%) | 0.57 |
| IVC (mm) (n=117) | 18.7 ± 5.1 | 21.5 ± 3.4 | 0.003 |
| Pericardial Effusion (n=120) | 13 (18%) | 8 (17%) | 0.72 |
| Organ Support | |||
| Non-invasive ventilation | 15 (13%) | 8 (14%) | 0.74 |
| Invasive ventilation | 106 (88%) | 52 (93%) | 0.36 |
| VV-ECMO | 2 (3%) | 2 (4%) | 0.64 |
| Renal replacement therapy | 33 (28%) | 25 (45%) | 0.02 |
| Outcomes | |||
| Length of stay (days) | 20.5 ±18.1 | 14.1 ±12.4 | 0.006 |
*Where measures are available only in a sub-sample this is shown in parenthesis.
¥ APACHE = Acute physiologic assessment and chronic health evaluation; CRP = C-reactive protein; Hs-TnT = high sensitive Troponin-T; IVC = inferior vena cava; LV = Left ventricular; LVEF = left ventricular ejection fraction; RV = Right ventricle; SOFA = sequential organ failure assessment TAPSE = Tricuspid Annular Plane Systolic Excursion; VV-ECMO = Venovenous extracorporeal membrane oxygenation.
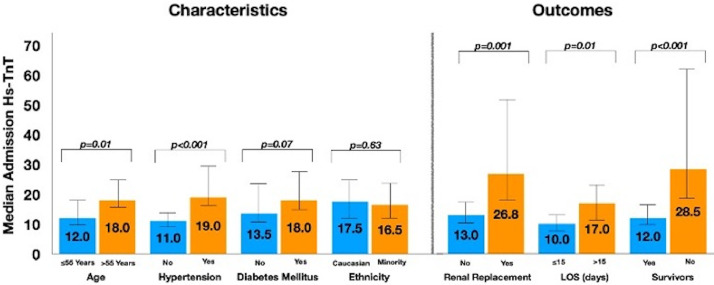
Admission hs-TnT was 16.5 (9.0 to 49.3) ng/L, with a negatively skewed distribution (Figure 2 ). 56% of patients had a hs-TnT concentration above the ULN. hs-TnT values were higher in patients with a history of hypertension than those who did not and in patients older than 55 years (the median age in the study cohort) than those who were younger; the levels were not different in diabetics compared with non-diabetics or in patients of Black, Asian and Minority Ethnic (BAME) ethnicities compared with Caucasians (Figure 3 ).
Figure 2.
Histogram of admission and peak high sensitivity Troponin-T measurements.
Figure 3.
Patient characteristics and outcomes by admission high-sensitivity Cardiac Troponin-T. Length of stay (LOS) analysis exclusively of patients who survived to discharge from intensive care unit.
Peak Troponin concentration was 53.0 (16.0 - 124.0) ng/L. Eighteen patients (10.2%) developed major myocardial injury by the pre-specified definition and had a peak hs-TnT concentration of 583.0 (332.0 to 1176.0) ng/L. Seventeen (94%) of this group were male, 67% had a history of hypertension, 67% were diabetic and 61% had hyperinflammation diagnosed during the admission (peak CRP 361 (327.75 to 451.5) mg/L, peak Ferritin 3989 (2527.75 to 5497.25) μg/L). In these patients with major myocardial injury, hs-TnT on admission to ICU was 65 (18.25 to 289.8) ng/L, SOFA score was 8.0 (6.25 to 10), APACHE-II score was 17.0 ± 5.6 and LEVF 53.0 ± 13.9% (5/18 had ≥Moderate LVSD).
Survivors were younger (52.2 ± 12.9 vs 61.1 ± 10.7 years, p < 0.001), had lower mean APACHE II score (12.9 ± 4.9 vs 16.3 ± 4.5, p < 0.001) and had a higher rate of hyperinflammation (45% vs 27%, p = 0.02) and more frequently utilized renal replacement therapy (45% vs 28% p = 0.02), compared with non-survivors.
Non-survivors had higher admission hs-TnT (28.5 (14.0 to 81.0) ng/L vs 12.0 (8.0 to 27.8) ng/L, p = 0.001) and peak hs-TnT (78.0 (39.0 to 188.0) ng/L vs 34.5 (11.25 to 105.75) ng/L, p < 0.001) compared with survivors. In addition, amongst those who survived to discharge from ICU, those with length of stay >15-days (the median LOS for all survivors) had significantly higher hs-TnT concentration at admission to ICU than those with ≤15-day LOS (Figure 3).
