Abstract
Cerebral small vessel disease (SVD) and inflammation are increasingly recognized as key contributors to Alzheimer's disease (AD), although the timing, trajectory, and relation between them early in the disease process is unclear. Therefore, to investigate very early-stage changes, we compared 158 healthy midlife adults with and without inherited AD predisposition (APOE4 carriership (38% positive), parental family history (FH) of dementia (54% positive)) on markers of SVD (white matter hyperintensities (WMH), cerebral microbleeds), and inflammation (C-reactive protein (CRP), fibrinogen), cross-sectionally and longitudinally over two years. While WMH severity was comparable between groups at baseline, longitudinal progression of WMH was greater in at-risk groups (APOE4+ and FH+). Topographically, APOE4 was associated exclusively with deep, but not periventricular, WMH progression after adjusting for FH. Conversely, APOE4 carriers displayed lower CRP levels than noncarriers, but not fibrinogen. Furthermore, interaction analysis showed that FH moderated the effect of SVD and inflammation on reaction time, an early feature of SVD, but not episodic memory or executive function. Findings suggest that vascular and inflammatory changes could occur decades before dementia onset, and may be of relevance in predicting incipient clinical progression.
Keywords: Cerebral small vessel disease, White matter hyperintensities, Cerebral microbleeds, Inflammation, Alzheimer's disease, APOE4, Risk factors
Highlights
-
•
Heritable risk factors related to SVD progression, despite lower levels of inflammation.
-
•
SVD progression had more pronounced adverse effects on reaction time in at-risk individuals.
-
•
Association between SVD and inflammation was stronger in those at-risk.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder traditionally characterized by aberrant protein accumulation in the brain. In recent years, however, our understanding of AD etiology has expanded, implicating cerebral small vessel disease (SVD) and inflammation as key players in its pathogenesis (Bos et al., 2018; Heneka et al., 2015; Kinney et al., 2018; Wardlaw et al., 2013). However, the timing and contribution of these alterations early in the disease process remain unclear. This is an important gap to fill, as developing our understanding of vascular and inflammatory changes in the preclinical phase sheds light on the early trajectory and mechanistic pathways leading up to dementia, and thus facilitates early detection and disease management.
In AD, subclinical biological changes are detectable many years before observable symptom onset (Ritchie et al., 2016). Given the heritable nature of AD, one can identify at-risk individuals decades before disease onset would occur and thereby examine early alterations in asymptomatic individuals at higher risk of developing AD. Using this approach, we investigate longitudinal changes in SVD and inflammation in relation with established heritable risk factors, apolipoprotein ε4 (APOE4) (Strittmatter and Roses, 1996), and parental family history (FH) of dementia (Scarabino et al., 2016).
Radiological findings of SVD are common in the elderly population, and their presence predicts future dementia and risk of stroke (Debette et al., 2019). However, associations between inherited risk of dementia and SVD have been predominantly examined in cross-sectional studies, reporting largely discrepant findings depending on age cohorts. For instance, 2 studies of older adults, with mean ages of 64 and 74 years, respectively, reported significant associations between heritable risk factors and SVD (Stamm et al., 2019; Wolters et al., 2017). Yet, a study of younger adults—aged 52 years old on average—did not find evidence of such an association (Stefaniak et al., 2018). These contrasting reports suggest that the effect of inherited risk on vascular alterations may emerge only in older age and suggests a need to consider the longitudinal progression of neurovascular injury.
Inflammation is increasingly implicated in neurodegenerative diseases such as AD (Gabin et al., 2018; Gong et al., 2016; Heneka et al., 2015; Kinney et al., 2018; Tao et al., 2018). Under normal circumstances, inflammation is a protective biological response to infections and injuries. However, prolongation of an inflammatory response can have deleterious effects on surrounding tissue. Although the immune system is independent of the central nervous system, the two are engaged in constant bidirectional communication. In pathologic states, the central nervous system can be affected by systemic inflammation. Chronic systemic inflammation can compromise the integrity of the blood–brain barrier, thereby allowing the entry of toxins and pathogens into the brain, and activating glial cells (Bendorius et al., 2018; Kempuraj et al., 2017). Blood markers of inflammation have been linked to neurodegenerative conditions including AD (Gabin et al., 2018; Gong et al., 2016; Tao et al., 2018), cognitive decline (Noble et al., 2010; Watanabe et al., 2016), and SVD (Low et al., 2019). However, there has been a lack of research considering longitudinal changes in systemic inflammation in relation to heritable AD risk.
To address the existing gaps in literature, the core objective of our study was to investigate the influence of inherited dementia risk on the longitudinal progression of SVD and inflammation in cognitively healthy midlife adults. There is also significant merit in elucidating the pathologic contributions to early cognitive decline, as evidenced by recent findings that even subtle cognitive decline in preclinical AD has robust clinical utility in predicting disease progression and conversion (Papp et al., 2020). Therefore, we further examined the interaction between these pathologic markers and inherited risk factors on neuropsychological measures. In addition, we tested the association between SVD and inflammation and whether these relationships would be moderated by inherited risk. We hypothesized that SVD and inflammation would differ by APOE4 carriership and FH in terms of progression, but not cross-sectionally, and that these pathologies would be more detrimental to clinical measures in individuals with inherited predisposition to dementia, relative to those without.
2. Material and methods
2.1. Participants
The protocol of the PREVENT-Dementia study has been described in detail previously (Ritchie and Ritchie, 2012). Cognitively healthy, midlife (age 40–59 years) participants were recruited through multiple sources. Majority of participants with parental family history of dementia (FH+) were recruited from the dementia register database held at West London Mental Health National Health Service Trust, which holds information on patients with dementia and cognitive impairment who have consented to be approached for clinical research, and their careers (often offspring), or through memory clinic referrals—most FH+ participants therefore had parents with a confirmed diagnosis of dementia. On the other hand, majority of FH− participants were spouses/friends/other relatives of FH+ participants. Other participants were recruited via the Join Dementia Research website (https://www.joindementiaresearch.nihr.ac.uk/) or by registering their interest through the PREVENT-Dementia website (https://preventdementia.co.uk/) and public presentations and engagement sessions.
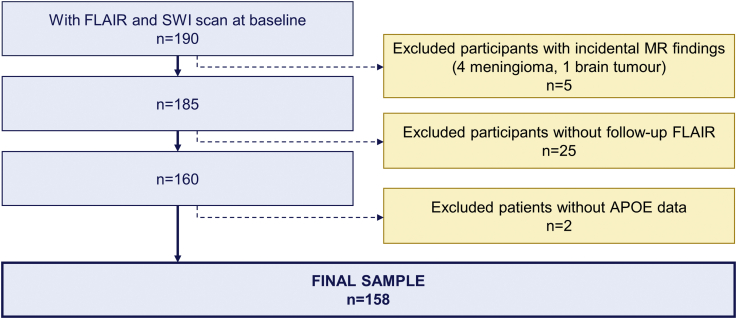
This study included 158 participants who underwent clinical assessment and magnetic resonance imaging (MRI) at both baseline and two-year follow-up (mean = 2.03 years; range = 1.91–2.48 years) (Fig. 1). The research was approved by the London-Camberwell St Giles National Health Service Ethics Committee, and all subjects provided written informed consent. Family history of dementia was defined as positive (FH+) if participants reported at least one parent having clinically diagnosed dementia, while APOE4 status was regarded positive for ≥1 ε4 allele (APOE4+). Sixty were APOE4 carriers (38.0%), whereas 85 had a FH of dementia (53.8%), more often on the maternal (35.4%) rather than paternal side (13.3%), while 5.0% had both mothers and fathers with dementia. In terms of parental dementia subtype, majority of FH+ subjects reported Alzheimer's or mixed (Alzheimer's/vascular) dementia (n = 66, 76%), 13 reported vascular dementia (15%), and 8 were unsure or reported other dementia subtypes (9%). Four participants carrying both the APOE2 and APOE4 allele were analyzed as APOE4 carriers. Given that FH and APOE4 may have independent contributions to dementia risk (Donix et al., 2012), the two were analyzed as separate risk factors.
