Abstract
Patients with membranous nephropathy have an increased risk of malignancy compared to the general population, but the target antigen for malignancy-associated membranous nephropathy is unknown. To explore this, we utilized mass spectrometry for antigen discovery in malignancy-associated membranous nephropathy examining immune complexes eluted from frozen kidney biopsy tissue using protein G bead immunoglobulin capture. Antigen discovery was performed comparing cases of membranous nephropathy of unknown and known type. Mass spectrophotometric analysis revealed that nerve epidermal growth factor-like 1 (NELL1) immune complexes were uniquely present within the biopsy tissue in membranous nephropathy. Additional NELL1-positive cases were subsequently identified by immunofluorescence. In a consecutive series, 3.8% of PLA2R- and THSD7A-negative cases were NELL1-positive. These NELL1-positive cases had segmental to incomplete IgG capillary loop staining (93.4%) and dominant or co-dominant IgG1-subclass staining (95.5%). The mean age of patients with NELL1-positive membranous nephropathy was 66.8 years, with a slight male predominance (58.2%) and 33% had concurrent malignancy. Compared with PLA2R- and THSD7A-positive cases of membranous nephropathy, there was a greater proportion of cases with malignancies in the NELL1-associated group. Thus, NELL1-associated membranous nephropathy has a unique histopathology characterized by incomplete capillary loop staining, IgG1-predominance, and is more often associated with malignancy than other known types of membranous nephropathy.
Keywords: Membranous nephropathy, segmental membranous nephropathy, malignancy-associated membranous nephropathy, neural epidermal growth factor-like 1, NELL1, malignancy
Graphical Abstract
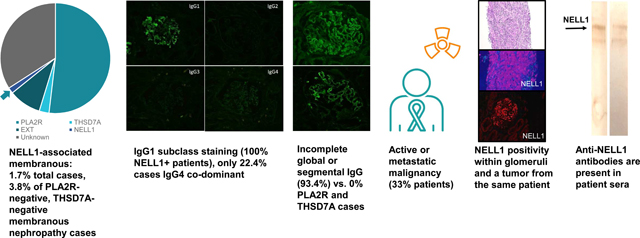
CONCLUSION: NELL1 is a target antigen in malignancy-associated membranous nephropathy. Nerve epidermal growth factor-like 1 (NELL1), a recently identified antigen in membranous nephropathy, is enriched in patients with malignancy-associated membranous nephropathy, and anti-NELL1 antibodies can be detected within serum.
INTRODUCTION.
Membranous nephropathy (MN) is the most common cause of idiopathic nephrotic syndrome in adults. It is a disease caused by the deposition of immune complexes within a glomerular distribution. It can be caused by antibodies directed against podocyte antigens (primary MN) or through secondary deposition of circulating immune complexes (or alternatively in situ immune complex formation) within glomeruli. The most common causes of secondary MN are autoimmune diseases, of which systemic lupus erythematosus accounts for the majority of cases (membranous lupus nephritis). The second most common secondary etiology is malignancy. Other causes include infections (such as viral hepatitis), graft-versus-host disease, allergic responses (such as against bovine serum albumin), and drug reactions.
Membranous nephropathy has been reported to occur concurrently with malignancy in a subset of patients and is the most common glomerular disease occurring coincidently with neoplasms. It can be the first sign of an occult malignancy in up to 20% of cases1. This association was first reported by Lee et al. in 1966, where 11% of patients with nephrotic syndrome had an underlying carcinoma, and enriched in patients with membranous nephropathy (73%)2. The risk of cancer in patients with MN is 2 to 3-fold higher than the age-matched general population up to 4 years following the diagnosis of MN3.
While malignancy is a known “secondary” association of MN, determining who should undergo a thorough workup to exclude a co-existent cancer is a subject of debate. Risk factors for concurrent malignancy include older age (greater than 65 years), a 20 pack-year or greater smoking history, use of chronic immunosuppression, or presence of thrombotic complications (such as renal vein thrombosis, deep venous thrombosis, or a pulmonary embolism)4. In a study by Ronco et al., as many as 22% of patients with MN who are 60 years of age or older had malignancy, which is 10-fold higher than age-matched controls5. Other factors characteristic of malignancy-associated MN include PLA2R-negativity, and IgG1 and/or IgG2-restricted subclass predominance6, 7
Neural epidermal growth factor-like 1 (NELL1) is a recently described antigen in MN8. Our findings suggest that NELL1-associated MN is commonly associated with malignancy and has unique histopathologic findings that may aid in identification of cases in everyday clinical practice.
RESULTS
Mass spectrometry analysis revealed NELL1 as an antigen in membranous nephropathy.
Protein G was used to isolate IgG-containing immune complexes from frozen tissue remnants from each of 59 renal biopsies. These affinity-purified immune complexes were then analyzed by mass spectrometry to determine their proteomic composition. Two groups were compared: the first group contained MN cases of known antigen types, including PLA2R (n=4), THSD7A (n=3), and EXT1/2 (n=4). Diabetic glomerulopathy and arterionephrosclerosis samples with no positive immunofluorescence staining were utilized as negative controls (n=7). The second group contained cases negative for PLA2R, THSD7A, and EXT1/2 (n=41). By comparing proteomes from the known with the unknown MN types, it was possible to discern unique proteins.
