Introduction
Alzheimer's disease (AD) is the most common cause of dementia. The disease is associated with the presence of plaques and neurofibrillary tangles in the brain, which leads to synaptic and neuronal degeneration and progressive learning and memory impairment (Spires-Jones and Hyman, 2014). Work of the last decades has produced a variety of diagnostic tools and treatment alternatives, yet the mechanisms underlying the disease are poorly understood, and interventions are still minimally effective, possibly in part because they are started after irreversible damage has been done. Identifying early markers of AD might therefore advance therapeutic development and effectiveness.
A likely source of early AD biomarkers is hippocampal network activity. The hippocampus, an important region for acquisition and consolidation of memory, is particularly affected in AD (Braak and Braak, 1991). Normal hippocampal memory function relies on a variety of electrophysiological phenomena, perhaps the most notable of which are sharp wave ripples (SWRs) (Buzsáki, 2015). The spectrum of SWR oscillations spans from the γ (30-80 Hz) to the ripple band (100-250 Hz). During SWRs, excitatory neurons and GABAergic interneurons engage in a delicate interaction that, if disrupted, can lead to pathologic forms of activity, including fast ripples (>250 Hz), very large amplitude ripples, and hyperexcitability (Aivar et al., 2014). SWRs emerge from the dynamical interaction between pyramidal cells and local circuit GABAergic interneurons. While the dynamics of SWRs are altered in various ways in AD models (Fig. 1) (Gillespie et al., 2016; Iaccarino et al., 2016; Witton et al., 2016; Jura et al., 2019), the role that each neuronal type plays in these changes is unclear. Therefore, a recent study by Caccavano et al. (2020) examined the mechanisms underlying disruption of the hippocampal network activity in a mouse model of AD.
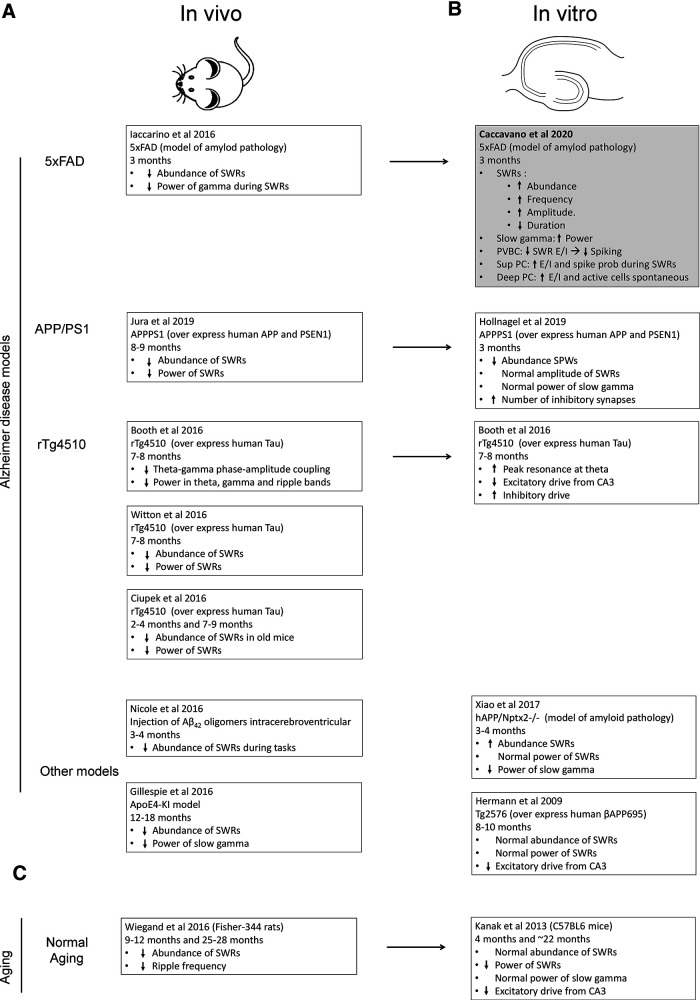
Figure 1.
