Abstract
Background and aims
The idea of treating COVID-19 with statins is biologically plausible, although it is still controversial. The systematic review and meta-analysis aimed to address the association between the use of statins and risk of mortality in patients with COVID-19.
Methods
Several electronic databases, including PubMed, SCOPUS, EuropePMC, and the Cochrane Central Register of Controlled Trials, with relevant keywords up to 11 November 2020, were used to perform a systematic literature search. This study included research papers containing samples of adult COVID-19 patients who had data on statin use and recorded mortality as their outcome of interest. Risk estimates of mortality in statin users versus non-statin users were pooled across studies using inverse-variance weighted DerSimonian-Laird random-effect models.
Results
Thirteen studies with a total of 52,122 patients were included in the final qualitative and quantitative analysis. Eight studies reported in-hospital use of statins; meanwhile, the remaining studies reported pre-admission use of statins. In-hospital use of statin was associated with a reduced risk of mortality (RR 0.54, 95% CI 0.50–0.58, p < 0.00001; I2: 0%, p = 0.87), while pre-admission use of statin was not associated with mortality (RR 1.18, 95% CI 0.79–1.77, p = 0.415; I2: 68.6%, p = 0.013). The funnel plot for the association between the use of statins and mortality were asymmetrical.
Conclusion
This meta-analysis showed that in-hospital use of statins was associated with a reduced risk of mortality in patients with COVID-19.
Keywords: Statins, In-hospital, Pre-admission, COVID-19, SARS-CoV-2, Mortality
Introduction
Many believe we are now approaching the end of the coronavirus disease 2019 (COVID-19) pandemic by the ultimate discovery of effective SARS-CoV-2 vaccination. However, there is still a time gap before the global vaccination could finally be completed. In the meantime, we still need to take preventive measures for COVID-19 and its fatal complications by not only controlling specific chronic comorbidities [1–4], but also seeking the possible adjunctive treatment for patients already in severe COVID-19 condition [5].
Acute respiratory distress syndrome (ARDS) and thromboembolism are the two notorious complications that cause a high mortality rate among hospitalized patients with severe COVID-19 [6, 7]. Current evidence suggests that lethal complications of SARS-CoV-2 infection originate from the “overactive” inflammatory response, which culminates in cytokine storms [8]. The initial inflammatory response occurs after the SARS-CoV-2 invasion cause disruption of both the epithelial and endothelial cells in alveoli [9, 10]. The subsequent endothelial dysfunction, release of inflammatory cytokines, thrombin generation, and fibrin clot deposition ultimately conclude in ARDS and catastrophic hypercoagulable state in COVID-19 [11].
Statins appear to hold the rationale as adjunctive therapy of COVID-19. Statins are more than lipid-lowering agents; they wield a pleiotropic effect with anti-inflammatory, immunomodulatory, and antithrombotic properties [12–14]. Studies have shown that statins could improve the outcomes of patients with community-acquired pneumonia [15, 16]. However, the use of statins in COVID-19 patients is still controversial. Cariou et al. reported an increased risk of mortality among statin users [17], while Zhang et al. reported otherwise [18]. Therefore, we aimed to address the association between the use of statins and risk of mortality in patients with COVID-19.
Materials and methods
Data sources
Several electronic databases, including PubMed, SCOPUS, EuropePMC, and the Cochrane Central Register of Controlled Trials, with relevant keywords (“COVID-19” OR “SARS-CoV-2”) AND (“statin” or “HMG-CoA reductase inhibitors” or “simvastatin” or “atorvastatin” or “rosuvastatin” or “pitavastatin” or “lovastatin” or “fluvastatin”) AND “mortality”, were used to perform a systematic literature search by three independent authors (HP, IH, and NS). We restricted the time of published articles from 1 December 2019 until our search finalization, which was 11 November 2020. Any duplicate records were removed after obtaining the initial results. By screening the title and abstracts, potential articles were then sorted. Afterwards, the full texts of the remaining articles were assessed for relevance based on eligibility criteria. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was carried out as the core protocol in this study.
