Abstract
Water scarcity is a known and major issue throughout the world. To tackle water scarcity, there is an urgent need for water re-use and recycling through wastewater treatment. This study is an attempt to re-used industrial effluent after treatment with gamma irradiation. The main drain of the industrial estate was sampled and analyzed for Physico-chemical parameters. For treatment, irradiation dose 13 Kilo Gray (kGy) cobalt (Co60) was applied. The treated water was re-analyzed for comparison with pre-analysis and compliance with the National environmental quality standard (NEQS). A decrease was observed in TSS, BOD5, and COD with 79%, 81%, and 85% respectively. The results achieved are within the permissible limits of NEQS. It was concluded that gamma radiation is an instant method for industrial effluent and is herein recommended.
Keywords: Industrial estate, Wastewater, TSS, BOD, COD, Gamma irradiation
Industrial estate, Wastewater, TSS, BOD, COD, Gamma irradiation
1. Introduction
Worldwide water bodies have been affected by toxic effluents of industries and are continuously deteriorating as industrialization coupled with urbanization has caused the excess release of wastewater (Salvatore et al., 2009). Since the last decade, the focal area of research is to remediate industrial pollution (Bui et al., 2016). Different sorts of hazardous material discharge from industries have caused severe environmental contamination (Liu et al., 2016). Aquatic biota is harmed by these toxic effluents (Luo et al., 2014). Industrial wastewater leads to contamination of surface water but also contaminates groundwater aquifers (Azizullah et al., 2011). Mostly in industrial processes freshwater is used instead of wastewater because of the bad smell, color, and turbidity of wastewater (Liu et al., 2016). The rising pressure of freshwater demand has caused severe water pollution and scarcity (Rehman and Adnan, 2013). Therefore, the treatment of wastewater has been recognized as an important issue across the globe, and through wastewater treatment, the issue of water scarcity can be addressed (Toze, 2006; Salgot et al., 2001).
A variety of physical, chemical, biological, and combination of processes are under study and are developed for the treatment of wastewater in the past few years (Lee et al., 2015; Ahmed et al., 2017). The various study suggested that conventional and non-conventional treatment processes adopted for wastewater cannot sufficiently reduce pollutants to the limit where it can be reused also the reduction in pathogen removal is insufficient (Verde et al., 2004). However, due to the partial degradation and removal of pollutants, the effluent wastewater still poses serious threats to ground and surface water from where it can enter the food chain and affect humans as well as the riverine ecosystem (Rivier et al., 2019). To minimize health risks posed by pollutants present in industrial wastewater advance and new technologies need to be introduced (Rai et al., 2019). Irradiation technology is one of the most advanced ways of degradation of pollutants from wastewater by the use of ion forming radiations which are emitted from electron beam accelerators (beta rays) and generates gamma-ray (Lajayer et al., 2018) as well as non-ionizing less energetic radiations, Ultraviolet rays (UV) (Lee et al., 2015). Irradiation of wastewater with gamma rays is considered to be an efficient and rapid method for industrial effluent treatment. Studies suggested that among many different types of radiations, the performance of gamma rays in the degradation of wastewater pollutants is widely reported (Limam et al., 2018; Wang et al., 2019).
Gamma rays are preferred for pollutants degradation as they are reported to have deeper penetration in comparison to beta rays. Gamma rays have excellent at penetration and are reliable in the process of ion formation (Chmielewski, 2007) and most importantly it requires a lower dose of radiations for removal of pathogens in comparison to beta rays. The dose required for gamma is far less than the lethal dose required for achieving the removal of the same pathogens from wastewater (EI-Motaium, 2006). Also, the application of gamma rays for pollutant degradation is a physical process which has several advantages like no chemical additives (Verde et al., 2004), also environmental conditions do not affect gamma irradiation performance (Tahri et al., 2010), and no by-products are produced (Chu et al., 2010). Interaction of gamma rays water leads to the production of several powerful oxidizing and reducing ions e.g. (OH, eaq, H) also with the production of molecular products (e.g. H2, H2O2). These species result in the degradation of various types of organic and inorganic pollutants (Wang and Chu 2016). Production of reducing and oxidizing species is at the same approximate amount in water radiolysis (Kim et al., 2019), which is documented to have a beneficial role in the degradation of contaminants present in effluent which are easily removed through oxidation and reduction pathways. Irradiation of wastewater results in the oxidation of organic matter and causes breakdown in the structure of pollutants. Breakdown and oxidation of various pollutants enhance degradation of pollutants in which major reductions are reported in pH, biochemical oxygen demand (BOD), and chemical oxygen demand (COD) (Rathod et al., 2011; Madureira et al., 2017).