Echocardiography was performed in 68% of the study cohort (n = 120), at a median of 3 days (1 - 3) from admission. There was no difference between non-survivors and survivors when LVEF was considered as a continuous variable (54.9 ± 14.3% vs 57.7 ± 9.3%, p = 0.24) but a higher proportion of the former had at least moderate left ventricular impairment than the latter (22% vs 10%, p = 0.051). In addition, there was no difference in tricuspid annular plane systolic excursion (TAPSE) measurements, or the proportion classified as having right ventricular impairment between non-survivors and survivors.
Independent predictors of mortality were age (hazard ratio (HR) per 5-year increment 1.32, 95% Confidence Interval (CI) 1.16 - 1.5, p < 0.001), APACHE-II Score (HR 1.08, CI 1.03 to 1.14, p = 0.002) and admission hs-TnT (HR 1.73, CI 1.16 - 2.57, p = 0.007). Multivariate Cox Regression based on characteristics available at admission demonstrated that age (HR 1.33, CI 1.16 - 1.51, p < 0.01) and admission hs-TnT (HR 1.94, CI 1.22 to 3.10, p = 0.006) were the only independent predictors of mortality (Table 3 ).
Table 3.
Univariate and multivariate cox regression of data available at admission
| Variable | Univariate |
Multivariate (Model 1: n=175) |
||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | p | HR | CI (95%) | p | |
| Age (per 5 years) | 1.32 | 1.16 – 1.51 | <0.001 | 1.33 | 1.16 – 1.51 | <0.001 |
| Male sex | 1.07 | 0.58 – 1.98 | 0.82 | |||
| Ethnicity (Black and Asian) | 1.00 | 1.00 – 1.01 | 0.93 | |||
| Body mass index | 1.00 | 0.96 – 1.04 | 0.93 | |||
| Diabetes mellitus | 0.99 | 0.58 – 1.71 | 0.98 | |||
| Hypertension | 1.33 | 0.78 – 2.26 | 0.29 | |||
| SOFA Score | 1.04 | 0.93 – 1.17 | 0.49 | |||
| APACHE II Score | 1.08 | 1.03 – 1.14 | 0.002 | 1.03 | 0.97 – 1.10 | 0.33 |
| Admission Hs-TnT | 1.73 | 1.16 – 2.57 | 0.007 | 1.94 | 1.22 – 3.10 | 0.006 |
| Admission CRP | 1.84 | 0.83 – 4.06 | 0.13 | 0.96 | 0.42 –2.19 | 0.92 |
| Admission Ferritin | 0.97 | 0.50 – 1.87 | 0.97 | |||
¥ APACHE = Acute physiologic assessment and chronic health evaluation; CRP = C-reactive protein; Hs-TnT = high sensitive Troponin-T; SOFA = sequential organ failure assessment.
ROC analysis (Figure 4 ) showed that admission hs-TnT had an under the curve (AUC) of 0.71, while for age, APACHE-II score and SOFA score these were 0.68, 0.67, and 0.60 respectively. The optimal cut-off value of hs-TnT to predict mortality was >17 ng/L, at a sensitivity of 69.6% and specificity of 62.5%. The sensitivity and specificity of the ULN (13 ng/L) were 80.4% and 50.8% respectively and for 2 xULN 53.6% sensitivity and 74.2%.
Figure 4.
Receiver operating characteristic curve of mortality. Area under the curve (AUC): Hs-TnT 0.71, Age 0.68, APACHE-II score 0.67 and SOFA score 0.60. Optimal cut-off value of hs-TnT to predict mortality was >17 ng/L, at a sensitivity of 69.6% and specificity of 62.5%.
Kaplan-Meier survival analysis of mortality by multiples of the hs-TnT ULN mortality are shown in Figure 5 . Compared with patients with hs-TnT ≤ULN, those with values between 1- 2 x ULN or 2 xULN had significantly higher mortality (Log-rank p < 0.001).
Figure 5.
Kaplan-Meier curves of death rate stratified by multiples of the upper limit of normal for high sensitivity Troponin-T.
Discussion
In this study of patients with severe COVID-19 pneumonitis requiring management on an intensive care unit, hs-TnT concentrations were above the 99th centile at admission to the unit in the majority of cases (56%) but the degree of elevation was minimal, when compared with populations with acute coronary syndromes or myocarditis. Yet, even minimal elevation in hs-TnT proved to be a powerful predictor of survival to discharge from ICU and a better predictor of survival than conventional ICU risk scores, such as the SOFA or APACHE-2 scores, or other biomarkers that have come to be regarded as reflective of severe COVID-19 infection, such as CRP or Ferritin. Whilst COVID-19 has been postulated to directly cause myonecrosis via different mechanisms, including myocarditis (due to direct entry of SARS-CoV-2 into myocardial cells or as part of the systemic inflammatory response to the infection) or microvascular damage due to disseminated intravascular coagulation (causing ischaemia despite unobstructed epicardial arteries)11, 12, 13, in our study, modest peak Troponin concentrations and relatively well preserved left ventricular function indicate that most patients do not suffer major myocardial injury. These findings suggest that the modest elevation in hs-TnT concentration does not herald contractile dysfunction or other fatal sequelae of direct cardiac involvement but, rather, the heart appears to be acting as a sensitive and integrated detector of hypoxic, inflammatory and circulatory stress.