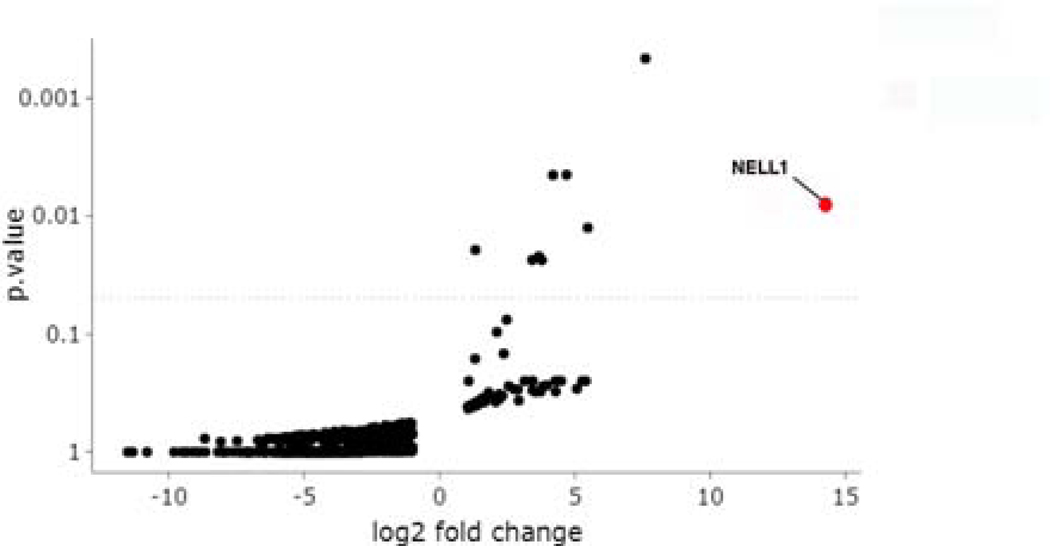
This technique was highly sensitive and specific for identification of antigenic targets in MN of known type. PLA2R was the only protein that immunoprecipitated with IgG in 4/4 PLA2R-positive MN biopsies and 0/55 remaining biopsies (Supplemental Figure 1A). THSD7A was the only protein identified in 3/3 THSD7A-positive MN cases and 0/56 remaining biopsies (Supplemental Figure 1B). EXT was identified in 4/4 EXT-positive MN cases and 0/55 remaining biopsies (Supplemental Figure 1C). There were no proteins that uniquely immunoprecipitated with IgG within diabetic nephropathy or arterionephrosclerosis samples. Thus, as a proof of principle, this approach specifically identified the autoantigen in cases with MN of known type. When the same analysis was applied to cases of MN of unknown antigenic type, there were 2 of 41 cases in which NELL1 was identified (Figure 1). No membranous lupus nephritis cases (which included 16 of the 41 PLA2R-negative, THSD7A-negative, and EXT1/2-negative MN cases) submitted for mass spectrometry were NELL1-positive. This analysis confirms that IgG is bound to NELL1 in a subset of MN cases, which were also confirmed to be positive by tissue immunofluorescence staining.
Figure 1.
Immunoglobulin capture from frozen kidney biopsy followed by MS identifies NELL1 as a target antigen. Volcano plot showing NELL1 as a potential protein of interest, with a high fold-change and low p-value compared to other protein candidates.
NELL1 colocalizes with IgG in subepithelial glomerular immune deposits.
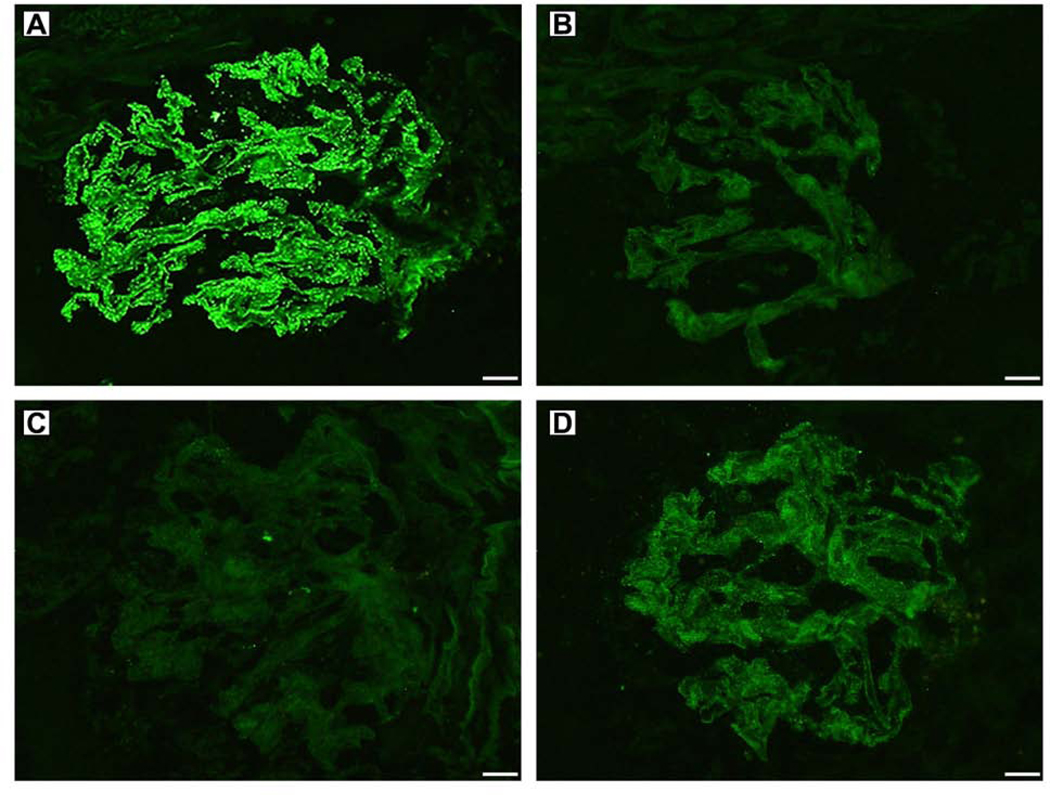
Dual NELL1-Rhodamine Red X and IgG-FITC labeling showed near complete co-localization of NELL1 with IgG by confocal microscopy (86.7 ± 9.4%, Supplemental Figure 2, top panel). There was no significant co-localization of NELL1 and IgG in PLA2R-positive MN cases used as controls (25.1 ± 14.4%) (Supplemental Figure 2, bottom panel). A graphical representation of the co-localization data is provided in Supplemental Figure 3. There was a significant increase in NELL1/IgG co-localization in NELL1+ MN cases compared to PLA2R+ MN controls (p<0.0001).
Frequency data from NELL1 staining in a consecutive case series of MN
To determine the frequency of NELL1 positivity in membranous nephropathy, we stained 349 consecutive MN cases with tissue available for PLA2R, THSD7A, EXT2, and NELL1. This series included 83 pure class V membranous lupus nephritis (no proliferation) and 266 primary MN cases. Among the 349 total cases, 181 were PLA2R-positive (51.9%),10 were THSD7A-positive (2.9%), 33 were EXT2 positive (9.5%), and 6 were NELL1 positive (1.7%). Among the 266 primary MN cases only, there were 180 PLA2R-positive (67.7%), 7 THSD7A-positive (2.6%), 6 EXT2 positive (2.3%), and 5 NELL1 positive (1.9%). Among the 83 class V membranous lupus nephritis cases, there were 1 PLA2R-positive (1.2%), 3 THSD7A-positive (3.6%), 27 EXT2 positive (32.5%), and 1 NELL1 positive (1.2%). As there were too small of a number of cases from the 9 month consecutive case series to identify potential clinical or histologic associations of NELL1-associated MN, NELL1-associated MN cases were identified from over the course of 5 years of MN cases. In total, 91 NELL1-associated MN cases were identified.