Summary of SWR alterations in AD models in vivo (A) and in vitro (B). Summary of Hermann et al. (2009); Ciupek et al. (2015); Booth et al. (2016); Gillespie et al. (2016); Iaccarino et al. (2016); Nicole et al. (2016); Witton et al. (2016); Xiao et al. (2017); Hollnagel et al. (2019); Jura et al. (2019); and Caccavano et al. (2020). C, Findings for normal aging studies are also included. Summary of Kanak et al. (2013) and Wiegand et al. (2016).
The authors used the 5xFAD model of familial AD, which is characterized by heavy amyloid β accumulation in hippocampus and associated cortical areas. After confirming the presence of amyloid β plaques in the subiculum and hippocampal area CA1 of 3-month-old 5xFAD mice and demonstrating these animals' impaired performance on the Barnes maze, the authors turned to an in vitro preparation to study CA1 microcircuits using electrophysiological and calcium imaging techniques.
Caccavano et al. (2020) recorded the local field potential in CA1 in slices containing the medial hippocampus, where spontaneous SWRs emerge most consistently in vitro. They found that SWRs were more abundant, had larger amplitudes, slightly faster intraripple frequencies, and were of shorter duration in 5xFAD mice than in controls. More strikingly, the spectral organization of the events was altered in 5xFAD mice. While the power of the ripple band was relatively similar to control, the slow-γ band was increased in recordings from these mice.
Multicellular imaging in 5xFAD mice crossed with Thy1-GCaMP6f mice, in which a calcium indicator is expressed in a subset of CA1 neurons, revealed differences in the ensembles of activated cells during SWR activity. In slices from 5xFAD/1;Thy1-GCaMP6f/1 mice, ensembles were larger and less similar compared with control Thy1-GCaMP6f/1 littermates, suggesting that aberrant cell participation may disrupt the neuronal orchestra in AD mice. This is consistent with a recent report by Poll et al. (2020), which showed that activation of additional ensembles interfered with memory recall in another model of AD. Interestingly, Caccavano et al. (2020) observed different alteration of firing dynamics of deep and superficial CA1 pyramidal cells. During SWRs, superficial cells fired more in 5xFAD mice than controls, whereas firing of deep neurons was similar in 5xFAD mice and controls. Voltage-clamp recordings confirmed cell-type-specific changes and provided additional hints on the mechanisms: in superficial pyramidal cells from 5xFAD mice, although the frequency and charge of spontaneous IPSCs were reduced, the inhibitory charge during SWRs was not significantly different from controls. In contrast, the inhibitory charge increased during SWRs in deep neurons in 5xFAD mice. Consistent with these findings, only superficial CA1 pyramidal neurons from 5xFAD mice showed larger ratios of excitation to inhibition during SWRs.
To better understand the mechanisms underlying these changes, the authors performed loose cell-attached and patch-clamp recordings in CA1 parvalbumin-expressing (PV+) neurons, which are the most active interneurons during SWRs in vivo. They classified PV+ interneurons as basket cells (PVBCs), bistratified cells, or axo-axonic cells based on morphology and firing dynamics during SWRs. 5xFAD mice showed abnormal activity patterns in PVBCs during SWRs. The firing window of PVBCs was narrower and the firing rate was lower in 5xFAD than in controls. According to the authors, the firing of bistratified cells from 5xFAD remained relatively preserved, although there was a trend to widen their firing window. They also noted that axo-axonic interneurons from 5xFAD mice had a nonstatistically significant trend to reduce their firing and to shift their phase preference during SWRs. Together, these data by Caccavano et al. (2020) suggest a major role of PVBC activity underlying hyperexcitability and cell ensemble disruption in the hippocampus of 5xFAD mice, leading to distortion of SWR dynamics.
