Abstract
Cognitive impairments are a core feature of first-episode psychosis (FEP), arising before illness onset and antipsychotic exposure. Individuals with chronic psychosis experience poorer physical health while taking antipsychotic medication, but health disparities may be evident at FEP onset, prior to antipsychotic exposure. Given the links between cognition and physical health in healthy populations, the aim was to explore whether cognition and physical health are associated in FEP, which could inform early physical health interventions for cognition in FEP. Participants were aged 15 to 25 and included 86 individuals experiencing FEP with limited antipsychotic exposure and duration of untreated psychosis of ≤six months, and 43 age- and sex-matched controls. Individuals with FEP performed significantly poorer than controls in most cognitive domains (Cohen's d = 0.38 to 1.59). Groups were similar in metabolic health measures, excluding a significantly faster heart rate in FEP (d = 0.68). Through hierarchical regression analyses, we found that in the overall sample, BMI was negatively related to current IQ after controlling for education and group (FEP/control). Relationships between BMI and cognition were consistent across the FEP and healthy control groups. In FEP, current IQ and working memory were negatively correlated with lipid profiles. Findings suggest that in FEP, impaired cognition is exhibited earlier than physical health problems, and that compared to controls, similar relationships with cognition are demonstrated. Causal pathways and trajectories of relationships between health and cognition in FEP require investigation, especially as antipsychotic medications are introduced. The findings have implications for cognitive and health interventions.
Keywords: First episode psychosis, Cognition, Body mass index, Heart rate, Metabolic syndrome, Lipids
1. Introduction
Cognitive impairments across multiple domains are an early core feature of first-episode psychosis (FEP). The severity of impairments is comparable to that observed in chronic schizophrenia (Mesholam-Gately et al., 2009). Impairments are apparent regardless of exposure to antipsychotics, though antipsychotics introduce a potentially confounding factor (Ballesteros et al., 2018; Mesholam-Gately et al., 2009). Cognitive impairments generally remain stable despite symptomatic remission and significantly explain variance in functional outcomes (Sheffield et al., 2018). Despite group-level findings, significant heterogeneity in cognitive functioning in FEP is observed (Uren et al., 2017). Cognitive impairment in FEP is hypothesised as the outcome of abnormal neurodevelopment (Sheffield et al., 2018). Though, physical health may be a modifiable factor that increases risk for cognitive impairment, as there is evidence in healthy populations of negative relationships between body weight and cognitive function (Liang et al., 2014; Smith et al., 2011). It remains unclear whether this relationship applies to people with psychosis, who may be prone to socio-economic disadvantage or lifestyle factors that increase propensity for poor physical health.
It is recognised that people with chronic schizophrenia have several risk factors for cardiovascular disease, including dyslipidaemia, hypertension, impaired glucose tolerance/type 2 diabetes mellitus, non-alcoholic fatty liver disease and obesity (Gates et al., 2015; Xu and Zhuang, 2019). Many of these issues are associated with antipsychotic medication. Though, poor health may be observed among antipsychotic-naïve individuals with FEP due to other potential contributors including modifiable lifestyle factors (e.g., poor diet, sedentary lifestyle, smoking and substance use), common underlying biological features (e.g., altered neural reward systems and shared genetic variants with diabetes), and potentially, duration of untreated psychosis (DUP). A systematic review by Foley and Morley (2011) found that in FEP patients with minimal antipsychotic exposure, several health parameters did not differ from control samples. Subsequent studies have shown that compared to healthy controls, people with FEP have higher waist-to-hip ratios (Shah et al., 2019); higher proportions of overweight/obesity independent of medication (Kolenic et al., 2018); and higher levels of insulin resistance, total and low-density lipoprotein cholesterol, and triglycerides (Keinänen et al., 2018). Participant inclusion criteria varied across studies, especially antipsychotic exposure and participant ages (up to around 50 years; Strassnig et al., 2008), potentially leading to these discrepant results. Jensen et al. (2017) demonstrated that compared to healthy controls, young people (12–17 years) with FEP with little antipsychotic exposure displayed double the frequency of waist circumferences >90th percentile and had significantly higher total and low-density lipoprotein cholesterol. Few studies have analysed FEP samples who are younger, antipsychotic-naïve, and have relatively short DUP. These factors should be considered as older age and antipsychotic exposure are associated with worse physical health, while longer DUP could indirectly impact health via lifestyle factors.
Overall, the literature suggests that poorer physical health may emerge early in psychosis, representing one malleable factor potentially associated with cognitive impairment. These two previously independently studied features of psychosis warrant simultaneous investigation. They may have distinct aetiologies but could reinforce each other or be associated, where greater cognitive impairments are related to poorer health. This possibility has clinical relevance for monitoring physical health and delivering interventions in FEP. Otherwise healthy samples demonstrate relationships between cognitive performance, particularly cognitive flexibility, attention, memory, and current IQ, and aspects of physical health, including body mass index (BMI) and weight (Gray et al., 2020; Steenbergen and Colzato, 2017), metabolic syndrome (Batty et al., 2008; Yau et al., 2012), and diabetes (Han et al., 2013). Clinical studies have shown that in individuals with bipolar disorder, those with comorbid obesity displayed poorer sustained attention, processing speed, memory, and reasoning/problem-solving (Depp et al., 2014). In individuals with schizophrenia, comorbid metabolic syndrome was associated with more severe impairments in IQ, immediate and delayed memory, speed of processing, and vigilance (de Nijs and Pet, 2016), while comorbid diabetes was associated with lower overall cognition, especially in processing speed, visuospatial ability, and memory (Dickinson et al., 2008). We are aware of only one study that has examined relationships between cognition and physical health in FEP. Chen et al. (2016) found that individuals with FEP and comorbid impaired glucose tolerance did not have greater cognitive impairments compared to those with normal glucose tolerance. Sampling limitations, including participant ages ranging up to 45 years and long DUP (ranging up to 60 months), may have limited conclusions concerning physical health.
In the present study we analysed cognitive and physical health data simultaneously collected at baseline from healthy controls and a selective sample of young (15 to 25 years) antipsychotic-naïve individuals with FEP with short DUP (6 months or less; Cechnicki et al., 2014) who were enrolled in a clinical trial (see Method). The three aims were to compare the FEP and healthy control groups on (1) cognitive ability and (2) metabolic health, and (3) to explore relationships between cognition and BMI and whether associations differ according to FEP versus healthy control status. An exploratory aim was to investigate relationships between blood measures of metabolic health (glucose, triglycerides, cholesterol, high-density lipoprotein, and low-density lipoprotein) and cognition in the FEP group.
