Abstract
Older adults (OA) evaluate faces to be more trustworthy than do younger adults (YA), yet the processes supporting these more positive evaluations are unclear. This study identified neural mechanisms spontaneously engaged during face perception that differentially relate to OA’ and YA’ later trustworthiness evaluations. We examined two mechanisms: salience (reflected by amygdala activation) and reward (reflected by caudate activation) – both of which are implicated in evaluating trustworthiness. We emphasized the salience and reward value of specific faces by having OA and YA evaluate ingroup male White and outgroup Black and Asian faces. Participants perceived faces during fMRI and made trustworthiness evaluations after the scan. OA rated White and Black faces as more trustworthy than YA. OA had a stronger positive relationship between caudate activity and trustworthiness than YA when perceiving ingroup, but not outgroup, faces. Ingroup cues might intensify how trustworthiness is rewarding to OA, potentially reinforcing their overall positivity.
Keywords: trustworthiness, reward, caudate, race, aging
The increasing number of senior citizens worldwide has elicited a growing interest in whether older adults (OA) are more susceptible to deception than younger adults (YA; for reviews, see Bailey & Leon, 2019; Ruffman, Murray, Halberstadt, & Vater, 2012). A proposed explanation for potentially increased deception susceptibility is that OA perceive others as being more trustworthy than do YA. Consistent with this assertion, OA rate younger and older racial ingroup faces as being more trustworthy than do YA (Cassidy, Boucher, Lanie, & Krendl, 2019; Castle, Eisenberger, Seeman, Moons, & Boggero, 2012; Ng, Zebrowitz, & Franklin Jr., 2014; Zebrowitz, Boshyan, Ward, Gutchess, & Hadjikhani, 2017; Zebrowitz, Franklin Jr., Hillman, & Boc, 2013). Perceiving people as highly trustworthy facilitates positive social contact that is central to well-being (for a review, see Ryff & Singer, 2000). Indeed, OA often rely on positive social contact to maintain their health (e.g., Seeman, 2000). Although there are many ways in which OA could seek positive social contact (Carstensen, Isaacowitz, & Charles, 1999), the current study focused on initial face perception. Here, we sought to characterize mechanisms engaged during face perception that differentially relate to the positivity of OA’ and YA’ subsequent trustworthiness evaluations.
Facial trustworthiness evaluations are made spontaneously (Klapper, Dotsch, van Rooij, & Wigboldus, 2016), persist over time (Ambady & Rosenthal, 1992; Willis & Todorov, 2006), and relate to myriad real-world outcomes (e.g., criminal sentencing; Wilson & Rule, 2016). Having higher facial trustworthiness, for example, enables people making emotional appeals to seem more sincere to perceivers (Baker, Porter, ten Brinke, & Mundy, 2016), which may increase misattributed credibility. Although OA generally perceive faces to be more trustworthy than do YA (e.g., Zebrowitz et al., 2013), it is unclear why this age difference emerges. Identifying processes relating to OA perceiving higher facial trustworthiness relative to YA is critical to understand positivity biases in OA’ face perception (e.g., Cassidy et al., 2019). Further, it may inform why some OA are more likely to be deceived by others (James, Boyle, & Bennett, 2014), providing theoretical groundwork for interventions to reduce the negative consequences of deception.
In the current study, we characterized OA’ and YA’ spontaneous brain activity when they perceived faces naturally varying in trustworthiness (Oosterhof & Todorov, 2008). Of interest was to identify brain regions whose activation differentially related to the positivity of OA’ and YA’ later trustworthiness evaluations of those same faces. This approach differs from ones used in functional magnetic resonance imaging (fMRI) tasks that measure brain activity during online trust evaluations (e.g., Hughes, Ambady, & Zaki, 2017), and conferred three key benefits. First, this approach allowed us to extend work in YA showing reliability between brain activity during spontaneous face evaluation and later trustworthiness evaluations (Rule, Krendl, Ivcevic, & Ambady, 2013) to OA. Second, it bypassed age-related task complexity effects on brain activity (Park & Reuter-Lorenz, 2009). Third, this approach tested whether brain activity tracks subjective trustworthiness evaluations. Although people show evaluative consensus (Todorov, Said, Engell, & Oosterhof, 2008), using subjective evaluations bypassed the possibility that evaluative norms from one age group (e.g., YA) might unduly affect the results.
A neuroscience approach is also beneficial because brain regions engaged during trustworthiness evaluations are well-characterized (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). One explanation for age differences in these evaluations might be that higher trustworthiness cues are more salient for OA than for YA. Supporting this possibility, trustworthiness- versus untrustworthiness-related concepts are more salient for OA than YA during face perception (Cassidy et al., 2019). Amygdala activity has been widely implicated in enhancing the perception of emotionally salient stimuli (Cunningham & Brosch, 2012). OA may thus have higher amygdala activation than YA when perceiving higher trustworthiness cues during face perception. Indeed, OA, but not YA, have higher amygdala activation when perceiving positive versus negative pictures (Mather et al., 2004). OA (versus YA) also have higher amygdala activation to faces normed by other raters as being highly trustworthy (Zebrowitz, Ward, Boshyan, Gutchess, & Hadjikhani, 2018). Although the same study found that amygdala activation was similar when OA and YA evaluated low and medium trustworthy faces, other studies have shown higher amygdala activation among YA to faces decreasing in their trustworthiness (Engell, Haxby, & Todorov, 2007; Winston, Strange, O’Doherty, & Dolan, 2002). This work suggests that whereas higher trustworthiness cues might be more salient for OA versus YA, lower trustworthiness cues might be more salient for YA versus OA. Following these patterns, a stronger positive relationship should emerge between OA’ versus YA’ spontaneous amygdala activation during face perception and their later trustworthiness evaluations of the same faces.
Although some work has shown linear effects of facial trustworthiness on amygdala activation (e.g., Winston et al., 2002), quadratic effects revealing higher responsivity toward highly trustworthy and highly untrustworthy faces have also been shown (e.g., Said, Baron, & Todorov, 2009). Past work has suggested that OA may exhibit a similar quadratic effect in amygdala responsivity toward highly trustworthy and untrustworthy faces (Zebrowitz, Ward, et al., 2018). We thus examined linear and quadratic relationships between OA’ and YA’ spontaneous amygdala activation during face perception and their later trustworthiness evaluations.
An alternative explanation is that more trustworthy faces might be more rewarding to OA than YA. Indeed, facial cue positivity (e.g., higher trustworthiness) positively relates to reward processing (Mende-Siedlecki, Said, & Todorov, 2013; Zebrowitz, Boshyan, et al., 2018; Zebrowitz, Ward, et al., 2018). Suggesting that such cues might be especially rewarding to OA, OA have more reward-related brain activity than YA when perceiving positive facial cues (e.g., happy faces; Rademacher, Salama, Grunder, & Spreckelmeyer, 2014). The caudate, a brain region whose activation reflects expected reward value (Delgado, Stenger, & Fiez, 2004; Fareri, Chang, & Delgado, 2015) and intentions to trust others (King-Casas et al., 2005), might specifically have increased activation in OA to higher trustworthiness cues. Indeed, OA versus YA have higher caudate activity toward faces normed by other raters as being more versus less trustworthy (Zebrowitz, Ward, et al., 2018). If higher trustworthiness cues are more rewarding for OA than they are for YA, a stronger positive relationship should emerge between OA’ spontaneous caudate activation during face perception and their later trustworthiness evaluations than it would for YA.
