Highlights
-
•
Reward reduced RT interference during reactive control, but increased RT interference during proactive control in male adolescents.
-
•
Increased reward-related cue-locked theta power was associated with increased RT interference on proactive trials.
-
•
Increased reward-related stimulus-locked theta inter-channel phase synchrony was related to facilitated performance on proactive trials.
Keywords: Proactive control, Reactive control, Theta, Adolescence, Cognitive control
Abstract
Adolescence is marked by increased reward-seeking, which can alter cognitive control abilities. Previous research found that rewards actually improve cognitive control in children, adolescents, and adults, but these studies only investigated reactive control. The goal of the current study was to elucidate reward’s influence on both proactive and reactive control during adolescence. To this end, 68 (Mean age = 13.61, SD = 2.52) male adolescents completed a rewarded cued flanker paradigm while electroencephalogram (EEG) was collected. Theta power and inter-channel phase synchrony, both implicated in cognitive control, were quantified after cues and stimuli to understand their role during reward-cognitive control interactions. The data suggest that reward reduced interference during reactive control; however, reward increased interference during proactive control in this sample of adolescent males. Reward-related increases in cue-locked theta power predicted more reward-related RT interference on proactive trials. In contrast, increases in stimulus-locked theta ICPS were associated with better performance on rewarded proactive trials. The pattern of results show that reward differentially impacted proactive and reactive control in adolescence, which may have implications for the increased risk-taking behaviors observed during adolescence.
1. Introduction
Adolescence is a time period of marked physiological and neural development and typically includes increases in risky decision making and sensation seeking (Cauffman et al., 2010; Shulman et al., 2016; Steinberg et al., 2008). Increases in risk-taking have been posited to be related to an imbalance in the development of reward processes compared to cognitive control processes. While reward centers of the brain, including striatal and limbic regions, peak in their development in adolescence (Silverman et al., 2015; Van Leijenhorst et al., 2010), areas of the brain involved in cognitive control, including cingulate and prefrontal cortices, continue to develop throughout adolescence into early adulthood (Galvan et al., 2006; Somerville and Casey, 2010a). Additionally, connectivity within and between brain networks changes during adolescence (Kelly et al., 2009; Larsen et al., 2018; Supekar et al., 2009). Reward can motivate cognitive control, so understanding reward-cognitive control interactions may reveal important insights into the neural underpinnings of risk-taking in adolescence.
Cognitive control, the ability to monitor salient events and respond advantageously to reach a certain goal, develops through adolescence into adulthood (Durston et al., 2006, 2002; Luna et al., 2004, Luna et al., 2001). Reward enhances cognitive control in children, adolescents, and adults (Chung et al., 2011; Zhai et al., 2015). Adolescents perform just as well as adults on rewarded trials of antisaccade tasks (Geier et al., 2010; Hallquist et al., 2018; Padmanabhan et al., 2011). Though performance levels are similar between adolescents and adults, adolescents still show signs of neural immaturity during rewarded cognitive control tasks. Adolescents display increased striatal activity, not observed in children or adults, during anticipation and receipt of rewards (Ernst et al., 2005; Geier et al., 2010; Padmanabhan et al., 2011). Moreover, control-related regions continue developing and connectivity between regions strengthens throughout adolescence (Luna et al., 2001; Ordaz et al., 2013). Recent work has emphasized the importance of network connectivity in the development of cognitive control and reward-cognitive control interactions (Somerville and Casey, 2010a). Corticostriatal connectivity between reward-related striatal areas and control-related cortical areas is weaker in adolescents compared to adults, especially under high stakes (Insel et al., 2017). Thus, both cortical activation and network integration are undergoing important changes during rewarded cognitive control in adolescence.
According to the Dual Mechanisms of Control theory, cognitive control can operate via proactive and/or reactive control (Braver, 2012). Reactive control involves later reflexive processing of information in a just-in-time manner after the appearance of an event (stimulus) requiring the allocation of control; whereas, proactive control involves early, future-oriented, and planful strategies allocated prior to an event (stimulus) requiring control. Thus, proactive control involves allocating control prior to stimulus presentation in order to avoid or reduce conflict, while reactive control involves resolving conflict after it occurs. In particular, proactive control can be engaged after an informative cue (e.g., a certain shape indicates what type of trial is next) as a preparatory mechanism. Both proactive and reactive control develop as early as 4–5 years old (Elke and Wiebe, 2017; Grammer et al., 2014); however, younger children tend to favor reactive control with proactive control engagement developing into adolescence (Chevalier et al., 2015; Munakata et al., 2012; Troller‐Renfree et al., 2020).
In the presence of rewards, adults tend to enhance proactive control in preparation for events of interest (Soutschek et al., 2014), an effect driven by areas in the prefrontal cortex (Jimura et al., 2010). Indeed, after informative cues adults upregulate proactive control to reduce interference (Chiew and Braver, 2013, 2016). Critically, the majority of research surrounding reward’s influence on cognitive control during adolescence has focused on reactive control leaving an important gap in the literature. The few studies investigating adolescents’ use of proactive control under rewarded conditions have found that reward does promote the use of a proactive control strategy (Jin et al., 2020) and increase neural activity in preparatory phases before a stimulus (Magis-Weinberg et al., 2019; Strang and Pollak, 2014); however, these studies have not explicitly tested proactive control after informative cues. While it is clear that the balance between proactive and reactive control is sensitive to motivational factors, further elucidating how rewards impact preparation after informative cues could lend insight into interactions between reward and cognitive control circuitry in adolescence and serve as proxy measurements of why adolescents engage in more risky behaviors (Luciana and Collins, 2012; Luna et al., 2013; Somerville and Casey, 2010b; Steinberg, 2008).
While most studies examining the development of cognitive control have focused on fMRI, electroencephalogram (EEG) can measure theta oscillations (4−8 Hz), which are widely implicated in cognitive control processes in adults (Cavanagh and Frank, 2014; Cavanagh et al., 2012; Cohen and Donner, 2013; Nigbur et al., 2012; van Driel et al., 2015) and have only recently begun to be investigated in adolescence (Bowers et al., 2018; Buzzell et al., 2019; Crowley et al., 2014; Liu et al., 2014). Theta oscillations can index both proactive and reactive control processes by measuring theta activity to informative cues that allow for proactive control or to non-cued stimuli that engage reactive control. During proactive control, informative cues produce an increased theta power response in adults (Cavanagh et al., 2012; Cooper et al., 2016) and in adolescents (Mazaheri et al., 2014). Theta inter-channel phase synchrony (ICPS) between frontal sites increases after informative cues that facilitate proactive control (Cooper et al., 2015). During reactive control, un-cued stimuli with increased levels of conflict elicit increased theta power (Cohen and Donner, 2013; Hanslmayr et al., 2008; Harper et al., 2014; Lavallee et al., 2014). There is also evidence that theta ICPS is increased after high conflict stimuli (Aviyente et al., 2017; Gulbinaite et al., 2014), reflective of reactive control. Quantifying theta power and ICPS, both to cues and stimuli, could reveal important distinctions regarding how rewards affect these neural mechanisms differently during proactive vs reactive control.