2. Method
2.1. Design, procedure, and participants
The study involved analysis of baseline data collected for a triple-blind randomised placebo-controlled trial named Staged Treatment and Acceptability Guidelines in Early Psychosis Study (STAGES; Francey et al., 2020; O'Donoghue et al., 2019). The trial, conducted within the Early Psychosis Prevention and Intervention Centre (EPPIC), a sub-program of Orygen Youth Health, Melbourne, Australia included baseline and follow-up assessments of cognition and physical health among other measures. Informed consent was gained from 129 participants (86 FEP and 43 healthy controls), aged 15 to 25 years. The FEP participants had a first-episode of any DSM-defined psychotic disorder (4th ed.; DSM-IV; American Psychiatric Association, 1994), and had at least one of the following positive symptoms measured by the expanded Brief Psychiatric Rating Scale version four (exBPRS; Ventura et al., 1993): Suspiciousness, Hallucinations, Unusual Thought Content, and Conceptual Disorganisation. Other eligibility criteria for FEP participants included relatively short DUP (≤6 months) and being antipsychotic-naïve. Additional criteria are outlined by O'Donoghue et al. (2019). Forty-three healthy controls with no history of mental disorder were recruited via flyers, snowballing, and social media advertisements to match FEP participants on age, sex, and socioeconomic status. The study received ethics approval in 2007 from Melbourne Health Human Research Ethics Committee (MHREC; HREC 2007.616).
2.2. Measures
2.2.1. Demographic and clinical characteristics
Demographic information was collected from all participants. The Social and Occupational Functioning Assessment Scale (SOFAS; Morosini et al., 2000) was used to assess functioning. The Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I; First et al., 1995) was used to determine psychotic and comorbid diagnoses in FEP participants, and screen healthy controls. All participants were administered the BPRS extended version (Ventura et al., 1993) to determine the severity/frequency of general psychiatric symptoms, as well as psychotic symptoms specifically via a subscale based on the aforementioned operational criteria for psychosis. Severity of negative symptomatology was assessed in FEP participants using the Scale for the Assessment of Negative Symptoms (SANS; Andreason, 1984).
2.2.2. Cognition
A battery of cognitive tests was administered to all participants. These included estimated premorbid and current IQ (evaluated respectively via the Wording Reading subtest of the WRAT-4; Wilkinson and Robertson, 2006, and a composite of Picture Completion and Information of the WAIS-III; Wechsler, 1997), visual perception and reasoning (Picture Completion: WAIS-III), acquired general knowledge (Information subtest of WAIS-III), attention and working memory (Digit Span subtest of WAIS-III), speed of processing (Digit Symbol – Coding of WAIS-III), inhibition (The Stroop Test Golden Version; Golden, 1978), verbal fluency (The Controlled Oral Word Association Test [COWAT]; Strauss et al., 2006), and immediate and delayed verbal memory (Paired Associate Learning Test [PAL]; Savage et al., 2002).
2.2.3. Physical Health
Non-invasive measures of physical health (including heart rate, blood pressure, weight, waist circumference, height, and BMI) were obtained from all participants. Blood levels of glucose (random if fasting was not available), triglycerides, cholesterol, high-density lipoprotein, and low-density lipoprotein were obtained from the FEP participants only. International Diabetes Foundation (2006) guidelines were used to create categorical variables for systolic and diastolic blood pressure, triglycerides, high-density lipoprotein, fasting plasma glucose, and waist circumference. For adult participants (≥18 years), BMI was categorised according to the National Health and Medical Research Council (2013). For participants <18 years, BMI was categorised into healthy weight, overweight, and obese, according to the participant's age and sex, using a conversion chart developed by Cole et al. (2000). The chart does not include cut-offs for underweight BMI, so the adult standard was used. Supplementary Table A.1 displays categorical variables.
2.3. Statistical analyses
Data were analysed using IBM® SPSS® Statistics Version 25 (IBM Corp, 2017). The dataset was examined for improbable values, normality, univariate outliers, and missingness. For aims 1 and 2, FEP participants were compared to healthy controls on all cognitive and physical health variables with independent samples t-tests if continuous and Chi-squared tests (χ2) if categorical. Mann-Whitney U tests were conducted for continuous variables where assumptions of normality were violated. Bonferroni corrections were applied to adjust for multiple comparisons. Cohen's d provided estimates of effect sizes, where d = 0.3 was defined as small, d = 0.5 as moderate, and d = 0.8 as a large effect (Cohen, 1988). Due to the small sample sizes in some BMI subcategories, the underweight category was removed and the overweight and obese categories were combined, resulting in comparisons between ‘healthy’ BMI versus ‘overweight/obese’ BMI.
Regarding aim 3, Spearman correlations were conducted as distributional assumptions were not met. All selected cognitive variables were significantly correlated with education (except attention/working memory) and FEP/control group membership. Thus, the two potential confounds (education and FEP/healthy control status) were included in four separate four-block hierarchical linear regressions predicting cognitive performance. The following cognitive variables were chosen as independent variables based on previous literature: current IQ (WAIS-III Estimated Full Scale IQ); working memory (WAIS-III Digit Span Backwards); speed of processing (WAIS-III Digit Symbol – Coding); and immediate verbal learning (PAL Total of Three Trials). The effects of years of education and psychosis status (FEP versus healthy control) were controlled for in Blocks 1 and 2, respectively. BMI was entered in Block 3 to examine whether it predicted cognition in the overall sample. Block 4 included an interaction term between BMI and Group (FEP/control) to explore whether relationships differed according to group.
For the exploratory aim, as distributional assumptions were not met, Spearman rank-order correlations explored relationships between blood measures and cognitive performance in FEP.
3. Results
3.1. Participant inclusion, demographic and clinical information
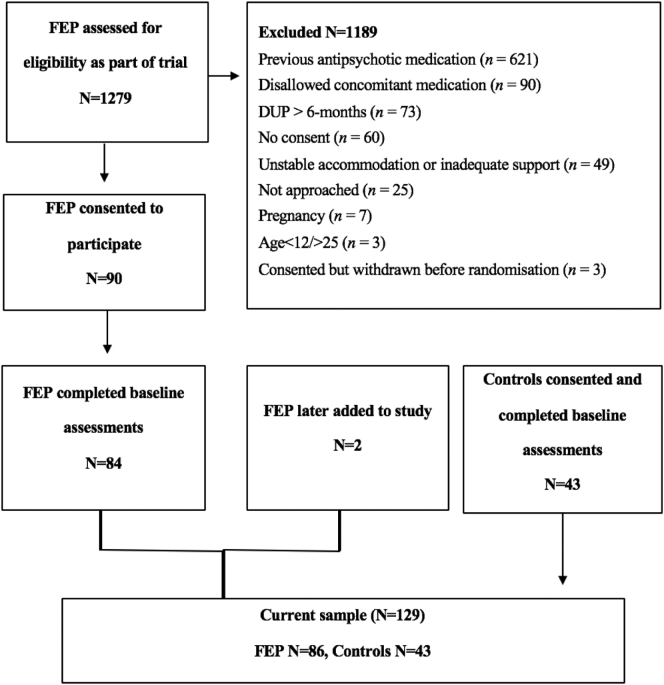
Fig. 1 presents the flow of the 86 FEP participants into the current study (mean age = 18.5 years, female = 55.3%) and 43 healthy controls (mean age = 19.2 years, female = 67.4%), resulting in a total sample of 129 participants. Table 1 displays the sample characteristics and group comparisons. The groups were well-matched on age, sex, and race, although FEP participants had significantly fewer average years of education. The average SOFAS score in FEP participants indicated moderate difficulty in functioning. Psychotic disorders in the sample with FEP included: psychosis not otherwise specified (n = 20; 23.5%), schizophreniform disorder (n = 18; 21.2%), schizophrenia (n = 12; 14.1%), major depressive disorder with psychotic features (n = 16; 18.8%), delusional disorder (n = 5; 5.9%), substance-induced psychosis (n = 13; 15.3%) and schizoaffective disorder (n = 1; 1.2%).
