Abstract
Concurrent infection with Schistosoma mansoni and Salmonella species is not uncommon in the endemic area of sub-Saharan Africa, although its prevalence may have regional variations. We discuss such coinfection and associated factors in an Ethiopian context. We assessed the prevalence of S. mansoni and Salmonella coinfections among patients attending two hospitals in southern Ethiopia. A facility-based cross-sectional study was carried out between 1 October and 30 November 2019. In total 271 participants with gastrointestinal complaints were selected through a systematic sampling technique. S. mansoni was detected using direct microscopy and formalin–ether concentration techniques, whereas Salmonella was identified by conventional culture methods and the Widal test. Antibiotic susceptibility test for Salmonella isolates was performed. The prevalence rates of S. mansoni and Salmonella infections were 17.30% and 7.70% respectively. The prevalence of S. mansoni–Salmonella coinfection was 7.7%. Of the factors analysed in connection with coinfection, male sex, age and frequency of exposure to contaminated water bodies were found to be statistically significant. S. mansoni–Salmonella coinfections pose a grave health problem in the study area, especially among children. Our conclusions can be used by the medical community to frame and implement intervention strategies for the management of S. mansoni–Salmonella coinfections.
Keywords: Coinfection, Ethiopia, jinka, Salmonella spp., salmonellosis, Schistosoma mansoni, schistosomiasis
Highlights
-
•
The individual prevalences of Schistosoma mansoni and Salmonella were 17.30% and 7.70%, respectively.
-
•
The overall prevalence of S. mansoni–Salmonella coinfection was 7.70%.
-
•
Coinfection was significantly associated with male sex, younger age and frequency of exposure to contaminated water bodies.
-
•
Multidrug resistance was detected in 50% of Salmonella isolates.
Introduction
Schistosomiasis is a neglected tropical disease and it remains the second most afflicting parasitic illness especially in sub-Saharan African countries [1]. According to some recent reports, schistosomiasis has been documented in more than 40 nations in Africa, specifically in poor communities without safe drinking water or adequate sanitation [1]. The distribution and prevalence of schistosomiasis have increased over the past few decades, mainly because of construction of hydroelectric projects and dams as well as the existence of floating populations [2]. A recent estimate in this context by the World Health Organization (WHO) indicated that about 240,000,000 people are infected worldwide, another 700,000,000 are at greater risk in Africa alone and more than a quarter of a million perish annually [3]. It has been reported that in the second largest continent, per year, loss of half a billion US dollars is due to the menace of schistosomiasis [4]. Also, it has been estimated that about 54,000,000 are infected with, and more than seven times this number are living with an immediate risk of being affected by, Schistosoma mansoni [5].
The coinfection of Schistosoma with other microorganisms is a common phenomenon and often presents a clinical picture of schistosomiasis [6]. Among them, enterobacterial coinfections by Salmonella species has been reported for more than 50 years, causing morbidity [6,7]. Salmonella is a frequently found bacteria which results in invasive infection, especially in the least developed nations of Africa and their poor healthcare systems, including Ethiopia [8]. The WHO estimates that 16,000,000–33,000,000 cases of typhoid fever with almost a quarter of a million deaths happen annually, and those who die are mostly juveniles [9].
S. mansoni–Salmonella coinfection is considered to be a public health problem and a challenge to clinical management [10]. It is estimated that more than 20 species of Salmonella of human or animal origin may be associated with schistosomes [11]. A recent study in coinfected patients hints at the symbiotic relationship existing between the bacteria and the parasite [12]. Concurrent infections occur when enteroinvasive Salmonella penetrates into the systemic circulation of the victim and adheres to the tegument of adult Schistosoma [13]. The coinfection produces a set of clinical manifestations, such as indolent febrile illness, weight loss, abdominal pain, pallor, edema of lower limbs, marked hepatomegaly and splenomegaly [13]. Therapy for Salmonella infection without treating the underlying schistosomiasis may later result in a relapse of bacteraemia and related symptoms [14]. In addition, Salmonella bacteraemia has been associated with glomerulonephritis and nephrotic syndrome in individuals infected with S. mansoni [15]. Some evidence substantiates the notion that that schistosomes and Salmonella share antigens responsible for immunologic tolerance to the bacterial infection [13]. Furthermore, latent bacteria in the parasite lead to ineffective antibiotic therapy, and such bacteria cannot be completely eliminated, eventually resulting in drug resistance and the emergence of multidrug-resistant Salmonella [11,16].
Recently it has become clear that coinfection with S. mansoni–Salmonella is common in endemic areas where people are exposed to both of the pathogens, resulting in high morbidity and mortality [16]. Schoolchildren younger than 15 in deprived communities are the most affected; however, people of all ages can become infected [17]. In several schools, it is one of the commonest reasons for students' poor academic and physical performance as well as absenteeism [18]. A fragile healthcare system combined with lack of adequate supply of potable water, unsanitary conditions and poor personal hygiene in African countries make S. mansoni–Salmonella coinfection difficult to contain [19].