Fig. 1.
Participant selection flowchart.
2.2. Imaging analysis
All participants underwent structural MRI at baseline and two-year follow-up. MRI scans were acquired on a 3T Siemens Verio. Three-dimensional T1-weighted MPRAGE parameters were: 160 slices, repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, flip angle = 9°, voxel size = 1 × 1 × 1mm3. Fluid-attenuated inversion recovery (FLAIR) parameters were: 27 slices, TR = 9000 ms, TE = 94 ms, flip angle = 150°, voxel size = 0.43 × 0.43 × 4mm3. Susceptibility-weighted imaging (SWI) parameters were: 72 slices, TR = 28 ms, TE = 20 ms, flip angle = 15°, voxel size = 0.72 × 0.72 × 1.2 mm3.
2.3. Quantification of white matter hyperintensities
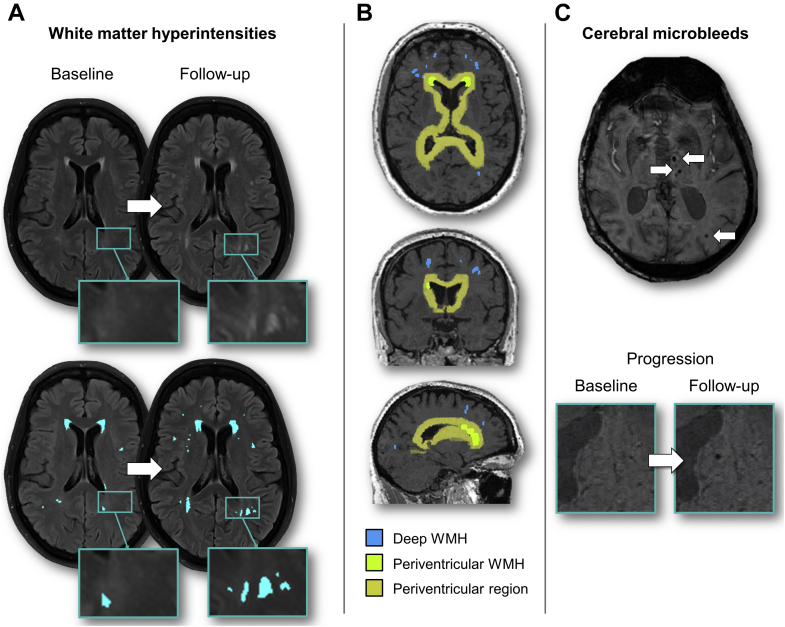
White matter hyperintensities (WMH) lesion maps were obtained using an automated script on the Statistical Parametric Mapping 8 (SPM8) suite (http://www.fil.ion.ucl.ac.uk/spm/) on FLAIR MRI; details on the procedures involved have been described previously (Firbank et al., 2003). Briefly, SPM8 was used to perform segmentation of T1-weighted images into gray matter (GM), white matter, and cerebrospinal fluid, based on prior probability maps. Using the GM and WM maps, a brain mask was created and used to perform removal of nonbrain matter from the FLAIR images. WMH segmentation was then conducted in FLAIR native space. Initial WMH maps were obtained using threshold-based segmentation at a threshold of 1.2 times the median pixel intensity, i.e., lesions with pixel intensity more than 1.2 times the median intensity of the whole brain were included in the WMH map. All lesion maps from both baseline and follow-up visits were reviewed by a single-experienced rater (A.L.) blinded to all clinical information, including FH, APOE4 status, and blood inflammatory levels (Fig. 2). Lesion maps obtained from the segmentation procedure were used as starting points for manual WMH delineation. Baseline and follow-up FLAIR images were evaluated side by side during delineation to ensure consistency. WMH volumes were normalized by total intracranial volume to account for individual differences in head size ((WMH/total intracranial volume) ∗ 100%) and transformed using cube root transformation due to skewness.
Fig. 2.
Radiological markers of cerebral small vessel disease (SVD). (A) White matter hyperintensities (WMH) were manually delineated on FLAIR MRI at baseline and two-year follow-up; (B) Classification of periventricular and deep WMH based on threshold distance from ventricles, derived from morphological ventricle dilation; (C) Cerebral microbleeds were identified on 3T SWI scans, and progression was defined as the occurrence of new microbleeds at follow-up, which were confirmed to be absent at baseline.
WMH were classified into periventricular and deep WMH based on threshold distance from ventricles. Binary masks of ventricles underwent 4 iterations of morphological dilation in MNI space. This dilated ventricle mask was then transformed to subject space to define the boundary between periventricular and deep WMH. Because of the transformation, this is a variable distance accounting for individual brain size and is approximately 10 mm, in line with recommendations (Griffanti et al., 2018).
2.4. Cerebral microbleed assessment
Cerebral microbleeds (CMB) were identified on 3T SWI following the Microbleed Anatomical Rating Scale (MARS) which quantifies CMB lesion count per location (Gregoire et al., 2009). In keeping with the MARS, lobar regions were defined according to Stark and Bradley (Stark and Bradley Jr, 1999), comprising cortical and subcortical regions, while deep regions included the basal ganglia, thalamus, internal capsule, external capsule, corpus callosum, and deep and periventricular WM (anatomical diagram available in Gregoire et al., 2009). Following the recommendations made by Greenberg et al. (2009), CMB were defined as areas of round/ovoid black signals, excluding tubular or linear structures (Greenberg et al., 2009) (Fig. 2). Suspected microbleeds were cross-validated on T1- and T2-weighted scans to exclude microbleed “mimics”. In instances of uncertainty, microbleeds were labeled as “possible microbleeds”—this includes situations whereby microbleeds cannot be distinguished from vascular flow voids. Such cases of “possible microbleeds” were excluded from analysis, and only “definite microbleeds” were analyzed. All CMB identified at follow-up were cross-checked against baseline scans to confirm their presence at baseline, and new CMB were flagged. Microbleed progression was defined as the presence of any new CMB at follow-up that were confirmed to be absent at baseline and was analyzed as a binary variable (new CMB vs. no new CMB). Ratings were performed blinded to all clinical information including FH, APOE4 carriership, and blood analysis.
2.5. Neuropsychological measures
Participants underwent neuropsychological assessment at baseline and two-year follow-up. As part of the COGNITO battery (Ritchie et al., 2014), participants were assessed on reaction time, episodic memory, and executive function. Reaction time was measured using a simple reaction time task administered through a touchscreen which records responses and response latencies, and mean reaction time across 12 trials was computed. Episodic memory was assessed using a delayed free recall task of 9 names, and executive function was evaluated using the Stroop test item of COGNITO.
2.6. Genotyping
TaqMan genotyping on QuantStudio12 K Flex was used to establish APOE variants. Genomic DNA was isolated from whole blood and genotyping was performed in 384 well plates, using the TaqMan polymerase chain reaction (PCR)-based method. The final volume PCR was 5 μL using 20 ng of genomic DNA, 2.5 μL of TaqMan Master Mix, and 0.125 μL of 40× Assay by Design Genotyping Assay Mix, or 0.25 μL of 20× Assay on Demand Genotyping Assay. The cycling parameters were 95° for 10 minutes, followed by 40 cycles of denaturation at 92° for 15 seconds and annealing/extension at 60° for one minute. PCR plates were then read on Thermo Fisher QuantStudio 12K Flex Real-Time System instrument with QuantStudio 12K Flex Software or TaqMan Genotyper Software v1.3.