The present study is designed to investigate the wastewater pollution load of the industrial estate. Treatment of effluents by advanced oxidation process (gamma radiation Co60) as it’s a novel technology and has not been previously adopted for the treatment of wastewater in the study area.
2. Material and methods
2.1. Study area
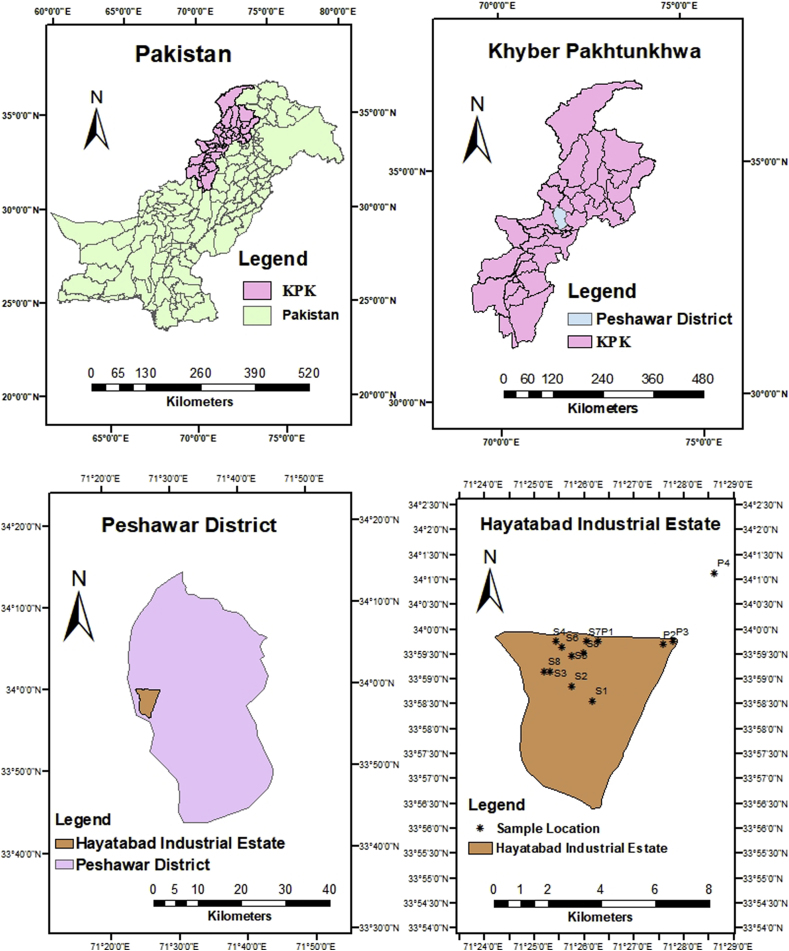
The Hayatabad Industrial Estate is situated in the northwest of Peshawar from 6-7 Km on main Jamrud Road. It is the famous and largest industrial estate in Khyber Pakhtunkhwa (Jan et al., 2010). It was established in 1963 (Nafees et al., 2015). Hayatabad Industrial estate comprises up to 372 installed factories. 246 out of 372 are working on a full scale and are entitled as large industries (SDA, 2016), which includes paper, marble, food, iron, ghee, and steel mills, also these are major contributors to pollution (Tariq at al., 2006). A separate main drain of water is allocated which passes throughout the industries and carries away effluents generated from the industrial estate (HIE, Peshawar). Effluents are drained into a separate main drain away from industries, known as Malakandar Nala/Budni Nala which later on joins Shalam River and Kabul River (Jan et al., 2010). Industrial wastewater severely impacts the water quality of River Kabul which includes deteriorating quality of water for fish breeding, for animal watering, and also for irrigation purposes (Inamullah and Alam, 2014).
2.2. Wastewater sampling
A detailed field survey was conducted to identify the sampling points for industrial wastewater collection. 4 sampling points were identified o the main drain collecting the wastewater of all industrial estate. Main Effluent drain near Rahman medical institute Peshawar was abbreviated as P1, Main effluent drain near phase 3 chowk as P2, Main Effluent drain near Nasir Bagh as P3, Main Effluent drain near Agriculture colony as P4 shown in Figure 1. A total of 16 composite samples were collected from different mentioned points (P1, P2, P3, P4) on the main industrial drain. Each composite sample was composed of 4 grab samples collected at a one-hour interval. Samples were collected in clean plastic bottles of volume 1.5 L from each sampling site pre-rinsed with distilled water. The samples were immediately transported to the laboratory and stored at 4 °C for further analyses.