Eligibility criteria
The inclusion criteria were as follows: (1) research articles (either observational or interventional studies), (2) in adults patients with confirmed COVID-19 based on reverse transcriptase—polymerase chain reaction (RT-PCR) test, (3) which had data on the use of statins (either pre-admission or in-hospital use of statins), and (4) reported all types of mortality. In-hospital use of statins was defined as the use of statins during hospitalization. In contrast, statins that were chronically or routinely taken before hospital admission were defined as pre-admission use of statins. The latter also included the use of statins, which was not explicitly stated as being continued during hospitalization. The outcome of interest was all types of mortality, including in-hospital mortality and 14- or 28-days (30-days) mortality. Non-research letters, review articles, case reports, research samples < 18 years old, and non-English language articles, were excluded from this study.
Data collection and extraction
Information from the included studies was collected by three independent authors (HP, IH, NS) using a pre-specified form. This form consisted of the first author, design of the study, location, samples, age, sex, use of statins (pre-admission or in-hospital), type and dose of statins, mortality, adjusted estimate, and covariates adjustment.
Study quality assessments
The Newcastle–Ottawa Scale (NOS), which is quality assessment tool for non-randomized studies [19], was used independently by the three authors (HP, IH, and NS) for the included studies. Any disagreements were decided through discussion.
Statistical analysis
Meta-analysis of included studies was conducted using STATA 16 (StataCorp LLC, Texas, US). In this analysis, hazard ratio (HR), odds ratio (OR), and risk ratio were regarded as comparable measures of risk estimates and defined as relative risk (RR) in this study. RRs of mortality in statin users versus non-statin users were pooled across studies using inverse-variance weighted DerSimonian-Laird random-effect models. Statistically significant values were considered if the P-values were < 0.05. Between-study heterogeneity was assessed using I-square (I2) statistic, with a value of > 50 percent or P-values < 0.10 suggesting heterogeneity. Any significant heterogeneity was further assessed with leave-one-out sensitivity analysis. Funnel-plot asymmetry test was used to assess the presence of publication bias.
Results
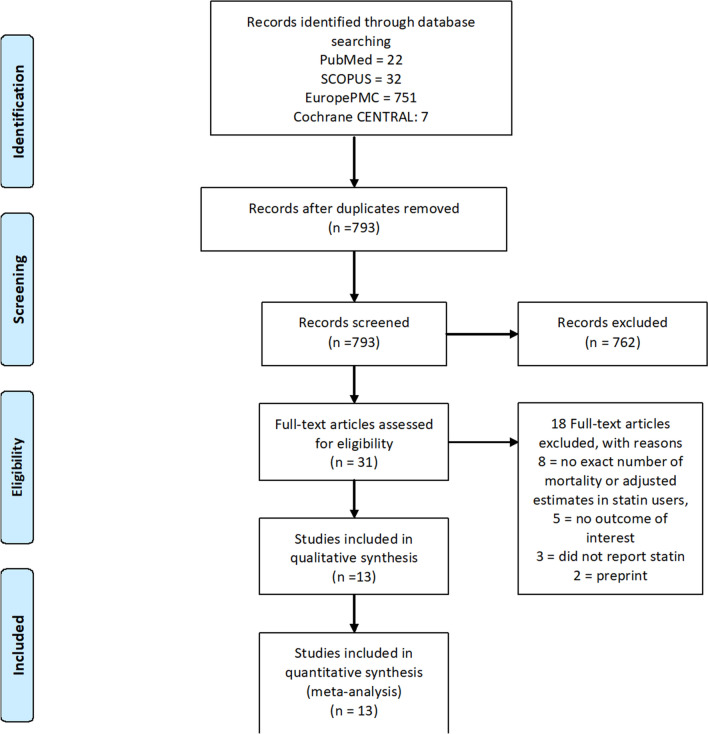
There were 812 records from our initial searches, which was reduced to 793 after duplicates removal. We excluded 762 records after title and abstract screening. After assessing 31 full texts of the remaining articles for eligibility, eight articles were excluded, because they did not report either number of mortality or adjusted estimates of mortality in the statin group. Five studies were also excluded because they did not report mortality as outcome of interest. Three other articles did not report the use of statin, while two articles were preprints. Thereby, 13 studies with a total of 52,122 patients met our inclusion criteria and were included in the final qualitative and quantitative analysis (Fig. 1) [17, 18, 20–30]. Eight studies reported in-hospital use of statins; meanwhile, the remaining studies reported pre-admission use of statins. The baseline characteristics of the included studies can be seen in Table 1.
Fig. 1.
Study flow diagram
Table 1.