What are the mechanisms underlying SWR alterations in AD? Under physiological conditions, firing of pyramidal cells and PVBCs during SWRs is appropriately coordinated (Somogyi and Klausberger, 2005), and ripples rarely accelerate because most neurons are under strong inhibitory control, which is reflected by the rhythmic positive deflection of the local field potential (Aivar et al., 2014). Loss of this perisomatic inhibition by PVBCs in the hippocampus leads to massive bursting of pyramidal neurons and the generation of pathologic ripples (Gulyás and Freund, 2015). Perisomatic disinhibition may slightly accelerate cycles that are now dominated by excitation, triggering large-amplitude ripples that reflect synchronous in-phase firing, or fast ripples reflecting out-of-phase firing of pyramidal neurons (Aivar et al., 2014). In addition, enlarged-amplitude events that occur in 5xFAD mice may result from stronger excitatory transmission from CA3 pyramidal neurons during SWRs. Indeed, alterations in excitatory and inhibitory transmission in the CA3 and dentate gyrus have been described in other AD models (Palop et al., 2007; Booth et al., 2016; Hollnagel et al., 2019). Perisomatic disinhibition also has major effects on SWR duration, by favoring abnormal bursting with prominent after-hyperpolarization that may shorten the oscillation, as happens in 5xFAD mice. Similar effects have been shown in experiments with optogenetic silencing of PV+ interneurons during SWRs, which shortened the oscillation (Schlingloff et al., 2014). Interestingly, tasks demanding strong memory load have been shown to require long SWRs, and prolongation of SWRs improves memory (Fernandez-Ruiz et al., 2019), whereas interruption of SWRs results in memory deficits (Roux et al., 2017). Thus, these mechanisms may contribute to AD-associated cognitive deficits.
While PVBCs seem to be the most affected interneuronal type in 5xFAD mice, Caccavano et al. (2020) did not discard the possibility that mild alterations in PV axo-axonic cells may also contribute to hyperexcitability, given the strategic location of their synapses in the axon initial segment for controlling pyramidal cell firing (Somogyi et al., 1983). Indeed, loss of axo-axonic cells and their synapses in cerebral cortex and hippocampus are known to contribute to epileptogenesis (DeFelipe, 1999; Alhourani et al., 2020), which is noteworthy because seizures are up to 6 times more prevalent in AD than in age-matched controls (Pandis and Scarmeas, 2012). Seizures and hyperexcitability emerge early during AD progression (Palop et al., 2007), and alterations of the normal SWR patterns may be informative about disease progression. Thus, it is possible that broad populations of PV+ GABAergic cells are affected in AD.
Changes in the firing patterns of PV neurons might stem from several factors. For instance, intrinsic factors, such as downregulation of Nav1.1 channels, may contribute, as shown in other AD models (Verret et al., 2012). Caccavano et al. (2020) also suggest that loss of perineuronal nets surrounding PV+ interneurons could explain their reduced excitability, given that PV cells from genetic KO and knockdown of the perineuronal net protein brevican receive fewer excitatory synapses (Favuzzi et al., 2017). In this regard, it has recently been shown that microglia facilitate the extensive loss of perineuronal nets in 5xFAD mice and in human tissue (Crapser et al., 2020). Perineuronal nets surround preferentially PVBCs compared with the rest of PV interneurons (Yamada and Jinno, 2015), potentially explaining why PVBCs seem to be the most affected PV population in the experiments by Caccavano et al. (2020). Potential treatments might target voltage-dependent sodium channels or perineuronal net proteins to restore the inhibitory activity (Xu et al., 2020). Indeed, early restoration of PV+ interneuron activity prevents network hyperexcitability and memory loss in a mouse model of AD (Hijazi et al., 2020). Importantly, although changes in PV+ interneurons likely contribute to the hyperexcitability observed by Caccavano et al. (2020), other factors, including impairment of interneuronal types not examined by the authors, may play roles as well. For instance, lower dendritic inhibition mediated by oriens-lacunosum moleculare interneurons has been linked to the increased excitability (Cossart et al., 2001).