Fig. 1.
Flow of participants into the current study.
Table 1.
Demographic information and comparisons between FEP participants and healthy controls.
| Characteristic | Statistic | Total (N = 129) |
FEP (n = 86) |
Controls (n = 43) |
Statistic | Value | df | p |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age | M (SD) | 18.7 (2.9) | 18.5 (2.8) | 19.2 (3.0) | t | 1.29 | 125 | 0.200 |
| Sex | ||||||||
| Males | % (N) | 40.6 (52) | 44.7 (38) | 32.6 (14) | χ2 | 1.75 | 1 | 0.186 |
| Females | % (N) | 59.4 (76) | 55.3 (47) | 67.4 (29) | ||||
| Ethnicity | ||||||||
| Caucasian | % (N) | 82.8 (106) | 84.7 (72) | 79.1 (34) | χ2 | 0.64 | 3 | 0.425 |
| Other | % (N) | 17. 2 (22) | 15. 3 (13) | 20.9 (9) | ||||
| Education | ||||||||
| Total number of years of education/training | M (SD) | 12.6 (2.2) | 12.2 (2.1) | 13.3 (2.2) | t | 2.85 | 116 | .009a |
| Symptomatology | ||||||||
| BPRS total score | M (SD) | 49.2 (14.9) | 58.2 (9.4) | 31.6 (3.6) | t | 23.00 | 117.98 | <.001a |
| BPRS psychotic subscale | M (SD) | 11.1 (5.8) | 14.7 (3.4) | 4.1 (0.3) | t | 28.30 | 85.40 | <.001a |
| SANS total scoreb | M (SD) | 35.8 (18.3) | ||||||
| Functioning | ||||||||
| SOFAS | M (SD) | 63.2 (16.7) | 54.3 (11.9) | 80.7 (9.0) | t | 14.02 | 107.75 | <.001a |
Notes. Ns vary due to missing data. N = Valid number of participants; % = Valid percent of participants, M = Mean; SD; Standard deviation.
BPRS = Brief Psychiatric Rating Scale; SOFAS = Social and Occupational Functioning Assessment Scale. Average scores of BPRS Total and BPRS Psychotic among controls indicate few to no clinical symptoms. Overall sample SOFAS score within range of 61–70, indicating some difficulty in functioning but generally functioning well. Sample with FEP in range of 51–60, indicating moderate difficulty in functioning; and control sample in range of 81–90 (when rounded to nearest whole number), indicating good functioning in all areas.
Independent Samples Mann-Whitney U test (non-parametric) p values for variables not meeting normality assumptions of parametric t-test.
SANS (Scale for the Assessment of Negative Symptoms) was not administered to healthy controls.
3.2. Cognition
Healthy controls significantly outperformed the FEP group on most cognitive domains assessed (Table 2). Effect sizes were large, especially in acquired general knowledge (d = 1.59), current and premorbid IQ (d = 1.36 and 0.88), immediate verbal learning and memory (d = 0.92 and 0.80) and delayed verbal memory (d = 0.97), working memory (d = 0.79), and speed of processing (d = 0.77). After Bonferroni corrections, the differences in visual perception and reasoning, and attention span were no longer significant.
Table 2.
Cognitive performance in the overall sample and differences between FEP participants and healthy controls.
| Test | Subtest | Domain |
M (SD) |
t | df | p | Effect size estimate | ||
|---|---|---|---|---|---|---|---|---|---|
| Total (N = 129) |
FEP (n = 86) |
Controls (n = 43) |
|||||||
| WRAT-4 WAIS-III |
Word Readinga | Premorbid IQ | 101.6 (15.4) | 97.2 (14.5) | 109.6 (13.6) | 4.55 | 116 | <0.001 | 0.88 |
| Full Scale IQa | Current IQ | 97.3 (14.6) | 91.5 (13.5) | 107.6 (10.0) | 7.36 | 106.58c | <.001b | 1.36 | |
| Picture Completiona | Visual perception and reasoning | 9.1 (2.7) | 8.7 (2.7) | 9.9 (2.4) | 2.55 | 118 | .010b | 0.50 | |
| Informationa | Acquired general knowledge | 10.1 (3.3) | 8.7 (3.0) | 12.6 (2.0) | 8.84 | 112.83c | <.001b | 1.59 | |
| Digit Span Forward | Attention span | 9.7 (2.0) | 9.4 (2.0) | 10.1 (1.9) | 1.97 | 117 | .043b | 0.38 | |
| Digit Span Backwards | Working memory | 6.8 (2.1) | 6.3 (2.0) | 7.8 (1.9) | 4.13 | 117 | <.001b | 0.79 | |
| Digit Symbol – Codinga | Speed of processing | 9.0 (3.1) | 8.1 (2.8) | 10.3 (3.1) | 3.90 | 104 | <0.001 | 0.77 | |
| Stroop | Stroop Colour-Word | Inhibition | 45.6 (12.1) | 43.0 (12.0) | 50.1 (11.0) | 3.17 | 113 | 0.002 | 0.62 |
| COWAT | Phonemic Total | Verbal fluency | 36.5 (11.7) | 33.8 (11.0) | 41.4 (11.5) | 3.51 | 116 | <.001b | 0.67 |
| Semantic Total | Verbal fluency | 21.6 (5.8) | 20.5 (6.3) | 23.6 (4.1) | 3.17 | 112.44c | 0.002 | 0.58 | |
| PAL | First Trial | Immediate verbal memory | 3.1 (2.3) | 2.5 (2.1) | 4.2 (2.2) | 4.22 | 117 | <.001b | 0.80 |
| Total of Three Trials | Immediate verbal learning | 14.2 (6.0) | 12.5 (5.8) | 17.5 (5.1) | 4.72 | 117 | <.001b | 0.92 | |
| Delayed Recall | Delayed verbal memory | 5.7 (2.1) | 5.1 (2.0) | 6.9 (1.6) | 5.02 | 100.70c | <.001b | 0.97 |
Notes. WRAT = Wide Range Achievement Test; WAIS = Wechsler Adult Intelligence Scale; COWAT = Controlled Oral Word Association Test; PAL = Paired Associate Learning Test. Bonferroni Correction = 0.05/13 = 0.0038. p values that remained significant after Bonferroni corrections are bolded. Effect size estimate = Cohen's d.
Refers to tests using scores scaled according to participants' age (for Estimated Premorbid IQ and Estimated Full Scale IQ: population M = 100, and SD = 15; for Picture Completion, Information, and Digit Symbol – Coding: population M = 10, and SD = 3).
Independent Samples Mann-Whitney U test (non-parametric) p values for variables not meeting normality assumptions of parametric t-test.
Equal variances not assumed.
3.3. Physical health
Physical health did not differ between the FEP group and controls (Table 3), with one exception. A significantly faster resting heart rate was observed among participants with FEP (mean heart rate = 78.8 in FEP compared to 70.7 in controls), with a moderate effect size (d = 0.68).
Table 3.
