Abstract
Anisakidae, marine nematodes, are underrecognized fish-borne zoonotic parasites. Studies on factors that could trigger parasites to actively migrate out of the fish are very limited. The objective of this study was to assess the impact of different environmental conditions (temperature, CO2 and O2) on larval motility (in situ movement) and mobility (migration) in vitro. Larvae were collected by candling or enzymatic digestion from infected fish, identified morphologically and confirmed molecularly. Individual larvae were transferred to a semi-solid Phosphate Buffered Saline agar, and subjected to different temperatures (6 ℃, 12 ℃, 22 ℃, 37 ℃) at air conditions. Moreover, different combinations of CO2 and O2 with N2 as filler were tested, at both 6 °C and 12 °C. Video recordings of larvae were translated into scores for larval motility and mobility. Results showed that temperature had significant influence on larval movements, with the highest motility and mobility observed at 22 ℃ for Anisakis spp. larvae and 37 ℃ for Pseudoterranova spp. larvae. During the first 10 min, the median migration of Anisakis spp. larvae was 10 cm at 22 ℃, and the median migration of Pseudoterranova spp. larvae was 3 cm at 37 ℃. Larval mobility was not significantly different under the different CO2 or O2 conditions at 6 °C and 12 ℃. It was concluded that temperature significantly facilitated larval movement with the optimum temperature being different for Anisakis spp. and Pseudoterranova spp., while CO2 and O2 did not on the short term. This should be further validated in parasite-infected/spiked fish fillets.
Subject terms: Parasite physiology, Applied microbiology
Introduction
Marine ascaridoids, particularly of the family Anisakidae, are underrecognized zoonotic foodborne parasites which may have a substantial influence on public health. They lead to human anisakidosis1–3, which is mainly caused by Anisakis simplex sensu stricto, Anisakis pegreffii and Pseudoterranova decipiens4–6, while other members of the Anisakidae and Raphidascaridae family are less commonly responsible for human infections7,8. High prevalence rates of Anisakidae have been found in most wild commercial marine fish species (some species close to 100%), with a worldwide distribution9,10. The occurrence of Anisakidae varies between fish species and fishing sea. For example, Mercken et al. reported a mean prevalence of respectively 95% and 5% in Pollack and Atlantic salmon, with fish caught in the Northeast Atlantic having the highest infection rate (68%) from all investigated fishing zones11. Human infection after the consumption of fish infected with the L3 larvae may lead to a variety of symptoms, ranging from mild to severe abdominal pain and mild allergic reactions to anaphylaxis7,12. The latter is caused by specific allergens of the larvae, of which some are heat and freeze resistant13. An increase in fish and human cases has occurred in the past few decades14. Globally, the number of human cases was estimated to be 20,000 per year prior to 2010, with the majority attributable to Japan15. The consumption of sushi and sashimi (raw fish dishes) is an important source of human infection in Japan14,16, with 2000–3000 cases of anisakiasis being reported per year17. However, in a study of Bao and colleagues18, the number of cases needing hospitalization in Spain was estimated to be 8,320 per year, excluding mild infections, which indicates that the earlier mentioned global figures are probably seriously underestimated18. In addition to the impact on public health, these parasites are also an important cause of economic cost for the sector. Because of the public health risk, the European Union Regulation No 853/2004 stipulates that all fish destined for raw consumption, cold-smoking processing with internal temperature below 60 ℃, marinated or salted process should be frozen at a temperature of no higher than -20 ℃ for at least 24 h19. This leads to an important cost for the industry and has consequences on the quality of the fish. In addition, the sale of fresh fish has led to a growing number of consumer complaints, because the larva can become very mobile and therefore very visible for the consumer on top of the fillets9. The problem was reported to be more pronounced with the introduction of modified atmosphere packaging (MAP). This packaging system, including a mixture of oxygen (O2), carbon dioxide (CO2) and nitrogen (N2) in the headspace of a package, guarantees the extension of the shelf life of fresh products and is intensively applied by the fish industry to present packaged fresh fish to the consumer at the retail level. However, increased carbon dioxide levels have been reported to activate parasitic larvae20, resulting in more consumer complaints, which have a negative impact on the sales and therefore sector.
Recent risk models showed that inactivating or removing infectious larvae would greatly reduce the number of human cases18. The most commonly used techniques to inactivate larvae, such as freezing21,22 or heating23, also change the organoleptic properties of the fish. Moreover, the fish is no longer considered as “fresh” and the effects of certain allergens may still exist12, so the allergic public health risk persists. The fish industry tries to identify infected fish / fish fillets and remove the larvae manually in the case of light infections, while heavily infected fish are being discarded. The routinely used diagnostic method is candling, by which the fish fillets are placed on a transparent plate above a light source. Visible larvae can be removed from the fillets during this process. However, this technique is very labor intensive and lacks sensitivity24, thereby neither eliminating the public health risks, nor consumers' complaints.
An applicable method which can remove larvae quickly from infected fish, without affecting its quality and without increasing the microbiological contamination should therefore be developed. Currently, there is no such technique available. To allow the development of such a method, more insight is necessary in the triggers for larvae to actively migrate out of the fish flesh. According to Simat et al.25, certain biochemical changes in dead fish trigger post mortem migration of larvae from viscera to flesh. Examples of such biochemical changes are the production of biogenic amine25, the accumulation of fatty acid, lactic acid or phosphoric acid, which decrease the pH of flesh26,27. But those factors are not implementable in practice. As mentioned above, Pascual et al. studied larval motility, mobility and behavior after 3 days of storage and found that MAP (CO2, O2) affected the migration of larvae20. Cipriani et al. studied larval migration after storage at low temperature (2 ℃, 5 ℃, 7 ℃) for 24 h, 48 h and 72 h, concluding that temperature plays an important role in larval post mortem mobility26. Moreover, there is an association between temperature and gene expression levels of some antigenic and functional proteins released by zoonotic species of Anisakis, which may be involved in the host tissue migration of the parasite in the hostile target tissues of the fish host28. Long treatment periods are unsuitable for the fish processing industry considering the short shelf-life of fresh fish. There are no studies that have systematically evaluated short-term effects (in a timeframe useful for the industry) of temperature, CO2 and O2 on the motility/mobility of larvae. The objectives of this study were therefore to investigate the short-term effects of temperature, CO2 and O2 on larval motility and mobility. For this, Pseudoterranova spp. larvae and Anisakis spp. larvae were subjected to different conditions of temperature, CO2 and O2 with N2 as filler in vitro. Larval movements were video recorded, transcribed and analyzed. The study will provide information about the potential of the above-mentioned factors to trigger larvae to actively move, which could then potentially be applied to quickly remove larvae from fish in an industrial setting.