Figure 1.
Map of the Sampling sites.
2.3. Chemical analysis
The samples were analyzed for physicochemical properties such as pH, electrical conductivity (EC), Total suspended solids (TSS), Total dissolved solids (TDS), Biological Oxygen demand (BOD5), and Chemical oxygen demand (COD) according to the standard method of water and wastewater treatment (APHA, 2005). PH and was measured through pH meter Jenco Electronics & vision data logger 6091 and electrical conductivity (EC) with a conductivity meter (Electrical conductivity measuring bridge) calibrated with their buffer KCl solutions of 0.01M. Suspended solids were measured by the gravimetric method and dissolved solids were also examined by a conductivity meter. BOD was identified by the Iodometric method and COD was determined by the closed reflux method with (Hanna COD analyzer). After analysis results were compared with the permissible limits given for industrial discharge (Pak-NEQs, 2010).
2.4. Gamma irradiation
The irradiation was carried out at room temperature by using a Co-60 gamma rays source (CoS-44 HH, Purchased from the Institute of Isotopes Co., Ltd., Budapest, Hungary) installed at Nuclear Institute for Food and Agriculture (NIFA) Peshawar Khyber Pakhtunkhwa, Pakistan. Wastewater samples were irradiated at a dose of 13 kGy without any further treatment. The samples were immediately re-analyzed after irradiation to know the effect of gamma irradiation on the characteristics of industrial wastewater.
2.5. Statistical analysis
A paired sample t-test was carried out to compare the difference before and after irradiation.
2.6. Percent reduction of pollution
The efficiency of Gamma radiation was calculated based on percent reduction in the level of pollutants that is
| % Removal = [(C1–C2)/C1] ×100 |
C1 is the concentration of parameter before irradiation (non-irradiated samples) while C2 is the concentration of parameter after irradiation (irradiated sample) (Sivakumar and Nouri, 2015).
3. Result and discussion
3.1. Analytical results before irradiation
The Physico-chemical characteristic of the samples collected from different points of the main industrial drain varied from point to point. Maximum pH was found for P1 with an average value of 8.5. For sampling points P2, P3, and P4 it was observed 7.9, 7.2, and 7.4 respectively. Electrical conductivity (EC) was observed with an average value of 1200 for P1 and 1240 uS/cm for P3. The higher value of total dissolved solids was found with an average value of 690 mg/L for the sample collected from P3. The total suspended load varied along with the sampling points. Maximum suspended solids were observed with the average value of 615 mg/l for P2, while its minimum value was found for P1 as 520 mg/L. Moreover, the values of suspended solids were found beyond the permissible limits of Pak-NEQS. This maximum value of the suspended load is due to the discharge of marble effluents in the main drain. The maximum value of BOD was found 275 mg/L for P2 and its minimum value was observed for P4. However, the BOD values exceeded than permissible limits for all sampling points. COD was determined with the average value of 376, 369, 285, and 370 mg/l for P1, P2, P3, and P4 respectively. The observed COD was beyond the acceptable limits, as described by Pak-NEQS (Table 1).
Table 1.
Industrial Wastewater characteristics.
| S.No | Sampling sites | Conc. | pH | EC | TDS | TSS | BOD | COD |
|---|---|---|---|---|---|---|---|---|
| 1 | P1 | Min | 7.7 | 1100 | 650 | 460 | 255 | 370 |
| Max | 8.6 | 1300 | 730 | 580 | 275 | 382 | ||
| Avg | 8.4 | 1200 | 672 | 520 | 263 | 376 | ||
| P2 | Min | 7.3 | 1100 | 610 | 563 | 270 | 350 | |
| Max | 8.3 | 1300 | 700 | 650 | 285 | 375 | ||
| Avg | 7.9 | 1200 | 659 | 615 | 275 | 369 | ||
| 3 | P3 | Min | 6.6 | 1280 | 620 | 470 | 140 | 270 |
| Max | 7.8 | 1300 | 770 | 661 | 160 | 290 | ||
| Avg | 7.2 | 1240 | 690 | 540 | 149 | 285 | ||
| 4 | P4 | Min | 6.6 | 1000 | 640 | 490 | 175 | 361 |
| Max | 7.9 | 1300 | 710 | 679 | 185 | 380 | ||
| Avg | 7.4 | 1200 | 660 | 590 | 180 | 370 | ||
| Pak – NEQS | 06–10 | -- | 3500 | 150 | 80 | 150 | ||
The main effluent drain of Hayatabad Industrial Estate is called North Nala or Malakandair which carries the effluent load of Industrial Estate and finally joins Budnai Nala. The Budnai Nala further joins the Shalam River and finally Kabul River. A study carried out by Nafees et al. (2015) also showed a higher concentration of BOD, COD, and TSS in industrial wastewater. There is no appropriate treatment facility inside the industries to reduce the pollution load. Contamination level is increasing over time due to the lack of wastewater maintenance facility creating a problem for the environment (Nafees et al., 2010).