Though work in adults implicates reward as a strong influencer of proactive and reactive control, less work has addressed this interaction from a developmental perspective. The interaction between reward and cognitive control is especially important in adolescence when control circuits are still developing but reward sensitivity is heightened, which has implications for risk-taking in adolescence. The current study examined the impact of reward’s influence on proactive control after informative cues and reactive control after uninformative cues throughout adolescence. Moreover, the current study investigated reward’s influence on theta dynamics during proactive and reactive control. To this end, a sample of male adolescents completed a rewarded cued flanker paradigm while EEG was collected. Males only were recruited in order to remove possible effects of differences between reward sensitivity or pubertal development between sexes. We hypothesized that the presence of rewards would yield an increase in reactive control, as indicated by smaller flanker effects following un-cued stimuli. Additionally, we hypothesized that rewards would also lead to enhanced proactive control after informative cues in adolescents based on prior work in adults (Chiew and Braver, 2016; Jimura et al., 2010). We also tested whether cue- or stimulus-locked theta power and ICPS were associated with reward-related changes in proactive and reactive control.
2. Materials and methods
2.1. Participants
Male participants aged 9–17 were recruited to participate in the study. To mitigate the possibility of heterogeneous effects due to gender differences in reward sensitivity and pubertal development, the sample was restricted to only male because males, in particular, display increased risky behaviors (Li et al., 2007; Torrubia et al., 2001; van Hemel-Ruiter et al., 2015). Male children and adolescents were recruited using the Infant and Child Studies database at the University of Maryland. The University of Maryland Institutional Review Board approved all procedures.
The sample consisted of 76 male participants. Parents of all participants reported no birth defects or current diagnoses, no visual/uncorrected visual impairment, and no allergies to salts/plastics/latex. Eight participants were excluded for various reasons including developmental delays not reported at screening (n = 2), poor (<60 %) accuracy on task baseline (n = 5), and too few stimulus-locked trials (<8) after EEG cleaning (n = 1). The final sample consisted of 68 neurotypical males (Mage = 13.61, SD = 2.52, Range = 9.09–17.84 years). Puberty scores were collected, but because they were highly correlated with age in this sample of male adolescents, r = 0.83, p < .001, exploratory analyses involving puberty are in the supplement. Table 1 details information about demographics of the final sample.
Table 1.
Sample Demographics, N = 68.
| Mean Age (Years) | 13.61 (2.52) |
|---|---|
| Race/Ethnicity | |
| Black/African American | 17 (25.0 %) |
| Asian | 5 (7.4 %) |
| Caucasian | 30 (44.1 %) |
| Hispanic | 4 (5.9 %) |
| Biracial | 12 (17.6 %) |
| Mother’s Education Level | |
| High School Graduate | 2 (2.9 %) |
| Associates Degree | 3 (4.4 %) |
| College Graduate | 16 (23.5 %) |
| Graduate Degree | 45 (66.2 %) |
| Other | 1 (1.5 %) |
| Unknown | 1 (1.5 %) |
| Median Annual Household Income | $135,000 |
2.2. Procedures
All parents of child and adolescent participants provided informed consent and all child and adolescent participants provided assent. Participants were seated about 70 cm in front of the presentation computer. Then, participants completed two blocks of a rewarded cued flanker paradigm: a baseline block without any possibility of reward and a reward block. All tasks were presented in E-Prime 2.0.10 (Psychology Software Tools, Pittsburgh, PA). EEG was recorded throughout both blocks using a 128-channel HydroCel Geodesic net, a NetAmps 400 Amplifier, and Netstation 5.2 software (EGI, Inc; Eugene, OR).
2.3. Rewarded cued flanker paradigm
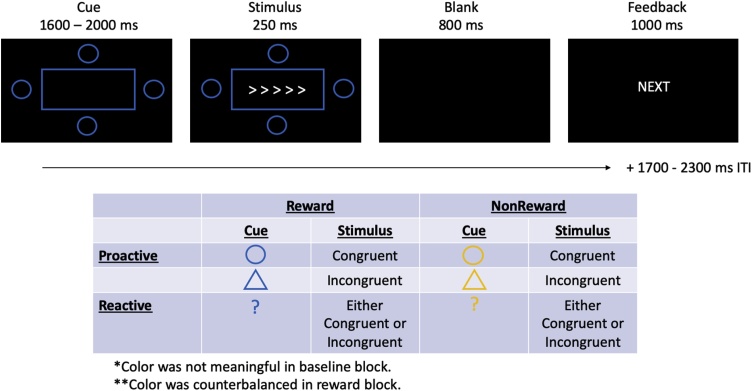
The rewarded cued flanker paradigm (Fig. 1) was adapted from Chiew and Braver (2016). In this paradigm, participants were presented with an array of arrows. They were instructed to press a button on a button box depending on the direction of the center arrow as quickly and as accurately as possible. In 50 % of the trials, the arrows were congruent and in the other 50 % of trials, the arrow was incongruent, or the middle arrow was facing a direction than the flanking arrows.
Fig. 1.
Rewarded Cued Flanker Paradigm.
Before the arrows were presented, the participants were given informative cues to indicate which trial type (i.e., congruent or incongruent) was about to be presented. A box was presented in the center of the screen with a cue image on each side of the box. If a circle was presented, the upcoming trial was congruent. If a triangle was presented, the upcoming trial was incongruent. If a question mark was presented, then the participant was unaware of what trial type was to be presented. Trials with informative cues that were predictive of the type of stimulus that was shown (i.e., circle and triangle) indexed proactive control because participants could prepare for the upcoming stimulus. In particular, participants could narrow the focus of attention to the central arrows on incongruent trials (preventing/reducing conflict), but widen the focus of attention to include peripheral arrows on congruent trials (increase facilitation). On the other hand, trials with non-informative question mark cues indexed reactive control, as participants had no knowledge of the upcoming stimulus identity, and therefore were unable to prepare control in a planful manner, instead requiring them to reflexively react once the stimulus was presented. The mapping of the cue shape and stimulus type was explicitly stated to the participant and the participant completed a cue “quiz” before the start of the experiment to ensure correct cue mapping.
In addition to the informative nature of the cue shape, the cue color informed the participant about the potential for reward. Cues (circle, triangle, question mark) could either be blue or orange, denoting reward possible or no reward possible. These colors were counterbalanced across participants. Participants received a reward of $0.10 on rewarded trials if they were both accurate and fast enough (below baseline RT cutoff).
Participants completed a practice block, a baseline block, and a reward block. In the practice and baseline blocks, both blue and orange cues were presented, but the participant was not told about the meaning of the colors. First, the participants practiced and did not advance to the baseline block until they reached 60 % accuracy to ensure that they understood the task. In the baseline block, a mean RT of correct trials was calculated and used as a RT cutoff in the reward block. Next, participants completed the reward block, which contained both a trial-level reward manipulation and the trial-level information manipulation. The participants were notified that they could earn rewards on some trials, while on other trials, no reward was possible. Reward was designated by color – either blue or orange depending on counterbalance – and participants were told that they must respond to the flanker array correctly and quickly (e.g., before the RT cutoff) in order to collect the reward. The participant did not know their actual reaction time cutoff; they just knew that they needed to respond quickly. The baseline block consisted of 96 trials and the reward block consisted of 288 trials.
The participants were given trial-level feedback based on their responses. In the baseline block, if the response was incorrect (error of commission), feedback read “WRONG” in red font. If the trial was skipped (error of omission), feedback read “SLOW” in red font. If the participant made the correct response, feedback read “NEXT” in white font was presented. In the reward block, “WRONG” and “SLOW” feedback were still presented for errors of commission and omission, respectively. For the non-rewarded trials, “NEXT” was presented when the response was correct below the RT cutoff or correct above the RT cutoff. For rewarded trials, “$$$$” was presented in green when the response was correct and below the RT cutoff. Otherwise, “NEXT” was presented when the response was correct, but above the RT cutoff. “$$$$” denoted that the participant had earned $0.10. Participants were told how much money they had earned during their breaks.