Myocardial injury was recognized early during the pandemic in the pathophysiological response to COVID-19. It was observed in 5 out of the first 41 patients with COVID-19 in Wuhan 14 and in 7.2% of patients from a later patient series from Wuhan 15, with higher cardiac biomarker elevation in ICU patients. A larger observational study demonstrated that cardiac Troponin-I and N-terminal pro-BNP were independent predictors of in-hospital death by a multivariable Cox regression analysis in 671 patients with COVID-19 16. These findings have been confirmed by further studies in Europe and USA.4 , 17 , 18 Recently, a study of 2,736 patients (with varying degrees of illness) admitted to 5 hospitals in New York City demonstrated that 36% of patients had a Troponin I concentration greater than the ULN.4 Mortality during hospitalization was 18.5% and admission Troponin I concentration was an independent predictor of survival. However, in another study, restricted to patients with COVID-19 requiring treatment on ICU, Troponin (T or I) checked within 24 hours of admission was associated with mortality but did not remain predictive when adjusted for other factors. Interestingly, the prevalence of Troponin elevation above the ULN was similar to what we found in our study (51% compared with 56%) as was mortality on ICU (36.2% compared with 31.8%). There are several notable differences between the two studies. Perhaps the most pertinent are differences in Troponin assays: the study from Baltimore included both Troponin T and I assays and only 1 out of 4 Troponin-I assays were high-sensitivity. The populations also appear to be different: our cohort were younger, had a lower BMI, and lower prevalence of diabetes and hypertension.
Our institution is a large tertiary center in London with 988 inpatient bed capacity with one of the largest ICUs in the UK and serves as one of the 5 national ECMO centers. During the “first wave” of COVID-19 there was an institutional upscaling of ventilated patient ICU capacity – at its peak our institution had 189 patients in ventilated beds, compared with approximately 90 ventilated beds prior to COVID-19 pandemic. COVID-19 surge teams comprising doctors, nurses and allied health professionals were formed to manage increased ICU admissions. The majority of patients treated with VV-ECMO were excluded due to Troponin on admission to ICU being unavailable as these patients were transferred from another ICU for advanced care. Consequently, excluded patients were younger and had higher left ventricular ejection fraction compared with included patients, as eligibility for VV-ECMO tend to be younger patients with pre-specified risk profile (Table 1). Despite this there was no difference in other variables including baseline, biochemistry, echocardiogram and outcomes between included and excluded patients. There was no difference in proportion of survivors between those included and excluded (survival in the whole population was 69%).
In hospitalized patients, severe illness secondary to COVID-19 is more common in older patients, males, ethnic minorities and those with underlying cardiovascular co-morbidities.19, 20, 21 Our study population characteristics are similar to recently published report of 10,834 patients with COVID‑19 in England, Wales and Northern Ireland.22 In this large study, severe ARDS was observed in 37% and hospital mortality was 42%. Moreover, conventional severity scoring appeared not to adequately reflect their acute severity of critically ill COVID-19 patients.22
The findings of this study should be interpreted in light of some limitations. First, this was a retrospective observational study and hence it is not possible to exclude selection bias; while survival was similar amongst patients for whom we had access to admission Troponin concentrations (and hence were included in the study) there were some notable differences when compared with the excluded population, such as a greater use of VV-ECMO in the latter. Second, we did not systematically perform serial echocardiography to assess longitudinal left and right ventricular function. Third, our sample size is relatively small, although it is one of the largest cohorts of severe COVID-19 pneumonitis to be reported, to date, and the fact that it is a single center study ensures standardization of biomarker assays, management and investigation of these patients. Fourth, treatment decisions may have been influenced by subjective factors, for example in utilization of renal replacement therapy. Finally, our regression models are limited to the data available and we are unable to comment on the utility of novel biomarkers such as interleukin-6 concentrations.
In conclusion, in critically ill patients with COVID-19 pneumonitis, the hs-TnT level at admission is a powerful independent predictor of the likelihood of surviving to discharge from ICU. In most cases, hs-TnT elevation does not represent clinically relevant myocardial injury but acts as a sensitive integrated biomarker of global stress. Whether stratification based on admission Troponin level could be used to guide prognostication and management warrants further evaluation.