Materials and methods
Collection of larvae
Fish and larvae (the latter collected during industrial candling) were supplied by a fish whole-sale company in Belgium and transported to the laboratory on ice on a weekly basis until the end of the experiment. Fish (mainly mackerel, blue whiting, cod, herring and pollack29) were skinned and filleted upon arrival and digested the following day to collect the Anisakidae larvae, which were subsequently defined as one batch. Anisakidae larvae collected during the industrial candling were rinsed and cleaned, and digested if too much fish tissue was present, and such collected larvae were defined as another batch. Digestion was done as described by Jackson et al. with a minor modification30. Briefly, fish muscle and viscera were separately put in a pepsin and HCl digestion solution at 37 ℃ and stirred for 30 min. The digested fish solution was poured over a sieve where after the contents of the sieve were rinsed into a petri dish placed on the candling table. Larvae were collected, visually assessed for integrity and viability and identified according to the macroscopic morphological features of larvae31,32 into macroscopically assumed Pseudoterranova spp. and Anisakis spp.. After the in vitro experiment, subsets of larvae were identified molecularly (see 2.5). Larvae from the same batch were allocated to each of the conditions under evaluation (see 2.3), so that larvae from the same source were used for pairwise comparison of different conditions. Grouped larvae were stored in Phosphate Buffered Saline (PBS) at 4 °C until use within 24 h.
In vitro experimental setup
Medium preparation
To observe larval movement under different temperature, CO2 and O2 conditions, Phosphate Buffered Saline (PBS) agar medium was prepared as a carrier. To determine the optimal agar concentration for this study, the migration and ease of visual inspection of larvae in 2, 2.5, 3, 3.5 g/L agar were compared in a preliminary experiment. Finally, 2.5 g/L of agar was chosen as the most optimal concentration as larvae could actively move or coil in 2.5 g/L agar and PBS medium and were easily visible.
Firstly, 1000 mL PBS solution was prepared using 5 PBS tablets (Sigma-Aldrich, St. Louis, MO, USA), then 2.5 g agar was added. The solution was mixed and dissolved by heating with frequent agitation. Lastly the solution was boiled for 1 min and transferred to a water bath at 45 °C to cool down for further use.
Preparation of larvae within the medium
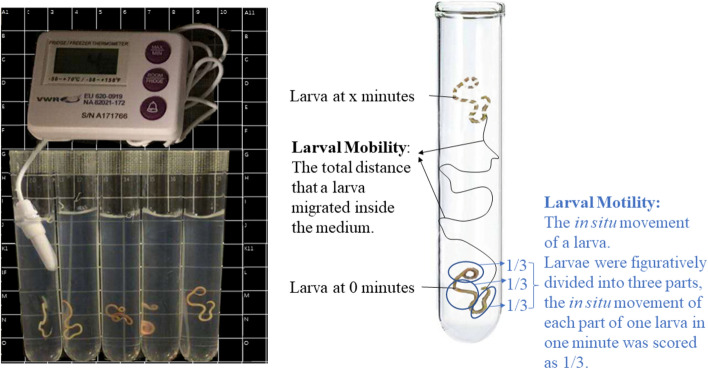
Tubes and larvae were placed at 4 ℃ to cool down for 10 min. One mL of medium was poured into each tube and solidified immediately. Then, one larva was transferred in each of the tubes, after which another 9 mL of the medium was poured into each tube. The tubes were then placed in the fridge at 4 °C to ensure a rapid solidification of the agar medium. The temperature of the medium inside the tubes was monitored by a thermometer. Once the temperature of the medium reached 4 ℃, the tubes were ready for further use. Five tubes from which one contained a thermometer were positioned in front of a black background as visualized in Fig. 1. This setup was repeated for a total of 4 times per condition, for both Pseudoterranova spp. and Anisakis spp., resulting in a total of 20 replicates per condition and per genus, except that 21 larvae were investigated for the effect of temperature on Anisakis spp. larvae and 22 larvae were investigated for the effect of CO2 on Pseudoterranova spp. larvae at 6 ℃.
Figure 1.
Experimental setup (left) and measurements of larval movements (right).
Larval treatments at different temperatures and gas atmospheres
To evaluate the effect of temperature, 6 ℃, 12 ℃, 22 ℃ and 37 ℃ were chosen based on previous studies26 on the one hand, as those temperature ranges may contribute to increasing the movement of larvae and on the other hand, in a more arbitrary way, aiming to cover a sufficiently wide temperature range including 37 ℃ which approaches the temperature of marine mammals, the final hosts of larvae. The tubes with background were put in a ventilated refrigerator or incubator at respectively 6 ℃, 12 ℃, 22 ℃ and 37 ℃ with a timer and thermometer recording the time and real-time medium temperature. Larval movements were registered by video recording using a tablet (Apple, A1822, USA) with a light source. After 30 min, the recording was stopped.
For the CO2 and O2 experiment, modified atmosphere packaging was applied. Different CO2 and O2 concentrations were evaluated at two different temperatures, 6 °C and 12 °C. Tubes with larvae, background and thermometer were placed in a plastic packaging tray (Decapac, HERENTALS, Belgium) and transferred to the fridge at 6 or 12 ℃. When the temperature of the medium reached the destinated temperature, silica gel (Type II, 3.5 mm bead size) (Sigma-Aldrich, St. Louis, MO, USA) was put into the tray to eliminate the moisture outside the tubes and trays were immediately modified atmosphere packaged at the required gas mixture with a MAP tray sealer machine (Deca, GROSCHOPP, Germany). A top film (Bemis Packaging Benelux, MONCEAU-SUR-SAMBRE, Belgium) with the specifications OPALEN/HB/65/AF/PP/PL was used. For the CO2 experiment, 20%, 40%, 60%, 80%, or 90% CO2 was used for the experiment at 12 °C and 0%, 20%, 60% or 90% CO2 for the experiment at 6 °C. In all packages 5% O2 was added together with N2 as a compensation gas. For the O2 experiment at 6 and 12 ℃, 0%, 5%, 21% or 80% O2 was used compensated with N2. After packaging, trays were put back into the corresponding fridge with a timer recording the time. Larval movements were registered by video recording during the first 30 min. Additionally, for the experiments at 6 ℃, the movements were further recorded during 1-min recordings every 30 min for 6 h.
Assessment of larval movements
Larval movements were assessed through video recording (see “Larval treatments at different temperatures and gas atmospheres”). The video recordings were analyzed to define larval motility and mobility to evaluate the changes of larval movements, which is shown in Fig. 1.
Assessment of larval motility
Larval motility was defined as the in situ movement of a larva. To quantify this motility, larvae were figuratively divided into three parts: the head part, the middle part and the tail part. In 1 min, the in situ movement of each part of one larva was scored as 1/3. For example, if only one part of the larva moved in 1 min, the motility of this larva is 1/3; if two body parts moved, the motility of larva for that minute is 2/3; if all three parts of the larva moved in 1 min, the motility of the larva was recorded as 1. The motility of larvae was determined for each video recorded minute. For the 30-min movies, the motility of one larva in 30 min was calculated as the sum of larval motility of each minute.
Assessment of larval mobility
Larval mobility is defined as the total distance that a larva migrated inside the medium. To determine the migration distance of larvae, the tubes were placed on a black background with white lines; the distance between each line was 1 cm. For larvae that had moved out of the medium, their migration distance inside the medium was divided by the time during which larvae were inside the medium and the value was multiplied by 30. For the effect of temperature, larval mobility during the first five and ten minutes was also analyzed. Similar as for 30 min, for larvae that had moved out of the medium during the first five or ten minutes, the migration distance inside the medium was divided by the time during which larvae were inside the medium in the first five or ten minutes and the value was multiplied by five or ten, respectively.