In both blocks, the timing of the trials was consistent. The cue was presented for 1600–2000 ms, followed by the flanker array for 250 ms. The arrows disappeared after 250 ms, but the participant could still respond during a blank screen for the next 800 ms. Then, the feedback appeared for 1000 ms, followed by an ITI of 1700–2300 ms.
2.4. Characterization of proactive and reactive control
Behavioral data were cleaned by removing any anticipatory responses (<150 ms RT) and any trials that were deemed outliers – having an RT two standard deviations above the mean. Accuracy was scored as percent errors and RT was scored as mean RT. Studies of flanker tasks show that typical behavior is to slow down and respond less accurately on incongruent trials compared to congruent trials (Eriksen and Eriksen, 1974). This phenomenon is known as the “flanker effect” and is indicative of larger interference from the flanking arrows on incongruent trials. Thus, the dependent measure for analyses was the flanker effect, or interference, a difference score between incongruent and congruent trials for the measure of interest. After calculating interference scores, outliers +/−3 SD above the mean were removed for accuracy and RT by condition (reward proactive, reward reactive, nonreward proactive, nonreward reactive).
2.5. Task effort survey
After both the baseline block and the reward block, participants answered six questions about their performance. The first two questions asked the participant about overall motivation in that block: “How motivated were you to do well?” and “How motivated were you to pay attention?” on a scale from 0 (not motivated) to 10 (extremely motivated). For the following four questions, the participant used a scale from 0 (not hard) to 10 (extremely hard). The four questions were: “How hard did you try to be correct after blue shapes?”, “How hard did you try to be correct after orange shapes?”, “How hard did you try to go fast after blue shapes?”, and “How hard did you try to go fast after orange shapes?”
2.6. Electroencephalography (EEG)
2.6.1. Acquisition and preprocessing
During the rewarded cued flanker paradigm, continuous EEG was recorded from a 128-channel HydroCel Geodesic Sensor Net. The data were sampled at 500 Hz and referenced to Cz online. All electrode impedances were below 50 kΩ prior to data collection. EEG analysis was conducted off-line using The Maryland Analysis of Developmental EEG (MADE) Pipeline (Debnath et al., 2020), a standardized EEG pipeline based on MATLAB 2014b (MathWorks, Inc., Natick, MA) and the EEGLab Toolbox (Delorme and Makeig, 2004). Data were high-pass filtered at 0.3 Hz and low-pass filtered at 49 Hz. The FASTER plugin for EEGLab (Nolan et al., 2010) identified bad channels (MeanNumber of Bad Channels = 5.32, SD = 2.69, Range = 1–14). No participant had greater than 12 % bad channels. To identify artifacts in the data, independent components analysis (ICA) was performed on a copy of the dataset that was filtered with a 1 Hz high-pass filter. Prior to ICA decomposition, the copied data were epoched into arbitrary 1 s epochs for the purpose of detecting and removing portions of the EEG data contaminated with significant artifact. An initial rejection of noisy EEG data was performed using a combined voltage threshold rejection of ±1000 μV to remove disconnected channels and a spectral threshold rejection using a 30 dB threshold within the 20–40 Hz band to remove EMG-like activity (EEGLAB pop_rejspec function; Delorme and Makeig, 2004). If artifact rejection rejected >20 % of epochs for a given channel, this channel was removed from both the 1 Hz high-pass dataset and the 0.1 Hz high-pass ERP dataset. ICA weights from the ICA run on the copied (1 Hz) dataset were then copied back to the continuous 0.3 Hz high-passed data. The adjusted-ADJUST Matlab scripts (Leach et al., 2020; Mognon et al., 2011) identified artifactual independent components, which were then removed from the data. The data were epoched from −1000 ms before to 2000 ms after both the cue and the stimulus. A rejection threshold of +/−125 μV based on ocular electrodes (E8, E25 E127, E126) identified and rejected any ocular artifacts that may have been missed during previous processing steps. After rejection of epochs containing residual ocular artifacts, epochs containing channels with voltage +/−125 μV were interpolated at the channel level unless more than 10 % of channels exceeded this threshold within a given epoch, in which case the epoch was rejected instead. Channels that exceeded the ±125 μV threshold for greater than 20 % of epochs were removed from the dataset. Finally, any missing or removed channels were interpolated using a spherical spline interpolation and data were re-referenced to the average of all electrodes. The epoched data were filtered with a surface Laplacian filter in order to minimize volume conduction over the scalp by filtering out spatially broad features of the data (Cohen, 2014) in order to improve both spatial and functional specificity of brain activity (Kamarajan et al., 2015; Tenke and Kayser, 2012). For both cue-locked and stimulus-locked epochs, each condition had to have at least 8 trials to be included.
2.6.2. Time frequency analyses
2.6.2.1. Theta power
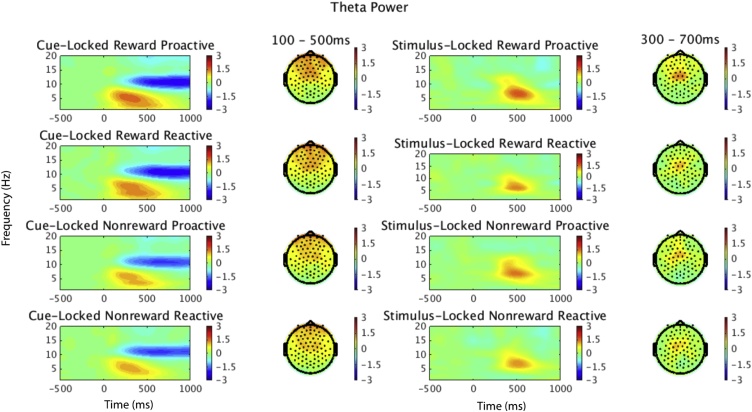
Theta power in each epoch of interest (cue-locked and stimulus-locked) was computed using custom MATLAB scripts (Mike X Cohen, 2014). Event related spectral perturbation (ERSP) was calculated for the epoched data. ERSP provides an estimate of average changes in spectral power (in dB) relative to a baseline period (Delorme and Makeig, 2004). Each CSD converted epoch was convolved with Morlet wavelets, which estimated spectral power in the frequency range 1–30 Hz. To optimize the time-frequency resolution, wavelet cycles were set at 3 cycles at the lowest frequency (1 Hz) increasing to 10 cycles at the highest frequency (30 Hz). ERSPs were computed for all channels and separately for the four cue-locked conditions (proactive reward, reactive reward, proactive nonreward, reactive nonreward) and for the eight stimulus-locked conditions (proactive reward incongruent and congruent, reactive reward incongruent and congruent, proactive nonreward incongruent and congruent, reactive nonreward incongruent and congruent). ERSPs were calculated for each epoch relative to a baseline period of −400 to −100 ms before the onset of either the cue or the stimulus (Fig. 2).
Fig. 2.
Theta Power. Time frequency surfaces and topographic plots for each condition. Topoplots are 4-8 Hz in the time ranges denoted. ROIs were determined based on an average of all conditions. Note that stimulus-locked epochs are flanker effects (incongruent - congruent).
