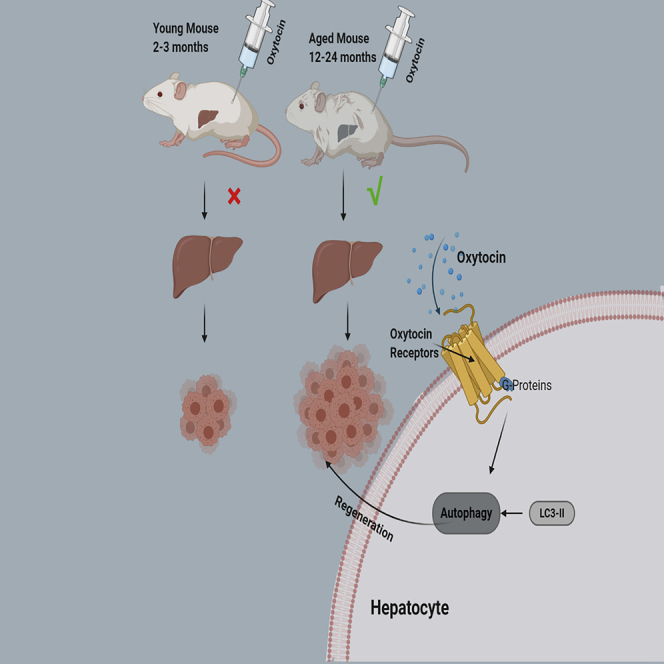
Summary
Liver aging impairs the ability of hepatocyte regeneration. Recent studies have found that oxytocin (OT) plays an important role in promoting tissue repair and maintaining differentiation and regeneration of stem cells. Here, we reported that OT receptors, which are specifically located in hepatocytes, decrease with aging in human and mice. Interestingly, the level of serum OT also decline with age. Notably, OT promotes hepatocyte regeneration only in aged mice but not in young mice in vitro and in vivo. Further studies reveal that OT promotes autophagy in either AML12 mouse hepatocytes or aged mice after partial hepatectomy or with CCl4-induced acute liver injury. In conclusion, OT promotes liver regeneration, especially in aged mice, which may be achieved by promoting autophagy. All these results support the possibility of OT and its analog being a potent anti-aging drug and promote liver rejuvenation.
Subject areas: age, molecular physiology, molecular biology, endocrinology
Graphical abstract
Highlights
-
•
The levels of oxytocin (OT) and its receptor OTR decrease with age
-
•
OT is a hepatotrophic factor for regeneration in aged liver
-
•
OT promotes hepatocyte autophagy in aged mice
Age; molecular physiology; molecular biology; endocrinology
Introduction
With the increase of age, organ aging is an irresistible process. Aging leads to an imbalance in mammalian tissue homeostasis and a great decline of organ regeneration ability. Geriatric science hypothesis suggests that aging is the main modifiable risk factor for most chronic diseases (Hodes et al., 2016). As the largest parenchymal organ, many studies have suggested that the liver showed significant differences in physiological functions, as well as disease development and prognosis in different age. Aging liver decreases in volume, besides the quality of functional hepatocytes also decline (Wakabayashi et al., 2002). Aging liver causes synthetic and metabolic dysfunction (Tietz et al., 1992), which is also a risk factor for fibrosis progression of hepatitis C and poor prognosis of alcoholic hepatitis (Poynard et al., 2001; Forrest et al., 2005). It rises up the incidence of nonalcoholic fatty liver disease (Amarapurkar et al., 2007; Park et al., 2006). What's more, risk of certain senile-related diseases, such as diabetes, arteriosclerosis, arthritis, and neurodegenerative diseases, can increase due to dysfunction of hepatic sinusoid endothelial cells (Lsecs) in aging liver (Baynes, 2001). In aged liver, the incidence of complications of alcoholic liver disease is higher than that in other age groups. In addition, aging liver accelerates the progression of liver fibrosis, cirrhosis, and hepatocellular carcinoma after hepatitis C virus (HCV) infection (Poynard et al., 2001; Thabut et al., 2006; Ryder et al., 2004). In the field of liver transplantation, the incidences of primary liver dysfunction and delayed postoperative nonfunction in elderly donors are relatively high, and the one-year survival rate of patients receiving liver transplantation from elderly donors is distinctly lower than that of controls (Serrano et al., 2010). The above differences indicate that the elderly liver and young liver have different effects on the clinical outcomes of diseases. Therefore, it is of great significance to promote the regeneration of liver aging cells to improve the elderly liver mass and delay the progression of liver-related diseases.
Oxytocin (OT) is a nonapeptide containing one internal disulfide bond. It is traditionally thought to be synthesized by the supraoptic nucleus and paraventricular nucleus of the hypothalamus, transported along the axon to the posterior pituitary and released into the blood circulation, promoting contraction of smooth muscle between the uterus and breast lobule to regulate delivery and lactation. Recent studies have found that OT can be synthesized and released by many peripheral organs such as the heart (Jankowski et al., 1998), uterus (Friebe-Hoffmann et al., 2007), and thymus (Geenen et al., 1987); besides, the central nervous system and OT receptors (OTRs) are expressed on cell surfaces in these organs. Moreover, OT can promote the repair of ischemic myocardium (Houshmand et al., 2009), the differentiation and regeneration of fibroblast stem cells (Elabd et al., 2008) and skeletal muscle stem cells (Elabd et al., 2014). Subcutaneous injection of OT can restore muscle regeneration in aged mice by improving the function of aged muscle stem cells (Elabd et al., 2014). Another study also demonstrates that subcutaneous administration of OT can reverse bone loss and reduce bone marrow adiposity in ovariectomized mice and rats (Elabd et al., 2008). Therefore, we speculate that OT may play a role in promoting the regeneration of aging hepatocytes. To test this hypothesis, we utilize cultured mouse liver organoids and two commonly used mouse models to explore whether OT can promote liver regeneration and reverse the liver cellular senescence.