Tariq et al. (2006) also determined high contents of TSS, chemical oxygen demand, and biological oxygen demand in industrial effluents of Hayatabad Industrial Estate. The wastewater produced from industries not only contaminates surface water but also the groundwater reservoirs (Azizullah et al., 2011). In Pakistan production rate of wastewater from industries is 4432.35 million m3, before its disposal only 1% of this wastewater is treated (Nafees et al., 2015). The industrial estates of Khyber Pakhtunkhwa such as Hayatabad industrial estate Peshawar, Gadoon amazai Swabi, and Hattar industrial estate Haripur release their industrial effluents into water bodies without any treatment and thus affecting the water quality and aquatic biota (Rashed, 2013; Nasrullah et al., 2006). Contamination of industrial processes becomes a serious problem to aquatic life (Amin et al., 2013).
3.2. Radiolysis of water
When gamma irradiation reacts with water a chain of reaction takes place. Several reactive species are produced during the process. The reactive species include both molecular products and radical products. The result of hydrolysis can be presented as (Makuuchi, 2003)
Molecular products include hydrogen molecule (H2) and hydrogen peroxide (H2O2), while radical species include hydrated electron (e-eq), a hydrogen atom (H), hydroxyl radical (OH), and per hydroxyl radical (H2O). These species are responsible for oxidation and reduction. Hydrated electron and hydrogen radical are reducing radicals. While the hydroxyl radical and per hydroxyl radical are oxidizing radicals. Hydrated electron, the hydrogen atom, and hydroxyl radicals are produced in sufficient amount and are the most reactive species; therefore play a key role in the radiolysis of water and aqueous solution (Mahmood, 2007).
3.3. Effect of gamma-irradiation on pH
The change in physicochemical parameters of wastewater before and after irradiation is summarized in (Table 2). In comparing pH values of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant difference was observed in the value of pH after irradiation at a dose of 13 kGy.
Table 2.
Effect of Gamma irradiation on selected water quality parameters.
| Parameter | Sample No | Reading Before Treatment |
Reading After Treatment |
% Change |
||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Average | Min | Max | Average | |||
| pH | P1 | 7.7 | 8.6 | 8.4 | 7.5 | 8.2 | 7.9 | 5% |
| P2 | 7.3 | 8.3 | 7.9 | 6.5 | 7.3 | 6.9 | 1% | |
| P3 | 6.6 | 7.8 | 7.2 | 6.2 | 7.3 | 6.9 | 4% | |
| P4 | 6.6 | 7.9 | 7.4 | 6.5 | 7.4 | 6.8 | 8% | |
| EC | P1 | 2200 | 2070 | 2050 | 2642 | 2665 | 2658 | 23% |
| P2 | 2530 | 2570 | 2558 | 2845 | 2879 | 2862 | 12% | |
| P3 | 1410 | 1460 | 1444 | 1635 | 1658 | 1642 | 14% | |
| P4 | 1490 | 1525 | 1508 | 1685 | 1705 | 1696 | 13% | |
| TDS | P1 | 1100 | 1035 | 1025 | 1265 | 1285 | 1279 | 21% |
| P2 | 1265 | 1285 | 1279 | 1425 | 1444 | 1431 | 11% | |
| P3 | 705 | 730 | 722 | 830 | 850 | 840 | 14% | |
| P4 | 745 | 762 | 754 | 840 | 862 | 849 | 11% | |
| TSS | P1 | 460 | 580 | 520 | 101 | 128 | 115 | 78% |
| P2 | 563 | 650 | 615 | 115 | 139 | 126 | 80% | |
| P3 | 470 | 661 | 540 | 105 | 127 | 118 | 78% | |
| P4 | 490 | 679 | 590 | 100 | 133 | 120 | 80% | |
| BOD | P1 | 255 | 275 | 263 | 30 | 45 | 38 | 85% |
| P2 | 270 | 285 | 275 | 40 | 55 | 49 | 82% | |
| P3 | 140 | 160 | 149 | 20 | 35 | 28 | 81% | |
| P4 | 175 | 185 | 180 | 30 | 42 | 36 | 80% | |
| COD | P1 | 370 | 382 | 376 | 47 | 62 | 51 | 86% |
| P2 | 350 | 375 | 369 | 40 | 56 | 49 | 87% | |
| P3 | 270 | 290 | 285 | 38 | 45 | 42 | 85% | |
| P4 | 361 | 380 | 370 | 48 | 65 | 51 | 86% | |