To choose the time window of the region of interest (ROI), cue-locked theta power was averaged over all conditions. Because stimulus-locked theta power was analyzed as a flanker effect (incongruent – congruent), that subtraction was averaged over the four conditions (reward proactive, reward reactive, nonreward proactive, nonreward reactive) to choose the ROI. The ROIs of interest for theta (4−8 Hz) activity were 100−500 ms for cue-locked theta power and 300−700 ms for stimulus-locked theta power at a frontocentral cluster (E12, E5, E6, E13, E112, E7, E106; see Fig. S1).
2.6.2.2. Theta inter-channel phase synchrony (ICPS)
Theta ICPS is a measure of the consistency of phase oscillations between two channels (or clusters of channels) over time and frequency (Cohen, 2014). Here, ICPS was calculated as follows:
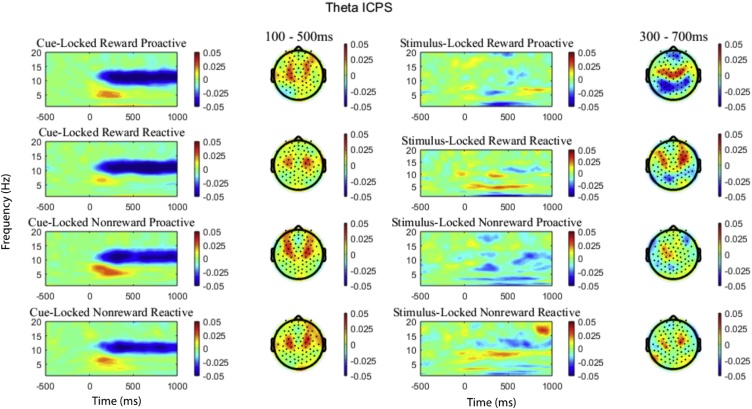
where n is the number of trials for each time and each frequency band, x and x are the phase angles of electrodes x and y at frequency f and time t. ei is from Euler's formula and provided complex polar representation of phase angle difference (Cohen, 2014). Phase angles were calculated from two electrodes and then subtracted. An ICPS value closer to 1 indicated that the phase angles from two channels were completely synchronized, whereas an ICPS value close to 0 indicated random phase angle difference between two channels (Cavanagh et al., 2009). We chose to keep the ROIs for theta ICPS consistent with the time windows used for theta power: 100−500 ms for cue-locked and 300−700 ms for stimulus-locked. Those ROIs were extracted at channel clusters overlying medial and lateral frontal areas in both the left and right hemispheres (see Fig. S1). Fig. 3 depicts time frequency and topographic plots for ICPS in each condition.
Fig. 3.
Theta ICPS. Time frequency surfaces and topographic plots for each condition. Topoplots are 4-8 Hz in the time ranges denoted. ROIs were determined based on an average of all conditions.
Additionally, outliers +/-3 SD above the mean were removed. For theta power, the outliers were removed within condition, while for theta ICPS, outliers were removed based on both condition and hemisphere. Paired t-tests revealed that right and left lateralized cue-locked and stimulus-locked ICPS did not differ based on condition (p’s > 0.1). Thus, right and left theta ICPS were averaged within each condition to create a measure of medial-lateral theta ICPS (Buzzell et al., 2019).
2.7. Statistical approach
To investigate task effects, a series of 2-level multilevel models (MLMs) were performed using the lme4 package in R (Package “lme4,” 2020). In these analyses, a random intercept for each participant was estimated for each model using a variance components covariance. Behavioral and neural measurements had ICCs ranging from 0.23 to 0.68, which are above the recommended level (0.15–0.30), which suggested that MLMs are appropriate (Mathieu et al., 2012).
2.7.1. Confirming expected task effects
2.7.1.1. Subjective ratings of motivation
Analysis of the task effort survey data was done with a series of MLMs. First, responses about how hard participants tried to be correct and fast were compared for the reward and nonreward trials during the baseline block (note that these trials are designated reward and nonreward based on the participant’s assigned color for the reward block, but they did not know about the rewards during the baseline block). Reward was a fixed effect (−1 for nonreward and 1 for reward). Age was grand mean-centered and added as a predictor to quantify any age-related effects. A RewardxAge interaction was also examined. Within the baseline block, we expected no differences for nonreward and reward trials, as there was no meaning assigned to the colors in the baseline block.
Second, MLMs of responses to the two questions about overall motivation in the baseline block vs reward block were performed. Block was a fixed effect (−1 for baseline and 1 for reward). Again, grand mean-centered age and a BlockxAge interaction were added as predictors. We expected higher motivation ratings for the reward block compared to the baseline block.
A third series of MLMs was used to compare ratings of how hard participants tried to be correct and fast for rewarded trials to nonrewarded trials within the reward block. Nonreward trials were coded -1 and reward trials were coded 1. Grand mean-centered age and a RewardxAge interaction were examined. Within the reward block, we expected responses to indicate that participants tried harder to be correct and fast on rewarded trials compared to nonrewarded trials. We expected that these results would confirm that the rewarded trials in the reward block significantly increase self-reported motivation. Consistent with prior work, we anticipated that there would be no age-related changes in self-reported motivation (Geier and Luna, 2012; Paulsen et al., 2015).
2.7.2. Age-related changes in influence of reward on proactive and reactive control
Two MLMs were conducted to examine behavioral performance, one for accuracy and one for reaction time. The dependent variables were accuracy (percent errors) flanker effect and RT flanker effect. Reactive trials were designated -1 and proactive trials were coded as 1; Nonreward trials were coded -1 and reward trials were coded 1. Age was grand-mean centered and used as a continuous predictor. Interactions between all predictors were included in the model. Post-hoc tests of simple effects were used to explore significant interactions. MLMs exploring the effects of reward, control, and age on neural measures and exploring effects of puberty can be found in the supplement.
2.7.3. Brain-behavior relations
The next set of analyses explored relations between the four neural measures (cue-locked theta power, cue-locked theta ICPS, stimulus-locked theta power, and stimulus-locked theta ICPS) and task performance. To quantify the effect of reward, reward-related measures were calculated by subtracting the variable of interest in the nonrewarded trials from the variable of interest in the rewarded trials:
For cue-locked theta power and theta ICPS, a positive reward-related value denoted higher theta activity in the reward trials compared to the nonrewarded trials, indicating more reward-related theta activity. However, reward-related RT and stimulus-locked theta activity were calculated by subtracting flanker effects of the nonreward trials from the flanker effect of the reward trials. Thus, the interpretation of a positive reward-related value for these measures was more reward-related interference.
Two hierarchical regressions were used. Proactive and reactive trials were investigated separately to avoid a triple difference score. The first regression tested the predictive power of age and reward-related neural activity during proactive trials when predicting reward-related task performance on proactive trials. The second regression analyses tested how age and reward-related neural activity during reactive trials predicted reward-related task performance on reactive trials.
For each hierarchical regression, centered age was the first predictor entered. The second block of predictors were the neural measures and R2 change was assessed. The third block of predictors included the interactions between each neural measure and age and, again, R2 change was assessed. Outliers +/−3SD of reward-related scores were removed prior to hierarchical regressions; thus, listwise deletion was employed for these specific analyses due to missingness from outlier removal.