Results
OTRs were specifically expressed on hepatocytes, meanwhile both serum OT and OTR levels decline with age in mice and human
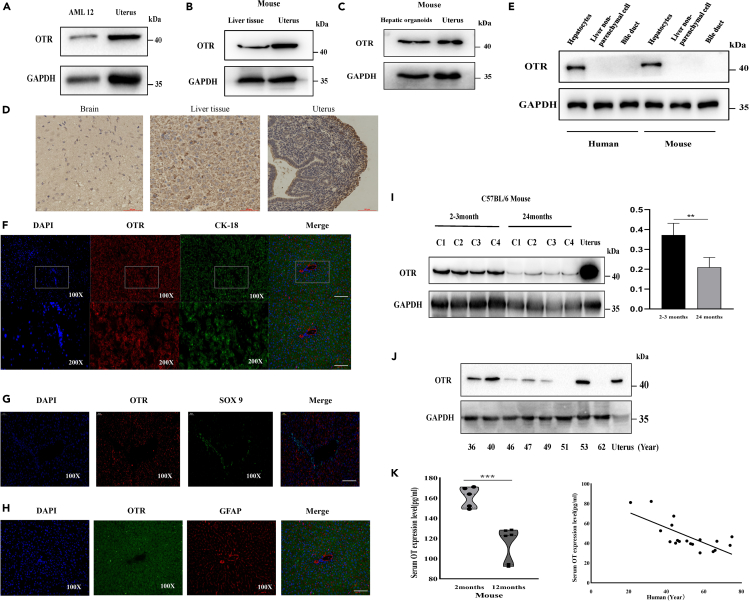
In order to detect whether OTRs are specifically expressed in hepatocytes, we tested the expression of OTR in C57BL/6 male mice and human liver hepatocytes, AML12 cell line (mouse hepatocytes), and mouse hepatic organoids. Western blot analysis suggested that OTRs were specifically expressed in hepatocytes but not either non-parenchymal cells or bile duct epithelial cells (Figures 1A–1D). Then, we tested the expression characteristics of OTRs by immunofluorescence. OTRs were mainly distributed on the hepatocytes (red fluorescence) but not SOX9+ bile duct epithelial cells (green fluorescence) (Figures 1E and 1F) or GFAP + hepatic stellate cells (red fluorescence) (Figure 1G). There is increasing evidence which showed that OT is an age-specific circulating hormone, that is to say, as the age increases, the level of serum OT and expression of OTRs on muscle stem cells also decrease (Elabd et al., 2014). Therefore, we inferred that serum OT or OTRs in the liver had the same characteristics as those in the muscle. To confirm this hypothesis, we analyzed the serum OT level and the expression of liver OTRs, from human (21–74 years old) and mice (2-3 months and 24 months old). The results show that the expression of OTRs in 24-month-old mice was significantly lower than that in 2-month-old mice and that the expression of liver OTRs declined with age in human (Figures 1H and 1I). Similarly, the level of OT in 24-month-old mice was significantly lower than that in 2-month-old mice and so was the level of OT in human serum (Figure 1I). These results suggest that liver aging is associated with a decrease in OT/OTR signal strength.
Figure 1.
OTRs are specifically expressed in hepatocytes, and the levels of serum OT and OTR expression decline with age in mice and human
(A) Western blot in mouse AML12 cell line.
(B)Western blot in liver tissue of mice.
(C)Western blot in hepatic organoid of mice.
(D) Immunohistochemistry in various components of mouse. Scale bar is 25μm.
(E) Western blot in various components of mouse liver.
(F) Immunofluorescence localization of OTRs and CK-18 in mouse liver tissue (red, OTRs; green, CK-18; blue, DAPI).
(G) Immunofluorescence localization of OTRs and SOX9+ in mouse liver tissue (red, OTRs; green, SOX9+; blue, DAPI).
(H) Immunofluorescence localization of OTRs and GFAP in mouse liver tissue (red, GFAP; green, OTRs; blue, DAPI). 100×, Scale bar is 50μm. 200×, Scale bar is 100μm.
(I) Western blot in liver of C57BL/6 male mice from 2 to 3months or 24 months (five mice per group), gray value analysis by Image J.
(J) Western blot in the liver from 36- to 62-year-old humans, gray value analysis by Image J.
(K) Serum OT levels were quantified using enzyme immunoassay from 2–3- or 24-month-old mice (five mice per group) and 21- to 74-year-old humans. Values represent the mean ± Standard error of mean (SEM). ∗p < 0.05; ∗∗p < 0.01; t test. OT: oxytocin.