Characteristics of the included studies
| First author | Design | Location | Total samples (statin vs non-statin) | Age | Male (%) | Statins use | Type of statins (%) | Mortality (statin vs non-statin)* | Adjusted estimate (95% CI) | Adjusted covariates | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cariou [17] | RC, Multi-center | France | 2449 (1192 vs 1257) | 71.7 (10.8) vs 70.2 (13.9) | 67.8 vs 60.5 | Pre-admission | NA | 28 days (NA) | aOR: 1.46 (1.08–1.95) | Age, Gender, ethnicity, BMI, HTN, DM complications, HF, OSA or COPD, use of any of the following drugs/drug classes on admission: metformin, DPP-4 inhibitors, GLP-1 RA; insulin; ezetimibe; RAS blockers and MRA, CCB, anticoagulant agents and corticosteroids | 8 |
| Lala [23] | RC, Single-Center | USA | 2736 (984 vs 1752) | 66.4 (15.8) | 59.6 | In-hospital | NA | In-hospital: 506 (18.5, NA) | aHR: 0.57, (0.47–0.69) | Age, gender, race, ethnicity, BMI, CAD, DM, HF, HTN, AF, CKD; CURB-65 score, ACEI or ARB use | 8 |
| De Spiegeleer [24] | RC, Multi-Center | USA | 154 (31 vs 123) | 85.6 (5.3) vs 85.9 (7.6) | 32.3 vs 33.3 | Pre-admission | NA | 14 days after symptoms onset (NA) | aOR: 0.51 (0.14–1.35) | Age, gender, functional status, DM, HTN | 9 |
| Zhang [18] | RC, Multi-Center | China | 13,981 (After Propensity- matched: 861 vs 3444 = 4305) | 65.0 (57–71) vs 65.0 (57–72) | 47.7 vs 48.0 | In-Hospital |
Atorvastatin: 84.8 Rosuvastatin: 13.1 Simvastatin: 1.9: Pravastatin: 1.3 Pitavastatin: 7 0.8 |
28 days: 45 (5.2) vs 325 (9.4) | aHR: 0.58, (0.43–0.80) | Age, gender, and O2 saturation at admission | 9 |
| Daniels [30] | RC, Single-Center | USA | 170 (46 vs 124) | 59 (19) | 58.0 | Pre- admission | NA | In-Hospital (NA) | aOR: 0.45 (0.11–1.87) | Age, gender, and comorbid conditions including obesity, HTN, DM, CVD, and CKD | 8 |
| Krishnan [25] | RC, Single-Center | USA | 152 (81 vs 71) | 66 (13) | 62.5 | Pre-admission | NA | In-Hospital: 60 (39.5, NA) | OR: 2.443 (1.225–4.756) | No adjustment of covariates | 6 |
| Mallow [26] | RC, Multi-Center | USA | 21,676 (5313 vs 16,363) | 64.9 (17.2) | 52.8 | In Hospital | NA | In-Hospital: 4936 (22.8, NA) | aOR: 0.54 (0.49–0.60) | Age, gender, CDC Risk Factors (chronic lung disease, asthma, heart condition, immunocompromised, obesity, DM, CKD on dialysis, liver disease, HTN) DNR status, Insurance, Teaching hospital status, Hospital bed size | 8 |
| Masana [28] | RC, Multi-Center | Spain | 2157 (581 vs 1576) before 1:1 genetic matching | 73 (65–80) vs 74 (64–84) | 356 (61.3) vs 336 (57.9) |
In-Hospital Statin Withdrawn:241 (42.2) vs Statin maintained: 327 (57.8) |
30% on high intensity (80 mg/day atorvastatin or 20 mg/day rosuvastatin) 70% on low-moderate intensity | In-hospital: 115 (19.8) vs 148 (25.4) | aHR:0.58 (0.39–0.89) | Age, gender, baseline comorbidities (HTN, hyperlipidemia, DM, obesity, CAD, Stroke, PAD, HF, COPD/Asthma, CLD, CKD, RD, Cancer) | 8 |
| Rodriguez-Nava et. [22] | RC, Single-Center | USA | 87 (47 vs 40) | 68 (58–75) | 56 (64.4) | In-Hospital | Atorvastatin 40 mg | In-hospital: 23 (47.9) vs 24 (61.5) | aHR: 0.38 (0.18–0.77) | Age, number of comorbidities, hypertension, cardiovascular disease, severity, invasive mechanical ventilation, and antibiotics other than azithromycin | 8 |