3. Results
3.1. Subjective motivation
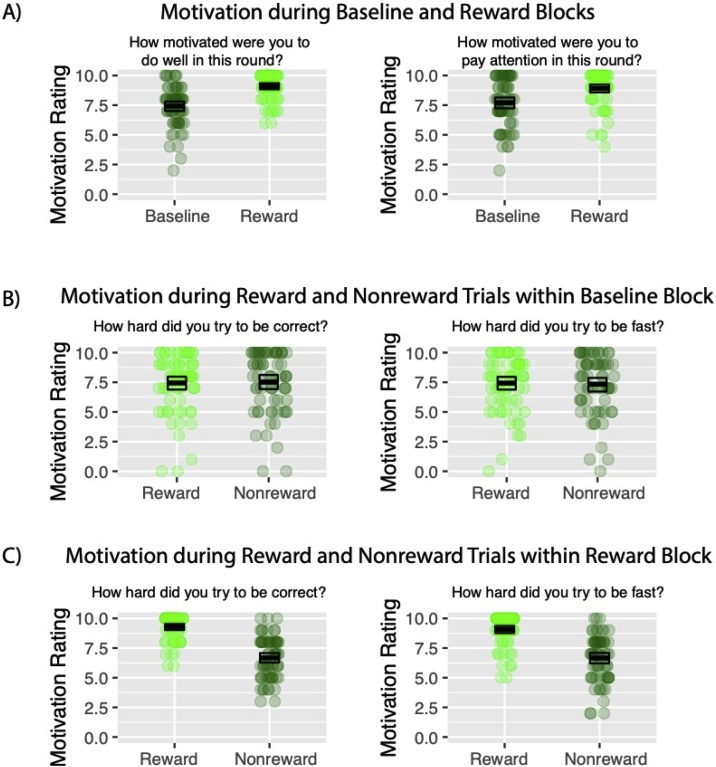
To assess differences in motivation during the task, responses on the Task Effort Survey were analyzed. During the baseline block, there were no differences between how hard participants tried to be correct, b = −0.04, t(66) = −0.58, p = 0.56, or to be fast, b = 0.04, t(66) = −0.69, p = 0.49, for reward compared to nonreward cues (Fig. 4B). Additionally, there were no effects of age or interactions between age and reward on ratings of motivation during the baseline block.
Fig. 4.
Results from the Task Effort Survey. A) Comparison of overall motivation during baseline block compared to reward block. B) Comparison of rewarded trials and nonrewarded trials in the baseline block. C) Comparison of rewarded trials vs nonrewarded trials during the reward block.
Comparing motivation in the baseline block to the reward block, participants reported that they were more motivated to do well in the reward block compared to the baseline block, t(66) = 9.87, p < 0.001 (Fig. 4A). Further, there was a BlockxAge interaction for how motivated the participants were to do well in the block, t(66) = −3.83, p < 0.001. Specifically, higher ratings of motivation to do well were associated with increasing age in the baseline block only, r(66) = .42, p < 0.001, while there was no association between age and motivation to do well in the reward block, r(66) = 0.05, p = 0.66. Moreover, participants were more motivated to pay attention in the reward block compared to the baseline block, t(66) = 5.78, p < 0.001 (Fig. 4A). These effects were not moderated by age.
Finally, within the reward block, participants reported that they tried harder to be correct on the rewarded trials compared to the nonrewarded trials, t(66) = 11.46, p < 0.001 (Fig. 4C). Moreover, there was a marginal RewardxAge interaction, t(66) = −2.00, p = 0.05. Though follow-up correlations were not significant, they suggested that increasing age was associated with trying less hard to be correct in the reward condition, r(66) = −0.19, p = 0.12, but with trying harder to be correct in the nonreward condition, r(66) = 0.14, p = 0.24. In addition, participants tried to be faster on the rewarded trials compared to the nonrewarded trials, t(66) = 10.00, p < 0.001 (Fig. 4C). This effect did not change with age. Overall, results from the task effort survey suggest that the participants were more motivated in the reward block compared to the baseline block and by the rewarded trials compared to nonrewarded trials.
3.2. Behavioral results
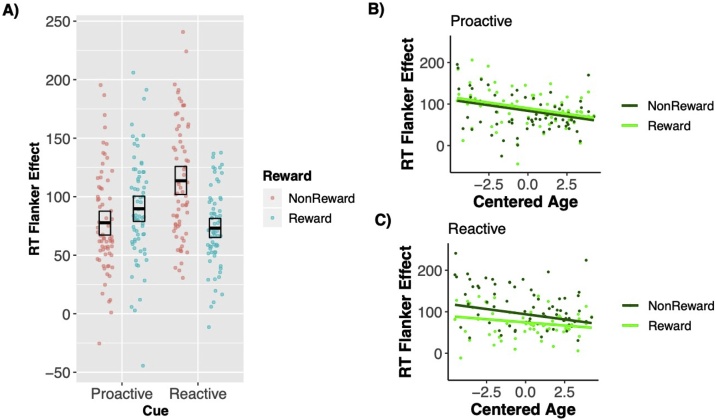
To examine the impact of reward, control (proactive vs reactive), and age on cognitive control, we focused on the flanker effect of RT and accuracy within the reward block only. For RT flanker effect, results revealed main effects of both reward, b = −6.77, t(196.85) = −5.35, p < 0.001, control, b = −5.16, t(196.85) = −4.08, p < 0.001, and age, b = −5.21, t(196.85) = −2.85, p = 0.006. Further, there was also a significant interaction between reward and control, b = 12.78, t(196.85) = 10.09, p < .001. As shown in Fig. 5A, in the reactive context, there was less RT interference on reward trials compared to nonreward trials, t(196.90) = −10.89 p < .001. However, in the proactive context, there was more interference on reward trials compared to nonreward trials, t(196.80) = 3.36, p < .001. In the nonreward condition, the proactive condition decreased RT interference compared to the reactive condition, t(196.80) = −10.04, p < 0.001. Whereas, in the reward condition, the proactive condition increased RT interference, t(196.90) = 4.24, p < 0.001.
Fig. 5.
Interaction between reward and control. A) In nonrewarded conditions, there was less interference on proactive trials compared to reactive trials. However, in rewarded conditions, there was more interference on proactive trials compared to reactive trials. B) No difference in the relation between age and RT flanker for reward vs nonreward conditions in the proactive context. C) Interaction between age and reward when predicting RT flanker effect.
Additionally, there was a marginal RewardxControlxAge interaction, b = −1.00, t(195.96) = −1.98, p = 0.05. As displayed on Fig. 5B and C, when probing this marginal three-way interaction, proactive and reactive were investigated separately. In proactive contexts, there was a main effect of age, b = −5.36, t(66)=−2.81, p = 0.007, and a main effect of reward, b=6.00, t(66) = 3.02, p = 0.004. These main effects suggest that proactive RT interference decreases with age but is increased in rewarded conditions. However, in reactive contexts, there was a significant reward x age interaction, b = 1.99, t(64.55) = 2.53, p = 0.01 (Fig. 5C). As seen in Fig. 5C, RT interference during nonrewarded reactive condition decreased with age, r(66)=−.36, p < 0.001, but there was no statistically significant relation between RT interference and age in the rewarded reactive condition, r(65)=−0.17, p = 0.17. This result provides some evidence that reward is more impactful in reducing reactive interference at younger ages.
Finally, we examined the same effects for the accuracy flanker effect, but there were no significant effects of reward, control, or age nor significant interactions.
