Abstract
Release of neuronal transmitters from nerve terminals is triggered by the molecular Ca2+ sensor synaptotagmin 1 (Syt1). Syt1 is a transmembrane protein attached to the synaptic vesicle (SV), and its cytosolic region comprises two domains, C2A and C2B, which are thought to penetrate into lipid bilayers upon Ca2+ binding. Before fusion, SVs become attached to the presynaptic membrane (PM) by the four-helical SNARE complex, which is thought to bind the C2B domain in vivo. To understand how the interactions of Syt1 with lipid bilayers and the SNARE complex trigger fusion, we performed molecular dynamics (MD) simulations at a microsecond scale. We investigated how the isolated C2 modules and the C2AB tandem of Syt1 interact with membranes mimicking either SV or PM. The simulations showed that the C2AB tandem can either bridge SV and PM or insert into PM with its Ca2+-bound tips and that the latter configuration is more favorable. Surprisingly, C2 domains did not cooperate in penetrating into PM but instead mutually hindered their insertion into the bilayer. To test whether the interaction of Syt1 with lipid bilayers could be affected by the C2B-SNARE attachment, we performed systematic conformational analysis of the C2AB-SNARE complex. Notably, we found that the C2B-SNARE interface precludes the coupling of C2 domains and promotes their insertion into PM. We performed the MD simulations of the prefusion protein complex positioned between the lipid bilayers mimicking PM and SV, and our results demonstrated in silico that the presence of the Ca2+ bound C2AB tandem promotes lipid merging. Altogether, our MD simulations elucidated the role of the Syt1-SNARE interactions in the fusion process and produced the dynamic all-atom model of the prefusion protein-lipid complex.
Significance
Neuronal transmitters are packed in synaptic vesicles (SVs) and released by fusion of SVs with the presynaptic membrane. SVs are attached to the presynaptic membrane by the SNARE protein complex, and fusion is triggered by the Ca2+ sensor synaptotagmin 1 (Syt1). Although Syt1 and SNARE proteins have been extensively studied, it is not yet fully understood how the interactions of Syt1 with lipids and the SNARE complex induce fusion. To address this fundamental problem, we took advantage of Anton2, a unique computational environment, which enables simulating the dynamics of molecular systems at a scale of microseconds. Our simulations produced a dynamic all-atom model of the prefusion protein-lipid complex and demonstrated in silico how the Syt1-SNARE complex triggers fusion.
Introduction
Neuronal transmitters are released from nerve terminals in response to Ca2+ influx. Calcium binds synaptotagmin 1 (Syt1), a synaptic vesicle (SV) protein that drives the fusion of SVs with the presynaptic membrane (PM) and triggers rapid transmitter release (1).
Syt1 comprises two Ca2+ binding domains, C2A and C2B, connected by a flexible linker and attached to SV by a transmembrane helix (2). Each domain has two loops forming a Ca2+ binding pocket, and in each pocket, Ca2+ ions are chelated by five aspartic acids (3, 4, 5). Both domains C2A (3,6) and C2B (4) can bind phospholipids, and the binding properties of C2 domains depend on the lipid composition. In particular, the association of the C2B domain with the lipid membrane depends on the presence of phosphatidylinositol 4,5-bisphosphate (PIP2) in the bilayer (7).
It is generally agreed that SV-PM fusion depends on the interaction of Syt1 into phospholipids (8); however, it is still debated how the protein-lipid interactions occur at the atomistic level. One possible scenario is that Syt1 bridges PM and SV by inserting the Ca2+-bound tips of its C2 domains into the PM and SV phospholipid bilayers (9,10). Alternatively, Syt1 could trigger the fusion by penetrating into PM with the tips of both its domains, promoting PM curvature and generating the lipid stalk followed by the pore opening (11,12). It was also proposed that these two mechanisms could be combined and that Syt1 could bridge the membranes via a heteromerization (13,14). Finally, different configurations of Syt1 domains could control different modes of transmitter release, such as synchronous, asynchronous, and spontaneous fusion (15).
The attachment between SV and PM is maintained by the protein complex termed SNARE (16,17). The SNARE proteins form a coil-coiled four-helical bundle, which consists of the SV-associated protein synaptobrevin (Syb) and the PM-associated proteins syntaxin (Syx) and SNAP25, also known as t-SNARE. Syt1 can interact with t-SNARE proteins, and multiple studies suggest an important role for Syt1-SNARE interactions during fusion (18, 19, 20, 21, 22, 23). However, other studies have argued against this possibility (24,25), and it is still debated how the Syt1-SNARE complex is formed in vivo and how its formation affects the fusion process (26, 27, 28, 29, 30, 31, 32).
Fusion is regulated by the cytosolic protein complexin (Cpx), which attaches to the SNARE complex (33), forming a five helical SNARE-Cpx bundle. Cpx promotes Ca2+-dependent fusion (34, 35, 36), and several structural (37,38), genetic (39), and biochemical (40,41) studies suggest that Cpx may directly interact with Syt1 on the SNARE bundle (but see also (42)).
To elucidate the atomistic detail of Syt1 interactions with the SNARE-Cpx complex and lipid bilayers, we performed all-atom molecular dynamics (MD) simulations at a microsecond scale. We took advantage of unique capabilities of the specialized Anton supercomputer designed for MD simulations (43,44) that enabled a breakthrough in simulating the protein dynamics at a timescale of microseconds (45,46). We have recently employed these computational tools to investigate the conformational dynamics of Syt1 in the solution (47,48). Here, we employed this approach to elucidate how Syt1 interactions with PM, SV, and the SNARE-Cpx complex could drive synaptic fusion.
Methods
System setup
All the molecular systems were constructed using Visual Molecular Dynamics Software (VMD; Theoretical and Computational Biophysics Group, National Institutes of Health Center for Macromolecular Modeling and Bioinformatics, at the Beckman Institute, University of Illinois at Urbana-Champaign, Champaign, IL). All the simulations were performed in water-ion environment with explicit waters. Potassium and chloride ions were added to neutralize the systems and to yield 150-mM concentration of KCl. Water boxes with added ions were constructed using VMD.
The palmitoyl oleyl phosphatidylcholine (POPC) lipid bilayers were generated using VMD. The initial structure of the anionic lipid bilayer containing phosphatidylserine (POPS) and PIP2, POPC/POPS/PIP2 (75:20:5) (49), was kindly provided by Dr. J. Wereszczynski (Illinois Institute of Technology). In all the systems, the lipid bilayers were positioned in the x-y plain.
The initial structures for the isolated C2A (50) and C2B (51) domains in their Ca2+-bound forms were obtained from crystallography studies (PDB: 1BYN and PDB: 1TJX, respectively, in the Protein Data Bank). The initial structures of the isolated domains in their Ca2+-free forms were obtained by removing Ca2+ ions from the Ca2+-bound structures. For the initial structure of the Syt1 C2AB tandem, we took a transient state with uncoupled domains from the MD trajectory obtained in our earlier study (48).
To build the initial model of the C2AB-SNARE-Cpx complex (I1), we combined two structures: PDB: 5CCG (C2AB-SNARE complex) obtained by crystallography (27) and the SNARE-Cpx complex extracted from our earlier MD simulations (52). We have superimposed the SNARE bundles in the two models by minimizing their root mean-square deviation (RMSD) distances. Subsequently, one of the SNARE bundles (from PDB: 5CCG) was removed, and the structure of the resulting Syt1-SNARE-Cpx complex was optimized, employing energy minimization. Chelated Ca2+ ions were kept within the Ca2+ binding pockets as in the original PDB: 5CCG structure. To build the structure of the C2B-SNARE-Cpx complex, we have removed the C2A domain (residues 140–270) from the Syt1-SNARE-Cpx complex.
The additional starting models of the Syt1-SNARE-Cpx complex (I3 and I4) were built as follows. The starting configuration for the I3 complex was derived from the PDB: 3N1T C2B-SNARE complex (28) combined with the C2AB tandem (33,48) and the SNARE-Cpx complex (52). The structures were combined subsequently. First, the C2B domain from C2B-SNARE (PDB: 3N1T) complex was superimposed with the C2B domain from the C2AB tandem employing the RMSD minimization, and then the C2B module from PDB: 3N1T was removed. Subsequently, the SNARE bundle from the resulting C2AB-SNARE complex was superimposed with the SNARE-Cpx complex employing the same method, and the first SNARE bundle was removed. This procedure was followed by the energy minimization of the resulting C2AB-SNARE-Cpx complex.
The starting configuration for the I4 complex was obtained by docking of the C2AB tandem to the SNARE-Cpx complex. We have positioned the SNARE-Cpx complex and the C2AB tandem in such a way that the Syt1-interacting stretch of SNAP25 (32–40 (53)) was facing the SNARE-interacting surface of the C2B domain (47). Out of the two possible orientations of the C2B domain, we chose the one with negatively charged residues of Syt1 facing the positively charged residues of SNAP25 and vice versa. Subsequently, we performed the Monte-Carlo/Minimization docking procedure (54) for this molecular system employing ZMM software (www.zmmsoft.com (55)).
All the molecular systems, including their sizes and the lengths of respective MD trajectories, are summarized in Table S1.
MD
The MD simulations were performed, employing a CHARMM36 force field (56) modified to include the parameters for PIP2 as described in (49). The simulations were performed with periodic boundary conditions and Ewald electrostatics in NPT ensemble at 310 K.
The heating (20 ps) and equilibration (100 ns) phases were performed, employing NAMD (57) Scalable Molecular Dynamics (Theoretical and Computational Biophysics Group, National Institutes of Health Center for Macromolecular Modeling and Bioinformatics, at the Beckman Institute, University of Illinois at Urbana-Champaign) at Extreme Science and Engineering Discovery Environment Stampede cluster (Texas Advanced Computing Center). The NAMD simulations were performed with a flexible cell and with a time step of 1.5 fs, employing Langevin thermostat and Berendsen barostat.
Production runs were performed at Anton2 supercomputer (43,44) with Desmond software through the National Center for Multiscale Modeling of Biological Systems, Pittsburgh Supercomputing Center, and D. E. Shaw Research Institute. All the Anton2 simulations were performed in a semi-isotropic regime, with a time step of 2.5 fs, employing the multigrator (58) to maintain constant temperature and pressure.
Analysis and visualization
The trajectory analysis was performed, employing VMD and Vega ZZ (Drug Design Laboratory) software. All the parameters along all the trajectories were computed with a time step of 2.4 ns. The number of Van der Waals (VdW) contacts between two molecules was computed as the number of the pairs of atoms separated by the distance of less than 3 Å. The penetration of a protein into the lipid bilayer was computed as p = , where is the maximal atomic Z coordinate of the lipid bilayer, and is the minimal atomic Z coordinate of the protein (Fig. S1). The distributions of the parameters along MD trajectories were compared using the Kolmogorov-Smirnov (K.-S.) test.
Results
The interactions of the C2B domain of Syt1 with phospholipid bilayers and the SNARE complex
We started from simulating the interactions of the C2B domain with lipid bilayers because these interactions were shown to be critical for the fusion process (8,59,60). First, we simulated the Ca2+-free and Ca2+-bound (Ca2+C2B) forms of the C2B domain positioned near a homogeneous neutral lipid bilayer (POPC). In the initial configuration (Fig. S2 A), the protein was positioned in such a way that it did not form any VdW contacts with the bilayer. This initial configuration was also used in all the subsequent simulations of the isolated C2 domains.