| Rossi [27] | PC, Single-Center | Italy | 71 (42 vs 29) | 71 (64–92) vs 73 (63–90) | 57.1 vs 55.2 | In-Hospital |
Hydrophilic Statin: 38.1 (Rosuvastatin 33.3, Pravastatin 4.8) Liphophilic Statin: 61.9 (Atorvastatin 52.3, Simvastatin 9.6) High intensity Statin: 42.8 |
In-Hospital Death: 9 (21.4) vs 10 (34.5) | OR:0.52 (0.18–1.50) | No adjustment of covariates | 6 |
| Saeed [21] | RC, Single-Center | USA | 4252 (1355 vs 2897) | 69 (12) vs 63 (17) | 54 vs 53 | In-Hospital |
Atorvastatin: 76.0 Pravastatin 5.0 Rosuvastatin: 1.0 Simvastatin:18.0 |
In-hospital: 23.0 vs. 27.0 In Diabetic samples: 24.0 vs 39.0 |
aHR: 0.51 (0.43–0.61) in diabetic samples | Age, gender, history ASHD, CCI, DBP, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine and intravenous antibiotic use during hospitalization | 8 |
| Song [29] | RC, Single-Center | USA | 249 (123 vs 126) | 71 (60–79) vs 54.5 (42–67) | 57.0 | In Hospital | NA | In-hospital death: 27 (22.0) vs 15 (11.9) | aOR: 0.88 (0.37–2.08) | Age, gender, race, CVD, chronic pulmonary disease, DM, obesity | 9 |
| Grasseli [20] | RC, Single-Center | Italy | 3988 (479 vs 3509 | 63 (56–69) | 79.9 | Pre-Admission | Atorvastatin, fluvastatin, pravastatin, simvastatin, rosuvastatin | In Hospital: 1926 (48.3, NA) | aHR: 0.98 (0.81–1.20) | Age, gender, respiratory support, Comorbidities (HTN, hypercholesterolemia, Heart Disease, T2DM, COPD, cancer), Medications (ACEI, ARB, Diuretic), PEEP, FiO2, and PaO2/FiO2 at admission | 8 |
RC Retrospective Cohort, PC Prospective Cohort, NA Not Available, ASHD Atherosclerotic Heart Disease, AF Atrial Fibrillation, ACEI Angiotensin Converting Enzyme Inhibitor, ARB Angiotensin Receptor Blocker, BP Blood Pressure, BMI Body-mass Index, CAD Coronary artery disease, CCB calcium-channel blockers, CCI Charlson Comorbidity Index, COPD Chronic Obstructive Pulmonary Disease, CKD Chronic Kidney Disease, CLD Chronic Liver Disease, CVD Cardiovascular Disease, COVID-19 Coronavirus disease 2019, DBP Diastolic Blood Pressure, DPP-4 Dipeptidyl Peptidase-4, DM Diabetes Mellitus, T1DM Type 1 DM, T2DM Type 2 DM, DNR Do-Not-Resuscitate, FiO2 fraction of inspired oxygen, HTN Hypertension, HF Heart Failure, GLP-1 RA Glucagon-like Peptide-1 Receptor Agonist, MRA Mineralocorticoid receptor antagonist, OSA Obstructive Sleep Apnea, PAD Peripheral Artery Disease, PaO2 arterial partial pressure of oxygen, PEEP positive end-expiratory pressure, RD Rheumatic Disease, RAS Renin–angiotensin–aldosterone system, aOR Adjusted Odds Ratio, aHR Adjusted Hazard Ratio, eGFR Estimated Glomerular Filtration Rate
*Data are presented as total mortality in Statin (%) vs Non-Statin group (%), if not available, total mortality in both groups will be presented
In-hospital use of statins and mortality
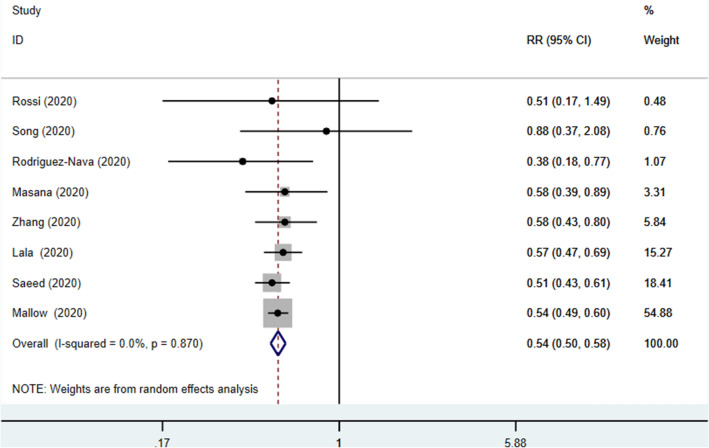
In-hospital use of statin was associated with a reduced risk of mortality (RR 0.54, 95% CI 0.50–0.58], p < 0.00001; I2: 0%, p = 0.87) (Fig. 2).