Overall sample physical health and differences between FEP participants and healthy controls.
| Measure | Statistic | Total (N = 129) |
FEP (N = 86) |
Controls (N = 43) |
Statistic | Value | df | p | Effect size estimate |
|---|---|---|---|---|---|---|---|---|---|
| Resting heart rate | M (SD) | 75.1 (12.4) | 78.8 (11.4) | 70.7 (12.4) | t | 3.25 | 91 | 0.002 | 0.68 |
| Systolic blood pressure | M (SD) | 117.9 (13.4) | 119.8 (14.4) | 114.8 (11.1) | t | 1.93 | 107 | 0.056 | 0.39 |
| Raised systolic blood pressure | % (N) | 22.0 (24) | 26.9 (18) | 14.3 (6) | χ2 | 2.38 | 1 | 0.123 | |
| Diastolic blood pressure | M (SD) | 75.7 (9.6) | 76.5 (10.1) | 74.5 (8.6) | t | 1.10 | 107 | 0.275 | 0.22 |
| Raised diastolic blood pressure | % (N) | 18.3 (20) | 22.4 (15) | 11.9 (5) | χ2 | 1.89 | 1 | 0.169 | |
| Weight | M (SD) | 70.3 (16.2) | 71.3 (17.3) | 68.6 (14.3) | t | 0.89 | 112 | .535b | 0.18 |
| Waist circumference | M (SD) | 81.5 (11.5) | 82.6 (12.3) | 80.5 (10.7) | t | 0.82 | 79 | .702b | 0.18 |
| Dysregulated waist circumference | % (N) | 35.8 (29) | 33.3 (13) | 38.1 (16) | χ2 | 0.20 | 1 | 0.655 | |
| Height | M (SD) | 171.8 (9.2) | 171.7 (9.4) | 172.0 (9.0) | t | 0.13 | 107 | 0.895 | 0.03 |
| BMI | M (SD) | 24.0 (5.1) | 24.5 (5.6) | 23.1 (4.1) | t | 1.38 | 107 | .356b | 0.28 |
| BMI categorya | |||||||||
| Healthy weight | % (N) | 66.3 (69) | 60.9 (39) | 75.0 (30) | χ2 | 2.18 | 1 | 0.140 | |
| Overweight or obese | % (N) | 33.7 (35) | 39.1 (25) | 25.0 (10) | |||||
Notes. % = Valid percent of participants.
Bonferroni Correction = 0.05/12 = 0.0042. p values that remained significant after Bonferroni corrections are bolded. Effect size estimate = Cohen's d.
Dysregulated Waist Circumference = Circumference greater or equal to cut offs (International Diabetes Foundation, 2006); Raised Systolic Blood Pressure ≥ 130 mmHg; Raised Diastolic Blood Pressure ≥ 85 mmHg (International Diabetes Foundation, 2006).
Underweight category was excluded due to inadequate sample size.
Independent Samples Mann-Whitney U test (non-parametric) p values for variables not meeting normality assumptions of parametric t-test.
3.4. Cognition and physical health
3.4.1. Regressions predicting cognitive performance from BMI
Table 4 displays the four hierarchical linear regression analyses. Regressions revealed that education significantly predicted processing speed (at Block 3: β = 0.21, p = .028), but no other cognitive variable. The greatest improvements in R2 across all four models occurred at Block 2 when accounting for FEP/control membership, with FEP membership being a consistent significant negative predictor of cognitive functioning. BMI was significantly negatively related to current IQ (β = −0.17, p = .045), uniquely accounting for 2% of variation. The change in F ratio when including BMI was 4.13, p = .045, indicating significant model improvement. At Block 3, the model accounted for 33% of variance in current IQ, constituting the strongest model according to R2 values. BMI did not significantly predict any other cognitive variable. The interaction term between BMI and FEP/healthy control status was tested in all models at Block 4 but was not significant, suggesting that the association between BMI and IQ did not differ according to group membership.
Table 4.
Hierarchical regressions predicting current IQ, working memory, processing speed, and verbal learning and memory in the overall sample.
| Outcome variable | Block | Predictor variables | b | SE b | β | sr2 | p | R | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Current IQ | Block 1 | Constant | 74.30 | 8.07 | <0.001 | 0.28 | 0.08 | 0.07 | ||
| Years of Education/ Training | 1.82 | 0.63 | 0.28 | 0.28 | 0.005 | |||||
| Block 2 | Constant | 95.06 | 7.90 | <0.001 | 0.55 | 0.31 | 0.29 | |||
| Years of Education/ Training | 0.98 | 0.57 | 0.15 | 0.14 | 0.087 | |||||
| FEP Membership | −15.26 | 2.64 | −0.50 | −0.48 | <0.001 | |||||
| Block 3 | Constant | 105.01 | 9.19 | <0.001 | 0.58 | 0.33 | 0.31 | |||
| Years of Education/ Training | 1.07 | 0.56 | 0.16 | 0.16 | 0.060 | |||||
| FEP Membership | −14.47 | 2.63 | −0.47 | −0.45 | <0.001 | |||||
| BMI | −0.48 | 0.24 | −0.17 | −0.17 | 0.045 | |||||
| Working memory | Block 1 | Constant | 4.89 | 1.18 | <0.001 | 0.16 | 0.03 | 0.02 | ||
| Years of Education/ Training | 0.15 | 0.09 | 0.16 | 0.16 | 0.099 | |||||
| Block 2 | Constant | 6.91 | 1.26 | <0.001 | 0.37 | 0.13 | 0.12 | |||
| Years of Education/ Training | 0.07 | 0.09 | 0.08 | 0.07 | 0.427 | |||||
| FEP Membership | −1.48 | 0.42 | −0.34 | −0.33 | 0.001 | |||||
| Block 3 | Constant | 7.56 | 1.49 | <0.001 | 0.37 | 0.14 | 0.11 | |||
| Years of Education/ Training | 0.08 | 0.09 | 0.08 | 0.08 | 0.394 | |||||
| FEP Membership | −1.43 | 0.43 | −0.33 | −0.31 | 0.001 | |||||
| BMI | −0.03 | 0.04 | −0.08 | −0.08 | 0.418 | |||||
| Processing speed | Block 1 | Constant | 3.65 | 1.74 | 0.038 | 0.30 | 0.09 | 0.08 | ||
| Years of Education/ Training | 0.42 | 0.14 | 0.30 | 0.30 | 0.003 | |||||
| Block 2 | Constant | 6.30 | 1.87 | 0.001 | 0.42 | 0.18 | 0.16 | |||
| Years of Education/ Training | 0.31 | 0.13 | 0.23 | 0.22 | 0.021 | |||||
| FEP Membership | −1.94 | 0.63 | −0.30 | −0.29 | 0.002 | |||||
| Block 3 | Constant | 4.45 | 2.19 | 0.045 | 0.44 | 0.20 | 0.17 | |||
| Years of Education/ Training | 0.30 | 0.13 | 0.21 | 0.21 | 0.028 | |||||
| FEP Membership | −2.09 | 0.63 | −0.32 | −0.31 | 0.001 | |||||
| BMI | 0.09 | 0.06 | 0.15 | 0.15 | 0.116 | |||||
| Verbal learning and memory | Block 1 | Constant | 6.28 | 3.38 | 0.066 | 0.23 | 0.05 | 0.04 | ||
| Years of Education/ Training | 0.63 | 0.26 | 0.23 | 0.23 | 0.019 | |||||
| Block 2 | Constant | 12.60 | 3.55 | 0.001 | 0.42 | 0.18 | 0.16 | |||
| Years of Education/ Training | 0.38 | 0.26 | 0.14 | 0.13 | 0.146 | |||||
| FEP Membership | −4.65 | 1.19 | −0.37 | −0.35 | <0.001 | |||||
| Block 3 | Constant | 14.12 | 4.21 | 0.001 | 0.43 | 0.18 | 0.16 | |||
| Years of Education/ Training | 0.39 | 0.26 | 0.14 | 0.14 | 0.135 | |||||
| FEP Membership | −4.53 | 1.21 | −0.36 | −0.34 | <0.001 | |||||
| BMI | −0.07 | 0.11 | −0.06 | −0.06 | 0.501 |
Note. Block 4 not displayed here due to non-significance of interaction between BMI and FEP/healthy control group membership.
