Highlights
-
•
Vapor pressure deficit strongly influenced VOC emission of tundra shrubs.
-
•
Evergreens had a higher leaf-to-air temperature difference than deciduous shrubs.
-
•
Leaf traits affected VOC emission of deciduous tundra shrubs.
-
•
All shrubs had a higher temperature optimum for photosynthesis than expected.
Keywords: Salix myrsinites, Betula nana, Cassiope tetragona, Rhododendron lapponicum, Leaf temperature, VOC, Tundra, Photosynthesis, MEGAN
Abstract
Temperature is one of the key abiotic factors during the life of plants, especially in the Arctic region which is currently experiencing rapid climate change. We evaluated plant traits and environmental variables determining leaf temperature in tundra shrubs and volatile organic compound (VOC) emissions with field measurements on deciduous tundra shrubs, Salix myrsinites and Betula nana, and evergreen Cassiope tetragona and Rhododendron lapponicum. Higher leaf-to-air temperature difference was observed in evergreen, compared to deciduous shrubs. Evergreen shrubs also showed continuously increasing photosynthesis with increasing temperature, suggesting high thermal tolerance. For the deciduous species, the optimum temperature for net photosynthesis was between our measurement temperatures of 24 °C and 38 °C. Air temperature and vapor pressure deficit were the most important variables influencing leaf temperature and VOC emissions in all the studied plants, along with stomatal density and specific leaf area in the deciduous shrubs. Using climate data and emission factors from our measurements, we modelled total seasonal tundra shrub VOC emissions of 0.3–2.3 g m−2 over the main growing season. Our results showed higher-than-expected temperature optima for photosynthesis and VOC emission and demonstrated the relative importance of plant traits and local environments in determining leaf temperature and VOC emissions in a subarctic tundra.
1. Introduction
Temperature is one of the most important factors affecting the distribution and activity of plants (Berry and Bjorkman, 1980). Temperature is especially important for the plants growing in the Arctic, the region of the world with the average air temperature in July at 10 °C or lower, and an area which experiences the most rapid increase in temperature with climate change (Post et al., 2019). Helliker and Richter (2008) found that plants from subtropical to boreal biomes maintain their leaf temperature at constant 21 °C during maximal carbon assimilation, which means that leaf temperature of arctic tundra plants can be much higher than the surrounding air temperature to maximize carbon uptake (Körner, 2003b; Lindwall et al., 2016). Nevertheless, we have poor understanding of quantified relationships between leaf temperature and ecophysiological processes in tundra shrubs.
Volatile organic compounds (VOCs) are a large group of reactive hydrocarbons that can impact atmospheric chemistry (Glasius and Goldstein, 2016). While some VOCs are produced anthropogenically, the majority are biogenic and released by living organisms, mainly plants (Kesselmeier and Staudt, 1999). Isoprene, C5H8, is the most emitted biogenic VOC (Guenther et al., 2012) and a building block for monoterpenes, C10H16, oxygenated monoterpenes, C10H16On, and sesquiterpenes, C15H24, collectively known as terpenoids or isoprenoids. VOCs are important for coping with biotic and abiotic stresses and play an important role in communication with other plants and other organisms(Laothawornkitkul et al., 2009; Peñuelas and Staudt, 2010; Pollastri et al., 2014).
Inside plant leaves, VOCs fluctuate between liquid and gaseous phase according to their Henry’s law constant (kH) which is considerably high (kH ∼ 7500 Pa m3 mol−1 for isoprene at 25 °C; Niinemets et al., 2004). Compounds de-novo synthesized in the leaf mesophyll, like isoprene and certain terpenoids, are usually directly linked to photosynthetic activity (Loreto and Sharkey, 1990; Delwiche and Sharkey, 1993; Loreto et al., 1996; Laothawornkitkul et al., 2009). However, some other terpenoids can be stored in specific leaf structures, like ducts or glandular trichomes (Laothawornkitkul et al., 2009; Schollert et al., 2015). The strong temperature dependency of VOC emission is a direct result of faster enzymatic reactions and compound diffusion from stored pools. The coupled responses of photosynthesis and VOC emission to leaf temperature have been documented for lower latitude herbs (Monson et al., 1992) and evergreen trees (Filella et al., 2007), but we do not know how tundra shrubs respond.
Tundra shrubs possess a set of physiological adaptations to their harsh living environment (Körner, 2003c). Their photosynthetic apparatus is highly specialized to the overall low growth temperature (Körner, 2003a). However, experimental studies suggest strong temperature responses of VOC emissions at branch and ecosystem levels (Kramshøj et al., 2016; Lindwall et al., 2016), directly coupled to plant ecophysiology. Tang et al. (2016) also suggested stronger temperature sensitivity for VOC emissions from tundra vegetation than currently implemented in models. According to the classic review by Berry and Bjorkman (1980), we would expect tundra plants to have lower than the global average optimum temperature for photosynthesis, but we lack empirical data e.g. in the form of temperature response curves to estimate optimum temperatures of photosynthesis and VOC emissions.
Studies investigating the influence of climate change on VOC emission from tundra plants have found that the warmer climate generally leads to higher VOC emission (Adams et al., 2001; Faubert et al., 2010; Schollert et al., 2015; Lindwall et al., 2016; Li et al., 2019). Like any other biological processes, temperature drives photosynthesis and VOC emissions, but the optimum (Topt) for the two processes may be different in deciduous and evergreen species. For example, Topt for isoprene synthase activity has been shown to be 5–10 °C higher than Topt for photosynthesis in velvet bean, Mucuna pruriens L. (Monson et al., 1992). Evergreen shrubs have thicker leaves that can survive the long winter, compromising stomatal conductance and photosynthesis (Chapin and Shaver, 1985; Baddeley et al., 1994) as compared to deciduous species. Due to their darker color and specialized leaf shape (Schollert et al., 2015, 2017) they heat up more efficiently in the sun and are not limited by low leaf longevity and senescence (Johnson and Tieszen, 1976).
Shoot height is important for light competition (Westoby et al., 2002; Wright et al., 2004) and convective heat loss (Molau, 1997). Specific leaf area (SLA) and leaf dry mass content (LDMC) are important traits for relative growth rate (Garnier, 1992; Wright et al., 2004) and also potentially influence leaf temperature. Water use efficiency (WUE), a ratio between photosynthesis rate and transpiration, describes in more detail the relationship plants have with water (Condon et al., 2002). Environmental factors like vapor pressure deficit (VPD), which combines air temperature and humidity, can have profound effects on photosynthetic rates (Lin et al., 2012), wind speed can increase the convective heat loss (Molau, 1997) and soil moisture and soil temperature strongly influence the ecophysiology of tundra shrubs (Pattison and Welker, 2014).
In this study, we assessed factors determining leaf temperature and evaluated the importance of plant traits and near-surface environmental variables driving VOC emission in two evergreen and two deciduous tundra shrubs. We also compared temperature response curves for photosynthesis, transpiration and VOC emission. Finally, the obtained VOC emission factors (EFs), i.e. emission rates normalized to the temperature of 30 °C and light level of 1000 μmol m−2 s−1 (Guenther et al., 1995), for the four shrub species were compared with the original values from a widely used VOC emission model, Model of Emissions of Gases and Aerosols from Nature (MEGAN, Guenther et al. (2012). MEGAN was adjusted with measurement-based EFs, plant traits and weather data to estimate VOC emissions from early June to early September for this tundra ecosystem.
2. Materials and methods
2.1. Study site and species
This study was conducted on a mesic tundra heath, in the village of Abisko, northern Sweden (68°20'47" N, 18°49'32" E) from June 8 to September 2, 2018. Mean annual air temperature and precipitation in the Abisko region were 0.2 °C and 337 mm, respectively (30-year mean 1986–2015, ANS, 2020). The growing season usually starts after snowmelt in June and ends with senescence in late August or early September. The vegetation of the area mainly consists of deciduous and evergreen shrubs, graminoids and mosses with some herbs and horsetails (Tiiva et al., 2008). The bedrock is covered by highly organic soil with a pH of 6.9 (Rinnan et al., 2008).
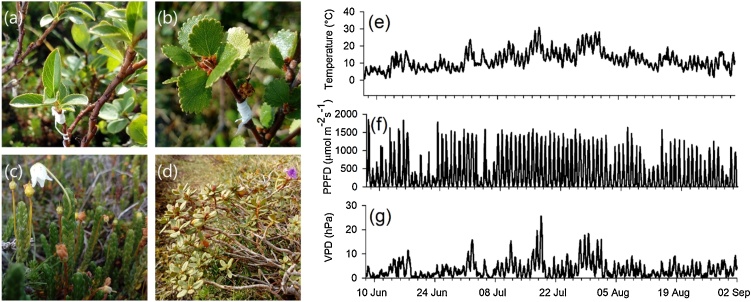
The plant species in this study consisted of the most common species in the area, the deciduous dwarf shrubs Salix myrsinites L., and Betula nana L. and the evergreen dwarf shrubs Cassiope tetragona (L.) D. Don, and Rhododendron lapponicum (L.) Wahlenb. (Fig. 1a–d). We did not distinguish between the sexes in Salix.