The 3.2-μs production run of the Ca2+-free C2B module showed that the protein was largely detached from the bilayer over the entire length of the MD trajectory, making infrequent contacts with lipids (Fig. 1 A; Fig. S2, B.1 and B.2). The analysis of energy components demonstrated that electrostatic energy prevails in the C2B-lipid interactions and also that the energy of C2B-ion interactions drastically exceeds the energy of C2B-lipid interactions (Fig. S2 B.3). This result suggests that the Ca2+-free C2B module has a preference for a water-ion environment versus lipids, and it explains the limited number of protein-lipid contacts observed over the course of the MD trajectory (Fig. S2 B.1). In contrast, the Ca2+C2B domain largely interacted with the bilayer over the course of the 4.0-μs trajectory, and these interactions were predominantly maintained by its α-helix and the adjacent β-sheets (residues 380–410), as well as by its C2AB linker (Fig. 1 B; Fig. S2, C.1 and C.2). The analysis of energy components showed that the energies of Ca2+C2B-ion interactions were shifted toward more positive values when compared with the Ca2+-free module (Fig. S1 D, top), possibly because of the reduced electrostatic attraction to K+ ions. Respectively, the energies of Ca2+C2B-lipid interactions were shifted toward more negative values (Fig. S2 D, bottom), in agreement with consistent protein-lipid contacts observed for the Ca2+C2B module (Fig. S2 C) but not for its Ca2+-free form.
Figure 1.
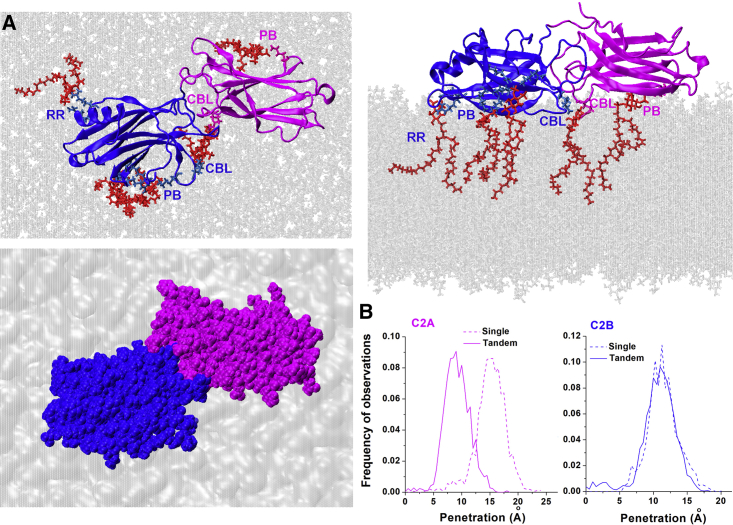
Interactions of the C2B domain with lipid bilayers. (A) The Ca2+-free C2B module is detached from the SV bilayer. Shown is a representative conformational state at the end of the C2B-POPC trajectory. Note that the Ca2+ binding loops (CBLs) do not interact with lipids. (B) The Ca2+C2B module interacts with the SV bilayer via its α-helix at the opposite tip from CBL. Shown is a representative state at the end of the Ca2+C2B-POPC trajectory. Green spheres depict Ca2+ ions. (C) The C2B module is anchored to a PIP2 molecule via its PB and CBL motifs, whereas the RR motif anchors to another PIP2 molecule. Shown is a representative state at the end of the C2B-POPC/POPS/PIP2 trajectory. (D) The CBL motif of the Ca2+C2B module is inserted into the bilayer, being anchored to a PIP2 molecule, whereas Ca2+ ions form coordination bonds with two POPS molecules. In addition, PB is anchored to another PIP2 molecule. (E) Shown is the penetration of the CBL and PB motifs of the C2B and Ca2+C2B modules into the POPC/POPS/PIP2 bilayer mimicking PM. The images (top) show the penetration levels (marked with lines) corresponding to the peaks of the frequency distributions (bottom) for the Ca2+-free and Ca2+-bound forms of the C2B module. Ca2+ binding significantly promotes the penetration (p < 0.01 per K.-S. test). To see this figure in color, go online.
The interactions of the C2B module with neutral phospholipids described by these simulations would likely define the dynamics of the attachment of the C2B domain to SV because SVs are predominantly composed out of neutral lipids (61,62). Our results suggest that the Ca2+-free C2B domain would likely float in a cytosol being detached from SV, whereas Ca2+ binding would promote a weak C2B-SV association. We next asked how would the C2B module interact with the PM, which has a high content of anionic lipids and also incorporates PIP2 (8,63), which is critical for the attachment of Syt1 (7,10,25). To model the PM composition, we used the atomic model of the membrane patch containing anionic lipids (POPS, 20%) and PIP2 (5%).
The simulations demonstrated that both Ca2+-free and Ca2+-bound forms of the C2B domain rapidly attached to the POPC/POPS/PIP2 (75:20:5) bilayer and stayed attached over the entire course of respective trajectories (Fig. 1, C and D; Fig. S3 A). The analysis of energy components demonstrated the C2B-lipid attraction was largely defined by the interactions of the C2B module with PIP2 (Fig. S3 B). The interactions with PIP2 were maintained by the three sites of the C2B domain (Fig. S3 C): 1) the polybasic stretch (PB; residues K321-327), 2) two basic residues of the second Ca2+-binding loop (CBL; residues K366 and K369), and 3) two basic residues at the opposite tip (RR, residues R398 and R399). The PB and CBL motifs consistently maintained salt bridges with PIP2 for both Ca2+-free and Ca2+-bound forms, whereas the RR motif only formed dynamic salt bridges with PIP2 for the Ca2+-free C2B module (Fig. 1, C and D; Fig. S3 D). For Ca2+C2B, strong attraction between Ca2+ ions and anionic lipids was observed (Fig. S3 E), and two POPS molecules completed the coordination of chelated Ca2+ in the end of the trajectory (Fig. 1 D).
Thus, both Ca2+-free and Ca2+-bound forms of the C2B module became strongly attached to the bilayer mimicking PM; however, the attachment configurations differed in the two forms. For the Ca2+-free C2B module, the three motifs (CBL, PB, and RR) contributed to dynamic anchoring to the bilayer, producing broad surface attachment (Fig. 1 C). In contrast, the Ca2+C2B module was tilted, with the Ca2+-bound tip inserting into the bilayer (Fig. 1 D), Ca2+ ions forming coordination bonds with POPS, the CBL motif anchoring to PIP2, and the PB region serving as the second anchor. To evaluate how deeply the Syt1 motifs penetrated into the bilayer, we measured the distance between the most exposed atom(s) of the lipid monomers and the most submerged atom(s) of the protein (see Methods; Fig. S1). Our results (Fig. 1 E) show that the hydrophobic residues of both Ca2+-free and Ca2+-bound forms of the C2B domain consistently inserted into the POPC/POPS/PIP2 bilayer; however, the penetration was significantly deeper for the Ca2+-bound form (Fig. 1 E).
Can the C2B domain simultaneously attach to PM and to the SNARE complex? To address this question, we next simulated the interactions of Ca2+C2B with the coil-coiled four-helical motif of the SNARE complex and the lipid bilayer mimicking PM. For the initial approximation of the Syt1-SNARE complex, we relied on the crystallography study (27), which identified a large interface between the C2B domain and the SNARE bundle. Because the SNARE complex in its prefusion state is thought to be associated with the fusion affecter Cpx, we generated a model of the Ca2+C2B-SNARE-Cpx complex. As described in the Methods, we combined the structures of Ca2+C2B-SNARE (27) with SNARE-Cpx (33,52) and then positioned the Ca2+C2B-SNARE-Cpx above the POPC/POPS/PIP2 bilayer (Fig. S4 A, left) in such a way that the PM and CBL motifs of the C2B module were facing the bilayer.
The protein complex rapidly attached to the membrane (Fig. 2 A; Fig. S4 A). Over the course of the 4.8-μs MD trajectory, PIP2 molecules redistributed toward the protein complex (Fig. S4 B), forming multiple salt bridges with t-SNARE (Fig. 2 B) and the C2B module (Fig. 2 C). The energy of the interactions (Fig. 2 D) and the number of VdW contacts (Fig. 2 E) between PIP2 and the proteins plateaued after ∼3 μs of the simulation and remained at the plateau level until the end of the trajectory. Notably, the tight interface between the C2B domain and the SNARE bundle was not disrupted (Fig. 2, D–F; Fig. S4 C). The salt bridges and tight VdW contacts between the C2B module and the SNARE bundle revealed by crystallography (27) were retained in the end of the trajectory, and in addition, new salt bridges between the RR motif of the C2B module and two aspartate residues of t-SNARE (D58 of SNAP25 and D231 of Syx; Fig. 2 F) were formed. These results show that Ca2+C2B can simultaneously attach to the SNARE bundle and anchor to the PM component PIP2.
Figure 2.
The Ca2+C2B-SNARE-Cpx complex anchored at the lipid bilayer mimicking PM. (A) The final point of the MD trajectory is two perpendicular views showing the protein complex in VdW representation on the top of the POPC/POPS/PIP2 bilayer (surface representation). Syb, red; Syx, cyan; SNAP25, lime; Cpx, orange; C2B, blue. Note the tight extensive contacts between the protein complex and the bilayer. (B) Shown is the protein complex anchored to multiple PIP2 molecules (red). The top view shows PIP2 in VdW representation (top), and the side view shows PIP2 in bond representation (bottom). (C) The C2B module is anchored to PIP2 via its PB and CBL motifs, as was the case for the isolated C2B domain. Ca2+ ions are removed for clarity. (D) Shown is the energy of the interactions within the protein-lipid complex along the MD trajectory. Note the energy of PIP2-SNARE (cyan) and PIP2-C2B (red) decreasing along the trajectory (toward tighter interactions) and then reaching a plateau. Also note a constant energy of C2B-SNARE interactions (dark green) showing that the C2B-SNARE interface largely stayed intact. (E) Shown is the number of VdW contacts within the protein-lipid complex along the MD trajectory. Note numerous contacts between PIP2 and SNARE (cyan), PIP2 and C2B (red), and C2B and SNARE (dark green) in the end of the trajectory. (F) Shown are the residues forming salt bridges and tight VdW interactions (shown in the VdW representation) between the C2B module and t-SNARE proteins in the end of the trajectory. Two views show the opposite surfaces of the protein complex. Ca2+ ions are shown as green spheres. To see this figure in color, go online.