Fig. 2.
Forest plot showing overall effect estimates of in-hospital use of statins and mortality in patients with COVID-19. RR Relative Risk, CI Confidence Interval
Pre-admission use of statins and mortality
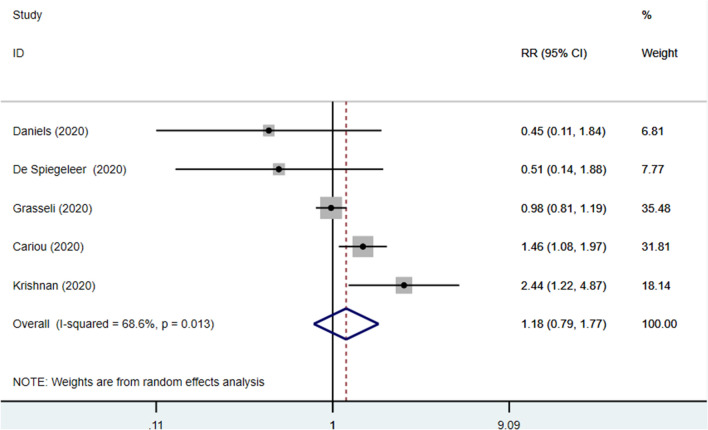
Pre-admission use of statin was not associated with mortality (RR 1.18, 95% CI 0.79–1.77], p = 0.415; I2: 68.6%, p = 0.013) (Fig. 3). Leave-one out sensitivity analysis could not significantly reduce heterogeneity.
Fig. 3.
Forest plot showing overall effect estimates of pre-admission use of statins and mortality in patients with COVID-19. RR Relative Risk, CI Confidence Interval
Quality assessment and risk of bias
Most studies included in this analysis were considered as high quality (Table 1). The risk of bias was mainly due to insufficient data validators and inadequate data reporting. The funnel plot for the association between the use of statins, both in-hospitals and pre-admission, and mortality were asymmetrical (Fig. 4a, b).
Fig. 4.
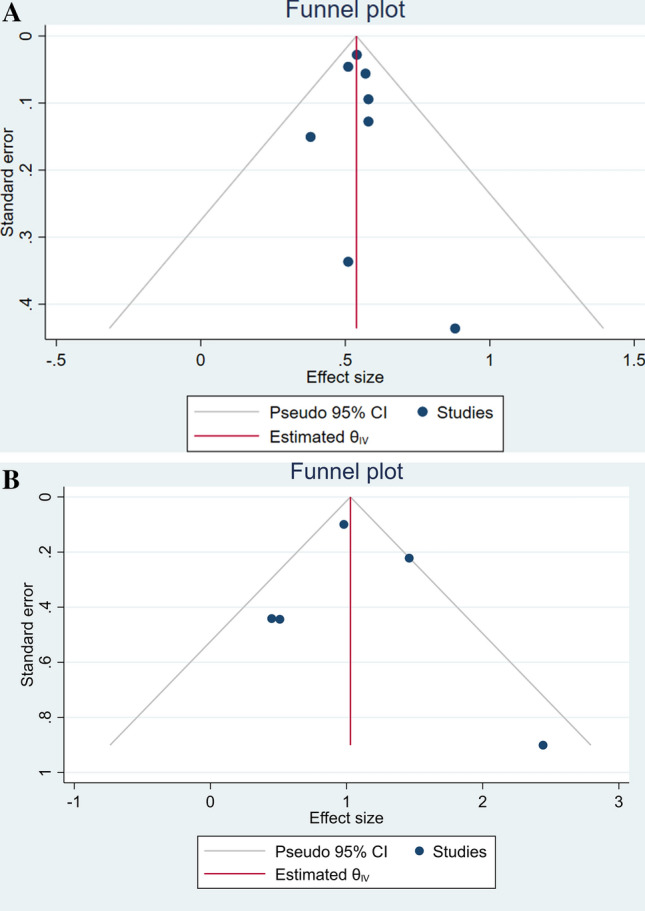
Funnel-plot analysis. a In-hospital and b Pre-admission use of statins and mortality in patients with COVID-19. CI Confidence Interval
Discussion
The differentiation between in-hospital and pre-admission statins use proved to be the key explanation that illuminates the conflicting evidence of previous studies. The association between statins and increased risk of mortality among hospitalized diabetic patients with COVID-19 was previously reported by Cariou et al. [17]; however, the authors explicitly stated that they lacked information on whether statins were continued during hospitalization. Suppose the study sample on diabetic patients was used as a justification for this occurrence. In that case, it could not explain the results of Saeed et al., which showed a significant reduction of in-hospital mortality among diabetic patients with COVID-19 receiving statins [21].