OT promoted the proliferation of mouse hepatocytes and enhanced the growth of mouse hepatic organoids
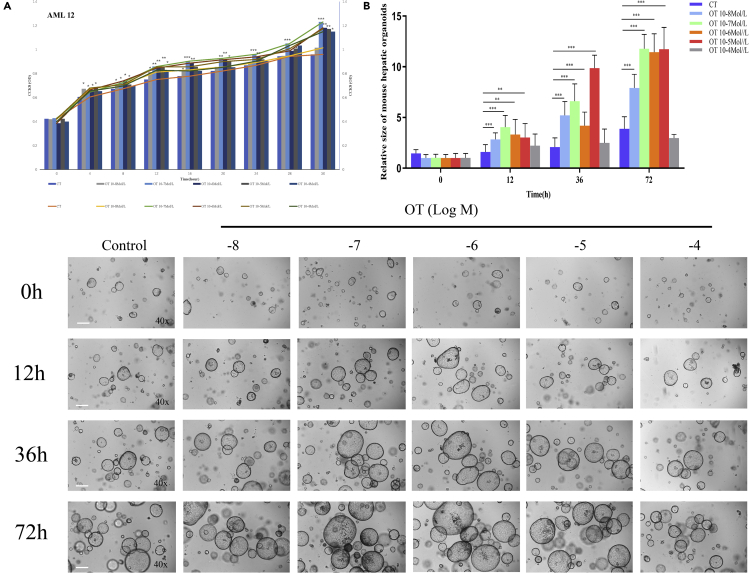
To help elucidate the role of OT in liver regeneration, first of all, we investigated the effects of different concentrations of OT (10−8 Mol/L~10−4 Mol/L) on the proliferation of AML12 cell line by CCK-8 assay. Compared with the control group, OT promoted the proliferation of AML12 cells in the concentration range of (10−7 Mol/L ∼ 10−4 Mol/L, Figure 2A). Next, we generated 3-D mouse hepatic organoids and observed the effects of different doses of OT (10−8 Mol/L ∼ 10−4 Mol/L) on liver regeneration at 0h, 12h, 36h, and 72h (5-6 wells/group). These results show that compared with the control group, OT promotes the growth of mouse liver organoids in the concentration range of (10−8Mol/L ∼ 10−5Mol/L); however, high concentration of OT (10−4 Mol/L) has no effect on organoid growth (Figure 2B). In a word, our results indicate that OT can promote the proliferation of hepatocytes and enhance the growth of hepatic organoids in vitro.
Figure 2.
OT promotes the proliferation of AML12 cell line and enhances the growth of mouse hepatic organoid
(A) Growth curve of AML12 cell line under different concentrations of OT treatment (10−8 Mol/L~10−4Mol/L) respectively for 0h, 4h, 8h, 12hr, 16hr, 20hr, 24hr.
(B)3-D mouse hepatic organoids from 2- to 3-month-old C57BL/6 mice were cultured in matrigel and administered with PBS (Control) or OT (10−8 Mol/L~10−4Mol/L) for 0 hr, 12 hr, 36 hr, 72 hr (5-6 wells/group). The pictures showed the size of mouse hepatic organoids (×40). At least 4 image fields per each sample were quantified. Bottom: The quantification of the liver organoids size in the 2- to 3-month-old C57BL/6 mice by Image-Pro Plus 6.0. Original magnifications: ×40 (B); scale bar: 200μm. Values represent the mean ± Standard error of mean (SEM). ∗∗p < 0.01; ∗∗∗p < 0.001; t test. OT: oxytocin; OD: optical density (450 nm).
OT enhances liver regeneration after partial hepatectomy in aged mice
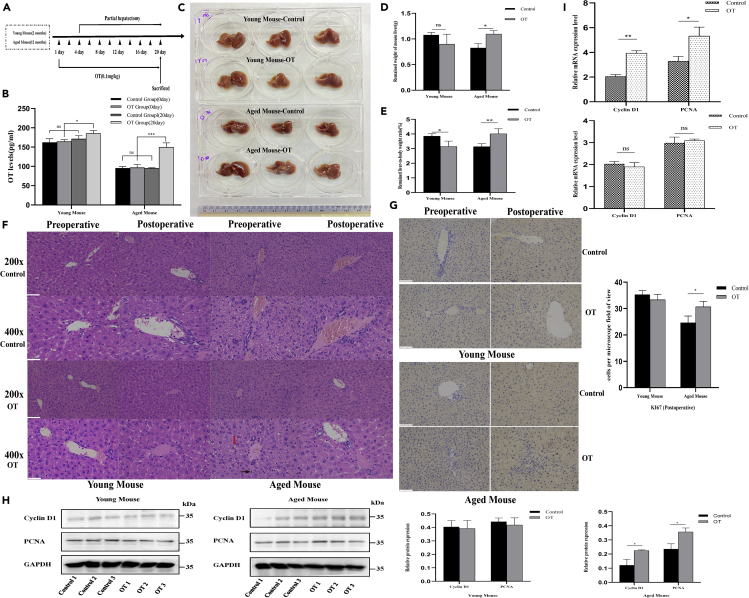
It is known that OT has great effects on promoting tissue repair, maintaining stem cell differentiation, and regeneration (Houshmand et al., 2009; Elabd et al., 2008, 2014). We are wondering whether OT can enhance liver regeneration and reverse the liver cellular senescence. As shown in the schematic diagram of Figure 3A, C57BL/6 male mice of 2–3 months and 12 months were used to construct a partial hepatectomy-liver regeneration model. The serum OT concentrations of young and old mice were increased after intraperitoneal injection of OT (Figure 3B). Interestingly, exogenous OT had no significant effect on liver regeneration in young mice after 20 consecutive days of treatment. However, exogenous OT can enhance the weight of remained liver of aged mice (Figures 3C–3E). Hematoxylin-eosin (HE) staining also shows that exogenous OT can improve the liver status of aged mice (Figure 3F). After exogenous OT treatment, the infiltration of inflammatory cells in the liver of old mice was reduced, and the degree of steatosis was reduced. Next, we examined the effects of exogenous OT on the proliferation and cell cycle after mouse partial hepatectomy. The results show that exogenous OT can affect the liver proliferation and cell cycle in elderly mice after surgery but has no effect on young mice (Figures 3G and 3H). These results suggest that exogenous OT increases residual liver weight by enhancing residual liver regeneration in aged mice.