How comparable are these observations across AD models? Different rodent models have been used to elucidate changes and mechanisms underlying hippocampal dysfunction in AD, but in vivo and in vitro studies often yield contradictory results. For instance, in vivo recordings consistently report a decrease in the rate of SWR events in 5xFAD (Iaccarino et al., 2016), APPPS1 (Jura et al., 2019), APoE4 (Gillespie et al., 2016), and rTf4510 models of AD (Ciupek et al., 2015; Witton et al., 2016) (Fig. 1A), whereas in vitro recordings from the same models show variable results, including increases, decreases, and no change in SWR rate compared with controls (Hermann et al., 2009; Xiao et al., 2017; Hollnagel et al., 2019) (Fig. 1B). Furthermore, ripples recorded in vivo in most AD models show reduced power, especially at the slow γ band (Fig. 1C), whereas in vitro recordings of these very same models reflect disparate trends (Fig. 1B,C). How can these disparate observations be explained?
The slice preparation is useful for dissecting microcircuit mechanisms that are currently elusive in vivo, but extrapolation is challenging. First, most in vivo recordings are obtained from the dorsal hippocampus, while slices are typically prepared from horizontal sections of the ventral hippocampus, and molecular and electrophysiological differences have been described along the dorsoventral axis (Lee et al., 2014; Kouvaros and Papatheodoropoulos, 2017). Second, the composition of the extracellular medium, as well as the level of oxygenation and recording conditions (i.e., interface vs submerged chambers) all affect the type of SWR events that spontaneously emerge from slices (Hájos and Mody, 2009; Aivar et al., 2014). Simply modifying the balance between calcium and magnesium has a major effect in the excitation to inhibition ratio and the physiological or pathologic types of SWR recorded in slices prepared from normal rodents (Aivar et al., 2014). To facilitate comparison and enhance replicability, in vitro SWRs should match the properties of in vivo SWRs.
It is still unknown how different excitatory sources and the interaction of diverse neuronal subpopulations determine the spectral properties of SWRs in vivo. Caccavano et al. (2020) propose that spike rate reduction of PVBC firing explains distortion of SWR-associated activity in 5xFAD mice. Yet, spike rate alterations in PVBC as well as the consistent decrease of slow γ power seen in vivo, among other changes, can only be fully understood after the many microcircuits underlying SWR events are identified (de la Prida, 2020). For instance, emerging data suggest that not only interneuronal subtypes, but also deep and superficial pyramidal cells, have critical impact on SWR dynamics (Valero et al., 2015; Wu et al., 2015), given specific connectivity between PVBC, superficial, and deep pyramidal cells (Lee et al., 2014). Since different proportions of CA1 deep and superficial pyramidal cells exist along the septotemporal axis of the hippocampus, comparisons between dorsal and ventral hippocampal slices of 5xFAD mice might help to further clarify the issue.
In conclusion, Caccavano et al. (2020) shed light on an early and important disturbance in the hippocampal CA1 microcircuit in vitro of AD-model mice. The critical role of interneurons and specific subcircuits innervating deep and superficial CA1 pyramidal cells suggests that early alterations operate at the microcircuit level. A major readout of local microcircuit function (Buzsáki, 2015), SWR dynamics, was significantly altered in the AD model. More work is required to gain additional insights into the in vivo mechanisms to put together the emerging advances (Fig. 1) and better understand the role of SWRs in AD's pathophysiology.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/jneurosci-journal-club.
A.S.-A. was supported by Spanish Ministry of Science, Innovation and Universities Juan de la Cierva-Formación Fellowship FJCI-2017-32719, and Consejo Superior de Investigaciones Científicas. We thank Liset Menendez de la Prida for critical review of the manuscript and feedback.
The authors declare no competing financial interests.