This current meta-analysis showed that in-hospital use of statins was significantly associated with reduced risk of mortality in patients with COVID-19. The mortality risk reduction was almost 50% compared to non-statin users with good consistency across all the included studies. Meanwhile, pre-admission use of statins did not exert the same benefit. Previous two meta-analyses in smaller scales showed conflicting results. Kow et al. reported that the use of statins significantly reduced risk for fatal or severe disease in COVID-19 [31], whereas Hariyanto et al. showed the insignificant associations between statins and poor outcome [32]. Interestingly, both of the studies did not differentiate between in-hospital and pre-admission use of statins. This differentiation further translated into low levels of heterogeneity and statistically significant association.
Statins are widely known as lipid-lowering agents with pleiotropic effects on inflammation, immunomodulation, and oxidative stress [13]. Despite their pharmacodynamic properties, statins are readily available, inexpensive, and have well-established safety profiles, which contribute to the ongoing use of these drugs to improve disease outcome in influenza and other viral illnesses [33]. The use of statins in reducing mortality among hospitalized patients infected with influenza was reported previously by Vandermeer et al. and Laidler et al. [34, 35], although the latter concluded that the unmeasured variables may confound the effect. However, the evidence that statins reduce mortality in influenza patients has led to their consideration as potential adjunctive treatments to reduce mortality in emerging viral diseases likely to cause significant epidemics [36].
Numerous experts postulated that the benefits of statin in COVID-19 might be related to the angiotensin-converting enzyme 2 (ACE2) expressions [13, 37]. ACE2 is the tissue receptor that facilitates the entry of SARS-CoV-2 into host cells [38]. This receptor is expressed not only in the lungs but is also found throughout the body, including the heart, vasculature, brain, gastrointestinal tract, and the kidneys [39]. ACE2 plays an important role in counter-regulating the renin–angiotensin system, especially in the conversion of angiotensin (AT) II to AT-(1–7). AT-(1–7) counteracts the effects of AT II, which induces inflammation, increased oxidative stress, vasoconstriction, and fibrosis, leading to tissue injuries [40]. A previous study reported that statins increase the expression of ACE2 by approximately two-fold [41], and the increased activity of ACE2 has been associated with decreased incidence of ARDS [42]. Nevertheless, whether there is an association between ACE2 activities and the severity of COVID-19 is still a matter of debate. A recent trial of medication known to upregulate ACE2 expressions showed no effects on the outcome of hospitalized patients with COVID-19 [43, 44].
This study's novel finding, which was the mortality risk reduction only among hospitalized COVID-19 patients with in-hospital use of statins, might be due to the drug's direct antiviral effects (Table 2). Statins may prevent SARS-CoV-2 entry to the respiratory epithelial cells through the modulation of CD-147, a cell surface protein with an essential role in facilitating viral access to the host cells [45]. Furthermore, the drugs could inhibit SARS-CoV-2 replication by directly interacting with the viral main protease (Mpro), which serves as a key enzyme in proteolytic maturation [46]. The direct nature of antiviral actions of statins in COVID-19 theoretically conveys its benefit only when the drugs are actively taken; thus, pre-admission use of statins could not wield the same effects.
Table 2.