Identification of larvae at species level
After the movies were taken, larvae were stored in ethanol for a subgroup identification by Polymerase Chain Reaction combined with Restriction Fragment Length Polymorphism (PCR/RFLP). For each tested condition, four larvae were selected for Anisakis spp. identification (one larva from each tray) and one larva for Pseudoterranova spp. identification. In total, 110 larvae were selected and identified. DNA extraction from larvae and PCR amplification for the Internal Transcribed Spacer (ITS) fragment were implemented according to Zhu et al.33. The amplified ITS fragments were digested by endonucleases HinfI and HhaI following the protocol from the European Reference Laboratory (ISS, 2018). The PCR/RFLP profiles were confirmed, followed by a species identification of larvae34–36.
Statistical analysis
Video recordings were transcribed into Excel (Microsoft office 2016, Microsoft, USA) according to the definition of assessment of larval movements (motility and mobility). In total, 11 larvae were excluded from the analyses because they were dead (n = 2) or because they were on top of the medium at the beginning of the experiment (n = 9). Conditions were only compared within experiments that used larvae from the same batch(es) to account for a potential batch effect. Since the data were not normally distributed, non-parametric Kruskal–Wallis rank sum tests were carried out to check if the movements were the same for each of the conditions. If at least one condition differed (p < 0.05), pairwise comparisons between conditions were performed using Wilcoxon rank sum tests (= Mann–Whitney tests) with Bonferroni corrections for multiple testing. R version 3.6.2 was used for all statistical analyses (R Core Team, 2019).
Results
Larvae identification
After the preliminary morphological identification of larvae, molecular analysis was conducted on respectively a total of 88 and 22 macroscopically assumed Anisakis spp. and Pseudoterranova spp. larvae. For Anisakis spp., 86 larvae were identified as A. simplex, and two as A. pegreffii. The latter two larvae were both from the CO2 experiment, one from 0% CO2 at 6 ℃ and the other from 90% CO2 at 12 ℃. All Pseudoterranova spp. larvae were identified as P. decipiens s.s.
Effect of temperature on larval movements
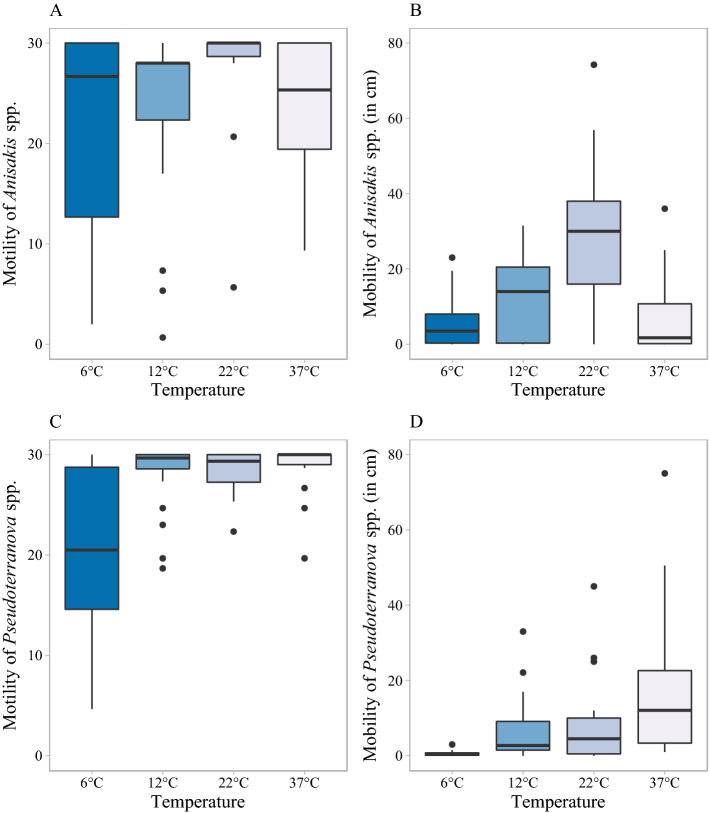
The effects of four different temperatures on larval motility and mobility are shown in Fig. 2. For Anisakis spp., temperature had a significant effect on larval motility (Kruskal–Wallis test: χ2-value = 11.1, df = 3, p = 0.011), with the highest motility at 22 °C. At this temperature, the median value for motility was 30, which indicates that over 50% of larvae were moving from head to tail during the full 30 min. During the 30 min, the motility of Anisakis spp. larvae at 22 °C was significantly higher compared to larval motility at 12 °C (p = 0.007). All Anisakis spp. larvae were motile in at least 1 min of the 30 min at all temperatures, but there was a variation between larvae. For example, 7 out of 21 Anisakis spp. larvae were motile during the full 30 min at 6 ℃, but there were also 3 Anisakis spp. larvae that had a motility less than 5.
Figure 2.
Effect of temperature on larval motility and mobility (in cm) during the first 30 min. (A) Motility of Anisakis spp., (B) mobility of Anisakis spp., (C) motility of Pseudoterranova spp. and (D) mobility of Pseudoterranova spp. under different temperature conditions. The box consists of the upper quartile, median and lower quartile. The whiskers are drawn up to the highest or lowest observed point from the dataset that falls within 1.5 times the interquartile range. Observations beyond the end of the whiskers are plotted individually.
There were respectively 9 out of 21 and 3 out of 20 Anisakis spp. larvae that migrated out of the medium at 22 ℃ and 37 ℃. Similar as the motility of Anisakis spp. larvae, also the mobility of Anisakis spp. larvae differed at the four temperatures (χ2 = 22.6, df = 3, p < 0.001). Mobility of Anisakis spp. larvae was significantly higher at 22 ℃ compared to the mobility at other temperatures (p ≤ 0.02). At 22 ℃, the median migration of Anisakis spp. larvae was 30 cm, but mobility varied greatly between the different larvae, ranging between 0 and 74 cm (interquartile range, IQR = 22). Only one out of 21 Anisakis spp. larvae didn’t migrate at 22 °C, while 5 Anisakis spp. larvae didn’t migrate at 6 °C and 37 °C (Table 1). Mobility of Anisakis spp. larvae at 6 ℃ was significantly lower compared with larval mobility at 22 ℃ (W = 54.5; p < 0.001). At 6 °C, Anisakis spp. larvae migrated between 0 and 23 cm (IQR = 7.7), with a median migration of 3.5 cm. At 12 ℃, Anisakis spp. larvae had a median migration of 14 cm. Mobility of Anisakis spp. larvae at 37 ℃ decreased significantly compared with larval mobility at 22 ℃ (W = 356.5; p < 0.001). At 37 °C, Anisakis spp. larvae had a median migration of 1.7 cm (range between 0 and 36 cm; IQR = 10.6).
Table 1.