Characteristics of NELL1-associated MN (91 patients)
On light microscopy, NELL1-associated MN cases showed prominent glomerular capillary loops (Figure 2A). Forty one of 91 cases (45.1%) showed formation of spikes or holes on silver stains (Figure 2B), often within a segmental distribution within glomeruli. A segmental (involvement of <50% of glomerular tuft) to incomplete global (segmental loops uninvolved by staining) capillary loop staining pattern was seen in 93.4% of cases of NELL1-associated MN (Figure 2C + D) and in no cases of PLA2R (0/181) or THSD7A-associated MN (0/10). Complete (100% tuft involvement) capillary loop staining was seen in 6 of 91 (6.6%) of NELL1-associated MN, compared to 100% of PLA2R positive MN and 100% of THSD7A positive MN control cases (Supplemental Figure 4). The segmental pattern of immune deposits was also seen by the distribution of electron-dense immune-type deposits by electron microscopy, showing sparing of some glomerular capillary loops (Figure 2E), with other loops containing electron-dense immune-type deposits (Figure 2F).
Figure 2.
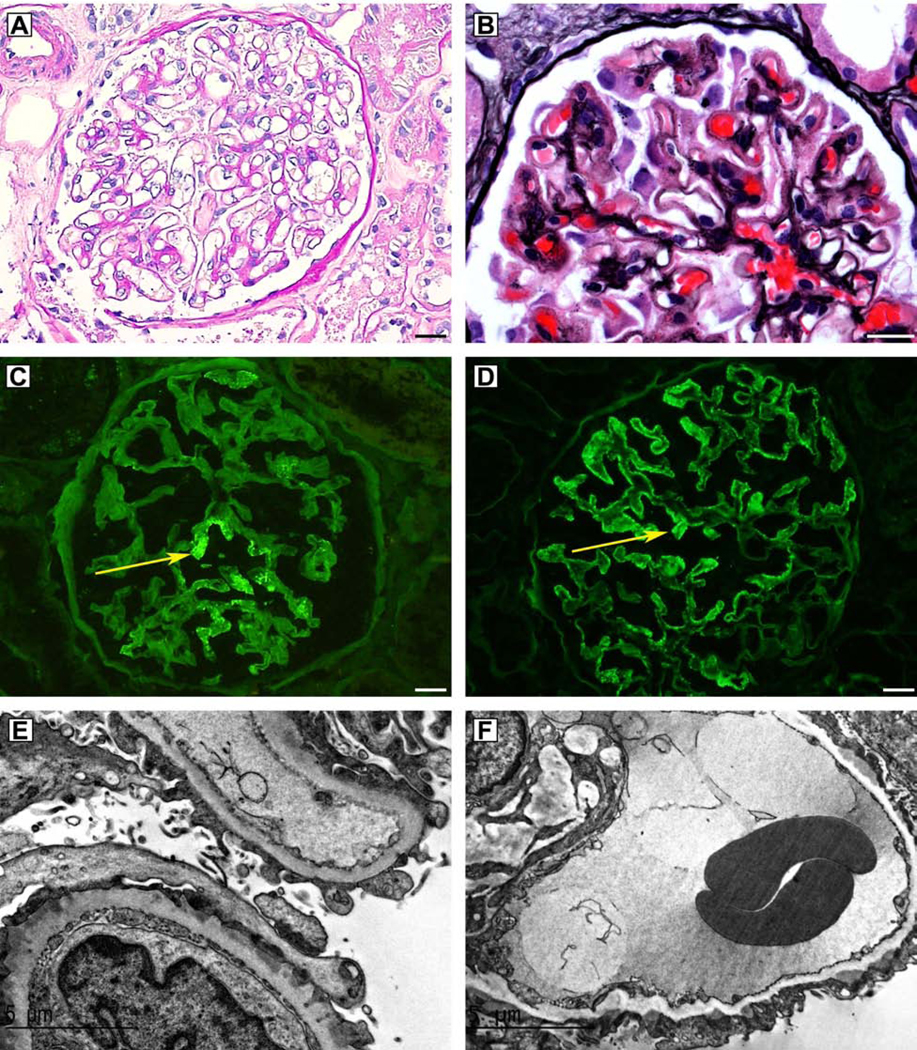
NELL1-associated MN shows unique histopathologic features. A) Glomerulus on Periodic Acid Schiff (PAS) stain, showing prominent glomerular capillary loops; B) Spikes and holes along the glomerular capillary loops seen on silver stain; C) Immunofluorescence microscopy demonstrating segmental IgG staining within glomeruli; D) Immunofluorescence microscopy demonstrating incomplete global IgG staining within glomeruli; E) Electron microscopy showing capillary loop sparing of electron-dense immune-type deposits; F) Electron microscopy showing electron-dense deposits along the glomerular capillary loops.
Biopsies from NELL1-associated MN patients showed no significant difference in IgA positivity (7.7% NELL1 versus 10% PLA2R, p= 0.52), IgM positivity (9.9% NELL1 versus 15.5% PLA2R, p= 0.26), C1q positivity (0% NELL1 versus 2% PLA2R, p=0.30), and lacked a full-house immunofluorescence pattern with expression of IgA, IgG, IgM, C3, and C1q (0% NELL1 versus <1% PLA2R, p=1.0), compared to PLA2R-positive MN (Table 1). C3 positivity was less common in NELL1-associated MN compared to PLA2R-positive MN (78.0% NELL1 versus 91% PLA2R, Fisher’s exact test =0.002). There was no significant extraglomerular staining in cases of NELL1-associated MN, with no cases demonstrating deposits along tubular basement membranes, Bowman’s capsule, or vessels. EXT2-positive membranous cases had a higher frequency of IgA, IgM, C3, C1q, full house immunofluorescence, and presence of mesangial electron-dense deposits than NELL1-associated, PLA2R-positive, or THSD7A-positive MN. A summary of the histopathologic features is included in Table 1.
Table 1.