Most of the African nations are known for coinfections by S. mansoni–Salmonella, both of which are waterborne pathogens. A literature survey indicates that the prevalence and distribution of S. mansoni–Salmonella coinfection in endemic countries of Africa has not been studied extensively so far. S. mansoni–Salmonella coinfections are endemic in many regions of Ethiopia too, but this fact remains unstudied. All the studies performed to date nationally in Ethiopia focus merely on infections caused by either S. mansoni or by Salmonella. Routine health surveillance is needed in the case of people living in endemic areas like Jinka, a deep southern Ethiopian valley, as they have poor living conditions and are often exposed to contaminated water bodies, which makes them highly vulnerable to coinfections. If the coinfection is not properly diagnosed or promptly treated, it can eventually become severe. Therefore, studies related to local prevalence and risk factors associated with S. mansoni–Salmonella coinfection are relevant.
We performed the present study to assess the prevalence rates of S. mansoni and Salmonella infections separately and to evaluate more precisely the coinfection rates among patients attending Jinka General Hospital and the Jinka Millennium Health Center, Jinka, a remote southern area in the Southern Nations, Nationalities and People's Region (SNNPR), a regional state of Ethiopia.
Methods
Study area, design, period and study population
A cross-sectional study was designed and carried out at two government health facilities in Ethiopia: Jinka General Hospital and the Jinka Millennium Health Center. These two primary healthcare institutions (located in Jinka, the capital of the South Omo Zone of the SNNPR) receive a large number of patients, approximately 50,000 annually. Jinka prefecture has a river with tributaries, some streams, several rain-fed ponds and shallow water bodies that are utilized commonly by humans as well as wild and domesticated animals. The study area is known for a large number of cases of schistosomiasis and typhoid fever as a result of poor sanitary conditions, with the former being more or less endemic. During the study period between 1 October and 30 November 2019, a total of 271 individuals were selected from a larger group of patients. The inclusion criteria were as follows: suspected cases (i.e. those with gastrointestinal manifestations) for which physicians recommended either stool examination alone or both stool examination and the Widal test. The exclusion criteria were patients (a) undergoing treatments for schistosomiasis and Salmonella infection, (b) severely affected but unable to communicate, (c) less than 5 year old, (d) residing outside the study site and (e) who did not submit a written consent to participate. The study was ethically approved by the institutional review board of the College of Medicine and Health Sciences, Arba Minch University (IRB/0203195/11/01/11).
Sample size determination and sampling technique
The sample size was calculated using a single population proportion formula. A p value of 0.2 was chosen from a study performed in Sudan [20]. After considering a confidence interval (CI) of 95% (z = 1.96) and a 5% marginal error (d = 0.05), the initial sample size was estimated to be 256; after computing a 10% nonresponse rate, the final sample size was 271. The selection of study participants from Jinka General Hospital and the Jinka Millennium Health Center was done by proportional allocation corresponding to each institution. A systematic random sampling technique was chosen to recruit the study participants. The sampling interval was calculated by dividing the total number of target patients by the sample size. The Kth value was inferred from the number of patients on a daily basis who used the stool examination facilities in these two health institutions during the study period. Individuals were selected by lottery.
Data and specimen collection
Before data and sample collection, written informed consent was obtained from all the participants, including the parents of children, after receipt of a clear briefing about the purpose of the study. Interviews were conducted with the aid of well-trained health professionals. Relevant details related to the sociodemographic (age, sex, occupation, educational level and residence) and environmental/behavioral (presence of latrine, source of drinking water) status of each participant were solicited by a face-to-face interview by means of a pretested structured questionnaire. Five grams of fresh stool specimen was collected from each participant aseptically into a clean, dry, sterile, wide-mouthed plastic container equipped with a spoon, labeled with an identification code. For the detection of S. mansoni eggs, each faecal sample was examined microscopically (10 × and 40 × objectives) using the direct wet mount and stool concentration technique (formalin–ether concentration technique), as described previously by Cheesbrough [21]. The Widal test was used for the diagnosis of enteric fever by using 5 mL of blood. Anti-Salmonella antibodies were detected according to the nationally approved standard using the Widal agglutination kit (Murex Biotech, UK) comprising the polyvalent somatic (O) and flagella (H) antigens of Salmonella enterica serovar Typhi and Salmonella enterica serotypes Paratyphi A, B and C. For the slide agglutination test, a drop of serum from each patient was placed on a clean tile and mixed with antigens, then rocked for 3 minutes. Antibody titration was performed only for slide-reactive samples. The titre values of 1:80 for O and 1:160 for H antigens in a single analysis were considered the cutoff points for a positive results as per standard recommendation [22]. All positive samples were reinspected by an experienced laboratory technologist at the Jinka Regional Public Health Laboratory for further confirmation. A third reader (an experienced microbiologist from the Medical Microbiology and Parasitology Laboratory, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University) also checked the results to eliminate any discordance, if present. To avoid any bias, all the laboratory technologists who were involved were unaware of the results.
To confirm the Salmonella infection, stool specimens were cultured [23]. A small portion of the freshly collected stool sample was inoculated into selenite F broth (Oxoid, UK) and incubated at 37°C for 24 hours. After the preenrichment period, the broth was subcultured onto xylose lysine deoxycholate (XLD) agar (Oxoid) and further incubated at 37°C for 24–48 hours. The pure cultures of bacterial isolates were subsequently subjected to species identification and confirmation. Morphologic, biochemical and physiologic characteristics of isolated bacteria were ascertained by using standard laboratory methods as described by Margot et al. [23]. Corresponding American Type Culture Collection strains were utilized as reference standards to validate the biochemical identification of Salmonella.