References
- Aivar P, Valero M, Bellistri E, Menendez de la Prida L (2014) Extracellular calcium controls the expression of two different forms of ripple-like hippocampal oscillations. J Neurosci 34:2989–3004. 10.1523/JNEUROSCI.2826-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhourani A, Fish KN, Wozny TA, Sudhakar V, Hamilton RL, Richardson RM (2020) GABA bouton subpopulations in the human dentate gyrus are differentially altered in mesial temporal lobe epilepsy. J Neurophysiol 123:392–406. 10.1152/jn.00523.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth XC, Witton XJ, Nowacki J, Tsaneva-Atanasova XK, Jones XM, Randall AD, Brown XJ (2016) Altered intrinsic pyramidal neuron properties and pathway-specific synaptic dysfunction underlie aberrant hippocampal network function in a mouse model of tauopathy. J Neurosci 36:350–363. 10.1523/JNEUROSCI.2151-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2015) Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25:1073–1188. 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccavano A, Bozzelli PL, Forcelli PA, Pak DT, Wu JY, Conant K, Vicini S (2020) Inhibitory parvalbumin basket cell activity is selectively reduced during hippocampal sharp wave ripples in a mouse model of familial Alzheimer's disease. J Neurosci 40:5116–5136. 10.1523/JNEUROSCI.0425-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciupek SM, Cheng J, Ali YO, Lu HC, Ji D (2015) Progressive functional impairments of hippocampal neurons in a tauopathy mouse model. J Neurosci 35:8118–8131. 10.1523/JNEUROSCI.3130-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben Ari Y, Esclapez M, Bernard C (2001) Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4:52–62. [DOI] [PubMed] [Google Scholar]
- Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohs LA, Green KN (2020) Microglia facilitate loss of perineuronal nets in the Alzheimer's disease brain. EBioMedicine 58:102919 10.1016/j.ebiom.2020.102919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Prida LM (2020) Potential factors influencing replay across CA1 during sharp-wave ripples. Philos Trans R Soc Lond B Biol Sci 375:20190236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J (1999) Chandelier cells and epilepsy. Brain 122:1807–1822. 10.1093/brain/122.10.1807 [DOI] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B (2017) Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95:639–655. 10.1016/j.neuron.2017.06.028 [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz A, Oliva A, Oliveira ED, Rocha-Almeida F, Tingley D, Buzsáki G (2019) Long-duration hippocampal sharp wave ripples improve memory. Science 364:1082–1086. 10.1126/science.aax0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie AK, Jones EA, Lin YH, Karlsson MP, Kay K, Yoon SY, Tong LM, Nova P, Carr JS, Frank LM, Huang Y (2016) Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90:740–751. 10.1016/j.neuron.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Freund TT (2015) Generation of physiological and pathological high frequency oscillations: the role of perisomatic inhibition in sharp-wave ripple and interictal spike generation. Curr Opin Neurobiol 31:26–32. 10.1016/j.conb.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Hájos N, Mody I (2009) Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods 183:107–113. 10.1016/j.jneumeth.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Both M, Ebert U, Gross G, Schoemaker H, Draguhn A, Wicke K, Nimmrich V (2009) Synaptic transmission is impaired prior to plaque formation in amyloid precursor protein-overexpressing mice without altering behaviorally-correlated sharp wave-ripple complexes. Neuroscience 162:1081–1090. 10.1016/j.neuroscience.2009.05.044 [DOI] [PubMed] [Google Scholar]