Fig. 1.
Studied plant species (a) Salix myrsinites, (b) Betula nana, (c) Cassiope tetragona, (d) Rhododendron lapponicum and air temperature at 2 m height (e), photosynthetic photon flux density (PPFD) (f), and vapor pressure deficit at 2 m height (g) in the study site between June 8 and September 2, 2018.
2.2. Weather station
A weather station was set up in the center of the measurement area of ca. 1200 m2 in late May 2018. It consisted of a wind speed anemometer (R3−50; 327 cm above ground; GILL, Lymington, UK), two shielded relative humidity and temperature sensors (CS215; 20 cm and 200 cm above ground; Campbell Scientific, Logan, UT, USA), an incoming photosynthetic photon flux density (PPFD) sensor (LI-190; 115 cm above ground; LI-COR, Lincoln, NE, USA), and PT-100 temperature probes (W-EYK, Heraeus, Kleinostheim, Germany) at different soil depths (3, 10, 15 and 20 cm) connected to a Campbell CR1000 data logger.
Air temperature, PPFD and VPD during the measurement period are presented in Fig. 1. Mean annual temperature in 2018 was 0.2 °C (ANS, 2020). Mean air temperature for the period between June 8 and September 2 measured at the site in 2018 was 11.7 ± 0.05 °C (Fig. 1e). Air temperature for the same period averaged over 100 years (1914–2013) taken at the Abisko Scientific Research Station (ANS, 2020), ca. 1 km away, was 9.9 ± 0.12 °C and over 30 years (1986–2015) was 9.7 ± 0.16 °C. Therefore, when taken a whole, 2018 was an ordinary year in Abisko in terms of air temperature, but with a very warm summer.
2.3. Leaf temperature and photosynthesis measurements
In order to understand the importance of leaf temperature on physiology of tundra shrubs, two types of gas exchange measurements were performed, 1) measurements at prevailing leaf temperature at varying environmental conditions across the growing season and 2) temperature response curves. At each sampling date, three plant individuals from each species were measured. For each measurement, new plant individuals were randomly selected from the heath area of approx. 100 m × 100 m. In each measurement, a twig containing 5–25 leaves from an unshaded, uppermost branch of the shrub was measured. The gas exchange measurements were conducted using a portable photosynthesis system, a LI-6400 XT (LI-COR, Inc, Lincoln, Nebraska, U.S.A) with a red/blue light leaf chamber (6400−02 B), which was used as a branch cuvette, rather than a leaf cuvette, due to the small size of the leaves.
Prior to the gas exchange measurements at prevailing leaf temperature, the leaf surface temperatures of each leaf in the measured twig were determined from the adaxial side using an Optris Laser Sight infrared thermometer (OPTRIS GmbH; Berlin, Germany) and averaged across all the leaves on the twig. The LI-6400 XT was used in-situ on living twigs of tundra shrubs to measure plant physiological variables such as net photosynthesis (An) and stomatal conductance (Gs). Twigs were carefully inserted to the leaf chamber to avoid physical stress, clamped around the stem and the flowrate through the chamber was maintained at 400 μmol s−1 (∼595 mL min-1). Photosynthetic photon flux density (PPFD) in the leaf chamber was set to 1000 μmol m-2 s-1, relative humidity (RH) was targeted to 60 % and the reference CO2 concentration in the leaf chamber was set to 400 μmol mol-1 using the LI-6400 XT’s mixer system. In order to correct the data for the leaf area and mass, the twigs were harvested after the finished measurements.
To produce temperature response curves, we measured gas exchange with the LI-6400 XT over five leaf temperature steps targeting 10 °C, 17 °C, 24 °C, 31 °C, and 38 °C, while the same twig was clamped inside the leaf chamber. This temperature range was chosen to reflect on the natural range of peak growing season temperatures the tundra plants experience. To increase the temperature range beyond that provided by the Peltier system of LI-6400 XT, which can alter temperature by ± 6 °C from the ambient, we used ice packs or hand warmers (The Heat Company; A - 5541 Altenmarkt, Austria) around the chamber. We measured temperature response curves for three replicates/species.
2.4. Sampling of VOC emissions
A charcoal filter (Supelco Inc., Bellefonte, Pennsylvania, USA) was installed on the air intake of the LI-6400 XT to filter the air entering the leaf chamber for hydrocarbons (Ortega et al., 2008). VOCs were then sampled in parallel to photosynthesis measurements using a trace gas kit (8100−664) as a flow divider attached to the outlet of the LI-6400 XT leaf chamber (Ekberg et al., 2009). Air was pulled from this flow divider with a pocket pump (TOUCH; SKC Ltd, Blandford Forum, UK) at 200 mL min−1 through a stainless steel cartridge (Markes International, Llantrisant, UK) containing Tenax TA and Carbograph 1TD adsorbents, which capture compounds with 5 or more C atoms. After sampling, the cartridges were sealed with Teflon-coated brass caps and stored at 4 °C until analysis. The VOC sampling and logging of the physiological parameters were started simultaneously following a period of 20 min after twig insertion to avoid stress-induced VOC emissions (Niinemets et al., 2011). Since the biological activity is expected to be lower at lower temperatures, sampling time for Betula, Salix and Rhododendron was 30 min at leaf temperatures between 10 °C and 17 °C, and 20 min at higher leaf temperatures. Sampling time for Cassiope was 30 min at all temperatures, as it is known to have low levels of photosynthetic activity (Baddeley et al., 1994). Sampled air volume varied accordingly, between 4 and 6 L. Blank measurements with an empty leaf chamber were made to determine the compounds originating from the sampling system. They were done every day under the same conditions the plants were exposed to and once at each step of the temperature response curve.
2.5. VOC analysis
The adsorbent cartridges were transported to Copenhagen and analyzed using gas chromatography-mass spectrometry (7890A Series GC coupled with a 5975C inert MSD/DS Performance Turbo EI System, Agilent) after thermal desorption (TD100-xr, Markes International Ltd, Llantrisant, UK). Helium was used as the carrier gas and the temperature of the oven was held at 40 °C for 1 min, followed by raising it to 210 °C at 5 °C min−1, and finally to 250 °C at a rate of 20 °C min−1. Individual compounds were separated by a HP-5 capillary column (50 m length, 0.2 mm diameter, 0.33 μm film thickness).
The chromatograms were analyzed using the software PARADISe (v3.87, Johnsen et al., 2017). The VOCs were identified according to 28 authentic standards (Table S1) and, for the compounds with no standards available, using the mass spectra in the NIST 14 library (National Institute of Standards and Technology, Gaithersburg, MD, USA). Compounds for which the NIST 14 library Match Factor was <800 were excluded. The sample concentrations were calculated based on the standards. The VOCs were classified into non-oxygenated monoterpenes (MTs), oxygenated monoterpenes (OMTs), sesquiterpenes (SQTs), and non-isoprenoid VOCs. Compounds for which no standard was available were quantified using α-pinene for MTs, 1,8-cineole for OMTs, trans-caryophyllene for SQTs and toluene for non-isoprenoid VOCs.
Concentrations of the VOCs in the blank samples were subtracted from the plant VOC samples. In addition, VOCs with high concentrations in the blanks were omitted as they were assessed to originate from the LI-6400 XT system.
The VOC emission rate was calculated according to Eq. 1 (Ortega and Helmig, 2008)
| (1) |
where Cout and Cin are the concentrations of VOCs in the outlet and inlet air and F is the flow rate into the leaf chamber. The concentration in the filtered inlet air was presupposed to be zero (Ortega et al., 2008; Niinemets et al., 2011).
2.6. Determination of specific leaf area and leaf dry mass content
Directly after VOC sampling and photosynthesis measurements, the measured twig was harvested, the leaves were separated from the stem, and the leaf area was scanned and calculated using ImageJ (v. 1.52a; Wayne Rasband, National Institutes of Health, Bethesda, USA). For Cassiope with square-based parallelepiped twigs (Fig. 1c), we kept the leaves on the twig, scanned from one side and doubled the projected area to obtain an estimate of the leaf area (Campioli et al., 2009). The fresh leaves were weighed, oven dried at 70 °C for 48 h, and weighed to determine dry mass. The specific leaf area (SLA, leaf area per dry weight) and leaf dry mass content (LDMC, dry weight divided by fresh weight) were calculated.
2.7. Plant height, soil moisture, chlorophyll fluorescence, and leaf surface structures
Plant height was measured using a ruler from the soil surface to the top of the measured twig. A portable chlorophyll fluorimeter, PAM-2100 (Heinz Walz GmbH, Effeltrich, Germany) was used to determine the light-adapted fluorescence and the quantum yield under ambient conditions of two leaves from the same plant, but from a twig adjacent to the one used for gas exchange measurements and VOC sampling. Electron transport rate (ETR) for each plant was calculated according to Genty et al. (1989) from the average of the two quantum yield measurements. Soil moisture was determined 10 cm from each measured plant using a Theta Probe ML3 (Delta T-Devices, Cambridge, UK) after the gas exchange measurements and VOC sampling.