2.7. Inflammatory markers
Systemic inflammation was quantified using serum measures of CRP and fibrinogen. Participants provided fasting blood samples on the morning of their first visit, the same day as clinical and cognitive assessments. Technicians were blinded to all clinical information. CRP concentration in serum was assessed using the Beckman Coulter AU System with a CRP Latex reagent. Following the latest recommendations of best practices, participants with CRP exceeding 10 mg/L (n = 6) were excluded from CRP analysis as these may be attributable to an acute immune response (Mac Giollabhui et al., 2020). Fibrinogen was measured using the Clauss method (Clauss, 1957) by determining the clotting time of diluted plasma after the addition of thrombin. CRP and fibrinogen data underwent cube root transformation due to right-tailed skewness.
2.8. Statistical analysis
Standard statistical techniques were used for descriptive analyses (Table 1). To test whether FH and APOE4 carriership differed in baseline levels of SVD (WMH and CMB) and inflammation (CRP and fibrinogen), we independently fitted regression models to each of these outcomes of interest (simple linear regression models fitted to WMH, CRP, and fibrinogen; logistic regression models fitted to binary CMB variable). In all models, we adjusted for sex (1 = male, 2 = female), age (centered at sample mean of 52.2 years), and years of education (centered at sample mean of 16.1 years) (corresponding results in Section 3.1). To assess whether FH and APOE4 carriership were associated with the trajectory of WMH, CRP, and fibrinogen, independent linear mixed effects models were fitted to each outcome of interest, allowing intercepts to vary between subjects and adjusted for the same covariates, while CMB progression was analyzed as a dichotomized variable (no new CMB vs. ≥1 new CMB) using logistic regression analysis (corresponding results in Section 3.2).
Table 1.
Participant characteristics
| Measures | Full sample158 | Family history |
APOE4 |
||||
|---|---|---|---|---|---|---|---|
| FH− | FH+ | p value (FH) | APOE4− | APOE4+ | p value (APOE4) | ||
| n | 158 | 73 | 85 | 98 | 60 | ||
| Baseline demographics | |||||||
| Sex (N, % females)c | 109, 69.0% | 49, 67.1% | 60, 70.6% | 0.639 | 68, 69.4% | 41, 68.3% | 0.889 |
| Age in yearse | 52.2 (5.4) | 51.1 (6.3) | 53.1 (4.4) | 0.113 | 52.7 (5.4) | 51.3 (5.5) | 0.083 |
| Education in yearse | 16.1 (3.4) | 16.4 (3.8) | 15.9 (3.0) | 0.206 | 16.0 (3.5) | 16.3 (3.1) | 0.581 |
| Family historyc | 53.8% | - | 100% | - | 46.9% | 65.0% | 0.027f |
| APOE4c | 38.0% | 28.8% | 45.9% | 0.027f | - | 100% | - |
| APOE2c | 8.2% | 8.2% | 8.2% | 0.997 | 9.2% | 6.7% | 0.576 |
| Hypertensionc | 16.5% | 11.0% | 21.2% | 0.084 | 16.3% | 16.7% | 0.955 |
| Baseline | |||||||
| WMH volumea,e | 0.10 (0.15) | 0.09 (0.13) | 0.11 (0.17) | 0.596 | 0.10 (0.17) | 0.10 (0.13) | 0.225 |
| CMB (% present)c | 21.5% | 21.9% | 21.2% | 0.910 | 17.3% | 28.3% | 0.103 |
| CRP (mg/L)e | 2.7 (1.5) | 2.7 (1.5) | 2.8 (1.6) | 0.586 | 2.9 (1.8) | 2.4 (0.9) | 0.030f |
| Fibrinogen | 3.0 (0.7) | 3.0 (0.7) | 3.1 (0.7) | 0.861 | 3.1 (0.7) | 3.0 (0.6) | 0.660 |
| Reaction time (ms) b,d | 341.1 (38.5) | 334.9 (41.0) | 346.5 (35.6) | 0.063 | 338.7 (40.9) | 345.0 (34.3) | 0.304 |
| Delayed recall | 7.0 (1.5) | 6.8 (1.6) | 7.1 (1.4) | 0.515 | 6.8 (1.6) | 7.2 (1.5) | 0.222 |
| Stroop test | 17.6 (3.1) | 18.0 (3.0) | 17.3 (3.2) | 0.048f | 17.7 (2.8) | 17.4 (3.5) | 0.549 |
| Follow-up | |||||||
| WMH volumea,e | 0.12 (0.19) | 0.10 (0.14) | 0.14 (0.23) | 0.182 | 0.12 (0.22) | 0.12 (0.14) | 0.035f |
| CMB (% present)c | 29.9% | 30.6% | 29.4% | 0.876 | 26.5% | 35.6% | 0.230 |
| CRP (mg/L)e | 2.8 (1.8) | 2.8 (1.8) | 2.9 (1.8) | 0.261 | 3.0 (2.0) | 2.6 (1.4) | 0.423 |
| Fibrinogen | 3.0 (0.7) | 2.9 (0.7) | 3.1 (0.7) | 0.027f | 3.1 (0.8) | 3.0 (0.7) | 0.605 |
| Reaction time (ms) b,d | 343.5 (38.9) | 339.2 (39.8) | 347.0 (38.0) | 0.262 | 344.3 (42.7) | 342.2 (32.2) | 0.732 |
| Delayed recall | 7.3 (1.5) | 7.4 (1.6) | 7.1 (1.4) | 0.143 | 7.3 (1.6) | 7.2 (1.4) | 0.454 |
| Stroop test | 17.8 (3.1) | 17.9 (3.5) | 17.7 (2.9) | 0.291 | 17.9 (3.5) | 17.5 (2.5) | 0.075 |
a Values expressed as percentages (%), b,c Values as mean (standard deviation).
Key: CMB = cerebral microbleeds, CRP = C-reactive protein; FH = parental family history of dementia, WMH = white matter hyperintensities.
Missing data: CMB at follow-up (n = 1), baseline CRP (n = 1 missing; n = 5 outliers excluded), follow-up CRP (n = 3 missing; n = 2 outliers excluded), fibrinogen (n = 13), follow-up fibrinogen (n = 11), baseline reaction time (n = 2), follow-up reaction time (n = 5).
Normalized volume adjusting for total intracranial volume ((WMH/TIV) ∗ 100%).
Longer reaction time indicates poorer performance.
Chi-square test of independence.
t-test.
Mann-Whitney U test.
p < 0.05.
To test associations between SVD (WMH and CMB) and inflammation (CRP and fibrinogen) at baseline, regression models were fitted to WMH and CMB in separate models, and to examine whether these associations were moderated by group (FH, APOE4 carriership), we repeated the analysis with addition of inflammation ∗ group interaction terms (corresponding results in Section 3.3).
To assess the relationship between neuropsychological measures and biomarkers (WMH, CMB, CRP, and fibrinogen), linear regression models were independently fitted to neuropsychological measures, and moderation by heritable risk factors was tested by repeating the analysis with biomarker ∗ group interaction terms (corresponding results in Section 3.4). To examine whether the trajectory of WMH, CMB, CRP, and fibrinogen related to changes in reaction time, linear mixed effects models were fitted to neuropsychological measures; to test if these associations were moderated by FH or APOE4, biomarker ∗ group ∗ interval interactions were tested (corresponding results in Section 3.4).