Number of larvae that were motile/mobile in 30 min of CO2 and O2 experiments.
| Parasite | Category | Temperature | CO2 at 6 ℃ | CO2 at 12 ℃ | O2 at 6 ℃ | O2 at 12 ℃ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 ℃ | 12 ℃ | 22 ℃ | 37 ℃ | 0% | 20% | 60% | 90% | 20% | 40% | 60% | 80% | 90% | 0% | 5% | 21% | 80% | 0% | 5% | 21% | 80% | ||
| Anisakis spp. larvae | MT | 21 | 21 | 21 | 20 | 20 | 19 | 19 | 19 | 15 | 19 | 18 | 18 | 15 | 15 | 17 | 16 | 8 | 12 | 12 | 13 | 10 |
| MB | 16 | 18 | 20 | 15 | 10 | 9 | 7 | 9 | 9 | 14 | 12 | 11 | 8 | 4 | 8 | 11 | 1 | 3 | 6 | 5 | 2 | |
| N | 21 | 21 | 21 | 20 | 20 | 20 | 19 | 20 | 18 | 19 | 19 | 20 | 19 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Pseudoterra-nova spp. larvae | MT | 20 | 20 | 20 | 18 | 17 | 21 | 22 | 17 | 20 | 19 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 19 |
| MB | 15 | 19 | 18 | 18 | 5 | 13 | 5 | 8 | 18 | 19 | 19 | 19 | 20 | 12 | 12 | 8 | 8 | 14 | 10 | 16 | 12 | |
| N | 20 | 20 | 20 | 18 | 17 | 22 | 22 | 17 | 20 | 19 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 19 | |
MT the number of larvae that were motile in 30 min, MB the number of larvae that were mobile in 30 min, N total number of larvae analyzed (excluding ineligible larvae).
Larval mobility under four different temperatures was also evaluated respectively during the first 5 and 10 min. During the first 10 min, mobility of Anisakis spp. larvae at 22 ℃ was already significantly higher than the mobility at other temperatures (p < 0.01). At 22 ℃, Anisakis spp. larvae had a median migration of 10 cm and a maximum value of 30 cm in 10 min. Also, 20 out of 21 Anisakis spp. larvae (95%) were mobile at 22 ℃ while only 59% to 67% of Anisakis spp. larvae were mobile at the other temperatures during the first 10 min. During the first 5 min, the mobility of Anisakis spp. larvae at 22 ℃ was already significantly higher than the mobility of Anisakis spp. larvae at 6 ℃ and 12 ℃ (p < 0.01), but the difference in the mobility of Anisakis spp. larvae between 22 ℃ and 37 ℃ was not significant (p = 0.057). After 5 min, Anisakis spp. larvae had a median migration of respectively 3 cm and 0.7 cm at 22 ℃ and 37 ℃ and at 22 ℃ 86% of Anisakis spp. larvae were mobile while only 57% to 65% of Anisakis spp. larvae were mobile at the other temperatures.
All Pseudoterranova spp. larvae were motile during at least 1 min of the 30 min at all temperatures. Motility of Pseudoterranova spp. larvae at 6 ℃ was significantly lower than larval motility at other temperatures with p < 0.01 (Fig. 2). There was no significant difference in motility of Pseudoterranova spp. larvae between 12, 22 and 37 ℃ and there were respectively 40%, 40% and 56% Pseudoterranova spp. larvae that had a motility value of 30 at 12, 22 and 37 °C, meaning that those larvae were fully motile during every minute of the 30 min.
For the mobility of Pseudoterranova spp. larvae, the highest migration was observed at 37 ℃ (Fig. 2). There were 6 out of 18 Pseudoterranova spp. larvae that migrated out of the medium at 37 ℃. All Pseudoterranova spp. larvae were mobile at 37 °C, with a median migration of 12 cm, and a maximum of 75 cm. Mobility of Pseudoterranova spp. larvae at 6 °C ranged between 0 and 3 cm and was significantly lower compared to mobility at 12 ℃ (W = 39; p-value < 0.001), 22 ℃ (W = 84.5; p-value = 0.01) and 37 ℃ (W = 8.5; p-value < 0.001). The differences of larval mobility between 12, 22 and 37 ℃ were not significant (p > 0.05). There were five Pseudoterranova spp. larvae that did not migrate at 6 ℃ with all Pseudoterranova spp. larvae having a mobility at 37 ℃ (Table 1). The same trend was already observed after 5 and 10 min, with the lowest mobility at 6 °C compared with other temperatures (p < 0.01). During the first 10 min, Pseudoterranova spp. larvae had a median migration of 3 cm at 37 ℃, with a maximum of 26 cm. Moreover, 90% of Pseudoterranova spp. larvae were mobile at 37 ℃ during the first 10 min, while 70% were mobile at 12 and 22 ℃ and only 30% were mobile at 6 ℃. During the first 5 min, Pseudoterranova spp. larvae had a median migration of 1.5 cm at 37 ℃, with a maximum of 11 cm. During the first 5 min, 83% of Pseudoterranova spp. larvae were mobile at 37 ℃, while only 65% and 60% of Pseudoterranova spp. larvae were mobile at 12 and 22 ℃ and 20% of Pseudoterranova spp. larvae were mobile at 6 ℃
Effect of CO2 on larval movements
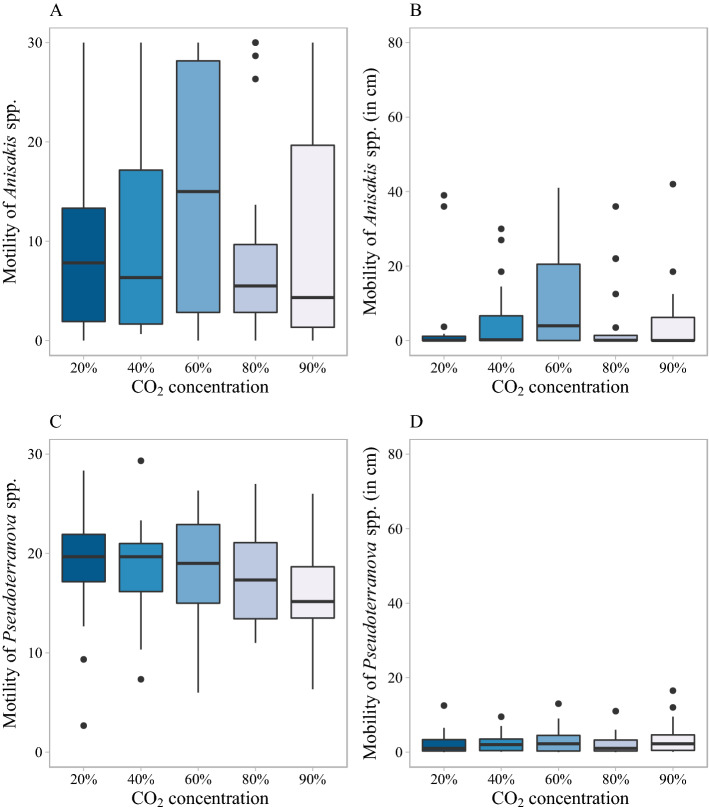
The effect of different CO2 concentrations on larval movement was tested at 6 °C and at 12 °C. At 6 ℃, as shown in Table 1, more than 95% of Anisakis spp. larvae showed a level of motility for each condition, but only 37% (at 60% CO2) to 50% (at 0% CO2) of Anisakis spp. larvae were mobile (see Supplementary Fig. 1). At 6 ℃, there was no significant difference in the motility or mobility of Anisakis spp. larvae between the different tested CO2 concentrations. The mobile larvae only migrated a very short distance with more than 96% of Anisakis spp. larvae having a mobility below 2.5 cm.
At 12 °C, as it is shown in Table 1, most Anisakis spp. larvae were motile under the different CO2 concentrations. The motility and mobility of Anisakis spp. larvae under 20%, 40%, 60%, 80% and 90% CO2 at 12 ℃ are shown in Fig. 3. There was no significant difference of larval motility for Anisakis spp. larvae at different CO2 concentrations at 12 °C, but the highest median motility (15) was observed at 60% CO2. Depending on the condition, between 30 to 50% of Anisakis spp. larvae did not migrate at 12 ℃, the median larval mobility was therefore very close to 0 at 20%, 40%, 80%, 90% CO2. Similar as for larval motility, Anisakis spp. larvae had the highest median (4 cm) at 60% CO2, but there were no significant differences of larval mobility between different CO2 concentrations for Anisakis spp. larvae at 12 °C.
Figure 4.
Effect of O2 on larval motility and mobility at 12 ℃ during the first 30 min. (A) Motility of Anisakis spp., (B) mobility of Anisakis spp., (C) motility of Pseudoterranova spp. and (D) mobility of Pseudoterranova spp. under different O2 conditions. The box consists of the upper quartile, median and lower quartile. The whiskers are drawn up to the highest or lowest observed point from the dataset that falls within 1.5 times the interquartile range. Observations beyond the end of the whiskers are plotted individually.
Figure 3.
Effect of CO2 on larval motility and mobility at 12 ℃ during the first 30 min. (A) Motility of Anisakis spp., (B) mobility of Anisakis spp., (C) motility of Pseudoterranova spp. and (D) mobility of Pseudoterranova spp. under different CO2 conditions. The box consists of the upper quartile, median and lower quartile. The whiskers are drawn up to the highest or lowest observed point from the dataset that falls within 1.5 times the interquartile range. Observations beyond the end of the whiskers are plotted individually.
For Pseudoterranova spp. at 6 ℃, only one larva at 20% CO2 was not motile, while all other Pseudoterranova spp. larvae were motile in at least 1 min of the total 30 min under all tested concentrations of CO2. The motility of Pseudoterranova spp. larvae at 20% CO2 was significantly higher compared to larval motility at 0% CO2 (p = 0.043) (see Supplementary Fig. 1). There were only 23% (60% CO2) to 59% (20% CO2) Pseudoterranova spp. larvae that were mobile at different CO2 concentration at 6 ℃ (Table 1) with a maximum migration of only 1.5 cm in 30 min. No significant differences in migration of Pseudoterranova spp. larvae between the different CO2 concentrations were observed at 6 ℃.
At 12 ℃ (Fig. 3), all Pseudoterranova spp. larvae were motile, with no significant differences in larval motility between different CO2 concentrations. More than 90% of Pseudoterranova spp. larvae were mobile under each CO2 condition, with the migration ranging between 0 and 16.5 cm, but similar as for motility, there were no significant differences in larval mobility between different CO2 concentrations.
For the effect of CO2 on larval movements measured every 30 min for 6 h at 6 ℃, there were only 1 Anisakis spp. larvae and 7 Pseudoterranova spp. larvae that were still mobile after 6 h. The number of larvae that are still motile every 30 min until 6 h at different CO2 conditions is shown in Table 2. For Anisakis spp. larvae, the number of larvae that were motile at 1 h decreased suddenly compared with the number of motile larvae at 0.5 h. More Pseudoterranova spp. larvae were still motile after 6 h compared with Anisakis spp. larvae at 0%, 20%, 60% and 90% CO2.
Table 2.
Number of larvae that were motile in 1-min recordings every half hour during 6 h.
| Parasite | Conditions | Time point (h) at which one-minute recording was performed within a total period of 6 h | N | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 5.5 | 6 | |||
| Anisakis spp. larvae | 0%CO2 | 16 | 16 | 4 | 3 | 5 | 4 | 2 | 5 | 3 | 3 | 3 | 0 | 2 | 20 |
| 20%CO2 | 15 | 13 | 8 | 4 | 2 | 5 | 2 | 6 | 2 | 5 | 1 | 4 | 2 | 20 | |
| 60%CO2 | 12 | 7 | 2 | 2 | 1 | 5 | 1 | 0 | 2 | 0 | 1 | 1 | 3 | 19 | |
| 90%CO2 | 13 | 12 | 2 | 0 | 0 | 3 | 1 | 2 | 0 | 1 | 4 | 0 | 1 | 20 | |
| 0%O2 | 7 | 6 | 4 | 1 | 4 | 4 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 20 | |
| 5%O2 | 5 | 9 | 5 | 4 | 4 | 4 | 2 | 0 | 3 | 4 | 0 | 1 | 1 | 20 | |
| 21%O2 | 8 | 12 | 5 | 7 | 8 | 7 | 6 | 7 | 6 | 5 | 6 | 5 | 5 | 20 | |
| 80%O2 | 4 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 3 | 4 | 2 | 0 | 2 | 20 | |
| Pseudoterranova spp. larvae | 0%CO2 | 12 | 10 | 9 | 7 | 11 | 5 | 8 | 10 | 8 | 8 | 10 | 9 | 6 | 17 |
| 20%CO2 | 16 | 18 | 14 | 14 | 14 | 14 | 13 | 11 | 12 | 12 | 5 | 9 | 7 | 22 | |
| 60%CO2 | 10 | 13 | 18 | 12 | 16 | 12 | 8 | 14 | 9 | 12 | 14 | 9 | 8 | 22 | |
| 90%CO2 | 11 | 14 | 5 | 9 | 6 | 11 | 14 | 14 | 11 | 10 | 11 | 11 | 9 | 17 | |
| 0%O2 | 18 | 11 | 16 | 18 | 14 | 15 | 8 | 16 | 14 | 14 | 13 | 7 | 4 | 20 | |
| 5%O2 | 16 | 18 | 14 | 19 | 12 | 14 | 17 | 13 | 13 | 10 | 12 | 14 | 16 | 20 | |
| 21%O2 | 16 | 14 | 11 | 13 | 14 | 15 | 15 | 14 | 15 | 16 | 11 | 12 | 12 | 20 | |
| 80%O2 | 8 | 15 | 11 | 9 | 9 | 10 | 12 | 12 | 5 | 12 | 8 | 10 | 11 | 20 | |
N total number of larvae analyzed (excluding ineligible larvae).
1-min recordings were taken every 30 min for 6 h at different conditions of CO2 and O2 experiments at 6 ℃.
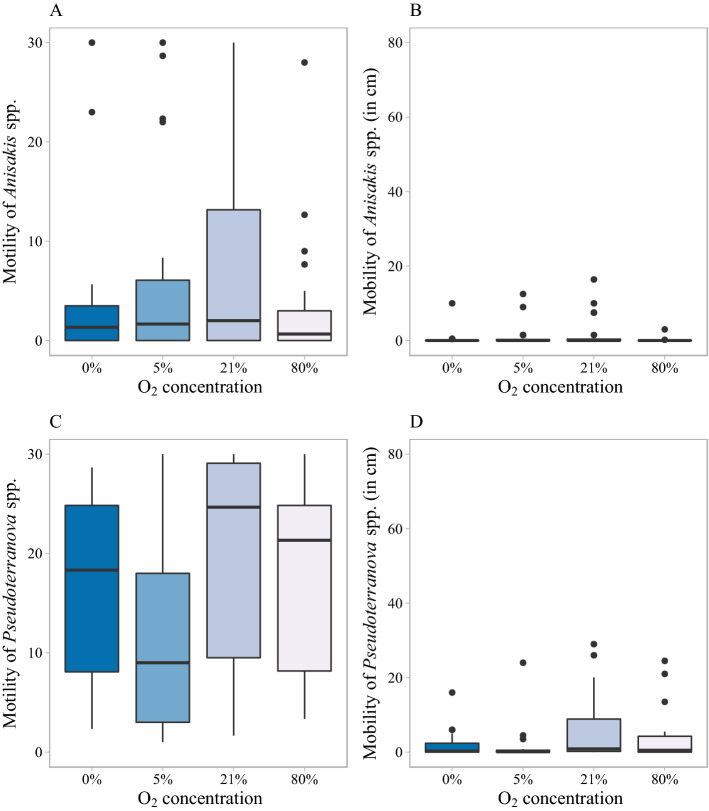
Effect of O2 on larval movements
Larval motility and mobility under 0%, 5%, 21% and 80% O2 were studied respectively at 6 ℃ (see Supplementary Fig. 2) and 12 ℃ (Fig. 4) in absence of CO2.
For Anisakis spp., at 6 ℃, as shown in Table 1, more than 75% of Anisakis spp. larvae were motile at 0%, 5% and 21% O2, whereas at 80% O2 only 8 out of 20 Anisakis spp. larvae were motile. The motility of Anisakis spp. larvae at 21% O2 was significantly higher than larval motility at 80% O2 (p = 0.011), and the motility of Anisakis spp. larvae at 80% O2 was also significantly lower than larval motility at 5% O2 with p = 0.031 (see Additional file 2). For the mobility of Anisakis spp. at 6 ℃, more Anisakis spp. larvae migrated at 21% O2 compared with other O2 concentrations (Table 1). Larval mobility at 21% O2 was higher compared to the mobility at 80% O2 (W = 300; p-value = 0.005). Under 21% O2, migration up to 19.5 cm was observed, but the median migration was only 0.3 cm in 30 min.
At 12 ℃, as shown in Table 1, more than 50% of Anisakis spp. larvae were motile. There was no significant difference of larval motility between the different O2 conditions (Fig. 4). For the mobility, the median migration of Anisakis spp. larvae was 0 cm at each of the O2 conditions and no significant differences of larval mobility were observed between different O2 concentrations (p > 0.05).
All Pseudoterranova spp. larvae at 6 ℃ and 12 ℃ were motile and there were no significant differences of larval motility between different O2 conditions at 6 ℃ and 12 ℃. For the mobility, at 6 ℃, the median migration of Pseudoterranova spp. larvae at the four different O2 concentrations ranged only from 0 to 0.3 cm in 30 min (see Additional file 2). At 12 ℃, similar as for Anisakis spp., there seemed to be a trend of higher larval motility and mobility at 21% O2, but the difference between the different O2 concentrations was not significant.
The number of larvae that are still motile over time until 6 h at different O2 conditions is shown in Table 2. After 6 h, there were very few Anisakis spp. larvae that were still motile, with five Anisakis spp. larvae being motile at 21% O2 while only one Anisakis spp. larvae was motile at 0% O2. More Pseudoterranova spp. larvae were motile after 6 h for all conditions with O2 (more than 55%) compared with Pseudoterranova spp. larvae at 0% O2 (only 20% larvae being motile).
Discussion
The number of papers studying the effect of physical or chemical stimuli on movement of Anisakidae larvae is very limited. The few studies related to larval motility/mobility mainly focused on post mortem migration of larvae from viscera to fillets during long-term storage25,26 or aimed at killing the larvae23. The innovative aspect of this study was that it systematically investigated the short-term effect of temperature, CO2 and O2 on larval motility/mobility in vitro, under controlled conditions, aiming to identify a method that could trigger larvae to actively migrate out of fillets on the short-term. In this study, temperature was found to have a positive influence on larval movement. Cipriani et al. studied the response of A. pegreffii to the storage temperature of European anchovies after 24, 48 and 72 h (long term) and found that larval migration from the viscera to the fillets was positively related to the increase of fish storage temperatures from 2 °C to 5 and 7 ℃26. Cipriani et al. also found no statistically significant variation in infection either in the fillets or the viscera after 72 h when anchovies was stored at 2 ℃ indicating the importance of temperature on the post mortem motility of A. pegreffii larvae in anchovies26. In the present study, different temperatures had varying degrees of influence on larval movement. The movement of Pseudoterranova spp. larvae at 6 ℃ was significantly lower than the movement at other temperatures, which showed that low temperature could indeed inhibit the movement of larvae. Arthur et al. studied the effect of post mortem handling on larval abundance in the musculature and also found that storage of pollock on ice for 7 days did not result in any detectable migration of parasites from the body cavity into the musculature37. Also no loss of parasites from the musculature or significant change in the abundance of parasites in the musculature occurred37. However, in the study presented here, high temperatures such as 22 ℃ for Anisakis spp. larvae and 37 ℃ for Pseudoterranova spp. larvae, could significantly increase larval movement in a short time (5, 10, 30 min as investigated in this study). In fact, the mobility of Anisakis spp. larvae at 22 ℃ significantly differed from mobility at other temperatures already after 5 min. In the first 5 and 10 min, the median migration of Anisakis spp. larvae at 22 ℃ reached 3 cm and 10 cm respectively, which corresponds with several times the thickness of fillets. Therefore, high temperatures could increase larval movement on the short-term, which could facilitate larvae to actively move out of infected fish flesh. In this study, the most optimum temperature with maximum movement of Anisakis spp. larvae was 22 ℃, while the optimum temperature for Pseudoterranova spp. was 37 ℃. For Anisakis spp., larval mobility at 37 ℃ (1.7 cm) was already significantly decreased compared with mobility at 22 ℃ (30 cm). This could complicate using short-term storage at increased temperature in practice as many fish species were infected with mixed Anisakidae genera. According to Mehrdana et al. and Mercken et al., simultaneous infection with Anisakis simplex s. s. and Pseudoterranova decipiens were recorded in cod38, haddock, plaice, pollack, whiting and gurnard11. On the other hand, for some fish species, such as salmon, halibut, leng, and dogfish, Anisakis spp. are normally more common than Pseudoterranova spp. larvae11. In addition, the median migration of Pseudoterranova spp. larvae at 22 °C was 4.5 cm in 30 min and 1.75 cm in 10 min, and an increased temperature of 22 ℃ already significantly increased the mobility of Pseudoterranova spp. larvae compared to lower temperatures. Short-term storage at 22 ℃ may thus be a promising method in practice for removing the infecting larvae.
For the effect of CO2 on larval movement at 6 ℃, there was no significant difference in larval migration between different CO2 concentrations in 30 min during which the migration of all larvae was very low. More long term effects were assessed at 6 ℃, with a longer observation of 1 min each 30 min until 6 h. After 6 h, only 8 out of 157 larvae (Anisakis spp. larvae and Pseudoterranova spp. larvae) were still mobile and the motility of larvae in the one-minute movies at the 6th hour were lower compared with larval motility in the first minute movies. In conclusion, CO2 did not have a significant effect on migration of both Anisakis spp. larvae and Pseudoterranova spp. larvae in 30 min and may thus not be applicable to trigger larvae to actively migrating out of fillets in an industrial setting either. At 12 ℃, there was no significant difference of larval motility and mobility found between different CO2 concentrations in 30 min both for Anisakis spp. larvae and Pseudoterranova spp. larvae. This means that a CO2 enriched atmosphere seems unsuitable in an industrial context to remove larvae from infected fishes on a short time (30 min). Although there was no meaningful impact of CO2 on larval mobility confirmed in 30 min and even 6 h, according to Pascual et al., if several days of storage would be implemented, the total migration of Anisakis spp. larvae at 60% CO2 maybe observed higher than the total migration at other CO2 concentrations (Fig. 3)20. Pascual et al. studied the behavior of Anisakis spp. larvae in fish stored under different CO2 modified atmospheres at 3 °C. In that study, Anisakis simplex sensu stricto stored at 20% CO2 tended to a quiescence and mostly coiled behavior at day 15, whereas Anisakis spp. larvae stored at 55%-90% CO2 were active, stretched and larval spontaneous migration occurred after 15 days of storage. This can explain why in practice, an increased presence of visible active larvae on the surface of fish is observed when MAP is used at retail level20.
Under different O2 conditions at 6 and 12 ℃, both Anisakis spp. larvae and Pseudoterranova spp. larvae had no or only a very low median mobility. There is no meaningful impact of O2 on larval mobility and an O2 enriched atmosphere cannot be used in an industrial context to trigger larvae to actively move out of infected fillets.
The batch of larvae may also affect larval movement, as different factors (such as the origin and age of the larvae) may influence larval behavior. To account for this potential batch effect, in this study conditions were only compared within experiments that used larvae from the same batch(es). Also, we observed a high variability between larvae. For example, Anisakis spp. larvae from the same batch at 22 ℃ in the temperature experiment had a maximum migration of 74 cm and a minimum migration of only 7 cm in 30 min. This may be due to the different ages and physical conditions of larvae. The variability should be taken into consideration when assessing the practicability of the technique. Larval mobility in fish may be very different from larval mobility in PBS agar as reported in this study, because the composition and texture of PBS agar are different from fish. So validation of the temperature experiments in real fish fillets is necessary and a common temperature for both Anisakis spp. larvae and Pseudoterranova spp. larvae should be further investigated. Finally, the impact of a rapid, short term increase in temperature followed by a rapid decrease to the temperature of melting ice on other pathogens or spoilage bacteria should be investigated.
Conclusions
In this study, the effect of temperature on larval movement of Anisakis spp. and Pseudoterranova spp. on a short-time exposure was clearly illustrated. Increasing temperature increased larval movement, but the optimal temperature for movement was different between the two investigated species. For Anisakis spp., the optimum temperature was around 22 ℃ whereas it was 37 ℃ for Pseudoterranova spp. Atmospheres enriched with CO2 or O2 could not facilitate larval movement significantly on a short-term. These results provide evidence for the effect of temperature on larval movements and could be a basis for further exploring a method for industrially removing larvae from infected fish with a short-term treatment.
Supplementary Information
Acknowledgements
This work was supported by the China Scholarship Council (CSC) and the Ghent University Special Research Funds (BOF.STG.2018.0015.01). The authors would like to thank the whole sale company for material assistance during this study.
Author contributions
A.G. participated in the design of the study, performed the experiments, carried out the laboratory work, drafted and revised the manuscript. I.V.D. participated in the design of the study, analyzed the data statistically with R, assisted in drafting the manuscript and reviewed the manuscript. F.D. and S.G. conceived the study, participated in its design, supervised the experiments and coordination and reviewed the written manuscript. All authors have read and approved the final manuscript.
Data availability
All data are available, upon reasonable request, from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Frank Devlieghere and Sarah Gabriël.
Contributor Information
Aiyan Guan, Email: Aiyan.Guan@UGent.be.
Sarah Gabriël, Email: sarah.gabriel@ugent.be.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83505-5.
References
- 1.Buchmann, K. & Mehrdana, F. Effects of anisakid nematodes Anisakis simplex (s.l.), Pseudoterranova decipiens (s.l.) and Contracaecum osculatum (s.l.) on fish and consumer health. Food Waterborne Parasitol.4, 13–22, 10.1016/j.fawpar.2016.07.003 (2016).
- 2.Deardorff, T. L., Raybourne, R. B. & Desowitz, R. S. Behavior and viability of third-stage larvae of Terranova sp. (type HA) and Anisakis simplex (type I) under coolant conditions. J. Food Protect.47, 49–52 (1984). [DOI] [PubMed]
- 3.Eiras J, et al. Potential risk of fish-borne nematode infections in humans in Brazil–current status based on a literature review. Food Waterborne Parasitol. 2016;5:1–6. doi: 10.1016/j.fawpar.2016.08.002. [DOI] [Google Scholar]
- 4.Colón-Llavina MM, et al. Some metazoan parasites from marine mammals stranded in California. Pac. Sci. 2019;73:461–473. doi: 10.2984/73.4.3. [DOI] [Google Scholar]
- 5.Aco Alburqueque R, Palomba M, Santoro M, Mattiucci S. Molecular identification of zoonotic parasites of the genus Anisakis (Nematoda: Anisakidae) from fish of the southeastern Pacific Ocean (Off Peru Coast) Pathogens. 2020;9:910. doi: 10.3390/pathogens9110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattiucci, S., Cipriani, P., Levsen, A., Paoletti, M. & Nascetti, G. in Advances in Parasitology Vol. 99 (eds D. Rollinson & J. R. Stothard) 93–263 (Academic Press, 2018). [DOI] [PubMed]
- 7.Hochberg NS, Hamer DH, Hughes JM, Wilson ME. Anisakidosis: Perils of the deep. Clin. Infect. Dis. 2010;51:806–812. doi: 10.1086/656238. [DOI] [PubMed] [Google Scholar]
- 8.Levsen A, et al. A survey of zoonotic nematodes of commercial key fish species from major European fishing grounds—Introducing the FP7 PARASITE exposure assessment study. Fish. Res. 2018;202:4–21. doi: 10.1016/j.fishres.2017.09.009. [DOI] [Google Scholar]
- 9.Pozio E. Integrating animal health surveillance and food safety: The example of Anisakis. Rev. Sci. Tech. 2013;32:487–496. doi: 10.20506/rst.32.2.2246. [DOI] [PubMed] [Google Scholar]
- 10.Karpiej K, Dzido J, Rokicki J, Kijewska A. Anisakid nematodes of Greenland halibut Reinhardtius hippoglossoides from the Barents Sea. J. Parasitol. 2013;99:650–654. doi: 10.1645/GE-2987.1. [DOI] [PubMed] [Google Scholar]
- 11.Mercken E, Van Damme I, Serradell A, Gabriël S. Presence of Anisakidae in commercial fish species imported into the Belgian food markets: A systematic review and meta-analyses. Int. J. Food Microbiol. 2020;318:108456. doi: 10.1016/j.ijfoodmicro.2019.108456. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwenhuizen NE, Lopata AL. Anisakis – A food-borne parasite that triggers allergic host defences. Int. J. Parasitol. 2013;43:1047–1057. doi: 10.1016/j.ijpara.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwenhuizen NE, Lopata AL. Anisakis–A food-borne parasite that triggers allergic host defences. Int. J. Parasitol. 2013;43:1047–1057. doi: 10.1016/j.ijpara.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Baird FJ, Gasser RB, Jabbar A, Lopata AL. Foodborne anisakiasis and allergy. Mol. Cell. Probes. 2014;28:167–174. doi: 10.1016/j.mcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Hazards, E. P. o. B. Scientific opinion on risk assessment of parasites in fishery products. EFSA J.8, 1543 (2010).
- 16.Jackson G. The, “new disease” status of human anisakiasis and North American cases: A review. J. Milk Food Technol. 1975;38:769–773. doi: 10.4315/0022-2747-38.12.769. [DOI] [Google Scholar]
- 17.Yorimitsu N, et al. Colonic intussusception caused by Anisakiasis: A case report and review of the literature. Intern. Med. 2013;52:223–226. doi: 10.2169/internalmedicine.52.8629. [DOI] [PubMed] [Google Scholar]
- 18.Bao M, et al. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci. Rep. 2017;7:43699. doi: 10.1038/srep43699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Council Regulation (EC) No 853/2004 of the European parliament and of the council of 29 April 2004 laying down specific hygiene rules for food of animal origin. J. Eur. Union139, 55–205 (2004).
- 20.Pascual S, Antonio J, Cabo M, Piñeiro C. Anisakis survival in refrigerated fish products under CO2 modified-atmosphere. Food Control. 2010;21:1254–1256. doi: 10.1016/j.foodcont.2010.03.002. [DOI] [Google Scholar]
- 21.Deardorff, T. L. & Throm, R. Commercial blast-freezing of third-stage Anisakis simplex larvae encapsulated in salmon and rockfish. J. Parasitol. 600–603 (1988). [PubMed]
- 22.Wharton D, Aalders O. The response of Anisakis larvae to freezing. J. Helminthol. 2002;76:363–368. doi: 10.1079/JOH2002149. [DOI] [PubMed] [Google Scholar]
- 23.Vidaček S, et al. Antigenicity and viability of Anisakis larvae infesting hake heated at different time-temperature conditions. J. Food Prot. 2010;73:62–68. doi: 10.4315/0362-028X-73.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Levsen A, Lunestad BT, Berland B. Low detection efficiency of candling as a commonly recommended inspection method for nematode larvae in the flesh of pelagic fish. J. Food Prot. 2005;68:828–832. doi: 10.4315/0362-028X-68.4.828. [DOI] [PubMed] [Google Scholar]
- 25.Šimat V, Miletić J, Bogdanović T, Poljak V, Mladineo I. Role of biogenic amines in the post-mortem migration of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) larvae into fish fillets. Int. J. Food Microbiol. 2015;214:179–186. doi: 10.1016/j.ijfoodmicro.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani P, et al. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: Implications to seafood safety. Food Control. 2016;59:148–157. doi: 10.1016/j.foodcont.2015.04.043. [DOI] [Google Scholar]
- 27.Hotez P, Cappello M, Hawdon J, Beckers C, Sakanari J. Hyaluronidases of the gastrointestinal invasive nematodes Ancylostoma caninum and Anisakis simplex: possible functions in the pathogenesis of human zoonoses. J. Infect. Dis. 1994;170:918–926. doi: 10.1093/infdis/170.4.918. [DOI] [PubMed] [Google Scholar]
- 28.Palomba M, et al. Differences in gene expression profiles of seven target proteins in third-stage larvae of Anisakis simplex (sensu stricto) by sites of infection in blue whiting (Micromesistius poutassou) Genes. 2020;11:559. doi: 10.3390/genes11050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClelland G, Martell D. Surveys of larval sealworm (Pseudoterranova decipiens) infection in various fish species sampled from Nova Scotian waters between 1988 and 1996, with an assessment of examination procedures. NAMMCO Sci. Publ. 2001;3:57–76. doi: 10.7557/3.2959. [DOI] [Google Scholar]
- 30.Jackson G, Bier J, Payne W, McClure F. Recovery of parasitic nematodes from fish by digestion or elution. Appl. Environ. Microbiol. 1981;41:912–914. doi: 10.1128/AEM.41.4.912-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst R. Identification and description of larval Anisakis simplex and Pseudoterranova decipiens (Anisakidae: Nematoda) from New Zealand waters. NZ J. Mar. Freshwat. Res. 1984;18:177–186. doi: 10.1080/00288330.1984.9516040. [DOI] [Google Scholar]
- 32.Berland B. Nematodes from some Norwegian marine fishes. Sarsia. 1961;2:1–50. doi: 10.1080/00364827.1961.10410245. [DOI] [Google Scholar]
- 33.Zhu X, Gasser RB, Podolska M, Chilton NB. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int. J. Parasitol. 1998;28:1911–1921. doi: 10.1016/S0020-7519(98)00150-7. [DOI] [PubMed] [Google Scholar]
- 34.Farjallah, S. et al. Occurrence and molecular identification of Anisakis spp. from the North African coasts of Mediterranean Sea. Parasitol. Res. 102, 371 (2008). [DOI] [PubMed]
- 35.D'Amelio S, et al. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000;30:223–226. doi: 10.1016/S0020-7519(99)00178-2. [DOI] [PubMed] [Google Scholar]
- 36.La Rosa, G., D’Amelio, S. & Pozio, E. Molecular identification of Nematode worms from seafood (Anisakis spp. and Pseudoterranova spp.) and meat (Trichinella spp.). in Food-Borne Pathogens 217–232 (Springer, 2006).
- 37.Arthur J, Margolis L, Whitaker D, McDonald T. A quantitative study of economically important parasites of walleye pollock (Theragra chalcogramma) from British Columbian waters and effects of postmortem handling on their abundance in the musculature. Can. J. Fish. Aquat. Sci. 1982;39:710–726. doi: 10.1139/f82-100. [DOI] [Google Scholar]
- 38.Mehrdana F, et al. Occurrence of zoonotic nematodes Pseudoterranova decipiens, Contracaecum osculatum and Anisakis simplex in cod (Gadus morhua) from the Baltic Sea. Vet. Parasitol. 2014;205:581–587. doi: 10.1016/j.vetpar.2014.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available, upon reasonable request, from the corresponding author.