The interactions of the C2A domain and the C2AB tandem with phospholipid bilayers
Next, we asked how the C2A domain of Syt1 adds to the protein-lipid interactions. First, we simulated the interactions of the isolated C2A domain with the phospholipid bilayers POPC and POPC/POPS/PIP2. The initial position of the C2A module relative to the bilayer was in all the cases similar to that for the C2B module (Fig. S2 A). The Ca2+-free C2A module showed relatively weak but consistent interactions with neutral lipids over the entire course of the 3.8-μs MD trajectory, predominantly attaching to the bilayer via its Ca2+-binding tip (Fig. 3 A; Fig. S5 A). These interactions were more consistent for the Ca2+C2A module, with the Ca2+-bound tip being in contact with the bilayer over the major part of the 3.7-μs trajectory (Fig. 3 B; Fig. S5 B). The analysis of energy components showed that the energy of Ca2+C2A-POPC interactions was largely defined by the attraction of Ca2+ ions to the POPC bilayer (Fig. S5 C), whereas the analysis of Ca2+ coordination revealed that oxygen atoms of phosphate groups of two POPC molecules completed the coordination of the chelated Ca2+ ions (Fig. S5 D).
Figure 3.
The interactions of the C2A domain with the lipid bilayer. (A and B) The C2A module interacts with the SV bilayer via its Ca2+-binding tip. Shown are representative states in the end of respective trajectories of the Ca2+-free or Ca2+-bound C2A modules. Green spheres depict Ca2+ ions. (C) The C2A module is anchored to PIP2 via its PB and CBL motifs. (D) The CBL of the Ca2+C2A module is immersed into the bilayer, being anchored to PIP2, whereas Ca2+ ions formed coordination bonds with two POPS molecules. PB is also anchored to PIP2. (E) Shown is the penetration of the CBL and PB motifs of the C2A and Ca2+C2A modules into lipid bilayers. The images (top and middle) show the penetration levels corresponding to the peaks of the frequency distributions for the Ca2+-free and Ca2+-bound forms of the C2A module interacting with the PM bilayer (black and green lines, respectively). Ca2+ binding significantly promotes the penetration (p < 0.01 per K.-S. test for the C2A-PM versus Ca2+C2A-PM distributions). The Ca2+-bound tip of Ca2+C2A also penetrates into the POPC bilayer mimicking SV (olive line). To see this figure in color, go online.
Next, we simulated the interactions of the C2A domain with the POPC/POPS/PIP2 bilayer mimicking PM. Both Ca2+-free and Ca2+-bound forms of the C2A module rapidly attached to the bilayer (Fig. 3, C and D; Fig. S6 A) and remained attached over the entire lengths of respective trajectories. The Ca2+-lipid interactions were largely defined by the POPS lipid component (Fig. S6 B, purple), and two POPS molecules completed Ca2+ coordination in the end of the trajectory (Fig. 3 D). The electrostatic attraction of Ca2+ ions to the bilayer added to the energy of the Ca2+C2A interactions with lipids (Fig. S6 C, green).
The C2A domain has a short polybasic stretch (PB, K189–192; Fig. S6 D), and over the course of the trajectory, the PB motif of the C2A domain formed salt bridges with PIP2 molecules (Fig. 3, C and D; Fig. S6 E). The basic residues of the CBL motif of the C2A domain (residues R233 and K236) served as a second anchor attaching to PIP2 (Fig. 3, C and D; Fig. S6, D and E), whereas the Ca2+-bound tip of Ca2+C2A immersed into the bilayer (Fig. 3 E, green). Some penetration into the POPC/POPS/PIP2 bilayer was also observed for the Ca2+-free C2A module Fig. 3 E, black), but it was significantly below the penetration level of Ca2+C2A. Interestingly, Ca2+C2A also showed some penetration into the POPC membrane (Fig. 3 A, olive).
Thus, our simulations showed that the affinity of the C2A domain to lipid bilayers, as well as the penetration of the C2A module into lipids, were collectively promoted by chelated Ca2+ ions, anionic lipids, and PIP2 molecules (Fig. 3), as was the case for the C2B domain (Fig. 1). Notably, the Ca2+ binding loops of the C2A domain had some affinity to neutral lipids, in contrast to the C2B domain. For both C2A and C2B modules, chelated Ca2+ ions formed coordination bonds with anionic lipids and promoted the immersion of the Ca2+-bound tips into the bilayer, whereas several basic residues anchored to PIP2. However, the C2A domain had fewer anchors than the C2B domain because its PB stretch is shorter, and its structure does not have an analog for the RR motif of the C2B domain.
We next asked how these properties of the isolated domains would define the interactions of the C2AB tandem with lipid bilayers. First, we simulated the dynamics of the C2AB tandem at the POPC bilayer. The initial structures of the Ca2+-free and Ca2+-bound forms of the C2AB tandem were taken from our earlier MD simulations of the C2AB tandem in the solution (48). To minimize the initial influence of the interdomain coupling, for the starting, we took a transient state with maximally separated domains (Fig. S7 A) extracted from the MD trajectory obtained for Ca2+C2AB. Only two Ca2+ ions were chelated by the C2A domain in the C2AB tandem, as was suggested by earlier experimental (64,65) and computational (48) studies. The initial state of the Ca2+-free C2AB tandem was obtained by removing the Ca2+ ions.
During the course of the 4.7-μs trajectory of the Ca2+-free C2AB tandem, its C2B domain went through multiple configurations (Fig. S7 B), occasionally interacting with the membrane briefly (Fig. S7 C), and these transitions were accompanied by several interdomain rotations (Fig. S7 D). The Ca2+-binding tip of the C2A domain attached to the bilayer within the initial 1 μs of the trajectory (Fig. S7, B and C) and continued interacting with lipids until the end of the simulation (Fig. 4 A), consistently penetrating into the bilayer (Fig. 4 B). We next investigated the Ca2+C2AB dynamics at the POPC bilayer (Fig. 4, C and D; Fig. S7, E–G). After initial interdomain rotations, Ca2+C2AB adopted a conformation in which Ca2+-binding tips of C2 domains were at the opposite sides of the tandem (Fig. S7 E), and this configuration remained stable for the entire length of the simulation. The C2A domain interacted with lipids via its Ca2+-bound tip, whereas the C2B domain interacted with the bilayer via its α-helix at the opposite tip (Fig. 4 C) in line with the dynamics of the isolated Ca2+C2A and Ca2+C2B domains on the POPC bilayer. The tips of both domains were inserted into the membrane (Fig. 4 D).
Figure 4.
The interactions of the C2AB tandem with neutral lipids (POPC). (A) The Ca2+-free C2AB tandem has its C2A domain (magenta) attached to the bilayer via its Ca2+-binding tip, whereas the C2B domain (blue) is detached from the bilayer. The image shows a representative time point at the end of the trajectory. (B) The tip of the Ca2+-free C2A domain consistently penetrates into the lipid bilayer. (C) Ca2+C2AB is attached to the bilayer via the Ca2+-bound tip of its C2A domain and the opposite tip of its C2B domain. Green spheres depict Ca2+ ions. (D) The tips of both domains within the Ca2+C2AB tandem consistently penetrate into the bilayer. To see this figure in color, go online.
We next investigated the interactions of the C2AB tandem with anionic lipids containing PIP2. First, we performed MD simulations of the Ca2+-free C2AB tandem interacting with the POPC/POPS/PIP2 bilayer. The 5.1-μs MD run was started from the same initial state as the previous simulations of the C2AB tandem (Fig. S7 A). During the initial 0.5 μs of the simulation, the tandem underwent two conformational transitions (Fig. S8, A and B) and attached to the bilayer (Fig. S8 C). The attached tandem had tightly coupled C2 domains, and each domain was anchored to PIP2 in the same way as was observed for the isolated C2 modules (Fig. 5 A). The C2B domain was anchored via its three motifs (PB, CBL, and RR), and the C2A domain was anchored via its two motifs (CBL and PB). The energies of the protein-lipid interactions (Fig. S8 D) showed a strong attraction of the C2B domain to the bilayer, and the penetration of the C2B domain into lipids within the tandem was similar to the penetration observed for the isolated C2B domain (Fig. 5 B, blue). In contrast, the C2A domain in a tandem did not insert into the bilayer to the level observed for the isolated C2A domain (Fig. 5 B, magenta). Thus, the tight coupling of the C2 domains in a tandem restricted the interactions of the C2A domain with the bilayer.
Figure 5.
The interactions of the Ca2+-free C2AB tandem with the POPC/POPS/PIP2 bilayer. (A) Shown are three views of the protein-lipid complex at the end of the trajectory. PIP2 anchors (red) bind the CBL and PB motifs of both domains. The VdW representation (bottom) shows a tight coupling between the C2 domains. (B) The penetration into lipids is significantly reduced for the C2A domain in the tandem compared with the isolated C2A module (p < 0.001). In contrast, the C2B domain penetrates into the lipid bilayer to the same extent, either as a part of the tandem or in isolation. To see this figure in color, go online.
Next, we investigated the Ca2+C2AB tandem attaching to the POPC/POPS/PIP2 bilayer. After undergoing several conformation transitions within the initial 1.3 μs of the simulation (Fig. S8, E and F), the tandem adopted a conformation with perpendicularly oriented domains and maintained this conformation until the end of the 5.1-μs trajectory (Fig. S8, F and G). Both domains were attached to the bilayer and tightly coupled (Fig. 6 A), and the energy of the protein-lipid interactions showed a strong attraction, especially for the C2B domain (Fig. S8 H). As in the case of the isolated domains, each module was anchored to PIP2 via two motifs: PB and CBL (Fig. 6 A). However, the Ca2+ ions of both domains remained positioned above the surface of the bilayer (Fig. 6 A, right) and did not form coordination bonds with POPS. Accordingly, neither of the C2 domains within the tandem penetrated into the lipid bilayer to the extent observed for the isolated modules (Fig. 6 B).
Figure 6.
The interactions of Ca2+C2AB with the POPC/POPS/PIP2 bilayer. (A) Three views of the protein-lipid complex in the end of the trajectory show PB and CBL anchors attached to PIP2 (red). The VdW representation (bottom) shows that C2 domains are tightly coupled. (B) The penetration into the lipid bilayer is significantly reduced for each of the C2 domains in a tandem compared with the isolated C2 modules (p < 0.001 for the C2A domain and p < 0.01 for the C2B domain). To see this figure in color, go online.
These results show that the coupling of the C2 domains would counteract the immersion of each domain into the POPC/POPS/PIP2 lipid bilayer. This surprising finding suggests that each of the Ca2+-bound C2 modules would be more efficient in immersing into PM in isolation compared with the C2 domains coupled within the C2AB tandem, raising the question of why both domains are critical for triggering fusion (8,59,66,67). One possibility is that in the prefusion protein-lipid complex, the C2 domains of Syt1 become uncoupled. Another possibility is that the immersion of the C2 domains into PM is not the major mechanism by which Syt1 triggers fusion. For example, the coupled C2 domains could trigger fusion by bridging and merging PM and SV via simultaneously penetrating into the PM and SV bilayers.
To investigate the latter possibility, we simulated the dynamics of the C2AB tandem between two bilayers mimicking PM and SV. To simulate the two bilayers, we took advantage of the periodic boundary conditions. The advantage of such a system is that the distance between the bilayers is fixed and the size of the system is kept at a minimum. For the starting configuration, we took the trajectory end point for Ca2+CAB tandem on the POPC bilayer. One of the bilayer leaflets was substituted by the POPC/POPS/PIP2 monolayer, and Z-dimension of the cell was adjusted to allow the protein to make VdW contacts with the POPC/POPS/PIP2 periodic image (Fig. 7 A.1). The overall configuration of this system and the distance between the opposing membranes (47 Å) largely agreed with those obtained in a spin-labeling study (68).