After irradiation at 13 kilo Gray (kGy) pH values were reduced from 8.35 to 7.01. Gamma irradiation reduced the pH of all the samples collected from industrial drains. The obtained values were within the permissible limits of NEQS. The OH is the main radical for the oxidation of organic compounds produced during the radiolysis of wastewater. The oxidation of organic compounds converts higher molecular weight compounds into lower weight compounds such as organic acid and further oxidation results in lowering the pH (Wang et al., 2006; Paul at al., 2011; Parvin et al., 2015). Following these results, Guo et al. (2008) also reported a decrease in pH of municipal wastewater at the 8 kGy absorbed dose.
3.4. Effect of gamma-irradiation on TDS
Total dissolved solids (TDS) of industrial effluent drain before and after irradiation are given in (Table 2). In comparing Total dissolved contents of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant difference was observed in the value of TDS after irradiation at a dose of 13 kGy.
When effluent samples were subjected to Gamma irradiation increase in concentration was observed with the increase of radiation dose. This increase is due to the conversion of the dissolved organic compound to simple molecular compounds or the formation of dimmers or trimmers. The suspended solids that were at the bottom of the container are dissolved by radiation dose thus increasing the concentration of TDS (Mohammad and Firas. 2006). When TDS values are above the permissible limits of NEQS it causes high turbidity in the water and restricts the light penetration and affects the growth of the biological population (Rehman et al., 2015). Results obtained are in agreement with the previous work of Bhuiyan et al. (2016).
3.5. Effect of gamma-irradiation on EC
Salinity in water is caused by the presence of chloride ions and inorganic salt cations (Fe3+, Ca2+, K+, Na+) which in return shows an elevated level of electrical conductivity (parveen et al., 2017). In comparing electrical conductivity of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant change was observed in the value of EC after irradiation at a dose of 13 kGy. The values of electrical conductivity before and after irradiation are summarized in Table 2. When the samples were irradiated electrical conductivity increased with irradiation dose. This is because EC is the function of dissolved salts in water Bhuiyan et al. (2016).
3.6. Effect of gamma-irradiation on TSS
Higher values of total suspended solids in water cause turbidity in water thus making it unfit for drinking and irrigation. In comparing suspended solids contents of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant difference was observed in the contents of TSS after irradiation at a dose of 13 kGy When effluent samples were treated with gamma irradiation at 13 kGy suspended solids were removed up 75%–80% shown in Table 2.
During irradiation OH radical produced from radiolysis of wastewater react with suspended solids, as a result, the organic substances and suspended material were degraded and converted into a precipitate form which causes a decrease in suspended solids (Selambakkannu et al., 2011). These results are in line with the previous work of Bhuiyan et al. (2016), they also observed a decrease in the value of TSS at an absorbed dose of 10 kGy, while treating textile wastewater with gamma radiation. After irradiation total, suspended solids were found below the permissible limits of Pak-NEQS.
3.7. Effect of gamma-irradiation on BOD
Biological oxygen demand in industrial effluents samples before and after irradiation is illustrated in Table 2. Exceeded values of BOD cause depletion in the oxygen level of water. When such effluent mixes with water stream it affects the aquatic life. In comparing Biological oxygen demand of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant difference was observed in the value of BOD after irradiation at a dose of 13 kGy After treatment of industrial wastewater with gamma radiation at 13 kGy Dose, the values of BOD reduced from 263 to 38 and from 275 to 74 mg/L with an average removal efficiency of 80%. After treatment results were found under the permissible limits defined by Pak-NEQS (Table 2).