Potential role of statins in COVID-19
| Potential role of statins | Molecular mechanism of action | References |
|---|---|---|
| Preventing SARS-CoV-2 Entry | Modulation of CD-147 expression | [45] |
| Inhibiting SARS-CoV-2 Replication | Interaction viral main protease (Mpro) | [46] |
| Attenuating hyperinflammatory response and cytokine storms |
Modulation of Toll-like receptor (TLR) and NOD-like receptor activities Reduction of IL-6 and IL-1β cytokines |
[49] [50] |
| Modulating hypercoagulability | Decreased plasma levels of von Willebrand factor antigen (vWF:Ag) and plasma endothelin-1 concentrations | [53, 54] |
| Mitigating lung injury | Increased expression of ACE2* | [41, 42] |
SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus-2, COVID-19 Coronavirus disease-2019, ARDS Acute Respiratory Distress Syndrome, ACE2 angiotensin-converting enzyme 2, IL-6 Interleukin-6, IL-1β Interleukin-1β
*Still controversial
Another potential biological hypothesis on the benefits of using statins to treat COVID-19 relates to their anti-inflammatory, immunomodulatory, and antithrombotic properties [12]. Hyper-inflammatory condition leading to cytokine storms along with hypercoagulable state are considered hallmarks of severe COVID-19 [47]. These conditions may ultimately culminate in MOF and even death [48]. Statins may confer their benefit in attenuating inflammation by the modulation of Toll-like receptor and NOD-like receptor activities [49], thus reducing the hyper-inflammatory state in severe COVID-19. It has been shown previously that high dose administration of simvastatin can reduce the production of IL-6 and IL-1β cytokines [50]. The advent of lung pathology has facilitated the observation of occlusion and micro-thrombosis in the small pulmonary vessels of critically ill COVID-19 patients [51]. It has elucidated the benefit of using anticoagulant therapy in hospitalized COVID-19 patients [52]. It seems that the antithrombotic activities of statins could also play a role. The use of statins is associated with decreased plasma levels of von Willebrand factor antigen (vWF:Ag) and plasma endothelin-1 concentrations [53, 54]. Reduced concentrations of both plasma vWF:Ag and endothelin-1 contribute to the antithrombotic properties of statins.
Although this meta-analysis showed preliminary evidence for in-hospital use of statins in reducing the risk of mortality among patients with COVID-19, there were several possible caveats. Any verdict regarding the benefits of statins on the outcome of COVID-19 patients could not be drawn conclusively due to the nature of retrospective and observational studies included in this analysis. These included studies could not accurately measure the strength of the association; thus, further randomized controlled trials are needed to confirm the benefits of statins. Moreover, cautious interpretation of the findings of this meta-analysis should take into account the possible presence of publication bias as denoted by the asymmetrical funnel plot. Lastly, the type and the dose of statins in more than half of the included studies were mostly not described.
Conclusion
In-hospital use of statins was associated with a reduced risk of mortality in patients with COVID-19. Meanwhile, pre-admission use of statins did not exert the same effect.
Author contributions
HP: conceptualization, methodology, validation, writing—original draft, writing—review and editing, supervision. IH: conceptualization, methodology, investigation, data curation, formal analysis, validation, resources, visualization, writing—original draft, writing—review and editing. AP: software, validation, data curation, formal analysis, visualization. NYK: writing—review and editing, supervision. TAS: writing—review and editing, supervision. EM: writing—review and editing, supervision. RW: writing—review and editing, supervision. NNMS: conceptualization, writing—review and editing, supervision.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The data used to support the findings of this study are included in the article.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hikmat Permana, Email: hikmat.permana@unpad.ac.id.
Ian Huang, Email: ianhuang2108@gmail.com.
Aga Purwiga, Email: aga.purwiga@gmail.com.
Nuraini Yasmin Kusumawardhani, Email: nuraini.yasmin.k@gmail.com.
Teddy Arnold Sihite, Email: teddysyhyte@yahoo.com.
Erwan Martanto, Email: erwanmartanto@yahoo.com.
Rudi Wisaksana, Email: rudiw98@gmail.com.