Figure 7.
The dynamics of the Ca2+C2AB tandem between the lipid bilayers mimicking PM and SV. (A) Shown are three conformations along the trajectory. (A.1) Shown is the initial configuration. The CBL motif of Ca2+C2A is immersed into the POPC bilayer. (A.2) Shown is the transient state (1 μs). The Ca2+C2B is anchored to PIP2 (red) via its CBL and PB motifs. This state is characterized by a prominent lipid protrusion (arrow). (A.3) Shown is the final state. Both C2 domains are anchored to PIP2 clusters via their CBL and PB motifs. (B) Shown is the number of contacts between lipids and CBLs of each C2 domain along the trajectory (residues 170–180 and 230–240 for the C2A domain and residues 300–310 and 360–370 for the C2B domain). Note the conformational transition after the initial 1 μs. (B.1) The CBLs of the C2A domain form numerous VdW contacts with the POPC bilayer during the initial 1 μs of the trajectory. Subsequently, the contacts are formed only with the opposing POPC/POPS/PIP2 bilayer. (B.2) The CBLs of the C2B domain form VdW contacts only with the POPC/POPS/PIP2 bilayer. Arrows in (B.1) and (B.2) point to the time points shown in (A.2). To see this figure in color, go online.
During the initial 1 μs of the MD run, the Ca2+C2B domain attached to the POPC/POPS/PIP2 bilayer (Fig. 7 A.2), being anchored to a PIP2 molecule via its CBL and PB motifs. The contacts between Ca2+C2A and POPC remained extensive (Fig. 7, A.2 and B), and these interactions created a prominent protrusion in the POPC lipid bilayer at the interface of C2 domains (Fig. 7 A.2, arrow). Hypothetically, such protrusions could promote the stalk formation and lipid merging. It should be also noted that the molecular system employed in this set of simulations would likely produce an underestimation of any out-of-plain deviations of the lipid bilayers, such as bulging and protrusions, because the leaflet coupling could produce artificial lateral membrane tensions.
The subsequent simulations, however, produced a conformational transition of the Ca2+C2A domain, followed by its attachment to the POPC/POPS/PIP2 bilayer (Fig. 7, A.3 and B; Video S1) via its CBL and PB motifs. Meanwhile, additional PIP2 molecules aggregated and attached to the Ca2+C2B domain (Fig. 7 A.3). This configuration proved to be stable for the subsequent 5.5 μs of the simulation, suggesting that the PM-SV bridging by the Ca2+C2AB tandem is a transient state and that both domains would tend to interact with PM.
The interval between subsequent frames is 2.5 ns. Note the Ca2+ binding loops of the C2A domain attached to SV in the beginning of the trajectory and interacting with PM in the end of the trajectory. The video supplements the Fig. 7.
Thus, our simulations revealed a transient state of the Ca2+C2AB tandem that could bridge the SV and PM bilayers and promote lipid protrusions potentially leading to a stalk formation. However, the simulations also showed that this state would compete with a more favorable and stable state in which both domains would attach to PM via their CBL and PB motifs. The question then still remains: how do the C2 domains cooperate in promoting fusion? To elucidate this issue, we investigated whether the attachment to the SNARE complex could alter the dynamics of the C2AB tandem and its interactions with PM.
Interactions of the C2AB tandem with the SNARE-Cpx complex and phospholipids
We started from the structure of the C2AB-SNARE complex obtained by crystallography (27), which has an extensive C2B-SNARE interface. Our simulations of the C2B-SNARE-Cpx complex demonstrated that this interface (which we term I1, shown in Fig. 2) is stable in the water-ion-lipid environment. To understand how the C2A domain adds to the protein-lipid interactions, we constructed the C2AB-SNARE-Cpx complex by combining C2AB-SNARE (37) with SNARE-Cpx (52) as described in Methods. The structure was then equilibrated, and the 9-μs MD run was performed (Fig. S9). Surprisingly, we observed a sharp increase in the energy of C2B-SNARE interactions during the initial 500 ns of the trajectory (Fig. S9 A), denoting weakened SNARE-C2B interactions. Notably, such an increase was not observed in the absence of the C2A domain (Fig. 2 D, olive). Furthermore, the energy distribution for C2B-SNARE interactions was shifted toward more positive values in the presence of the C2A domain (Fig. S9 B), suggesting that the interactions of the C2A domain destabilized the complex. The analysis of the trajectory showed that the complex underwent a conformational transition between 4.8 and 5.0 μs (Fig. S9 C) so that the C2B module shifted along the SNARE bundle toward its membrane-distal terminus, forming a new interface with t-SNARE proteins (we term this new interface I2; Fig. S9 C). This new conformation corresponded to a local minimum in the energy of the C2B-SNARE interactions (Fig. S9 A, I2). Together with the results presented in Fig. 2, these results indicate that although the C2B-SNARE interface I1 (37) is energetically favorable and likely stable in the water-ion environment, and the position of the C2A domain obtained by crystallography may not be energetically optimal at physiological ion concentrations. Furthermore, optical studies showed that the Syt1-SNARE complex samples multiple conformational states in the solution (69), and the NMR approach identified the structure of the Syt1-SNARE complex in which the PB motif of the C2B domain anchored onto the SNARE bundle (28). We therefore sought to employ MD simulations to investigate the conformational states of the C2AB-SNARE-Cpx complex more systematically.
We generated four initial approximations for the C2B-SNARE interface (Fig. S10): I1 interface obtained by crystallography (27), I2 interface produced by our initial MD simulations (Fig. S10), I3 interface obtained by NMR studies (28), and I4 interface obtained by docking of the C2B domain to the SNARE bundle basing on spectroscopy (53) and genetic (47) studies, as described in Methods. All the complexes had the conformation of the C2AB tandem with perpendicularly oriented C2 domains (Fig. S11 A) because both crystallography (64) and MD (48) studies suggest that this conformation would be energetically favorable. Each complex was equilibrated, and the production MD runs were performed. In all the four trajectories (Fig. S11 B), both C2 modules were attached to the SNARE-Cpx bundle (note negative energies for each of the C2 domains interacting with the SNARE-Cpx bundle); however, the interactions of the C2B domain prevailed (note lower energies for C2B). In each run, we ensured that the energies of the interactions of the SNARE-Cpx bundle with C2 domains reached a plateau (of at least 2 μs), and this required MD runs of 6–9 μs each (Fig. S11 B, I1–I3). The only exception was the state I4, which converged to the state similar to the final point of the I2 trajectory (I2f; Fig. S11 C). Respectively, the I4 run was terminated (at 2.7 μs) as being redundant. Thus, four initial approximations of the C2AB-SNARE-Cpx complex, built upon four different C2B-SNARE interfaces (Figs. S10 and S11 A), converged into three different conformational states (I1f, I2f, and I3f; Fig. 8 A; Fig. S10 C), which were stable at a microsecond scale.
Figure 8.
Three conformational states of the C2AB-SNARE-Cpx. (A) Shown is the overall topology of the conformational states I1f, I2f, and I3f. Syb, red; Syx, cyan; SNAP25, green; Cpx, orange; C2B, blue; C2A, magenta. (B) Shown is the interface between the C2B domain and the SNARE-Cpx bundle for the three states. The residues forming salt bridges and the strongest VdW attachments are shown in the VdW representation and marked. (C) Shown is the interface between the C2A domain and the SNARE-Cpx bundle. The locations of the CBL and PB motifs are marked for both C2 domains. (D) Shown are the energy distributions for the interactions of the C2A and C2B domain with the SNARE-Cpx bundle derived for each of the three complexes over the final 2 μs of the respective trajectories. For the comparison, the energy distributions for the interactions of the C2 domains with the lipid bilayer mimicking PM (POPC/POPS/PIP2) are shown (black lines; the same data as in Fig. S8D). Note that the PM distributions are shifted toward more negative energy values and largely do not overlap with the SNARE-Cpx distributions, suggesting a preference for each of the C2 domains to attach to PM versus the SNARE-Cpx bundle. To see this figure in color, go online.
The I1 interface between the C2B domain and the SNARE bundle (27) remained largely unchanged over the course of the trajectory (I1, Fig. S11 A vs. Fig. S11 C; note the unchanged position of the C2B domain). The final end-point complex (I1f, Fig. 8 A) had several stabilizing salt bridges between the C2B domain and t-SNARE (Fig. 8 B; R281 of C2B and D51 of SNAP25, E295 of C2B and K40 of SNAP25, and R398 of C2B and D231 of Syx), as well as multiple VdW contacts (note Y338 of C2B and H162 of SNAP25; Fig. 8 B, I1f). Notably, mutating the C2B residues R281, E295, and Y338 belonging to this interface produces Syt1 loss of function (47). The interactions of the C2A domain were weaker and only involved a single salt bridge with SNAP25 in the end of the trajectory (Fig. 8 C, I1f).
In the I2f complex, the C2B module was shifted toward the membrane-distal end of the SNARE bundle compared with the I2f complex. Interestingly, the C2A module underwent a conformational transition in the beginning of the I2 trajectory (at 1.7 μs) and formed two salt bridges with Cpx, which were stable until the end of the trajectory (for over 5 μs). The salt bridges were formed between Ca2+-binding loops of the C2A domain and the Cpx stretch, connecting its central and accessory helixes (Fig. 8 C, I2f). Thus, the MD simulations revealed a new tripartied interface between Syt1, Cpx, and the SNARE bundle in which the C2B domain forms a tight interface with t-SNARE, whereas the C2A domain forms salt bridges with Cpx (Fig. 8, A–C, I2f).
The I3 complex had the PB stretch of the C2B domain binding t-SNARE (28), and these interactions stabilized over the course of the 6-μs trajectory. In addition, the CBL motif of the C2B domain formed a salt bridge with Cpx (Fig. 8 B, I3f). The C2A domain, though, formed a salt bridge with Cpx via the third loop of its Ca2+-binding tip (K196 of C2A and D68 of Cpx; Fig. 8 C, I3f). This loop of the C2A domain also formed a salt bridge with Syx (R199 of C2A and D214 of Syx), whereas the first Ca2+-binding loop of the C2A domain formed a salt bridge with SNAP25 (D172 of C2A and R17 of SNAP25). Thus, both C2 domains in the I3f complex formed tight links with the SNARE-Cpx bundle, and these interactions involved the CBL and PB motifs of the C2B domain as well as the Ca2+-binding tip of the C2A domain.
The energy of the interactions between each of the C2 domains and the SNARE-Cpx bundle was shifted toward more negative values for the complex I3f (Fig. 8 D). This finding is in line with the results of the NMR studies, which suggested that the C2B-SNARE complex in the solution would be predominantly formed via the I3 interface (28). However, the proximity to PM may alter the interplay between the conformational states of the Syt1-SNARE-Cpx complex. Indeed, the enthalpy analysis indicates that the attraction to PM (Fig. 8 D, black lines) would be stronger for each of the C2 domains than the attraction to the SNARE-Cpx bundle. This result suggests that the conformational state(s) of the Syt1-SNARE-Cpx complex enabling simultaneous interactions of C2 domains with PM would likely prevail in the proximity to PM.