All statistical analyses were conducted using R. Models were estimated under a missing at random missing data assumption using maximum likelihood estimation. The lm function was used to fit linear regression models, and glm was used for logistic regression models. From the lme4 package (Bates et al., 2015), lmer was used to fit linear mixed effects models, and glmer was used for mixed effects logistic regression analysis. Statistical significance was set at p < 0.05.
3. Results
Sample characteristics are presented in Table 1. The sample was majority female (69%), had a mean age of 52.2 years (SD 5.4), and an average of 16.1 years of education (SD 3.4). Sex, age, and years of education did not differ by FH or APOE4 carriership.
3.1. Group differences at baseline
Using general linear models, APOE4 carriership was related to lower CRP levels and greater presence of CMB at baseline. On the other hand, WMH and fibrinogen did not differ by FH or APOE4 carriership (Table 2, Figure A.1).
Table 2.
Baseline and longitudinal group differences by family history and APOE4
| Measures | Standardized β | Family history |
Standardized β | APOE4 |
||
|---|---|---|---|---|---|---|
| t value | p value | t value | p value | |||
| Baseline | ||||||
| WMHa | 0.02 | 0.12 | 0.907 | 0.11 | 0.69 | 0.489 |
| CMBb | −0.09 | −0.44 | 0.660 | 0.41 | 2.03 | 0.042d |
| CRPa | −0.02 | −0.15 | 0.885 | −0.31 | −2.01 | 0.047d |
| Fibrinogena | −0.08 | −0.48 | 0.632 | −0.00 | −0.03 | 0.980 |
| Longitudinal change | ||||||
| WMHc | 0.05 | 2.24 | 0.026d | 0.08 | 3.48 | <0.001e |
| CMBb | −0.03 | −0.09 | 0.931 | 0.33 | 1.12 | 0.262 |
| CRPc | 0.01 | 0.10 | 0.924 | 0.06 | 0.74 | 0.458 |
| Fibrinogenc | 0.07 | 1.02 | 0.311 | −0.02 | −0.22 | 0.828 |
Note: All models adjusted for sex, age, and years of education.
General linear model.
Logistic regression model.
Linear mixed effects model.
p < 0.05.
p < 0.001.
3.2. Longitudinal group differences
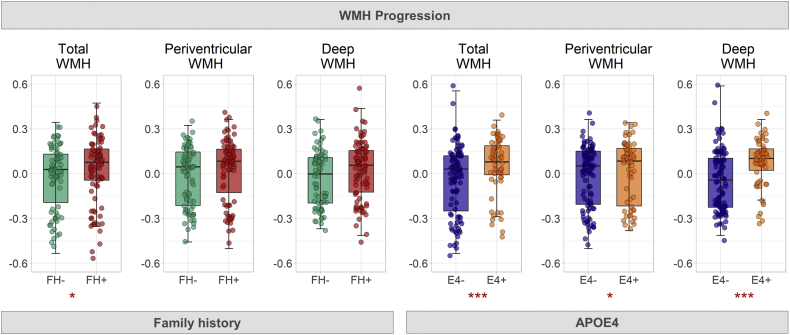
Significant FH ∗ interval interaction in linear mixed effects modeling showed greater WMH progression in FH+ than FH− (Table 2, Fig. 3, Figure A.1). Linear mixed effects modeling of group differences (FH−, maternal FH+, and paternal FH+) further showed that maternal FH+ (β = 0.06, t = 2.32, p = 0.022), but not paternal FH+ (β = 0.05, t = 1.43, p = 0.155) related to greater WMH progression than FH−. Similarly, interaction analysis of APOE4 ∗ interval showed that APOE4 carriers had greater WMH progression than noncarriers (Table 2); this was observed for both periventricular (β = 0.06, t = 2.19, p = 0.030) and deep WMH (β = 0.10, t = 3.65, p < 0.001). Adjusting for FH, APOE4 remained significantly associated with deep (β = 0.10, t = 3.47, p < 0.001), but not periventricular WMH progression (β = 0.05, t = 1.92, p = 0.056). On the other hand, changes in CMB, CRP, and fibrinogen did not differ by FH or APOE4 carriership (Table 2).
Fig. 3.
Boxplots depict greater WMH progression in participants with parental history of dementia (FH+) and APOE4 carriers (APOE4+). WMH volumes are residuals controlling for sex, age, and education. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.3. Association between SVD and inflammation
In logistic regression analysis, APOE4 ∗ CRP interaction on CMB was significant at follow-up, whereby the relationship between CRP and CMB was more pronounced in APOE4 carriers than in noncarriers (β = 0.68, t = 2.76, p = 0.006); this applied to lobar CMB (β = 0.68, t = 2.68, p = 0.007), but not deep CMB (β < 0.01, t = 0.01, p = 0.992). Separate regression models confirmed that the positive associations between CRP and CMB were present in APOE4 carriers but not noncarriers.
3.4. Reaction time
Interaction analysis in general linear models showed that FH moderated the association between WMH and longer reaction time, in that the relationship was more pronounced in FH+ rather than FH− (total WMH: β = 0.52, t = 3.11, p = 0.002; periventricular WMH: β = 0.58, t = 3.49, p < 0.001; deep WMH: β = 0.37, t = 2.25, p = 0.026). In terms of inflammation, significant interaction of CRP and FH on reaction time showed that the relationship between higher CRP levels and longer reaction time was stronger in FH− than FH+ (β = −0.38, t = −2.41, p = 0.017).
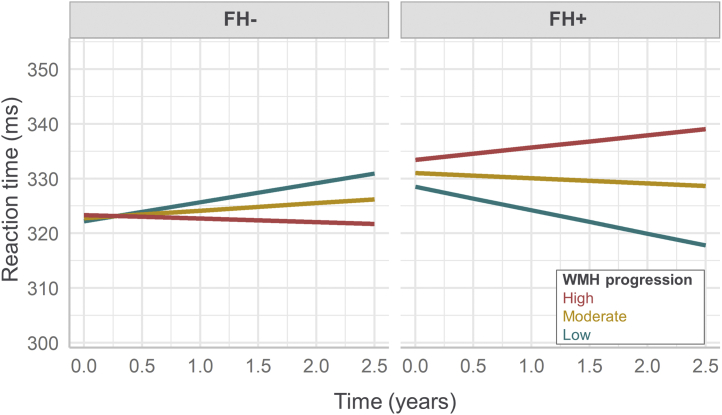
Longitudinally, significant FH ∗ WMH ∗ interval interaction in mixed effects analysis demonstrated that the association between WMH progression and increase in reaction time was stronger in FH+ than FH− (β = 0.15, t = 2.09, p = 0.038) (Fig. 4). This effect was observed in deep (β = 0.19, t = 2.77, p = 0.006) but not periventricular WMH (β = 0.08, t = 1.13, p = 0.262). Independent models fitted in the FH+ and FH− groups separately confirmed that the positive association was significant in FH+ but not the FH− group. Similarly, FH moderated the association between CMB progression and longitudinal change in reaction time, in that the relationship was stronger in FH+ than FH− (β = 0.15, t = 2.12, p = 0.036). These interaction effects were not observed in relation to episodic memory or executive function.
Fig. 4.
Plot of estimated marginal means of reaction time depicts a significant three-way interaction of time (scan interval), family history, and total WMH progression on reaction time from linear mixed effects regression model. Note: Increasing reaction time equates to poorer performance. High and low WMH progression was defined as +/−1 SD from mean (moderate WMH progression). Linear mixed model adjusted for sex, age, and education.