Histopathologic parameters of kidney biopsies with NELL1-associated membranous nephropathy, compared to PLA2R-associated, THSD7A-associated, and EXT2-associated membranous nephropathy.
| Parameter | NELL1 positive (n=91) | PLA2R positive (n=181) | THSD7A positive (n=10) | EXT2 positive (n=33) | Fisher’s exact test NELL1 vs. PLA2R |
|---|---|---|---|---|---|
| Incomplete global capillary loop (>50%) | 44/91 (48.3%) | 0/181 (0%) | 0/10 (0%) | 0/33 (0%) | p<0.0001 |
| Pure segmental (<50%) | 41/91 (45.0%) | 0/181 (0%) | 0/10 (0%) | 0/33 (0%) | p<0.0001 |
| Segmental or incomplete capillary loop staining | 85/91 (93.4%) | 0/181 (0%) | 0/10 (0%) | 0/33 (0%) | p<0.0001 |
| IgA positivity | 7/91 (7.7%) | 19/181 (10%) | 4/10 (40%) | 12/33 (36%) | 0.52 |
| IgG positivity | 91/91 (100%) | 181/181 (100%) | 10/10 (100%) | 33/33 (100%) | N/A |
| IgM positivity | 9/91 (9.9%) | 28/181 (15.5%) | 1/10 (10%) | 12/33 (36%) | 0.26 |
| C3 positivity | 71/91 (78.0%) | 166/181 (91%) | 6/10 (80%) | 31/33 (94%) | 0.002 |
| C1q positivity | 0/91 (0%) | 4/181 (2%) | 1/10 (10%) | 12/33 (36%) | 0.30 |
| Full house immunofluorescence | 0/91 (0%) | 1/181 (<1%) | 0/10 (0%) | 5/33 (15%) | 1.00 |
| Mesangial deposits | 21/88* (23.9%) | 19/181 (10%) | 4/10 (40%) | 26/33 (79%) | 0.01 |
| Subendothelial deposits | 0/88* (0%) | 2/181 (1%) | 1/10 (10%) | 6/33 (18%) | 1.00 |
Mesangial and subendothelial electron dense deposits are assessed from 88 total cases instead of 91 total cases, as 3 of the 91 cases did not have a glomerulus available for evaluation by electron microscopy.
IgG subclass staining was performed from 67 cases with residual frozen tissue available for testing (Table 2). There was IgG1 staining in all cases of NELL1-associated MN (67/67 cases, Figure 3 and Supplemental Figure 5), and 49 of 67 cases were IgG1 dominant (73.1%). Eighteen of 67 (26.9%) of cases had IgG1 restriction. Concurrent IgG2 staining was present in 25/67 cases (37.3%), and concurrent IgG4 staining was present in 36/67 cases (53.7%). Three of 67 cases (4.5%) were IgG1 and IgG2 co-dominant, and twelve of 67 cases (17.9%) were IgG1 and IgG4 co-dominant. Therefore, 95.5% of NELL1-associated MN cases had dominant or co-dominant IgG1 subclass staining (Figure 3). There were 3 of 67 cases with IgG1, IgG2, and IgG3 staining (4.5%) without IgG4, a pattern seen in autoimmune disease.
Table 2.
IgG subclass staining in NELL1-associated MN. Staining intensity trace or greater is included.
| IgG subclass | Number of cases | Percentage of total |
|---|---|---|
| IgG1 | 67/67 | 100% |
| IgG2 | 25/67 | 37.3% |
| IgG3 | 10/67 | 14.9% |
| IgG4 | 36/67 | 53.7% |
| IgG1 dominant | 49/67 | 73.1% |
| IgG2 dominant | 0/67 | 0% |
| IgG3 dominant | 0/67 | 0% |
| IgG4 dominant | 3/67 | 4.5% |
| IgG1/IgG2 co-dominant | 3/67 | 4.5% |
| IgG1/IgG4 co-dominant | 12/67 | 17.9% |
| IgG1/IgG2/IgG3 positive | 3/67 | 4.5% |
| IgG1 restriction | 18/67 | 26.9% |
Figure 3.
NELL1 positive MN cases show IgG1 staining (with variable IgG2 and IgG4 expression). A. IgG1; B. IgG2; C. IgG3; D. IgG4.
Electron microscopy was available for 88 of 91 cases of NELL1-associated MN. A majority of cases showed severe (>50%) podocyte foot process effacement (69/88 cases, 78.4%), with 11/88 cases (12.5%) showing moderate podocyte foot process effacement (25–50%), and 8/88 cases (9.1%) showing mild podocyte foot process effacement. NELL1-associated MN cases had a greater incidence of mesangial deposits (21/88 cases, 23.9% versus 19/181 cases for PLA2R, 10%, Fisher’s exact test p= 0.01), and no subendothelial deposits (0/88 cases, 0% versus 2/181 cases, 1%, p=1.0, Table 1).
Clinical associations with NELL1-associated membranous nephropathy
Patients with NELL1-associated MN were older than patients with PLA2R-positive MN (mean age 66.8 ± 10.8 years NELL1, versus 56.4 ± 13.9 years PLA2R), patients with THSD7-associated MN (mean age 45.1 ± 16.3 years), and EXT-associated MN (mean age 39.6 ± SD 16.1 years). Patients with NELL1-associated MN with malignancy were significantly older than patients without malignancy (71.0 ± 8.6 years vs. 65.0 ± 10.5 years, student’s t-test p=0.01). There was a slight male predominance (58.2%). Approximately one-quarter of NELL1-associated MN cases had a history of diabetes mellitus (22/91, 24.2%), however only 9% had changes of diabetic nephropathy on biopsy. Twenty of 91 patients with NELL1-associated MN had acute tubular injury on biopsy (22.0%), and two of 91 showed concurrent acute interstitial nephritis (2.2%). Additional disease associations comparing PLA2R, THSD7A, EXT2, and NELL1-positive MN are provided in Supplemental Table 1.
Two cases of NELL1-associated MN had a history of Crohn’s disease. To further explore this, we stained all cases of PLA2R negative, THSD7A negative MN in patients with a history of Crohn’s disease, ulcerative colitis, or inflammatory bowel disease from our database and found that 2 of 8 cases (25%) were NELL1-positive. While NELL1 was identified as a gene of interest in Crohn’s disease in a genome-wide association study 9, whether this represents a potential secondary etiology for development of NELL1-associated MN in a subset of patients is unclear.