3.4.2. Correlations between blood measures of health and cognition in FEP
Spearman rank order correlations (Supplementary Table A.2) showed that current IQ was significantly negatively correlated with triglycerides, rs = −0.42, while working memory was significantly negatively correlated with total cholesterol, rs = −0.31, and low-density lipoprotein, rs = −0.40. No other significant relationships were found.
4. Discussion
This study sought to compare the cognitive performance and metabolic health of healthy controls with young individuals with FEP who had minimal exposure to antipsychotic medication and short DUP. The study contributes to the limited literature by exploring relationships between cognition and physical health and investigating whether these relationships differ across these two populations. The key findings were that the FEP group had significantly poorer cognitive functioning across most domains but similar physical health to the control group, apart from a higher resting heart rate. After accounting for education and group membership, BMI significantly explained variance in IQ in the total sample. There was no significant interaction between BMI and FEP/control membership in predicting any cognitive variable. In the FEP group, current IQ was significantly negatively correlated with triglycerides, and working memory was significantly negatively correlated with total cholesterol and low-density lipoprotein.
4.1. Cognition
As expected, most cognitive domains were moderately to largely impaired in FEP versus controls, particularly in current IQ, acquired general knowledge, immediate and delayed verbal memory, working memory and speed of processing. The current sample with FEP had significantly fewer years of education than healthy controls, possibly owing to disruptions in study and training during adolescence and young adulthood either related to neurodevelopmental factors or the incipient development of psychosis (Davis et al., 2016), thus possibly contributing to their poorer general knowledge. Meta-analyses involving people with first-episode schizophrenia (Fatouros-Bergman et al., 2014; Mesholam-Gately et al., 2009) demonstrated comparatively larger effect sizes, especially in the areas of processing speed (standardised mean differences found by the two meta-analyses were 0.96 and 1.03), verbal memory and verbal fluency (1.20 for immediate and 0.85 for delayed according to Mesholam-Gately et al. (2009); 1.03 according to Fatouros-Bergman et al. (2014)), and working memory (0.79 and 0.97, respectively). The relatively smaller (albeit significant) effect sizes in the current study could stem from the stringent inclusion and exclusion criteria for the trial (O'Donoghue et al., 2019). Namely, current participants had to be living in stable accommodation, be at low risk of aggression, and they represented all psychotic disorders, which may have conferred better cognitive performance (Ahmed et al., 2018; Shamsi et al., 2011; Sheffield et al., 2018). Nonetheless, the current participants with short DUP and antipsychotic-naïve FEP demonstrated widespread large cognitive impairments, lending support to the neurodevelopmental model of schizophrenia (Bora, 2015).
4.2. Physical health
The current study addressed previous limitations in sampling characteristics which could influence physical health, such as antipsychotic exposure and older age (Foley and Morley, 2011; Jensen et al., 2017). Contrary to expectations, physical health did not differ between the FEP and control groups, apart from a significantly higher resting heart rate in FEP. Contrary to Jensen et al. (2017) who found a higher proportion of waist circumferences >90th percentile among individuals with FEP, in the present study there were no significant differences in BMI or waist circumference according to proportions or averages. The discrepant heart rate in FEP is consistent with evidence that increased heart rate in late adolescence is associated with risk for subsequent schizophrenia (Latvala et al., 2016). Increased heart rate may also be influenced by a range of hypothetical factors which are typical in populations with psychosis (Foley and Morley, 2011) and may impact heart rate (British Heart Foundation, 2013; Townsend et al., 2007; Valentini and Parati, 2009). These factors include autonomic dysfunction (Hattori et al., 2018), smoking and other substance use, other medications, more sedentary lifestyles, and greater levels of anxiety and stress. Modifiable lifestyle factors may impact heart rate at an earlier stage of psychosis, but could have a longer-term cumulative influence on other aspects of health. The aforementioned lifestyle factors were not explored in depth as determinants of physical health in FEP, constituting an avenue for longitudinal research and a target for interventions in FEP (British Heart Foundation, 2013). The results of otherwise comparable health among individuals with FEP suggest that metabolic abnormalities in chronic populations had not yet emerged in the antipsychotic-naïve FEP stage. Poorer health in chronic samples may result from antipsychotic exposure and longer-term cumulative effects of adverse lifestyle factors (Heald et al., 2017; Osborn, 2001). The present findings suggest an opportunity for intervention in FEP before physical health declines, such as education regarding the potential impacts of modifiable lifestyle factors, programs encouraging healthier choices, non-pharmacological treatments, and metabolically favourable antipsychotics paired with careful monitoring of health (Curtis et al., 2016; Firth et al., 2019).
4.3. Cognition and physical health
FEP significantly negatively predicted cognition in all models, while fewer years of education predicted slower processing speed, but no other cognitive domain. Consistent with Batty et al. (2008) and de Nijs and Pet (2016), higher BMI was associated with lower current IQ, above the effects of education and FEP status. However, BMI was not a significant predictor of the remaining cognitive domains in the overall sample, contrary to Gray et al. (2020) and Steenbergen and Colzato (2017). Participants with FEP showed similar relationships between BMI and cognition to the healthy sample.
Exploratory analyses found a significant negative correlation between current IQ and triglycerides. Poorer working memory was correlated with higher total cholesterol and low-density lipoprotein cholesterol. Notably, these latter two health variables were highly correlated (rs = 0.93). Nevertheless, the findings suggest that blood lipid profiles are relevant for cognitive performance in FEP. Consistent with Chen et al. (2016), glucose was not significantly associated with cognitive performance. Studies investigating individuals with chronic schizophrenia and comorbid diabetes have found exacerbated cognitive impairments in attention, memory (Han et al., 2013), processing speed, and visuospatial ability (Dickinson et al., 2008). In the later stages of psychosis, glucose dysregulation may be more advanced than in the current sample; the relationship between glucose and cognition may strengthen with chronicity or severity. This possibility could be investigated longitudinally in future work.