To emphasize the relative salience or reward value of specific faces, we examined positive relationships between YA’ and OA’ brain activation to faces and their later trustworthiness evaluations across racial ingroup and outgroup faces. Prior work examining age differences in trustworthiness evaluations has used racial ingroup faces (Castle et al., 2012), finding higher amygdala and caudate responses toward highly trustworthy faces among OA versus YA (Zebrowitz, Ward, et al., 2018). We examined relationships between brain activation toward faces and subsequent evaluations of their trustworthiness across racial ingroup and outgroup faces because the salience (e.g., Phelps et al., 2000) and reward value (e.g., Cikara, Botvinick, & Fiske, 2011) of faces varies by such group membership. We also considered that not all outgroups are similarly perceived (Crandall, Eshleman, & O’Brien, 2002). Relative to Black individuals, for example, Asian individuals have more positive conceptualizations (Lee, Wong, & Alvarez, 2009; F. Wong & Halgin, 2006; P. Wong, Lai, Nagasawa, & Lin, 1998), and are not stereotyped to be untrustworthy to the extent of Black individuals (Cassidy et al., 2017). Yet, ingroup preferences often emerge even when group distinctions are arbitrary (Tajfel, 1970; Tajfel & Turner, 1979). Age differences in brain activity to higher trustworthiness cues that positively relate to later evaluations might generalize across outgroups or be specific to certain outgroups. We thus examined age differences using ingroup White and outgroup Black and Asian faces.
If higher trustworthiness cues are more rewarding to OA than they are to YA, we would anticipate that OA would show a stronger positive relationship between caudate responsivity to higher trustworthiness than YA when faces hold additional reward cues. Thus, an expected age difference might be particularly pronounced for ingroup faces. Indeed, prior work suggests that ingroup faces might cue reward to a greater extent than do outgroup faces (e.g., Cikara et al., 2011; Hackel, Zaki, & Van Bavel, 2017). This possibility means that even if faces are evaluated similarly with regard to trustworthiness, a cue signaling additional reward (e.g., ingroup status) could further boost the positive relationship between caudate activity and facial trustworthiness. Notably, OA seek out more rewarding social interactions than do YA, often by limiting the scope of their relationships to focus on those that are most rewarding to them (English & Carstensen, 2014). We thus hypothesized a stronger positive relationship between OA’ versus YA’ caudate activation toward faces and their subsequent trustworthiness evaluations would emerge for ingroup White, but not outgroup Black or Asian, faces. Because OA’ enhanced reward sensitivity to positive facial cues relative to YA has only been shown using racial ingroup faces (Drueke et al., 2015; Zebrowitz, Ward, et al., 2018), examining how different group memberships constrain this difference extends the literature by identifying boundary conditions for this effect.
If higher trustworthiness cues are more salient to OA than YA, this relationship might also be expected to be confounded by other salient characteristics of faces (e.g., group membership). Supporting that some faces are more salient than others, people exhibit higher amygdala activity toward ingroup versus outgroup faces on the basis of having a shared social identity even when group membership is arbitrary (Van Bavel, Packer, & Cunningham, 2008). Because OA have fewer, but higher quality, relationships than do YA, one possibility is that perceiving increasing trustworthiness on faces might be most salient when faces belong to groups for whom OA are most likely to affiliate (e.g., ingroup versus outgroup faces; Tajfel, 1970). Because such higher amygdala activity emerges when group distinctions are arbitrary (Van Bavel et al., 2008), we hypothesized a stronger positive relationship between OA’ versus YA’ amygdala activation toward faces and their subsequent trustworthiness evaluations would emerge for ingroup White, but not outgroup Black or Asian, faces.
The current study characterized whether increased salience (reflected by amygdala activation) or reward (reflected by caudate activation) during face perception differentially related to the positivity of YA’ and OA’ later trustworthiness evaluations of the same ingroup faces. Identifying potential relationships may evince novel mechanisms for OA’ versus YA’ increased positivity toward faces.
Method
Participants
The Indiana University IRB approved this study. Forty YA (Mage=21.58 years, SD=2.81, age range=18–33, 25 female) and 35 OA (Mage=71.66 years, SD=6.09, age range=61–86, 22 female) from Indiana University and the surrounding community participated over two sessions. The first session consisted of behavioral measures (relevant measures are described below and are summarized in Table 1) and an fMRI screening. The second session was the fMRI study. Participants self-identified as White, were right-handed, did not have conditions potentially impacting cognitive function or brain activity, and provided written informed consent. The YA and OA sample sizes were selected on the basis of imaging work on trust disparities between ingroup and outgroup faces (e.g., Stanley et al., 2012), and the OA sample was more than double the size used in related work (e.g., Moran, Jolly, & Mitchell, 2012). OA had more years of education than YA and had higher vocabulary scores than YA (Shipley, 1986). YA had faster processing speed than OA, as measured by digit comparison (Hedden et al., 2002). Suggesting normal functioning, YA and OA did not differ on MMSE scores (Folstein, Folstein, & McHugh, 1975). Further, we measured participants explicit prejudice toward Black individuals using the Attitudes Toward Blacks questionnaire (Brigham, 1993) and their internal and external motivation to control prejudice (Plant & Devine, 1998). No age differences emerged on any measure, suggesting that any emergent age differences in neural activity with regard to trustworthiness and race might not be attributed to broader group differences in prejudice. Note, however, that these measures did not assess attitudes toward Asian individuals. See Table 1.
Table 1.
Means (standard deviations) for demographic and task data in YA and OA.
| YA | OA | t | p | Cohen’s d | |
|---|---|---|---|---|---|
| Years of education | 15.24 (1.88) | 16.96 (2.19) | 3.66 | <.001 | .84 |
| Vocabulary | 31.25 (4.48) | 36.63 (2.32) | 6.40 | <.001 | 1.46 |
| Processing speed | 79.00 (14.69) | 61.03 (11.69) | 5.81 | <.001 | 1.33 |
| Mini-Mental State Examination | 29.38 (.95) | 28.80 (1.62) | 1.90 | .06 | .44 |
| ATB | 36.23 (13.80) | 40.40 (11.88) | 1.39 | .17 | .32 |
| IMS | 40.53 (6.02) | 39.80 (6.99) | .48 | .63 | .11 |
| EMS | 23.40 (8.85) | 19.91 (9.23) | 1.69 | .10 | .39 |
Note. IMS: internal motivation to control prejudice; EMS: extremal motivation to control prejudice; ATB: explicit prejudice against Black individuals
Scanning session
Participants completed three fMRI tasks in a counterbalanced order. Relevant here was a face perception task. The others were an unrelated attitudes task and an unrelated theory of mind localizer. For the face perception task, 60 Black and 60 White young male faces with neutral expressions were drawn from the Eberhardt Face Database (https://web.stanford.edu/group/mcslab/cgi-bin/wordpress/examine-the-research/). This database, which has been used in fMRI studies on race perception (e.g., Cassidy & Krendl, 2016), includes attractiveness and stereotypicality ratings for each face. To determine whether brain activity positively relating to increased perceived trust emerged selectively or generalized across ingroup versus outgroup faces, 60 Asian young male faces with neutral expressions were drawn from the CAS-PEAL database (Gao et al., 2008). Fifteen undergraduates rated these faces for attractiveness and stereotypicality using the same scales (1 [not at all] to 7 [very much]) as the Eberhardt Face Database. The Black, Asian, and White faces did not differ in attractiveness, F(2, 177)=.27, p=.77, ηp2<.01. The Black, Asian, and White faces did not differ in stereotypicality, F(2, 177)=1.14, p=.32, ηp2=.01. The faces were not pre-normed with regard to trustworthiness, meaning that we were unaware how participants would rate these faces during the task. This choice was intentional given that our primary interest was in participants’ subjective ratings of the faces. In addition, 120 cars (60 black and 60 white) were selected from online searches and cropped to remove any background. No ratings on the cars were obtained. All stimuli were presented in greyscale.