3.3. Brain-behavior relations
Table 2 details the hierarchical regression predicting reward-related proactive RT interference. We chose to focus on RT interference only because there were no task effects on accuracy interference. Age alone was not a significant predictor. When adding the second block of predictors that included the four reward-related proactive EEG measures, R2 was significantly increased. Elevated reward-related proactive cue theta was associated with increased reward-related proactive RT interference, b = 12.66, p = 0.03. Additionally, increased reward-related proactive stimulus-locked theta ICPS interference predicts reduced reward-related proactive RT interference, providing evidence that increased theta IPCS to the stimulus after informative cues is associated with improvements in performance in rewarded vs nonrewarded conditions. The third block of predictors, including interactions with age, did not significantly increase R2, F(4,50) = 0.68, p = 0.61.
Table 2.
Hierarchical Multiple Regression Predicting Reward-Related Proactive RT Interference.
| Reward-Related Proactive RT Interference |
||||||
|---|---|---|---|---|---|---|
| Variable | Block 1 |
Block 2 |
||||
| B | SE (B) | B | SE(B) | |||
| Intercept | 13.53** | 3.93 | 0.00 | 12.09** | 3.97 | 0.00 |
| Age | −1.00 | 1.61 | −0.08 | −2.81† | 1.52 | −0.23 |
| Rew-Rel Pro Cue Power | 12.66* | 5.83 | 0.26 | |||
| Rew-Rel Pro Cue ICPS | −80.37 | 100.26 | −0.10 | |||
| Rew-Rel Pro Stim Power | −3.46 | 4.20 | −0.10 | |||
| Rew-Rel Pro Stim ICPS | −259.21** | 62.91 | −0.50 | |||
| F stat | 0.38 | 4.02** | ||||
| R2 % | 0.66 | 27.11 | ||||
| △ R2 F stat | 4.78** | |||||
R2 % is the percent of total variance explained. Block 3 is not displayed because it was not significant.
p < .10.
p < .05.
p < .01.
No block of predictors significantly predicted reward-related reactive RT interference (Table 3).
Table 3.
Hierarchical Multiple Regression Predicting Reward-Related Reactive RT Interference.
| Reward-Related Reactive RT Interference |
||||||
|---|---|---|---|---|---|---|
| Variable | Block 1 |
Block 2 |
||||
| B | SE(B) | B | SE(B) | |||
| Intercept | −35.46** | 3.98 | 0.00 | −34.82** | 4.38 | 0.00 |
| Age | 2.46 | 1.63 | 0.19 | 2.36 | 1.66 | 0.18 |
| Rew-Rel Re Cue Power | −0.84 | 5.86 | −0.02 | |||
| Rew-Rel Re Cue ICPS | 160.02 | 145.52 | 0.15 | |||
| Rew-Rel Re Stim Power | 1.74 | 4.02 | 0.06 | |||
| Rew-Rel Re Stim ICPS | 57.41 | 74.88 | 0.10 | |||
| F stat | 2.28 | 0.78 | ||||
| R2 | 3.78 | 6.77 | ||||
| △ R2 F stat | 0.44 | |||||
R2 is the percent of total variance explained. Block 3 is not displayed because it was not significant.
p < .01.
4. Discussion
Adolescence is a time period with marked development of both reward-related and cognitive control brain circuitry. Previous research has provided evidence that reward enhances reactive control in adolescence. The goal of the current study was to examine the effects of reward on proactive control after informative cues and reactive control after uninformative cues in males during adolescence. It was hypothesized that, similar to adults, reward would enhance proactive control after informative cues. Moreover, we expected such improvements in control would be facilitated by increases in theta power or ICPS. The current study provides evidence that reward impacts aspects of both proactive and reactive control in adolescent males, though in ways different than hypothesized. While reward reduced RT interference in reactive contexts, reward actually increased RT interference in proactive contexts. Further, increased reward-related cue theta power predicted increased RT interference in reward-related performance in proactive contexts.
Separately, reward and informative cues did reduce RT interference. Importantly, reward differentially impacted performance depending on which form of control was to be employed. In reactive contexts, reward upregulated performance, an effect that has been seen in children, adolescents, and adults (Geier et al., 2010), by reducing RT interference. However, in proactive contexts after informative cues, reward increased RT interference. Because there are no effects on accuracy flanker effect for either reward or control strategy, increased RT interference, which isolates the effects of conflict rather than RT in general, on proactive reward trials could be interpreted as less efficient responding. These results that male adolescents may be less efficient on reward proactive trials are contrary to the few studies of rewarded proactive control in childhood and adolescence. For instance, Strang and Pollak (2014) found that, in children, adolescents, and adults, reward blocks decreased RT compared to neutral blocks in an AXCPT task and activation in fronto-parietal regions were sustained in the reward blocks compared to neutral blocks. However, they did not directly test if reward creates a behavioral shift from reactivity to proactivity using commonly employed AXCPT metrics, like the Proactive Behavioral Shift Index (PBSI) or d’ context. In another study, children, adolescents, and adults showed sustained behavioral improvements in a reward block compared to a neutral block, suggesting a sustained task set and proactive control (Jin et al., 2020). However, by reliance on a block-wise design, this cannot rule out global changes in arousal that may occur in the context of reward, which may lead to a non-specific speeding of responses. In contrast, Magis-Weinberg et al. (2019) used a trial-wise manipulation of reward and found that reward enhanced proactive control via increased frontal activity after a set of consonants but before a letter probe; however, the set of consonants was not in and of itself informative because the letter probe may or may not have been in the set. In the current study, we manipulated both the rewarding and informative nature of the cue at the trial-level, perhaps leading to our novel results that reward increases RT interference in proactive contexts. Ultimately and contrary to initial hypotheses, our results suggest that, in male adolescents, reward increases RT interference during proactive control when preparing a response based on an informative cue but facilitates reactive control by reducing RT after uninformative cues.
Additionally, there was some evidence that how reward impacts reactive control changes with age. At younger ages, there was a larger difference between the reward and nonreward conditions during reactive control. Specifically, reward trials decreased behavioral deficits in younger male adolescents compared to nonreward trials. This result is in line with some prior work that also found that children and adolescents performed better in an antisaccade task when rewarded compared to adults (Geier et al., 2010; Padmanabhan et al., 2011). Unlike the increased RT interference seen in rewarded proactive control, rewarded reactive control is enhanced via reduced RT interference and that seems to be particularly true at younger ages for male adolescents.
Not only did the current study seek to characterize the effects of reward, control, and development on cognitive control, but also aimed to explore how theta dynamics, integral in cognitive control processes, predicted task behavior in rewarded situations compared to nonrewarded situations. The neural measures of theta power and ICPS did not support reactive reward-related performance. However, in proactive contexts, increased reward-related cue theta power was associated with increased reward-related RT interference during rewarded proactive control. This effect did not change with age, indicating that this relation is seen throughout adolescence. In proactive contexts, the rewarded cues may be increasing theta power of the participant such that they become distracted and cognitive resources are pulled away from cognitive control processes, resulting in increased RT interference. Another possibility is that participants did not have enough time to accurately process what the cue indicated on informative cues: reward/nonreward and congruent/incongruent, which resulted in increased RT interference. Though the cue was presented for 1600–2000 ms, a time range that should allow adequate preparatory time after informative cues (Wendt and Kiesel, 2011), adding another mapping, of reward vs nonreward, may have overwhelmed the cognitive resources of these adolescents.