Given the considerable cognitive impairments, yet little evidence for poorer physical health in FEP, the findings suggest a step-wise or staged onset of cognitive and physical impairments, with the former emerging earlier. While this suggests that cognitive and physical health difficulties may arise largely through differing aetiological mechanisms, our findings also suggest a role of physical health (BMI, triglycerides, cholesterol) in cognitive performance (or vice versa). Poor diet and obesity is associated with cognitive dysfunction in preclinical and clinical studies (Francis and Stevenson, 2013; Greenwood and Winocur, 2005; Smith et al., 2011). Psychosis may lead to decreased motivation and functioning, possibly influencing cognition and physical health. Negative associations between cognition and BMI and lipid parameters could also arise from the cognitive capacity to make healthier lifestyle choices. Healthy individuals demonstrate associations between psychosocial stress and cognitive impairments; stress additionally constitutes a potential risk factor for abdominal fat accumulation, metabolic syndrome, and obesity (Dye et al., 2017). Higher cognitive performance could lead to well-paying careers, allowing disposable income to afford a healthy diet, access to physical activity, and access to quality health education and services. Conversely, physical activity is protective against cognitive decline, with the best evidence in the elderly (Blondell et al., 2014). Presumably, multiple factors and pathways are implicated in bidirectional relationships between cognition and health in FEP. de Nijs and Pet (2016) theorised a reinforcing cycle in psychosis whereby poorer cognitive functioning may increase unhealthy behaviours, leading to altered metabolism, which circularly impacts cognition. The introduction of antipsychotics could contribute to this cycle by worsening physical health, which may interact with cognitive impairments. Further investigation is necessary regarding mechanistic associations between cognition and physical health in a larger sample of FEP. Longitudinal research should investigate causal pathways between cognition and health in psychosis, external factors and the introduction of antipsychotics, and trajectories over time.
The current study indicates that close monitoring and interventions for cognitive and physical health from the onset of psychosis treatment is likely beneficial. Indeed, studies have found improved cognition following exercise. Hallgren et al. (2019) found improved processing speed, visual learning, and visual attention after a 12-week exercise program for young adults with FEP. Few studies have combined cognitive training with exercise and diet modification. Though in first-episode schizophrenia patients, McEwen et al. (2017) found improved functional connectivity associated with gains in reasoning/problem-solving, above the effects of exercise alone. Physical health and cognitive impairments could be considered as co-primary outcomes following interventions in FEP.
4.4. Strengths and limitations
The strengths of the current study included addressing previous sampling limitations and our relatively novel assessment of relationships between cognition and a range of discrete components of metabolic health in FEP. Nevertheless, the cross-sectional design limits the extent that causal relationships between cognition and physical health can be determined. Future studies should collect invasive health data (e.g., glucose, lipids) from controls, as this limited the extent that potential abnormalities in blood measures of metabolic health could be investigated in FEP. Lastly, due to our relatively small sample and the number of variables under investigation, we did not explore additional potential confounding factors of cognitive and physical health, including cigarette smoking, alcohol and other substance use (Foley and Morley, 2011; Stramecki et al., 2018); stress and trauma (Mayo et al., 2017); sleep (Keshavan et al., 2011); and negative symptom severity (Orešič, 2012; Suvisaari et al., 2018), which should be investigated in future longitudinal research.
4.5. Conclusions
This study contributes to scholarship by examining relationships between cognition and physical health in FEP, using a selective sample of young antipsychotic-naïve participants with short DUP. Participants with FEP demonstrated cognitive impairments in most domains, although physical health was comparable to healthy controls, bar a faster heart rate. BMI predicted current IQ, but no other cognitive variable in the overall sample. Relationships between cognition and BMI did not differ according to FEP/healthy control group status. The early emergence of cognitive impairments in FEP implies a neurodevelopmental origin. Limited physical health disturbances prior to antipsychotic exposure in the current sample with FEP suggest that poor physical health demonstrated in previous samples of FEP is likely influenced by longitudinal cumulative effects of treatment, illness (e.g., cognition), and lifestyle factors). These findings advance the limited literature investigating modifiable factors associated with cognitive impairments in FEP, with implications for possible mutual benefits of cognitive and health interventions. Avenues for future research include longitudinal examination of the pathways, additional mechanisms, and confounding factors underlying relationships between cognition and health in FEP.
CRediT authorship contribution statement
KA, SW, BO and RH conceived the study. SW and SH conducted the statistical analysis. SW wrote the first draft of the manuscript. KA wrote parts of the second draft of the manuscript. LB, JG and MK conducted data collection, data scoring, cleaning and entry. All authors contributed to the final draft of the manuscript and approved the final version submitted for publication.
Declaration of competing interest
MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier – all unrelated to this work.
In the past 3 years, CP served on an advisory board for Lundbeck, Australia Pty Ltd. He has received honoraria for talks presented at educational meetings organized by Lundbeck. He received a grant from Lundbeck Foundation. His work is funded by grants and fellowship from the National Health & Medical Research Council (NHMRC) of Australia.
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Acknowledgements
The study was supported by the National Health and Medical Research Council (NHMRC) (1064704). KA is supported by a NHMRC Career Development Fellowship (1141207). MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). BN is supported by an NHMRC Senior Research Fellowship (1137687). CP was supported by a NHMRC Senior Principal Research Fellowship (1105825).
This study has been supported by a large number of clinical staff at Orygen Youth Health: Craig Macneil, Kingsley Crisp, Dylan Alexander, Tina Proffitt, Rachel Tindall, Jennifer Hall, Lisa Rumney, Franco Scalzo, Melissa Pane, Linda Kader, Frank Hughes, Clare Shelton, Ryan Kaplan, David Hallford, Bridget Moller, Rick Fraser, and research assistants: Daniela Cagliarini, Suzanne Wiltink, Janine Ward, and Sumudu Mallawaarachichi.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2021.100194.