Figure 3.
OT promotes liver regeneration in vivo
(A) Schematic of partial hepatectomy. The experimental period lasted for 20 days and 2–3- or 12-month-old C57BL/6 male mice were used. From the first day to the 20th day, the mice were administered with control (PBS) or OT (0.1mg/kg). On the fourth day, partial hepatectomy was performed. Liver regeneration in mice was detected 16 days after operation.
(B)The serum OT concentration at day 0 and day 20 in the control (PBS) group and the OT (0.1mg/kg) administration group of the 2- to 3-month-old C57BL/6 mice were shown (three mice per group).
(C) Mouse residual liver morphology.
(D) Liver weight remaining after 16 days of partial hepatectomy in mice (three mice per group).
(E) Liver-to-body weight ratio after 16 days of partial hepatectomy in mice (three mice per group).
(F) H & E staining image for different groups; red arrow: steatosis, black arrow: inflammatory cell. 200×, scale bar is 100μm. 400×, scale bar is 200μm.
(G) Ki67 staining image for different groups. 200×, scale bar is 100μm.
(H) Western blot in the liver of different groups; gray value analysis was used by Image J.
(I) qPCR in liver of different groups; values represent the mean ± Standard error of mean (SEM). ∗p < 0.05; t test. OT: oxytocin; NS: no significance.
Exogenous OT enhanced liver regeneration and improved CCl4-induced acute liver injury
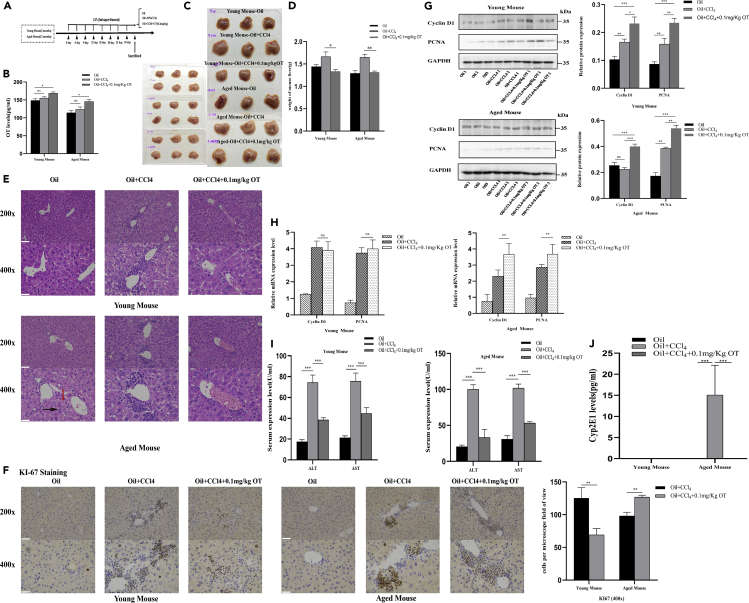
As shown in the schematic diagram of Figure 4A, C57BL/6 male mice of 2–3 months and 12 months were used to construct CCl4-induced liver injury model. The serum OT level in the OT group was higher than that in either control group or CCl4 group (Figure 4B). Compared with the control group and the OT group, the liver swelling becomes pale and the liver capsule tension becomes larger in the CCl4 group (Figures 4C and 4D). HE staining showed that the OT group had less liver steatosis, weaken liver cord structure damage, and inflammatory reaction than that in the CCl4 group (Figure 4E). Moreover, exogenous OT can promote liver proliferation and cell cycle after acute liver injury in mice, especially in 12 months (Figures 4F and 4G). In addition, OT also attenuated the CCl4-induced increase in the concentration of serum AST and ALT detected by aminotransferase assay kit (Figure 4H). It is known that CYP2E1 is considered to be an important effector molecule for CCL4-induced toxicity (Robin et al., 2002). In this study, we found that exogenous OT largely reduced CCL4-induced increase in CYP2E1 in elderly mice (Figure 4I).
Figure 4.
OT improves CCl4-induced acute liver injury in vivo
(A) Schematic of CCl4-induced acute liver injury model. Two- and 12-month-old C57BL/6 male mice were divided into three groups after four weeks of adaptation: oil (corn oil), CCl4 (corn oil as solvent), CCl4 + OT (0.1 mg/kg).