The task was modeled as an event-related design over two runs each lasting three minutes and 44 seconds (four 2s dummy scans followed by 108 scan-related TRs at 2s each). Participants viewed images (30 of each race and 30 of each car color in each run) for 1s each. Images were randomly presented. All conditions were equally represented in both runs. The order of stimuli and fixations were created using a random number generator. No two images of the same type appeared twice in succession.
Half of the images appeared on the right side of the display and half on the left. It was equally probable that images from all conditions would appear on either side across runs. Participants indicated via button press on which side of the display images appeared (as in Cassidy, Lee, & Krendl, 2016; Cunningham et al., 2004). Responses were monitored to ensure attention. On average, participants responded to 294.96 (SD=7.02) of the 300 trials for a response rate of 98.32% (SD=2.34%) with 99.24% (SD=.09%) accuracy. There were no age differences in response rate (MYA=98.56%, SD=2.34%; MOA=98.05%, SD=2.35%, t(73)=.94, p=.35, d=.22) or accuracy (MYA=99.34%, SD=.06%; MOA=99.12%, SD=1.15%, t(73)=1.05, p=.30, d=.28).
Periods of jitter, in the form of a fixation cross at the center of the display, ranged from 1s to 7s and were pseudorandomly presented in each run. There were seven 1s fixations, three 3s fixations, three 5s fixations, and two 7s fixations in each run (Mjitter=3s, SD=2.27) with 10s of fixation at the beginning and 11s of fixation at the end, for a total of 66s of fixation and 150s of stimulus presentation.
After the scanning session, participants were taken to a separate room where they made self-paced trustworthiness ratings on all faces from the face perception task (1 [not at all trustworthy] to 7 [very trustworthy]). Faces were presented in a random order. Two YA were not included in the below-described analyses because ratings were not obtained from them.
fMRI data acquisition.
Whole-brain imaging was performed on a Siemens 3.0T Prisma MRI scanner using a 20-channel phase arrayed head coil at the Indiana University Imaging Research Facility in Bloomington, Indiana. Stimuli were presented using a back projector and behavioral data were collected on a Dell laptop running Windows 7. The scanner was synced to the data collection equipment via scanner TTL.
Anatomical images were collected prior to the functional tasks in one run lasting three minutes and 52 seconds. These images were acquired with high-resolution 3-D magnetization prepared rapid gradient echo sequence (sagittal rotation; 160 slices, TE = 2.7ms, TR = 1800ms, TI = 900ms, flip angle = 9 degrees, 1.0mm isotropic voxels; with no fat suppression).
Functional images were collected using simultaneous multi-slice scanning, for which 54 slices 2.2mm thick were acquired with an echo-planar image (EPI) sequence sensitive to blood oxygen level dependent contrast (T2*; TE=30ms, TR=2000ms, flip angle=52 degrees, 2.2mm isotropic voxels, FOV=242mm, in-plane matrix size=110×110, A/P phase encoding direction). Slices were 2.2mm thick with no gap and collected in an interleaved order (multi-band acceleration factor=2). These slices provided partial-brain coverage (i.e., the entire cortex with partial cerebellum, but not brainstem). Four dummy scans were included at the start of each run to allow for stabilization of the scanner signal. Dummy scans were excluded from analyses.
fMRI data preprocessing and analyses.
Preprocessing and analyses of functional data were conducted in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were realigned to correct for motion, normalized to the MNI (Montreal Neurological Institute) template, and smoothed using an 8-mm FWHM isotropic Gaussian kernel. Data were resampled to 3mm-isotropic voxels.
A GLM model for each participant that incorporated the White, Black, and Asian face conditions and trustworthiness ratings as parametric modulators, as well as covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) computed parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel. Relevant parameter estimates were included in a group level analysis, treating participants as a random effect.
Because our a priori hypotheses concerned amygdala and caudate activity, we limited analyses to anatomically-defined left and right amygdala and caudate regions of interest (ROIs) defined by the WFU Pickatlas (Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Burdette, & Kraft, 2003; Tzourio-Mazoyer et al., 2002). We examined anatomical ROIs for two reasons. First, anatomical ROIs reflect an objective and conservative way to examine our hypotheses. Second, because past work had found age differences in amygdala and caudate responsivity to faces varying in trustworthiness using anatomical ROIs (Zebrowitz, Ward, et al., 2018), we used a similar approach to most closely replicate and extend past work. Further, we limited our examination of regions involved in reward to the caudate because past work has specifically found age differences in caudate responsivity to facial trustworthiness, but not age differences in other regions associated with reward (Zebrowitz, Ward, et al., 2018).
We characterized activations within the ROIs by extracting parameter estimates of each condition using Marsbar (Brett, Anton, Valabregue, & Poline, 2002). To inform future related work, we also include data from an exploratory whole-brain analysis thresholding the Age Group × Target Race interaction to p<.005 (k=50; Table 2). To explore if emergent activations in this whole-brain analysis (e.g., activations within caudate) paralleled the expected patterns from the anatomical ROIs, we extracted parameter estimates of each condition relative to baseline averaged across a 6-mm sphere centered on peaks identified by the Age Group× Target Race interaction and created using Marsbar.
Table 2.
Results from an exploratory whole-brain analysis of the interaction between Age Group and Target Race (p < .005 uncorrected, k = 50).
| Region | BA | k | F | MNI coordinates |
|---|---|---|---|---|
| Precuneus/posterior cingulate cortex | 29/30 | 65 | 9.18 | −9, −48, 15 |
| Left posterior cingulate cortex | 29/30 | * | 7.94 | −18, −48, 6 |
| Left posterior cingulate cortex | 29/30 | * | 5.91 | −27, −48, 0 |
| Right caudate/thalamus | 63 | 7.33 | 6, 0, 6 | |
| Right caudate | * | 6.92 | 12, 18, 3 | |
| Right caudate | * | 6.50 | 9, 6, 12 | |
| Right anterior cingulate cortex | 24 | 62 | 6.87 | 6, 36, 12 |
| Left anterior cingulate cortex | 24 | * | 6.78 | −6, 36, 12 |
sub-cluster of above-listed region
Note. No regions showed a main effect of Target Race or Age Group on brain activity to increasing trustworthiness, although regions did emerge when the threshold was further relaxed.