Antimicrobial susceptibility test
The antibiotic susceptibility profiles of all Salmonella isolates were determined by the Kirby-Bauer disc diffusion technique according to the criteria set by the Clinical and Laboratory Standards Institute (CLSI) using Oxoid antibiotic discs. Inocula were prepared in sterile normal saline and its density adjusted to 0.5 McFarland standard. The test organisms were inoculated over the Müller-Hinton agar (Oxoid), exposed to a concentration gradient of antibiotic diffusion from an impregnated paper disc and then incubated at 37°C for 24 hours. The diameters of the zones of inhibition around the discs were measured to the nearest millimeter and categorized as sensitive, intermediate and resistant according to the CLSI standardized table. Salmonella spp. which exhibited resistance to three classes of antibiotics were considered as multidrug resistant (MDR). A series of antibiotic discs such as ampicillin (10 μg), amoxicillin/clavulanic acid (30 μg), ceftriaxone (30 μg), cefotaxime (5 μg), gentamicin (10 μg), amikacin (30 μg), tetracycline (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), nalidixic acid (30 μg) and cotrimoxazole (25 μg) were used.
Statistical analysis
The data were cleaned, edited, checked for completeness, entered to Epi Info 3.5.3 (Centers for Disease Control and Prevention, USA) and exported to SPSS 20 (IBM, USA). It was then analysed by means of descriptive data; frequencies and percentages were used to describe patient characteristics. Outputs of statistical analysis were presented in the form of tables, charts and graphs. Bivariate logistic regression analysis was used to identify the associated factors among dependent and independent variables. The variables with statistical significance (p < 0.25) thus obtained were then subjected to a multivariate logistic regression analysis. The p values which were ≤0.05 at 95% CI were considered to indicate a statistical association.
Results
Sociodemographic characteristics
A total of 271 eligible participants were included in this study, with a response rate of 100%. The ages of participants ranged from 5 to 70 years with a mean ± standard deviation of 23 ± 9 years. Male-to-female ratio was 1.5:1. More than a third of the participants, 102 (37.60%), were within the age range of 5–15 years, and the student population was 76 (28%). A wafer-thin majority of participants, 141 (52%), were residents of a rural area, and 84.50% of the households in the study area, Jinka, had latrine facilities. A total of 53.5% of the participants had had contact with water bodies on at least some occasions (seldom). The detailed sociodemographic characteristics of individuals are summarized in Table 1.
Table 1.
Schistosoma mansoni–Salmonella coinfection: sociodemographic characteristics of the study participants, October–November 2019 (n = 271)
| Characteristic | Total samples |
S. mansoni–infected patients (n = 47) |
Salmonella-positive patients (n = 21) |
Salmonella and S. mansoni coinfection (n = 21) |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | p | n (%) | p | n (%) | Adjusted OR (95% CI) | p | ||
| Gender | ||||||||
| Male | 165 | 38 (23.30) | 0.014 | 17 (10.30) | 0.60 | 17 (10.30) | 2.51 (1.109–4.62) | 0.010 |
| Female | 106 | 9 (8.49) | Ref | 4 (3.70) | Ref | 4 (3.77) | Ref | |
| Age category | ||||||||
| 5–15 years | 102 | 31 (65.90) | 0.037 | 9 (8.80) | 0.27 | 9 (8.82) | 3.19 (1.10–9.20) | 0.008 |
| 16–30 years | 93 | 11 (23.40) | 0.27 | 9 (9.60) | 0.89 | 9 (9.67) | 2.03 (0.62–6.65) | NS |
| 31–45 years | 70 | 5 (10.60) | 0.29 | 3 (4.20) | 0.29 | 3 (4.28) | 1.23 (0.27–5.59) | NS |
| >46 years | 6 | 0 | Ref | 0 | Ref | 0 | Ref | |
| Place of residence | ||||||||
| Rural | 141 | 44 (31.20) | 0.75 | 13 (9) | 0.77 | 13 (28.88) | Ref | 0.177 |
| Urban | 130 | 3 (2.30) | Ref | 8 (6.10) | Ref | 8 (6.15) | 2.40 (0.84–6.81) | |
| Occupational status | ||||||||
| Employed | 7 | 0 | 0.29 | 0 | Ref | 0 | Ref | |
| Housekeeper | 55 | 2 (3.63) | Ref | 4 (7.20) | 0.776 | 4 (7.27) | 3.42 (1.12–10.43) | NS |
| Merchant | 68 | 1 (1.47) | 0.75 | 3 (8.60) | 0.37 | 3 (8.68) | 6.23 (0.57–67.12) | NS |
| Student | 76 | 39 (51.31) | 0.14 | 12 (15.70) | 0.64 | 12 (15.78) | 1.8 (0.40–6.90) | NS |
| Other | 65 | 5 (7.69) | 0.37 | 2 (3.07) | 0.77 | 2 (3.07) | NS | |
| Educational status | ||||||||
| Illiterate | 92 | 13 (14.13) | 0.08 | 8 (8.80) | 0.86 | 7 (7.6) | 0.25 (0.01–3.54) | NS |
| Can read and write | 136 | 28 (20.50) | 0.24 | 34 (65.30) | 0.53 | 11 (8.08) | 3.40 (0.59–19.50) | NS |
| Grade (1–10) | 37 | 6 (16.21) | 0.54 | 5 (11.60) | Ref | 3 (22.50) | Ref | NS |
| Tertiary-level education | 6 | 0 | Ref | 8 (8.80) | 0.86 | 0 | 0.717 (.12, 3.99) | NS |
| Habit and frequency of contact with water body | ||||||||
| Frequently | 57 | 34 (59.64) | 0.02 | 8 (8.80) | 0.86 | 13 (9) | 2.78 (0.81–9.68) | 0.001 |
| Seldom | 145 | 13 (8.96) | 0.72 | 34 (65.30) | 0.53 | 8 (5.50) | 0.25 (0.019–3.49) | NS |
| Not at all | 69 | 0 | Ref | 5 (11.60) | Ref | 0 | Ref | |
| Drinking water source | ||||||||
| Bono water | 86 | 0 | Ref | 0 | Ref | 0 | Ref | |
| Standpipe | 90 | 8 (8.80) | 0.64 | 5 (5.50) | 0.77 | 5 (5.50) | 3.54 (1.09–11.5) | NS |
| River water | 52 | 34 (65.38) | 0.58 | 13 (25) | 0.68 | 13 (25) | 0.04 (0.008–1.2) | NS |
| Well | 43 | 5 (11.62) | 0.81 | 3 (6.97) | 0.62 | 3 (6.97) | 2.69 (2.31–183.1) | NS |
| Latrine present | 42 | 38 (90.4) | 0.74 | 19 (90.40) | 0.66 | 19 (90.47) | 0.016 (0.001–0.8) | NS |
CI, confidence interval; OR, odds ratio; NS, not significant. Reference, p ≤ 0.05.