Five years of idiopathic MN biopsies from our database were studied to identify malignancy-associated MN cases. 111 malignancy-associated MN cases were identified, of which 35 were PLA2R positive, 4 were THSD7A positive, and 30 were NELL1 positive (Table 3). Given the much higher prevalence of PLA2R-positive MN as compared to NELL1 MN, patients with cancer are more likely to have PLA2R-positive MN. However, the prevalence of malignancy is much higher in patients with NELL1 MN as compared to other types of MN. For example, 33% of NELL1-positive patients had malignancy (30/91), while only 4.2% of PLA2R-positive patients (35/829) and 10.8% of THSD7A-positive patients had concurrent malignancy (4/37). Two THSD7A-positive patients and 4 NELL1-positive cases had two concurrent primary tumors (Table 3). The associated antigens for malignancy-associated MN was unknown in 42 patients, for which three patients had two primary tumors (Supplemental Figure 6).
Table 3.
Increased prevalence of malignancy in NELL1-associated MN, compared to PLA2R-associated MN, THSD7A-associated MN, and membranous nephropathy due to unknown antigens. The types and frequencies of tumors seen in patients with malignancy-associated membranous nephropathy are shown.
| Type of neoplasm | PLA2R positive (n=35 of 829) | THSD7A positive (n=4 of 37) | NELL1 positive (n=30 of 91) | Unknown antigen (n=42 of 421) |
|---|---|---|---|---|
| Prostate adenocarcinoma | 10 | 3 | 6 | 10 |
| Breast carcinoma | 12 | 1 | 5 | 8 |
| Gastric carcinoma | 0 | 0 | 1 | 2 |
| Soft tissue tumor | 0 | 0 | 1 | 0 |
| Glioma | 0 | 0 | 1 | 0 |
| Colon adenocarcinoma | 1 | 0 | 1 | 6 |
| Lung carcinoma | 1 | 0 | 3 | 4 |
| Bladder carcinoma | 5 | 0 | 2 | 0 |
| Renal cell carcinoma | 2 | 1 | 3 | 5 |
| Pancreatic carcinoma | 0 | 0 | 0 | 1 |
| Hepatocellular carcinoma | 0 | 0 | 0 | 1 |
| Thyroid carcinoma | 0 | 0 | 1 | 2 |
| Ovarian carcinoma | 0 | 0 | 1 | 0 |
| Uterine carcinoma | 0 | 0 | 0 | 1 |
| Cervical carcinoma | 1 | 0 | 1 | 0 |
| Testicular carcinoma | 1 | 0 | 0 | 0 |
| Skin (melanoma, BCC, SCC) | 2 | 0 | 4 | 1 |
| Squamous cell carcinoma (head/neck) | 0 | 1 | 1 | 2 |
| Laryngeal carcinoma | 0 | 0 | 0 | 1 |
| Nasopharyngeal carcinoma | 0 | 0 | 1 | 0 |
| Thymoma | 0 | 0 | 1 | 0 |
| Lymphoma | 0 | 0 | 1 | 1 |
| Total | 35 | 6* | 34** | 45*** |
While 4 patients in the THSD7A positive cohort had a history of malignancy, the number of tumors is >4 due to two patients with two primary tumors.
While 30 patients in the NELL1 positive cohort had a history of malignancy, the number of tumors is >30 due to four patients with two primary tumors.
42 patients with MN of unknown type had a history of malignancy, and the number of tumors is >42 due to three patients with two primary tumors.
Of note, the epidemiology of membranous nephropathy changed within this five year time period, where PLA2R-positive MN made up 87% of cases of MN at our institution at 2014, but only 42% in 2019. We attribute this to serum-based approaches to the diagnosis of primary membranous nephropathy 10, with anti-PLA2R and anti-THSD7A assays now widely available for screening of patients presenting with nephrotic syndrome.
A majority of NELL1-associated MN patients with a history of malignancy had concurrent proteinuria and ongoing malignancy (n=19/30), eight patients had the cancer identified prior to MN, one patient had malignancy identified in a work-up for causes of secondary MN, and in two patients the underlying malignancy preceded the diagnosis of MN but the time of diagnosis was unknown (Supplemental Table 2). Seven of these patients had metastatic disease at the time of diagnosis. We excluded patients in which the diagnosis of malignancy was remote compared to the diagnosis of membranous nephropathy or in complete remission (n=4 of 34 NELL1+ patients had a history of malignancy; the 30 patients in our cohort had active malignancy). The timing between the diagnosis of cancer and MN in our cohort (when known) is summarized in Supplemental Table 2.
Serum creatinine at the time of biopsy and quantitative 24-hour proteinuria values were similar in patients with NELL1-associated MN compared to PLA2R, THSD7A, or EXT-associated MN (serum creatinine: PLA2R 1.93 mg/dL ± 2.19, THSD7A 1.85 mg/dL ± 2.34, NELL1 1.33 mg/dL ± 0.86 (data available for 73/91 patients); and 24 hour proteinuria: PLA2R 8.03 ± 5.09 g, THSD7A 6.68 ± 4.76 g, NELL1 6.6 ± 4.1 g (for which data was available in 59 of 91 patients). A majority of patients in all groups had normal renal function at the time of biopsy, with the variation due to outliers with concurrent acute kidney injury. A majority of patients (71.2%) with NELL1-associated MN had nephrotic range proteinuria (≥3.5 g) at the time of biopsy (corresponding to 42/59 patients with available quantitative proteinuria data). Depressed albumin levels were seen in 74.5% patients (38/51 patients with serum albumin values available with <3.5 g/dL albumin, mean albumin = 2.84 g/dL ± 0.95 g/dL).
Tumor staining for NELL1
Tissue of the associated tumor was available form two patients with NELL1-associated MN. In one patient with NELL1-associated MN and concurrent invasive ductal carcinoma of the breast, NELL1 positivity by immunofluorescence and immunohistochemical staining was seen within the patient’s breast biopsy (Supplemental Figure 7A + B) and mastectomy specimen (Supplemental Figure 7C + D). A patient with active follicular lymphoma also demonstrated NELL1 staining on an excisional lymph node biopsy (Supplemental Figure 7E + F).
NELL1 antibodies are detected in sera from patients with NELL1-associated MN.
Twenty-eight patients with NELL1-associated MN had available blood samples to test for serum reactivity against NELL1 antigen. Twenty of 28 patients (71.4%) with NELL1-associated MN had serum reactivity to NELL1 recombinant protein under non-reducing conditions (representating western blots in Supplemental Figure 8). No control sera from PLA2R-positive MN showed reactivity (0/28 patients). Of note, 3 of the 8 NELL1-positive patients without serum reactivity were in clinical remission, with a >50 percent reduction in proteinuria at the time of serum collection compared to the time of biopsy.