4. Discussion
We examined the effect of inherited predisposition to dementia (parental history of dementia and/or the APOE4 allele) on SVD and systemic inflammation in healthy midlife adults at baseline and longitudinally. Inherited risk was associated with lower levels of CRP, higher incidence of CMB, and greater increase in WMH over two years. Furthermore, the detrimental effect of SVD on reaction time was more pronounced in those with FH relative to those without, cross-sectionally and longitudinally. Conversely, the relationship between elevated CRP levels on reaction time was stronger in those without FH.
Increased WMH can be detected years before estimated symptom onset in dominantly inherited AD, which suggests that WMH may be an early event in AD pathogenesis (Lee et al., 2016). In this study, although WMH severity did not differ by FH or APOE4 carriership at baseline, these risk factors were associated with greater longitudinal progression of WMH over a two-year period. Adjusting for FH, APOE4 was associated exclusively with deep (but not periventricular) WMH progression. This may be linked to etiological differences between periventricular and deep WMH. Some studies have found that only periventricular WMH is implicated in increased risk of AD (Kim et al., 2015; Van Straaten et al., 2008), whereas both periventricular and deep WMH are associated with subcortical vascular dementia (Kim et al., 2015). Therefore, the exclusive coupling of APOE4 and deep WMH could be taken to imply that APOE4 predisposes one to dementia via vascular pathways. This is aligned with earlier studies showing that associations between AD severity and WMH progression were diminished after adjusting for APOE4 status, suggesting that WMH accumulation may be driven by APOE4 rather than AD diagnosis (Sudre et al., 2017). On the other hand, the heightened predisposition to dementia associated with parental history extends beyond genetics, and may also be driven by concomitant nongenetic factors such as shared environment, lifestyle, and socioeconomic status (Scarabino et al., 2016). In addition, we observed that greater WMH progression was observed in those with maternal but not paternal family history of dementia, although unequal sample sizes warrant replication of this finding in a more balanced sample.
In our sample of healthy midlife adults, APOE4 carriers displayed lower levels of CRP, a sensitive marker of systemic inflammation (Pepys and Hirschfield, 2003). Given that SVD and CRP are known to be positively associated (Low et al., 2019), it is counterintuitive that a risk factor (in this case, APOE4) would be associated positively with one pathology but negatively with another. Although somewhat surprising at first glance, these results are corroborated by earlier studies similarly reporting lower levels of CRP in APOE4 carriers (Chasman et al., 2006; Grönroos et al., 2008; Haan et al., 2008; Hubacek et al., 2010; Mänttäri et al., 2001; März et al., 2004; Ukkola et al., 2009). The juxtaposition of these contradictory findings suggests that APOE4 and CRP could be operating along distinct mechanistic pathways in relation to SVD, and may offer novel insight into the causal pathways of APOE4 as a risk factor in neurodegeneration. Despite being well documented in the literature, the biological mechanisms responsible for the negative association remain to be elucidated, although some attribute it to the downregulation of the mevalonate pathway in APOE4 carriers (März et al., 2004). CRP and fibrinogen levels did not differ by family history—this is consistent with the only other study to our knowledge examining CRP in relation to parental history of dementia, which also reported nonsignificant findings (Van Exel et al., 2009).
Inherited dementia risk had a dual effect on disease progression, in that predisposed individuals not only experienced greater progression of SVD, but also more pronounced slowing of reaction time in relation to SVD progression. In other words, the same degree of pathologic progression was related to greater slowing of reaction time in a person with inherited risk, than in a person without. This suggests that at-risk individuals could have a heightened susceptibility to vascular alterations. An alternative explanation is that SVD progression linked to heritable risk factors may be accompanied by other risk-related pathologies contributing to the cascade of clinical deficits (Shi et al., 2017). In terms of inflammation, elevated CRP levels were associated with slower reaction times, particularly in those without parental history of dementia. The absence of this association in those with family history suggests that poorer clinical features in this group may be driven instead by incipient disease. Notably, these findings were observed only in relation to reaction time but not other neuropsychological measures, i.e., episodic memory and executive function. This may be due to reaction time being a particularly early clinical feature in SVD (Jouvent et al., 2015; Richards et al., 2019), compared with the cognitive measures of memory and executive function which may be affected in later disease stages.
Positive associations between SVD and CRP were present in individuals with inherited risk but not in those without. This is in line with existing literature demonstrating the moderating effect of APOE4 on the relationship between inflammation and SVD (Low et al., 2019; Romero et al., 2012; Walker et al., 2017). Specifically, APOE4 has been shown to moderate associations between CRP and WMH (Walker et al., 2017) and between lipoprotein phospholipase A2 and CMB (Romero et al., 2012). This may be attributed to the increased propensity of APOE4 to promote inflammation and the tendency for APOE4 carriers to produce stronger neuroinflammatory responses to peripheral systemic inflammation (Cash et al., 2012; Lynch et al., 2003; Ophir et al., 2005). However, due to the inability to ascribe directionality, alternative interpretations should also be considered, whereby CRP acts as a moderator, i.e., APOE4 relates to greater SVD severity, but only in individuals with high CRP levels. To that end, the finding that high CRP levels amplified the detrimental effect of APOE4 on SVD suggests differential vulnerability, whereby individuals with heritable risk are more susceptible to vascular risk factors and consequent parenchymal injury. This corresponds with existing evidence that APOE4 has a lower antioxidant capacity than other isoforms and is less effective in protecting against oxidative stress, both of which could exacerbate the deleterious effects of risk factors such as smoking and low-grade inflammation (Dose et al., 2016; Jofre-Monseny et al., 2008; Miyata and Smith, 1996). Although we are unable to conclude the genetic overlap between SVD and AD in this present study, past research suggests that AD shares some genetic overlap specifically with SVD-related stroke but not other stroke subtypes (Traylor et al., 2016). Nevertheless, the relationship between the genetics of SVD and AD remains poorly understood at present and warrants further investigation.
Biases in this study could have arisen from SVD ratings not being blinded to time point. Baseline and follow-up WMH lesion maps were compared side-by-side to improve consistency of WMH delineation, while SWI scans from both time points were compared with confirm that “new” CMB in follow-up scans were not present at baseline. While intended to improve accuracy, the lack of blinding to time point may have introduced implicit bias toward the detection of greater SVD burden at follow-up. That being said, such systemic biases would apply to the whole sample and should not have affected the results on the group differences observed. Furthermore, the degree of WMH and CMB progression observed falls within the range of annual progression in previous reports (Alber et al., 2019; Harper et al., 2018).
CMB incidence was higher than earlier reports in healthy populations, likely due to (1) more sensitive detection of CMB afforded by thin slice 3T SWI, which detects up to 3 times the number of CMB compared with conventional 1.5 T GRE (Nandigam et al., 2009), and (2) oversampling of participants with a family history of dementia and, by extension, APOE4 carriership, a well established risk factor for CMB in older adults (Poels et al., 2010). Majority of CMB positive cases had a single microbleed, while only a handful had more than one. Because of the dichotomization of CMB burden (present/absent), our ability to make inferences based on the degree of CMB severity was limited.