After the gas exchange measurements, the twig was harvested, and we took four leaves per plant from an adjacent twig to obtain replicas of the leaf surface structures. These leaves were put on a microscope slide in a drop of superglue (Loctite, Westlake, Ohio, USA), pressed for 1 min, and were then carefully removed to leave a print of the leaf surface in the glue (Valkama et al., 2003). Of these four leaves, two represented the abaxial and the other two the adaxial side of a leaf in Salix and Betula. Rhododendron leaves were separated into current year (2018) and previous year (2017) leaves, and only the adaxial side was used for surface structure analysis as the concave leaves prevented making abaxial leaf prints. Leaf structures of Cassiope are in within-leaf cavities (Schollert et al., 2015), and therefore this species was omitted from leaf surface structures analysis.
We counted stomatal density in Salix, stomatal density and glandular trichome density in Betula and glandular trichome density in Rhododendron. Stomatal densities were counted for areas of 0.6 mm2 and averaged between four measurements on two replicas of each side of the leaf. Glandular trichome densities were counted and averaged similarly to stomata, but the inspected areas were 3.14 mm2.
2.8. Statistical analyses
We used one-way ANOVA to test whether species and the evergreen vs. deciduous groups differed from each other in leaf-to-air temperature difference.
For each plant species and compound separately, partial least squares (PLS) regression analysis was used to assess the covariance between the emission rates of the two most emitted VOCs (dependent variables, Y) and explanatory plant trait and environmental variables (independent variables, X) using SIMCA 16.0.1. (Umetrics, version 13.0.3.0, Umeå, Sweden). We opted to explain the emission of the most dominant compounds instead of VOC groups, because compounds within a group are likely differently controlled. For example, we could not obtain significant models for Betula and Rhododendron using groups. The X variables included photosynthesis, stomatal conductance, SLA, LDMC, density of stomata and glandular trichomes (where applicable), intercellular CO2 concentration, transpiration, water use efficiency, ETR, twig height, leaf temperature as output from LI-6400 XT, VPD based on leaf temperature (obtained from the LI-6400 XT that uses calculations of Buck (1981)), soil moisture, ambient PPFD, air temperature and relative humidity at 2 m height and soil temperature at 10 cm. Variables with a VIP (Variable Influence on Projection) < 0.5 were excluded from the models. The extracted PLS models had one component and were tested using analysis of variance of the cross-validated residuals (CV-ANOVA, Eriksson et al., 2008).
One-component PLS models were also calculated to assess the covariance between the leaf temperature (Y) and the explanatory plant trait and environmental variables (X). The X variables were stomatal conductance, transpiration, density of stomata and glandular trichomes (where applicable), SLA, LDMC, twig height, VPD based on leaf temperature, PPFD from the weather station, air temperature at 2 m height, relative humidity at 2 m, wind speed, friction velocity as a proxy for turbulence (u*), soil moisture and soil temperature (at 10 cm depth).
2.9. Modelling of seasonal VOC emissions
An excel-based site version of MEGAN v3.1, mostly based on Guenther et al. (2012), was used to estimate seasonal emissions of VOCs for the four studied dwarf shrubs using the continuous weather data (at hourly resolution) and the species-specific emission factor (EF, μg m−2 leaf area h-1) derived from the measurement data as inputs. To model emissions from each single species, the model assumes a continuous canopy of one species at a time.
MEGAN accounts for radiation transfer within canopy environment and leaf-level energy balance. Radiation is partitioned into direct and diffuse light for both visible (PAR) and near infrared (NIR) ranges (equations used from Spitters et al., 1986a; and 1986b, Lizaso et al., 2005; Jacovides et al., 2007). In the canopy layer, reflection and scattering coefficients for PAR and NIR are specified as properties of plant functional type. The canopy in the model is divided into 5 layers, based on Gaussian distribution of leaf area from the top of canopy. For each canopy layer, the received 4 components of radiation decrease when going deeper into the canopy and the extinction coefficients are calculated based on Spitters et al. (1986b). After radiation partitioning, the canopy energy balance is solved to derive leaf temperature for both sunlit and shaded leaves based on Leuning (1989) and Goudriaan and Van Laar (1994). In the model, a spherical leaf angle distribution is assumed for canopy.
The model simulates emissions of 19 VOC groups based on a multiplication of EF with environmental response functions (Guenther et al., 1991, 2012). The EFs of 19 MEGAN compound groups for each plant species were extracted from the measurements, which had the measured leaf temperature within the range of 29−31 °C and standardized to uniform conditions (PPFD of 1000 μmol m−2 s-1 and temperature of 30 °C). In total, the numbers of samples used for parameterizing EFs were 5, 3, 4, and 5 for Salix, Betula, Cassiope, and Rhododendron, respectively. The measurements used for parameterizing EFs were excluded from model evaluation. The commonly-used EF values in MEGAN used for shrubs are also listed for comparison (Table 1). Furthermore, the hourly measured air temperature (at 20 cm and 2 m), PPFD, relative humidity and soil moisture from the weather station were used to drive the model. As all VOC samples were taken near the weather station, the same weather inputs were used for simulating the ecosystem emissions from these four species.
Table 1.
Emission factors§ (standardized emission rate ± SD, μg m−2 leaf area h-1) used in MEGAN based on the leaf-level measurements with leaf temperature in the range of 29-31 °C.
| Compound groups | Salix (n = 5) | Betula (n = 3) | Cassiope (n = 4) | Rhododendron (n = 5) | ME-GAN* |
|---|---|---|---|---|---|
| isoprene | 2534.1 ± 1505.2 | 9.2 ± 15.9 | 23.1 ± 46.2 | 25.5 ± 31.7 | 1471.4 |
| MBO | N/A | N/A | N/A | N/A | 0.6 |
| MT1: pinenes (α and β) | 12.4 ± 8.9 | 6.0 ± 6.8 | 100.0 ± 67.7 | 4.3 ± 3.2 | 112.8 |
| MT2: acyclic 3 bonds; myrcene; ocimenes | 10.4 ± 5.0 | 0.04 ± 0.07 | 21.2 ± 13.1 | 1.8 ± 1.7 | 54.0 |
| MT3: carene; camphene; others | 2.1 ± 3.0 | 206.0 ± 281.0 | 1544.0 ± 1442.0 | N/A | 44.1 |
| MT4: limonene; terpinenes; others | 50.7 ± 67.4 | 1.5 ± 1.4 | 6321 ± 4446 | 0.8 ± 0.8 | 78.5 |
| C10 aromatic: cymenes; cymenenes | 5.5 ± 3.1 | 1.3 ± 1.1 | 32.7 ± 19.8 | N/A | 77.3 |
| C8-C13 Oxygenated (e.g. camphor) | N/A | 55 ± 55 | 194 ± 135 | 9.0 ± 15.0 | 43.8 |
| highly reactive SQT (e.g. caryophyllene) | 421.1 ± 264.2 | 28.4 ± 22.5 | 37.0 ± 29.3 | 2825.0 ± 1450.0 | 7.4 |
| less reactive SQT (e.g. copaene) | 3.5 ± 3.9 | 30.0 ± 31.6 | 99.7 ± 53.8 | 8.9 ± 9.9 | 29.4 |
| methanol | N/A | N/A | N/A | N/A | 150.0 |
| acetone | N/A | N/A | N/A | N/A | 37.6 |
| acetaldehyde and ethanol | N/A | N/A | N/A | N/A | 29.16 |
| organic acids: formic, acetic, pyruvic | 0.04 ± 0.07 | 0.02 ± 0.04 | 65.20 ± 87.10 | 0.01 ± 0.01 | 28.0 |
| C2 to C4 HC (e.g.; ethene; ethane) | N/A | N/A | N/A | N/A | 102.5 |
| oxidation products: aldehydes | 168.2 ± 166.2 | N/A | 43.1 ± 44.1 | 92.2 ± 140.0 | 4.7 |
| Stress compounds (e.g.; linalool) | 746.0 ± 299.0 | 418.0 ± 64.0 | 364.0 ± 226.0 | 465.0 ± 731.0 | 5.6 |
| other VOCs (e.g., indole, pentane) | 9.1 ± 6.8 | 3.6 ± 5.8 | 304.3 ± 190.2 | 188.1 ± 155.3 | 22.9 |
| carbon monoxide | N/A | N/A | N/A | N/A | 0.3 |
Emission rate standardized to the temperature of 30 °C and PPFD of 1000 μmol m−2 s-1 based on Guenther et al. (1993).
MEGAN original values for shrub plant functional type; MTs: monoterpenes; N/A. means no data available from the measurements. MBO: Methylbutenol; HC: hydrocarbon.
Since there were no in situ measurements of leaf area index (LAI), a LAI value of 0.95 was used for Cassiope based on Campioli et al. (2009). For the other species, the LAI values were taken from the nearest grid of MODIS product (MCD15A2H Version 6, with LAI value for every 8 days). The MODIS LAI ranged from 0.9 to 1.7 m2/m2 from early June to the end of August. The model has been parameterized with the measured canopy height, canopy depth and leaf size based on the measurements. The detailed observation data used for the modelling part has been listed in Table S2.