In addition to the relations between reward-related cue theta power and behavior, increased reward-related stimulus-locked theta ICPS interference, or an increased differentiation between incongruent and congruent trials on reward compared to nonreward trials, was a predictor of improved reward-related behavior for proactive trials. Additionally, this relation did not change with age, suggesting that this effect is consistent throughout adolescence. Interestingly, reward-related stimulus-locked theta ICPS interference and reward-related cue theta power were related to behavioral performance in the proactive context in opposite directions. Elevated reward-related cue theta power was associated with increased reward-related RT interference, while elevated reward-related stimulus-locked theta ICPS interference was associated with reduced reward-related RT interference. Future studies with a larger sample might test how theta oscillations during different time periods of the task interact to predict behavioral performance.
It is important to note that, though the focus of the current study was rewards’ impact on proactive and reactive control, both approach and avoidance systems can motivate behavior. The paradigm does have trial-level feedback, including “WRONG” and “TOO SLOW.” Though money was not taken away as a form of punishment, avoiding negatively-valenced feedback could also be motivating the participants’ behavior during this task and future work could assess the interplay of both reward and punishment on proactive and reactive control.
The current study is not without limitations. First, the study would benefit from a larger sample size and the inclusion of young adults in order to test possible quadratic effects, since reward sensitivity tends to peak in adolescence (Cauffman et al., 2010; Shulman et al., 2016; Steinberg et al., 2009). Second, the families of the participants have highly educated mothers and are relatively affluent, limiting the generalizability to the population at large. This study would benefit from including participants from a more diverse socioeconomic background. Individuals with lower socioeconomic status, where money and resources are scarce, may perform differently in the presence of reward. Similarly, this sample was restricted to males, so reward could influence proactive and reactive control differently in females, especially during adolescence which involves sex-specific hormone changes during puberty. Finally, future work should analyze alpha suppression after rewarded and informative cues in adolescence. Though not the focus of this investigation, alpha suppression was observed after all types of cues and could be indicative of attention processes (Foxe and Snyder, 2011; van Driel et al., 2012).
Understanding reward’s impact on cognitive control is an essential step to understanding hallmark behaviors associated with males during adolescence. For example, adolescence is marked by an increase in reward sensitivity and reward seeking behaviors, like substance use or risky sexual behavior, especially in males. Our findings that reward was associated with increased RT interference during proactive control may lend insight into potential dysfunction in reward-cognitive control interactions in adolescence, when the prevalence of risky behaviors is particularly high. In addition to being a time period associated with engaging in risky behaviors, psychopathology also begins to manifest in adolescence. The interactions between reward networks and control networks are also implicated in a variety of psychopathologies, including depression (Forbes et al., 2010). Understanding the interplay between reward and control circuitry in novel contexts (e.g., proactive vs reactive) can also elucidate novel targets for intervention in clinical populations.
5. Conclusions
In conclusion, this study examined reward’s impact on both proactive and reactive control in male adolescents to understand the interactions of reward and cognitive control during a developmental period when both systems are undergoing developmental changes. While reward reduced RT interference during reactive control, reward increased RT interference during proactive control after an informative cue in adolescent males. Further, enhanced reward-related cue theta power is associated with these reward-related increased RT interference, specifically in proactive contexts, during adolescence in males.
Data availability statement
The data that support the findings of this study are available from the corresponding author, MEB, upon reasonable request.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors would like to acknowledge the funding sources for this project: the William Hodos Dissertation Assistantship through the Neuroscience and Cognitive Science Program at the University of Maryland to MEB and the National Institutes of Health Grant # U01MH093349 to NAF.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100934.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aviyente S., Tootell A., Bernat E.M. Time-frequency phase-synchrony approaches with ERPs. Int. J. Psychophysiol. 2017;111:88–97. doi: 10.1016/j.ijpsycho.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Bowers M.E., Buzzell G.A., Bernat E.M., Fox N.A., Barker T.V. Time-frequency approaches to investigating changes in feedback processing during childhood and adolescence. Psychophysiology. 2018;55(10):e13208. doi: 10.1111/psyp.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell G.A., Barker T.V., Troller-Renfree S.V., Bernat E.M., Bowers M.E., Morales S. Adolescent cognitive control, theta oscillations, and social observation. NeuroImage. 2019;198:13–30. doi: 10.1016/j.neuroimage.2019.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E., Shulman E.P., Steinberg L., Claus E., Banich M.T., Graham S., Woolard J. Age differences in affective decision making as indexed by performance on the Iowa gambling task. Dev. Psychol. 2010;46(1):193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Cohen M.X., Allen J.J.B. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Zambrano-Vazquez L., Allen J.J.B. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49(2):220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N., Martis S.B., Curran T., Munakata Y. Metacognitive processes in executive control development: the case of reactive and proactive control. J. Cogn. Neurosci. 2015;27(6):1125–1136. doi: 10.1162/jocn_a_00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Front. Psychol. 2013;4:15. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. Reward favors the prepared: incentive and task-informative cues interact to enhance attentional control. J. Exp. Psychol. Hum. Percept. Perform. 2016;42(1):52–66. doi: 10.1037/xhp0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T., Geier C., Luna B., Pajtek S., Terwilliger R., Thatcher D., Clark D.B. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depend. 2011;115(1–2):43–50. doi: 10.1016/J.DRUGALCDEP.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Mike X. 2014. Analyzing Neural Time Series Data: Theory and Practice.http://mikexcohen.com/book/Cohen_AnalyzingNeuralTimeSeriesData_TOC.pdf Retrieved from. [Google Scholar]
- Cohen Michael X., Donner T.H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013;110(12):2752–2763. doi: 10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- Cooper P.S., Wong A.S.W., Fulham W.R., Thienel R., Mansfield E., Michie P.T., Karayanidis F. Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. NeuroImage. 2015;108:354–363. doi: 10.1016/j.neuroimage.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Cooper P.S., Darriba Á., Karayanidis F., Barceló F. Contextually sensitive power changes across multiple frequency bands underpin cognitive control. NeuroImage. 2016;132:499–511. doi: 10.1016/j.neuroimage.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Crowley M.J., van Noordt S.J.R., Wu J., Hommer R.E., South M., Fearon R.M.P., Mayes L.C. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain Cogn. 2014;89:79–89. doi: 10.1016/j.bandc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R., Buzzell G.A., Morales S., Bowers M.E., Leach S.C., Fox N.A. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57(6) doi: 10.1111/psyp.13580. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Ulug A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002;5(4):F9–F16. doi: 10.1111/1467-7687.00235. [DOI] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development: commentary. Dev. Sci. 2006 doi: 10.1111/j.1467-7687.2005.00454.x. Blackwell Publishing Ltd. [DOI] [PubMed] [Google Scholar]