Results
Trustworthiness Ratings
We entered mean trustworthiness ratings into a 2 (Age Group: YA, OA)×3 (Target Race: White, Black, Asian) ANOVA. Replicating past work (e.g., Zebrowitz et al., 2013), a main effect of Age Group emerged such that OA (M=4.24, SD=.53) rated faces as being more trustworthy than did YA (M=3.87, SD=.54), F(1, 71)=8.63, p=.004, ηp2=.11. There was an interaction between Age Group and Target Race, F(2,142)=3.02, p=.05, ηp2=.04. OA rated White faces (M=4.37, SD=.66) more positively than did YA (M=3.79, SD=.68), t(71)=3.68, p<.002, d=.87. OA also rated Black faces (M=4.25, SD=.71) more positively than did YA (M=3.83, SD=.94), t(71)=2.17, p=.03, d=.50. OA and YA did not differ in their ratings for Asian faces (MOA: 4.10, SD=.54; MYA: 3.99, SD=.71; t(71)=.77, p=.44, d=.17). There was no main effect of Target Race, F(2, 142)=.11, p=.90, ηp2=.002. Note that the mean ratings for White, Black, and Asian faces were not at the extreme ends of the 7-point scale. This pattern suggests that emergent age differences in the strength of relationships between brain activity and increasing trustworthiness should not be attributed to general ceiling or floor effects of group membership.
Scale use.
We analyzed how YA and OA utilized the trustworthiness ratings scale to test for systematic age differences in scale use that could unduly affect parametric modulation analyses. We first examined the average trustworthiness ratings of the 180 faces. YA’ average ratings of faces ranged from 2.26 to 5.39. A Shapiro-Wilk test showed that YA’ average ratings were normally distributed, W(180)=.997, p=.99. OA’ average ratings of faces ranged from 2.51 to 6. A Shapiro-Wilk test showed that OA’ average ratings were normally distributed, W(180)=.995, p=.81. That these average ratings did not fall at the extreme ends of the scale (1 [not at all trustworthy] and 7 [very trustworthy]) suggests that the 180 faces were not evaluated as especially extreme with regard to trustworthiness.
To extend these analyses, each participant’s relative distribution of trustworthiness ratings was used to calculate a probability of differentiation (PD) for White, Black, and Asian faces (for details, see Linville, Fischer, & Salovey, 1989). A benefit of using PD is that it identifies the probability of distinguishing between two faces on trustworthiness (e.g., that two White faces will be rated differently on trustworthiness). In other words, PD reflects the extent to which individuals differentiate between faces. Thus, PD allowed us to determine whether the distributions of ratings above differed in systematic ways for OA and YA.
We entered PD values into a 2 (Age Group: YA, OA) × 3 (Target Race: White, Black, Asian) ANOVA. Consistent with past work (Ng et al., 2014), a main effect of Age Group, F(1,71)=4.48, p=.04, ηp2=.06, showed that OA (M=.72, SD=.09) had lower PD values than YA (M=.76, SD=.09). Lower PD values reflect less differentiation in scale use (e.g., a PD of 0 occurring with a 0% chance that a perceiver will rate faces differently on the scale). The main effect thus suggests that OA’ versus YA’ ratings were less differentiated on the scale. Also consistent with past work (e.g., Cassidy et al., 2017), there was a main effect of Target Race, F(2,142)=8.57, p<.001, ηp2=.11. Ratings of White faces (M=.76, SD=.09) were more differentiated than ratings of Black (M=.72, SD=.11), t(72)=4.06, p<.001, d=.48, or Asian (M=.73, SD=.10), t(72)=4.03, p<.001, d=.47, faces. Ratings of Black and Asian faces were similarly differentiated, t(72)=.82, p=.42, d=.10. Critically, there was no interaction, F(2,142)=1.30, p=.27, ηp2=.02. Thus, even though OA had less differentiated scale use than YA, the fact that this age effect was uniform across race does not suggest that systematic differences in scale use contributed to the interaction we predicted in brain responsivity (e.g., age differences in caudate activity in response to increasing trustworthiness as a function of target race).
Amygdala Activation and Subjective Trustworthiness Ratings
Linear effect.
Using parameter estimates extracted from anatomically defined amygdala ROIS, we tested for linear responses to increasing perceived trustworthiness in ingroup White and outgroup Black and Asian faces in a 2 (Age Group: YA, OA)×3 (Target Race: White, Black, Asian)×2 (Hemisphere: Right, Left) ANOVA. A two-way interaction between Age Group and Target Race was not significant, F(2, 142)=2.22, p=.11, ηp2=.03. A three-way interaction between Age Group, Target Race, and Hemisphere was also not significant, F(2, 142)=.05, p=.96, ηp2<.01. There were no main effects of Target Race, F(2, 142)=1.05, p=.35, ηp2=.02, or Age Group, F(1, 71)=1.20, p=.27, ηp2=.02 . An unanticipated interaction between Age Group and Hemisphere emerged, F(1, 71)=3.89, p=.05, ηp2=.05, although no simple effects comparing OA and YA emerged (ts<1.49, ps>.14). No other effects were significant (Fs<1.05, ps>.35).
Quadratic effect.
We also tested for quadratic responses using the above-described ANOVA. A two-way interaction between Age Group and Target Race was not significant, F(2, 142)=.85, p=.43, ηp2=.01. A three-way interaction between Age Group, Target Race, and Hemisphere was also not significant, F(2, 142)=.21, p=.81, ηp2<.01. There were no main effects of Target Race, F(2, 142)=.43, p=.65, ηp2<.01, or Age Group, F(1, 71)=.41, p=.52, ηp2<.01. No other effects were significant (Fs<2.68, ps>.11).
Caudate Activation and Subjective Trustworthiness Ratings
Using parameter estimates extracted from anatomically defined caudate ROIs, we tested for linear responses to increasing perceived trustworthiness in ingroup White and outgroup Black and Asian faces in a 2 (Age Group: YA, OA)×3 (Target Race: White, Black, Asian)×2 (Hemisphere: Right, Left) ANOVA.1 The predicted interaction between Age Group and Target Race was significant, F(2, 142)=3.74, p=.03, ηp2=.05. Across hemispheres, OA’ caudate activity to White faces positively related to their later trustworthiness evaluations more strongly than YA’ activity, t(71)=2.08, p=.04, d=.49 (Figure 1a). There was no age difference in the strength of this relationship for Black faces, t(71)=1.71, p=.09, d=.40, or for Asian faces, t(71)=1.48, p=.14, d=.35. The three-way interaction between Age Group, Target Race, and Hemisphere was not significant, F(2, 142)=.11, p=.89, ηp2<.01. There were no main effects of Target Race, F(2, 142)=1.66, p=.20, ηp2=.02, or Age Group, F(1, 71)=.86, p=.36, ηp2=.01. No other effects were significant (Fs<1.66, ps>.19).
Figure 1.