3. Results
3.1. Leaf temperature
According to our measurements in the field, the leaf temperature mean was not substantially different between the four plant species (ANOVA, p = 0.078, Table S3). However, relative to the air temperature, evergreens (Rhododendron and Cassiope) had significantly warmer leaves compared to the deciduous shrubs (Salix and Betula) (ANOVA, p = 0.012, Table S3).
According to the PLS regression, leaf temperature in Salix showed significant positive correlation to VPD, soil and air temperatures and transpiration rate (Fig. 2a). Leaf temperature in Salix correlated negatively with stomatal density. Leaf temperature in Betula correlated positively with VPD, soil and air temperature, PPFD, soil moisture, and transpiration. It correlated negatively with the SLA (Fig. 2b). Leaf temperature in Cassiope showed a positive correlation to air temperature and VPD but negative correlations with air humidity, soil moisture, and soil temperature (Fig. 2c). Leaf temperature in Rhododendron was positively correlated with VPD and air temperature and negatively with wind speed, but the model was not significant (Fig. 2d; p = 0.34, CV-ANOVA).
Fig. 2.
Regression coefficients from PLS regression models on leaf temperature in Salix (a), Betula (b), Cassiope (c) and Rhododendron (d). Error bars show ± 2 standard deviations of the regression coefficients. Significant variables are marked with grey bars. Significance of the model (CV-ANOVA) is shown p < 0.001 ***, p < 0.05 *.
3.2. VOC emissions
3.2.1. Salix myrsinites
VOC emission from Salix was on average 21.7 μg g−1 dw h−1 (Fig. 3a). Isoprene was the most emitted VOC (contributing 64 % of the total by mass), whilst non-isoprenoid VOCs, sesquiterpenes and monoterpenes constituted 24 %, 7%, and 5%, respectively (Fig. 3b).
Fig. 3.
(a) Total VOC emission rates (mean + SE) stacked by isoprene, monoterpenes, oxygenated monoterpenes, sesquiterpenes, and non-isoprenoid VOCs for S. myrsinites (n = 12), B. nana (n = 15), C. tetragona (n = 12) and R. lapponicum (n = 10) averaged over the measurement period. Mean leaf surface temperature for each species are shown. (b) The relative contribution of each compound group to the total VOC emission of each plant species.
Emission rates for all compounds across the measurement period are presented in the supplementary Table S4. On June 30, when air temperature was below 10 °C and leaf temperature approx. 13 °C, isoprene emission was low, around 0.04 μg g−1 dw h−1 (Table S4). Isoprene emission was much higher on July 17, at 23.4 μg g−1 dw h−1 and peaked on July 22 at 28.3 μg g-1 dw h-1, when leaf temperature was around 24 °C. Finally, the isoprene emission was again low, ca. 3.7 μg g-1 dw h-1 on August 15 measurement with leaf temperatures of 14 °C. Other VOC groups emitted by Salix followed a similar pattern (Table S4).
3.2.2. Betula nana
Averaged over the measurement period, Betula had a total VOC emission rate of 9.8 μg g−1 dw h-1 (Fig. 3a). Of all VOC groups, non-isoprenoid VOCs were the most abundant (77 % by mass), followed by non-oxygenated monoterpenes (21 %), with other groups contributing by 2% (Fig. 3b). The single most emitted VOC was 2-hexenal with 2.4 μg g-1 dw h−1, followed by the monoterpene 3-carene with 1.9 μg g−1 dw h−1 (See Table S5 for individual compounds).
On June 30, when the air temperature was below 10 °C and leaf temperature at 14 °C, the emission of non-isoprenoid VOCs was low, 3 μg g−1 dw h−1, but the emission of non-oxygenated monoterpenes was, compared to the rest of field season, relatively high with 4.6 μg g−1 dw h−1 (Table S5). With the higher air (16 °C) and leaf temperature (23 °C) on July 10, non-isoprenoid VOC emission reached a high of 18.5 μg g−1 dw h−1, while the MT emission was at its lowest, 0.006 μg g−1 dw h−1. Later measurements on July 20 and 24 were characterized by lower leaf temperatures and VOC emissions. On August 18, when the air and leaf temperatures were 16 °C and 18 °C, respectively, the non-isoprenoid VOC emission reached a low of 1.1 μg g−1 dw h−1, while the MT emission reached a peak of approximately 4.9 μg g−1 dw h−1.
3.2.3. Cassiope tetragona
Cassiope had a total VOC emission rate of 52.1 μg g−1 dw h-1 averaged over the measurement period (Fig. 3a). Non-oxygenated monoterpenes accounted for almost 85 % of the total VOCs (Fig. 3b) by mass. Γ-terpinene at 39.3 μg g−1 dw h−1 was the dominant compound and responsible for 82.5 % of the total VOC emission (Supplementary Table S6). MT emission was highest on July 2, at 121.9 μg g−1 dw h−1, when both air (21 °C) and leaf temperatures (28 °C) were highest as well (Table S6). On July 10, MT emission was 35.7 μg g−1 dw h−1, even though the air and leaf temperatures remained similar as in the previous measurement (18 °C and 26 °C, respectively). In the two last measurements, on July 20 and 24, MT emissions were low (7.0 and 12.0 μg g−1 dw h−1).
3.2.4. Rhododendron lapponicum
The total VOC emission from Rhododendron averaged over the measurement period was 38.9 μg g−1 dw h-1 (Fig. 3a). Sesquiterpenes dominated the VOC blend of this species (92 % by mass) followed by non-isoprenoid VOCs (8%; Fig. 3b). The most emitted sesquiterpene, responsible for over half of the total VOC emission, was β-elemene at 21.7 μg g−1 dw h−1, followed by α-selinene and α-humulene (Supplementary Table S7). Rhododendron emissions were only measured in July, through which sesquiterpene emission, together with leaf and air temperature, remained high (mean ± SE of 35.7 ± 7.1 μg g−1 dw h−1, Table S7).
3.3. Covariance between explanatory variables and VOC emission
We used PLS regression to test if plant traits and environmental factors (X variables) can explain the variation in the emissions of the most emitted individual VOCs (Y-variables) of the investigated plants species. Data on all the plant traits are shown in the Supplementary Table S8.
The dominating compounds we analyzed with the PLS regression were isoprene in Salix, 3-carene and 2-hexenal in Betula and γ-terpinene in Cassiope (Fig. 4).
Fig. 4.
Regression coefficients from PLS regression model of isoprene emission in Salix (a), 3-carene emission in Betula (b), 2-hexenal emission in Betula (c) and γ-terpinene emission in Cassiope (d). Error bars show ± 2 standard deviations of the regression coefficients. Significant variables are marked with grey bars. Significance of the model (CV-ANOVA) is shown p < 0.001 ***, p < 0.05 *.
The PLS regression model for Salix showed significant positive correlation between isoprene emission and soil, leaf and air temperatures, ETR and VPD (Fig. 4a). Isoprene emission also positively correlated with transpiration, but due to large variation in transpiration, this was not statistically significant. Isoprene emission correlated negatively with stomatal density of adaxial side of the leaves.
3-carene emitted by Betula correlated positively with SLA and the intercellular CO2 concentration and negatively with leaf, soil and air temperatures (Fig. 4b). 2-hexenal emission from Betula showed positive correlation with ETR, VPD and leaf temperature. It correlated negatively with intercellular CO2 concentration and SLA (Fig. 4c).
Γ-terpinene emission from Cassiope was positively correlated with leaf and ambient air temperature, as well as ambient PPFD and negatively correlated with transpiration, stomatal conductance, soil temperature and relative humidity of the ambient air (Fig. 4d).
No PLS models converged for the emissions of the dominant VOCs from Rhododendron.
3.4. Temperature response curves
In Salix, net photosynthesis (An) increased from 10 °C, plateaued around 24 °C and decreased at the higher temperatures (Fig. 5a). Isoprene emission increased exponentially within our temperature range of 10−38 °C (Fig. 5b).
Fig. 5.
Temperature response curves of net photosynthesis (An), emission rate (Er) of the main VOC group and the transpiration rate (Tr) for the investigated plant species. The symbols show three (two for Salix) independent measurements and the solid line shows the fitted average.
In Betula, An increased until our measurement temperature of 31 °C and was lower at our highest temperature (Fig. 5d). The emission of the dominant VOC group, non-isoprenoid VOCs, varied considerably for the three independent replicates with no clear temperature dependency (Fig. 5e).
Cassiope showed a nearly linear response of An to temperature increase until our highest measurement temperature of 38 °C (Fig. 5g). Monoterpene emission increased steadily until 31 °C and then sharply for the two replicates measured at 38 °C (Fig. 5h).
In Rhododendron, net photosynthesis increased until around our measurement temperature of 31 °C with no clear decrease at our highest temperature (Fig. 5j). The emission of sesquiterpenes increased exponentially with temperature (Fig. 5k).