Prevalence of S. mansoni and associated factors
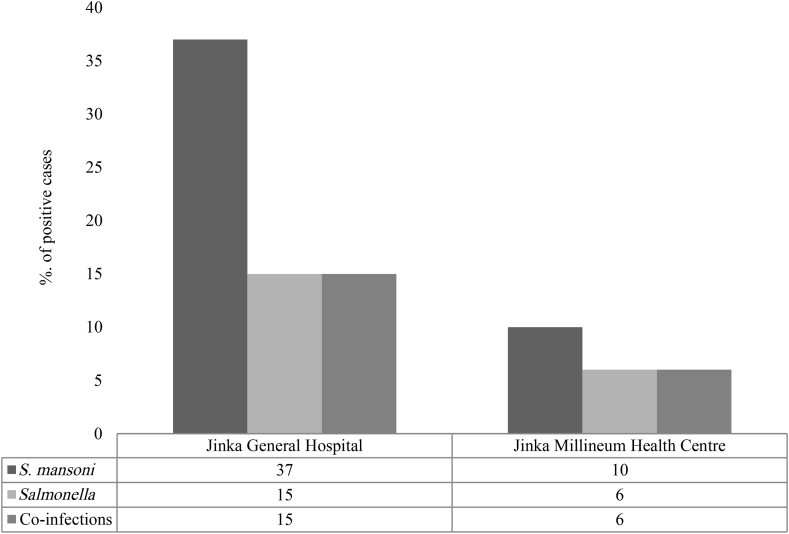
Results of the unstained wet mount examination revealed that of 271 stool samples, only 47 (17.30%) were positive for S. mansoni. A total of 37 (13.60%) stool samples from Jinka General Hospital and 10 (3.60%) from the Jinka Millennium Health Center were infected with S. mansoni (Fig. 1).
Fig. 1.
Distribution of Schistosoma mansoni, Salmonella and their coinfection in relation to health institutions. Out of 47 intestinal schistosomiasis patients, 37 (78.70%) were from Jinka General Hospital and ten (21.30%) were from Jinka Millennium Health Center. Results demonstrated that of 21 Salmonella-positive patients, 15 (71.40%) were from Jinka General Hospital and six (28.57%) were from Jinka Millennium Health Center. Number of coinfected patients was 15 from Jinka General Hospital and 6 from Jinka Millennium Health Center.
Aspects of the association among sociodemographic and other selected variables with respect to S. mansoni infection are summarized in Table 1. Among different factors analysed, gender, age and frequency of contact with contaminated water bodies were found to be independent predictors of infection. Bivariate logistic analysis revealed that S. mansoni infection in male participants was 2.70 times greater (Crude Odds Ratio (COD) 2.7 (95% CI, 1.20–6), p 0.14) than in female participants. Likewise, children were found to be 2.20 times more prone to be infected (COD 2.20 (95% CI, 1.05–4.90), p 0.37) with S. mansoni. In addition, it was found that those who had frequent contact with contaminated water had a tripled chance of being infected (COD 3.40 (95% CI, 1.30–36), p 0.02) compared to the reference category. However, no statistically significant associations were observed among S. mansoni infection and residential status, occupation or presence of a latrine at home.
Prevalence of Salmonella infection and associated factors
In the present study, the Widal agglutination test and stool culture techniques were used for the diagnosis of Salmonella infection (Table 1). This test had revealed that of the 271 blood samples screened, 21 (7.70%) were positive for Salmonella infection. The prevalence of Salmonella infection among S. mansoni–infected patients was 2.20% according to the results of the stool culture (Supplementary Data). However, compared to the results of the stool culture, the number of positive cases detected by the Widal test was quite high, making the Widal test a more effective method for the diagnosis of Salmonella in the present scenario. Extrapolation found the overall prevalence of Salmonella infection to be 7.70%. Regarding the detection of Salmonella infection, 15 cases (5.50%) from Jinka General Hospital and 6 (2.20%) from the Jinka Millennium Health Center were found to be positive (Fig. 1).