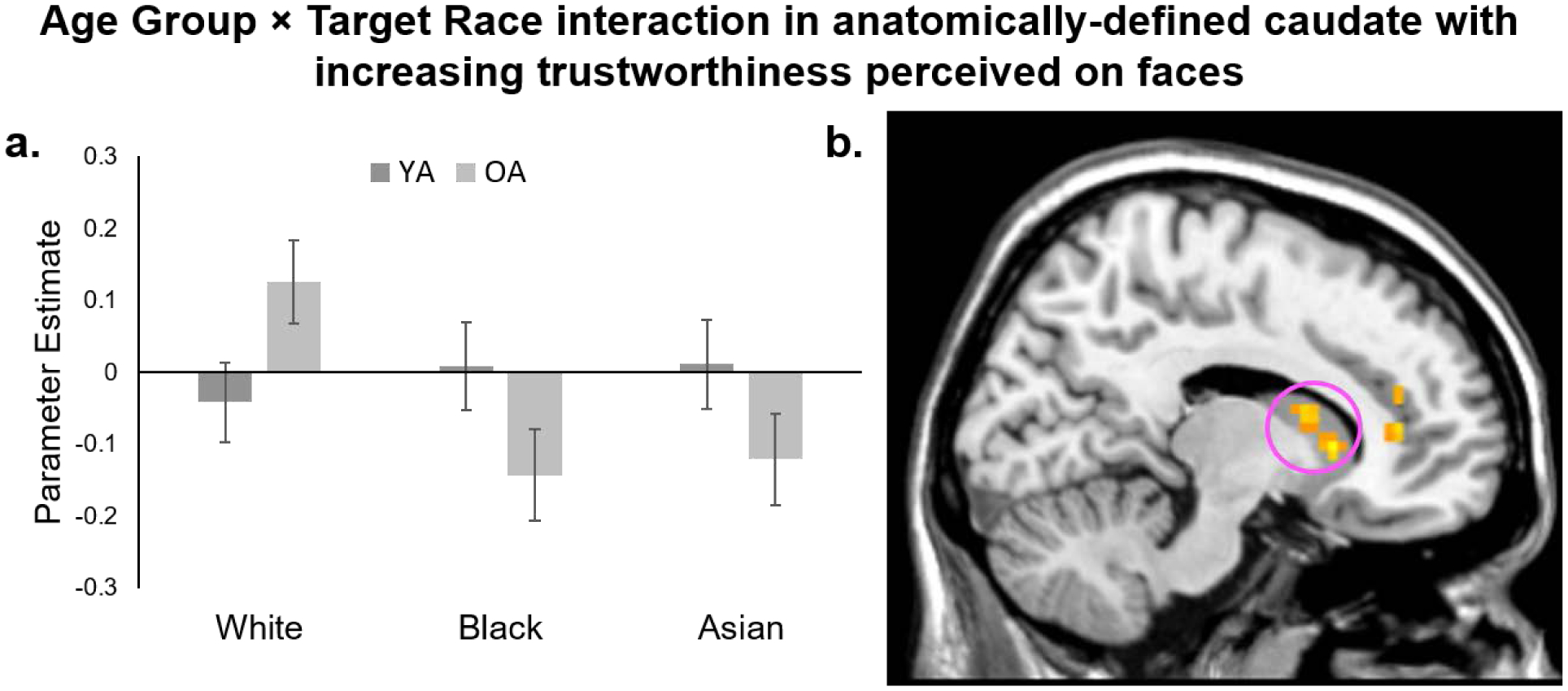
Characterizing parameter estimates based on anatomically-defined caudate across hemispheres revealed that OA’ caudate activation more strongly related to perceived trustworthiness than YA in White, but not Black or Asian faces (a). An exploratory whole-brain analyses revealed an Age Group × Target Race interaction in right caudate when perceiving increasing trustworthiness on faces (b).
The exploratory whole-brain analysis also revealed an interaction between Age Group and Target Race in right caudate (Figure 1b). We characterized this interaction to determine if it paralleled the pattern shown in our primary analyses of anatomically defined caudate. Parameter estimates extracted from a peak activation within caudate (MNI coordinates: 12, 18, 3) confirmed the interaction, F(2, 142)=5.17, p=.007, ηp2=.07. Paralleling the pattern across left and right anatomically defined caudate, OA’ right caudate activity to White faces positively related to their later trustworthiness evaluations more strongly than YA’ activity, t(71)=2.91, p=.005, d=.68. There was no age difference in the strength of this relationship for Black faces, t(71)=1.56, p=.12, d=.37, or for Asian faces, t(71)=1.47, p=.15, d=.35.
To justify our interpretation of this activation as reflecting reward-related responses to increasing facial trustworthiness, we used NeuroSynth (www.neurosynth.org; Yarkoni et al., 2011) to verify that similar activations from other work reflected reward processing. Using the search term “reward” in NeuroSynth returned a term-based meta-analytic association test map of activations from 922 studies on reward processing. The peak activation from within the caudate (MNI coordinates: 12, 18, 3) fell within activations on the association-test map from Neurosynth and had a z-value of 8.42. Z-values from association test maps determine whether specific activations more consistently emerge for experiments that do versus do not mention a given search term (e.g., reward), with higher values indicating more consistent emergence.
Discussion
A positive relationship between caudate activation to ingroup White faces and their later trustworthiness evaluations was stronger in OA than YA. This finding conceptually replicates and extends past work (Zebrowitz, Ward, et al., 2018) by linking OA’ caudate activity toward faces to their later subjective evaluations. By linking caudate activity to later evaluations, we show for the first time that spontaneous reward activity corresponds to OA’ subsequent trustworthiness evaluations. Because ingroup faces cue reward more than outgroup faces (Cikara et al., 2011), viewing highly trustworthy ingroup faces may elicit an especially strong reward response among OA. Supporting this interpretation, analyses of a right caudate peak identified in an exploratory whole-brain analyses verified that the caudate activity identified here has been found in other tasks more directly measuring reward processing.
One possibility as to why we observed age differences in the magnitude of caudate activity in response to rewarding social cues is that caudate activation specifically increases with intentions to trust others (King-Casas et al., 2005; see also Tricomi, Delgado, & Fiez, 2004). Perceiving people as trustworthy facilitates the positive social contact (for a review, see Ryff & Singer, 2000) that defines OA’ motivational goal to attend to socioemotional cues that enhance their daily emotional experiences (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000). YA, however, do not share that motivation to the same extent (English & Carstensen, 2014; Fredrickson & Carstensen, 1990). OA’ stronger positive relationship between caudate activation and subsequent trustworthiness ratings than YA may reflect the fact that OA’ expect the reward outcome of these faces to be greater. Interestingly, a region of anterior cingulate cortex, another region involved in reward processing (Bush et al., 2002), also exhibited this pattern (see Supplemental Materials). Speculatively, these findings suggest higher subjective trustworthiness evaluations elicit stronger reward responses in OA versus YA.
An open question regards whether OA’ versus YA’ stronger relationship between caudate responsivity and increasing facial trustworthiness is unique to social stimuli or extends to any stimuli OA subjectively evaluate as positive. Although the current study cannot disentangle these possibilities, prior work suggests that OA might have stronger caudate responses to any stimuli they subjectively evaluate as rewarding. Indeed, OA have higher caudate activity than YA toward smiling faces (Drueke et al., 2015), and also when tasting food when hungry (Jacobson, Green, & Murphy, 2010). Future work examining subjective evaluations of different kinds of stimuli (e.g., faces and objects) may determine the generalizability of the described effects.