The transpiration rate increased exponentially with leaf temperature in Salix and Cassiope (Fig. 5c, i), potentially causing water stress for plants. In Betula and Rhododendron (Fig. 5f, l), the transpiration rates at higher leaf temperatures differed between individual samples, and the fitted average reached a plateau, potentially indicating stomatal closure to reduce water loss through transpiration.
The percentage of carbon emitted as VOCs relative to the amount fixed in photosynthesis varied between species and temperature (Supplementary Table S9). In Betula and Salix, under 1% of carbon fixed was emitted as VOCs, except for the last temperature step in Salix with 3.5 ± 2.6 % C loss as VOCs. In Cassiope and Rhododendron, there was large variation between the replicates and a tendency of the percentages increasing with temperature. Photosynthetically fixed carbon emitted as VOCs in Cassiope was between -1.3 ± 1.7 % and 15.9 ± 4.5 % and between 0.8 ± 2.2 and 3.7 ± 2.5 % in Rhododendron.
With increasing leaf temperature, WUE decreased in deciduous shrubs, increased in Rhododendron and had no distinct pattern in Cassiope (Table S9). Stomatal conductance (gs) decreased throughout the temperature response curves in all species, except in Cassiope where it stayed around 0.05 mol H2O m−2 s-1 (Table S9). Supplementary Table S9 also contains information about photosynthesis and transpiration of the measured plants throughout temperature response curves.
3.5. Modelled VOC emissions
The studied species varied considerably in EFs for different compound groups. For some groups, the measured EFs were much higher than the original EFs used for shrubs in MEGAN (Table 1), for example, for isoprene from Salix, MT3 and MT4 from Cassiope, highly reactive SQT from Salix and Rhododendron, oxidation products from Salix and other VOCs from Rhododendron and Cassiope. Also, the EFs for the stress-related compounds were much higher than the original EFs in MEGAN.
The VOC emissions measured at ambient leaf temperature were matched to the modelled ones at corresponding time points and averaged over the same day for each species (Fig. 6). The wide spread of emission magnitudes of different compounds was generally captured by the model for Salix and Rhododendron (Fig. 6a,d). For Betula, the model largely underestimated the measured emissions for some groups, e.g., organic acids (less than 0.01 % of the observed), MT2 (13.2 % of the observed) and stress-related compounds (0.07 % of the observed) (Fig. 6b). For the compounds with high emission rates, the model overestimated the MT3 and MT4 (158.5 % and 71.0 % of the observed, respectively) emissions from Cassiope (Fig. 6c), but underestimated the isoprene emissions (32.2 % of the observed) and high reactive SQT (17.8 % of the observed) emissions from Salix and Rhododendron, respectively.
Fig. 6.
The measured and modelled VOC emissions (μg m−2 ground area h-1) for S. myrsinites (a), B. nana (b), C. tetragona (c), and R. lapponicum (d). The emissions were measured at the conditions of PPFD at 1000 μmol m−2 s-1 and leaf temperature comparable to ambient, and the modelled emissions were recalculated to the same PPFD level using the light response function in MEGAN. The 13 out of the 19 MEGAN VOC groups (see Table 1) with values are shown. MT1: α and β-pinenes; MT2: Acyclic 3 bonds; myrcene; ocimenes; MT3: carene, camphene, and others; MT4: sabinene, limonene, terpinenes, and others. HighR and LessR refer to highly reactive and less reactive, respectively.
After evaluating the modelled output at the time of the measurement, the seasonal variability in VOC emissions was also quantified (Fig. 7). Using 20 cm air temperature as input gave higher VOC emissions than using 2 m air temperature and there were generally larger absolute differences in warm days (Fig. 7). The largest daily increases by using 20 cm air temperature instead of the 2 m air temperature were 44 %, 33 %, 31 %, and 48 % for Salix, Betula, Cassiope and Rhododendron, respectively. When summing up the modelled emissions for the period June 8 to September 2 using 2 m air temperatures, the total VOC emissions for Salix, Betula, Cassiope and Rhododendron were 0.9, 0.3, 2.3 and 0.7 g m−2, respectively. By applying air temperature at 20 cm height instead, the modelled seasonal emissions increased by 21.4 %, 15.2 %, 14.1 % and 23.0 % for Salix, Betula, Cassiope and Rhododendron, respectively.
Fig. 7.
The growing season VOC emissions (mg m−2 ground area h-1) modelled using air temperature at 2 m height (left-side axis). The modelled difference ratio (in percentage, %) between using 20 cm and 2 m air temperature inputs is plotted on the right-side axis.
4. Discussion
4.1. Factors affecting leaf temperature and VOC emission in tundra shrubs
The main aim of our study was to assess the control of leaf temperature in four tundra shrub species and to study effects of plant traits, leaf temperature and other environmental factors on VOC emission from four common arctic shrubs. Despite high natural variation, we identified some plant traits linked to leaf temperature and VOC emission in the studied deciduous, but not in the evergreen shrub species.
The most important plant traits that correlated with leaf temperature in the studied deciduous shrubs were morphological leaf traits, LDMC and SLA, as well as stomata-related variables stomatal density, stomatal conductance and transpiration. SLA (ratio between leaf area and dry mass), differed largely between thicker and thinner leaves, which is important for determining the efficiency of heat retained inside the leaves (Leigh et al., 2012; Rosbakh et al., 2015). We observed that leaves with higher SLA were cooler, while plants with lower SLA had higher leaf temperatures. The findings of Schollert et al. (2015, 2017) suggest that the palisade parenchyma, epidermis and overall leaf thickness of the dwarf birch leaves might increase under a warmer climate. However, a trend of larger and thinner leaves at elevated temperatures has been observed for silver birch, Betula pendula, and other woody species at lower latitudes (Luomala et al., 2005; Hartikainen et al., 2009; Rasulov et al., 2015).
Leaf temperature naturally correlated with air (and soil) temperature, which is intercorrelated with PPFD. Solar radiation, here expressed as PPFD, effectively heats up the surface of leaves in low, dense tundra plant communities (Lindwall et al., 2015; Körner, 2019). Notwithstanding the structure of our setup, we observed that VPD was positively related to leaf temperature in all studied plant species. VPD has earlier been shown to be important for photosynthesis through increased stomatal closure at higher VPD (Lin et al., 2012). Increased air temperature was recently coupled to high VPD and increased terpenoid emission in an evergreen tree species (Duan et al., 2019). Furthermore, high air temperature and water deficit have been observed in earlier studies to strongly stimulate VOC emission of tundra plants (Kramshøj et al., 2016; Lindwall et al., 2016; Tang et al., 2018).
Our results showed that plant traits contributed to the measured VOC emission variations. We observed a positive correlation of ETR with isoprene emission in Salix. This correlation has been described before (Guenther et al., 1991; Monson et al., 1992; Sharkey and Monson, 2017) and reinforces the findings of Niinemets et al. (2004) that the ETR can be used for estimating isoprene emission. Isoprene emission provides protection against short-term periods of high leaf temperature (Singsaas and Sharkey, 1998; Singsaas et al., 1999) and is thought not to be limited by stomata (Monson and Fall, 1989). Our negative correlation between stomatal density of Salix leaves and isoprene emission is likely related to strong direct temperature response rather than an actual negative correlation.
The positive correlation between ETR and 2-hexenal emission in Betula suggests that, like isoprene, this compound is likely synthesized de-novo. The Betula leaves emitting most VOCs such as 2-hexenal were the ones with low SLA values (smaller and thicker leaves) and low intercellular CO2 concentration. Contrary, the plants emitting most 3-carene were the ones with larger, thinner leaves and high intercellular CO2 concentration. We suggest this is due to the natural physiological variation of this species. B. pendula and B. pubescens have been documented to exhibit large within-species variation in VOC emissions (Hakola et al., 2001; Maja et al., 2015).
We also observed a negative correlation between stomatal conductance/transpiration and γ-terpinene emission in Cassiope. We suggest that this relationship is due to leaf temperature, which was negatively correlated with stomatal conductance.
The lacking of correlation between VOC emission in R. lapponicum and the studied variables could be due to unquantified plant traits (for example number of flowers, leaf anatomy or N and P content in leaves) determining the relationship in this species or due to the technical challenges of sesquiterpene measurements (Duhl et al., 2008) leading to high variation in emission rates.
4.2. Temperature response of photosynthesis and VOC emission
We observed that the subarctic S. myrsinites photosynthesis increased with temperature until our measurement temperature of 24 °C and remained at a similar level still at our next measurement temperature of 31 °C, which suggests a higher Topt than the values derived for Salix polaris from the High Arctic (15 °C, Muraoka et al., 2002), for Salix myrsinifolia from a Finnish boreal forest (∼20 °C, Kaipiainen and Pelkonen, 2007), and even for Salix viminalis from southwest Sweden (20 °C, Cienciala and Lindroth, 1995). According to the work of Körner (2003a, d), the photosynthetic apparatus of alpine plants is optimized for their growth temperature. The higher Topt values in the low-statured tundra shrubs are likely related to the elevated leaf temperatures the plants experience.