The decrease resulted was due to the destruction of microorganisms responsible for the consumption of oxygen as microorganisms are sensitive to irradiation. Besides, radiation energy is also capable of mineralization of the organic compounds (Tahri et al., 2010; Parvin et al., 2015). Results of the study were further supported by Basfar and Rehim (2002), their results revealed that BOD up to 24% can be decreased with the application of 4 kGy absorbed dose of gamma irradiation.
3.8. Effect of gamma-irradiation on COD
In comparing Chemical oxygen demand of wastewater before and after Gamma irradiation using comparing sample t-test at P < 0.05 a significant difference was observed in COD after irradiation at a dose of 13 kGy. When effluent samples were treated with gamma radiation COD decreased from 460 mg/L to 80 mg/L with a removal efficiency of 82%. The reduction in COD after irradiation was irrespective of the sampling points.
Chemical oxygen demand (COD) is the measure of organic compound that is not degraded by microbial activity (Islam et al., 2014). For industrial wastewater studies and pollution control, it is an important parameter. A high level of COD indicates that the water is not feasible for the survival of aquatic organisms due to a reduction in DO content. For the decomposition of pollutant hydroxyl radical is a very important specie. It breaks the chemical bond because it has the potential to oxidize chemical compounds. The decrease in pollutant load is highly correlated to high irradiation doses (Getoff, 2002; Selambakkannu et al., 2011). The decrease in the amount of biodegradable material decreases the value of BOD and COD in irradiated wastewater (Bhuiyun et al., 2016). Lajayer et al. (2020) also reported that values of COD reduced with the increase of radiation dose while working on the effect of radiation on physicochemical and biological characteristics of wastewater effluents and sludge.
4. Conclusion
The effluent released from Hayatabad Industrial Estate joins nearby water bodies without any treatment. The toxic effluents released have an obnoxious consequence on the aquatic environment. The result obtained from the study revealed that Physico-chemical parameters varied for samples. pH, electrical conductivity, and total dissolved solids were found within the permissible limits of Pak-NEQS. Whereas Biological oxygen demand, chemical oxygen demand, total suspended solids were above the permissible limits. A massive amount of chemical reagents are required for chemical treatment. Radiation technology can be adopted to eliminate the use of chemical reagents. When the samples were subjected to gamma irradiation the parameter such as pH, COD, BOD, and suspended solids showed a significant reduction at a dose of 13 kGy. The removal efficiency of BOD, COD, and TSS was observed up to 80%. The results indicate that gamma irradiation is a quite effective and useful technique for wastewater treatment and its recycling.
Declarations
Author contribution statement
M. Nafees: Conceived and designed the experiments.
H.Hina: Analyzed and interpreted the data.
T. Ahmed: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Higher Education Commission, Pakistan.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ahmed M.B., Zhou J.L., Ngo H.H., Guo W., Thomaidis N.S., Xu J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J. Hazard Mater. 2017;323:274–298. doi: 10.1016/j.jhazmat.2016.04.045. [DOI] [PubMed] [Google Scholar]
- American Public Health Association (APHA) Vol. 21. 2005. p. 259. (Standards Method for the Examination of Water and Wastewater). [Google Scholar]
- Amin M.M., Hashemi H., Bina B., Hatamzadeh M., Abdellahi M. Disinfection of water and wastewater of Isfahan water and wastewater treatment plants by gamma irradiation. Int. J. Environ. Health Eng. 2013;2(1):16. [Google Scholar]
- Azizullah A., Khattak M.N.K., Richter P., Häder D.P. Water pollution in Pakistan and its impact on public health—a review. Environ. Int. 2011;37(2):479–497. doi: 10.1016/j.envint.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Basfar A.A., Rehim F.A. Disinfection of wastewater from a Riyadh wastewater treatment plant with ionizing radiation. Radiat. Phys. Chem. 2002;65(4-5):527–532. [Google Scholar]
- Bhuiyan M.R., Shaid A., Hossain M.A., Khan M.A. Decolorization and decontamination of textile wastewater by gamma irradiation in presence of H2O2. Desalin. Water Treat. 2016;57(45):21545–21551. [Google Scholar]