Out of the three conformational states (Fig. 8) identified by our simulations, the state I1f is the most likely candidate to satisfy this requirement. Indeed, the formation of the I3f complex involves the CBL and PB motifs of the C2B domain, which are also required for the attachment of the C2B domain to PM. Therefore, the formation of the I3f complex would preclude the attachment of the C2B domain to PM. The formation of the I2f complex does not involve either CBL or PB of the C2B domain; however, it involves CBL of the C2A domain. In contrast, the I1f complex does not involve either CBL or PB motifs of either of the C2 domains, suggesting that the I1f conformational state of the C2AB-SNARE-Cpx complex would allow Syt1 to simultaneously attach to lipid bilayers.
To test this suggestion, we positioned the I1f complex over the lipid bilayer mimicking PM (POPC/POPS/PIP2), substituted the Ca2+-free form of the C2AB tandem by its Ca2+-bound form, equilibrated this structure in water-ion environment, and performed the 8.4-μs MD run. Over the course of the trajectory, both Ca2+-bound tips immersed into the bilayer (Fig. 9, A and B). Notably, the I1 interface between the C2B domain and the SNARE bundle was not disrupted or even weakened. C2B domain remained tightly linked to the bundle via several salt bridges and hydrophobic interactions with t-SNARE proteins (Fig. 9 C). In contrast, the C2A domain separated from the bundle in the course of the simulation (Fig. 9, A and B). Notably, PIP2 molecules clustered around the protein complex over the course of the trajectory, anchoring both C2 domains and the SNARE bundle (Fig. 9 D). These results show that the interactions of the C2 domains with the bilayer do not compromise the ability of the C2B domain (but not the C2A domain) to form tight interactions with the SNARE bundle via the I1 interface.
Figure 9.
The association of the Ca2+C2AB tandem with the SNARE-Cpx bundle promotes the immersion of the Ca2+-bound tips of C2 domains into the lipid bilayer mimicking PM. (A) Given are two perpendicular views showing the Ca2+I1-PM complex at the trajectory end point. Note that the Ca2+-bound loops of both domains are inserted into the bilayer (top) and that the C2B domain is attached to t-SNARE (bottom). C2A, magenta; C2B, blue; Ca2+, green spheres; Syb, red; Syx, cyan; SNAP25, lime; Cpx, ochre; POPC/POPS/PIP2 bilayer, silver, surface representation. (B) Shown is the energy profile along the trajectory for the interactions of each of the C2 domains with the SNARE bundle and with the lipid bilayer. Note the steady level of the C2B-SNARE attractions. In contrast, the energy of the C2A-SNARE interactions increases to zero at the 4 μs time point, denoting the separation of the C2A domain from the SNARE-Cpx bundle. Also, note the decrease in the energies of C2-lipid interactions, denoting the insertion of C2 tips into the bilayer. (C) Given are two opposite views showing multiple salt bridges and tight VdW contacts between the C2B domain and the SNARE bundle. Ca2+ ions are removed for clarity. (D) PIP2 molecules (red; VdW representation) are clustered around the protein complex, anchoring the C2 domains and t-SNARE. (E) The Ca2+-bound tips of the C2 domains penetrate into the bilayers to a significantly larger extent than in the absence of the SNARE bundle (p < 0.01 for C2A; p < 0.001 for C2B). The graphs show the frequency distributions for the penetration into lipids. For comparison (thin lines), we re-plotted the distributions derived for the Ca2+C2AB tandem alone interacting with the POPC/POPS/PIP2 bilayer (the same data as in Fig. 6B for the tandem). To see this figure in color, go online.
We next assessed how the C2 domains penetrate into the bilayer within this protein-lipid complex (Ca2+I1-PM). Strikingly, we found that the penetration into lipids for each of the C2 domains within the Ca2+I1-PM complex was significantly deeper than the penetration for the C2 domains within the isolated C2AB tandem interacting with PM (Fig. 9 E). In other words, the attachment of the C2B domain to the SNARE bundle promoted the insertion of the tips of both C2 domains into the lipid bilayer. The likely explanation for this result is that the tight coupling between the C2B domain and the SNARE bundle within the Ca2+I1-PM complex uncouples the C2A and C2B domains within the C2AB tandem and allows them to deeper penetrate into lipids.
Thus, our results show that the interactions with the SNARE bundle promote the conformation of the C2AB tandem with uncoupled C2 domains. In the absence of the SNARE bundle, the C2 domains within the C2AB tandem mutually hinder their penetration into the bilayer (Fig. 6). However, the attachment of the C2B domain to the SNARE bundle via the I1 interface uncouples the C2 domains and abolishes their mutual hindering, thus enabling the insertion of the Ca2+-bound tips of the C2 domains into PM to the extent observed for the isolated C2 domains. Hypothetically, the PM distortions and tensions produced by these insertion events could drive fusion synergistically with SNARE zippering.
The model of the prefusion protein-lipid complex
We next investigated whether the Ca2+I1-PM complex could drive the SV-PM fusion. To test this, we added to the Ca2+I1-PM complex transmembrane domains of Syb and Syx embedded in the lipid bilayers mimicking SV and PM, respectively. To generate this molecular system, we performed the following steps (Fig. S12). First, we took the x-ray structure (70) of the SNARE complex with intact transmembrane domains of Syb and Syx (PDB: 3IPD; Fig. S11 A) and removed SNAP25. Next, we generated the Syxtm-Sybtm complex (Fig. S12 B) with bent transmembrane domains. This was done by keeping all the atoms of the coil-coiled region fixed, imposing distance constraints on C-terminus Cα atoms of Syxtm and Sybtm and performing the Monte-Carlo/minimization procedure with ZMM software (71). Next, the structures of Syx and Syb within the Ca2+I1-PM complex (Fig. 9) were sequentially replaced by Syxtm and Sybtm, respectively, employing the RMSD alignment. The resulting system (Fig. S12 C) had Syxtm partially embedded in the lipid bilayer mimicking PM. The lipid monomers overlapping with the Syxtm were removed from the system. Finally, the second membrane patch (POPC) mimicking SV was added to the system and positioned parallel to the POPC/POPS/PIP2 bilayer at a distance, allowing VdW contacts with the C2AB tandem (Fig. S12 D). The lipid monomers of the POPC patch overlapping with Sybtm were then removed from the system. Water and ions were added, and Z-dimension of the periodic cell was adjusted to allow a gap of 4.5 nm between the POPC patch and the periodic image of the POPC/POPS/PIP2 patch (Fig. S12 E, left). The size of the POPC patch was adjusted in such a way that its Y-dimension aligned with the POPC/POPS/PIP2 patch, whereas its X-dimension was shorter by 1.5 nm (Fig. S12 E, right). This was done to allow water molecules to circulate freely so that artificial pressures between the bilayers are not generated.
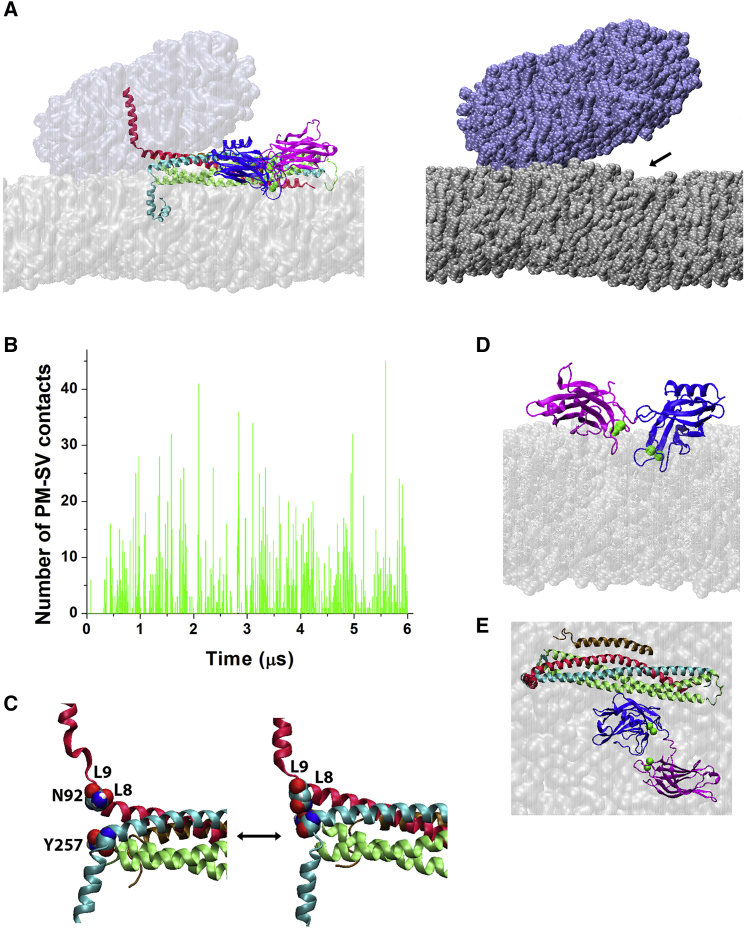
After equilibration of this system, we performed a 6-μs production MD run. Notably, in the course of the simulations, we observed multiple instances of the two bilayers approaching each other and making extensive contacts (Fig. 10, A and B; Video S2). Merging the membranes was usually associated with a transient increase in the curvature of the bilayer mimicking PM (Fig. 10 A, right, arrow). We also observed numerous transient zippering events for the layer 9 of the SNARE complex (Fig. 10 C), which was initially unzippered. Over the entire course of the trajectory, the Ca2+-bound tips of both C2 domains were immersed into the PM bilayer (Fig. 10 D), with a deeper penetration for the C2B domain, which remained attached to the SNARE bundle (Fig. 10 E). These simulations illustrate how the C2AB-SNARE complex brings together PM and SV and promotes their merging.
Figure 10.
The prefusion Ca2+Syt1-SNARE complex promotes lipid merging. (A) Shown are two views of the protein-lipid complex Ca2+I1-PM-SV in the end of the trajectory. The membranes mimicking PM (silver) and SV (ice blue) form extensive VdW contacts. Left: the Syb (red) TM domain spans through SV, and the Syx (cyan) TM domain spans through PM, whereas the Ca2+-bound tips of C2A (magenta) and C2B (blue) domains of Syt1 immerse into PM. Two membranes (surface representation) form extensive contacts. Right: the protein complex is removed for clarity, and the membranes are depicted in VdW representation to show numerous VdW contacts between SV and PM. The arrow points to the enhanced PM curvature observed at the site of Syt1 binding during several time frames corresponding to the transient event of lipid merging. (B) Numerous VdW contacts between the membrane patches mimicking PM and SV are repeatedly formed over the course of the trajectory. The points of zero contact denote the transient separations of the membrane patches. Note that the overall number of contacts increases over the initial 1 μs of the trajectory and then the system reaches an equilibrium, with the two membranes repeatedly forming VdW contacts and separating. (C) Shown is the zippering of the layer 9 of the SNARE complex. The unzippered layer 9 (left) has the residues N92 of Syb and Y257 of Syx (shown in VdW representation) being separated, whereas the complex with the fully zippered layer 9 has these residues forming a hydrogen bond. (D) In the end of the trajectory, the C2AB tandem has the Ca2+-bound tips of its C2 domains inserted into PM. (E) Shown is the top view of the Ca2+C2AB-SNARE-Cpx complex. The membrane patch mimicking SV is removed for clarity. To see this figure in color, go online.