Clinical follow-up.
Clinical follow-up with treatment response (evaluating remission of MN) and laboratory data was available from 59 of 91 (64.8%) patients. In addition to these 59 patients, 8 of the 91 patients in our cohort were not on active treatment at follow up as they were deceased or on dialysis (8 of 91, 12%), and 6 of 91 (6.6%) did not return for clinical follow-up after their biopsy. Mean clinical follow-up was 10.4 ± 13.5 months. The median proteinuria (± interquartile range) at follow-up (was 1.9± 4.1 g. The median change in proteinuria from the time of biopsy was 5.4 ± 6.6 g.
Twenty of 59 patients (34%) were in complete remission, with less than 300 mg proteinuria per day. Sixteen of 59 patients (27%) were in partial remission, with 300 mg to 3500 mg proteinuria per day or a greater than 50% reduction in proteinuria from the time of biopsy. Twenty-three of 59 patients (39%) had no remission, with less than 50 percent reduction in proteinuria and greater than 3.5 g proteinuria per day. A majority of patients had stable renal function at follow-up (median creatinine 1.1 ± 0.56 mg/dL.
Comparisons of the effects of various treatments were made from patients with available data (n=59), and sample medians are provided. For patients on renin-angiotensin system blockade (with angiotensin converting enzyme inhibitor and/or angiotensin receptor blockade therapy, n=32), the median proteinuria at time of biopsy was 4.9 ± 4.7 g, median proteinuria at follow-up 1.9 ± 4.0 g, and the median follow-up creatinine was 1.1 ± 0.4 mg/dL. In calcineurin-inhibitor treated patients (n=8), the median proteinuria at time of biopsy was 10 ± 9.1 g, proteinuria at follow-up 6.4 ± 10.4 g, and follow-up creatinine was 1.8 ± 3.0 mg/dL. In patients treated with cyclophosphamide (n=3), the median proteinuria at time of biopsy was 9.6 g, proteinuria at follow-up 3.9 g, and follow up creatinine 1.4 mg/dL (IQR not provided due to low ‘n’). In patients treated with mycophenolate and steroid therapy (n=3), the median proteinuria at time of biopsy was 8 g, proteinuria at follow-up 0.1 g, and creatinine at follow-up 0.9 mg/dL (IQR not provided due to low ‘n’). One patient was treated with rituximab, who presented with 10 g proteinuria, had >10 g proteinuria at follow-up, and creatinine at follow up was 1.0 mg/dL.
For patients undergoing active treatment of an underlying malignancy and not on concurrent immunosuppressive therapy (n=12), the median proteinuria at presentation was 5 ± 7.4 g, at follow-up was 0.1 ± 2.1 g, and follow-up creatinine was 1.0 ± 0.9 mg/dL. Due to the small number of patients on immunosuppressive therapy (n=15), an optimal treatment for NELL1-associated MN could not be ascertained, however patients with successful treatment for an underlying malignancy showed good response to therapy (with 9/12 patients treated for malignancy without concurrent immunosuppression in complete or partial remission at follow-up). The treatment and follow-up data following therapy is included in Supplemental Table 3.
Limitations.
Our study has several limitations. Complete clinical follow-up was not available in all patients. As patients with malignancy-associated MN can have cancer identified during a work-up following the diagnosis of their glomerular disease up to 2–4 years after membranous nephropathy and we did not have longitudinal follow up for 4 years or greater on all patients, our data likely underestimates the true frequency of malignancy in patients with MN.
DISCUSSION
NELL1 is a 140 kDa modular glycoprotein, which is expressed in low levels in adult tissues, but is active during development where it is essential for intramembranous and endochondral ossification. It shows sequence similarity to thrombospondin proteins, and its structure consists of a N-terminal laminin G-like domain, three VWF-C domains, a thrombospondin type 1 domain, and 5 tandem EGF domains (Kuroda et al, 1999) 11. NELL1, unlike PLA2R or THSD7A, does not contain a transmembrane domain, and has a secretory signal sequence.
Sethi et al recently shown that NELL1-positive membranous nephropathy was a specific subtype of membranous nephropathy, and was the responsible antigen in up to 16% of PLA2R-negative primary MN cases (Sethi et al, 2019) 8. We identified NELL1-associated MN in 3.8% of primary MN that were negative for PLA2R and THSD7A. An association with malignancy was higher in our cohort (33%) compared with their cohort malignancy association of 11.7%.
The histopathology of NELL1-associated MN was unique compared to other forms of MN. This includes segmental to incomplete global IgG staining, IgG1 subclass positivity, and lack of staining for other immune reactants (IgA, IgM, and C1q). Many of the histopathologic features observed in NELL1-associated MN have previously been described in reports of malignancy-associated MN within the literature. These include IgG1 and IgG2 subclass specificity, while primary PLA2R-positive MN cases more frequently had IgG4 dominant deposits (65%), malignancy-associated MN cases had a reduced frequency of IgG4 dominance (31% 6), a similar proportion within NELL1-associated MN cases (22.4%). Other features described in malignancy-associated MN also seen within NELL1-associated MN cases include segmental sparing of glomerular capillary loops, and the presence of mesangial immune deposits. In the NELL1-positive patients in our cohort, the presence of mesangial deposits did not correlate with malignancy. Previous reports have described endocapillary hypercellularity in malignancy-associated MN 12, and endocapillary hypercellularity or other proliferative changes were not present within NELL1-associated MN biopsies.
For a case of membranous nephropathy to be considered to be malignancy-associated, it must fulfill certain clinical criteria, which are often difficult to establish clinically within individual cases. First, the patient’s malignancy and glomerular disease must occur within a similar time frame4, generally within 2 years of one another. Second, the clinical and histologic remission of the glomerular disease should occur once the cancer is in remission12. Third, a relapse of the glomerular disease should occur upon cancer recurrence. Fourth, a pathophysiologic link is helpful, such as the same antigen expressed within the tumor and the glomerular disease 13. This temporal data was present in selected NELL1-positive patients, supporting that their MN was malignancy-associated.