Table 1 shows the association among sociodemographic status and other selected variables with the infection rate of Salmonella spp. Bivariate analysis revealed that the prevalence of Salmonella was significantly associated with S. mansoni (COD 2.50 (95% CI, 5–19), p 0.04). However, no significant association was found for sociodemographic variables (age, residential status, occupation and source of drinking water) and Salmonella spp. infection.
Prevalence of coinfection by S. mansoni–Salmonella spp. and associated factors
The number of coinfections reported from Jinka General Hospital and the Jinka Millennium Health Center were 15 (5.50%) and 6 (2.20%) respectively (Fig. 1). This means that the total number of coinfected cases from the present study is 21 (7.70%). The total number of cases of S. mansoni infection for both study sites is 47 (17.30%), whereas the combined figure for the positive cases of Salmonella infection is 21 (7.70%). It is evident from Fig. 1 that all positive cases of Salmonella (inclusive of both study settings) are invariably coinfected with S. mansoni.
Various factors were analysed to elucidate the association between S. mansoni and Salmonella spp. existing in coinfected individuals. In the logistic regression analysis, male sex (adjusted odds ratio (aOR) 2.50 (95% CI, 1.10–4.60), p 0.01), children (age ≤ 15 years) (aOR 3.19 (95% CI, 1.10–9.20), p 0.008) and frequency of contact with contaminated water bodies (aOR 2.78 (95% CI, 0.81–9.68), p 0.001) were the factors found to be statistically significant (Table 1).
Antibiotic susceptibility patterns
Antibiotic susceptibility patterns of all Salmonella isolates were confirmed using 11 antibiotics (Table 2). We found that the isolates varied considerably in their susceptibility to all the antimicrobials tested. The percentage of isolates resistant to ampicillin was 100%. However, 66% of the isolates showed resistance to both cotrimoxazole and tetracycline, and 50% exhibited resistance to ciprofloxacin. For the most part, all the isolates were susceptible to amoxicillin/clavulanic acid, cefotaxime and amikacin. In this study, we defined MDR as resistance to three or more groups of the antibiotics tested. A notable result obtained from the present study is that 50% (n = 3) of the isolates can be considered MDR.
Table 2.
Antibiotics susceptibility pattern of six Salmonella isolates
| Antimicrobial agent | Resistant | Intermediate | Susceptible |
|---|---|---|---|
| Ampicillin | 6 (100) | 0 | 0 |
| Amoxicillin/clavulanic acid | 0 | 0 | 6 (100) |
| Ceftriaxone | 2 (33) | 1 (16) | 3 (50) |
| Cefotaxime | 0 | 0 | 6 (100) |
| Gentamicin | 1 (16) | 2 (33) | 3 (50) |
| Amikacin | 0 | 0 | 6 (100) |
| Tetracycline | 4 (66) | 1 (16) | 1 (16) |
| Ciprofloxacin | 3 (50) | 1 (16) | 2 (33) |
| Chloramphenicol | 2 (33) | 2 (33) | 2 (33) |
| Nalidixic acid | 2 (33) | 1 (16) | 3 (50) |
| Cotrimoxazole | 4 (66) | 0 | 2 (33) |
Data are presented as n (%).
Discussion
Despite the control measures in place, the magnitude and impact of S. mansoni–Salmonella coinfections are gradually rising in Ethiopia. Our findings provide some baseline information on the individual prevalence of S. mansoni and Salmonella infections as well as the coinfection rates among patients attending Jinka General Hospital and the Jinka Millennium Health Center, Jinka, Ethiopia. It was found that the individual prevalence rates of S. mansoni and Salmonella and their coinfection rates differ only slightly as far as these two health institutions are concerned.
The rate of S. mansoni infection in the study area was 17.30% – a result similar to an earlier study conducted in the same locality with another study population [24]. Results of a previous meta-analysis showed that the pooled prevalence of S. mansoni among Ethiopians was 18.30%; also, it is region specific [24]. For instance, an earlier study conducted in southern Ethiopia had reported a higher regional prevalence of 33.60% [25]; this was comparatively much higher than our results. However, the prevalence rate we found is above the values reported from Nigeria (12%) [26] and Sudan (12%) [27]. These fluctuations could be connected to the method used for the diagnosis of S. mansoni, type of study participants and sample size. We have found that infection by S. mansoni had a statistical association with some variables. Male patients (n = 38) outnumbered female patients; this is in agreement with the findings of previous studies conducted in another region of Ethiopia [28] and also Sudan [27]. It was also found that a majority (n = 31) of positive cases occur in children aged 5 to 15; this finding is by and large consistent with the data in the existing literature [29,30]. A possible reason for the higher prevalence rate of S. mansoni in children might be attributed to their frequent recreational activities in muddy, contaminated and stagnant water bodies, which are laden with snails infected with S. mansoni. In addition, the rate of infection was pronounced among respondents who have frequent contact with contaminated water bodies; the extent is comparable to the results reported from other regions of Ethiopia [28,31]. Nevertheless, higher prevalence rates were observed among participants from rural areas without household latrines, although this result was not statistically significant.