A stronger relationship between OA’ caudate activity and increasing perceived trustworthiness relative to YA did not extend from racial ingroup to outgroup faces. This pattern is consistent with work showing that ingroup (versus outgroup) status is associated with higher caudate activity toward outcomes that reflect reward (Cikara et al., 2011; Hackel et al., 2017) and the likelihood of social contact (Islam & Hewstone, 1993). It also suggests that OA’ social reward sensitivity may be constrained to cues on faces belonging to groups to whom OA are more likely to affiliate (i.e., ingroup faces; Tajfel, 1970). Consistent with this interpretation, OA’ higher caudate activity than YA’ toward higher trustworthiness cues on White faces was paralleled by their more positive trustworthiness evaluations of White faces than YA (e.g., Zebrowitz et al., 2013).
Although neural response to outgroup faces was not of primary interest here, we offer a few speculative interpretations of these findings. Our finding that YA and OA had similar caudate activity to increasing trustworthiness on Asian faces suggests that higher trustworthiness cues on outgroup Asian faces might not be especially rewarding to OA. Paralleling this interpretation, OA did not have more positive evaluations of Asian faces relative to YA. YA and OA also had similar caudate activity to increasing trustworthiness on Black faces. OA, however, had higher overall trustworthiness evaluations of Black faces than YA. What might account for this inconsistency? One possibility is that OA may report positivity even when it might not hold social value for them, creating a potential mismatch between what OA say and their neural responses. Indeed, social norms suggest differential acceptable levels of bias toward different outgroups (Crandall et al., 2002), which could affect OA’ self-reported evaluations in an intergroup context. Speculatively, OA may report more positivity than YA toward Black, but not Asian, faces because they think it is less acceptable to outwardly express bias against Black than Asian individuals.
Alternatively, given the pervasive negative stereotypes associated with Black individuals (e.g., Stephan et al., 2002) the reward cue of facial trustworthiness might not be adequate to elicit higher trustworthiness evaluations of Black faces in OA. OA might, for example engage reward in tandem with other processes (e.g., control; Cassidy et al., 2016) to override prepotent negative responses. The extent that these processes are engaged in tandem may then positively relate to OA’ later trustworthiness evaluations of Black faces, eliciting more positive evaluations of Black faces in OA versus YA. Future work manipulating such processes may better pinpoint why OA evaluated Black faces more positively than YA overall. Such work might suggest a novel way that OA’ versus YA’ anti-outgroup bias (e.g., Gonsalkorale, Sherman, & Klauer, 2009) might manifest. Speculatively, differing reward cues elicited during face perception might accentuate group differences and propagate negative affect toward outgroup members. The above possibility, however, does not presuppose that YA do not have anti-outgroup bias. Rather, because YA are less sensitive than OA to social reward (Seaman et al., 2016), reward might not affect YA’ bias-related behavior to the extent that it might for OA. Overall, these possibilities highlight the utility of fMRI to understand OA’ social cognition because it speaks to identifying processes underlying OA’ evaluations rather than interpreting them at face value.
The above-described interpretations of the findings for outgroup faces are predicated on there being no significant differences in the strength of YA’ and OA’ caudate responsivity to increasing trustworthiness evaluations of faces. An interesting, albeit visual, pattern in these relationships, however, is a reversal of direction from ingroup faces. That is, YA appeared to have a stronger relationship between caudate activation and trustworthiness for outgroup faces than did OA. Although we are hesitant to overinterpret patterns that did not reach statistical significance, we note that prior work has found heightened caudate activation in response to both aversive and rewarding facial features (Liang, Zebrowitz, & Zhang, 2010). Thus, one possibility is that higher trustworthiness may attenuate the aversiveness of outgroup faces, thereby reducing aversive responses to these faces. Although no similar quadratic effects emerged in the present study1, future work manipulating the aversiveness (e.g., the extent to which faces are disfigured) and rewarding (e.g., the extent to which faces are attractive) nature of facial features on ingroup and outgroup faces can better characterize the nature of caudate responses to outgroup faces, and why age differences might emerge in response to these stimuli.
Although OA’ higher caudate activity than YA’ to increasing trustworthiness evaluated on White faces is consistent with past work (Seaman et al., 2016), it might seem surprising given that YA’ caudate activity positively relates to ingroup trust measured online during economic games (Hughes et al., 2017). Unlike the face perception task used here, past work found YA’ caudate activity tracked ingroup trust during a task with monetary incentives. Our finding that caudate activity in OA versus YA more strongly tracked ingroup trust is consistent with work suggesting that OA are motivated by social incentives, and that YA are motivated by monetary incentives (e.g., Seaman et al., 2016). One possibility is that monetary incentives activate YA’ caudate with increasing ingroup trust because it might be perceived as the optimal context for reward. Another possibility is that spontaneous caudate activity better tracks OA’ versus YA’ trustworthiness evaluations because it reflects the higher intrinsic value of trustworthiness to OA. YA may find trust valuable but engage reward processes when they are explicitly thinking about if they should trust others. Finally, other work has shown that YA’ caudate activates more when trusting lesser preferred groups in economic games (Stanley et al., 2012), a finding that has been interpreted as a reward for explicitly overcoming racial bias. It could be that these two processes operate in tandem within YA, attenuating caudate dissociation by race during face perception. It will be important for future work to disentangle these possibilities to better understand why age differences in trustworthiness evaluations widely emerge.
A limitation of the present study was that it used all younger male faces. Using younger faces removed the possibility that stereotype effects associated with other social categories (e.g., age) might unduly affect results. However, using younger faces meant that outgroup faces might be more distinct to OA versus YA. OA’ caudate activity, however, is sensitive to higher facial trustworthiness across younger and older faces (Zebrowitz, Ward, et al., 2018). Future work can disentangle if OA’ dissociation between increased perceived trustworthiness on ingroup versus outgroup faces changes when other ingroup characteristics (e.g., same-age) are represented in racial outgroup faces. Using same age ingroup faces, for example, might make higher trustworthiness cues especially salient to OA, potentially eliciting higher amygdala activity. Although no positive relationship between OA’ amygdala activity and perceived trustworthiness in ingroup faces was revealed here, manipulating additional demographic cues might be more likely to elicit such a relationship because conditions with enhanced motivational salience elicit higher amygdala activity (Cunningham & Brosch, 2012).
Other factors could also explain why linear and quadratic relationships with amygdala activation across age groups did not emerge. First, the faces used in the present study fell along a spectrum of trustworthiness. Thus, unlike other studies that have shown quadratic amygdala responses, the faces in the current work were not situated more extremely on the ends of a trustworthiness spectrum (e.g., Mattavelli, Andrews, Asghar, Towler, & Young, 2012). Second, some work suggests that trustworthiness evaluations aggregated across individuals better predict linear amygdala responses to facial trustworthiness than evaluations from individuals (Engell et al., 2007). Because we were interested in individual evaluations of trustworthiness, our approach may have been less likely to detect relationships. Future research can expand research on amygdala responsivity to facial trustworthiness by examining these possibilities.
The present study added to a growing literature identifying processes contributing to OA’ focus on positive socioemotional information (Carstensen & Mikels, 2005; Mather & Carstensen, 2005; Zebrowitz et al., 2017; Zebrowitz, Ward, et al., 2018) by showing that the positive relationship between caudate activity and later trustworthiness evaluations in ingroup White faces is stronger for OA than YA. Although this finding suggests that OA find positive facial cues on White faces especially rewarding, this study also revealed that relative group membership may provide a boundary for this effect. Increasing facial trustworthiness signals positive social contact (Todorov, 2008). Characterizing age effects on caudate activity to increasing facial trustworthiness thus has the potential to better understand OA’ interactions with an increasingly diverse United States population.