- Bui X.T., Vo T.P.T., Ngo H.H., Guo W.S., Nguyen T.T. Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci. Total Environ. 2016;563:1050–1067. doi: 10.1016/j.scitotenv.2016.04.191. [DOI] [PubMed] [Google Scholar]
- Chmielewski A.G. Practical applications of radiation chemistry. Russ. J. Phys. Chem. A. 2007;81(9):1488–1492. [Google Scholar]
- Chu L., Wang J., Wang B. Effects of aeration on gamma irradiation of sewage sludge. Radiat. Phys. Chem. 2010;79(8):912–914. [Google Scholar]
- EI-Motaium R.A. 2006. Application of Nuclear Techniques in Environmental Studies and Pollution Control. [Google Scholar]
- Getoff N. Factors influencing the efficiency of radiation-induced degradation of water pollutants. Radiat. Phys. Chem. 2002;65(4-5):437–446. [Google Scholar]
- Guo Z., Tang D., Liu X., Zheng Z. Gamma irradiation-induced Cd2+ and Pb2+ removal from different kinds of water. Radiat. Phys. Chem. 2008;77(9):1021–1026. [Google Scholar]
- InamUllah E., Alam A. Assessment of drinking water quality in Peshawar, Pakistan. Bulg. J. Agric. Sci. 2014;20(3):595–600. [Google Scholar]
- Islam M.S., Ahmed M.K., Habibullah-Al-Mamun M., Islam K.N., Ibrahim M., Masunaga S. Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Add. Contam. Part A. 2014;31(12):1982–1992. doi: 10.1080/19440049.2014.974686. [DOI] [PubMed] [Google Scholar]
- Jan F.A., Ishaq M., Khan S., Ihsanullah I., Ahmad I., Shakirullah M. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir) J. Hazard. Mater. 2010;179(1-3):612–621. doi: 10.1016/j.jhazmat.2010.03.047. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Lee S.H., Kim H.Y., Doudrick K., Yu S., Kim S.D. Decomposition of perfluorooctane sulfonate (PFOS) using a hybrid process with electron beam and chemical oxidants. Chem. Eng. J. 2019;361:1363–1370. [Google Scholar]
- Lajayer B.A., Najafi N., Moghiseh E., Mosaferi M., Hadian J. Removal of heavy metals (Cu 2+ and Cd 2+) from effluent using gamma irradiation, titanium dioxide nanoparticles, and methanol. J. Nanostruct. Chem. 2018;8(4):483–496. [Google Scholar]
- Lajayer B.A., Najafi N., Moghiseh E., Mosaferi M., Hadian J. Effects of gamma irradiation on physicochemical and biological characteristics of wastewater effluent and sludge. Int. J. Environ. Sci. Technol. 2020;17(2):1021–1034. [Google Scholar]
- Lee O.M., Kim H.Y., Park W., Kim T.H., Yu S. A comparative study of disinfection efficiency and regrowth control of microorganisms in secondary wastewater effluent using UV, ozone, and ionizing irradiation process. J. Hazard Mater. 2015;295:201–208. doi: 10.1016/j.jhazmat.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Limam R.D., Limam I., Clérandeau C., Khouatmia M., Djebali W., Cachot J., Chouari R. Assessment of the toxicity and the fertilizing power from the application of gamma-irradiated anaerobic sludge as fertilizer: effect on Vicia faba growth. Radiat. Phys. Chem. 2018;150:163–168. [Google Scholar]
- Liu B., Ma X., Ai S., Zhu S., Zhang W., Zhang Y. Spatial distribution and source identification of heavy metals in soils under different land use in a sewage irrigation region, northwest China. J. Soils Sediments. 2016;16(5):1547–1556. [Google Scholar]
- Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Madureira J., Pimenta A.I., Popescu L., Besleaga A., Dias M.I., Santos P.M., Margaça F.M. Effects of gamma radiation on cork wastewater: antioxidant activity and toxicity. Chemosphere. 2017;169:139–145. doi: 10.1016/j.chemosphere.2016.11.064. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Radiation induced decontamination of Cr (VI), Cu (II) and phenol in some tannery effluents. Nucl. Sci. Tech. 2007;18(4):212–217. [Google Scholar]
- Makuuchi K. 2003. Radiation processing of liquid with low energy electron accelerator. [Google Scholar]
- Nafees M., Shah W., Khan H., Ullah Z. Study of Paper Mill for Water Recycling, Hayatabad Industrial Estate, Peshawar. J. Sci. Technol. 2010;34(1) [Google Scholar]
- Nafees M., Nawab A., Shah W. Study on the performance of wastewater treatment plant designed for industrial effluents. J. Eng. Appl. Sci. 2015;34(1) [Google Scholar]
- Nasrullah R.N., Bibi H., Iqbal M., Durrani M.I. Pollution load in industrial effluent and ground water of Gadoon Amazai Industrial Estate (GAIE) Swabi, NWFP. J. Agric. Biol. Sci. 2006;1(3):18–24. [Google Scholar]
- National Environmental Quality Standard' 2010. http://epa.kp.gov.pk/page/national_environment_quality_standards on 2018 Retrieved from URL.