The prevalence rates of Salmonella observed by Widal agglutination and stool culture techniques were 7.70% and 2.20% respectively. By extrapolation, the overall prevalence of Salmonella infection was 7.70%. These data are more or less similar to the previous findings of two independent studies from Ethiopia and Nigeria [29,30] but is much lower than the extent of prevalence described by some other authors in Ethiopia [32,33]. Nevertheless, the rate of Salmonella infection currently observed was relatively lower than S. mansoni infection, and almost all Salmonella-infected patients were positive for S. mansoni, considering both health institutions. Indeed, Salmonella infection is statistically significant in patients already infected with S. mansoni. It has been reported that patients with Schistosoma infection are more vulnerable to concurrent infection with Salmonella than patients without Schistosoma infection [34].
Our results indicate a higher coinfection rate compared to that reported by a series of studies performed in the Democratic Republic of Congo and Sudan (2.20%, 3.40%, 3.80%) [12,27,35]. In contrast, the currently observed rate of coinfection is lower than that reported from Nigeria [16], with the latter showing a prevalence of 8.80%. In addition, another study performed earlier in Sudan showed a higher prevalence rate of coinfection (22.60%) [20]. Furthermore, a study performed in Nigeria about coinfection among patients with typhoid fever reported a prevalence of 12.50% [26]. The comparatively lower rate of coinfection observed by us could be attributed to variations in sample sizes, sociodemographic characteristics, degree of sanitation and hygiene among the study populations as well as the nonuniformity in studies duration. However, further comprehensive evaluations are required to arrive at the exact reason.
Another important aspect of our study is our finding that the utilization of molecular diagnostic techniques (e.g. quantitative PCR) for quantification of the extent of infection by S. mansoni or and Salmonella always resulted in rapid detection and accurate estimation, providing exact and higher rate of prevalence compared to conventional microscopic and serologic methods [36,37]. These techniques, particularly quantitative PCR, are more sensitive and provide an accurate measurement of the intensity of infections, especially in large-scale epidemiologic surveys; they are also useful in monitoring therapeutic responses [36].
We found that factors that influence coinfection are sex, age and frequency of contact with contaminated water bodies. Widely differing percentages of S. mansoni–Salmonella coinfections were observed when comparing the number of cases by sex, with 17 male (10.30%) and four female (3.70%) subjects, which is similar to the results reported from Sudan [27]. The reason for this male preponderance could be attributed to a higher degree of exposure among men as a result of their occupational, domestic and leisure activities [27]. In contrast with our results, a study conducted in Nigeria showed that the extent of coinfection was higher among female subjects (28.60%) [16]. Also, it can be inferred that the age of participants can affect the prevalence and severity of S. mansoni–Salmonella coinfection. We found that the coinfection rate was higher among schoolchildren; these data are comparable to the results of several studies conducted earlier in Nigeria [16,30]. The infection rate was pronounced among study subjects frequently exposed to contaminated water bodies. It is a well-acknowledged fact that lack of hygiene, frequent recreational activities in muddy riverbanks and contaminated, stagnant water bodies make humans vulnerable to infections [18]. Prevention of infection is highly related to improved sanitation, including effective sewage treatment, as well as avoiding contact with contaminated water bodies.
For the effective treatment of enteric fever, understanding the drug susceptibility patterns of Salmonella spp. is essential. Development of MDR among Salmonella isolates is a major crisis which limits the drug of choice for the treatment of enteric fever. In the present study, a high degree of resistance was found, particularly against three antibiotics (i.e. ampicillin, tetracycline and cotrimoxazole). This finding is similar to a recent meta-analysis performed on the drug resistance patterns of Salmonella spp. from different regions of Ethiopia. All the Salmonella isolates showed resistance to ampicillin. This result is in line with earlier Ethiopian studies [38,39]. In addition, 66% Salmonella spp. isolates exhibited resistance to both cotrimoxazole and tetracycline. This is in line with results documented in earlier studies performed in various regions of the country [[38], [39], [40], [41]]. According to the WHO, MDR exhibited by species of Salmonella is currently a serious global concern. In the present study, 50% of the isolates were found to be MDR; we think that this is likely to be correlated with the long-term use of the above mentioned antibiotics. In addition, a notable result of the present study is that all the isolates showed 100% sensitivity to amoxicillin/clavulanic acid, cefotaxime and amikacin, indicating the possibility of using these drugs to manage enteric fever in Jinka.
Conclusions
This is the first report on the prevalence of S. mansoni–Salmonella coinfection among patients attending two health institutions in Jinka, Ethiopia. The individual prevalence rate of S. mansoni and Salmonella infections in the present study were 17.30% and 7.70% respectively. The overall prevalence of S. mansoni–Salmonella coinfection was found to be 7.70%. It was identified that all cases of Salmonella infection at both health institutions had S. mansoni coinfection. In addition, sex, age and frequency of contact with contaminated water bodies were identified as independent predictors of coinfection. Fifty percent of Salmonella isolates were found to be MDR. Overall results indicate that S. mansoni–Salmonella coinfection is a growing health problem in the study area, especially among children. Therefore, large-scale control programs such as screening, diagnosis, treatment and surveillance as well as improved sanitary and hygiene practices, along with provision for safe water sources, are essential in the study area. Further, an in-depth and broader approach to research on disease epidemiology is also warranted.