Credit Author Statement
Original Research Submission: Impact and Determinants of High-Sensitivity Cardiac Troponin-T Concentration in Patients With COVID-19 Admitted to Critical Care. All authors fulfil the International Committee of Medical Journal Editors (ICMJE) recommendations for authorship. All authors approved the final manuscript. Conceptualization & Methodology: Ozan M. Demir, Matthew Ryan, Divaka Perera. Investigation: Ozan M. Demir, Matthew Ryan, Chiara Cirillo, Nishita Desai, Ana Pericao, Hannah Sinclair, Vasileios Stylianidis, Kelly Victor, Antonis N. Pavlidis. Formal analysis: Ozan M. Demir, Matthew Ryan, Bashir Alaour, Michael Marber, Divaka Perera. Writing - Original Draft: Ozan M. Demir, Divaka Perera. Writing - Review & Editing: Ozan M. Demir, Matthew Ryan, Andrew Jones, Antonis N. Pavlidis, Andrew Retter, Gerald Carr-White, Luigi Camporota, Nicholas Barrett, Michael Marber, Divaka Perera. Supervision: Divaka Perera.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relations that could have appeared to influence the work reported in this study.
Footnotes
Funding: The authors gratefully acknowledge funding from the British Heart Foundation (PG/19/9/34228 and FS/18/16/33396) and the UK National Institute for Health Research via the Biomedical Research Centre award to Guy's and St Thomas’ Hospital and King's College London.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK. Myocardial injury in severe COVID-19 compared to non-COVID acute respiratory distress syndrome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.050543. CIRCULATIONAHA.120.050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance 28. Who 2020:10. Available at: WHO/2019-nCoV/clinical/2020.5%0ACC BY-NC-SA 3.0 IGO%0AWHO/2019-nCoV/clinical/2020.5%0ACC BY-NC-SA 3.0 IGO%0Ahttps://apps.who.int/iris/handle/10665/330893e. [Google Scholar]
- 7.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 8.Vincent JL, De Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T, Khan E, Mehta P, Martin-Gutierrez L, Waddington KE, Robinson GA, Ribeiro Santos L, McLoughlin E, Snell A, Adeney C, Schim van der Loeff I, Baker KF, Duncan CJA, Hanrath AT, Lendrem BC, De Soyza A, Peng J, J'Bari H, Greenwood M, Hawkins E, Peckham H, Marks M, Rampling T, Luintel A, Williams B, Brown M, Singer M, West J, Jury EC, Collin M, Tattersall RS. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Retzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 11.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 12.Basso C, Leone O, Rizzo S, De Gaspari M, der Wal AC van, Aubry M-C, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani LR, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S, Shi S, Shi S, Qin M, Cai Y, Liu T, Liu T, Liu T, Shen B, Shen B, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Zhao Q, Zhao Q, Huang H, Huang H, Huang H, Yang B, Yang B, Yang B, Huang C, Huang C, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, De Marco A, Briani M, Bocciolone M, Bragato R, Corrada E, Gasparini GL, Marconi M, Monti L, Pagnotta PA, Panico C, Pini D, Regazzoli D, My I, Kallikourdis M, Ciccarelli M, Badalamenti S, Aghemo A, Reimers B, Condorelli G. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 18.Lorente-Ros A, Monteagudo Ruiz JM, Rincón LM, Ortega Pérez R, Rivas S, Martínez-Moya R, Sanromán MA, Manzano L, Alonso GL, Ibáñez B, Zamorano JL. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J. 2020;27:489–496. doi: 10.5603/CJ.a2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakeri R, Bendayan R, Ashworth M, Bean D, Dodhia H, Durbaba S, O Gallagher K, Palmer C, Curcin V, Aitken E, Bernal W, Barker R, Norton S, Gulliford M, Teo J, Galloway J, Dobson R, Shah A. A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. EClin Med. 2020;000 doi: 10.1016/j.eclinm.2020.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA - J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Kreutz R, Algharably EAEH, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for covid-19. Cardiovasc Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards-Belle A, Orzechowska I, Gould DW, Thomas K, Doidge JC, Mouncey PR, Christian MD, Shankar-Hari M, Harrison DA, Rowan KM, Banjo Y, Borowczak K, Cousins T, Cummins P, Dalemo K, Darnell R, Demissie H, Drikite L, Fleming A, Frederiksen D, Furnell S, Hussein A, Koelewyn A, Matthews T, Peters S, Samuels T, Saull M. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46:2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]