Supplementary Material
Acknowledgments
This research was supported in part by NIMH grant T32MH103213 to C.H., and grant numbers KL2TR002530 and UL1TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award to A.C.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
Footnotes
Because caudate responds to both aversive and rewarding facial characteristics (Liang et al., 2010), we also tested for quadratic effects of trustworthiness evaluations and caudate activity in YA and OA. The three-way interaction between Age Group, Target Race, and Hemisphere was not significant, F(2, 142)=.67, p=.52, ηp2=.009. The two-way interaction between Age Group and Target Race was not significant, F(2, 142)=.22, p=.80, ηp2=.003.
References
- Ambady N, & Rosenthal R (1992). Thin slices of expressive behavior as predictors of interpersonal consequences: A meta-analysis. Psychological Bulletin, 111(2), 256–274. [Google Scholar]
- Bailey P, & Leon T (2019). A systematic review and meta-analysis of age-related differences in trust. Psychology and Aging, 34(5), 674–685. [DOI] [PubMed] [Google Scholar]
- Baker A, Porter S, ten Brinke L, & Mundy C (2016). Seeing is believing: Observer perceptions of trait trustworthiness predict perceptions of honesty in high-stakes emotional appeals. Psychology, Crime, & Law, 22(9), 817–831. [Google Scholar]
- Brett M, Anton J, Valabregue R, & Poline J (2002). Region of interest analysis using an SPM toolbox. Paper presented at the International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Brigham J (1993). College students’ racial attitudes. Journal of Applied Psychology, 23, 1933–1967. [Google Scholar]
- Bush G, Vogt B, Holmes J, Dale A, Greve D, Jenike M, & Rosen B (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences, 99(1), 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen L, Isaacowitz D, & Charles S (1999). Taking time seriously: a theory of socioemotional selectivity. American Psychologist, 54(3), 165–181. doi: 10.1037//0003-066x.54.3.165 [DOI] [PubMed] [Google Scholar]
- Carstensen L, & Mikels J (2005). At the intersection of emotion and cognition: aging and the positivity effect. Current Directions in Psychological Science, 14(3), 117–121. doi: 10.1111/j.0963-7214.2005.00348.x [DOI] [Google Scholar]
- Carstensen L, Pasupathi M, Mayr U, & Nesselroade J (2000). Emotional experience in everyday life across the adult lifespan. Journal of Personality and Social Psychology, 79(4), 644–655. [PubMed] [Google Scholar]
- Cassidy B, Boucher K, Lanie S, & Krendl A (2019). Age effects on trustworthiness activation and trust biases in face perception. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 74(1), 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy B, & Krendl A (2016). Dynamic neural mechanisms underlie race disparities in social cognition. NeuroImage, 132, 238–246. [DOI] [PubMed] [Google Scholar]
- Cassidy B, Krendl A, Stanko K, Rydell R, Young S, & Hugenberg K (2017). Configural face processing impacts race disparities in humanization and trust. Journal of Experimental Social Psychology, 73, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy B, Lee E, & Krendl A (2016). Age and executive ability impact the neural correlates of race perception. Social Cognitive and Affective Neuroscience, 11(11), 1752–1761. doi: 10.1093/scan/nsw081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E, Eisenberger N, Seeman T, Moons W, & Boggero I (2012). Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences, 109(51), 20848–20852. doi: 10.1073/pnas.1218518109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Botvinick M, & Fiske S (2011). Us versus them: Social identity shapes neural responses to intergroup competition and harm. Psychological Science, 22(3), 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall C, Eshleman A, & O’Brien L (2002). Social norms and the expression and suppression of prejudice: The struggle for internalization. Journal of Personality and Social Psychology, 82(3), 359–378. doi: 10.1037//0022-3514.82.3.359 [DOI] [PubMed] [Google Scholar]
- Cunningham W, & Brosch T (2012). Motivational salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–59. [Google Scholar]
- Cunningham W, Johnson M, Raye C, Gatenby J, Gore J, & Banaji M (2004). Separable neural components in the processing of black and white faces. Psychological Science, 15(12), 806–813. [DOI] [PubMed] [Google Scholar]
- Delgado M, Stenger V, & Fiez J (2004). Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex, 14(9), 1022–1030. [DOI] [PubMed] [Google Scholar]
- Drueke B, Weichert L, Forkmann T, Mainz V, Gauiggel S, & Boecker M (2015). Neural correlates of positive and negative performance feedback in younger and older adults. Behavioral and Brain Functions, 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell A, Haxby J, & Todorov A (2007). Implicit trustworthiness decisions: Automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience, 19(9), 1508–1519. [DOI] [PubMed] [Google Scholar]
- English T, & Carstensen L (2014). Selective narrowing of social networks across adulthood is associated with improved emotional experience in daily life. International Journal of Behavioral Development, 38(2), 195–202. doi: 10.1177/0165025413515404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D, Chang L, & Delgado M (2015). Computational substrates of social value in interpersonal collaboration. The Journal of Neuroscience, 35(21), 8170–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, & McHugh P (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Jounral of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Fredrickson B, & Carstensen L (1990). Choosing social partners: How old age and anticipated endings make people more selective. Psychology and Aging, 5, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Cao B, Shan S, Chen X, Zhou D, Zhang X, & Zhao D (2008). The CAS-PEAL large-scale Chinese face database and baseline evaluations. IEEE Transactions on Systems, Man, and Cybernetics Part A: Systems and Humans, 38(1), 149–161. [Google Scholar]
- Gonsalkorale K, Sherman J, & Klauer K (2009). Aging and prejudice: Diminished regulation of automatic race bias among older adults. Journal of Experimental Social Psychology, 45, 410–414. doi: 10.1016/j.jesp.2008.11.004 [DOI] [Google Scholar]
- Hackel L, Zaki J, & Van Bavel J (2017). Social identity shapes social valuation: evidence from prosocial behavior and vicarious reward. Social Cognitive and Affective Neuroscience, 12(8), 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Park D, Nisbett R, Ji L, Jing Q, & Jiao S (2002). Cultural variation in verbal versus spatial neuropsychological function across the lifespan. Neuropsychology, 16, 65–73. [DOI] [PubMed] [Google Scholar]
- Hughes B, Ambady N, & Zaki J (2017). Trusting outgroup, but not ingroup members, requires control: Neural and behavioral evidence. Social Cognitive and Affective Neuroscience, 12(3), 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, & Hewstone M (1993). Dimensions of contact as predictors of intergroup anxiety, perceived out-group variability, and out-group attitude: An integrative model. Personality and Social Psychology Bulletin, 19(6), 700–710. [Google Scholar]
- Jacobson A, Green E, & Murphy C (2010). Age-related functional changes in gustatory and reward processing regions: an fMRI study. [DOI] [PMC free article] [PubMed]
- James B, Boyle P, & Bennett D (2014). Correlates of susceptibility to scams in older adults without dementia. Journal of Elder Abuse &Neglect, 26(2), 107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer C, Quartz S, & Montague P (2005). Getting to know you: reputation and trust in a two-person economic exchange. Science, 308(5718), 78–83. [DOI] [PubMed] [Google Scholar]