Shortcomings of the present work include the cross-sectional design with a relatively small sample size and short duration. In addition, in the clinical context, the results of the Widal test cannot be considered conclusive. Finally, for the antisera, species-level identification was not performed to differentiate among the Salmonella spp. isolates.
Acknowledgements
The authors thankfully acknowledge the Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University, for the facilities. We are also indebted to the staff of Jinka General Hospital and the Jinka Millennium Health Center as well as the study participants. Thanks are extended to K. R. Sabu for English-language editorial work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2021.100842.
Conflict of interest
None declared.
Credit author statement
| Conceptualization | Mohammed Seid, tsegaye yohanes and melat woldemariam |
|---|---|
| Methodology | Alebachew Marege, Bereket Boke, Samson Thomas, Muhammed Arage, Nebyt Mouze |
| Software | Mohammed Seid, Alebachew Marege |
| Validation | Mohammed Seid, Aseer Manilal |
| Formal analysis | Mohammed Seid, Alebachew Marege, Aseer Manilal |
| Investigation | Alebachew Marege, Bereket Boke, Samson Thomas, Muhammed Arage, Nebyt Mouze |
| Resources | Alebachew Marege, Bereket Boke, Samson Thomas, Muhammed Arage, Nebyt Mouze |
| Data Curation | Mohammed Seid, Alebachew Marege |
| Writing - Original Draft | Aseer Manilal, Mohammed Seid |
| Writing - Review & Editing | Aseer Manilal, Mohammed Seid |
| Visualization | Mohammed Seid, Aseer Manilal |
| Supervision | Mohammed Seid, Tsegaye Yohanes and Melat Woldemariam |
| Project administration | Mohammed Seid, Alebachew Marege |
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Adenowo A.F., Oyinloye B.E., Ogunyinka B.I., Kappo A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimes J.E.T., Croll D., Harrison W.E., Utzinger J., Freeman M.C., Templeton M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention The burden of schistosomiasis. 2011. http://www.cdc.gov/globalhealth/ntd/diseases/schisto_burden.html Available at:
- 4.Schistosomiasis Danso-Appiah T. Neglected tropical diseases: sub-Saharan Africa. In: Gyapong J., Boatin B., editors. Neglected tropical diseases: sub-Saharan Africa. Springer International; Amsterdam: 2016. pp. 253–254. [Google Scholar]
- 5.Chala B., Torben W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front Public Health. 2018;6(60) doi: 10.3389/fpubh.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chieffi P.P. The interrelationship between schistosomiasis and concomitant diseases. Mem Inst Oswaldo Cruz. 1992;87:291–296. doi: 10.1590/s0074-02761992000800045. [DOI] [PubMed] [Google Scholar]
- 7.Barsoum R.S. Urinary schistosomiasis: review. J Adv Res. 2013;4:453–459. doi: 10.1016/j.jare.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gal-Mor O., Boyle E.C., Grassl G.A. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Typhoid vaccines: weekly epidemiological record. Health section of the secretariat of the league of nations. 2008. https://www.who.int/wer/2008/wer8306.pdf 83:49–59. Available at:
- 10.Hsiao A., Toy T., Jin Seo H., Marks F. Interaction between Salmonella and schistosomiasis: a review. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud A.A.F. Schistosomiasis. In: Mahmoud A.A.F., editor. Tropical medicine: science and practice. Imperial College Press; London: 2001. [Google Scholar]
- 12.Mbuyi-Kalonji L., Barbé B., Nkoji G., Madinga J., Roucher C., Linsuke S. Non-typhoidal Salmonella intestinal carriage in a Schistosoma mansoni endemic community in a rural area of the Democratic Republic of Congo. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muniz-Junqueira M.I., Tosta C.E., Prata A. [Schistosoma-associated chronic septicemic salmonellosis: evolution of knowledge and immunopathogenic mechanisms] Rev Soc Bras Med Trop. 2009;42:436–445. doi: 10.1590/s0037-86822009000400015. Portuguese. [DOI] [PubMed] [Google Scholar]
- 14.Gendrel D., Kombila M., Beaudoin-Leblevec G., Richard-Lenoble D. Nontyphoidal Salmonella septicemia in Gabonese children infected with Schistosoma intercalatum. Clin Infect Dis. 1994;18:103–105. doi: 10.1093/clinids/18.1.103. [DOI] [PubMed] [Google Scholar]
- 15.Gendrel D., Richard-Lenoble D., Kombila M., Engohan E., Nardou M., Moussavou A. S. intercalatum and relapses of Salmonella infection in children. Am J Trop Med Hyg. 1984;33:1166–1169. doi: 10.4269/ajtmh.1984.33.1166. [DOI] [PubMed] [Google Scholar]
- 16.Modebe A., Nnachi N. Dual infections of enteric Salmonella species with S. mansoni among patients from two Hospitals in Jos, Nigeria. Appl Environ Microb. 2014;2:198–202. [Google Scholar]
- 17.Stothard J.R., Gabrielli A.F. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23:83–86. doi: 10.1016/j.pt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) WHO; Geneva: 2014. The control of schistosomiasis. Second report of a WHO Expert Committee. [Google Scholar]
- 19.Xu J., Yu Q., Tchuenté L.T., Bergquist R., Sacko M., Utzinger J. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 2016;16:376–383. doi: 10.1016/S1473-3099(15)00360-6. [DOI] [PubMed] [Google Scholar]