(B) After the mice were sacrificed, the serum OT level of each group was detected.
(C) The liver morphology of mice in the control group and the CCl4-induced acute liver injury model group.
(D) Comparison of liver weight in three groups (3 mice per group).
(E) H & E staining image for different groups of 2- and 12-month-old C57BL/6 male mice; red arrow: steatosis, black arrow: inflammatory cell. 200×, scale bar is 100μm. 400×, scale bar is 200μm.
(F) Ki67 staining image for different groups. 200×, scale bar is 100μm. 400×, scale bar is 200μm.
(G) Western blot in the liver of different groups; gray value analysis was used by Image J.
(H) qPCR in the liver of different groups.
(I) The AST and ALT levels were detected in serum of 2- and 12-month-old C57BL/6 male mice from different groups (three samples per group).
(J) After the mice were sacrificed, the serum Cyp2E1 level of each group was detected. Original magnifications: ×200; scale bar: 50μm. Values represent the mean ± Standard error of mean (SEM). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. t test. OT: oxytocin. ALT: alanine aminotransferase; AST: aspartate transaminase.
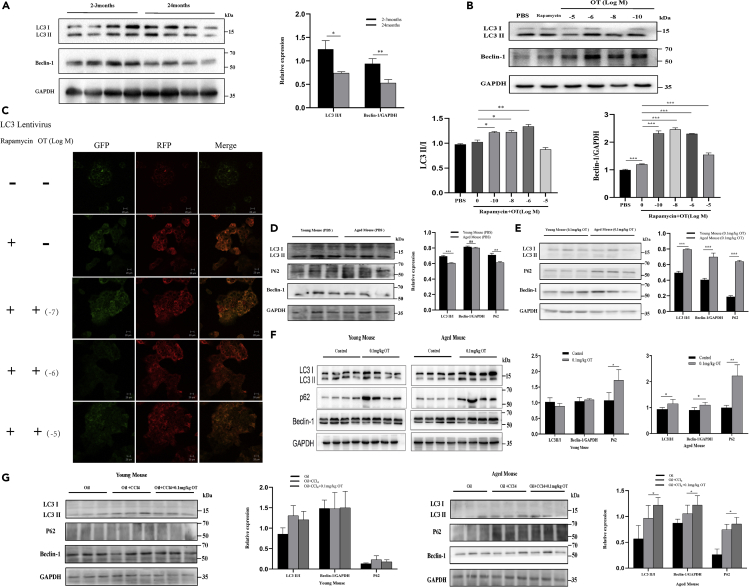
Effect of OT on autophagy activity in mouse liver
A large number of studies have shown that autophagy plays an important role in reversing cell aging and increase of autophagy can delay aging and extend longevity (Rubinsztein et al., 2011). First, we studied the basic levels of autophagy in liver tissues of young (2-3 months) and old mice (24 months). Consistent with the expression levels of serum OT and liver OTR in Figures 1H and 1G, the autophagy activity of mouse liver also decreases with age. Next, we examined the effect of OT on the autophagy activity of mouse liver cell lines. Compared to the control group, exogenous OT promoted the level of autophagy in AML12 cell lines (Figures 5B and 5C). Our in vivo studies, either partial hepatectomy-liver regeneration model or CCl4 acute liver injury model, also confirmed that exogenous OT largely improved the autophagic activity of the elderly liver than the liver of young mice (Figures 5D–5G). Taken together, the above results indicate that OT may promote liver cell regeneration by enhancing cell autophagy.
Figure 5.
OT promotes the increase of autophagy level in mouse liver
(A)Western blot in normal 2–3- or 24-month-old C57BL/6 mice (six mice per group), and gray value analysis by Image J.
(B) Western blot in AML12 cell line administered, respectively, with PBS, rapamycin (5 μMol/L), or OT (1010 Mol/L~10−5Mol/L) after rapamycin treatment. Gray value analysis by Image J.
(C) AML12 cell line was transfected with LC3 Lentivirus and administered, respectively, with PBS, rapamycin (5 μMol/L), or OT (10−7Mol/L~10−5Mol/L) after rapamycin treatment. GFP-RFP-LC3 results were observed by a confocal microscope (green: GFP, red: RFP). Original magnifications: ×400; scale bar: 20 μm.
(D) Western blot in control groups of 2–3- or 12-month-old C57BL/6 male mice and gray value analysis by Image J.
(E) Western blot in OT groups of 2–3- or 12-month-old C57BL/6 male mice and gray value analysis by Image J.
(F) Western blot in different groups of 2–3- or 12-month-old C57BL/6 male mice and gray value analysis by Image J.
(G) Western blot in different groups of 12-month-old C57BL/6 male mice and gray value analysis by Image J. At least three different ×400 image fields per each sample were quantified. Values represent the mean ± Standard error of mean (SEM). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; t test. OT: oxytocin.