Monson et al. (1992) determined that Topt for isoprene synthase activity for the velvet bean, Mucuna pruriens L. is usually 5–10 °C higher than Topt for photosynthesis. In our two samples, isoprene emission in S. myrsinites indeed increased to higher temperatures than photosynthesis. Thus, the temperature sensitivity for isoprene emission in tundra vegetation seems stronger than that for photosynthesis. Warming by 3.4 °C increases photosynthesis in boreal tree species when soil moisture is high (Reich et al., 2018). However, a large discrepancy has been earlier reported in response to warming between ecosystem-level isoprene emission and CO2 exchange: A field experiment conducted on a Greenlandic tundra heath dominated by Salix glauca showed that warming by approx. 3 °C caused a 240 % increase in isoprene emission (Kramshøj et al., 2016), while the same treatment significantly decreased gross primary production (Dahl et al., 2017), through enhanced soil respiration at higher temperatures.
We observed that the Topt of B. nana An was between 24 °C and 38 °C, which matches the findings of (Chapin and Shaver, 1996) for the same species, and is similar to the optimum of B. pendula (Wittmann and Pfanz, 2007). B. nana is known for high non-isoprenoid emission rates (Rinnan et al., 2011; Schollert et al., 2015; Vedel-Petersen et al., 2015; Li et al., 2019). However, the temperature responses of the emissions did not show a consistent pattern between the samples, probably as this is a complex group of compounds with different molecular structures or it can reflect the individual differences between plants.
Cassiope, together with other evergreen tundra shrubs, is known to have a low rate of photosynthesis compared to deciduous shrubs (Chapin and Shaver, 1985) with a clear temperature limitation to shoot growth (Havström et al., 1993; Campioli et al., 2013). In our temperature response curves, An of Cassiope increased with temperature until our last step, at 38 °C, suggesting good heat tolerance. Emission of monoterpenes, the most abundant VOC group, increased steadily with temperature and then drastically above 30 °C.
R. lapponicum showed increasing An towards our highest measurement temperatures, which suggests that it is also a very heat tolerant species. The Topt for R. lapponicum An may indeed be higher than the average surrounding air temperature, and even higher than the leaf Topt for An of more southern species (25 °C for Rhododendron hyperythrum from Taiwan and Rhododendron catawbiense from eastern USA, 20 °C for Rhododendron russatum from China; Ranney et al., 1995). The overall low rate of carbon assimilation compared to Salix and Betula may be due to that tundra Rhododendron spp. have high autotrophic respiration rates (plant metabolism) which creates a major electron sink (Huang et al., 2019). The emission rate of sesquiterpenes, which dominate the total VOC blend of Rhododendron, continued to rise through our temperature increment range. Sesquiterpene emissions are known to increase drastically at higher temperatures (Duhl et al., 2008).
The percentage of photosynthetically fixed carbon emitted as VOCs generally increased at higher temperatures. The changes in the loss of fixed carbon as VOC with leaf temperature are important when assessing the ecosystem carbon budget (Kesselmeier et al., 2002).
4.3. Modelled tundra ecosystem VOC emissions
The used VOC measurement technique only quantified VOCs with minimum five carbon atoms, and therefore six out of the 19 MEGAN groups could not be compared in this study (Table 1). For the 13 groups that were quantified, the studied four dwarf shrubs differed greatly from each other and also from the default MEGAN values, which demonstrates the diversity of emission profiles at species level. As VOC emissions vary between species, it is ultimately necessary to consider species composition and their contribution to emissions at community or regional levels (Valolahti et al., 2015). The sample size used for parametrizing EFs was small and there was high within-species variation, which means that the modelled magnitudes for different compound groups may change when including more samples.
The modelled total VOC emissions for the growing season 2018 are in the range 0.3–2.3 g m−2. These modelled seasonal totals are higher than the annual totals of isoprene and monoterpene emissions in the same region, obtained previously using a different ecosystem model (LPJ-GUESS, Tang et al., 2016). However, other issues could explain the discrepancy: 1) Stress-related compounds seem abundant for all the studied species but this compound group was not considered in the previous modelling study; 2) The summer 2018 was warm, some days with an air temperature at 2 m of 30 °C (Fig. 1e), and temperature differences between years of course contribute to modelled differences in different years. We cannot elucidate whether part of the stress compounds were emitted in response to this particularly hot summer. It should also be noted that, as it was not possible to derive significant temperature response curves for all compound groups used in MEGAN, the modelled seasonal values in this study were based on the standard G93 temperature response curve (Guenther et al., 1993), which may underestimate emissions as compared to using a temperature response curve derived from arctic species (Tang et al., 2016; Seco et al., 2020).
The modelled summer season emissions increased by 14–23 % when replacing the model input of 2 m air temperature with temperature measured close to the shrub canopy, at 20 cm height from the ground. The differences can be even larger if we also include the effect of wind speed contribution on leaf boundary layer conductance. At the canopy height (often 20 cm or less), the wind speed is typically lower than at 2 m height, indicating less efficient vertical mixing and decoupling between air temperature and leaf temperature. The conventional way of using 2 m air temperature for modelling inputs may cause large uncertainties in simulating ecosystem processes in this region.
4.4. Limitations and future directions
To our knowledge, this is the first study to assess the importance of leaf temperature on gas exchange and VOC emission from tundra shrubs on the branch scale. As such, it carried some limitations with it, mainly in the limited number of replicates, studied plant species and locations covered. A higher number of independent, replicate measurements would allow for more robust PLS models and more detailed analysis within a single plant species.
It would be relevant also to compare further plant species, including other growth forms important in the tundra, such as graminoids and bryophytes. Tundra ecosystems span an enormous area of our planet with plants that are adjusted to the local environmental conditions, and still the field studies focus on a few hotspot study locations, including our study site Abisko (Metcalfe et al., 2018). As this can bias our understanding, other studies across the Arctic would be appreciated.
5. Conclusions
We showed that air temperature and VPD were the most important environmental factors driving leaf temperature and VOC emission on the studied sub-arctic tundra, while plant traits affecting leaf temperature and VOC emissions of arctic tundra shrubs were species-specific. With the ongoing climate change, we expect increasing temperatures and alterations in cloudiness and soil water availability, which are likely to have a profound effect on both leaf temperature and VOC emission from tundra shrubs. Leaf temperature of these low-statured plants tends to be decoupled from the air temperature at 2 m height, which is commonly used as model input, leading to underestimation of VOC emission rates. Indeed, our study illustrated a higher-than-expected Topt for both photosynthesis and VOC emissions for the studied tundra species showing that they are well acclimated to a near-surface temperature that often exceeds the commonly used air temperature taken at a standard height. This suggests strong responses with climatic warming.