Appendix A. Supplementary data
Supplementary tables
References
- Ahmed A.O., Richardson J., Buckner A., Romanoff S., Feder M., Oragunye N., Ilnicki A., Bhat I., Hoptman M.J., Lindenmayer J.P. Do cognitive deficits predict negative emotionality and aggression in schizophrenia? Psychiatry Res. 2018;259:350–357. doi: 10.1016/j.psychres.2017.11.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th ed. Author; Washington D. C: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andreason N.C. University of Iowa; Iowa: 1984. The Scale for the Assessment of Negative Symptoms. [Google Scholar]
- Ballesteros A., Sánchez-Torres A.M., López-Ilundain J.M., Cabrera B., Lobo A., González-Pinto A.M., Díaz-Caneja C., Corripio I., Vieta E., de la Serna E. Is cognitive impairment associated with antipsychotic dose and anticholinergic equivalent loads in first-episode psychosis? Psychol. Med. 2018;48(13):2247–2256. doi: 10.1017/S0033291717003774. [DOI] [PubMed] [Google Scholar]
- Batty G.D., Gale C.R., Mortensen L.H., Langenberg C., Shipley M.J., Deary I.J. Pre-morbid intelligence, the metabolic syndrome and mortality: the Vietnam experience study. Diabetologia. 2008;51(3):436–443. doi: 10.1007/s00125-007-0908-5. [DOI] [PubMed] [Google Scholar]
- Blondell S.J., Hammersley-Mather R., Veerman J.L. Does physical activity prevent cognitive decline and dementia?: a systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14(1):510–521. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol. Med. 2015;45(1):1–9. doi: 10.1017/S0033291714001263. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation . BHF; UK: 2013. Heart Rythyms. [Google Scholar]
- Cechnicki A., Cichocki Ł., Kalisz A., Błądziński P., Adamczyk P., Franczyk-Glita J. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420–425. doi: 10.1016/j.psychres.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Chen D., Du X., Yin G., Yang K., Nie Y., Wang N., Li Y., Xiu M., He S., Yang F., Cho R., Kosten T., Soares J., Zhao J., Zhang X. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia: relationships with clinical phenotypes and cognitive deficits. Psychol. Med. 2016;46(15):3219–3230. doi: 10.1017/S0033291716001902. [DOI] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Lawrence Earlbaum Assciates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioural Sciences. [Google Scholar]
- Cole T.J., Bellizzi M.C., Flegal K.M., Dietz W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clinical research ed.) 2000;320(7244):1240–1245. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Watkins A., Rosenbaum S., Teasdale S., Kalucy M., Samaras K., Ward P.B. Evaluating an individualized lifestyle and life skills intervention to prevent antipsychotic-induced weight gain in first-episode psychosis. Early Intervention in Psychiatry. 2016;10(3):267–276. doi: 10.1111/eip.12230. [DOI] [PubMed] [Google Scholar]
- Davis J., Eyre H., Jacka F.N., Dodd S., Dean O., McEwen S., Debnath M., McGrath J., Maes M., Amminger P., McGorry P.D., Pantelis C., Berk M. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci. Biobehav. Rev. 2016;65:185–194. doi: 10.1016/j.neubiorev.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nijs J., Pet M.A. Metabolic syndrome in schizophrenia patients associated with poor premorbid school performance in early adolescence. Acta Psychiatr. Scand. 2016;133(4):289–297. doi: 10.1111/acps.12528. [DOI] [PubMed] [Google Scholar]
- Depp C.A., Strassnig M., Mausbach B.T., Bowie C.R., Wolyniec P., Thornquist M.H., Luke J.R., McGrath J.A., Pulver A.E., Patterson T.L., Harvey P.D. Association of obesity and treated hypertension and diabetes with cognitive ability in bipolar disorder and schizophrenia. Bipolar Disord. 2014;16(4):422–431. doi: 10.1111/bdi.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D., Gold J.M., Dickerson F.B., Medoff D., Dixon L.B. Evidence of exacerbated cognitive deficits in schizophrenia patients with comorbid diabetes. Psychosomatics. 2008;49(2):123–131. doi: 10.1176/appi.psy.49.2.123. [DOI] [PubMed] [Google Scholar]
- Dye L., Boyle N.B., Champ C., Lawton C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017;76(4):443–454. doi: 10.1017/S0029665117002014. [DOI] [PubMed] [Google Scholar]
- Fatouros-Bergman H., Cervenka S., Flyckt L., Edman G., Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr. Res. 2014;158(1–3):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- First M., Spitzer R.L., Gibbon M., Williams J.B. Institute; New, York, USA: 1995. Structured Clinical Interview for DSM-IV Axis 1 Disorders. [Google Scholar]
- Firth J., Siddiqi N., Koyanagi A., Siskind D., Rosenbaum S., Galletly C., Allan S., Caneo C., Carney R., Carvalho A.F., Chatterton M.L., Correll C.U., Curtis J., Gaughran F., Heald A., Hoare E., Jackson S.E., Kisely S., Lovell K., Maj M., McGorry P.D., Mihalopoulos C., Myles H., O’Donoghue B., Pillinger T., Sarris J., Schuch F.B., Shiers D., Smith L., Solmi M., Suetani S., Taylor J., Teasdale S.B., Thornicroft G., Torous J., Usherwood T., Vancampfort D., Veronese N., Ward P.B., Yung A.R., Killackey E., Stubbs B. The lancet psychiatry commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- Foley D.L., Morley K.I. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch. Gen. Psychiatry. 2011;68(6):609–616. doi: 10.1001/archgenpsychiatry.2011.2. [DOI] [PubMed] [Google Scholar]
- Francey, S.M., O'Donoghue, B., Nelson, B., Graham, J., Baldwin, L., Yuen, H.P., Kerr, M.J., Ratheesh, A., Allott, K., Alvarez-Jimenez, M., 2020. Psychosocial intervention with or without antipsychotic medication for first-episode psychosis: A randomized noninferiority clinical trial. Schizophrenia Bulletin Open 1(1), sgaa015.
- Francis H., Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–128. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Gates J., Killackey E., Phillips L., Alvarez-Jimenez M. Mental health starts with physical health: current status and future directions of non-pharmacological interventions to improve physical health in first-episode psychosis. Lancet Psychiatry. 2015;2(8):726–742. doi: 10.1016/S2215-0366(15)00213-8. [DOI] [PubMed] [Google Scholar]
- Golden J.C. Stoelting Company; Chicago, IL: 1978. Stroop Colour and Word Test. [Google Scholar]
- Gray J.C., Schvey N.A., Tanofsky-Kraff M. Demographic, psychological, behavioural, and cognitive correlates of BMI in youth: findings from the adolescent brain cognitive development (ABCD) study. Psychol. Med. 2020;50(9):1539–1547. doi: 10.1017/S0033291719001545. [DOI] [PubMed] [Google Scholar]
- Greenwood C.E., Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol. Aging. 2005;26(1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Skott M., Ekblom Ö., Firth J., Schembri A., Forsell Y. Exercise effects on cognitive functioning in youth adults with first-episode psychosis: FitForLife. Psychol. Med. 2019;49(3):431–439. doi: 10.1017/S0033291718001022. [DOI] [PubMed] [Google Scholar]
- Han M., Huang X.F., Chen D.C., Xiu M., Kosten T.R., Zhang X.Y. Diabetes and cognitive deficits in chronic schizophrenia: a case-control study. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Suda A., Kishida I., Miyauchi M., Shiraishi Y., Fujibayashi M., Tsujita N., Ishii C., Ishii N., Moritani T., Saigusa Y., Hirayasu Y. Association between dysfunction of autonomic nervous system activity and mortality in schizophrenia. Compr. Psychiatry. 2018;86:119–122. doi: 10.1016/j.comppsych.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Heald A., Pendlebury J., Anderson S., Narayan V., Guy M., Gibson M., Haddad P., Livingston M. Lifestyle factors and the metabolic syndrome in schizophrenia: a cross-sectional study. Ann. General Psychiatry. 2017;16:12. doi: 10.1186/s12991-017-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp., 2017. IBM Statistics for Windows, Version 25.0, Version 25.0 ed. IBM Corp., Armonk, NY.