Discussion
Liver aging is accompanied by a decrease in the total volume of the organ and blood flow, as well as changes at the cellular level, such as increased oxidative stress, reduction in the number and dysfunction of mitochondria, accelerated cell senescence, and reduced regenerative ability of all types of liver cells, including hepatocytes, hepatic stellate cells, Kupffer cells, liver sinusoidal endothelial cells. OT is known for its role in lactation, childbirth and social behavior that promotes trust and combination (Soloff et al., 1977; Lee et al., 2009). Recent work has suggested the role of OT in preventing osteoporosis and obesity (Beranger et al., 2014; Takayanagi et al., 2008). The OT level decreases after ovariectomy, which is similar to hormone aging (Takayanagi et al., 2008). Our present study also reveals that the level of serum OT declines with age. It is known that OT contributes to the rejuvenation of various cell types, including the stimulation of migration, the regenerative and therapeutic potential of different type of stem cells (Houshmand et al., 2009; Elabd et al., 2008, 2014). Interestingly, we found that OTRs were specifically located in hepatocytes from mouse and human. Notably, the expression of OTRs also decreases with age, hinting that OT signaling pathway may play a role in liver senescence. There are two types of liver regeneration in mammals. The first type of liver regeneration occurs after mild injury or partial hepatectomy. Massive remaining mature hepatocytes enter the cell cycle without an apparent dedifferentiation into a progenitor/stem cell-like state (Miyajima et al., 2014; Hu et al., 2018; Fausto et al., 2012). Genetic lineage tracking methods have demonstrated that hepatocytes around the portal vein can replenish liver mass after injury (Font-Burgada et al., 2015). The Axin2 protein lineage tracking allele shows that mature hepatocytes located around the central vein drive homeostatic hepatocyte self-renewal (Wang et al., 2015). The second type of liver regeneration occurs after massive or chronic liver injury (treatment of various compounds), and its regeneration is inhibited due to massive destruction of hepatocytes. At this time, liver regeneration is mainly mediated by hepatic progenitor cells (Zhang et al., 2008; Kung et al., 2010; Turner et al., 2011). In chronic liver injury, adult hepatocytes can be reprogrammed into proliferating bipotent progenitors (Tanimizu et al., 2014; Tarlow et al., 2014; Yimlamai et al., 2014; Yanger et al., 2014). In our study, we found that OT significantly promotes the hepatocytes growth. Interestingly, in either the model of partial hepatectomy or CCl4-induced acute liver injury, OT had no obvious effect in young mice but markedly promotes the regeneration of the remaining liver after partial hepatectomy or dramatically reduced CCl4-induced liver toxicity in aged mice. High serum Cyp2E1 concentration in aged mice suggested the elderly liver has weak detoxification ability but notably exogenous OT can improve its ability to deal with CCl4 toxic metabolites. In a word, OT promotes the regeneration of hepatocytes, especially in aged mice.
Autophagy is a highly conservative mechanism to maintain cell homeostasis under normal or stress conditions. It maintains cell homeostasis by degrading damaged organelles and protein aggregates and plays an important role in the process of cell senescence (Cuervo, 2008; Rubinsztein et al., 2011; Zhou et al., 2017; Garcia-Prat et al., 2016). It is well known that factors such as starvation, exercise, infection, oxidative stress can cause autophagy, and up-regulation of autophagy can slow down cell senescence, and inhibition of autophagy can promote cell senescence (Galluzzi et al., 2014). Current studies have shown that autophagy function damage caused by inactivation of various autophagy-related genes, together with other factors, is involved in the occurrence of age-related diseases such as neurodegenerative diseases, cancer, cardiac insufficiency, sarcopenia (Rubinsztein et al., 2011; Zhou et al., 2017; Hara et al., 2006). Muñoz-Cánoves P and Ho et al. revealed the key role of autophagy in cell metabolism and function maintenance, and impaired autophagy is related to cell failure and aging (Garcia-Prat et al., 2016; Ho et al., 2017).
In the process of autophagy, the non-activated cytoplasmic LC3 (LC3-I) is hydrolyzed and lipidated into activated LC3-II, and LC3-II binds to the autophagosome membrane. The content of LC3-II is directly proportional to the number of autophagic vesicles. When autophagy occurs in mammalian cells, the content of LC3 in the cell and the conversion of LC3-I to LC3-II are significantly increased. And Beclin-1 is a marker of phagocytic vesicles and autophagosomes. A recent study has shown that OT increases the expression of the autophagy-associated protein Beclin-1 in human ovarian cancer cell lines SKOV3 and MDAH-2774 (Mankarious et al., 2016). Our current research shows that the autophagy activity of mouse and human liver will decrease with age, and OT will increase the expression of autophagy markers in mouse liver. Consistent with this phenomenon, OTRs expression and the levels of serum OT decrease with age. All these results hint that the regulation of autophagy may underline the OT-exerted promotion on liver regeneration.
In our study, we found OT enhances basal- or rapamycin-induced autophagy of mouse hepatocytes in vitro or in vivo. Therefore, we believe that OT can induce autophagy to promote liver regeneration, but there is no relevant evidence for the relationship between OT/OTR signaling and autophagy-associated proteins. OTR is a G-protein-coupled receptor that activates proteinase C and induces intracellular calcium release when it binds to OT, thus, OTR acts as a second messenger to initiate cascade reactions, causing a series of activity changes in cells (Gimpl and Fahrenholz, 2001). Previous studies, including ours own, have proved that phosphorylated ERK1/2 is an effector downstream of OT (Strakova et al., 1998;Elabd et al., 2008;Ji et al., 2019). A recently published study shows that OT alleviates cellular senescence of aging cells in skin tissue through OTR-mediated ERK/Nrf2 signals (Cho et al., 2019). Furthermore, ERK1/2 also participated in the regulation of autophagy in the liver (Xiao et al., 2016). Therefore, we speculate that OT may promote autophagy of hepatocytes through activating phosphorylation of ERK1/2, thereby reversing the senescence of hepatocytes.