A key strength of our study was its longitudinal design, which enabled the investigation of pathologic progression across time. Furthermore, we focused on a relatively young subclinical sample of midlife adults, with and without heritable risk factors, which allowed us to identify early biomarker changes in individuals at risk of developing dementia. Several limitations warrant the replication of results, including the relatively modest sample size, lack of correction for multiple comparisons, missing values which may introduce bias in the estimation of parameters, and FLAIR slice thickness of 4 mm which may represent a limitation on WMH quantification. In addition, inflammation was measured using peripheral blood markers, which may not be reflective of inflammation in the central nervous system. CRP levels are known to vary widely over a short timescale, which could perhaps explain the presence of associations between CRP and APOE4 at baseline but not at follow-up. Furthermore, type of parental dementia was not limited to AD but included mixed and vascular dementia, in which inherited risk may propagate primarily vascular but not AD pathology, while diagnostic imprecision of parental dementia type is another limitation. While FH+ participants were largely recruited from dementia registries or through memory clinic referrals, FH information of participants recruited from other sources relied on self-report and was thus more susceptible to misreporting. Furthermore, those recruited from other sources, including FH− participants, were observed to be less motivated and less likely to return for follow-up assessments, resulting in greater dropout rates within this subset. Finally, as the sample was predominantly white (90%) (Ritchie et al., 2017), findings may not be applicable across other ethnicities. Future studies with larger, multiethnic cohorts and longer follow-up periods will be crucial to understand the interaction between these pathologic processes across the adult lifespan.
5. Conclusions
Our data demonstrate that cerebrovascular alterations can be detected decades before dementia onset would occur in those at risk. Individuals with parental history of dementia and/or APOE4 carriership experienced greater progression of cerebrovascular disease, despite showing lower levels of inflammation. Progression of cerebrovascular pathology also had more pronounced adverse effects on reaction time in at-risk individuals. Taken together, our findings suggest that inherited risk factors may accelerate the progression of cerebrovascular damage as a function of heightened vulnerability to vascular risk and low-grade inflammation, as opposed to direct causative mechanisms. Clinically, our findings signal the relevance of SVD and inflammation in relation to early clinical features and disease trajectory.
Disclosure statement
Authors declare no conflicts related to this study. Unrelated to this work, JOB has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai; has acted as a consultant for Lilly; and has received honorarium for talks from GE Healthcare and research support from Alliance Medical.
Acknowledgements
The authors thank all the PREVENT-Dementia participants for their enthusiastic participation in this study, the radiographers at the Clinical Imaging Facility, Imperial College London for their technical expertise and support in data acquisition, and the West London Mental Health National Health Service (NHS) Trust (now known as West London NHS Trust) for their help in subject recruitment.
Funding: Research grants from the UK Alzheimer's Society, the US Alzheimer's Association and philanthropic donations. This work was funded by a grant for the PREVENT-Dementia program from the UK Alzheimer's Society (grant numbers 178 and 264), and the PREVENT-Dementia study is also supported by the US Alzheimer's Association (grant number TriBEKa-17–519007) and philanthropic donations. AL is supported by the Lee Kuan Yew Fitzwilliam PhD Scholarship and the Tan Kah Kee Postgraduate Scholarship. EM is supported by Alzheimer's Society Junior Research Fellowship (RG 9611). JDS is a Wellcome clinical PhD fellow funded on grant 203914/Z/16/Z to the Universities of Manchester, Leeds, Newcastle and Sheffield. LS is supported by the Cambridge NIHR Biomedical Research Center (BRC) and Alzheimer's Research UK (ARUK-SRF2017B-1). HSM is supported by an NIHR Senior Investigator award. JOB and HSM receive infrastructural support from the Cambridge NIHR BRC.
Footnotes
Credit author statement: A.L. conducted the imaging analysis, performed the visual rating of cerebrovascular lesions, conducted the statistical analysis, and drafted the manuscript. L.S. developed the research question, assisted with the detailed design and implementation of the imaging protocol, supervised all aspects of the MR data design and collection, reviewed and provided critical feedback on the manuscript. J.D.S. performed visual rating of cerebrovascular lesions, reviewed and provided critical feedback on the manuscript. E.M. reviewed the data and manuscript and provided critical feedback. M.E.D. reviewed the data and manuscript and provided critical feedback. G.M.T. reviewed the manuscript and provided critical feedback. K.R. helped obtain funding, was involved with study design, and provided critical feedback on the manuscript. C.W.R. helped obtain funding, was involved with study design, provided critical feedback on the manuscript, and is Chief Investigator of the PREVENT-Dementia program. H.S.M. reviewed the drafts and provided critical feedback on the manuscript. J.O.B. helped obtain funding, led the design of the imaging protocol, supervised this study, and provided critical feedback on the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2020.10.029.
Appendix A. Supplementary data
References
- Alber J., Alladi S., Bae H.-J., Barton D.A., Beckett L.A., Bell J.M., Berman S.E., Biessels G.J., Black S.E., Bos I., Bowman G.L., Brai E., Brickman A.M., Callahan B.L., Corriveau R.A., Fossati S., Gottesman R.F., Gustafson D.R., Hachinski V., Hayden K.M., Helman A.M., Hughes T.M., Isaacs J.D., Jefferson A.L., Johnson S.C., Kapasi A., Kern S., Kwon J.C., Kukolja J., Lee A., Lockhart S.N., Murray A., Osborn K.E., Power M.C., Price B.R., Rhodius-Meester H.F.M., Rondeau J.A., Rosen A.C., Rosene D.L., Schneider J.A., Scholtzova H., Shaaban C.E., Silva N.C.B.S., Snyder H.M., Swardfager W., Troen A.M., van Veluw S.J., Vemuri P., Wallin A., Wellington C., Wilcock D.M., Xie S.X., Hainsworth A.H. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. 2019;5:107–117. doi: 10.1016/j.trci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Bendorius M., Po C., Muller S., Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos D., Wolters F.J., Darweesh S.K.L., Vernooij M.W., de Wolf F., Ikram M.A., Hofman A. Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018 doi: 10.1016/j.jalz.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Cash J.G., Kuhel D.G., Basford J.E., Jaeschke A., Chatterjee T.K., Weintraub N.L., Hui D.Y. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J. Biol. Chem. 2012;287:27876–27884. doi: 10.1074/jbc.M112.377549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasman D.I., Kozlowski P., Zee R.Y., Kwiatkowski D.J., Ridker P.M. Qualitative and quantitative effects of APOE genetic variation on plasma C-reactive protein, LDL-cholesterol, and apoE protein. Genes Immun. 2006;7:211–219. doi: 10.1038/sj.gene.6364289. [DOI] [PubMed] [Google Scholar]