Author contributions
T.S. performed field measurements, analyzed data, prepared graphs and wrote the original manuscript with contributions from all authors; J.T. performed modeling and prepared graphs; T.H. provided instrumentation and set up the weather station; R.R. designed the experiment and coordinated and supervised the research; All authors discussed the results and edited the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 771012) and the Danish National Research Foundation (Center for Permafrost, CENPERM DNRF100). Jing Tang was financially supported by European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 707187 and FORMAS grant (No. 2016-01580). We thank Abisko Scientific Research Station for being able to use their excellent facilities. We appreciate Prof. Alex Guenther’s instructions on the MEGAN model setup. We would also like to thank Marios Vazakas for the help in fieldwork preparation, Trine Mariager and Nanna Schrøder Baggesen for assistance in data collection, and Tatjana Simin for language revision.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.envexpbot.2021.104387.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adams J.M., Constable J.V., Guenther A.B., Zimmerman P. An estimate of natural volatile organic compound emissions from vegetation since the last glacial maximum. Chemosphere-Global Change Sci. 2001;3:73–91. [Google Scholar]
- ANS A.S.R.S. Polarforskningssekretariatet; 2020. Temperature and Precipitation Data 1913-2019. Abisko Scientific Research Station.http://polar.se/abisko [Google Scholar]
- Baddeley J., Woodin S., Alexander I. Effects of increased nitrogen and phosphorus availability on the photosynthesis and nutrient relations of three arctic dwarf shrubs from Svalbard. Funct. Ecol. 1994;8:676–685. [Google Scholar]
- Berry J., Bjorkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980;31:491–543. [Google Scholar]
- Buck A.L. New equations for computing vapor pressure and enhancement factor. J. Appl. Meteorol. 1981;20:1527–1532. [Google Scholar]
- Campioli M., Street L.E., Michelsen A., Shaver G.R., Maere T., Samson R., Lemeur R. Determination of leaf area index, total foliar N, and normalized difference vegetation index for Arctic ecosystems dominated by Cassiope tetragona. Arct. Antarct. Alp. Res. 2009;41:426–433. [Google Scholar]
- Campioli M., Schmidt N.M., Albert K.R., Leblans N., Ro-Poulsen H., Michelsen A. Does warming affect growth rate and biomass production of shrubs in the High Arctic? Plant Ecol. 2013;214:1049–1058. [Google Scholar]
- Chapin F.S., Shaver G.R. Physiological Ecology of North American Plant Communities. Springer; 1985. Arctic; pp. 16–40. [Google Scholar]
- Chapin F.S., III, Shaver G.R. Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology. 1996;77:822–840. [Google Scholar]
- Cienciala E., Lindroth A. Gas-exchange and sap flow measurements of Salix viminalis trees in short-rotation forest. Trees. 1995;9:295–301. [Google Scholar]
- Condon A.G., Richards R.A., Rebetzke G.J., Farquhar G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002;42:122–131. doi: 10.2135/cropsci2002.1220. [DOI] [PubMed] [Google Scholar]
- Dahl M.B., Priemé A., Brejnrod A., Brusvang P., Lund M., Nymand J., Kramshøj M., Ro-Poulsen H., Haugwitz M.S. Warming, shading and a moth outbreak reduce tundra carbon sink strength dramatically by changing plant cover and soil microbial activity. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-16007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C., Sharkey T. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993;16:587–591. [Google Scholar]
- Duan Q., Kleiber A., Jansen K., Junker-Frohn L.V., Kammerer B., Han G., Zimmer I., Rennenberg H., Schnitzler J.-P., Ensminger I. Effects of elevated growth temperature and enhanced atmospheric vapor pressure deficit on needle and root terpenoid contents of two Douglas fir provenances. Environ. Exp. Bot. 2019;166:103819. [Google Scholar]
- Duhl T., Helmig D., Guenther A. Sesquiterpene emissions from vegetation: a review. Biogeosciences. Eur. Geosci. Union. 2008;5(3):761–777. [Google Scholar]
- Ekberg A., Arneth A., Hakola H., Hayward S., Holst T. Isoprene emission from wetland sedges. Biogeosciences. 2009;6:601–613. [Google Scholar]
- Eriksson L., Trygg J., Wold S. CV‐ANOVA for significance testing of PLS and OPLS® models. J. Chemomet.: J. Chemomet. Soc. 2008;22:594–600. [Google Scholar]
- Faubert P., Tiiva P., Rinnan Å., Michelsen A., Holopainen J.K., Rinnan R. Doubled volatile organic compound emissions from subarctic tundra under simulated climate warming. New Phytol. 2010;187:199–208. doi: 10.1111/j.1469-8137.2010.03270.x. [DOI] [PubMed] [Google Scholar]
- Filella I., Wilkinson M.J., Llusia J., Hewitt C.N., Peñuelas J. Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol. Plant. 2007;130:58–66. [Google Scholar]
- Garnier E. Growth analysis of congeneric annual and perennial grass species. J. Ecol. 1992;80(4):665–675. [Google Scholar]
- Genty B., Briantais J.-M., Baker N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989;990:87–92. [Google Scholar]
- Glasius M., Goldstein A.H. Recent discoveries and future challenges in atmospheric organic chemistry. ACS Publ. 2016;50(6):2754–2764. doi: 10.1021/acs.est.5b05105. [DOI] [PubMed] [Google Scholar]
- Goudriaan J., Van Laar H. Modelling Potential Crop Growth Processes. Springer; 1994. Leaf energy balance and transpiration; pp. 121–148. [Google Scholar]
- Guenther A.B., Monson R.K., Fall R. Isoprene and monoterpene emission rate variability: observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. Atmos. 1991;96:10799–10808. [Google Scholar]
- Guenther A.B., Zimmerman P.R., Harley P.C., Monson R.K., Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 1993;98:12609–12617. [Google Scholar]
- Guenther A., Hewitt C.N., Erickson D., Fall R., Geron C., Graedel T., Harley P., Klinger L., Lerdau M., McKay W. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995;100:8873–8892. [Google Scholar]
- Guenther A., Jiang X., Heald C., Sakulyanontvittaya T., Duhl T., Emmons L., Wang X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2. 1): an extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 2012;5(6):1471–1492. [Google Scholar]
- Hakola H., Laurila T., Lindfors V., Hellén H., Gaman A., Rinne J. Variation of the VOC emission rates of birch species during the growing season. Boreal Environ. Res. 2001;6(3):237–249. [Google Scholar]
- Hartikainen K., Nerg A.-m., Kivimäenpää M., Kontunen-Soppela S., Mäenpää M., Oksanen E., Rousi M., Holopainen T. Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiol. 2009;29:1163–1173. doi: 10.1093/treephys/tpp033. [DOI] [PubMed] [Google Scholar]
- Havström M., Callaghan T.V., Jonasson S. Differential growth responses of Cassiope tetragona, an arctic dwarf-shrub, to environmental perturbations among three contrasting high-and subarctic sites. Oikos. 1993;66:389–402. [Google Scholar]
- Huang W., Yang Y.-J., Wang J.-H., Hu H. Photorespiration is the major alternative electron sink under high light in alpine evergreen sclerophyllous Rhododendron species. Plant Sci. 2019;289:110275. doi: 10.1016/j.plantsci.2019.110275. [DOI] [PubMed] [Google Scholar]
- Jacovides C., Tymvios F., Assimakopoulos V., Kaltsounides N. The dependence of global and diffuse PAR radiation components on sky conditions at Athens, Greece. Agric. For. Meteorol. 2007;143:277–287. [Google Scholar]
- Johnsen L.G., Skou P.B., Khakimov B., Bro R. Gas chromatography–mass spectrometry data processing made easy. J. Chromatogr. A. 2017;1503:57–64. doi: 10.1016/j.chroma.2017.04.052. [DOI] [PubMed] [Google Scholar]
- Johnson D.A., Tieszen L.L. Aboveground biomass allocation, leaf growth, and photosynthesis patterns in tundra plant forms in arctic Alaska. Oecologia. 1976;24:159–173. doi: 10.1007/BF00572757. [DOI] [PubMed] [Google Scholar]
- Kaipiainen E., Pelkonen P. Requirements for obtaining maximum indices of photosynthesis and transpiration in attached leaves of willow plants grown in short-rotation forest. Russ. J. Plant Physiol. 2007;54:309–313. [Google Scholar]
- Kesselmeier J., Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J. Atmos. Chem. 1999;33:23–88. [Google Scholar]
- Kesselmeier J., Ciccioli P., Kuhn U., Stefani P., Biesenthal T., Rottenberger S., Wolf A., Vitullo M., Valentini R., Nobre A. Volatile organic compound emissions in relation to plant carbon fixation and the terrestrial carbon budget. Global Biogeochem. Cycles. 2002;16:73-71–73-79. [Google Scholar]
- Körner C. Carbon limitation in trees. J. Ecol. 2003;91:4–17. [Google Scholar]
- Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer; 2003. The climate plants experience; pp. 31–46. [Google Scholar]
- Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer; 2003. Climatic stress; pp. 101–119. [Google Scholar]
- Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer; 2003. Uptake and loss of carbon; pp. 171–200. [Google Scholar]
- Körner C. 2019. Plant Adaptations to Alpine Environments. [Google Scholar]
- Kramshøj M., Vedel-Petersen I., Schollert M., Rinnan Å., Nymand J., Ro-Poulsen H., Rinnan R. Large increases in Arctic biogenic volatile emissions are a direct effect of warming. Nat. Geosci. 2016;9:349. [Google Scholar]
- Laothawornkitkul J., Taylor J.E., Paul N.D., Hewitt C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009;183:27–51. doi: 10.1111/j.1469-8137.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- Leigh A., Sevanto S., Ball M.C., Close J.D., Ellsworth D.S., Knight C.A., Nicotra A.B., Vogel S. Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol. 2012;194:477–487. doi: 10.1111/j.1469-8137.2012.04058.x. [DOI] [PubMed] [Google Scholar]
- Leuning R. Leaf energy balances: developments and applications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1989;324:191–206. [Google Scholar]