In this study, we also found that OT has a dual effect on liver regeneration. (1) OT has a linear effect on liver regeneration in the lower concentration range. (2) High concentrations of OT inhibit the growth of liver organoids. (3) Compared with old mice, young mice already have a higher OT concentration baseline, and injection of OT to increase blood OT content does not promote the regeneration of their liver.
For these unusual results, we suspect that high concentrations of OT will saturate OTRs and produce negative feedback. Or an increase in the concentration of OT is accompanied by an up-regulation of OTRs. When the concentration of OT rises above the critical point, it will in turn inhibit the ability of OTRs. These are also the directions that we need to study in depth in our future work.
In conclusion, OTRs are expressed in hepatocytes, and the expression of OTR and its ligand OT decrease with age. Interestingly, OT promotes hepatocyte regeneration and reverses hepatocyte senescence in aged mice that may be achieved by increasing autophagy level.
Limitations of the study
Our study reported that OT promoted liver regeneration in aging mice. But the role of autophagy in liver regeneration needs further research.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact: Jingxin Li (ljingxin@sdu.edu.cn).
Materials availability
All data generated or analyzed in this study are included in this published article and its supplemental information.
Data and code availability
All data generated or analyzed during this study are included in this published article. Any additional material is available from the corresponding author.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFC1302203), Taishan Scholars Program, Shandong Key Research and Development Program (2017GSF218032, 2019GSF108012), and Rongxiang Regenerative Medicine Foundation of Shandong University (No. 2019SDRX-13).
Author contributions
D.L., X.Z., and J.L. performed research, analyzed the data, and helped the draft manuscript; B.J. provided the human tissues; C.L., W.G., and J.L. were responsible for the design of the study, interpretation of data, and writing of the manuscript.
Declaration of interests
The authors declare no potential conflicts of interest.
Published: February 19, 2021
Contributor Information
Wei Guo, Email: guowei_182@126.com.
Jingxin Li, Email: ljingxin@sdu.edu.cn.
References
- Amarapurkar D., Kamani P., Patel N., Gupte P., Kumar P., Agal S., Baijal R., Lala S., Chaudhary D., Deshpande A. Prevalence of non-alcoholic fatty liver disease: population based study. Ann. Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- Baynes J.W. The role of AGEs in aging: causation or correlation. Exp. Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Beranger G.E., Pisani D.F., Castel J., Djedaini M., Battaglia S., Amiaud J., Boukhechba F., Ailhaud G., Michiels J.F., Heymann D. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology. 2014;155:1340–1352. doi: 10.1210/en.2013-1688. [DOI] [PubMed] [Google Scholar]
- Cho S.Y., Kim A.Y., Kim J., Choi D.H., Son E.D., Shin D.W. Oxytocin alleviates cellular senescence through oxytocin receptor-mediated ERK/Nrf2 signalling. Br. J. Dermatol. 2019;181:1216–1225. doi: 10.1111/bjd.17824. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C., Basillais A., Beaupied H., Breuil V., Wagner N., Scheideler M., Zaragosi L.E., Massiera F., Lemichez E., Trajanoski Z. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26:2399–2407. doi: 10.1634/stemcells.2008-0127. [DOI] [PubMed] [Google Scholar]
- Elabd C., Cousin W., Upadhyayula P., Chen R.Y., Chooljian M.S., Li J., Kung S., Jiang K.P., Conboy I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. J. Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M.A., Ye L. Hybrid Periportal hepatocytes regenerate the injured liver without Giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest E.H., Evans C.D., Stewart S., Phillips M., Oo Y.H., Mcavoy N.C., Fisher N.C., Singhal S., Brind A., Haydon G. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe-Hoffmann U., Baston D.M., Hoffmann T.K., Chiao J.P., Rauk P.N. The influence of interleukin-1beta on oxytocin signalling in primary cells of human decidua. Regul. Pept. 2007;142:78–85. doi: 10.1016/j.regpep.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Pietrocola F., Levine B., Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prat L., Martinez-Vicente M., Perdiguero E., Ortet L., Rodriguez-Ubreva J., Rebollo E., Ruiz-Bonilla V., Gutarra S., Ballestar E., Serrano A.L. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- Geenen V., Legros J.J., Franchimont P., Defresne M.P., Boniver J., Ivell R., Richter D. The thymus as a neuroendocrine organ - synthesis of vasopressin and oxytocin in human thymic epithelium. Ann. N Y Acad. Sci. 1987;496:56–66. doi: 10.1111/j.1749-6632.1987.tb35746.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Ho T.T., Warr M.R., Adelman E.R., Lansinger O.M., Flach J., Verovskaya E.V., Figueroa M.E., Passegue E. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543:205–210. doi: 10.1038/nature21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes R.J., Sierra F., Austad S.N., Epel E., Neigh G.N., Erlandson K.M., Schafer M.J., Lebrasseur N.K., Wiley C., Campisi J. Disease drivers of aging. Ann. N Y Acad. Sci. 2016;1386:45–68. doi: 10.1111/nyas.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshmand F., Faghihi M., Zahediasl S. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides. 2009;30:2301–2308. doi: 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Hu H.L., Gehart H., Artegiani B., Lopez-Iglesias C., Dekkers F., Basak O., Van Es J., Lopes S.M.C.D., Begthel H., Korving J. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175:1591. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Jankowski M., Hajjar F., Kawas S.A., Mukaddam-Daher S., Hoffman G., Mccann S.M., Gutkowska J. Rat heart: a site of oxytocin production and action. Proc. Natl. Acad. Sci. U S A. 1998;95:14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Liu N., Li J., Chen D., Luo D., Sun Q., Yin Y., Liu Y., Bu B., Chen X., Li J. Oxytocin involves in chronic stress-evoked melanoma metastasis via beta-arrestin 2-mediated ERK signaling pathway. Carcinogenesis. 2019;40:1395–1404. doi: 10.1093/carcin/bgz064. [DOI] [PubMed] [Google Scholar]
- Kung J.W., Currie I.S., Forbes S.J., Ross J.A. Liver development, regeneration, and carcinogenesis. J. Biomed. Biotechnol. 2010;2010:984248. doi: 10.1155/2010/984248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Macbeth A.H., Pagani J.H., Young W.S. Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankarious A., Dave F., Pados G., Tsolakidis D., Gidron Y., Pang Y., Thomas P., Hall M., Karteris E. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int. J. Oncol. 2016;48:1805–1814. doi: 10.3892/ijo.2016.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Tanaka M., Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Park S.H., Jeon W.K., Kim S.H., Kim H.J., Park D.I., Cho Y.K., Sung I.K., Sohn C.I., Keum D.K., Kim B.I. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2006;21:138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- Poynard T., Ratziu V., Charlotte F., Goodman Z., Mchutchison J., Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J. Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Robin M.A., Anandatheerthavarada H.K., Biswas G., Sepuri N.B., Gordon D.M., Pain D., Avadhani N.G. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J. Biol. Chem. 2002;277:40583–40593. doi: 10.1074/jbc.M203292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Ryder S.D., Irving W.L., Jones D.A., Neal K.R., Underwood J.C., Trent Hepatitis C.S.G. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M.T., Garcia-Gil A., Arenas J., Ber Y., Cortes L., Valiente C., Araiz J.J. Outcome of liver transplantation using donors older than 60 year of age. Clin. Transplant. 2010;24:543–549. doi: 10.1111/j.1399-0012.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Soloff M.S., Schroeder B.T., Chakraborty J., Pearlmutter A.F. Characterization of oxytocin receptors in the uterus and mammary gland. Fed. Proc. 1977;36:1861–1866. [PubMed] [Google Scholar]
- Strakova Z., Copland J.A., Lolait S.J., Soloff M.S. ERK2 mediates oxytocin-stimulated PGE2 synthesis. Am. J. Physiol. 1998;274:E634–E641. doi: 10.1152/ajpendo.1998.274.4.E634. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y., Kasahara Y., Onaka T., Takahashi N., Kawada T., Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Nishikawa Y., Ichinohe N., Akiyama H., Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J. Biol. Chem. 2014;289:7589–7598. doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow B.D., Pelz C., Naugler W.E., Wakefield L., Wilson E.M., Finegold M.J., Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabut D., Le Calvez S., Thibault V., Massard J., Munteanu M., Di Martino V., Ratziu V., Poynard T. Hepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease? Am. J. Gastroenterol. 2006;101:1260–1267. doi: 10.1111/j.1572-0241.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- Tietz N.W., Shuey D.F., Wekstein D.R. Laboratory values in fit aging individuals--sexagenarians through centenarians. Clin. Chem. 1992;38:1167–1185. [PubMed] [Google Scholar]
- Turner R., Lozoya O., Wang Y., Cardinale V., Gaudio E., Alpini G., Mendel G., Wauthier E., Barbier C., Alvaro D., Reid L.M. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H., Nishiyama Y., Ushiyama T., Maeba T., Maeta H. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: Comparison with CT volumetry. J. Surg. Res. 2002;106:246–253. doi: 10.1006/jsre.2002.6462. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhao L.D., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Liu H., Yu J., Zhao Z., Xiao F., Xia T., Wang C., Li K., Deng J., Guo Y. Activation of ERK1/2 Ameliorates liver steatosis in leptin receptor-deficient (db/db) mice via stimulating ATG7-dependent autophagy. Diabetes. 2016;65:393–405. doi: 10.2337/db15-1024. [DOI] [PubMed] [Google Scholar]
- Yanger K., Knigin D., Zong Y.W., Maggs L., Gu G.Q., Akiyama H., Pikarsky E., Stanger B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D., Christodoulou C., Galli G.G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B.Z., Camargo F.D. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.L., Theise N., Chua M., Reid L.M. The stem cell niche of human livers: Symmetry between development and regeneration. Hepatology. 2008;48:1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- Zhou J., Chong S.Y., Lim A., Singh B.K., Sinha R.A., Salmon A.B., Yen P.M. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging (Albany NY) 2017;9:583–599. doi: 10.18632/aging.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Any additional material is available from the corresponding author.