- Clauss A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Debette S., Schilling S., Duperron M.G., Larsson S.C., Markus H.S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M., Ercoli L.M., Siddarth P., Brown J.A., Martin-Harris L., Burggren A.C., Miller K.J., Small G.W., Bookheimer S.Y. Influence of alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. Am. J. Geriatr. Psychiatry. 2012;20:565–573. doi: 10.1097/JGP.0b013e3182107e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose J., Huebbe P., Nebel A., Rimbach G. APOE genotype and stress response - a mini review. Lipids Health Dis. 2016 doi: 10.1186/s12944-016-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank M.J., Minett T., O’Brien J.T. Changes in DWI and MRS associated with white matter hyperintensities in elderly subjects. Neurology. 2003;61:950–954. doi: 10.1212/01.wnl.0000086375.33512.53. [DOI] [PubMed] [Google Scholar]
- Gabin J.M., Saltvedt I., Tambs K., Holmen J. The association of high sensitivity C-reactive protein and incident Alzheimer disease in patients 60 years and older: the HUNT study, Norway. Immun. Ageing. 2018;15:4. doi: 10.1186/s12979-017-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C., Wei D., Wang Y., Ma J., Yuan C., Zhang W., Yu G., Zhao Y. A meta-analysis of C-reactive protein in patients with Alzheimer’s disease. Am. J. Alzheimers. Dis. Other Demen. 2016 doi: 10.1177/1533317515602087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S.M., Vernooij M.W., Cordonnier C., Viswanathan A., Al-Shahi Salman R., Warach S., Launer L.J., Van Buchem M.A., Breteler M.M. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire S.M., Chaudhary U.J., Brown M.M., Yousry T.A., Kallis C., Jager H.R., Werring D.J. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Jenkinson M., Suri S., Zsoldos E., Mahmood A., Filippini N., Sexton C.E., Topiwala A., Allan C., Kivimäki M., Singh-Manoux A., Ebmeier K.P., Mackay C.E., Zamboni G. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: a study in older adults. Neuroimage. 2018;170:174–181. doi: 10.1016/j.neuroimage.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Grönroos P., Raitakari O.T., Kähönen M., Hutri-Kähönen N., Marniemi J., Viikari J., Lehtimäki T. Association of high sensitive C-reactive protein with apolipoprotein E polymorphism in children and young adults: the Cardiovascular Risk in Young Finns Study. Clin. Chem. Lab. Med. 2008;46:179–186. doi: 10.1515/CCLM.2008.033. [DOI] [PubMed] [Google Scholar]
- Haan M.N., Aiello A.E., West N.A., Jagust W.J. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiol. Aging. 2008;29:1774–1782. doi: 10.1016/j.neurobiolaging.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A.M., Clayson L., Wardlaw J.M., Valdés Hernández M.D.C. Considerations on accuracy, pattern and possible underlying factors of brain microbleed progression in older adults with absence or mild presence of vascular pathology. J. Int. Med. Res. 2018;46:3518–3538. doi: 10.1177/0300060518755623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., Khoury J. El, Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubacek J.A., Peasey A., Pikhart H., Stavek P., Kubinova R., Marmot M., Bobak M. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum. Immunol. 2010;71:304–308. doi: 10.1016/j.humimm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre-Monseny L., Minihane A.M., Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol. Nutr. Food Res. 2008 doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Jouvent E., Reyes S., De Guio F., Chabriat H. Reaction time is a marker of early cognitive and behavioral alterations in pure cerebral small vessel disease. J. Alzheimer’s Dis. 2015;47:413–419. doi: 10.3233/JAD-150083. [DOI] [PubMed] [Google Scholar]
- Kempuraj D., Thangavel R., Selvakumar G.P., Zaheer S., Ahmed M.E., Raikwar S.P., Zahoor H., Saeed D., Natteru P.A., Iyer S., Zaheer A. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017 doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Choi S.H., Lee Y.M., Kim M.J., Kim Y.D., Kim J.Y., Park J.H., Myung W., Na H.R., Han H.J., Shim Y.S., Kim J.H., Yoon S.J., Kim S.Y., Kim D.K. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int. Psychogeriatr. 2015;27:2069–2077. doi: 10.1017/S1041610215001076. [DOI] [PubMed] [Google Scholar]
- Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018 doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Viqar F., Zimmerman M.E., Narkhede A., Tosto G., Benzinger T.L.S., Marcus D.S., Fagan A.M., Goate A., Fox N.C., Cairns N.J., Holtzman D.M., Buckles V., Ghetti B., McDade E., Martins R.N., Saykin A.J., Masters C.L., Ringman J.M., Ryan N.S., Förster S., Laske C., Schofield P.R., Sperling R.A., Salloway S., Correia S., Jack C., Weiner M., Bateman R.J., Morris J.C., Mayeux R., Brickman A.M. White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 2016;79:929–939. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A., Mak E., Rowe J.B., Markus H.S., O’Brien J.T. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res. Rev. 2019;53:100916. doi: 10.1016/j.arr.2019.100916. [DOI] [PubMed] [Google Scholar]
- Lynch J.R., Tang W., Wang H., Vitek M.P., Bennett E.R., Sullivan P.M., Warner D.S., Laskowitz D.T. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N., Ellman L.M., Coe C.L., Byrne M.L., Abramson L.Y., Alloy L.B. To exclude or not to exclude: considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mänttäri M., Manninen V., Palosuo T., Ehnholm C. Apolipoprotein E polymorphism and C-reactive protein in dyslipidemic middle-aged men. Atherosclerosis. 2001 doi: 10.1016/s0021-9150(01)00480-4. [DOI] [PubMed] [Google Scholar]
- März W., Scharnagl H., Hoffmann M.M., Boehm B.O., Winkelmann B.R. The apolipoprotein E polymorphism is associated with circulating C-reactive protein (the Ludwigshafen risk and cardiovascular health study) Eur. Heart J. 2004;25:2109–2119. doi: 10.1016/j.ehj.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Miyata M., Smith J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- Nandigam R.N.K., Viswanathan A., Delgado P., Skehan M.E., Smith E.E., Rosand J., Greenberg S.M., Dickerson B.C. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am. J. Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J.M., Manly J.J., Schupf N., Tang M.X., Mayeux R., Luchsinger J.A. Association of C-reactive protein with cognitive impairment. Arch. Neurol. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir G., Amariglio N., Jacob-Hirsch J., Elkon R., Rechavi G., Michaelson D.M. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiol. Dis. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Papp K.V., Buckley R., Mormino E., Maruff P., Villemagne V.L., Masters C.L., Johnson K.A., Rentz D.M., Sperling R.A., Amariglio R.E. Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimers Dement. 2020;16:552–560. doi: 10.1016/j.jalz.2019.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels M.M.F., Vernooij M.W., Ikram M.A., Hofman A., Krestin G.P., Van Der Lugt A., Breteler M. Stroke. Stroke. 2010. Prevalence and risk factors of cerebral microbleeds: an update of the rotterdam scan study. [DOI] [PubMed] [Google Scholar]
- Richards E., Bayer A., Tree J.J., Hanley C., Norris J.E., Tales A. Subcortical ischemic vascular cognitive impairment: insights from reaction time measures. J. Alzheimers Dis. 2019;72:845–857. doi: 10.3233/JAD-190889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C.W., Ritchie K. The PREVENT study: a prospective cohort study to identify mid-life biomarkers of late-onset Alzheimer’s disease. BMJ Open. 2012;2:1–6. doi: 10.1136/bmjopen-2012-001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K., Carrière I., Berr C., Amieva H., Dartigues J.F., Ancelin M.L., Ritchie C.W. The clinical picture of Alzheimer’s disease in the decade before diagnosis: clinical and biomarker trajectories. J. Clin. Psychiatry. 2016;77:e305–e311. doi: 10.4088/JCP.15m09989. [DOI] [PubMed] [Google Scholar]