SNAP25 is removed for clarity. Purple: Syb; violet: Syx; orange: Syt1. The interval between subsequent frames is 0.5 ns. Note the two membranes gradually approaching each other, forming contacts, separating, forming contacts again, and separating. The video supplements the Fig. 10.3
To delineate the specific role of Ca2+ chelation by Syt1 in this in silico system, we repeated the above simulations for the Ca2+-free form of the C2AB tandem. We started from the same initial approximation (Fig. S12) but removed chelated Ca2+ ions. The system was subsequently neutralized and re-equilibrated, and the 6-μs production MD run was performed. We observed that over the course of the trajectory, the edges of the two membrane patches periodically approached each other; however, they usually did not form VdW contacts (Fig. S13 A). Over the entire course of the simulation, we observed only several frames in which VdW contacts were present, and these contacts were not extensive (Fig. S13 B). The overall number of contacts was drastically diminished for the Ca2+-free form of the C2AB tandem, compared with its Ca2+-bound form (Fig. 11 A). Furthermore, the system with the Ca2+-bound form of the C2AB tandem consistently showed relatively long-lasting (for tens of nanoseconds) stretches of the trajectory with the two patches being in contact (Fig. 11 B, green). In contrast, in the absence of Ca2+, these contacts were very brief (Fig. 11 B, violet).
Figure 11.
Chelation of Ca2+ by Syt1 promotes lipid merging. (A) The number of contacts between the PM and SV membrane patches over the course of the trajectory is drastically diminished when chelated Ca2+ ions are removed from the system. The graph depicts the distributions of the number of VdW contacts observed over the course of the two trajectories. The Ca2+-free system mostly shows zero contacts (separated patches), whereas the system with chelated Ca2+ ions shows numerous VdW contacts over hundreds of trajectory points. The inset shows the average number of contacts per frame (p < 0.0001). (B) The contacts between PM and SV are significantly prolonged in the presence of Ca2+. PM-SV contacts plotted over the final 0.3 μs of the trajectory show several continuous intervals lasting for 10–20 ns (thick green lines below the graph) for the Ca2+-bound complex. This is not the case for the Ca2+-free complex, which only shows VdW contacts in a single time point (for less than 2.4 ns). The inset shows the distributions of the contact duration, with significantly increased contact durations for the Ca2+-bound form (green; note PM-SV contacts lasting for up to 24 ns). In contrast, the contacts are brief (lasting for less than 5 ns) for the Ca2+-free complex. To see this figure in color, go online.
Thus, the ability of the Ca2+C2AB-SNARE complex to drive lipid merging in our molecular system is not reproduced for the Ca2+-free complex. These results suggest that the force generated by zippering of the SNARE bundle is sufficient to bring SV and PM in a close proximity but insufficient to overcome the repulsion between the bilayers and to induce the formation of extensive VdW contacts. The additional force needed to bring the membranes into the closer contact is provided by the Ca2+CAB tandem. We questioned whether the action of the Ca2+C2AB tandem is solely due to its interactions with the lipid bilayer or whether the tandem also affects zippering of the layer 9 of the SNARE complex. To test this, we quantified zippering of the layer 9 of the SNARE complex by measuring the distance between its interacting residues (N92 of Syb forming a hydrogen bond with Y257 of Syx) and found that zippering of the layer 9 was not significantly altered by the presence of Ca2+ in the complex (Fig. S13 C). This result suggests that the additional force needed to merge the two membranes is produced by the interactions of Ca2+C2AB with PM. Indeed, the number of VdW contacts formed between each of the C2 domains and PM was significantly higher for the of Ca2+-bound form of the tandem (Fig. S13 D), and each C2 domain penetrated deeper into the PM when being in its Ca2+-bound form (Fig. S13 E). The out-of-plain deviations of the PM bilayer were significantly increased for the system incorporating the Ca2+C2AB tandem (Fig. S13 F), and the number of VdW contacts between PM and t-SNARE proteins was also significantly higher for the system incorporating Ca2+C2AB (Fig. S13 G). These results suggest that Syt1 can serve as an additional anchor for the t-SNARE bundle and transiently promote PM curvature, thus bringing PM in a tighter contact with SV.
Altogether, our results demonstrate in silico that the interactions of Syt1 with the SNARE bundle promote the penetration of Ca2+-bound tips of Syt1 into PM, and this in turn, synergistically with SNARE zippering, triggers the formation of extensive VdW contacts between PM and SV, which can lead to SV-PM fusion.
Discussion
We performed the first microsecond-scale, all-atom MD simulations of the neuronal Ca2+ sensor Syt1 interacting with lipid bilayers and SNARE proteins. The employed timescale of several microseconds allowed us to observe unconstrained conformational transitions, including Syt1 interdomain rotations, the formation of contacts between Syt1 and the SNARE-Cpx bundle, and the immersion of C2 domains of Syt1 into the lipid bilayer.
We started from simulating the interactions of isolated C2 domains with lipid bilayers and subsequently extended our simulations to the C2AB tandem. We next simulated the dynamic interaction of the tandem with the SNARE-Cpx complex and, finally, modeled the prefusion complex comprising the C2AB tandem attached to the SNARE-Cpx bundle positioned between lipid bilayers mimicking PM and SV. These simulations produced the model of the prefusion complex in which Syt1 adopted a conformation with Ca2+-bound tips of both domains immersed into PM. The C2B domain was attached to the SNARE bundle, and this enabled the cooperative action of SNARE zippering and Syt1 penetration into PM, which led to the formation of VdW contacts between the PM and SV lipid bilayers in silico.
In support of this model, extensive evidence suggested that Ca2+-bound tips of both Syt1 domains insert into PM (7,8,15), possibly driving the fusion by promoting PM curvature (11,12,72). However, some controversy remained in reconciling these studies with crystallography data (64) supported by computations (48), which suggested that Syt1 adopts a conformation with perpendicularly oriented domains, and therefore, the Ca2+-bound tips of Syt1 would likely face perpendicular planes. Furthermore, spin-labeling studies suggested that membrane-associated Syt1 would adopt a conformation with Ca2+-bound tips of its C2 domains facing opposite surfaces of the tandem and likely penetrating into opposite bilayers (68). Our MD simulations resolved this apparent controversy by demonstrating that the interactions of Syt1 with the SNARE complex and with the PM component PIP2 collectively promote the conformation of Syt1 in which Ca2+-bound tips of both domains can penetrate into PM.
The importance of PIP2 in anchoring Syt1 to lipid bilayers has been already established (25,73, 74, 75, 76). Our MD simulations identified the Syt1 residues interacting with PIP2 and determined the three-dimensional configurations for the attachment of each of the C2 modules to lipid bilayers. In particular, the simulations identified three PIP2-binding sites for the C2B domain: 1) the PB, 2) the basic residues (K366 and K369) of the second Ca2+ binding loop, CBL, and 3) the basic residues of the opposite tip (R398 and R399), RR. These residues interchange in forming salt bridges with several PIP2 molecules, enabling dynamic and reliable anchoring to a PIP2 cluster. In agreement with (73), the simulations showed that such attachment of the C2B domain to a PIP2 cluster occurs even in the absence of Ca2+. The simulations also showed that upon Ca2+ binding, the C2B module tilts and its Ca2+-bound tip immerses deeper into the bilayer. In this configuration, Ca2+ ions form coordination bonds with anionic lipids, but the RR motif is unable to form a salt bridge with PIP2 molecules. Thus, both Ca2+-free and Ca2+-bound forms of the C2B domain anchor to anionic PIP2-containing lipids; however, the Ca2+-bound form penetrates deeper into the bilayer. These results agree with quantitative thermodynamic studies (74) that observed the Ca2+-induced membrane penetration experimentally. Notably, a similar configuration for the attachment of the C2B module to PIP2 was demonstrated for the Syt1 analog DOC2B (77), suggesting a general mechanism for the membrane anchoring of C2B domains.
The anchoring mechanism described above can account for the enhanced Ca2+ binding observed for the C2B domain of Syt1 in the presence of PIP2 (65,78). Indeed, the attachment of the CBL motif to PIP2 would likely open the Ca2+-binding pocket and allow Ca2+ ions to enter the pocket more freely. In addition, the salt bridges formed between PIP2 and CBL would neutralize the positive charges of the CBL basic residues and further promote Ca2+ entry into the binding pocket. This anchoring mechanisms also agrees with the established role of the PB stretch of the C2B domain (74) and explains the loss of function in Syt1 mutants K366Q (79,80) and R398Q/R399Q (22).
For the C2A module, our MD simulations revealed only two sites for the interactions with PIP2: 1) basic residues of the second Ca2+-binding loop (R233 and K236), CBL, and 2) a very short PB stretch containing only three basic residues: K189, K190, and K192. These findings account for the lesser affinity to PIP2 observed for the C2A domain versus the C2B domain (81). The MD simulations also revealed that the Ca2+-binding tip of the C2A domain is rather hydrophobic and forms weak contacts with neutral lipids, unlike the Ca2+-binding tip of the C2B domain. The latter result seems to be in an apparent contradiction with liposome-binding studies (6), which detected the Ca2+-dependent binding of the C2A domain to anionic (POPC/POPS) and PIP2-containing liposomes but did not detect a significant attachment of the C2A module to neutral POPC liposomes. However, in agreement with the latter study, our simulations show that the interactions of the C2A domain with the bilayer containing POPS and PIP2 are indeed much stronger than the interactions of the C2A module with POPC. Unfortunately, a direct comparison of MD simulations with the results of in vitro experiments is not feasible because MD approach considers a closed system with all the components positioned in a close proximity to each other. In such a system, even weak affinities between molecules would be detectable, which may be not necessarily the case for in vitro assays. It should be noted, however, that the closed system utilized by the MD approach closely mimics the in vivo system whereby Syt1 is attached to SV and positioned in a rather narrow gap between SV and PM. At these conditions, even weak interactions could affect the system dynamics. In particular, even weak affinity of the C2A module to neutral lipids would prompt its attachment to SV as long as PM is not situated in the immediate proximity.
Consistently, the simulations of the C2AB tandem suggested that at a distance from the PM, the C2A domain would likely have its Ca2+-binding tip attached to the SV bilayer, whereas the C2B domain would likely float in a cytosol. This configuration would be favorable for C2B anchoring to PIP2 molecules of PM. Alternatively, the C2B domain could anchor to the SNARE complex.