- International Diabetes Foundation . 2006. The IDF Consesus Worldwide Definition of the Metabolic Syndrome. (IDF.) [Google Scholar]
- Jensen K.G., Correll C.U., Ruda D., Klauber D.G., Stentebjerg-Olesen M., Fagerlund B., Jepsen J.R.M., Fink-Jensen A., Pagsberg A.K. Pretreatment cardiometabolic status in youth with early-onset psychosis: baseline results from the TEA trial. J. Clin. Psychiatry. 2017;78(8):e1035–e1046. doi: 10.4088/JCP.15m10479. [DOI] [PubMed] [Google Scholar]
- Keinänen J., Suvisaari J., Reinikainen J., Kieseppä T., Lindgren M., Mäntylä T., Rikandi E., Sundvall J., Torniainen-Holm M., Mantere O. Low-grade inflammation in first-episode psychosis is determined by increased waist circumference. Psychiatry Res. 2018;270:547–553. doi: 10.1016/j.psychres.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Montrose D.M., Miewald J.M., Jindal R.D. Sleep correlates of cognition in early course psychotic disorders. Schizophr. Res. 2011;131(1–3):231–234. doi: 10.1016/j.schres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenic M., Franke K., Hlinka J., Matejka M., Capkova J., Pausova Z., Uher R., Alda M., Spaniel F., Hajek T. Obesity, dyslipidemia and brain age in first-episode psychosis. J. Psychiatr. Res. 2018;99:151–158. doi: 10.1016/j.jpsychires.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Latvala A., Kuja-Halkola R., Ruck C., D’Onofrio B.M., Jernberg T., Almqvist C., Mataix-Cols D., Larsson H., Lichtenstein P. Association of resting heart rate and blood pressure in late adolescence with subsequent mental disorders: a longitudinal population study of more than 1 million men in Sweden. JAMA Psychiatry. 2016;73(12):1268–1275. doi: 10.1001/jamapsychiatry.2016.2717. [DOI] [PubMed] [Google Scholar]
- Liang J., Matheson B.E., Kaye W.H., Boutelle K.N. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. 2014;38(4):494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D., Corey S., Kelly L.H., Yohannes S., Youngquist A.L., Stuart B.K., Niendam T.A., Loewy R.L. The role of trauma and stressful life events among individuals at clinical high risk for psychosis: a review. Frontiers in Psychiatry. 2017;8:55. doi: 10.3389/fpsyt.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen S., Jarrahi B., Subotnik K., Ventura J., Nuechterlein K. 396. Neuroplasticity benefits of adding aerobic exercise to cognitive training in first-episode schizophrenia patients. Biol. Psychiatry. 2017;81(10):S161–S162. [Google Scholar]
- Mesholam-Gately R.I., Giuliano A.J., Goff K.P., Faraone S.V., Seidman L.J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Morosini P.L., Magliano L., Brambilla L., Ugolini S., Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000;101(4):323–329. [PubMed] [Google Scholar]
- National Health and Medical Research Council . National Health and Medical Research Council; Melbourne: 2013. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia. [Google Scholar]
- O’Donoghue B., Francey S.M., Nelson B., Ratheesh A., Allott K., Graham J., Baldwin L., Alvarez-Jimenez M., Thompson A., Fornito A., Polari A., Berk M., Macneil C., Crisp K., Pantelis C., Yuen H.P., Harrigan S., McGorry P. Staged treatment and acceptability guidelines in early psychosis study (STAGES): a randomized placebo controlled trial of intensive psychosocial treatment plus or minus antipsychotic medication for first-episode psychosis with low-risk of self-harm or aggression. Study protocol and baseline characteristics of participants. Early Intervention in Psychiatry. 2019;13(4):953–960. doi: 10.1111/eip.12716. [DOI] [PubMed] [Google Scholar]
- Orešič M. Obesity and psychotic disorders: uncovering common mechanisms through metabolomics. Dis. Model. Mech. 2012;5(5):614–620. doi: 10.1242/dmm.009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn D.P. Topics in review: the poor physical health of people with mental illness. West. J. Med. 2001;175(5):329. doi: 10.1136/ewjm.175.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage G.R., Saling M.M., Davis C.W., Berkovic S.F. Direct and indirect measures of verbal relational memory following anterior temporal lobectomy. Neuropsychologia. 2002;40(3):302–316. doi: 10.1016/s0028-3932(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Shah P., Iwata Y., Caravaggio F., Plitman E., Brown E.E., Kim J., Chan N., Hahn M., Remington G., Gerretsen P., Graff-Guerrero A. Alterations in body mass index and waist-to-hip ratio in never and minimally treated patients with psychosis: a systematic review and meta-analysis. Schizophr. Res. 2019;208:420–429. doi: 10.1016/j.schres.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Lau A., Lencz T., Burdick K.E., DeRosse P., Brenner R., Lindenmayer J.P., Malhotra A.K. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr. Res. 2011;126(1–3):257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J.M., Karcher N.R., Barch D.M. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol. Rev. 2018;28(4):509–533. doi: 10.1007/s11065-018-9388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E., Hay P., Campbell L., Trollor J.N. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes. Rev. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen L., Colzato L.S. Overweight and cognitive performance: high body mass index is associated with impairment in reactive control during task switching. Frontiers in Nutrition. 2017;4:51. doi: 10.3389/fnut.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Stramecki F., Kotowicz K.D., Piotrowski P., Frydecka D., Rymaszewska J., Beszlej J.A., Samochowiec J., Jablonski M., Wronski M., Moustafa A.A., Misiak B. Assessment of the association between cigarette smoking and cognitive performance in patients with schizophrenia-spectrum disorders: a case-control study. Frontiers in Psychiatry. 2018;9:642. doi: 10.3389/fpsyt.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M., Miewald J., Keshavan M., R., G. Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons. Schizophr. Res. 2008;93(1–3):90–98. doi: 10.1016/j.schres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New York: 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- Suvisaari J., Mantere O., Keinanen J., Mantyla T., Rikandi E., Lindgren M., Kieseppa T., Raij T.T. Is it possible to predict the future in first-episode psychosis? Frontiers in Psychiatry. 2018;9:580. doi: 10.3389/fpsyt.2018.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M.H., Baier M.G., Becker J.E., Ritchie M.A. Blood pressure, heart rate, and anxiety in schizophrenia. Psychiatry Res. 2007;151(1–2):155–157. doi: 10.1016/j.psychres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Uren J., Cotton S.M., Killackey E., Saling M.M., Allott K. Cognitive clusters in first-episode psychosis: overlap with healthy controls and relationship to concurrent and prospective symptoms and functioning. Neuropsychology. 2017;31(7):787–797. doi: 10.1037/neu0000367. [DOI] [PubMed] [Google Scholar]
- Valentini M., Parati G. Variables influencing heart rate. Prog. Cardiovasc. Dis. 2009;52(1):11–19. doi: 10.1016/j.pcad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Ventura J., Green M.F., Shaner A., Liberman R.P. Training and quality assurance with the brief psychiatric rating scale: “the drift buster”. Int. J. Methods Psychiatr. Res. 1993;34:221–244. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) [Google Scholar]
- Wilkinson G.S., Robertson G.J. Professional Manual Lutz, FL. Inc; Psychological Assessment Resources: 2006. WRAT-4: Wide range achievement test. [Google Scholar]
- Xu H., Zhuang X. Atypical antipsychotics-induced metabolic syndrome and nonalcoholic fatty liver disease: a critical review. Neuropsychiatr. Dis. Treat. 2019;15:2087. doi: 10.2147/NDT.S208061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau P.L., Castro M.G., Tagani A., Tsui W.H., Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables