Abstract
Introduction
Older patients with early breast cancer (EBC) derive modest survival benefit from chemotherapy but have increased toxicity risk. Data on the impact of chemotherapy for EBC on quality of life in older patients are limited, but this is a key determinant of treatment acceptance. We aimed to investigate its effect on quality of life in older patients enrolled in the Bridging the Age Gap study.
Materials and methods
A prospective, multicentre, observational study of EBC patients ≥70 years old was conducted in 2013–2018 at 56 UK hospitals. Demographics, patient, tumour characteristics, treatments and adverse events were recorded. Quality of life was assessed using the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaires (EORTC-QLQ) C30, BR23 and ELD 15 plus the Euroqol-5D (eq-5d) over 24 months and analysed at each time point using baseline adjusted linear regression analysis and propensity score-matching.
Results
Three thousand and four hundred sixteen patients were enrolled in the study; 1520 patients undergoing surgery and who had high-risk EBC were included in this analysis. 376/1520 (24.7%) received chemotherapy. At 6 months, chemotherapy had a significant negative impact in several EORTC-QLQ-C30 domains, including global health score, physical, role, social functioning, cognition, fatigue, nausea/vomiting, dyspnoea, appetite loss, diarrhoea and constipation. Similar trends were documented on other scales (EORTC-QLQ-BR23, EORTC-QLQ-ELD15 and EQ-5D-5L). Its impact was no longer significant at 18–24 months in unmatched and matched cohorts.
Conclusions
The negative impact of chemotherapy on quality-of-life is clinically and statistically significant at 6 months but resolves by 18 months, which is crucial to inform decision-making for older patients contemplating chemotherapy.
Trial registration number ISRCTN
46099296.
Keywords: Breast cancer, Older patients, Adjuvant chemotherapy, Quality of life
Highlights
-
•
This is a multicentre, cohort study of 3416 women (aged >70 years) with breast cancer.
-
•
In older women with high-risk, early breast cancer, chemotherapy reduces quality of life.
-
•
The relevant affected domains include cognition, fatigue, physical, role and social functioning.
-
•
Chemotherapy QoL impacts are transient and largely resolve completely by 18–24 months.
1. Introduction
Almost half of all breast cancer cases are diagnosed in patients aged ≥65 years [1]. Nonetheless, older adults are under-represented in clinical trials [2]. Moreover, standard trial end-points may not be appropriate for older individuals and quality of life (QoL), functional status and cognition may be as important as chance of cure [3]. These knowledge gaps contribute to considerable variation in treatment in this age group [4].
Curative chemotherapy is associated with a survival benefit only in patients with node-positive and oestrogen receptor (ER)–negative disease [5,6]. Older adults have higher risk of treatment toxicities due to comorbidities and reduced organ function, while benefits are mitigated by competing risks [7]. The impact of chemotherapy on QoL may influence clinicians' and patients' perspectives [8].
Therefore, the effect of anticancer treatments on QoL is essential to inform treatment decisions in this cohort. The CALGB 49907 study documented better QoL for patients aged ≥65 receiving capecitabine versus standard regimens, but no QoL differences persisted at 1 year [9]. Patients receiving chemotherapy within clinical trials had better QoL improvements compared with those treated off study [10]. Nonetheless, prospective data on QoL for older patients with early breast cancer (EBC) receiving standard chemotherapy are lacking.
Comorbidities, literacy, symptoms and compliance may influence patient-reported outcomes [11], but the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires have been validated to evaluate QoL generically in cancer patients [12] and, specifically, in older individuals [13] and in those diagnosed with breast cancer [14].
We aimed to investigate the impact of chemotherapy on QoL in real-world EBC patients aged ≥70 recruited to the Bridging the Age Gap study [15]. Matching survival outcomes for the cohort are reported separately.
2. Methods
2.1. Regulatory approval
Ethics approval (IRAS: 12 LO 1808) and research governance approval were obtained. All patients (or their proxies, if cognitively impaired) gave written informed consent.
2.2. Study design
Bridging the Age Gap is a prospective multicentre, observational cohort study. Patients were recruited from 56 UK centres in England and Wales (Table S1). Eligible patients were women ≥70 years at diagnosis of operable invasive breast cancer (tumour-node-metastasis stages: T1-3, plus some operable T4b, N0-1, M0). Those unsuitable for surgery or with previous EBC within five years were not eligible.
2.3. Baseline data collection
Patients were recruited at the time of diagnosis and could participate at three levels: full, partial (no requirement to complete QoL assessments) or proxy (simple third-party data collection for those with cognitive impairment).
Primary tumour characteristics were collected at baseline. Staging was performed if indicated. Surgery, radiotherapy and systemic treatment data were also collected.
Baseline geriatric assessments included comorbidities (Charlson comorbidity index [CCI]) [16], nutrition (Abridged Patient Generated Subjective Global Assessment [aPG-SGA]) [[17], [18], [19]], functional status (Eastern Cooperative Oncology Group Performance status [ECOG PS], activities of daily living [ADL] [20], instrumental activities of daily living [IADL]) [21], cognition (Mini-Mental State Examination [MMSE]) [22] and medications. Patients were classified as high risk based on ≥1 of the following criteria: 1) Human epidermal growth factor receptor type 2 (HER2)-positive status; 2) ER-negative status; 3) grade III; 4) ≥1 malignant lymph node; 5) recurrence score (RS) ≥30 (Table S2).
QoL was evaluated using four questionnaires. The EORTC-QLQ-C30 includes five functional domains (physical, role, emotional, cognitive and social), nine symptoms (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties) and global health status [12]. The EORTC-QLQ-BR23 comprises 23 questions evaluating body image, sexual functioning and enjoyment, future perspective, systemic therapy side-effects, breast symptoms, arm symptoms and frustration with hair loss [14]. The EORTC-QLQ-ELD15 contains five scales (functional independence, relationships with family and friends, worries about the future, autonomy and burden of illness) [13]. The EQ-5D-5L was used in this analysis to assess overall QoL [23] and individual questions were scored separately from 1 to 5.
Patients were followed up at 6 weeks, 6, 12, 18 and 24 months and QoL and side-effects, based on the Common Terminology Criteria for Adverse Events (CTCAE v4.0), were assessed at each visit.
2.4. Statistical analyses
Analyses were performed in IBM SPSS statistics version 24 and R version 3.6.3 [24,25]. A p < 0.05 was considered statistically significant.
The questionnaires were scored according to the EORTC Scoring Manual (3rd Edition) [13]. Missing data were managed accordingly. The analysis included patients with high-risk EBC where QoL questionnaires were available. The mean difference (95% confidence interval [CI]) of the domain scores at each time point, adjusted for baseline scores, was calculated with linear regression models for high-risk participants. Effect sizes after analyses of the EORTC-QLQ-C30 were categorised as either trivial, small, medium or large according to pre-specified thresholds for each domain [26].
The chemotherapy effect on the global health score over time for high-risk patients was estimated using a mixed-effect linear model. The model allowed for time, treatment, treatment–time interaction, and baseline global health status. Differences between the chemotherapy and non-chemotherapy groups were derived at each time point using linear contrasts. The model was fitted to high-risk patients and to the propensity score–matched patients only. For the unmatched analysis the model also adjusted for age and baseline functionality scores.
Propensity score matching was performed to compare the EORTC-QLQ-C30 global health score and the EQ-5D-5L usual activities score in a matched cohort receiving chemotherapy versus patients not receiving it. Logistic regression was used to calculate propensity scores for treatment allocation in high-risk patients. These were used to match chemotherapy patients to those who did not receive chemotherapy based on ADL, IADL, MMSE, ECOG, aPG-SGA, CCI, number of medications and age. The ratio and calliper widths of the propensity scores were chosen based on examination of propensity score overlaps for several combinations of ratios and callipers. A 1:3 ratio for chemotherapy to no chemotherapy and a calliper of 0.25 times the propensity scores standard deviation was used to optimally match quality and numbers. Participants were matched on the Nottingham prognostic index category (good: ≤3.4, moderate: 3.5–5.4, poor: >5.4) and HER2 status.
3. Results
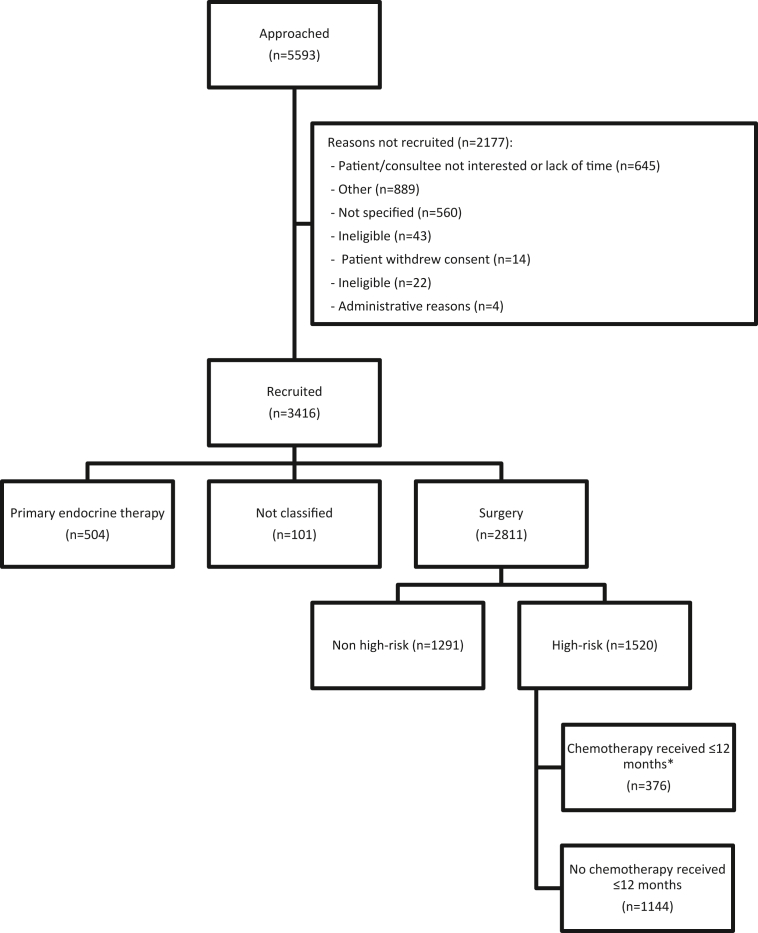
Between January 2013 and June 2018, 3456 women were recruited from 56 hospitals in England and Wales, and 3416 included in the analysis. 2811/3416 (82.3%) underwent surgery within 6 months of diagnosis, 1520/2811 (54.1%) had high-risk EBC and 376/1520 (24.7%) received chemotherapy (Fig. 1) [27]. The time frames for treatments received in each cohort are shown in Fig. S1 wherein the slight offset in timing of endocrine therapy and radiotherapy between the chemotherapy and no chemotherapy groups can be seen and should be considered when interpreting the findings.
Fig. 1.
The STROBE flow diagram for the chemotherapy versus no chemotherapy analyses. ∗ Patients who only received palliative chemotherapy regimens where not counted as having received chemotherapy. STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
Patients had a median age of 76.9 years, had a median CCI of 1 (range: 0–9), and took a median of four medications (0–18); 1063 (69.9%) were independent in their ADLs and 1091 (71.8%) in their IADLs, 1346 (88.6%) had a normal MMSE, 1168 (76.8%) had a low aPG-SGA score and 1379 (90.7%) had ECOG PS of 0–1 (Table 1).
Table 1.
Baseline postoperative tumour and patient characteristics by receipt of chemotherapy.
| Variable | Category | Chemotherapy |
No chemotherapy |
Total |
|---|---|---|---|---|
| N = 376 | N = 1144 | N = 1520 | ||
| Participation level | Full | 304 (80.9%) | 816 (71.3%) | 1120 (73.7%) |
| Partial | 68 (18.1%) | 284 (24.8%) | 352 (23.2%) | |
| Consultee | 4 (1.1%) | 44 (3.8%) | 48 (3.2%) | |
| Main side | Right | 169 (44.9%) | 545 (47.6%) | 714 (47.0%) |
| Left | 207 (55.1%) | 599 (52.4%) | 806 (53.0%) | |
| Tumour size (mm) | n | 375 | 1143 | 1518 |
| Mean (SD) | 32.9 (20.7) | 29.0 (17.5) | 29.9 (18.4) | |
| Median (IQR) | 29.0 (21.0, 40.0) | 25.0 (18.0, 35.0) | 25.0 (18.2, 36.0) | |
| Min, Max | 0, 210 | 0, 155 | 0, 210 | |
| Tumour size (mm) | ≤20 | 93 (24.7%) | 399 (34.9%) | 492 (32.4%) |
| 21–50 | 233 (62.0%) | 644 (56.3%) | 877 (57.7%) | |
| >50 | 49 (13.0%) | 100 (8.7%) | 149 (9.8%) | |
| Unknown | 1 (0.3%) | 1 (0.1%) | 2 (0.1%) | |
| Grade | Grade I | 2 (0.5%) | 77 (6.7%) | 79 (5.2%) |
| Grade II | 122 (32.4%) | 447 (39.1%) | 569 (37.4%) | |
| Grade III | 247 (65.7%) | 617 (53.9%) | 864 (56.8%) | |
| Unknown | 5 (1.3%) | 3 (0.3%) | 8 (0.5%) | |
| Histology | Ductal NST | 270 (71.8%) | 813 (71.1%) | 1083 (71.2%) |
| Lobular carcinoma | 52 (13.8%) | 110 (9.6%) | 162 (10.7%) | |
| Tubular carcinoma | 0 (0.0%) | 5 (0.4%) | 5 (0.3%) | |
| Mucinous carcinoma | 1 (0.3%) | 13 (1.1%) | 14 (0.9%) | |
| Other | 29 (7.7%) | 97 (8.5%) | 126 (8.3%) | |
| Unknown | 24 (6.4%) | 106 (9.3%) | 130 (8.6%) | |
| ER positive? | No | 132 (35.1%) | 240 (21.0%) | 372 (24.5%) |
| Yes | 241 (64.1%) | 893 (78.1%) | 1134 (74.6%) | |
| Unknown | 3 (0.8%) | 11 (1.0%) | 14 (0.9%) | |
| HER2 status | Negative | 210 (55.9%) | 908 (79.4%) | 1118 (73.6%) |
| Inconclusive | 3 (0.8%) | 7 (0.6%) | 10 (0.7%) | |
| Positive | 159 (42.3%) | 173 (15.1%) | 332 (21.8%) | |
| Unknown | 4 (1.1%) | 56 (4.9%) | 60 (3.9%) | |
| Oncotype Dx test performed | No | 35 (9.3%) | 150 (13.1%) | 185 (12.2%) |
| Yes | 5 (1.3%) | 16 (1.4%) | 21 (1.4%) | |
| Not Applicable | 252 (67.0%) | 434 (37.9%) | 686 (45.1%) | |
| Unknown | 84 (22.3%) | 544 (47.6%) | 628 (41.3%) | |
| Breast surgery | Wide local excision (non wire localised) | 113 (30.1%) | 412 (36.0%) | 525 (34.5%) |
| Wire localised wide local excision | 43 (11.4%) | 150 (13.1%) | 193 (12.7%) | |
| Therapeutic mammoplasty/breast reshaping after WLE | 18 (4.8%) | 14 (1.2%) | 32 (2.1%) | |
| Mastectomy | 186 (49.5%) | 549 (48.0%) | 735 (48.4%) | |
| Mastectomy and reconstruction | 12 (3.2%) | 11 (1.0%) | 23 (1.5%) | |
| Other | 4 (1.1%) | 8 (0.7%) | 12 (0.8%) | |
| Axillary surgery | Axillary sample | 11 (2.9%) | 38 (3.3%) | 49 (3.2%) |
| Axillary clearance | 136 (36.2%) | 247 (21.6%) | 383 (25.2%) | |
| Sentinel lymph node biopsy | 200 (53.2%) | 725 (63.4%) | 925 (60.9%) | |
| Internal mammary node biopsy | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | |
| No axillary surgery | 7 (1.9%) | 27 (2.4%) | 34 (2.2%) | |
| Unknown | 22 (5.9%) | 106 (9.3%) | 128 (8.4%) | |
| Nodal status | pN0-1mi | 175 (46.5%) | 508 (44.4%) | 683 (44.9%) |
| pN1 | 117 (31.1%) | 494 (43.2%) | 611 (40.2%) | |
| pN2 | 52 (13.8%) | 95 (8.3%) | 147 (9.7%) | |
| pN3 | 32 (8.5%) | 46 (4.0%) | 78 (5.1%) | |
| pNx | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | |
| Nottingham Prognostic Index | n | 371 | 1139 | 1510 |
| Mean (SD) | 5.1 (1.0) | 4.7 (0.9) | 4.8 (1.0) | |
| Median (IQR) | 4.9 (4.4, 5.7) | 4.5 (4.3, 5.3) | 4.6 (4.3, 5.4) | |
| Min, Max | 2.4, 10.2 | 2.1, 8.1 | 2.1, 10.2 | |
| Age | n | 376 | 1144 | 1520 |
| Mean (SD) | 73.65 (3.33) | 77.97 (5.19) | 76.90 (5.14) | |
| Median (IQR) | 73.00 (71.00, 76.00) | 78.00 (74.00, 81.00) | 76.00 (72.00, 80.00) | |
| Min, Max | 69, 87 | 69, 95 | 69, 95 | |
| Charlson comorbidity index (no age) | n | 365 | 1103 | 1468 |
| Mean (SD) | 0.79 (1.08) | 1.11 (1.38) | 1.03 (1.32) | |
| Median (IQR) | 0.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | |
| Min, Max | 0, 6 | 0, 9 | 0, 9 | |
| Charlson calculated probability | n | 365 | 1103 | 1468 |
| Mean (SD) | 0.56 (0.26) | 0.43 (0.29) | 0.46 (0.29) | |
| Median (IQR) | 0.77 (0.21, 0.77) | 0.53 (0.21, 0.77) | 0.53 (0.21, 0.77) | |
| Min, Max | 0, 0.77 | 0, 0.77 | 0, 0.77 | |
| Number of concurrent medications | n | 314 | 1021 | 1335 |
| Mean (SD) | 3.66 (2.51) | 4.30 (2.69) | 4.15 (2.66) | |
| Median (IQR) | 3.00 (2.00, 5.00) | 4.00 (2.00, 6.00) | 4.00 (2.00, 6.00) | |
| Min, Max | 0, 14 | 0, 18 | 0, 18 | |
| ADL category | No dependency | 303 (80.6%) | 760 (66.4%) | 1063 (69.9%) |
| Mild dependency | 33 (8.8%) | 146 (12.8%) | 179 (11.8%) | |
| Moderate/severe dependency | 16 (4.3%) | 136 (11.9%) | 152 (10.0%) | |
| Unknown | 24 (6.4%) | 102 (8.9%) | 126 (8.3%) | |
| IADL category | No dependency | 315 (83.8%) | 776 (67.8%) | 1091 (71.8%) |
| Mild dependency | 26 (6.9%) | 124 (10.8%) | 150 (9.9%) | |
| Moderate/severe dependency | 10 (2.7%) | 136 (11.9%) | 146 (9.6%) | |
| Unknown | 25 (6.6%) | 108 (9.4%) | 133 (8.7%) | |
| MMSE category | Normal function | 342 (91.0%) | 1004 (87.8%) | 1346 (88.6%) |
| Mild impairment | 28 (7.4%) | 111 (9.7%) | 139 (9.1%) | |
| Moderate impairment | 4 (1.1%) | 14 (1.2%) | 18 (1.2%) | |
| Severe | 2 (0.5%) | 15 (1.3%) | 17 (1.1%) | |
| APG SGA category | Low | 299 (79.5%) | 869 (76.0%) | 1168 (76.8%) |
| Moderate | 38 (10.1%) | 125 (10.9%) | 163 (10.7%) | |
| High | 4 (1.1%) | 19 (1.7%) | 23 (1.5%) | |
| Unknown | 35 (9.3%) | 131 (11.5%) | 166 (10.9%) | |
| ECOG performance status | Fully active | 296 (78.7%) | 740 (64.7%) | 1036 (68.2%) |
| Restricted in physically strenuous activity | 59 (15.7%) | 284 (24.8%) | 343 (22.6%) | |
| Ambulatory and capable of all self-care | 3 (0.8%) | 43 (3.8%) | 46 (3.0%) | |
| Capable of only limited self-care | 2 (0.5%) | 18 (1.6%) | 20 (1.3%) | |
| Unknown | 16 (4.3%) | 59 (5.2%) | 75 (4.9%) |
SD, standard deviation; IQR, interquartile range; NST, no special type; ADL, activities of daily living; IADL, instrumental activities of daily living; MMSE, Mini–Mental State Examination; APG SGA, Abridged Patient-Generated Subjective Global Assessment; ECOG, Eastern Cooperative Oncology Group.
Chemotherapy data were available for 360 patients: 124 (34.4%) received anthracycline and taxanes, 119 (33.1%) a taxane alone and 116 (32.2%) an anthracycline alone; one patient received cyclophosphamide, methotrexate, fluorouracil. Three-hundred thirty-two patients (21.8%) had HER2-positive disease: 150 (45.2%) received chemotherapy plus trastuzumab, 13 (3.9%) received trastuzumab alone and 9 (2.7%) chemotherapy alone. EBC was ER-positive in 1134 patients (75.3%), with 1079 (95.1%) receiving endocrine therapy (Fig. S1).
Of these high-risk patients, 1120 (73.7%) enrolled with full participation in the protocol (necessary for completion of QoL questionnaires) and 304/1120 (27.1%) had chemotherapy. Figs. S2–S4 and Tables S3–5 show completion rates of QoL questionnaires.
3.1. Impact on QoL domains (EORTC-QLQ-C30)
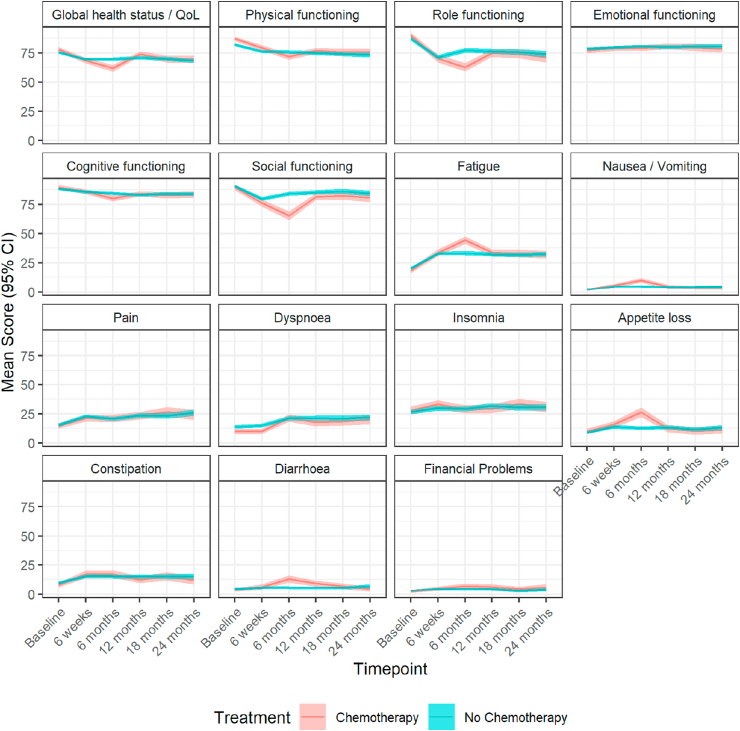
1049/1120 patients (93.7%) completed the global health-status questions included in the EORTC-QLQ-C30 questionnaire at baseline (Table S6a; Fig. 2). After adjustment for baseline scores, at 6 weeks the differences in the mean scores on some EORTC-QLQ-C30 domains were statistically significant between patients undergoing chemotherapy compared with those of patients not receiving it, including global health (adjusted mean difference: −2.81, 95% CI: −5.17 to −0.44, p = 0.020), social functioning (−3.57, CI: −6.71 to −0.43, p = 0.026) and constipation (3.43, CI: 0.23 to 6.62, p = 0.035). The impact of chemotherapy remained significant on most domains at 6 months, including global health which was both statistically and clinically significant but small (−9.20, CI: −11.95 to −6.44, p < 0.001), physical functioning (medium difference: −8.05, CI: −10.21 to −5.89, p < 0.001), role functioning (small difference: −17.59, CI: −21.24 to −13.95, p < 0.001), cognitive functioning (small difference: −5.55, CI: −7.97 to −3.13, p < 0.001), social functioning (large difference: −18.72, CI: −22.17 to −15.27, p < 0.001), and financial problems (small difference: 3.28, CI: 1.16 to 5.39, p = 0.002). At 12 months statistically significant differences persisted in physical functioning (trivial difference: −2.76, CI −4.95 to −0.57, p = 0.014), role functioning (trivial difference: −4.41, CI: −8.17 to −0.64, p = 0.022), social functioning (trivial difference: −3.78, CI: −7.00 to −0.56, p = 0.022), diarrhoea (small difference: 4.15, CI: 1.62 to 6.68, p = 0.001) and financial problems (trivial difference: 2.50, CI: 0.27 to 4.73, p = 0.028). Chemotherapy was no longer impactful in any of these domains at 18 and 24 months.
Fig. 2.
Mean (95% CI) scores over time points for the chemotherapy versus no chemotherapy population measured on the EORTC-QLQ-C30 scale. CI, confidence interval; EORTC-QLQ, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaires; QoL, quality of life.
The analyses were repeated on a propensity score–matched subgroup of 410 patients (150 chemotherapy, 260 no chemotherapy) with similar findings (Figs. S5–7; Table S6b).
3.2. Impact on breast cancer–specific QoL domains (EORTC-QLQ-BR23)
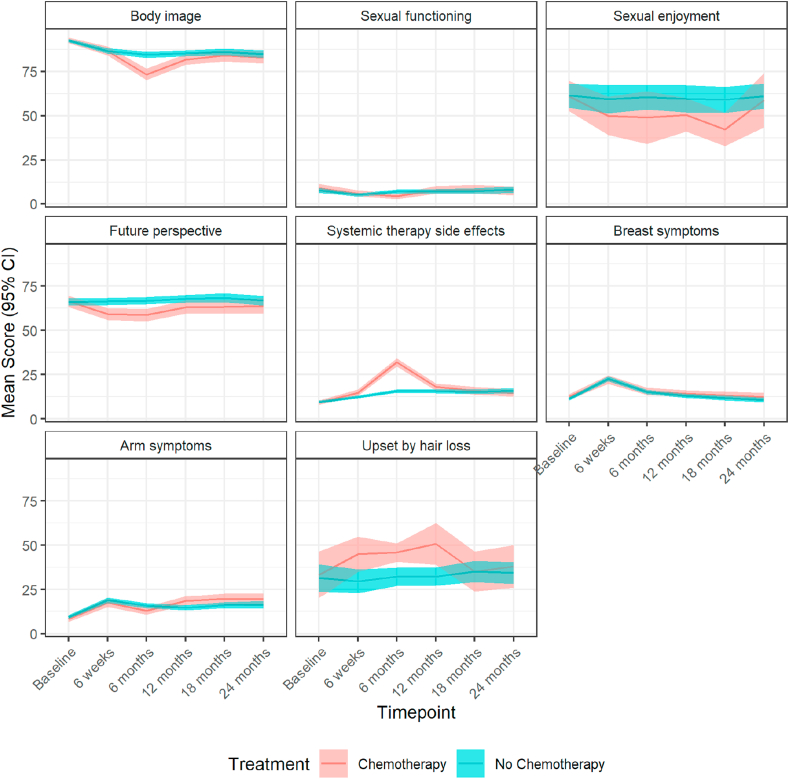
1054/1120 patients (94.1%) completed some or all of the EORTC-QLQ-BR23 questionnaire at baseline (Fig. 3; Table S7). After adjustment for baseline measurements patients given chemotherapy experienced a significant decline of some EORTC-QLQ-BR23 mean scores at 6 weeks compared with those not receiving it in future perspective (adjusted mean difference: −7.20, 95% CI: −10.72 to −3.68, p < 0.001) and systemic therapy side-effects (3.04, CI: 1.47 to 4.61, p < 0.001). At 6 months, mean scores were significantly different in future perspectives (−7.54, CI −11.28 to −3.80, p < 0.001) and systemic therapy side-effects (16.97, CI: 15.00 to 18.94, p < 0.001). At 12 months, the mean scores between the two groups differed in future perspectives (−4.96, CI: −8.89 to −1.03, p = 0.013), systemic therapy side-effects (3.32, CI: 1.41 to 5.22, p = 0.001) and the effect of chemotherapy became significant in arm symptoms (4.94, CI: 2.18 to 7.69, p < 0.001). At 18 months, the differences remained significant in future perspective (−4.97, CI: −9.37 to −0.57, p = 0.027) and arm symptoms (3.27, CI: 0.01 to 6.54, p = 0.049), and at 24 months only in arm symptoms (4.02, CI: 0.13 to 7.90, p = 0.043).
Fig. 3.
Mean (95% CI) scores over time points for the chemotherapy versus no chemotherapy population measured on the EORTC-QLQ-B23 scale. CI, confidence interval; EORTC-QLQ, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaires.
3.3. Impact on older adults-specific QoL domains (EORTC-QLQ-ELD15)
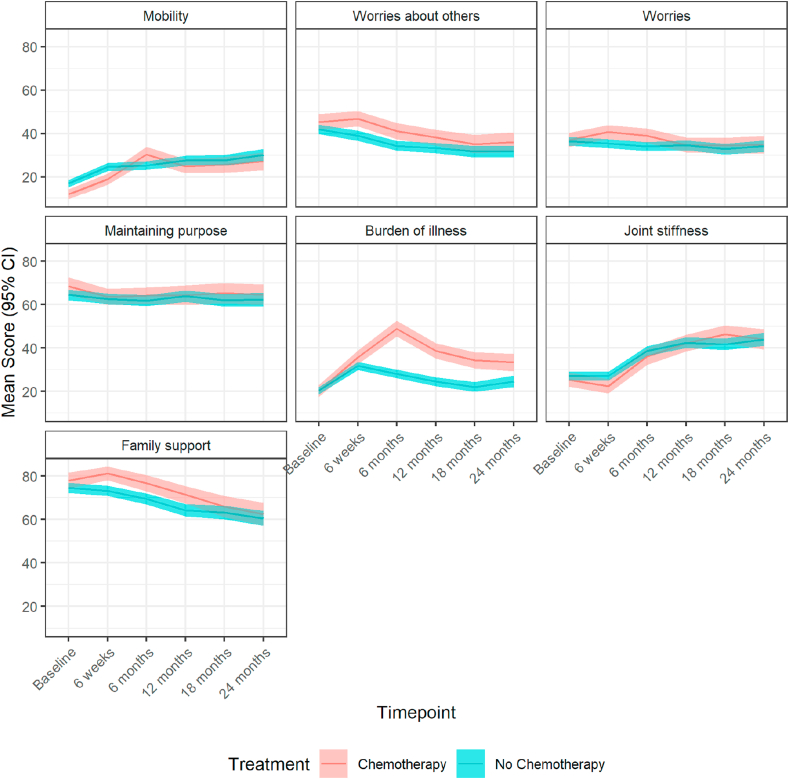
Some or all of the EORTC-QLQ-ELD15 questionnaire was completed at baseline by 1048/1120 patients (Table S8; Fig. 4). At 6 weeks scores were significantly different between patients given chemotherapy and those not treated in worries about others (adjusted mean difference: 5.31, 95% CI: 1.55 to 9.07, p = 0.006), worries (4.09, CI: 0.92 to 7.27, p = 0.011) and burden of illness (4.68, CI: 1.25 to 8.11, p = 0.007). These differences persisted at 6 months (worries about others [6.19, CI: 2.44 to 9.95, p = 0.001]; worries [4.18, CI: 0.89 to 7.46, p = 0.013]; burden of illness [21.60, CI: 17.82 to 25.39, p < 0.001]); the impact on mobility also became significant (9.82, CI: 6.87 to 12.78, p < 0.001). At 12 months, changes remained significant regarding worries about others (4.47, CI: 0.42 to 8.52, p = 0.031) and burden of illness (15.21, CI: 11.30 to 19.12, p < 0.001), which was the only domain significantly influenced also at 18 months (12.99, CI: 8.81 to 17.17, p < 0.001) and 24 months (8.80, CI: 3.93 to 13.66, p < 0.001).
Fig. 4.
Mean (95% CI) scores over time points for the chemotherapy versus no chemotherapy population measured on the EORTC-QLQ-ELD15 scale. CI, confidence interval; EORTC-QLQ, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaires.
Maintaining purpose did not differ throughout the follow-up period, whereas chemotherapy had a positive impact on family support mean scores at 6 weeks (6.21, CI: 2.26 to 10.17, p = 0.002), at 6 months (4.91, CI: 0.26 to 9.56, p = 0.038) and at 12 months (5.43, CI: 0.39 to 10.46, p = 0.035).
3.4. Impact on EQ-5D-5L score and questions
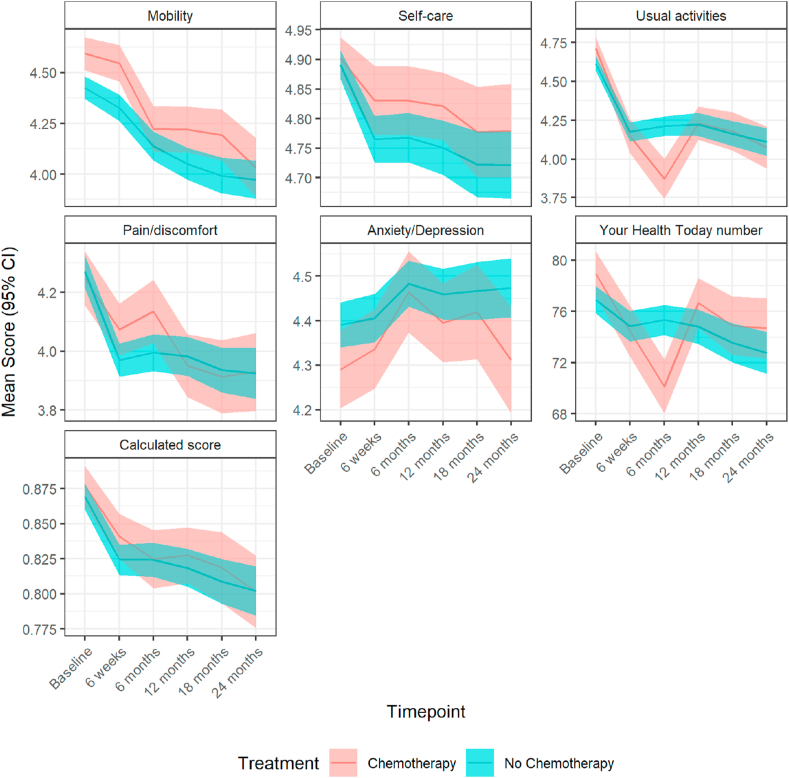
Among the high-risk patients, an EQ-5D-5L score was calculated in 1315 patients (86.5%) at baseline. Health utilities were similar with estimated mean differences less than 0.02 units (p > 0.1), whereas the visual analogue scale measures were significantly worse at 6 months in patients receiving chemotherapy versus not (adjusted mean difference: −6.57, 95% CI: −8.74 to −4.40, p < 0.001). Changes were subsequently no longer significant (Table S9; Fig. 5).
Fig. 5.
Mean (95% CI) scores over time points for the chemotherapy versus no chemotherapy population measured on the EQ-5D-5L scale. The calculated score is a single summary number (index value) which reflects the health state in the context of the preferences of the general population of a country/region and is derived by applying a formula attaching weights to each of the levels in each dimension as per the EQ-5D-5L User Guide. CI, confidence interval.
A similar pattern on EQ-5D-5L usual activities score was seen in 520 (118 chemotherapy, 332 no chemotherapy) propensity score–matched patients (Fig. S8).
4. Discussion
This study demonstrates that chemotherapy has both a clinically and statistically significantly negative impact at 6–12 months on several QoL domains (physical, role, cognitive and social functioning, financial problems), symptom scores (fatigue, nausea, dyspnoea, appetite loss, constipation, diarrhoea), and perceived global health. These changes are clinically meaningful and involve key domains for this population [28] for whom even low-grade toxicities may be challenging [29].
Reassuringly, this effect resolves for most items over 18–24 months, which is consistent with previous QoL data reported in younger cohorts: for example, in 280 EBC patients many domains improved within 12 months after diagnosis, with the exception of cognitive function and financial problems [30], and similar improvements in role functioning were seen in a study of 817 EBC patients [31]. A registry-based analysis documented better physical functioning, role-physical, role-emotional and fatigue scales at 15 years in EBC patients including 46.9% aged ≥65 [32]. Similarly, 588 EBC patients enrolled in the Moving Beyond Cancer study had improved physical and psychosocial functioning after radical treatment regardless of chemotherapy use [31]. Neuropsychological analyses also confirmed improving cognitive function during the first four years after radical therapy for EBC [33,34], although data on financial impact are limited [30]. The CANTO study confirmed the transient nature of the impact of chemotherapy on QoL in a large population [35]. Nonetheless, these analyses have either focused on younger patients, where the risk/benefit ratio is different, or addressed the impact of breast cancer treatments (and not specifically of chemotherapy) on QoL in this age group. Our findings are consistent with a previous study in 109 patients aged 70 or older, of whom 57 received adjuvant docetaxel/cyclophosphamide chemotherapy [36].
To our knowledge, this is the largest study to evaluate the impact of contemporary chemotherapy regimens in older adults with EBC in real-world patients. QoL is a meaningful end-point for older patients, who typically derive less survival benefit and increased toxicities on systemic anticancer treatments [37,38]. These benefits need to be carefully balanced with the detrimental impact on QoL and treatment side-effects [39].
Our analysis included baseline geriatric assessments characterising patients in relevant health domains for this age group, such as functional status, comorbidity, cognition, nutrition and concurrent medications which may impact QoL. A comprehensive geriatric assessment can help achieve the required balance between treatment benefits and side-effects and is recommended by guidelines from the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the International Society for Geriatric Oncology [28,40]. In a randomised study, integrated oncogeriatric care has recently been shown to improve QoL in older patients with cancer being considered for systemic anticancer therapy [41]. Of particular interest was our finding that in patients ≥80 the negative impact on QoL does not resolve, which suggests a lack of resilience in this cohort.
The study has several limitations. Selection bias may have influenced our findings despite its inclusive entry criteria and the different levels of participation. The recruited population was slightly skewed toward younger individuals compared with the general UK EBC patient population [42]. Moreover, we did not include socio-economic factors that might influence frailty nor the effect of endocrine therapy or radiotherapy on QoL, owing to multiple confounders to such an analysis. We did not capture the impact of chemotherapy on QoL outcomes beyond 24 months, and missing data on longitudinal QoL assessments may have influenced findings. Other factors not measured by our analysis may also impact on chemotherapy decisions; therefore, the propensity score matching does not adjust for all differences between the groups. Furthermore, some effects of chemotherapy on QoL documented in our analysis might be statistically significant but not clinically relevant, although for the majority of domains clinically meaningful changes are seen at the six-month time point, which represents the time when most women would have been on chemotherapy. Finally, it was not possible to categorise chemotherapy effects on QoL measured on BR23, ELD15 and EQ-5D-5L domains as thresholds have not been established for these specific tools, and the latter is a utility scale.
In conclusion, our analysis shows that chemotherapy has an impact on several QoL domains in older EBC patients compared with a matched cohort who did not receive cytotoxics. Nonetheless, these effects are temporary and largely resolve within two years. This is essential information for older women to use in decision-making because individualised decisions on treatment options should be based on their values.
Sponsorship
The study was sponsored by Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust. Clinical Research Office, First Floor 'C' Block, Doncaster Royal Infirmary, Armthorpe Road, Doncaster, DN2 5LT, UK.
Conflict of interest statement
The authors declare no conflict of interest. Professors Stephen Walters and Thompson Robinson are National Institute for Health Research (NIHR) Senior Investigators; Jenna Morgan is a NIHR Clinical Lecturer; and Kate Lifford is funded by the NIHR as part of this project. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care.
Acknowledgements
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference number RP-PG-1209-10071). In addition, NB and AR would like to acknowledge the support of the Cridlan Ross Smith Charitable Trust and the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London.