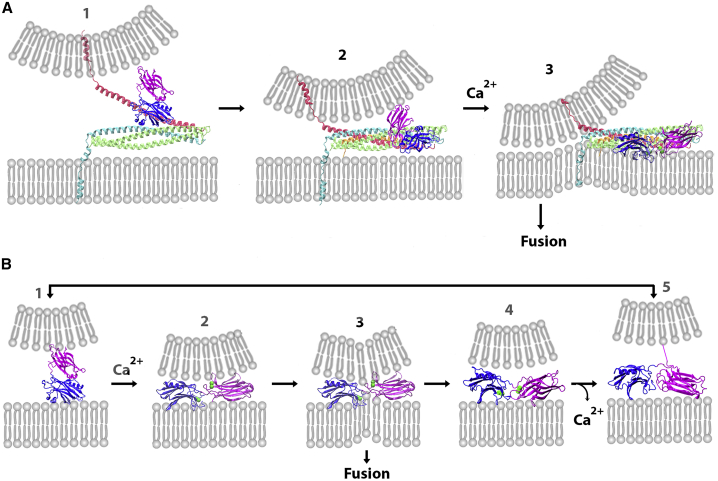
In agreement with (25), our simulations suggest that the attractions of the C2B domain to PIP2 would be energetically more favorable than the attractions to the SNARE complex. However, besides energetic favorability, other factors should be considered for the molecular system in vivo. One important factor is the electrostatic repulsion of SV and PM, which brings up the requirement for molecular anchors. The conformational analysis of Syt1 presented here and in our earlier study (48) suggests that Syt1 could only serve as a relatively short-distance molecular anchor, reaching within a distance of 4–5 nm, because the C2 domains are predominantly coupled and the C2A domain tends to attach to SV. Such a distance range between PM and SV would be reached when the assembly of the SNARE bundle is initiated (Fig. 12 A1), and this initial prefusion state would be favorable for Syt1 anchoring to t-SNARE.
Figure 12.
Two hypothetical pathways for the Syt1-mediated fusion. (A) Shown is the synergistic action of the SNARE complex and Syt1. The C2B domain anchors to t-SNARE (1). Subsequently, Syt1 forms an intermediate complex with the SNARE bundle (2). Upon Ca2+ binding, the tips of both C2 domains immerse into PM and drive fusion synergistically with SNARE zippering (3). (B) If the SNARE complex is not formed, Syt1 can anchor to PM and drive fusion with a low probability. The C2B domain anchors to PM (1), and upon Ca2+ binding, the tips of the C2A and C2B domains insert into the SV and PM bilayers, respectively, (2), induce lipid budging (3), and can drive lipid merging. However, a conformational transition would likely produce the state with both domains being attached to PM (4), which could stabilize (5) and keep SV anchored to PM. The states 1 and 5 could also transition to each other directly. To see this figure in color, go online.
The Syt1-SNARE complex is structurally heterogeneous, and it samples multiple conformational states (69). Our MD simulations identified three such states with comparable energies for the Syt1-SNARE interactions. The state (I3, Fig. 8) matching the C2B-SNARE complex identified by the NMR approach (28) had the C2B domain anchored to the SNARE complex via several salt bridges formed by its PB stretch, and this conformational state is a likely candidate for the initial anchoring of the C2B domain onto the SNARE bundle. However, this conformational state is unlikely to stabilize in proximity to PM because PIP2 molecules of PM would compete with the SNARE bundle for the attachment to the PB motif of Syt1 and the interactions with PIP2 would likely prevail. Therefore, a subsequent conformational transition would likely occur, leading to a new state of the Syt1-SNARE-Cpx complex. This new conformational state should allow Syt1 to interact with PM and the SNARE bundle simultaneously. Our simulations identified a new conformation of the complex (I2, Fig. 8), which appears to be a likely candidate for this intermediate state (Fig. 12 A2). The C2B-SNARE interface of this Syt1-SNARE-Cpx complex does not involve the PB motif, thus enabling simultaneous interactions of the C2B domain with the SNARE bundle and PM. Interestingly, this complex has the C2A domain of Syt1 directly interacting with Cpx on the SNARE bundle, thus providing a potential explanation for the role of Cpx in promoting evoked fusion via the interaction with Syt1 (39,40,82). However, structural studies coupled with mutagenesis also propose other prefusion states of the Syt1-SNARE-Cpx complex (37), and a sequence of several intermediate states is possible.
Our simulations produced the atomic model of the final prefusion state of the Ca2+Syt1-SNARE-Cpx complex attached to PM (Figs. 9, 10, and 12 A3). This is the minimal model of the prefusion complex, which includes the C2AB tandem of Syt1, as well as the coil-coiled motif and TM domains of the SNARE proteins and the SNARE-binding domain of Cpx. The model excludes 1) the N-terminal Habc domain of Syx, 2) the flexible loops of SNAP25, 3) the TM domain and the TM-C2A linker of Syt1, and 4) the N-terminal and C-terminal domains of Cpx. Although the protein domains listed above, as well as other effector proteins interacting with SNARE machinery, may affect synaptic fusion (1,16), we now focus on the interactions of C2 modules of Syt1 with lipid bilayers and with the coil-coiled SNARE motif.
Multiple lines of evidence support our molecular model of the prefusion complex (Figs. 9, 10, and 12 A3). First, it has an extensive interface between the C2B domain and t-SNARE, which was identified by crystallography (27) and proved stable in silico at a microsecond scale at physiological ion concentrations (this study). Second, the mutations disrupting this interface impaired evoked synaptic transmission (37,47). Third, this study showed that the formation of this complex would promote the immersion of the tips of both C2 domains of Syt1 into PM, which is thought to be the trigger for evoked Ca2+-dependent fusion (8,11,15). Finally, the simulations performed in this study (Fig. 10) showed that this complex would drive the formation of VdW contacts between the lipid bilayers.
Notably, our simulations elucidated the role of the attachment of the C2B domain of Syt1 to the SNARE bundle. We showed that in the absence of this attachment, the C2 domains mutually hinder their immersion into PM even though they simultaneously anchor to PM. More specifically, the simulations demonstrated that each of the C2 domains in its isolated state would penetrate into PM deeper than within the tandem. This result was surprising because the penetration into the bilayer and its distortion by one or both C2 domains is thought to drive the synaptic fusion (8,11,72). However, our simulations also demonstrated that the C2B-SNARE attachment uncouples the C2 domains and enables their tips to immerse into PM deeper. Together with a recent study (32) that demonstrated that Syt1 induces a conformational change in the SNARE complex and promotes its fusion activity, our study supports the cooperative synergistic action of Syt1 and the SNARE complex, suggesting that the Syt1-SNARE interactions mutually promotes the penetration of the Ca2+-bound tips of Syt1 into PM and SNARE zippering.
Altogether, our simulations favor the scenario for the evoked fusion (Fig. 12 A) in which the C2B domain would anchor onto t-SNARE (Fig. 12 A1) and, upon the zippering of Syb and the attachment of Cpx, would transition through intermediate state(s), likely involving an interaction between Syt1 and Cpx (Fig. 12 A2). Upon Ca2+ binding, the complex would transition to its final prefusion state (Fig. 12 A3), and the immersion of the tips of C2 domains into PM would drive fusion synergistically with full SNARE zippering.
Syt1 could also anchor to PM independently of the SNARE complex (Fig. 12 B). Being a transmembrane protein attached to SV and having a high affinity to the PM component PIP2, Syt1 would serve as an efficient anchor for docking SVs to PM (24,25). In addition, our simulations identified a potentially fusogenic state of Ca2+Syt1, which has the Ca2+-bound tip of the C2A domain attached to the SV bilayer and the Ca2+-bound tip of the C2B domain inserted into the PM bilayer (Figs. 7 and 12 B3). This finding agrees with a spin-labeling study (10) that suggested that Syt1 could bridge membranes by penetrating the opposing bilayers with the Ca2+-bound tips of its C2A and C2B domains.
Our simulations showed that such a state of the C2AB tandem would promote lipid bulging, and this could potentially lead to fusion. Importantly, the simulations also showed that unless the lipid merging occurs rapidly, such a state of the C2AB tandem would not stabilize because Syt1 would likely undergo a conformational transition so that the Ca2+-bound tip of the C2A domain would attach to PM (Fig. 12 B4). It should be noted, however, that the stability of the fusogenic state of the PM-Ca2+C2AB-SV complex would likely depend on the composition of the SV bilayer. Indeed, the affinity of the C2A module to membranes is significantly enhanced by the presence of anionic lipids (6), and therefore, the presence of anionic lipids in SVs would promote C2A-SV binding and stabilize the Syt1 state bridging SV and PM. We simulated the SV membrane as a neutral POPC bilayer because a recent study suggested that SVs are largely composed out of neutral lipids, with the content of anionic lipids being below 1% (61). However, an earlier study suggested a higher content of anionic lipids in SVs, possibly up to 10% (62). We cannot exclude the possibility that SV composition may vary between synapses or even between different functional pools of SVs within a single synapse, and this could define the prominence of the SNARE-independent fusion pathway (Fig. 12 B), which can serve to promote the SV-PM docking and mediate low-probability fusion, such as asynchronous or spontaneous transmitter release (83). This model (Fig. 12) agrees with the finding that different conformational states of Syt1 control different components of synaptic fusion (15).
Acknowledgments
This study was supported by the National Institute of Mental Health grant R01 MH099557. MD simulations were performed at the Anton supercomputer (D. E. Shaw Research and Pittsburgh Supercomputer Center) and at Extreme Science and Engineering Discovery Environment resources (Stampede supercomputer at Texas Advanced Computing Center). We thank Dr. J. Wereszczynski for kindly sharing the model of the POPC/POPS/PIP2 membrane.
Editor: Michael Grabe.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.12.025.