- Hijazi S, Heistek TS, Scheltens P, Neumann U, Shimshek DR, Mansvelder HD, Smit AB, van Kesteren RE (2020) Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer's disease. Mol Psychiatry 25:3380–3398. 10.1038/s41380-019-0483-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollnagel JO, Elzoheiry S, Gorgas K, Kins S, Beretta CA, Kirsch J, Kuhse J, Kann O, Kiss E (2019) Early alterations in hippocampal perisomatic GABAergic synapses and network oscillations in a mouse model of Alzheimer's disease amyloidosis. PLoS One 14:e0209228 10.1371/journal.pone.0209228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai LH (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540:230–235. 10.1038/nature20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura B, Macrez N, Meyrand P, Bem T (2019) Deficit in hippocampal ripples does not preclude spatial memory formation in APP/PS1 mice. Sci Rep 9:12 10.1038/s41598-019-56582-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanak DJ, Rose GM, Zaveri HP, Patrylo PR (2013) Altered Network Timing in the CA3-CA1 Circuit of Hippocampal Slices from Aged Mice. PLoS One 8:e61364 10.1038/s41598-019-56582-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C (2017) Prominent differences in sharp waves, ripples and complex spike bursts between the dorsal and the ventral rat hippocampus. Neuroscience 352:131–143. 10.1016/j.neuroscience.2017.03.050 [DOI] [PubMed] [Google Scholar]
- Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I (2014) Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82:1129–1144. 10.1016/j.neuron.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O, Hadzibegovic S, Gajda J, Bontempi B, Bem T, Meyrand P (2016) Soluble amyloid beta oligomers block the learning-induced increase in hippocampal sharp wave-ripple rate and impair spatial memory formation. Sci Rep 6:12 10.1038/srep22728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55:697–711. 10.1016/j.neuron.2007.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandis D, Scarmeas N (2012) Seizures in Alzheimer disease: clinical and epidemiological data. Epilepsy Curr 12:184–187. 10.5698/1535-7511-12.5.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll S, Mittag M, Musacchio F, Justus LC, Giovannetti EA, Steffen J, Wagner J, Zohren L, Schoch S, Schmidt B, Jackson WS, Ehninger D, Fuhrmann M (2020) Memory trace interference impairs recall in a mouse model of Alzheimer's disease. Nat Neurosci 23:952–958. 10.1038/s41593-020-0652-4 [DOI] [PubMed] [Google Scholar]
- Roux L, Hu B, Eichler R, Stark E, Buzsáki G (2017) Sharp wave ripples during learning stabilize the hippocampal spatial map. Nat Neurosci 20:845–853. 10.1038/nn.4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingloff D, Káli S, Freund TF, Hájos N, Gulyás AI (2014) Mechanisms of sharp wave initiation and ripple generation. J Neurosci 34:11385–11398. 10.1523/JNEUROSCI.0867-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562:9–26. 10.1113/jphysiol.2004.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Nunzi MG, Gorio A, Smith AD (1983) A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res 259:137–142. 10.1016/0006-8993(83)91076-4 [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron 82:756–771. 10.1016/j.neuron.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM (2015) Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci 18:1281–1290. 10.1038/nn.4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–721. 10.1016/j.cell.2012.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand JL, Gray DT, Schimanski LA, Lipa P, Barnes CA, Cowen SL (2016) Age is associated with reduced sharp-wave ripple frequency and altered patterns of neuronal variability. J Neurosci 36:5650–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witton J, Staniaszek LE, Bartsch U, Randall AD, Jones MW, Brown JT (2016) Disrupted hippocampal sharp-wave ripple-associated spike dynamics in a transgenic mouse model of dementia. J Physiol 594:4615–4630. 10.1113/jphysiol.2014.282889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Stark E, Ku PC, Wise KD, Buzsáki G, Yoon E (2015) Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies. Neuron 88:1136–1148. 10.1016/j.neuron.2015.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon D, Brewer J, Edland S, Wegiel J, Tycko B, Savonenko A, Reeves RH, Troncoso JC, McBain CJ, Galasko D, Worley PF (2017) NPTX2 and cognitive dysfunction in Alzheimer's disease. Elife 6:e23798 10.7554/eLife.23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhao M, Han Y, Zhang H (2020) GABAergic inhibitory interneuron deficits in Alzheimer's disease: implications for treatment. Front Neurosci 14:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Jinno S (2015) Subclass-specific formation of perineuronal nets around parvalbumin-expressing GABAergic neurons in Ammon's horn of the mouse hippocampus. J Comp Neurol 523:790–804. 10.1002/cne.23712 [DOI] [PubMed] [Google Scholar]