- 20.Elfaki T.E.M., Abdalla E.J. Association between intestinal schistosomiasis and enteric fever in New Halfa City, Sudan. Int J Novel Res Healthc Nurs. 2015;2:45–52. [Google Scholar]
- 21.Cheesbrough M. 2nd ed. Cambridge University Press; Cambridge: 2005. District laboratory practice in tropical countries. part 1. [Google Scholar]
- 22.Birhanie M., Tessema B., Ferede G., Endris M., Enawgaw B. Malaria, typhoid fever, and their co-infection among febrile patients at a rural health center in Northwest Ethiopia: a cross-sectional study. Adv Med. 2014;2014:531074. doi: 10.1155/2014/531074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margot H., Stephan R., O’Mahony E., Iversen C. Comparison of rapid cultural methods for the detection of Salmonella species. Int J Food Microbiol. 2013;163:47–50. doi: 10.1016/j.ijfoodmicro.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Girma M., Agedew E., Gedeamu G., Haftu D., Yohannes T. Prevalence of S. mansoni infection and associated factors in irrigation workers in Gamo Gofa and South Omo Zone, 2017: a community based parasitological survey. J Parasitol Vector Biol. 2018;10:121–128. [Google Scholar]
- 25.Hussen S., Assegu D., Shimelis T. 2019. Prevalence of S. mansoni infection in Ethiopia: a systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishaleku D., Umeh E.U., Amali O., Gberikon G.M. Enteric Salmonella species and intestinal helminthes co-infection among typhoid fever patients attending PHC clinics in the Northern Zone of Nasarawa State, Nigeria. Asian J Appl Sci. 2015;3:355–359. [Google Scholar]
- 27.Mohager M.O., Mohager S.O., Kaddam L.A. The association between schistosomiasis and enteric fever in a single Schistosoma endemic area in Sudan. Int J Pharm Sci Res. 2014;5:2181–2184. [Google Scholar]
- 28.Mengistu M., Shimelis T., Torben W., Terefe A., Kassa T., Hailu A. Human intestinal schistosomiasis in communities living near three rivers of jimma town, South Western Ethiopia. Ethiop J Health Sci. 2011;21:111–118. doi: 10.4314/ejhs.v21i2.69051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerfu B., Medhin G., Mamo G., Getahun G., Tschopp R., Legesse M. Community-based prevalence of typhoid fever, typhus, brucellosis and malaria among symptomatic individuals in Afar Region, Ethiopia. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lar P.M., Emojevwe M.E., Onah J.A. Incidence of mixed infections of Schistosoma and Salmonella the federal capital territory, Abudja. Afr J Nat Sci. 2006;2:19–24. [Google Scholar]
- 31.Tefera A., Belay T., Bajiro M. Epidemiology of S. mansoni infection and associated risk factors among school children attending primary schools nearby rivers in Jimma town, an urban setting, Southwest Ethiopia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasihun A.G., Wlekidan L.N., Gebremariam S.A., Welderufael A.L., Muthupandian S., Haile T.D. Diagnosis and treatment of typhoid fever and associated prevailing drug resistance in northern Ethiopia. Int J Infect Dis. 2015;35:96–102. doi: 10.1016/j.ijid.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Deksissa T., Gebremedhin E.Z. A cross-sectional study of enteric fever among febrile patients at Ambo hospital: prevalence, risk factors, comparison of Widal test and stool culture and antimicrobials susceptibility pattern of isolates. BMC Infect Dis. 2019;27:19. doi: 10.1186/s12879-019-3917-3. 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madbouly N.H., Mira A.A., Hassan E.M., Abdel Ghany S.M. Correlation between schistosomiasis and chronic salmonellosis. J Trop Med. 1993;2:57–62. [Google Scholar]
- 35.Salem A., Bilal N., Ibrahim M. Frequent carriage of invasive salmonellae amongst patients infected with schistosomiasis in Sudan. Afr J Microbiol Res. 2015;9:543–548. [Google Scholar]
- 36.Weerakoon K.G., Gordon C.A., McManus D.P. DNA Diagnostics for schistosomiasis control. Trop Med Infect Dis. 2018;3:81. doi: 10.3390/tropicalmed3030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heymans R., Vila A., van Heerwaarden C.A.M., Jansen C.C.C., Castelijn G.A.A., van der Voort M. Rapid detection and differentiation of Salmonella species, Salmonella typhimurium and Salmonella enteritidis by multiplex quantitative PCR. PLoS One. 2018;25:13. doi: 10.1371/journal.pone.0206316. e0206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garedew L., Solomon S., Worku Y., Worku H., Gemeda D., Lelissa G. Diagnosis and treatment of human salmonellosis in addis ababa city, Ethiopia. Biomed Res Int. 2018;6406405 doi: 10.1155/2018/6406405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tosisa W., Mihret A., Ararsa A., Eguale T., Abebe T. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Pediatr. 2020;20:91. doi: 10.1186/s12887-020-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awol N., Nigusse D., Ali M. Prevalence and antimicrobial susceptibility profile of Salmonella and Shigella among food handlers working in food establishment at Hawassa city, southern Ethiopia. BMC Res Notes. 2019;12:712. doi: 10.1186/s13104-019-4725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mengist A., Mengistu G., Reta A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. Int J Infect Dis. 2018;75:74–79. doi: 10.1016/j.ijid.2018.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.