- Klapper A, Dotsch R, van Rooij I, & Wigboldus D (2016). Do we spontaneously form stable trustworthiness impressions from facial appearance? Journal of Personality and Social Psychology, 111(5), 655–664. [DOI] [PubMed] [Google Scholar]
- Lee S, Wong N, & Alvarez A (2009). The model minority and the perpetual foreigner: Stereotypes of Asian Americans. In Tewari N & Alvarez A (Eds.), Asian American Psychology: Current Perspectives (pp. 69–84). New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Liang X, Zebrowitz L, & Zhang Y (2010). Neural activation in the “reward circuit” shows a nonlinear response to facial attractiveness. Social Neuroscience, 5(3), 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville P, Fischer G, & Salovey P (1989). Perceived distributions of the characteristics of in-group and out-group members: Empirical evidence and a computer simulation. Journal of Personality and Social Psychology, 57(2), 165–188. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, & Burdette J (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, & Kraft R (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, … Carstensen L (2004). Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science, 15(4), 259–263. doi: 10.1111/j.0956-7976.2004.00662.x [DOI] [PubMed] [Google Scholar]
- Mather M, & Carstensen L (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. doi: 10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mattavelli G, Andrews T, Asghar A, Towler J, & Young A (2012). Response of face-selective brain regions to trustworthiness and gender of faces. Neuropsychologia, 50, 2205–2211. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said C, & Todorov A (2013). The social evaluation of faces: A meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience, 8(3), 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Jolly E, & Mitchell J (2012). Social-cognitive deficits in normal aging. The Journal of Neuroscience, 32(16), 5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S, Zebrowitz L, & Franklin R Jr. (2014). Age differences in the differentiation of trait impressions from faces. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof N, & Todorov A (2008). The functional basis of face evaluation. Proceedings of the National Academy of Sciences, 105(32), 11087–11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E, O’Connor D, Cunningham W, Funayama E, Gatenby J, Gore J, & Banaji M (2000). Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience, 12(5), 729–738. [DOI] [PubMed] [Google Scholar]
- Plant E, & Devine P (1998). Internal and External Motivation to Respond Without Prejudice. Journal of Personality and Social Psychology, 75(3), 811–832. [Google Scholar]
- Rademacher L, Salama A, Grunder G, & Spreckelmeyer K (2014). Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Social Cognitive and Affective Neuroscience, 9(6), 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Murray J, Halberstadt J, & Vater T (2012). Age-related differences in deception. Psychology and Aging, 27(3), 543–549. doi: 10.1037/a0023380 [DOI] [PubMed] [Google Scholar]
- Rule N, Krendl A, Ivcevic Z, & Ambady N (2013). Accuracy and consensus in judgments of trustworthiness from faces: Behavioral and neural correlates. Journal of Personality and Social Psychology, 104(3), 409–426. doi: 10.1037/a0031050 [DOI] [PubMed] [Google Scholar]
- Ryff C, & Singer B (2000). Interpersonal flourishing: A positive health agenda for the new millennium. Personality and Social Psychology Review, 4(1), 30–44. [Google Scholar]
- Said C, Baron S, & Todorov A (2009). Nonlinear amygdala response to face trustworthiness: Contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience, 21(3), 519–528. [DOI] [PubMed] [Google Scholar]
- Seaman K, Gorlick M, Vekaria K, Hsu M, Zald D, & Samanez-Larkin G (2016). Adult age differences in decision making across domains: Increased discounting of social and health-related rewards. Psychology and Aging, 31(7), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T (2000). Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion, 14(6), 362–370. [DOI] [PubMed] [Google Scholar]
- Shipley W (1986). Shipley Institute of Living Scale. In. Los Angeles: Western Psychological Services. [Google Scholar]
- Stanley D, Sokol-Hessner P, Fareri D, Perino M, Delgado M, Banaji M, & Phelps E (2012). Race and reputation: perceived racial group trustworthiness influences the neural correlates of trust decisions. Philosophical Transactions of the Royal Society B, 367(744–753). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan W, Boniecki K, Ybarra O, Bettencourt A, Ervin K, Jackson L, … Renfro C (2002). The role of threats in the racial attitudes of Blacks and Whites. Personality and Social Psychology Bulletin, 28(9), 1242–1254. [Google Scholar]
- Tajfel H (1970). Experiments in intergroup discrimination. Scientific American, 223, 96–102. doi: 10.1038/scientificamerican1170-96 [DOI] [PubMed] [Google Scholar]
- Tajfel H, & Turner J (1979). An integrative theory of intergroup conflict. In Organizational Identity: A Reader (pp. 56–65). [Google Scholar]
- Todorov A (2008). Evaluating faces on trustworthiness: An extention of systems for recognition of emotions signaling approach/avoidance behaviors. Annals of the New York Academy of Sciences, 1124, 208–224. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said C, Engell A, & Oosterhof N (2008). Understanding evaluation of faces on social dimensions. Trends in Cognitive Sciences, 12(12), 455–460. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Delgado M, & Fiez J (2004). Modulation of caudate activity by action contingency. Neuron, 41, 281–292. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Van Bavel J, Packer D, & Cunningham W (2008). The neural substrates of in-group bias: a functional magnetic imaging investigation. Psychological Science, 11, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Willis J, & Todorov A (2006). First impressions: making up your mind after a 100-ms exposure to a face. Psychological Science, 17(7), 592–598. [DOI] [PubMed] [Google Scholar]
- Wilson J, & Rule N (2016). Hypothetical sentencing decisions are associated with actual capital punishment outcomes: the role of facial trustworthiness. Social Psychological and Personality Science. [Google Scholar]
- Winston J, Strange B, O’Doherty J, & Dolan R (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience, 5(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Wong F, & Halgin R (2006). The “model minority”: bane or blessing for Asian Americans? Journal of Multicultural Counseling and Development, 34(1), 38–49. [Google Scholar]
- Wong P, Lai C, Nagasawa R, & Lin T (1998). Asian Americans as a model minority: self-perceptions and perceptions by other racial groups. Sociological Perspectives, 41(1), 95–118. [Google Scholar]
- Yarkoni T, Poldrack R, Nichols T, Van Essen D, & Wager T (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz L, Boshyan J, Ward N, Gutchess A, & Hadjikhani N (2017). The older adult positivity effect in evaluations of trustworthiness: emotion regulation or cognitive capacity. PLOS one. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz L, Boshyan J, Ward N, Hanlin L, Wolf J, & Hadjikhani N (2018). Dietary dopamine depletion blunts reward network sensitivity to face trustworthiness. Journal of Psychopharmacology, 32(9), 965–978. [DOI] [PubMed] [Google Scholar]
- Zebrowitz L, Franklin R Jr., Hillman S, & Boc H (2013). Older and younger adults’ first impressions from faces: similar in agreement but different in positivity. Psychology and Aging, 28(1), 202–212. doi: 10.1037/a0030927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz L, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2018). Older adults’ neural activation in the reward circuit is sensitive to face trustworthiness. Cognitive, Affective, & Behavioral Neuroscience, 18(1), 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