- Li T., Holst T., Michelsen A., Rinnan R. Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat. Plants. 2019;5:568–574. doi: 10.1038/s41477-019-0439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-S., Medlyn B.E., Ellsworth D.S. Temperature responses of leaf net photosynthesis: the role of component processes. Tree Physiol. 2012;32:219–231. doi: 10.1093/treephys/tpr141. [DOI] [PubMed] [Google Scholar]
- Lindwall F., Faubert P., Rinnan R. Diel variation of biogenic volatile organic compound emissions-a field study in the sub, low and high arctic on the effect of temperature and light. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123610. e0123610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall F., Schollert M., Michelsen A., Blok D., Rinnan R. Fourfold higher tundra volatile emissions due to arctic summer warming. J. Geophys. Res. Biogeosci. 2016;121:895–902. [Google Scholar]
- Lizaso J., Batchelor W., Boote K., Westgate M. Development of a leaf‐level canopy assimilation model for CERES‐Maize. Agron. J. 2005;97:722–733. [Google Scholar]
- Loreto F., Sharkey T.D. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- Loreto F., Ciccioli P., Cecinato A., Brancaleoni E., Frattoni M., Tricoli D. Influence of environmental factors and air composition on the emission of [alpha]-pinene from Quercus ilex leaves. Plant Physiol. 1996;110:267–275. doi: 10.1104/pp.110.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luomala E.M., Laitinen K., Sutinen S., Kellomäki S., Vapaavuori E. Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 2005;28:733–749. [Google Scholar]
- Maja M.M., Kasurinen A., Holopainen T., Kontunen-Soppela S., Oksanen E., Holopainen J.K. Volatile organic compounds emitted from silver birch of different provenances across a latitudinal gradient in Finland. Tree Physiol. 2015;35:975–986. doi: 10.1093/treephys/tpv052. [DOI] [PubMed] [Google Scholar]
- Metcalfe D.B., Hermans T.D., Ahlstrand J., Becker M., Berggren M., Björk R.G., Björkman M.P., Blok D., Chaudhary N., Chisholm C. Patchy field sampling biases understanding of climate change impacts across the Arctic. Nat. Ecol. Evol. 2018;2:1443–1448. doi: 10.1038/s41559-018-0612-5. [DOI] [PubMed] [Google Scholar]
- Molau U. Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: cassiope tetragona and Ranunculus nivalis. Glob. Change Biol. 1997;3:97–107. [Google Scholar]
- Monson R.K., Fall R. Isoprene emission from aspen leaves: influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 1989;90:267–274. doi: 10.1104/pp.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson R.K., Jaeger C.H., Adams W.W., Driggers E.M., Silver G.M., Fall R. Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol. 1992;98:1175–1180. doi: 10.1104/pp.98.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka H., Uchida M., Mishio M., Nakatsubo T., Kanda H., Koizumi H. Leaf photosynthetic characteristics and net primary production of the polar willow (Salix polaris) in a High Arctic polar semi-desert, Ny-Ålesund, Svalbard. Can. J. Bot. 2002;80:1193–1202. [Google Scholar]
- Niinemets Ü., Loreto F., Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets U., Kuhn U., Harley P.C., Staudt M., Arneth A., Cescatti A., Ciccioli P., Copolovici L., Geron C., Guenther A., Kesselmeier J., Lerdau M.T., Monson R.K., Penuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Ortega J., Helmig D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques–part A. Chemosphere. 2008;72:343–364. doi: 10.1016/j.chemosphere.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Ortega J., Helmig D., Daly R.W., Tanner D.M., Guenther A.B., Herrick J.D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques - part B: applications. Chemosphere. 2008;72:365–380. doi: 10.1016/j.chemosphere.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Pattison R.R., Welker J.M. Differential ecophysiological response of deciduous shrubs and a graminoid to long-term experimental snow reductions and additions in moist acidic tundra, Northern Alaska. Oecologia. 2014;174:339–350. doi: 10.1007/s00442-013-2777-6. [DOI] [PubMed] [Google Scholar]
- Peñuelas J., Staudt M. BVOCs and global change. Trends Plant Sci. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Pollastri S., Tsonev T., Loreto F. Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J. Exp. Bot. 2014;65:1565–1570. doi: 10.1093/jxb/eru033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E., Alley R.B., Christensen T.R., Macias-Fauria M., Forbes B.C., Gooseff M.N., Iler A., Kerby J.T., Laidre K.L., Mann M.E. The polar regions in a 2° C warmer world. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw9883. eaaw9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney T.G., Blazich F.A., Warren S.L. Heat tolerance of selected species and populations of Rhododendron. J. Am. Soc. Hortic. Sci. 1995;120:423–428. [Google Scholar]
- Rasulov B., Bichele I., Hüve K., Vislap V., Niinemets Ü. Acclimation of isoprene emission and photosynthesis to growth temperature in hybrid aspen: resolving structural and physiological controls. Plant Cell Environ. 2015;38:751–766. doi: 10.1111/pce.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P.B., Sendall K.M., Stefanski A., Rich R.L., Hobbie S.E., Montgomery R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature. 2018;562:263–267. doi: 10.1038/s41586-018-0582-4. [DOI] [PubMed] [Google Scholar]
- Rinnan R., Michelsen A., Jonasson S. Effects of litter addition and warming on soil carbon, nutrient pools and microbial communities in a subarctic heath ecosystem. Appl. Soil Ecol. 2008;39:271–281. [Google Scholar]
- Rinnan R., Rinnan Å., Faubert P., Tiiva P., Holopainen J.K., Michelsen A. Few long-term effects of simulated climate change on volatile organic compound emissions and leaf chemistry of three subarctic dwarf shrubs. Environ. Exp. Bot. 2011;72:377–386. [Google Scholar]
- Rosbakh S., Römermann C., Poschlod P. Specific leaf area correlates with temperature: new evidence of trait variation at the population, species and community levels. Alp. Bot. 2015;125:79–86. [Google Scholar]
- Schollert M., Kivimäenpää M., Valolahti H.M., Rinnan R. Climate change alters leaf anatomy, but has no effects on volatile emissions from arctic plants. Plant Cell Environ. 2015;38:2048–2060. doi: 10.1111/pce.12530. [DOI] [PubMed] [Google Scholar]
- Schollert M., Kivimäenpää M., Michelsen A., Blok D., Rinnan R. Leaf anatomy, BVOC emission and CO2 exchange of arctic plants following snow addition and summer warming. Ann. Bot. 2017;119:433–445. doi: 10.1093/aob/mcw237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seco R., Holst T., Matzen M.S., Westergaard-Nielsen A., Li T., Simin T., Jansen J., Crill P., Friborg T., Rinne J., Rinnan R. Volatile organic compound fluxes in a subarctic peatland and lake. Atmos. Chem. Phys. Discuss. 2020;2020:1–32. [Google Scholar]
- Sharkey T.D., Monson R.K. Isoprene research–60 years later, the biology is still enigmatic. Plant Cell Environ. 2017;40:1671–1678. doi: 10.1111/pce.12930. [DOI] [PubMed] [Google Scholar]
- Singsaas E., Sharkey T. The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ. 1998;21:1181–1188. [Google Scholar]
- Singsaas E.L., Laporte M.M., Shi J.-Z., Monson R.K., Bowling D.R., Johnson K., Lerdau M., Jasentuliytana A., Sharkey T.D. Kinetics of leaf temperature fluctuation affect isoprene emission from red oak (Quercus rubra) leaves. Tree Physiol. 1999;19:917–924. doi: 10.1093/treephys/19.14.917. [DOI] [PubMed] [Google Scholar]
- Spitters C., Toussaint H., Goudriaan J. Separating the diffuse and direct component of global radiation and its implications for modeling canopy photosynthesis Part I. Components of incoming radiation. Agric. For. Meteorol. 1986;38:217–229. [Google Scholar]
- Spitters C., Toussaint H., Goudriaan J. Separating the diffuse and direct component of global radiation and its implications for modeling canopy photosynthesis Part II. Calculation of canopy photosynthesis. Agric. For. Meteorol. 1986;38:231–242. [Google Scholar]
- Tang J., Schurgers G., Valolahti H., Faubert P., Tiiva P., Michelsen A., Rinnan R. Challenges in modelling isoprene and monoterpene emission dynamics of Arctic plants: a case study from a subarctic tundra heath. Biogeosciences. 2016;13:6651–6667. [Google Scholar]
- Tang J., Valolahti H., Kivimäenpää M., Michelsen A., Rinnan R. Acclimation of biogenic volatile organic compound emission from subarctic heath under long‐term moderate warming. J. Geophys. Res. Biogeosci. 2018;123:95–105. [Google Scholar]
- Tiiva P., Faubert P., Michelsen A., Holopainen T., Holopainen J.K., Rinnan R. Climatic warming increases isoprene emission from a subarctic heath. New Phytol. 2008;180:853–863. doi: 10.1111/j.1469-8137.2008.02587.x. [DOI] [PubMed] [Google Scholar]
- Valkama E., Salminen J.P., Koricheva J., Pihlaja K. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann. Bot. 2003;91:643–655. doi: 10.1093/aob/mcg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valolahti H., Kivimäenpää M., Faubert P., Michelsen A., Rinnan R. Climate change‐induced vegetation change as a driver of increased subarctic biogenic volatile organic compound emissions. Glob. Change Biol. 2015;21:3478–3488. doi: 10.1111/gcb.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel-Petersen I., Schollert M., Nymand J., Rinnan R. Volatile organic compound emission profiles of four common arctic plants. Atmos. Environ. 2015;120:117–126. [Google Scholar]
- Westoby M., Falster D.S., Moles A.T., Vesk P.A., Wright I.J. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002;33:125–159. [Google Scholar]
- Wittmann C., Pfanz H. Temperature dependency of bark photosynthesis in beech (Fagus sylvatica L.) and birch (Betula pendula Roth.) trees. J. Exp. Bot. 2007;58:4293–4306. doi: 10.1093/jxb/erm313. [DOI] [PubMed] [Google Scholar]
- Wright I.J., Reich P.B., Westoby M., Ackerly D.D., Baruch Z., Bongers F., Cavender-Bares J., Chapin T., Cornelissen J.H., Diemer M. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.