- Elke S., Wiebe S.A. Proactive control in early and middle childhood: an ERP study. Dev. Cogn. Neurosci. 2017;26:28–38. doi: 10.1016/j.dcn.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–1291. doi: 10.1016/J.NEUROIMAGE.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(2):162–172. doi: 10.1016/J.JAAC.2009.11.006. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe J.J., Snyder A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2(July):154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier Charles F., Luna B. Developmental effects of incentives on response inhibition. Child Dev. 2012;83(4):1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/J.DCN.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbinaite R., van Rijn H., Cohen M.X. Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Front. Hum. Neurosci. 2014;8(September):761. doi: 10.3389/fnhum.2014.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Geier C.F., Luna B. Incentives facilitate developmental improvement in inhibitory control by modulating control-related networks. NeuroImage. 2018;172:369–380. doi: 10.1016/J.NEUROIMAGE.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Pastötter B., Bäuml K.-H., Gruber S., Wimber M., Klimesch W. The electrophysiological dynamics of interference during the stroop task. J. Cogn. Neurosci. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Harper J., Malone S.M., Bernat E.M. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clin. Neurophysiol. 2014;125(1):124–132. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C., Kastman E.K., Glenn C.R., Somerville L.H. Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nat. Commun. 2017;8(1):1605. doi: 10.1038/s41467-017-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K., Locke H.S., Braver T.S. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc. Natl. Acad. Sci. U. S. A. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Auyeung B., Chevalier N. External rewards and positive stimuli promote different cognitive control engagement strategies in children. Dev. Cogn. Neurosci. 2020;44 doi: 10.1016/j.dcn.2020.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C., Pandey A.K., Chorlian D.B., Porjesz B. The use of current source density as electrophysiological correlates in neuropsychiatric disorders: a review of human studies. Int. J. Psychophysiol. 2015;97(3):310–322. doi: 10.1016/j.ijpsycho.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M.C., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex March. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Larsen B., Verstynen T.D., Yeh F.-C., Luna B. Developmental changes in the integration of affective and cognitive corticostriatal pathways are associated with reward-driven behavior. Cereb. Cortex. 2018;28:2834–2845. doi: 10.1093/cercor/bhx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallee C.F., Meemken M.T., Herrmann C.S., Huster R.J. When holding your horses meets the deer in the headlights: time-frequency characteristics of global and selective stopping under conditions of proactive and reactive control. Front. Hum. Neurosci. 2014;8:994. doi: 10.3389/fnhum.2014.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach S.C., Morales S., Bowers M.E., Buzzell G.A., Debnath R., Beall D., Fox N.A. Adjusting ADJUST: optimizing the ADJUST algorithm for pediatric data using geodesic nets. Psychophysiology. 2020:e13566. doi: 10.1111/psyp.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.R., Huang C.-Y., Lin W., Sun C.-W.V. Gender differences in punishment and reward sensitivity in a sample of Taiwanese college students. Pers. Individ. Dif. 2007;43(3):475–483. doi: 10.1016/J.PAID.2006.12.016. [DOI] [Google Scholar]
- Liu Z.X., Woltering S., Lewis M.D. Developmental change in EEG theta activity in the medial prefrontal cortex during response control. NeuroImage. 2014;85:873–887. doi: 10.1016/j.neuroimage.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Luciana M., Collins P.F. Incentive motivation, cognitive control, and the adolescent brain: is it time for a paradigm shift? Child Dev. Perspect. 2012;6(4):392–399. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Paulsen D.J., Padmanabhan A., Geier C. The teenage brain: cognitive control and motivation. Curr. Dir. Psychol. Sci. 2013 doi: 10.1177/0963721413478416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magis-Weinberg L., Custers R., Dumontheil I. Rewards enhance proactive and reactive control in adolescence and adulthood. Soc. Cogn. Affect. Neurosci. 2019 doi: 10.1093/SCAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J.E., Aguinis H., Culpepper S.A., Chen G. Understanding and estimating the power to detect cross-level interaction effects in multilevel modeling. J. Appl. Psychol. 2012;97(5):951–966. doi: 10.1037/a0028380. [DOI] [PubMed] [Google Scholar]
- Mazaheri A., Fassbender C., Coffey-Corina S., Hartanto T.A., Schweitzer J.B., Mangun G.R. Differential oscillatory electroencephalogram between attention-deficit/ hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry. 2014;76(5):422–429. doi: 10.1016/j.biopsych.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48(2):229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Munakata Y., Snyder H.R., Chatham C.H. Developing cognitive control: three key transitions. Curr. Dir. Psychol. Sci. 2012 doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigbur R., Cohen M.X., Ridderinkhof K.R., Stürmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J. Cogn. Neurosci. 2012;24(5):1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33(46) doi: 10.1523/JNEUROSCI.1741-13.2013. http://www.jneurosci.org/content/33/46/18109.short Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Package “lme4” (2020). Retrieved from https://github.com/lme4/lme4/.
- Padmanabhan A., Geier C.F., Ordaz S.J., Teslovich T., Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev. Cogn. Neurosci. 2011;1(4):517–529. doi: 10.1016/J.DCN.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D.J., Hallquist M.N., Geier C.F., Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev. Cogn. Neurosci. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J., Steinberg L. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 2016;17:103–117. doi: 10.1016/J.DCN.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M.H., Jedd K., Luciana M. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/J.NEUROIMAGE.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Casey B. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20(2):236–241. doi: 10.1016/J.CONB.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek A., Strobach T., Schubert T. Motivational and cognitive determinants of control during conflict processing. Cogn. Emot. 2014;28(6):1076–1089. doi: 10.1080/02699931.2013.870134. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev.: DR. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Graham S., O’brien L., Woolard J., Cauffman E., Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. http://psych.colorado.edu/∼mbanich/p/AgeDiffFutureOrientation.pdf Retrieved from. [DOI] [PubMed] [Google Scholar]
- Strang N.M., Pollak S.D. Developmental continuity in reward-related enhancement of cognitive control. Dev. Cogn. Neurosci. 2014;10:34–43. doi: 10.1016/j.dcn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke C.E., Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol. 2012;123(12):2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R., Avila C., Molto J., Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers. Individ. Differ. 2001;31(6):837–862. doi: 10.1016/S0191-8869(00)00183-5. [DOI] [Google Scholar]
- Troller‐Renfree S.V., Buzzell G.A., Fox N.A. Changes in working memory influence the transition from reactive to proactive cognitive control during childhood. Dev. Sci. 2020 doi: 10.1111/desc.12959. [DOI] [PubMed] [Google Scholar]
- van Driel J., Ridderinkhof K.R., Cohen M.X. Not all errors are alike: theta and alpha EEG dynamics relate to differences in error-processing dynamics. J. Neurosci. 2012;32(47):16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel J., Swart J.C., Egner T., Ridderinkhof K.R., Cohen M.X. (No) time for control: frontal theta dynamics reveal the cost of temporally guided conflict anticipation. Cogn. Affect. Behav. Neurosci. 2015;15(4):787–807. doi: 10.3758/s13415-015-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemel-Ruiter M.E., de Jong P.J., Ostafin B.A., Wiers R.W. Reward sensitivity, attentional bias, and executive control in early adolescent alcohol use. Addict. Behav. 2015;40:84–90. doi: 10.1016/J.ADDBEH.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A.R.B., Westenberg P.M., Crone E.A. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/J.NEUROIMAGE.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wendt M., Kiesel A. Conflict adaptation in time: foreperiods as contextual cues for attentional adjustment. Psychon. Bull. Rev. 2011;18(5):910–916. doi: 10.3758/s13423-011-0119-4. [DOI] [PubMed] [Google Scholar]
- Zhai Z.W., Pajtek S., Luna B., Geier C.F., Ridenour T.A., Clark D.B. Reward-modulated response inhibition, cognitive shifting, and the orbital frontal cortex in early adolescence. J. Res. Adolesc. 2015;25(4):753–764. doi: 10.1111/jora.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MEB, upon reasonable request.