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.11.022.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M. 2018. Global cancer observatory: cancer today.https://gco.iarc.fr/today Lyon, France; Available from: [Google Scholar]
- 2.Hurria A., Levit L.A., Dale W., Mohile S.G., Muss H.B., Fehrenbacher L. Improving the evidence base for treating older adults with cancer: American Society of clinical oncology statement. J Clin Oncol. 2015;33(32):3826–3833. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 3.Wildiers H., Mauer M., Pallis A., Hurria A., Mohile S.G., Luciani A. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society of Geriatric Oncology position article. J Clin Oncol. 2013;31(29):3711–3718. doi: 10.1200/JCO.2013.49.6125. [DOI] [PubMed] [Google Scholar]
- 4.Battisti N.M.L., Wallington M., Ring A., Payne S., Birch R., Bomb M. Is age a barrier to chemotherapy? Rates of treatment in older patients treated with breast, colon and lung cancer in England in 2014: a national registry study. Ann Oncol. 2018;29 p. viii562–viii575. [Google Scholar]
- 5.Giordano S.H., Duan Z., Kuo Y.F., Hortobagyi G.N., Goodwin J.S. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 6.Biganzoli L., Wildiers H., Oakman C., Marotti L., Loibl S., Kunkler I. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA) Lancet Oncol. 2012;13(4):e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim J., Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw. 2013;11(12):1494–1502. doi: 10.6004/jnccn.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ring A., Harder H., Langridge C., Ballinger R.S., Fallowfield L.J. Adjuvant chemotherapy in elderly women with breast cancer (AChEW): an observational study identifying MDT perceptions and barriers to decision making. Ann Oncol. 2013;24(5):1211–1219. doi: 10.1093/annonc/mds642. [DOI] [PubMed] [Google Scholar]
- 9.Kornblith A.B., Lan L., Archer L., Partridge A., Kimmick G., Hudis C. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29(8):1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandelblatt J.S., Makgoeng S.B., Luta G., Hurria A., Kimmick G., Isaacs C. A planned, prospective comparison of short-term quality of life outcomes among older patients with breast cancer treated with standard chemotherapy in a randomized clinical trial vs. an observational study: CALGB #49907 and #369901. J Geriatr Oncol. 2013;4(4):353–361. doi: 10.1016/j.jgo.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotté F., Bossi P., Carola E., Cudennec T., Dielenseger P., Gomes F. Addressing the quality of life needs of older patients with cancer: a SIOG consensus paper and practical guide. Ann Oncol. 2018;29(8):1718–1726. doi: 10.1093/annonc/mdy228. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C., Fitzsimmons D., Gilbert J., Arrarras J.I., Hammerlid E., Bredart A. Development of the European Organisation for Research and Treatment of Cancer quality of life questionnaire module for older people with cancer: the EORTC QLQ-ELD15. Eur J Canc. 2010;46(12):2242–2252. doi: 10.1016/j.ejca.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Sprangers M.A., Groenvold M., Arraras J.I., Franklin J., te Velde A., Muller M. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 15.Collins K., Reed M., Lifford K., Burton M., Edwards A., Ring A. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open. 2017;7(7):e015133. doi: 10.1136/bmjopen-2016-015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Ottery F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1 Suppl):S15–S19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 18.Read J.A., Crockett N., Volker D.H., MacLennan P., Choy S.T., Beale P. Nutritional assessment in cancer: comparing the mini-nutritional assessment (MNA) with the scored patient-generated subjective global assessment (PGSGA) Nutr Canc. 2005;53(1):51–56. doi: 10.1207/s15327914nc5301_6. [DOI] [PubMed] [Google Scholar]
- 19.Vigano A.L., di Tomasso J., Kilgour R.D., Trutschnigg B., Lucar E., Morais J.A. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet. 2014;114(7):1088–1098. doi: 10.1016/j.jand.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney F.I., Barthel D.W. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 21.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Team R.C. 2020. R: A language and environment for statistical computing.https://www.R-project.org/ Vienna, Austria; Available from: [Google Scholar]
- 25.Corp I. IBM Corp.; Armonk, NY: 2016. IBM SPSS statistics for windows. Version 24.0 ed. [Google Scholar]
- 26.Cocks K., King M.T., Velikova G., Martyn St-James M., Fayers P.M., Brown J.M. Evidence-based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol. 2011;29(1):89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Mohile S.G., Dale W., Somerfield M.R., Hurria A. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology summary. J Oncol Pract. 2018;14(7):442–446. doi: 10.1200/JOP.18.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalsi T., Babic-Illman G., Fields P., Hughes S., Maisey N., Ross P. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Canc. 2014;111(12):2224–2228. doi: 10.1038/bjc.2014.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu T., Ennis M., Hood N., Graham M., Goodwin P.J. Quality of life in long-term breast cancer survivors. J Clin Oncol. 2013;31(28):3540–3548. doi: 10.1200/JCO.2012.48.1903. [DOI] [PubMed] [Google Scholar]
- 31.Ganz P.A., Kwan L., Stanton A.L., Bower J.E., Belin T.R. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein D., Mercier M., Abeilard E., Puyraveau M., Danzon A., Dalstein V. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Canc Res Treat. 2011;129(1):125–134. doi: 10.1007/s10549-011-1408-3. [DOI] [PubMed] [Google Scholar]
- 33.Schagen S.B., Muller M.J., Boogerd W., Rosenbrand R.M., van Rhijn D., Rodenhuis S. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002;13(9):1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- 34.Ahles T.A., Saykin A.J., McDonald B.C., Li Y., Furstenberg C.T., Hanscom B.S. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira A.R., Di Meglio A., Pistilli B., Gbenou A.S., El-Mouhebb M., Dauchy S. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30(11):1784–1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 36.Quinten C., Kenis C., Hamaker M., Coolbrandt A., Brouwers B., Dal Lago L. The effect of adjuvant chemotherapy on symptom burden and quality of life over time; a preliminary prospective observational study using individual data of patients aged ≥70 with early stage invasive breast cancer. J Geriatr Oncol. 2018;9(2):152–162. doi: 10.1016/j.jgo.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Extermann M., Boler I., Reich R.R., Lyman G.H., Brown R.H., DeFelice J. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer. 2012;118(13):3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 38.Hurria A., Togawa K., Mohile S.G., Owusu C., Klepin H.D., Gross C.P. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H.S., Harden J.K. Symptom burden and quality of life in survivorship: a review of the literature. Canc Nurs. 2015;38(1):E29–E54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 40.Wildiers H., Heeren P., Puts M., Topinkova E., Janssen-Heijnen M.L., Extermann M. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soo W.-K., King M., Pope A., Parente P., Darzins P., Davis I.D. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol. 2020;38(15_suppl):12011. doi: 10.1016/S2666-7568(22)00169-6. [DOI] [PubMed] [Google Scholar]
- 42.Todd A., Martin C., Morgan J., Herbert E., Bradburn M., Burton M. Age specific recruitment and retention to a large multicentre observational breast cancer trial in older women: the Age Gap Trial. J Geriatr Oncol. 2020 doi: 10.1016/j.jgo.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.