Supporting material
References
- 1.Südhof T.C. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perin M.S., Brose N., Südhof T.C. Domain structure of synaptotagmin (p65) J. Biol. Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- 3.Ubach J., Zhang X., Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez I., Araç D., Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Chacón R., Königstorfer A., Südhof T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 6.Davletov B.A., Südhof T.C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 7.Bai J., Tucker W.C., Chapman E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 8.Chapman E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 9.Lin C.C., Seikowski J., Walla P.J. Control of membrane gaps by synaptotagmin-Ca2+ measured with a novel membrane distance ruler. Nat. Commun. 2014;5:5859. doi: 10.1038/ncomms6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo W., Herrick D.Z., Cafiso D.S. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry. 2011;50:2633–2641. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens S., Kozlov M.M., McMahon H.T. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 12.Hui E., Johnson C.P., Chapman E.R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui E., Gaffaney J.D., Chapman E.R. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat. Struct. Mol. Biol. 2011;18:813–821. doi: 10.1038/nsmb.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishnan S., Bera M., Rothman J.E. Synaptotagmin oligomers are necessary and can be sufficient to form a Ca2+ -sensitive fusion clamp. FEBS Lett. 2019;593:154–162. doi: 10.1002/1873-3468.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai H., Xue R., Chapman E.R. Different states of synaptotagmin regulate evoked versus spontaneous release. Nat. Commun. 2016;7:10971. doi: 10.1038/ncomms10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Südhof T.C., Rothman J.E. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz R.W., Zimmerberg J. Dynamic relationship of the SNARE complex with a membrane. Biophys. J. 2019;117:627–630. doi: 10.1016/j.bpj.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch K.L., Gerona R.R., Martin T.F. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol. Biol. Cell. 2008;19:5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schupp M., Malsam J., Sørensen J.B. Interactions between SNAP-25 and synaptotagmin-1 are involved in vesicle priming, clamping spontaneous and stimulating evoked neurotransmission. J. Neurosci. 2016;36:11865–11880. doi: 10.1523/JNEUROSCI.1011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang Z.P., Shin O.H., Südhof T.C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chicka M.C., Hui E., Chapman E.R. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat. Struct. Mol. Biol. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue M., Ma C., Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 2008;15:1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshihara M., Guan Z., Littleton J.T. Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 2010;107:14869–14874. doi: 10.1073/pnas.1000606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahn R., Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y., Seo J.B., Jahn R. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat. Struct. Mol. Biol. 2015;22:815–823. doi: 10.1038/nsmb.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyubimov A.Y., Uervirojnangkoorn M., Brunger A.T. Advances in X-ray free electron laser (XFEL) diffraction data processing applied to the crystal structure of the synaptotagmin-1 / SNARE complex. eLife. 2016;5:e18740. doi: 10.7554/eLife.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q., Lai Y., Brunger A.T. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 2015;525:62–67. doi: 10.1038/nature14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer K.D., Bacaj T., Rizo J. Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat. Struct. Mol. Biol. 2015;22:555–564. doi: 10.1038/nsmb.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiter M., Kádková A., Sørensen J.B. An electrostatic energy barrier for SNARE-dependent spontaneous and evoked synaptic transmission. Cell Rep. 2019;26:2340–2352.e5. doi: 10.1016/j.celrep.2019.01.103. [DOI] [PubMed] [Google Scholar]
- 30.Brunger A.T., Choi U.B., Zhou Q. The pre-synaptic fusion machinery. Curr. Opin. Struct. Biol. 2019;54:179–188. doi: 10.1016/j.sbi.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grushin K., Wang J., Krishnakumar S.S. Structural basis for the clamping and Ca2+ activation of SNARE-mediated fusion by synaptotagmin. Nat. Commun. 2019;10:2413. doi: 10.1038/s41467-019-10391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiessling V., Kreutzberger A.J.B., Tamm L.K. A molecular mechanism for calcium-mediated synaptotagmin-triggered exocytosis. Nat. Struct. Mol. Biol. 2018;25:911–917. doi: 10.1038/s41594-018-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Tomchick D.R., Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 34.Mohrmann R., Dhara M., Bruns D. Complexins: small but capable. Cell. Mol. Life Sci. 2015;72:4221–4235. doi: 10.1007/s00018-015-1998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trimbuch T., Rosenmund C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 2016;17:118–125. doi: 10.1038/nrn.2015.16. [DOI] [PubMed] [Google Scholar]
- 36.Trimbuch T., Xu J., Rosenmund C. Re-examining how complexin inhibits neurotransmitter release. eLife. 2014;3:e02391. doi: 10.7554/eLife.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q., Zhou P., Brunger A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. doi: 10.1038/nature23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnakumar S.S., Radoff D.T., Rothman J.E. A conformational switch in complexin is required for synaptotagmin to trigger synaptic fusion. Nat. Struct. Mol. Biol. 2011;18:934–940. doi: 10.1038/nsmb.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorquera R.A., Huntwork-Rodriguez S., Littleton J.T. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J. Neurosci. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokumaru H., Shimizu-Okabe C., Abe T. Direct interaction of SNARE complex binding protein synaphin/complexin with calcium sensor synaptotagmin 1. Brain Cell Biol. 2008;36:173–189. doi: 10.1007/s11068-008-9032-9. [DOI] [PubMed] [Google Scholar]
- 41.Chicka M.C., Chapman E.R. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry. 2009;48:657–659. doi: 10.1021/bi801962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J., Brewer K.D., Rizo J. Subtle Interplay between synaptotagmin and complexin binding to the SNARE complex. J. Mol. Biol. 2013;425:3461–3475. doi: 10.1016/j.jmb.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw D.E., Dror R., Towles B. Association for Computing Machinery; 2009. Millisecond-scale molecular dynamics simulations on Anton. In Proceedings of the Conference on High Performance Computing, Networking, Storage and Analysis; pp. 1–11. [Google Scholar]
- 44.Shaw D.E. Abstracts of Papers of the American Chemical Society. 2014. Long molecular dynamics simulations of proteins: Progress, promise, and problems; p. 247. [Google Scholar]
- 45.Zanetti-Domingues L.C., Korovesis D., Martin-Fernandez M.L. The architecture of EGFR’s basal complexes reveals autoinhibition mechanisms in dimers and oligomers. Nat. Commun. 2018;9:4325. doi: 10.1038/s41467-018-06632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan A.C., Jacobson D., Shaw D.E. Atomic-level characterization of protein-protein association. Proc. Natl. Acad. Sci. USA. 2019;116:4244–4249. doi: 10.1073/pnas.1815431116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan Z., Bykhovskaia M., Sutton R.B. A synaptotagmin suppressor screen indicates SNARE binding controls the timing and Ca2+ cooperativity of vesicle fusion. eLife. 2017;6:e28409. doi: 10.7554/eLife.28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bykhovskaia M. Calcium binding promotes conformational flexibility of the neuronal Ca(2+) sensor synaptotagmin. Biophys. J. 2015;108:2507–2520. doi: 10.1016/j.bpj.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alwarawrah M., Wereszczynski J. Investigation of the effect of bilayer composition on PKCα-C2 domain docking using molecular dynamics simulations. J. Phys. Chem. B. 2017;121:78–88. doi: 10.1021/acs.jpcb.6b10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao X., Fernandez I., Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Y., Sequeira S.M., Patel D.J. Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin I C2B domain. Protein Sci. 2004;13:2665–2672. doi: 10.1110/ps.04832604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bykhovskaia M., Jagota A., Littleton J.T. Interaction of the complexin accessory helix with the C-terminus of the SNARE complex: molecular-dynamics model of the fusion clamp. Biophys. J. 2013;105:679–690. doi: 10.1016/j.bpj.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch K.L., Gerona R.R., Martin T.F. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell. 2007;18:4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Scheraga H.A. Monte Carlo-minimization approach to the multiple-minima problem in protein folding. Proc. Natl. Acad. Sci. USA. 1987;84:6611–6615. doi: 10.1073/pnas.84.19.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garden D.P., Zhorov B.S. Docking flexible ligands in proteins with a solvent exposure- and distance-dependent dielectric function. J. Comput. Aided Mol. Des. 2010;24:91–105. doi: 10.1007/s10822-009-9317-9. [DOI] [PubMed] [Google Scholar]
- 56.Vanommeslaeghe K., Hatcher E., Mackerell A.D., Jr. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lippert R.A., Predescu C., Shaw D.E. Accurate and efficient integration for molecular dynamics simulations at constant temperature and pressure. J. Chem. Phys. 2013;139:164106. doi: 10.1063/1.4825247. [DOI] [PubMed] [Google Scholar]
- 59.Littleton J.T., Bai J., Chapman E.R. Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishiki T., Augustine G.J. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis K.T., Maddipati K.R., Jena B.P. Unique lipid chemistry of synaptic vesicle and synaptosome membrane revealed using mass spectrometry. ACS Chem. Neurosci. 2017;8:1163–1169. doi: 10.1021/acschemneuro.7b00030. [DOI] [PubMed] [Google Scholar]
- 62.Takamori S., Holt M., Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 63.Aoyagi K., Sugaya T., Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 64.Fuson K.L., Montes M., Sutton R.B. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry. 2007;46:13041–13048. doi: 10.1021/bi701651k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radhakrishnan A., Stein A., Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2009;284:25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Striegel A.R., Biela L.M., Reist N.E. Calcium binding by synaptotagmin’s C2A domain is an essential element of the electrostatic switch that triggers synchronous synaptic transmission. J. Neurosci. 2012;32:1253–1260. doi: 10.1523/JNEUROSCI.4652-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans C.S., He Z., Chapman E.R. Functional analysis of the interface between the tandem C2 domains of synaptotagmin-1. Mol. Biol. Cell. 2016;27:979–989. doi: 10.1091/mbc.E15-07-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrick D.Z., Kuo W., Cafiso D.S. Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: evidence for bilayer bridging. J. Mol. Biol. 2009;390:913–923. doi: 10.1016/j.jmb.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai A.L., Huang H., Cafiso D.S. Synaptotagmin 1 and SNAREs form a complex that is structurally heterogeneous. J. Mol. Biol. 2011;405:696–706. doi: 10.1016/j.jmb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein A., Weber G., Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/nature08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tikhonov D.B., Zhorov B.S. In silico activation of KcsA K+ channel by lateral forces applied to the C-termini of inner helices. Biophys. J. 2004;87:1526–1536. doi: 10.1529/biophysj.103.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McMahon H.T., Kozlov M.M., Martens S. Membrane curvature in synaptic vesicle fusion and beyond. Cell. 2010;140:601–605. doi: 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Bradberry M.M., Bao H., Chapman E.R. Phosphatidylinositol 4,5-bisphosphate drives Ca2+-independent membrane penetration by the tandem C2 domain proteins synaptotagmin-1 and Doc2β. J. Biol. Chem. 2019;294:10942–10953. doi: 10.1074/jbc.RA119.007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pérez-Lara Á., Thapa A., Jahn R. PtdInsP2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. eLife. 2016;5:e15886. doi: 10.7554/eLife.15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nyenhuis S.B., Thapa A., Cafiso D.S. Phosphatidylinositol 4,5 bisphosphate controls the cis and trans interactions of synaptotagmin 1. Biophys. J. 2019;117:247–257. doi: 10.1016/j.bpj.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin T.F. Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion. Subcell. Biochem. 2012;59:111–130. doi: 10.1007/978-94-007-3015-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michaeli L., Gottfried I., Ashery U. Phosphatidylinositol (4, 5)-bisphosphate targets double C2 domain protein B to the plasma membrane. Traffic. 2017;18:825–839. doi: 10.1111/tra.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Bogaart G., Meyenberg K., Jahn R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 2012;287:16447–16453. doi: 10.1074/jbc.M112.343418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li L., Shin O.H., Rosenmund C. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J. Biol. Chem. 2006;281:15845–15852. doi: 10.1074/jbc.M600888200. [DOI] [PubMed] [Google Scholar]
- 80.Wang P., Wang C.T., Chapman E.R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 2003;278:47030–47037. doi: 10.1074/jbc.M306728200. [DOI] [PubMed] [Google Scholar]
- 81.Guillén J., Ferrer-Orta C., Corbalán-García S. Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 2013;110:20503–20508. doi: 10.1073/pnas.1316179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhara M., Yarzagaray A., Bruns D. Complexin synchronizes primed vesicle exocytosis and regulates fusion pore dynamics. J. Cell Biol. 2014;204:1123–1140. doi: 10.1083/jcb.201311085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaeser P.S., Regehr W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014;76:333–363. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The interval between subsequent frames is 2.5 ns. Note the Ca2+ binding loops of the C2A domain attached to SV in the beginning of the trajectory and interacting with PM in the end of the trajectory. The video supplements the Fig. 7.
SNAP25 is removed for clarity. Purple: Syb; violet: Syx; orange: Syt1. The interval between subsequent frames is 0.5 ns. Note the two membranes gradually approaching each other, forming contacts, separating, forming contacts again, and separating. The video supplements the Fig. 10.3