Nanny Natalia M. Soetedjo, Email: nsoetedjo0@gmail.com, Email: n.natalia@unpad.ac.id
References
- 1.Pranata R, Supriyadi R, Huang I, Permana H, Lim MA, Yonas E, et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420959165. doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andhika R, Huang I, Wijaya I. The severity of COVID-19 in end-stage kidney disease patients on chronic dialysis. Ther Apher Dial. 2020;50(1744–9987):13597. doi: 10.1111/1744-9987.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijaya I, Andhika R, Huang I. Hypercoagulable state in COVID-19 with diabetes mellitus and obesity: is therapeutic-dose or higher-dose anticoagulant thromboprophylaxis necessary? Diabetes Metab Syndr Clin Res Rev. 2020;14:1241–1242. doi: 10.1016/j.dsx.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, et al. Effect of heart failure on the outcome of COVID-19—a meta analysis and systematic review. Am J Emerg Med. 2020;155:104743. doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb. 2020 doi: 10.1177/1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant H, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14:1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29–30:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;1:1–4. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med. 2020;9:1909. doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Hear J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KCH, Sewa DW, Phua GC. Potential role of statins in COVID-19. Int J Infect Dis. 2020;96:615–617. doi: 10.1016/j.ijid.2020.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Grudzinska FS, Dosanjh DP, Parekh D, Dancer RC, Patel J, Nightingale P, et al. Statin therapy in patients with community-acquired pneumonia. Clin Med (Northfield Il) 2017;17:403–407. doi: 10.7861/clinmedicine.17-5-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cariou B, Goronflot T, Rimbert A, Boullu S, Le May C, Moulin P, et al. Routine use of statins and increased mortality related to COVID-19 in inpatients with type 2 diabetes: Results from the CORONADO study. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32(176–187):e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. (2020) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 17 Aug 2020
- 20.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy: Jama; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeed O, Castagna F, Agalliu I, Xue X, Patel SR, Rochlani Y, et al. Statin use and in-hospital mortality in diabetics with COVID-19. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The effects of ARBs, ACEIs and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020 doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan S, Patel K, Desai R, Sule A, Paik P, Miller A, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67:110005. doi: 10.1016/j.jclinane.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID-19 patients by risk factors: results from a united states hospital claims database. J Heal Econ Outcomes Res. 2020;7:165–175. doi: 10.36469/jheor.2020.17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi R, Talarico M, Coppi F, Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. 2020;15:1573–1576. doi: 10.1007/s11739-020-02504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masana L, Correig E, Rodríguez-Borjabad C, Anoro E, Arroyo JA, Jericó C, et al. Effect of statin therapy on SARS-CoV-2 infection-related. Eur Hear J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, et al. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020;9:759. doi: 10.3390/pathogens9090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariyanto TI, Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bifulco M, Gazzerro P. Statins in coronavirus outbreak: It’s time for experimental and clinical studies. Pharmacol Res. 2020;156:104803. doi: 10.1016/j.phrs.2020.104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 35.Laidler MR, Thomas A, Baumbach J, Kirley PD, Meek J, Aragon D, et al. Statin treatment and mortality: propensity score-matched analyses of 2007–2008 and 2009–2010 laboratory-confirmed influenza hospitalizations. Open Forum Infect Dis. 2015;2:ofv028. doi: 10.1093/ofid/ofv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedson DS. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med. 2016 doi: 10.21037/atm.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe covid-19 infection. MBio. 2020;11:1–3. doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280):e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Wösten-Van Asperen RM, Bos AP, Bem RA, Dierdorp BS, Dekker T, Van Goor H, et al. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med. 2013;14:438–441. doi: 10.1097/PCC.0b013e3182a55735. [DOI] [PubMed] [Google Scholar]
- 43.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 44.Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-santander NR, Medina C, et al. Continuation versus discontinuation of renin—angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;2:1–10. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 46.Reiner Ž, Hatamipour M, Banach M, Pirro M, Al-Rasadi K, Jamialahmadi T, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7:1–12. doi: 10.1177/2054358120938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. 2020 doi: 10.1007/s12016-020-08791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moutzouri E, Tellis CC, Rousouli K, Liberopoulos EN, Milionis HJ, Elisaf MS, et al. Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide—induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis. 2012;225:381–387. doi: 10.1016/j.atherosclerosis.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 51.Luo W, Yu H, Gou J, Li X, Sun Y, Li J, et al. (2020) Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints 2020:1–18. https://www.preprints.org/manuscript/202002.0407/v4. Accessed 19 July 2020
- 52.Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GYH, et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen: systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost. 2016;115:520–532. doi: 10.1160/TH15-08-0620. [DOI] [PubMed] [Google Scholar]
- 54.Sahebkar A, Kotani K, Serban C, Ursoniu S, Mikhailidis DP, Jones SR, et al. Statin therapy reduces plasma endothelin-1 concentrations: a meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015;241:433–442. doi: 10.1016/j.atherosclerosis.2015.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.