The age-adjusted malignancy incidence rate for 65 years of age of both genders is 1.9% of the general population14. With 30 of 91 patients with NELL1-associated MN having malignancy, the standardized incidence ratio is 17.4 times that expected for the general population. Although NELL1-associated MN was enriched in patients with malignancy, there were many patients with MN and malignancy without an identified autoantigen. Therefore, the lack of NELL1 positivity would not exclude a patient with membranous of unknown type from undergoing a thorough workup for malignancy. The recommended workup to screen for neoplasia in the United States includes breast cancer screening by mammography in women, pap testing for cervical cancer in women, prostate cancer screening by prostate-specific antigen testing in men, colon cancer screening by colonoscopy, and a low-dose CT scan for screening of lung cancer in individuals with a history of smoking. Other guidelines also recommend serum testing for tumor-associated antigens (including CA-125, CEA, CA19–9, and AFP4). Further data is needed to determine if serum levels of NELL1 correlate with the patient’s underlying malignancy. If so, serological testing could be utilized to monitor cancer recurrence.
Questions remain regarding the pathophysiology of NELL1 in malignancy-associated MN. The types of tumors seen in patients with NELL1-associated MN are common (to include many carcinomas including prostate cancer, lung cancer, and breast cancer), and it is reported that there is high NELL1 expression within these tumors from data in the Human Protein Atlas15. However, only a very small percentage of patients with these malignancies will go on to develop MN. Therefore, further work is required to determine the pathophysiologic mechanism for anti-NELL1 antibody production and subsequent development of malignancy-associated MN.
In summary, NELL1-associated membranous nephropathy has histopathologic features that aid in the identification of cases, including a segmental to incomplete global granular capillary loop pattern for IgG and IgG1 subclass staining. One-third of patients with NELL1-associated MN have a history of malignancy. Anti-NELL1 antibodies are detected within patient sera, however further studies are required to elucidate whether antibody titers correlate with proteinuria and/or underlying malignancy. Nonetheless, a work-up to evaluate for underlying malignancy is recommended in patients with NELL1-associated membranous nephropathy.
MATERIALS AND METHODS.
Case selection.
Cases of MN were identified in our case archives. All data were collected according to a study protocol approved by Solutions IRB and all ethical principles and guidelines for the protection of human subjects in research were followed. A 9 month consecutive case series was used to determine the prevalence of NELL1 among all cases of MN. This cohort included a total of 404 membranous cases, of which 349 had residual available tissue for staining (Supplemental Figure 9). This cohort was screened to determine the frequency of NELL1-associated MN among primary MN and membranous lupus nephritis. Additional NELL1 cases, which were non-consecutive, were identified from NELL1 staining of renal biopsies with membranous nephropathy in our clinical practice after NELL1 staining was validated in our laboratory, as well as from screening cases within our biorepository, which is described below. The proportion of malignancy-associated MN cases in association with each antigen was evaluated.
Serum samples were obtained from the Arkana Laboratories biorepository. Patients with PLA2R-negative MN consented to have serum samples banked in a research protocol. The majority of serum samples were collected at the first clinical follow-up visit following diagnosis and most patients were not treatment naïve at the time of serum collection. We identified patients with NELL1-associated MN in our biorepository by immunofluorescence staining for NELL1 on residual FFPE biopsy tissue, for which we found 28 NELL1-positive patients and utilized serum samples to evaluate for serum reactivity by western blotting as described below.
Renal biopsy processing techniques.
Standard renal biopsy analytic processing methods were used including light, immunofluorescence, and electron microscopy.16, 17 All light microscopy samples were stained with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and Periodic Acid-Schiff (PAS) reagent. All direct immunofluorescence sections were cut at 3 μm and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C1q, fibrinogen, and κ-, and λ-light chains for 30 minutes, rinsed, and a coverslip applied using aqueous mounting media. For electron microscopy, thin sections were examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan).
Immunoglobulin G (IgG) subclass typing was performed on cases of MN with residual frozen tissue for evaluation (n=67 cases). IgG antibodies were all goat polyclonal direct FITC conjugates from Jackson Immunoresearch (IgG1 #115–095-205, IgG2 #115–095-207, IgG3 #F4641, and IgG4 #F9889).
Protein G tissue immunoprecipitation.
IgG co-immunoprecipitation was performed from fresh frozen tissue of renal biopsies. To do this, the residual OCT frozen tissue from archived renal biopsies was thawed, washed four times in phosphate buffered saline (PBS), and lysed by mechanical disruption of tissue cores in Pierce IP lysis buffer by bead beating. Protein extracts were incubated with 50 μl of protein G magnetic Dynabeads (Invitrogen) at room temperature for 1 hour, with shaking. Samples were then washed four times with PBS to reduce non-specific binding interactions. Proteins were digested from the beads using trypsin prior to mass spectrophotometric analysis.
Mass spectrometry.
Digested peptides were analyzed by NanoLC/MS/MS using a Thermo Orbitrap Fusion Lumos mass spectrometer. The peptides were loaded onto a reverse phase trap column (Integra-frit, New Objective, MA) containing 2.5 μm Waters XSelect CSH resin coupled to a 150 mm X 0.075 mm analytical column containing the same reverse phase resin as used in the trap. A nanoAcquity UPLC system (Waters Corp, Milfrd, MA) was then used to generate a 60 min gradient from 98:2 to 60:40 buffer A:B ratio. (Buffer A=0.1% formic acid, 0.5% acetonitrile; buffer B=0.1% formic acid, 99.9% acetonitrile.) Peptides were eluted from the column with an integrated spray tip (picofrit, New Objective) and ionized by electrospray (2.0 kV) followed by MS/MS analysis using higher energy collision induced dissociation (HCD). Survey scans of peptide precursors were performed at 240K resolution (at 400 m/z) with a 5 × 105 ion count target. Tandem MS was performed by isolation at 1.6 Th with the quadrupole, HCD fragmentation with normalized collision energy of 30, and rapid scan MS analysis in the ion trap. The obtained MS/MS data was searched against the most recent Uniprot human database containing both the Swiss Prot and the TREMBL entries using MaxQuant. Visualization of data was done using Scaffold v4.6. The false discovery rate was set at 1% for the peptide-to-spectrum matches. Normalized iBAQ values from MaxQuant were used for quantitation. iBAQ distributions for each sample were adjusted to control for differences in loading. IBAQ values equal to zero were removed from the data set. For statistical hypothesis testing, a two-sample t-test was performed for each protein using normalized iBAQ values for the two groups. If a protein was only detected in one group, a one-sample t-test was performed, using the smallest detected iBAQ value as the null hypothesis.