- Ritchie K., Carrière I., Su L., O’Brien J.T., Lovestone S., Wells K., Ritchie C.W. The midlife cognitive profiles of adults at high risk of late-onset Alzheimer’s disease: the PREVENT study. Alzheimers Dement. 2017;13:1089–1097. doi: 10.1016/j.jalz.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Ritchie K., de Roquefeuil G., Ritchie C.W., Besset A., Poulain V., Artero S., Ancelin M.-L. COGNITO: computerized assessment of information processing. J. Psychol. Psychother. 2014;4:136. [Google Scholar]
- Romero J.R., Preis S.R., Beiser A.S., DeCarli C., Lee D.Y., Viswanathan A., Benjamin E.J., Fontes J., Au R., Pikula A., Wang J., Kase C.S., Wolf P.A., Irrizary M.C., Seshadri S. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham heart study. Stroke. 2012;43:3091–3094. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabino D., Gambina G., Broggio E., Pelliccia F., Corbo R.M. Influence of family history of dementia in the development and progression of late-onset Alzheimer’s disease. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016;171B:250–256. doi: 10.1002/ajmg.b.32399. [DOI] [PubMed] [Google Scholar]
- Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W., Tsai R.M., Spina S., Grinberg L.T., Rojas J.C., Gallardo G., Wang K., Roh J., Robinson G., Finn M.B., Jiang H., Sullivan P.M., Baufeld C., Wood M.W., Sutphen C., McCue L., Xiong C., Del-Aguila J.L., Morris J.C., Cruchaga C., Fagan A.M., Miller B.L., Boxer A.L., Seeley W.W., Butovsky O., Barres B.A., Paul S.M., Holtzman D.M. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm B.C., Lao P.J., Rizvi B., Colon J., Igwe K., Chesebro A.G., Maas B., Schupf N., Mayeux R., Manly J.J., Brickman A.M. Parental history of dementia is associated with increased small vessel cerebrovascular disease. J. Gerontol. Ser. A. 2019 doi: 10.1093/gerona/glz291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D.D., Bradley W.G., Jr. third ed. Mosby; St. Louis: 1999. Magnetic Resonance Imaging. [Google Scholar]
- Stefaniak J.D., Su L., Mak E., Sheikh-Bahaei N., Wells K., Ritchie K., Waldman A., Ritchie C.W., O’brien J.T. Cerebral small vessel disease in middle age and genetic predisposition to late-onset Alzheimer’s disease. Alzheimers Dement. 2018;14:253–258. doi: 10.1016/j.jalz.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Strittmatter W.J., Roses A.D. Apolipoprotein E and Alzheimer’s disease. Annu. Rev. Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Sudre C.H., Cardoso M.J., Frost C., Barnes J., Barkhof F., Fox N., Ourselin S. APOE4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiol. Aging. 2017;53:67–75. doi: 10.1016/j.neurobiolaging.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Tao Q., Ang T.F.A., DeCarli C., Auerbach S.H., Devine S., Stein T.D., Zhang X., Massaro J., Au R., Qiu W.Q. Association of chronic low-grade inflammation with risk of alzheimer disease in ApoE4 carriers. JAMA Netw. Open. 2018;1:e183597. doi: 10.1001/jamanetworkopen.2018.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor M., Adib-Samii P., Harold D., Dichgans M., Williams J., Lewis C.M., Markus H.S., Fornage M., Holliday E.G., Sharma P., Bis J.C., Psaty B.M., Seshadri S., Nalls Mike A., Devan W.J., Boncoraglio G., Malik R., Mitchell B.D., Kittner S.J., Ikram M.A., Clarke R., Rosand J., Meschia J.F., Sudlow C., Rothwell P.M., Levi C., Bevan S., Kilarski L.L., Walters M., Thijs V., Slowik A., Lindgren A., De Bakker P.I.W., Lambert J.C., Ibrahim-Verbaas C.A., Naj A.C., Sims R., Bellenguez C., Jun G., Destefano A.L., Beecham G.W., Grenier-Boley B., Russo G., Thornton-Wells T.A., Jones N., Smith A.V., Chouraki V., Thomas C., Zelenika D., Vardarajan B.N., Kamatani Y., Lin C.F., Gerrish A., Schmidt H., Kunkle B., Dunstan M.L., Ruiz A., Bihoreau M.T., Choi S.H., Reitz C., Pasquier F., Hollingworth P., Ramirez A., Hanon O., Fitzpatrick A.L., Buxbaum J.D., Campion D., Crane P.K., Baldwin C., Becker T., Gudnason V., Cruchaga C., Craig D., Amin N., Berr C., Lopez O.L., De Jager P.L., Deramecourt V., Johnston J.A., Evans D., Lovestone S., Letenneur L., Morón F.J., Rubinsztein D.C., Eiriksdottir G., Sleegers K., Goate A.M., Fiçvet N., Huentelman M.J., Gill M., Brown K., Kamboh M.I., Keller L., Barberger-Gateau P., McGuinness B., Larson E.B., Green R., Myers A.J., Dufouil C., Todd S., Wallon D., Love S., Rogaeva E., Gallacher J., St George-Hyslop P., Clarimon J., Lleo A., Bayer A., Tsuang D.W., Yu L., Tsolaki M., Bossù P., Spalletta G., Proitsi P., Collinge J., Sorbi S., Sanchez-Garcia F., Fox N.C., Hardy J., Deniz Naranjo M.C., Bosco P., Brayne C., Galimberti D., Mancuso M., Matthews F., Moebus S., Mecocci P., Del Zompo M., Maier W., Hampel H., Pilotto A., Bullido M., Panza F., Caffarra P., Nacmias B., Gilbert J.R., Mayhaus M., Lannfelt L., Hakonarson H., Pichler S., Carrasquillo M.M., Ingelsson M., Beekly D., Alvarez V., Zou F., Valladares O., Younkin S.G., Coto E., Hamilton-Nelson K.L., Gu W., Razquin C., Pastor P., Mateo I., Owen M.J., Faber K.M., Jonsson P.V., Combarros O., O’Donovan M.C., Cantwell L.B., Soininen H., Blacker D., Mead S., Mosley T.H., Bennett D.A., Harris T.B., Fratiglioni L., Holmes C., De Bruijn R.F.A.G., Passmore P., Montine T.J., Bettens K., Rotter J.I., Brice A., Morgan K., Foroud T.M., Kukull W.A., Hannequin D., Powell J.F., Nalls M.A., Ritchie K., Lunetta K.L., Kauwe J.S.K., Boerwinkle E., Riemenschneider M., Boada M., Hiltunen M., Martin E.R., Schmidt R., Rujescu D., Wang L.S., Dartigues J.F., Mayeux R., Tzourio C., Hofman A., Nöthen M.M., Graff C., Jones L., Haines J.L., Holmans P.A., Lathrop M., Pericak-Vance M.A., Launer L.J., Farrer L.A., Van Duijn C.M., Van Broeckhoven C., Moskvina V., Schellenberg G.D., Amouyel P. Shared genetic contribution to ischemic stroke and Alzheimer’s disease. Ann. Neurol. 2016;79:739–747. doi: 10.1002/ana.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkola O., Kunnari A., Jokela M., Päivänsalo M., Kesäniemi Y.A. ApoE phenotype is associated with inflammatory markers in middle-aged subjects. Inflamm. Res. 2009;58:54–59. doi: 10.1007/s00011-008-8215-2. [DOI] [PubMed] [Google Scholar]
- Van Exel E., Eikelenboom P., Comijs H., Frölich M., Smit J.H., Stek M.L., Scheltens P., Eefsting J.E., Westendorp R.G.J. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch. Gen. Psychiatry. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- Van Straaten E.C.W., Harvey D., Scheltens P., Barkhof F., Petersen R.C., Thal L.J., Jack C.R., DeCarli C. Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J. Neurol. 2008;255:1302–1308. doi: 10.1007/s00415-008-0874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K.A., Power M.C., Hoogeveen R.C., Folsom A.R., Ballantyne C.M., Knopman D.S., Windham B.G., Selvin E., Jack C.R., Jr., Gottesman R.F. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the Atherosclerosis Risk in Communities Study. Stroke. 2017;48:3196–3202. doi: 10.1161/STROKEAHA.117.018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith C., Dichgans M. Mechanisms underlying sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kitamura K., Nakamura K., Sanpei K., Wakasugi M., Yokoseki A., Onodera O., Ikeuchi T., Kuwano R., Momotsu T., Narita I., Endo N. Elevated C-reactive protein is associated with cognitive decline in outpatients of a general hospital: the project in sado for total health (PROST) Dement. Geriatr. Cogn. Dis. Extra. 2016;6:10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters F.J., Van Der Lee S.J., Koudstaal P.J., Van Duijn C.M., Hofman A., Ikram M.K., Vernooij M.W., Ikram M.A. Parental family history of dementia in relation to subclinical brain disease and dementia risk. Neurology. 2017;88:1–8. doi: 10.1212/WNL.0000000000003871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.