- Parvin F., Ferdaus Z., Tareq S.M., Choudhury T.R., Islam J.M., Khan M.A. Effect of gamma-irradiated textile effluent on plant growth. Int. J. Recycl. Org. Waste Agric. 2015;4(1):23–30. [Google Scholar]
- Paul J., Rawat K.P., Sarma K.S.S., Sabharwal S. Decoloration and degradation of Reactive Red-120 dye by electron beam irradiation in aqueous solution. Appl. Radiat. Isot. 2011;69(7):982–987. doi: 10.1016/j.apradiso.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Lee S.S., Zhang M., Tsang Y.F., Kim K.H. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Rashed M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Organic Poll. Monitor. Risk Treat. 2013;7:167–194. [Google Scholar]
- Rathod P.H., Patel J.C., Jhala A.J. Potential of gamma-irradiated sewage sludge as fertilizer in radish: evaluating heavy-metal accumulation in sandy loam soil. Commun. Soil Sci. Plant Anal. 2011;42(3):263–282. [Google Scholar]
- Rehman M.Z.U., Rizwan M., Ghafoor A., Naeem A., Ali S., Sabir M., Qayyum M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its Phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Control Ser. 2015;22(21):16897–16906. doi: 10.1007/s11356-015-4883-y. [DOI] [PubMed] [Google Scholar]
- Rehman S., Adnan M. Department of Environmental Sciences, University of Peshawar; 2013. Evaluation of wastewater treatment plant through physico-chemical Analysis of industrial water of Hayatabad Industrial Estate; pp. 17–21. Unpublished MSc Thesis. [Google Scholar]
- Rivier P.A., Havranek I., Coutris C., Norli H.R., Joner E.J. Transfer of organic pollutants from sewage sludge to earthworms and barley under field conditions. Chemosphere. 2019;222:954–960. [Google Scholar]
- Salgot M., Campos C., Galofré B., Tapias J.C. Biological control tools for wastewater reclamation and reuse. A critical review. Water Sci. Technol. 2001;43(10):195–201. [PubMed] [Google Scholar]
- Salvatore M.D., Carratù G., Carafa A.M. Assessment of heavy metals transfer from a moderately polluted soil into the edible parts of vegetables. J. Food Agric. Environ. 2009;7(2):683–688. [Google Scholar]
- Selambakkannu S., Bakar K.A., Ming T.T., Sharif J. Effect of gamma and electron beam irradiation on textile waste water. Jurnal Sains Nuklear Malaysia. 2011;23(2):67. [Google Scholar]
- Sarhad Development Authority (SDA) Arbab Road; Peshawar: 2016. PIA Building. [Google Scholar]
- Sivakumar D., Nouri J. Removal of contaminants in a paper mill effluent by Azolla caroliniana. Glob. J. Environ. Sci. Manag. 2015;1(4):297–304. [Google Scholar]
- Tahri L., Elgarrouj D., Zantar S., Mouhib M., Azmani A., Sayah F. Wastewater treatment using gamma irradiation: Tétouan pilot station, Morocco. Radiat. Phys. Chem. 2010;79(4):424–428. [Google Scholar]
- Tariq M., Ali M., Shah Z.J.S.E. Characteristics of industrial effluents and their possible impacts on quality of underground water. Soil Environ. 2006;25(1):64–69. [Google Scholar]
- Toze S. Reuse of effluent water—benefits and risks. Agric. Water Manag. 2006;80(1-3):147–159. [Google Scholar]
- Verde S.C., Tenreiro R., Botelho M.L. Sanitation of chicken eggs by ionizing radiation: HACCP and inactivation studies. Radiat. Phys. Chem. 2004;71(1-2):29–33. [Google Scholar]
- Wang J., Chu L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an over- view. Radiat. Phys. Chem. 2016;125:56–64. [Google Scholar]
- Wang S., Li H., Xie S., Liu S., Xu L. Physical and chemical regeneration of zeolitic adsorbents for dye removal in wastewater treatment. Chemosphere. 2006;65(1):82–87. doi: 10.1016/j.chemosphere.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhuan R., Chu L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview. Sci. Total Environ. 2019;646:1385–1397. doi: 10.1016/j.scitotenv.2018.07.415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.