Immunostaining for membranous antigens
NELL1, EXT2, and PLA2R, THSD7A immunostaining was performed on formalin-fixed paraffin-embedded (FFPE) cut at a thickness of 3 μm sections. Rabbit polyclonal antibodies directed against human EXT2 (AbCam, catalog #AB102843), human PLA2R (Sigma, HPA012657), and human NELL1 (Thermo Fisher Scientific, PA5–27958) were followed by a Rhodamine red X-conjugated goat anti-rabbit secondary which was solid-phase adsorbed to ensure minimal cross-reaction with human IgG (1:100; Jackson Immunoresearch, cat# 111–295-144). A mouse monoclonal antibody directed against THSD7A (Atlas, AMAb91234) was followed by a FITC conjugated goat anti-mouse secondary (Jackson Immuno Research, catalog #115–095-205). Each case was run with positive and negative controls. The stain was evaluated by immunofluorescence microscopy. Each stain was judged to be positive if there was granular capillary loop staining within glomeruli of trace staining or greater.
Confocal microscopy.
Immunofluorescence staining was performed on 3 μm FFPE tissue sections. FFPE sections were deparaffinized and antigen retrieval was performed by heating the slides to 99°C. The sections were dual labeled with rabbit polyclonal NELL1 antibody and FITC-conjugated anti-human IgG antibody. NELL1 and IgG co-staining was performed on tissue sections from patients with PLA2R MN as negative controls. Negative controls were performed to ensure antibody specificity by omitting primary antibodies.
Co-localization of NELL1 with IgG was performed on a Leica DMI8 confocal microscope. A total of 10 glomeruli were analyzed per group. Confocal images were captured using sequential scanning of Z-stack images that were overlayed for maximal projection. Co-localization was quantified using the co-localization Leica image analysis software, where the percentage of co-localization of Rhodamine Red X and FITC was determined within glomeruli. Pierson correlation coefficients were determined for each glomerulus. Student’s t-tests were then used to determine differences and percent co-localization between groups.
NELL1 staining of tumor tissue.
Three tumor samples from two patients, including one biopsy and mastectomy specimen from a patient with invasive ductal carcinoma of the breast, and one lymph node resection from a patient with follicular lymphoma were stained with rabbit polyclonal anti-NELL1 antibody. Both patients had concurrent membranous nephropathy at the time of an active malignancy. Immunohistochemical staining for NELL1 was performed on 4 μm sections of tumor tissue on the Leica BOND platform at 1:100 primary dilution with anti-NELL1 rabbit polyclonal antibody (Thermo Fisher Scientific, PA5–27958). Rhodamine red X-conjugated goat anti-rabbit IgG (1:100; Jackson Immunoresearch, cat# 111–295-144) was used as the secondary antibody.
Western blotting.
NELL1 recombinant protein (R & D Systems, cat #5487-NL) was electrophoresed on NuPage 4–10% Bis-Tris gels (Invitrogen) at 0.2 μg/lane and transferred to PVDF membranes under non-reducing conditions. The membranes were blocked with 5% bovine serum albumin (BSA) solution in phosphate buffered saline containing 0.1% Tween (PBST), and then incubated with patient sera at 1:50 dilution in 2% BSA in PBS for 2 hours at room temperature. The membranes were washed with PBST, then incubated with anti-human IgG-HRP at 1:1000 dilution in 2% BSA in PBST for 1 hour at room temperature. The membranes were washed in PBST, with a final wash with PBS alone, and developed using 3,3’-diaminobenzidine substrate (DAB). The rabbit polyclonal anti-NELL1 antibody (Thermo Fisher Scientific, PA5–27958) was used as a positive control. A positive serum result is a band of the same molecular weight provided by the anti-NELL1 antibody. A total of 28 NELL1 positive serum samples and 28 PLA2R positive serum samples were used for evaluation.
Supplementary Material
ACKNOWLEDGMENTS:
We thank Sudhir Joshi and Lilli Barnum for their technical assistance. Research reported in this publication was supported by the National Institute On Minority Health and Health Disparities of the National Institutes of Health under Award Number R43MD014110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Beck J L. Membranous nephropathy and malignancy. Semin Nephrol 2010; 30: 635–644. [DOI] [PubMed] [Google Scholar]
- 2.Lee JC, Yamauchi H, Hopper J Jr., The association of cancer and the nephrotic syndrome. Ann Intern Med 1966; 64: 41–51. [DOI] [PubMed] [Google Scholar]
- 3.Birkeland SA, Storm HH. Glomerulonephritis and malignancy: a population-based analysis. Kidney Int 2003; 63: 716–721. [DOI] [PubMed] [Google Scholar]
- 4.Pani A, Porta C, Cosmai L, et al. Glomerular diseases and cancer: evaluation of underlying malignancy. J Nephrol 2016; 29: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int 1999; 56: 355–377. [DOI] [PubMed] [Google Scholar]
- 6.Lonnbro-Widgren J, Ebefors K, Molne J, et al. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J 2015; 8: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 2004; 19: 574–579. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S DH, Madden B, Charlesworth MC, Morelle J, Gross LA, Ravindran A, Buob D, Jadoul M, Fervenza FC, Ronco P. Neural epidermal growth factor-like 1 protein (NELL1) associated membranous nephropathy. Kidney International 2019. [DOI] [PubMed] [Google Scholar]
- 9.Franke A, Hampe J, Rosenstiel P, et al. Systematic association mapping identifies NELL1 as a novel IBD disease gene. PloS one 2007; 2: e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vriese AS, Glassock RJ, Nath KA, et al. A Proposal for a Serology-Based Approach to Membranous Nephropathy. J Am Soc Nephrol 2016; 28: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda S, Oyasu M, Kawakami M, et al. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun 1999; 265: 79–86. [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur C, Stengel B, Nochy D, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int 2006; 70: 1510–1517. [DOI] [PubMed] [Google Scholar]
- 13.Cambier JF, Ronco P. Onco-nephrology: glomerular diseases with cancer. Clin J Am Soc Nephrology 2012; 7: 1701–1712. [DOI] [PubMed] [Google Scholar]
- 14.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. National Cancer Institute 2020. [Google Scholar]
- 15.Uhlen M, Zhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science 2017; 357. [DOI] [PubMed] [Google Scholar]
- 16.Walker PD, Cavallo T, Bonsib SM. Practice guidelines for the renal biopsy. Mod Pathol 2004; 17: 1555–1563. [DOI] [PubMed] [Google Scholar]
- 17.Walker PD. The renal biopsy. Arch Pathol Lab Med 2009; 133: 181–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.